Abstract
Purpose
To evaluate the diagnostic yield and concordance of color duplex ultrasound (CDU) of the superficial temporal artery (STA), temporal artery biopsy (TAB), and American College of Rheumatology (ACR) criteria in the diagnosis of giant cell arteritis (GCA).
Methods
Prospective, masked study of all patients evaluated in one institution suspected of having GCA. All patients with a suspected diagnosis of GCA were admitted for pulsed intravenous corticosteroids. Patients underwent serologic work-up and ACR criteria were documented. All patients had a CDU and TAB performed within 3 days of initiation of systemic corticosteroid therapy. Main outcome measure: Concordance of CDU and TAB. Secondary outcome measures: Concordance between unilateral and bilateral CDU and TAB by side and segment, concordance between TAB and ACR criteria, and statistical analysis of serologic markers for GCA.
Results
The diagnosis of biopsy-proven GCA was found in 14 of 71 (19.7%) patients. The sensitivity of CDU compared to the reference standard of TAB ranged between 5.1% and 30.8% depending on the signs studied on CDU and correlation of specific TAB parameters. Of the serologic studies, a platelet count threshold of 400,000μL had the highest positive (18.32) and lowest negative (0.37) likelihood ratios for a diagnosis of GCA.
Conclusions
In this study, CDU showed minimal value in diagnosing GCA compared to TAB. There was poor correlation between CDU results and ACR criteria for GCA. The threshold platelet count had higher positive and negative predictive values for GCA than CDU and is a useful serologic marker for GCA.
INTRODUCTION
Giant cell arteritis (GCA) is a systemic vasculitis with the potential for severe, irreversible loss of vision. The prevalence of GCA is 19.6–27.9 per 100,000 persons, but varies markedly depending on age, sex, race, and ethnicity.1–3 The disease affects individuals over the age of 50 years, peaking between 70–80 years.3–5 One half of all patients with GCA will present with ocular symptoms, usually visual loss (88.6%); of note, in one series of 170 patients with biopsy-proven GCA, visual loss occurred in 48.8%.6 Other studies have found an incidence of visual loss in GCA between 8 and 70%.7–17 At present, a long course of corticosteroids, lasting months to years, is the only therapy and has been proven to be effective in preventing contralateral visual loss at correct dosages and durations;6,18–20 one study noted a mean of 7.5 months needed to taper a patient’s prednisone dose to 5 mg/d.19 Significant adverse effects occur regularly, especially in the elderly population. Proven and colleagues reported at least one adverse event in 86% of patients undergoing corticosteroid therapy for GCA, with diabetes mellitus noted in 9%, fracture in 38%, infection in 31%, and gastrointestinal bleeding in 4%.19 Nesher and associates reported that 58% of patients with GCA developed at least one major corticosteroid-related adverse event over a 3 year period.21 Correct diagnosis is therefore of paramount importance to both treat a patient with GCA in a timely fashion6 and to avoid corticosteroids in patients without GCA.3,19,22,23
Several studies have attempted to define an accurate diagnostic definition for GCA based on a variety of parameters,4,11,24,25 including the American College of Rheumatology (ACR) classification criteria,26 temporal artery biopsy (TAB),27 and/or color duplex ultrasound (CDU) of the superficial temporal artery (STA).28
The current gold standard for the diagnosis of GCA is superficial temporal artery biopsy of adequate length,18,28–33 a reasonably straightforward surgical procedure with a sensitivity ranging between 76 and 100%, a specificity approaching 100%, and a low complication rate, especially when the parietal branch is biopsied.3,7,22,23,27,28,34–37 The procedure can be safely performed in either an office or ambulatory surgery center and no cessation of anticoagulant or antiplatelet therapy is necessary, minimizing the exacerbation of other comorbidities.38 There are potential drawbacks to TAB, including surgical complications, difficulty in timely scheduling of the procedure, and discordance between sides, among others.22
Alternative methods have been proposed in the hopes of circumventing the need for TAB in the diagnosis of GCA. In 1995, Schmidt and colleagues reported the first use of color duplex ultrasound (color Doppler ultrasound, CDU) for the diagnosis of GCA, describing a perivascular, hypoechoic “halo sign” around the STA,39 possibly representing periarterial edema secondary to vascular inflammation.39,40 Other ultrasonographic findings, including arterial stenosis and occlusion, have also been studied in GCA.40 In a subsequent study, Schmidt and co-workers reported a halo sign sensitivity of 76% and a specificity of 92% for the diagnosis of GCA when compared to TAB.40 When the three ultrasonographic signs (halo sign, stenosis, occlusion) were combined, a sensitivity of 76% (vs 95%) and a specificity of 92% (vs 85%) were noted when compared to TAB (of note, cases of “biopsy-negative GCA” were included in the analysis).40 However, stenosis and occlusion also occur in patients with atherosclerosis and no histopathologic evidence of GCA,41–44 with some authors concluding that only the halo sign should be used as an accurate sign of GCA.43–45
Studies on the value of CDU in the diagnosis of GCA have heterogeneous results. One recurring difficulty in analyzing the efficacy of CDU in the diagnosis of GCA is lack of a standardized methodology.28 Many studies are small or retrospective with limitations in available data for analysis.46,47 Some series are not masked or only partially masked.40,41,43,48 Other studies lack important specifics, including a strict definition of GCA,41,43,49–51 length of corticosteroid therapy prior to CDU and TAB,30,43,52 unrecorded or widely varying length of TAB obtained,40,41,43,49,50,52 correlation between segment of positive CDU and TAB,53 experience of the ultrasonographer and interpreting radiologist,41 and continuation or cessation of corticosteroids depending on result of CDU, TAB, and ACR criteria.
In this series, we studied three diagnostic modalities (TAB, CDU, ACR criteria) in a prospective, masked fashion and their concordance was analyzed. The hypothesis for this thesis is that CDU correlates with TAB results for the diagnosis of GCA. Secondary outcomes include concordance between unilateral and bilateral TAB and CDU for side and segment, correlation between CDU and ACR, and a statistical analysis of serologic markers for GCA with CDU/TAB/ACR.
MATERIALS AND METHODS
After Institutional Review Board approval was obtained, the study was conducted in accordance with the principles of the Declaration of Helsinki and conformed to Health Insurance Portability and Accountability Act regulations. All patients with suspected GCA over a 14-month period who were admitted for pulsed intravenous corticosteroid therapy were approached prospectively for possible inclusion in the study and informed consent was obtained. Pulsed intravenous corticosteroid therapy (methylprednisolone 250mg every 6 hours for 12 doses) was initiated immediately on admission. Demographic, clinical, serologic (Westergren erythrocyte sedimentation rate (ESR), c-reactive protein (CRP), platelet count), and ACR criteria data were collected at baseline. Interleukin-6 (IL-6) was also measured in some patients. CDU was performed within 3 days of admission. TAB was performed on the same day as the CDU. Patients were excluded if they had received corticosteroid therapy within 6 months of presentation, did not have CDU within 3 days of starting corticosteroids, or if the TAB was not performed on the same day as the CDU.
AMERICAN COLLEGE OF RHEUMATOLOGY CLASSIFICATION CRITERIA
The American College of Rheumatology 1990 criteria26 specify that the diagnosis of GCA may be made when patients meet 3 of the 5 parameters:
Age over 50 years;
New onset of localized headache;
Temporal artery tenderness or decreased temporal artery pulse;
Erythrocyte sedimentation rate greater than or equal to 50 mm/hr;
A positive temporal artery biopsy.
These five data points were collected prospectively on each patient. After TAB, if three or more of the ACR criteria were present, the patient was considered “ACR positive”; if two or fewer of the criteria were present, the patient was labeled “ACR negative”.
COLOR DUPLEX ULTRASOUND
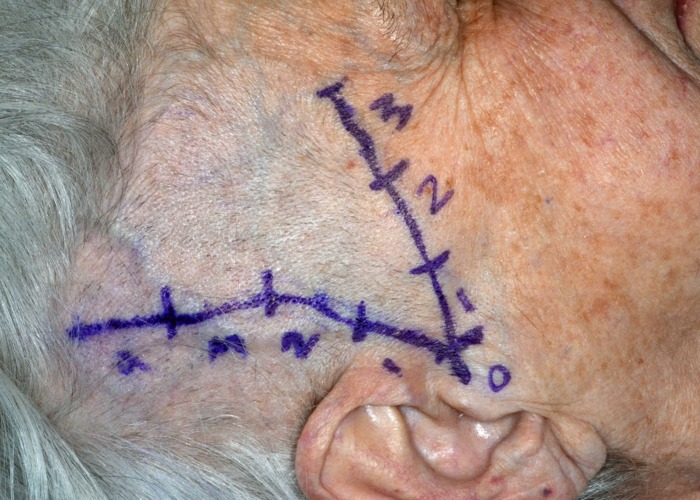
All patients included in the study underwent bilateral CDU of the STAs to evaluate each side by segment for the halo sign, stenosis, and/or occlusion. With the patient in a supine position, mapping and marking of segments were performed by an oculoplastic surgery fellow in conjunction with the ultrasonographer prior to the CDU. Marking was performed on both sides for the main trunk of the STA as well as both the frontal and parietal branches using the CDU and a standard surgical marking pen, beginning just anterior to the tragus of the ear (Figure 1).
FIGURE 1.
Clinical photograph of a patient after marking for CDU and TAB. CDU=Color duplex ultrasonography; TAB=temporal artery biopsy.
The first 2-centimeter segment was labeled as segment 0, with each subsequent 2-centimeter segment labeled from 1 to 3 or 4, depending on the length of STA that could be studied. A certified ultrasonographer with experience in head and neck vascular ultrasonography using a high frequency (linear 10–17 MHz) transducer for adequate penetration performed CDU. Multiple long and short axis images were obtained using grey scale, color Doppler, and spectral Doppler modes along the main trunk and both branches of the STA for at least 5 cm on each side. Although the ultrasonographer was aware of the concern for GCA, he/she was blinded to all clinical and serologic findings. One attending radiologist (LN) specializing in vascular ultrasonography and blinded to the clinical, serologic, and histopathologic findings interpreted all studies. CDU results were noted for each STA by side and segment, including any areas of hyperechoic wall thickening (halo sign), vessel stenosis, or vessel occlusion.
TEMPORAL ARTERY BIOPSY
All TABs were performed in the same fashion by one of two oculoplastic surgeons (JRB, APM) blinded to the CDU findings and the results of ACR criteria. TABs were performed within hours of the CDU in all patients. The procedure was performed in the operating room under intravenous sedation using lidocaine with epinephrine as local anesthetic infiltration. Any antiplatelet and anticoagulant therapies were continued normally and did not delay surgery. The previously described mapped course of the STAs was used in all cases. The details of the surgical technique are summarized in a previous publication.38 Each marked segment of the STA was removed and labeled for separate histopathologic evaluation. Prefixation length was measured for each excised segment,54 with at least 2cm total length excised on each side. The length of each segment excised was recorded prefixation. Bilateral biopsies were attempted in all patients.
HISTOPATHOLOGY
The diagnosis of GCA was made based on the histopathologic findings on TAB. The histopathology was evaluated by one ophthalmic pathologist (RCE) with extensive experience in the assessment and diagnosis of TAB specimens. The pathologist was masked to the clinical, serologic, and ultrasonographic findings. All specimens were at least 19mm in total postfixation length per side. Each segment of STA was sectioned transversely in a “breadloaf” fashion into 8 or more segments that were processed routinely for light microscopy, embedded in single paraffin block, and stained with hematoxylin-eosin and periodic acid-Schiff. Three slides containing several levels cut from each block were prepared. In selected cases, additional levels were prepared. Histopathologic criteria for the diagnosis of GCA included the presence of chronic granulomatous inflammation in the vessel wall with presence of epithelioid histiocytes, segmental loss or disruption of internal elastic lamina, compromise and/or obliteration of the arterial lumen, atrophy and scarring of the muscularis, and adventitial fibrosis. Giant cells were not required for the diagnosis.
CORTICOSTEROID THERAPY
After completion of 12 doses of pulsed intravenous therapy, oral corticosteroids were continued in patients with a positive TAB, regardless of the ACR and CDU results. Corticosteroids were stopped in all patients with a negative TAB, regardless of the ACR and CDU results.
STATISTICAL METHODS
The performance of CDU in diagnosing GCA as determined by TAB and by ACR criteria was evaluated using sensitivity, specificity, predictive values, likelihood ratios, and area under the receiver operating characteristics curve (ROC AUC). For patient level data, estimates and exact 95% confidence intervals (CIs) were calculated using the diagt procedure in Stata 14 (StataCorp LP, College Station, TX). For side-level data, estimates and 95% CIs were calculated using logistic random effects models to account for correlation among sides from the same subject.55 Logistic random effects models were fit using SAS PROC GLIMMIX in SAS/STAT 13.2 (SAS Institute, Cary, NC). The results of serologic testing were analyzed in an identical fashion.
RESULTS
Seventy-three patients were enrolled in the study and data from 71 patients with 131 TABs was included in the analysis. One patient was excluded because the CDU and TAB were not performed on the same day. One patient was excluded after the patient recalled having had corticosteroid therapy prior to admission. Demographic data is summarized in Table 1. The average age was 73 years (standard deviation = 8.5). Patients were mostly female (76.1%). The race/ethnicity distribution was mostly white (76.1%). Primary symptoms are listed in Table 2a; 49.3% of patients presented with visual loss as their primary complaint. The results of pupillary testing on presentation are listed in Table 2b, with an equal incidence of afferent pupillary defects in TAB positive and negative patients. All patients underwent CDU and TAB within a mean of 1.18 days of beginning pulsed intravenous corticosteroids (median 0 days, range 0–3 days). All patients underwent CDU and TAB on the same day. Sixty-three patients had bilateral biopsy results. The remaining 8 patients had only unilateral tissue sampling despite attempted bilateral biopsies. The length of each STA segment is summarized in Table 3. At least 2cm54 total postfixation length of the STA was obtained in all but one case (19mm) in which the contralateral artery could not be identified. There were no postoperative complications. The discordance rate between TAB sides was 1.6% (1/63).
TABLE 1.
DEMOGRAPHIC DATA
| VARIABLE | ALL PATIENTS (N=71) | TAB POSITIVE PATIENTS (N=14) | TAB NEGATIVE PATIENTS (N=57) |
|---|---|---|---|
| Age in years (mean, median, range) | 73.1, 72.0, 55–90 | 75.79, 76, 58–85 | 72.39, 71, 55–90 |
| Female sex | 76.1% | 85.7% | 73.7% |
| Ethnicity (number and %) | |||
| White | 54 (76.1) | 12 (85.7) | 42 (73.7) |
| Black | 12 (16.9) | 2 (14.3) | 10 (17.5) |
| Hispanic/Latino | 4 (5.6) | 0 (0) | 4 (7.0) |
| Asian | 1 (1.4) | 0 (0) | 1 (1.8) |
| Other | 0 (0) | 0 (0) | 0 (0) |
TABLE 2A.
PRIMARY PRESENTING SYMPTOMS
| PRESENTING SYMPTOM | NUMBER (%) |
|---|---|
| Vision loss | 35 (49.3) |
| Headache | 14 (19.7) |
| Blurred vision | 11 (15.5) |
| Diplopia | 11 (15.5) |
TABLE 2B.
AFFERENT PUPILLARY DEFECT ON CLINICAL PRESENTATION.
| TAB POSITIVE (N=14) | TAB NEGATIVE (N=57) | |
|---|---|---|
| Afferent Pupillary Defect Present | 8 | 28 |
| Afferent Pupillary Defect Not Present | 6 | 29 |
Fisher’s two-tailed test P=.7668
TABLE 3.
POSTFIXATION LENGTH OF TEMPORAL ARTERY BIOPSY BY SEGMENT.
| LOCATION OF TAB | RIGHT SEGMENT 1 | RIGHT SEGMENT 2 | RIGHT SEGMENT 3 | RIGHT TOTAL | LEFT SEGMENT 1 | LEFT SEGMENT 2 | LEFT SEGMENT 3 | LEFT TOTAL | TOTAL (RIGHT + LEFT)* |
|---|---|---|---|---|---|---|---|---|---|
| Mean (mm) | 14.79 | 13 | 14.71 | 29 | 14.61 | 9.5 | 14.13 | 26.82 | 56 |
| Median (mm) | 15 | 13 | 13.75 | 28.5 | 13 | 9.5 | 13.5 | 27 | 57 |
| Range (mm) | 10–28 | 16–22 | 9–25 | 19–50 | 9–29 | 9–10 | 9–21 | 18–43 | 34–93 |
Includes only cases with successful bilateral TABs
Corticosteroids were continued in all patients with positive TAB results and discontinued in all patients with negative TAB results, regardless of the CDU and ACR criteria results; no patient who discontinued corticosteroids experienced progressive visual loss.
Of the 71 patients, 14 (19.7%) were found to have biopsy-confirmed GCA. Frequencies of CDU findings compared to TAB findings are summarized in Tables 4a–d and 5a–d. Table 4e presents the performance of the diagnosis from CDU when any of three signs (halo, stenosis, occlusion) were present against the reference standard of TAB. Table 5e presents the performance of only the halo sign on CDU compared to TAB. When any abnormality on CDU was found, the sensitivity and specificity across all patients was 28.6% (95% CI 8.4, 58.1) and 87.7% (95% CI 76.3, 94.9), respectively, and 30.8% (95% CI 9.1, 61.4) and 86.0% (95% CI 73.3, 94.2), respectively, in patients who underwent bilateral TABs. When only the halo sign was considered, the sensitivity and specificity across all patients was 14.3% (95% CI 1.8, 42.8) and 96.5 (95% CI 87.9, 99.6), respectively, and 15.4% (95% CI 1.9, 45.4) and 96.0% (95% CI 86.3, 99.5), respectively, in patients who underwent bilateral TABs. Side-level and segment level correlation for CDU and TAB results as sensitivity and specificity are also listed, with lower sensitivities but higher specificities when compared to all patients and bilateral TABs. A comparison with previously two published meta-analyses is summarized in Table 6.
TABLE 4A.
PATIENT LEVEL DATA (N=71).
| CDU POSITIVE (N=11) | CDU NEGATIVE (N=60) | |
|---|---|---|
| TAB Positive (N=14) | 4 | 10 |
| TAB Negative (N=57) | 7 | 50 |
Positive CDU defined as any positive CDU finding
TABLE 4B.
PATIENT LEVEL DATA FOR BILATERAL TABS ONLY (N=63).
| CDU POSITIVE (N=11) | CDU NEGATIVE (N=52) | |
|---|---|---|
| TAB Positive (N=13) | 4 | 9 |
| TAB Negative (N=50) | 7 | 43 |
Positive CDU defined as any positive CDU finding.
TABLE 4C.
RESULTS BY SIDE (N=134).
| CDU POSITIVE (N=17) | CDU NEGATIVE (N=117) | |
|---|---|---|
| TAB Positive (N=26) | 6 | 20 |
| TAB Negative (N=108) | 11 | 97 |
Positive CDU defined as any positive CDU finding.
Fisher’s exact test P=.0149.
TABLE 4D.
RESULTS BY SEGMENT (N=252).
| CDU POSITIVE (N=17) | CDU NEGATIVE (N=235) | |
|---|---|---|
| TAB Positive (N=44) | 7 | 37 |
| TAB Negative (N=208) | 10 | 198 |
Positive CDU defined as any positive CDU finding.
Fisher’s exact test P=.0149.
TABLE 5A.
CDU (HALO SIGN ONLY) VS. TAB. PATIENT LEVEL DATA (N=71)
| HALO POSITIVE (N=4) | HALO NEGATIVE (N=67) | |
|---|---|---|
| TAB Positive (N=14) | 2 | 12 |
| TAB Negative (N=57) | 2 | 55 |
TABLE 5B.
CDU (HALO SIGN ONLY) VS. TAB. PATIENT LEVEL DATA (BILATERAL TABS ONLY, N=63)
| HALO POSITIVE (N=4) | HALO NEGATIVE (N=59) | |
|---|---|---|
| TAB Positive (N=13) | 2 | 11 |
| TAB Negative (N=50) | 2 | 48 |
TABLE 5C.
CDU (HALO SIGN ONLY) VS. TAB. RESULTS BY SIDE (N=134)
| HALO POSITIVE (N=5) | HALO NEGATIVE (N=129) | |
|---|---|---|
| TAB Positive (N=26) | 3 | 23 |
| TAB Negative (N=108) | 2 | 106 |
TABLE 5D.
CDU (HALO SIGN ONLY) VS. TAB
| HALO POSITIVE (N=5) | HALO NEGATIVE (N=247) | |
|---|---|---|
| TAB Positive (N=44) | 3 | 41 |
| TAB Negative (N=208) | 2 | 206 |
Results by segment (N=252).
Fisher’s exact test P=.0383.
TABLE 4E.
CDU PERFORMANCE (ANY ABNORMALITY) AT PATIENT, SIDE, AND SEGMENT LEVEL COMPARED TO TAB RESULT.
| PATIENT LEVEL ALL PATIENTS | PATIENT LEVEL BILATERAL | SIDE LEVEL | SEGMENT LEVEL | ||
|---|---|---|---|---|---|
| Sensitivity (%) (CI) | 28.6 (8.4,58.1) | 30.8 (9.1, 61.4) | 19.8 (5.7, 50.5) | 12.5 (3.5, 35.7) | |
| Specificity (%) (CI) | 87.7 (76.3,94.9) | 86.0 (73.3, 94.2) | 92.2 (83.7, 96.5) | 95.9 (91.8, 98.0) | |
| Positive predictive value (%) | (CI) | 36.4 (10.9,69.2) | 36.4 (10.9,69.2) | 32.6 (6.1,78.2) | 35.4 (6.6, 81.0) |
| Negative predictive value (%) (CI) | 83.3 (71.5,91.7) | 82.7 (69.7, 91.8) | 86.7 (75.8, 93.1) | 91.8 (83.7, 96.1) | |
| Positive likelihood ratio (CI) | 2.33 (0.79, 6.85) | 2.20 (0.76, 6.38) | 2.56 (1.55, 4.22) | 3.06 (1.99, 4.69) | |
| Negative likelihood ratio (CI) | 0.81 (0.58, 1.15) | 0.81 (0.55, 1.18) | 0.87 (0.69, 1.10) | 0.91 (0.78, 1.07) | |
| ROC AUC (CI) | 0.58 (0.45, 0.71) | 0.58 (0.45, 0.72) | 0.56 (0.45, 0.67) | 0.54 (0.47, 0.61) | |
CI = Confidence interval.
All confidence intervals at 95%.
ROC AUC = Area under the receiver operating characteristic curve.
TABLE 5E.
CDU PERFORMANCE (HALO SIGN ONLY) AT PATIENT, SIDE, AND SEGMENT LEVEL COMPARED TO TAB RESULT
| PATIENT LEVEL ALL PATIENTS | PATIENT LEVEL-BILATERAL | SIDE LEVEL | SEGMENT LEVEL | |
|---|---|---|---|---|
| Sensitivity (%) (CI) | 14.3 (1.8, 42.8) | 15.4 (1.9, 45.4) | 8.8 (1.6, 37.0) | 5.1 (0.9, 24.0) |
| Specificity (%) (CI) | 96.5 (87.9, 99.6) | 96.0 (86.3, 99.5) | 98.1 (92.7, 99.6) | 99.0 (96.2, 99.8) |
| Positive predictive value (%) (CI) | 50.0 (6.8, 93.2) | 50.0 (6.8, 93.2) | 56.0 (0, 100) | 56.0 (0, 100) |
| Negative predictive value (%) (CI) | 82.1 (70.8, 90.4) | 81.4 (69.1, 90.3) | 86.5 (76.0, 92.9) | 92.0 (83.7, 96.2) |
| PATIENT LEVEL ALL PATIENTS | PATIENT LEVEL BILATERAL | SIDE LEVEL | SEGMENT LEVEL | |
|
| ||||
| Positive likelihood ratio (CI) | 4.1 (0.6, 26.4) | 3.85 (0.6, 24.8) | 4.76 (2.96, 7.68) | 5.31 (3.60, 7.82) |
| Negative likelihood ratio (CI) | 0.89 (0.71, 1.11) | 0.88 (0.69, 1.12) | 0.93 (0.81, 1.07) | 0.96 (0.88, 1.04) |
| ROC AUC (CI) | 0.55 (0.46, 0.65) | 0.56 (0.45, 0.66) | 0.53 (0.47, 0.60) | 0.52 (0.48, 0.56) |
CI = Confidence interval. All confidence intervals at 95%.
ROC AUC = Area under the receiver operating characteristic curve.
TABLE 6.
COMPARISON OF DATA BETWEEN PRESENT STUDY AND TWO META-ANALYSES
| SENSITIVITY (95%CI) | SPECIFICITY (95%CI) | ISH | PPV (95%CI) | NPV (95%CI) | LR+ (95%CI) | LR− (95%CI) | ROC AREA (95%CI) | |
|---|---|---|---|---|---|---|---|---|
| Present study (63 patients with bilateral TABs) | ||||||||
| CDU (halo) vs TAB | 15 (2, 45) | 96 (86, 100) | N/A | 50 (7, 93) | 81 (69, 90)) | 3.85 (0.6, 24.8) | 0.88 (0.69, 1.12) | 0.56 (0.45, 0.66) |
| CDU (any abnormality) vs TAB | 31 (9, 61) | 86 (73, 94) | N/A | 36 (11, 69) | 83 (70, 92) | 2.20 (0.76, 6.38) | 0.81 (0.55, 1.18) | 0.58 (0.45, 0.72) |
| CDU (halo) vs post-biopsy ACR | 3 (0, 16) | 90 (74, 98) | N/A | 25 (1, 81) | 48 (34, 61) | 0.32 (0.04, 2.94) | 1.07 (0.94, 1.22) | 0.47 (0.41, 0.53) |
| CDU (any abnormality) vs post-biopsy ACR | 9 (2, 25) | 74 (55, 88) | N/A | 27 (6, 61) | 44 (31, 59) | 0.36 (0.11, 1.24) | 1.22 (0.97, 1.55) | 0.42 (0.32, 0.51) |
| Karassa and coworkers | ||||||||
| CDU (halo) vs TAB (14 studies, 532 patients) | 69 (57, 79) | 82 (75, 87) | Significant | NR | NR | NR | NR | NR |
| CDU (any abnormality) vs TAB (7 studies, 332 patients) | 88 (74, 95) | 78 (71, 84) | None | NR | NR | NR | NR | NR |
| CDU (halo) vs ACR (7 studies, 1092 patients) | 55 (36, 73) | 94 (82, 98) | Significant | NR | NR | NR | NR | NR |
| CDU (any abnormality) vs ACR (3 studies, 853 patients) | 87 (80, 91) | 96 (89, 98) | None | NR | NR | NR | NR | NR |
| Ball coworkers | ||||||||
| CDU halo vs TAB (9 studies, 357 patients) | 75 (67, 82) | 83 (78, 88) | None | NR | NR | NR | NR | NR |
| CDU (any abnormality) vs TAB (9 studies, 397 patients) | 83 (77,89) | 82 (77, 87) | Significant | NR | NR | NR | NR | NR |
| CDU halo vs ACR (6 studies, 401 patients) | 69 (60, 77) | 89 (84,92) | Significant | NR | NR | NR | NR | NR |
| CDU (any abnormality) vs ACR (7 studies, 571 patients) | 78 (72, 84) | 88 (84, 91) | Significant | NR | NR | NR | NR | NR |
ISH = Interstudy heterogeneity.
The data and analysis of post-biopsy ACR criteria and TAB result are summarized in Table 7a–d. The sensitivity and specificity of the ACR criteria vs TAB were 85.7% (95% CI 57.2, 98.2) and 57.9% (95% CI 44.1, 70.9), respectively, for all patients and 84.6% (95% CI 54.6, 98.1) and 58% (43.2, 71.8), respectively, for patients who had successful bilateral TABs.
TABLE 7A.
POST-BIOPSY ACR VS. TAB. PATIENT LEVEL DATA (N=71)
| ACR POSITIVE (N=36) | ACR NEGATIVE (N=35) | |
|---|---|---|
| Biopsy Positive (N=14) | 12 | 2 |
| Biopsy Negative (N=57) | 24 | 33 |
TABLE 7B.
POST-BIOPSY ACR VS. TAB. PATIENT LEVEL DATA (BILATERAL TABS ONLY, N=63)
| ACR POSITIVE (N=32) | ACR NEGATIVE (N=31) | |
|---|---|---|
| Biopsy Positive (N=13) | 11 | 2 |
| Biopsy Negative (N=50) | 21 | 29 |
TABLE 7C.
POST-BIOPSY ACR VS. TAB. RESULTS BY SIDE (N=134)
| ACR POSITIVE (N=68) | ACR NEGATIVE (N=66) | |
|---|---|---|
| Biopsy Positive (N=26) | 22 | 4 |
| Biopsy Negative (N=108) | 46 | 62 |
TABLE 7D.
POST-BIOPSY ACR PERFORMANCE AT PATIENT, SIDE, AND SEGMENT LEVEL COMPARED TO TAB RESULT.
| PATIENT LEVEL ALL PATIENTS | PATIENT LEVEL BILATERAL | SIDE-LEVEL | SEGMENT-LEVEL | |
|---|---|---|---|---|
| Sensitivity (%) (CI) | 85.7 (57.2,98.2) | 84.6 (54.6,98.1) | 90.2 (55.6,98.5) | 94.0 (65.4,99.2) |
| Specificity (%) (CI) | 57.9 (44.1,70.9) | 58.0 (43.2,71.8) | 59.4 (41.9,74.8) | 60.2 (38.3,78.6) |
| Positive predictive value (%)(CI) | 33.3 (18.6,51.0) | 34.4 (18.6,53.2) | 27.8 (13.4,49.0) | 19.6 (7.7,41.8) |
| Negative predictive value (%)(CI) | 94.3 (80.8,99.3) | 93.5 (78.6,99.2) | 96.4 (85.3,99.2) | 98.0 (91.6,99.6) |
| Positive likelihood ratio (CI) | 2.04 (1.40,2.95) | 2.01 (1.35,3.00) | 2.22 (1.63,3.03) | 2.36 (1.65,3.37) |
| Negative likelihood ratio (CI) | 0.25 (0.07,0.91) | 0.27 (0.07,0.97) | 0.16 (0.09,0.29) | 0.10 (0.06,0.17) |
|
| ||||
| PATIENT LEVEL ALL PATIENTS | PATIENT LEVEL BILATERAL | SIDE-LEVEL | SEGMENT-LEVEL | |
|
| ||||
| ROC AUC (CI) | 0.72 (0.60,0.83) | 0.71 (0.59,0.84) | 0.75 (0.63,0.86) | 0.77 (0.65,0.89) |
CI = Confidence interval. All confidence intervals at 95%.
ROC AUC = Area under the receiver operating characteristic curve.
Analysis of serologic results is listed in Tables 10–12. There was no statistically significant difference in the mean ESR and CRP values between TAB-positive and TAB-negative patients. However, a statistically significant difference in platelet count and IL-6 was noted between these two groups (Table 11). No significant difference in ESR, CRP, platelet count, or IL-6 was found between patients who were CDU-positive and CDU-negative, or ACR-positive and ACR-negative.
TABLE 10.
ANALYSIS OF SEROLOGIC RESULTS.
| ESR MEAN (MEDIAN, RANGE), n | CRP MEAN (MEDIAN, RANGE), n | PLATELETS MEAN (MEDIAN, RANGE), n | IL-6 MEAN (MEDIAN, RANGE), n | |
|---|---|---|---|---|
| All patients | 48.75 (48.0, 5–108), 71 | 3.09 (1.3, 0.1–24.35), 71 | 283.81 (263, 2.47–502), 71 | 4.64 (2.65, 0.31–17.92), 48 |
| TAB + | 53.64 (45, 9–105),14 | 3.59 (2.65, 0.1–10.16), 14 | 394.14 (408.5, 220–500), 14 | 9.09 (9.61, 0.31–16.0), 11 |
| TAB− | 47.54 (45, 5–108), 57 | 2.97 (1.2, 0.1–24.35), 57 | 256.71 (252, 2.47–502), 57 | 3.32 (1.98, 0.31–17.92), 37 |
| CDU+ | 58.09 (65, 24–90), 11 | 3.16 (2.3, 0.31–7.8), 11 | 289 (241, 171–441), 11 | 7.43 (7.96, 171–12.27), 6 |
| CDU− | 47.03 (40, 5–108), 60 | 3.08 (1.2, 0.1–24.35), 60 | 282.86 (267, 2.47–502), 60 | 4.24 (1.26, 0.31–17.92), 42 |
| ACR+ | 52.31 (54, 6–108), 36 | 2.93 (1.6, 0.1–10.16), 36 | 308.26 (297, 2.47–500), 36 | 5.49 (3.35, 0.31–17.92), 25 |
| ACR− | 45.09 (40, 5–108), 35 | 3.26 (0.9, 0.1–24.35), 35 | 258.66 (229, 100–502), 35 | 3.71 (1.83, 0.55–12.96), 23 |
ESR = Westergren erythrocyte sedimentation rate
CRP = c-reactive protein
IL-6 = interleukin-6
TAB = temporal artery biopsy
CDU = color duplex ultrasonography
ACR = American College of Rheumatology criteria.
All platelets values in thousands. Normal ranges: ESR = 0–22mm
CRP = <3.0mg/dL; platelets = 150,000–450,000/μL
IL-6 = 0.31–5.00pg/mL.
TABLE 11.
P VALUES BETWEEN DIAGNOSTIC MODALITIES FOR MEAN SEROLOGIC RESULTS.
| ESR | CRP | PLATELETS | IL-6 | |
|---|---|---|---|---|
| TAB+ vs TAB− | 0.4953 | 0.6158 | <0.0001 | <0.0001 |
| TAB+ vs CDU+ | 0.7171 | 0.7236 | 0.0088 | 0.4694 |
| TAB+ vs CDU− | 0.4817 | 0.6762 | 0.0004 | 0.0021 |
| TAB+ vs ACR+ | 0.8994 | 0.4569 | 0.0101 | 0.0473 |
| TAB+ vs ACR− | 0.3513 | 0.8241 | <0.0001 | 0.0009 |
| CDU+ vs CDU− | 0.2595 | 0.9547 | 0.8565 | 0.1020 |
| ACR+ vs ACR− | 0.3095 | 0.7404 | 0.0405 | 0.1703 |
P values <0.05 in bold.
ESR = Westergren erythrocyte sedimentation rate;
CRP = c-reactive protein
IL-6 = interleukin-6
TAB = temporal artery biopsy
CDU = color duplex ultrasonography
ACR = American College of Rheumatology criteria.
All platelets values in thousands.
TABLE 12.
STATISTICAL COMPARISONS BETWEEN ABNORMAL SEROLOGIES AND POSITIVE TAB.
| ABNORMAL SEROLOGY | SENSITIVITY (%) | SPECIFICITY (%) | PPV (%) | NPV (%) | POSITIVE LIKELIHOOD RATIO | NEGATIVE LIKELIHOOD RATIO |
|---|---|---|---|---|---|---|
| ESR (≥50mm/hr) | 57.14 | 56.14 | 24.24 | 84.21 | 1.30 | 0.76 |
| ESR (Miller) | 57.14 | 43.86 | 20.00 | 80.65 | 1.02 | 0.98 |
| CRP (>3.0mg/dL) | 57.14 | 73.68 | 34.78 | 87.50 | 2.17 | 0.58 |
| Platelets (>400,000/μL) | 64.29 | 96.49 | 81.82 | 91.67 | 18.32 | 0.37 |
| IL-6 (>5.00pg/mL) | 45.45 | 94.59 | 71.43 | 85.37 | 8.41 | 0.58 |
| ESR or CRP | 78.57 | 43.86 | 25.58 | 89.29 | 1.40 | 0.49 |
| ESR (Miller) or CRP | 71.43 | 35.09 | 21.28 | 83.33 | 1.10 | 0.81 |
| CRP or plts | 78.57 | 71.93 | 40.74 | 93.18 | 2.80 | 0.30 |
| Plts or IL-6 | 85.71 | 71.93 | 42.86 | 95.35 | 3.05 | 0.20 |
| CPR or IL-6 | 85.71 | 75.44 | 46.15 | 95.56 | 3.49 | 0.19 |
| CDU (any sign) or plts | 68.75 | 84.21 | 55.0 | 90.57 | 4.35 | 0.37 |
| CDU (halo only) or plts | 35.71 | 91.23 | 50.0 | 85.25 | 4.07 | 0.70 |
Abnormal ESR (Miller) defined as >age/2 for males and >(age+10)/2 for females55.
ESR = Westergren erythrocyte sedimentation rate
CRP = c-reactive protein; plts = platelets
IL-6 = interleukin-6
CDU = color duplex ultrasonography.
Table 12 details the statistical analysis of each serologic test compared to TAB result. The results for age-adjusted and age independent ESR were similar. A platelet count threshold of 400,000/μL had the highest positive (18.32) and lowest negative (0.37) likelihood ratios of any single serologic test, followed by IL-6 level (8.41 and 0.58, respectively). No other listed combination of serologic testing (ESR/CRP, ESR (Miller)56/CRP, CRP/platelets, platelets/IL-6, or CRP/IL-6) improved the positive likelihood ratios when compared to the threshold platelet count of 400,000/μL. CRP/platelets, platelets/IL-6, and CRP/IL-6 improved the negative likelihood ratios for GCA slightly (0.30, 0.20, 0.19, respectively). When abnormal CDU (any sign or halo sign only) was combined with platelet count only to see whether an abnormality in either would improve predictability for GCA, both the positive and negative likelihood ratios were worse than for platelet count alone.
DISCUSSION
Temporal artery biopsy is recommended in suspected GCA as the definitive test to either rule in or rule out the disease.3,57,58 Critics of the procedure cite multiple reasons for the limitations of TAB, including surgical complications, loss of histopathologic features with corticosteroid therapy, skip lesions, and discordance, among others; other modalities (including CDU) have been recommended as a better alternative.22,47,51,52,59
TAB is routinely described as the gold standard in the diagnosis of GCA, even by investigators who recommend other modalities to avoid TAB.28,49,60 However, the definition of “gold standard” is itself debatable. In the strictest sense, a gold standard test would have 100% sensitivity and specificity for a given disease,27 a combination that does not exist in medicine.61 A less rigid definition of a gold standard is a modality that, albeit imperfect, is the best test available to diagnose a given pathology and is usually referred to as the “reference standard”. As noted by Savino, any new test that attempts to supplant the accepted reference standard as “the new gold standard” for a specific diagnosis must exceed the sensitivity and specificity of the older test.22 Few would argue that the specificity of TAB is nearly 100%.7,22 A pathologist may on occasion “overcall” the histopathologic features in a TAB. However, as Niederkohr and Levin point out, it is highly unlikely that a clinician would opt to withhold systemic corticosteroid therapy based on other criteria in the face of a positive TAB, resulting in a 100% specificity for a positive result in clinical practice.27
The major issue with TAB in the diagnosis of GCA is its sensitivity, which varies widely in the literature mainly because of the methodologies used. In a Bayesian meta-analysis, Niederkohr and Levin calculated a sensitivity of unilateral TAB of 87.1% (95% CI 81.8–91.7%) using four published series of bilateral simultaneous (rather than sequential) TABs.27 An earlier publication by the same authors reported the sensitivity of unilateral TAB across three studies as 69–91.7% and of bilateral TAB (simultaneous or sequential) across seven studies of 76.7–100%, with a mean of 94.3 % (95% CI 90.4–98.1%). Hedges and colleagues reported that 5 of 63 (8%) patients with a negative TAB (>1cm in length) in a cohort of 91 patients had a diagnosis of GCA on clinical grounds.23 However, the diagnosis of GCA was later reversed in two of these patients. Of note, one of the three remaining patients had unequivocal histopathologic proof of GCA on subsequent postmortem examination, supporting the contention that biopsy-negative GCA does exist. Despite the less than perfect sensitivity for TAB in their study, the authors also concluded that no combination of clinical or serologic findings was as sensitive as TAB for the diagnosis of GCA.23 More recently, El-Dairi and coworkers calculated a sensitivity of 91.4% for TAB in the diagnosis of GCA, although specifics on the average length of STA obtained were not listed.7
The reasons for false-negative TABs vary and, as already noted, are generally ascribed to skip areas of normal tissue in the TAB,18,33,62 inadequate length of arterial specimen,33,63 discordance between sides,27,63 and variations in histopathologic criteria for definitive diagnosis. In addition, some studies define GCA based on various clinical parameters (including the ACR criteria), resulting in up to 19% of patients with negative TABs being diagnosed with biopsy-negative GCA.64 Because of this lack of truly objective criteria for a false-negative TAB, the actual sensitivity of a bilateral, simultaneous TAB is difficult to assess, but should equal or exceed that of a unilateral TAB, especially if an adequate specimen is obtained in a timely fashion (with regards to the initiation of corticosteroid therapy) and reviewed by an experienced pathologist.
In this cohort, corticosteroids were stopped in all patients with a negative TAB, regardless of CDU and ACR results. Multiple studies have documented the high incidence of progressive visual loss in patients with GCA who are either untreated or undertreated with corticosteroids. In Hayreh and coworkers’s study on the ocular manifestations of GCA, of the patients with bilateral visual loss secondary to TAB-proven GCA, 16.7% presented with bilateral, simultaneous visual loss and an additional 45.8% of patients developed bilateral, sequential visual loss within 1 week of unilateral visual loss.6 Other investigators have found visual loss rates in GCA between 8–70%.10,65,66 In our cohort, 28 patients with a negative TAB had evidence of an optic neuropathy on presentation, and an additional 29 did not (Table 2b). Assuming a false-negative rate of 12.9% for TAB,27 an 8–70% incidence of visual loss in GCA,7–12,14–17,65–67 and a 45.8% risk of progressive visual loss in undertreated GCA,20 we would have expected to see 0.3–2.6 patients develop an optic neuropathy and 1.7 patients with an optic neuropathy to develop contralateral visual loss because of a false-negative TAB and lack of corticosteroid therapy. No patient with a negative TAB suffered additional visual loss after cessation of therapy, regardless of CDU and ACR results.
Given the high specificity of TAB for GCA, the scenario of a negative CDU trumping a positive TAB to rule out GCA cannot be defended. In two large meta-analyses on CDU, neither group of authors concluded that a negative CDU rules out GCA when the TAB is positive.28,43 The relevant argument between the utility of CDU versus TAB in the diagnosis of GCA comes down to the perceived sensitivities and specificities of CDU in the diagnosis of GCA to avoid TAB. As an example, Landau and colleagues suggested that TAB could be avoided in cases of high clinical suspicion for GCA and a positive CDU, or in cases of low clinical suspicion and a negative CDU.22 In their meta-analysis, Karassa and associates reported that when the probability of GCA is <10%, a positive CDU increases the probability of GCA 25–30% when compared to a positive TAB, while a negative test “practically excludes” the probability of GCA (2–5%).28 Karassa and coworkers concluded that a negative CDU in a low-risk patient precludes the need for a TAB.28 However, they also note that TAB is necessary in essentially all other scenarios, including a positive CDU in a high-risk patient, arguing that the morbidities of long-term corticosteroid use necessitate a definitive histopathologic diagnosis. These scenarios, however, are highly dependent on what exactly constitutes a low and high clinical suspicion for GCA, which can be defined using a variety of parameters,3,68 leaving an unacceptably large grey area for the clinician. Younge and coworkers analyzed multiple clinical and serologic parameters to predict TAB result.68 Their study concentrated on the need for pre-TAB corticosteroids (TABs were perforemd in all patients with suspected GCA, regardless of their calculated risk). The authors listed a variety of statistical results for each parameter, including LRs. As an example, they found that the combination of jaw claudication and decreased vision had a positive LR of 60 for GCA, while a normal ESR decreased the likelihood by 40. The practical problem with such nomograms arises when an individual patient presents with multiple clinical findings and serologic results that may push the patient to both positive and negative likelihoods of disease. The authors also noted that the most compelling symptom for GCA – jaw claudication – was present in only 50% of patients with positive TABs and that 7.1% of their low-risk cohort (<10% probability of GCA) had positive TABs.68 Importantly, using their nomograms, the authors were able to categorize 279 patients as either high-risk or low-risk for GCA, leaving the remaining 842 patients (76% of the total) in the intermediate-risk category that, according to other authors,22,28 would require a TAB, regardless of CDU result. In addition, the interpretation of statistical results depends on several practical issues that vary dramatically when comparing various pathologies.3,27,61 First, what are the consequences of a false negative result? For suspected GCA, this includes the possibility of devastating bilateral visual loss, stroke, myocardial infarction, and a host of other complications.20 Given the gravity of morbidities associated with untreated GCA, the 7.1% incidence of GCA in Younge and colleagues “low-risk” cohort would be unacceptable to physicians treating suspected GCA. Second, what are the consequences of a false positive result? For suspected GCA, long-term systemic corticosteroid therapy would be necessary in an elderly patient, with a significant probability of adverse events.19
COLOR DUPLEX ULTRASONOGRAPHY
In this study, the prevalence of GCA was 19.7% based on TAB. Tables 4e (any CDU findings) and 5e (halo sign only) present the performance of CDU against the reference standard of TAB for the diagnosis of GCA. Most measures of the predictive accuracy of CDU showed minimal evidence of its value in diagnosing GCA. CDU performed poorly in terms of sensitivity.
For any positive CDU finding at the patient level (Table 4f), sensitivity was 28.6% (95% CI 8.4, 58.1) among all patients and 30.8% (95% CI 9.1, 61.4) among the subset with bilateral TAB results. Sensitivity was even lower at the segment (12.5%, 95% CI 3.5, 35.7) and side (19.8%, 95% CI 5.7, 50.5) levels. The positive predictive value of the test was low in all analyses (32.6–36.4%) and ROC AUCs (in this case, an average of sensitivity and specificity for the single test cutoff) were less than 0.6 with 95% CIs, including 0.5, a finding that indicates no value of the test. The positive likelihood ratio values were greater than 2 in all cases and reached statistical significance at the side and segment level. This suggests that any positive CDU finding increases the likelihood of actual GCA from pre-test to post-test; however, given the relatively low probability of positive GCA expected pre-test (<20% of biopsies in this sample were positive), this would still not increase the likelihood of positive TAB to much more than 50%.73 Furthermore, stenosis and occlusion on CDU are nonspecific signs that may occur in atherosclerosis and have been reported to occur in 1 of 5 of patients with negative TABS.28
Table 5e shows the results for CDU (halo sign only) compared with reference standard of TAB. Results for CDU (halo sign only) differ slightly from CDU (any sign) in that sensitivity is much lower; however, the rate of false positives in patients without GCA is very low (specificity ranging from 96–99%). This translated into a positive predictive value of 50% or greater and LR+ values of 3.85 or greater. These results should be interpreted with caution due to the low incidence of a positive halo sign (4 patients, 5 segments, and 5 sides). Further, as an overall assessment of accuracy, ROC AUC values were again close to 0.5 and 95% CIs included 0.5. Based on nomograms published by McGee, the positive LR ranges for CDU in our study would increase the probability of GCA by 15–30%, while the negative LRs would decrease the probability by <15%.69 Of note, only two previous papers on CDU in GCA reported LRs, and their results generally mirrored ours.45,46 Black and colleagues reported a positive and negative LR of 2.1 and 0.7 respectively, while Maldini and colleagues noted positive and negative LRs of 1.7 and 0.5.45,46 The clustering of the positive and negative LRs near 1.0 in both studies point to a low ability of CDU to either rule in or rule out GCA.69 Both groups concluded that a TAB is still necessary in most cases of suspected GCA, regardless of CDU findings.
Tables 8d (any CDU sign) and 9d (halo sign only) show similar results for CDU findings vs. post-biopsy ACR result. There was no evidence that CDU (any sign) performed well in comparison to a GCA diagnosis based on post-biopsy ACR criteria. Sensitivity was less than 10%, specificity was low except at the segment level, and predictive values were all below 50%. LR+ values suggested that a positive CDU finding at the side or segment level actually decreased the likelihood of GCA. ROC AUCs were all below 0.5. When compared to ACR, results for CDU (halo only only) were largely consistent with CDU (any sign) findings (Tables 9a–9e). Table 7d show results for ACR vs. TAB. Post-biopsy ACR had reasonably high sensitivity and modest specificity. PPV was low, but NPV was high. LR+ values were similar to CDU, but were significant. LR-values were quite low, meaning a negative post-biopsy ACR result greatly reduced the post-test probability of GCA. ROC AUC values were all greater than 0.7 with 95% CIs excluding 0.5, suggesting at least modest value of the tool in detecting GCA after TAB. However, these results cannot be applied to pre-biopsy ACR results. In addition, this analysis should be interpreted with caution, since a positive TAB is included as one of the five ACR criteria for GCA and may therefore skew the results.
TABLE 8A.
CDU VS. POST-BIOPSY ACR. PATIENT LEVEL DATA (N=71).
| CDU POSITIVE (N=11) | CDU NEGATIVE (N=60) | |
|---|---|---|
| Post-biopsy ACR Positive (N=36) | 3 | 33 |
| Post-biopsy ACR Negative (N=35) | 8 | 27 |
Positive CDU defined as any positive CDU finding.
TABLE 8B.
CDU VS. POST-BIOPSY ACR. PATIENT LEVEL DATA (BILATERAL TABS ONLY, N=63).
| CDU POSITIVE (N=11) | CDU NEGATIVE (N=52) | |
|---|---|---|
| Post-biopsy ACR Positive (N=32) | 3 | 29 |
| Post-biopsy ACR Negative (N=31) | 8 | 23 |
Positive CDU defined as any positive CDU finding.
TABLE 8C.
CDU VS. POST-BIOPSY ACR. RESULTS BY SIDE (N=142).
| CDU POSITIVE (N=17) | CDU NEGATIVE (N=125) | |
|---|---|---|
| Post-biopsy ACR Positive (N=26) | 4 | 68 |
| Post-biopsy ACR Negative (N=108) | 13 | 57 |
Positive CDU defined as any positive CDU finding.
TABLE 8D.
CDU PERFORMANCE (ANY ABNORMALITY) AT PATIENT, SIDE, AND SEGMENT LEVEL COMPARED TO POST-BIOPSY ACR RESULT.
| PATIENT LEVEL ALL PATIENTS | PATIENT LEVEL BILATERAL | SIDE-LEVEL | SEGMENT-LEVEL | |
|---|---|---|---|---|
| Sensitivity (%)(CI) | 8.3 (1.8,22.5) | 9.4 (2.0,25.0) | 4.6 (1.4,14.0) | 2.8 (1.2,6.1)* |
| Specificity (%)(CI) | 77.1 (59.9,89.6) | 74.2 (55.4,88.1) | 85.0 (69.6,93.4) | 94.5 (89.4,97.3) |
| Positive predictive value (%)(CI) | 27.3 (6.0,61.0) | 27.3 (6.0,61.0) | 21.3 (3.4,67.7) | 21.4 (3.2,69.3) |
| Negative predictive value (%)(CI) | 45.0 (32.1,58.4) | 44.2 (30.5,58.7) | 44.4 (29.3,60.6) | 46.7 (27.8,66.7) |
| Positive likelihood ratio (CI) | 0.37 (0.11,1.26) | 0.36 (0.11,1.24) | 0.31 (0.21,0.45) | 0.51 (0.41,0.62) |
| Negative likelihood ratio (CI) | 1.19 (0.97,1.46) | 1.22 (0.97,1.55) | 1.12 (0.98,1.28) | 1.03 (0.98,10.7) |
| ROC AUC (CI) | 0.43 (0.34,0.51) | 0.42 (0.32,0.51) | 0.45 (0.39,0.51) | 0.49 (0.47,0.51) |
CI = Confidence interval. All confidence intervals at 95%.
ROC AUC = Area under the receiver operating characteristic curve.
The random effects analysis for this sensitivity at the segment level did not converge.
TABLE 9D.
CDU (HALO SIGN ONLY) VS. POST-BIOPSY ACR. RESULTS BY SEGMENT (N=423)
| HALO POSITIVE (N=5) | HALO NEGATIVE (N=418) | |
|---|---|---|
| ACR Positive (N=216) | 1 | 215 |
| ACR Negative (N=207) | 4 | 203 |
TABLE 9A.
CDU (HALO SIGN ONLY) VS. POST-BIOPSY ACR. PATIENT LEVEL DATA (N=71)
| HALO POSITIVE (N=4) | HALO NEGATIVE (N=60) | |
|---|---|---|
| ACR Positive (N=36) | 1 | 35 |
| ACR Negative (N=35) | 3 | 32 |
TABLE 9B.
CDU (HALO SIGN ONLY) VS. POST-BIOPSY ACR. PATIENT LEVEL DATA (BILATERAL TABS ONLY, N=63)
| HALO POSITIVE (N=11) | HALO NEGATIVE (N=52) | |
|---|---|---|
| ACR Positive (N=32) | 1 | 31 |
| ACR Negative (N=31) | 3 | 28 |
TABLE 9C.
CDU (HALO SIGN ONLY) VS. POST-BIOPSY ACR. RESULTS BY SIDE (N=142)
| HALO POSITIVE (N=5) | HALO NEGATIVE (N=137) | |
|---|---|---|
| ACR Positive (N=26) | 1 | 71 |
| ACR Negative (N=108) | 4 | 66 |
TABLE 9E.
CDU PERFORMANCE (HALO SIGN ONLY) AT PATIENT, SIDE, AND SEGMENT LEVEL COMPARED TO POST-BIOPSY ACR RESULT.
| PATIENT LEVEL ALL PATIENTS | PATIENT LEVEL BILATERAL | SIDE-LEVEL | SEGMENT-LEVEL | |
|---|---|---|---|---|
| Sensitivity (%) (CI) | 2.8 (0.1,14.5) | 3.1 (0.1,16.2) | 1.4 (0.2,9.9) | 0.5 (0.1,3.3) |
| Specificity (%) (CI) | 91.4 (76.9,98.2) | 90.3 (74.2,98.0) | 95.3 (95.5,98.6) | 98.4 (95.1,99.5) |
| Positive predictive value (%) (CI) | 25.0 (0.6,80.6) | 25.0 (0.6,80.6) | 19.1 (0,100) | 19.1 (0,100) |
| Negative predictive value (%) (CI) | 47.8 (35.4,60.3) | 47.5 (34.3,60.9) | 47.7 (32.6,63.3) | 47.1 (27.8,67.4) |
| Positive likelihood ratio (CI) | 0.32 (0.04,2.97) | 0.32 (0.04,2.94) | 0.29 (0.21,0.41) | 0.28 (0.23,0.34) |
| Negative likelihood ratio (CI) | 1.06 (0.95,1.19) | 1.07 (0.94,1.22) | 1.03 (0.97,1.10) | 1.01 (0.99,1.03) |
| ROC AUC (CI) | 0.47 (0.42,0.53) | 0.47 (0.41,0.53) | 0.48 (0.45,0.51) | 0.49 (0.48,0.50) |
CI = Confidence interval.
All confidence intervals at 95%. ROC
AUC = Area under the receiver operating characteristic curve.
As already mentioned, one major issue in determining the efficacy of CDU in either ruling in or ruling out GCA is the heterogeneity in methodologies between studies. Some studies have concluded that CDU is superior to TAB in diagnosing GCA,44,52 but vary in their design as well as their definition of GCA. Karahaliou and colleagues concluded that the presence of a bilateral halo sign is 100% specific for GCA, precluding the need for TAB, based on their findings in 9 patients.44 On the other hand, Maldini and colleagues found that both the positive and negative LRs for CDU are poor in the diagnosis of GCA.45 Multiple studies have also included cases of “biopsy-negative GCA” or “GCA without biopsy”, with the diagnosis of GCA based on ACR or other clinical criteria, raising questions about the validity of the diagnosis of GCA in these studies.22,43 An additional problem with most published studies is that they lack the actual measurements of the TAB. In their seminal paper on CDU, Schmidt and colleagues include no data on length of STA specimens.39 Muratore co-workers state that all TABs were at “least 0.5cm” in length, a minimum which may be inadequate.49
Some experts have also proposed that CDU may have utility in guiding the surgeon toward a high yield area in the STA for biopsy.44,51 Karahaliou and colleagues concluded that a “directional TAB” in the area of a halo sign on CDU improves the yield of a positive TAB.44 In this study, the sensitivity of CDU (with any sign or halo sign only) decreased at the segment-level analysis when compared to all patient or bilateral TAB analysis (Tables 4e and 5e) and only 3 halo signs were identified in 44 positive TAB segments (7%) (Table 5d), raising significant doubts as to whether a “directional TAB” guided by CDU has any validity.53
As already noted, one disadvantage of TAB in the diagnosis of GCA is the loss of histopathologic features with corticosteroid use, although they may be present after weeks to months of corticosteroid therapy.70 Of note, this shortcoming applies to CDU as well. Schmidt and colleagues reported that the halo sign disappeared after a mean 16 days of corticosteroid use, comparable to the loss of histopathologic features, but may also be absent as soon as 7 days after initiation of corticosteroids.40,70 Hauenstein and associates noted that the sensitivity of CDU compared to positive TAB dropped rapidly from 92% with one day of corticosteroid use to 80% after 2–4 days, and only 50% with more than 4 days of therapy.30,70 Karahaliou co-workers reported that the halo sign disappeared in 50% of patients after 2 weeks of corticosteroid therapy and in all patients at 4 weeks.44
Two additional important issues have been noted in multiple studies on the use of CDU for the diagnosis of GCA. First, the accuracy of CDU depends on the technology and specific setting used.41,43,71 Second, the validity of CDU for GCA is highly dependent on the examiner,43,45,60 and the usefulness of CDU in the absence of an experienced examiner has been questioned.22,45 In a retrospective study by Black and colleagues, seven of 43 CDUs initially read as negative for the halo sign were reinterpreted as positive when reviewed by an “expert” reader, doubling the prevalence of a positive halo sign when compared to the initial CDU results.46 Despite this finding, the authors found no improved utility of CDU for the diagnosis of GCA when read by an expert radiologist. In this study, all CDUs were read by one radiologist with extensive experience in this technology. The need for specific technology and an experienced reader may not be available in all hospitals and may lead to as much of a delay as that in scheduling a TAB.
Table 6 compares the results of CDU in our study with two meta-analyses; there was an overlap of studies in both meta-analyses and some of the studies included “TAB-negative GCA” in their data analysis. The sensitivity of CDU (halo sign only) in our study (using the reference standard of positive TAB as the definition of GCA) was markedly lower than in the two meta-analyses (15% vs 69–75%) and the specificity was higher (96% vs 82–83%). Similarly, the sensitivity of CDU (halo sign only) when compared to the ACR criteria was lower (3% vs 55–69), but the specificity was similar (90% vs 89–96%). The marked difference in the results can be explained by high interstudy heterogeneity (Table 6) in methodologies of the studies included in the meta-analyses when compared to the stricter methodology used in the present manuscript, as well as the relatively low number of positive halo signs in our study.
In conclusion, the results of this study refute the hypothesis for the thesis that CDU correlates with TAB result in the diagnosis of GCA.
AMERICAN COLLEGE OF RHEUMATOLOGY CRITERIA FOR GCA
The ACR criteria for the diagnosis of GCA have a reported sensitivity and specificity of 93.5% and 91.2%.72 However, the ACR criteria for GCA were initially developed for research purposes as a method to distinguish GCA from other vasculitides, and not as a diagnostic tool for GCA; therefore, the published sensitivity and specificity cannot be applied to patients suspected of having GCA, since this represents a markedly different population compared to that studied by the ACR.7,57,63,72,73 Unfortunately, the ACR criteria have entered the methodologies of multiple studies and are not infrequently used to diagnose “biopsy-negative GCA”. This may then produce downstream issues in the analysis of sensitivities and specificities of a variety of serologic and radiographic testing (including CDU), as well as TAB.57 Multiple publications have questioned the use of solely clinical criteria, including those from the ACR, in the diagnosis of GCA.3,23,57,73 A recent retrospective study from our center comparing ACR criteria with unilateral or bilateral TAB found that 25.7% of patients with a positive TAB did not meet the ACR criteria for GCA, and 28.2% with a negative TAB did meet the criteria.74 Diagnostic agreement between TAB results alone and ACR criteria without biopsy was only 51.4% (none of the patients in the previous publication were used in the present analysis). Kermani at al found a better concordance between ACR and positive TAB, noting that only 12 of 177 patients (6.8%) with a positive TAB did not meet the ACR criteria for GCA, while El-Dairi reported that 23.1% of their cohort with a negative TAB met the ACR criteria.7,16 Table 7a–d summarizes the analyses of post-biopsy ACR criteria and TAB. The sensitivity of the post-biopsy ACR criteria (range 84.6–94.0) was better compared to CDU for the diagnosis of GCA, but the specificity (range 57.9–60.2) was worse.
SEROLOGIC TESTING
The utility of serologic testing as a predictor of GCA is a controversial subject. Niederkohr and Levin noted a higher positive likelihood ratio for the diagnosis of GCA as the ESR trends upwards.3 Lopez-Diaz and associates have reported an ESR between 70 and 100mm/hr as a predictor of ischemic visual loss from GCA,75 a finding not replicated in other cohorts.65 Parikh and co-workers reported that an age- and sex-adjusted ESR had a sensitivity of 76–86% in biopsy-proven GCA, but did not list specificities; sensitivity increased to 99% when combined with CRP.32 However, a more recent study reported no positive correlation between ESR and positive TAB, regardless of whether the ESR was adjusted for age.7
Data on the utility of CRP is more limited than for ESR.16,25,76 Parikh and associates found that CRP was more sensitive than age-adjusted ESR for biopsy-proven GCA, with a sensitivity of 97.5%, but specificity was not reported because of the study’s methodology.32 One large study by Kermani and colleagues concluded that CRP (odds ratio 2.94; 95% CI 1.83, 4.71) was a slightly more sensitive marker for a positive TAB (and therefore for GCA) than ESR (odds ratio 2.22; 95% CI 1.43, 3.46), quoting a sensitivity of 86.9%.16 In the same study, the authors found that a combined elevation of ESR and CRP resulted in an odds ratio of 3.06 (95% CI 2.03, 4.62), but a positive predictive value of only 29.3% for a positive TAB. The specificity of both tests was low (29.5% and 30.5% for ESR and CRP, respectively). El-Dairi and associates concluded that an elevated CRP increased the relative risk for GCA by 3.3 (p=0.037).7 Hayreh and colleagues also concluded that CRP was more predictive than ESR alone for GCA, and that the combination of elevated ESR and CRP resulted in a specificity of 97% for GCA.25 Multiple studies have also found a higher predictability of serologic testing for GCA when ESR and CRP were combined, with a sensitivity of 97–99%.16,25,32 Other investigators have commented that both ESR and CRP trend to lower levels in patients with GCA and visual complications.10,14,65,77
Niederkohr and Levin published pooled results for a variety of clinical and laboratory findings while constructing a decision tree for the management of GCA.3 They noted that thrombocytosis was a more sensitive and specific marker for GCA (positive LR = 5.982, negative LR = 0.565) than anemia (positive LR = 1.221, negative LR = 0.923) or ESR (positive LR = 0.551–2.446, negative LR = 0.775–1.581). The likelihood ratios for ESR varied because of stratification of results (<50mm/hr, 50–100mm/hr, and >100mm/hr). The authors did not calculate LRs for CRP and IL-6 because of a paucity of published data. They concluded that a platelet count is the preferred blood test in patients with either low or high pretest probabilities. Foroozan and colleagues reported a better specificity, positive predictive value, and negative predictive value of thrombocytosis (platelet count >400,000/μL) compared to ESR for the diagnosis of GCA, but a lower sensitivity (57% vs 79%).31 A recent analysis by El-Dairi and associates concluded that a platelet count of >400,000/μL had the highest correlation with GCA (relative risk 3.3, P=.0072) when compared to ESR and CRP.7
In our study, no significant difference in either ESR or CRP mean values was noted between TAB positive and TAB negative patients (Tables 10 and 11). However, highly significant differences were noted for both platelet and IL-6 levels. The utility of CDU vs TAB can therefore be studied indirectly. Significant differences in platelet count were noted between positive TABs and negative CDU or ACR. However, significant differences were also noted in patients with positive TABs and positive CDU or ACR, albeit at a lesser degree. If CDU result correlated closely with TAB result, the difference between platelet counts in cases where both were positive should not have been significant. Comparison of serologic results between CDU positive and negative patients showed no statistically significant difference in platelet count or Il-6 level, in contradistinction to the results for TABs; for post-biopsy ACR positive and negative patients, significance was only found for platelet count, suggesting that in this study, the post-biopsy ACR criteria may have been more accurate than CDU in the diagnosis of GCA, as already noted; again, this finding must be interpreted with caution, since a positive TAB result is one of the 5 parameters in the ACR criteria for GCA. Similar findings were noted for IL-6, except that no significant difference was noted between TAB and CDU positive results; however, because of the lower number of patients with IL-6 serology, these results must also be interpreted with caution.
Sensitivities, specificities, and other predictive analyses of serologic testing are listed in Table 12. There was no difference in ESR likelihood ratios when a level of ≥50mm/hr (an ACR criterion) was compared to an age-adjusted ESR using Miller’s formula,56 consistent with a previous study.7 Although other formulae for patient-specific ESR exist, the two aforementioned methods were analyzed, as they represent the most common thresholds for studying ESR in GCA. As already mentioned, Niederkohr and Levin did note improved positive and negative likelihood ratios when the ESR was stratified into three groups.3 In our study, the combination of either elevated ESR or CRP resulted in a sensitivity of 71.43–78.57 for biopsy-positive GCA (depending on the ESR formula used), significantly lower than that reported in previous studies.16,25,32 Positive and negative LRs for this combination of testing ranged between 1.10–1.40 and 0.49–0.81, respectively. The highest positive and lowest negative likelihood ratios of any serologic testing was a platelet threshold level of 400,000μL (18.32 and 0.37, respectively). CDU (any finding) and CDU (halo only) were combined with platelet count to analyze whether sensitivity and specificity for the diagnosis of GCA improved when either was abnormal (Table 12). This combination improved the positive and negative ratios for CDU marginally, but markedly decreased the likelihood ratios compared to the reported platelet count threshold. In other words, the combination of abnormal/normal CDU or platelet count had a lower potential for ruling in or ruling out GCA than the platelet count threshold alone.
There are several limitations in this study. The first is that we did not consider the possibility of biopsy-negative GCA patients who may have been diagnosed with GCA by either CDU or the ACR criteria. This is unlikely to have had any significant effect on the analysis for two reasons. Biopsy-negative GCA following bilateral TABs of adequate length reviewed by an experienced pathologist is likely a rare event.22 Second, no patient in this study experienced progressive visual loss, despite the cessation of corticosteroids in all patients with negative TABs.57
ESR was analyzed using two common methods (ACR criterion of ≥50mm/hr and Miller’s formula).26,56 Other formulae have been reported, including one by Hayreh and colleagues.25 Studies have reported that ESR is affected by abnormal hematocrit, rising significantly in cases of anemia, and alternative methods for ESR calculation have been recommended.78,79 Systemic medications (statins, nonsteroidal anti-inflammatory drugs) also impact ESR measurement, resulting in a lower reading.80 We did not record hematocrit or the use of systemic medications in our patient cohort. The use of other methodologies for ESR calculation may have resulted in different statistical results.
The most significant limitation of this study is the overall low number of patients with positive halo signs (Table 5a–d), making any statistical analysis regarding the halo sign difficult to interpret with accuracy. However, the paucity of halo signs despite documenting 26 sides and 44 segments of STA as positive Table5c and 5d) speaks to the low sensitivity of this CDU finding in positive TABs.
At a prevalence of 19.7%, the accuracy of our data would be more influenced by specificity than sensitivity, potentially leading to an overinterpretation of the accuracy of the test.81 For this reason, overall accuracy calculations were not performed.
ACKNOWLEDGEMENTS
Funding/Support: Wills Eye Innovation Grant #14-033.
-
Financial Disclosures:
Jurij R. Bilyk, MD: Speakers’ Bureau, Stryker Corporation.
Ann P. Murchison, MD, MPH: None.
Benjamin T. Leiby, PhD: None.
Robert C. Sergott, MD: Consultant for Merck & Co., Inc.; H. Lundbeck A/S; Alexion Pharmaceuticals, Inc.; Novartis International AG; GenSight Biologics SA; Janssen Pharmaceutical. Speakers’ Bureau for Biogen, Inc.; Teva Pharmaceutical Industries, Ltd.; Merck-Serono; Sanofi Genzyme; Novartis International AG.
Ralph C. Eagle, MD: Book royalties, Lippincott Williams & Wilkins.
Laurence Needleman, MD: None.
Peter J. Savino MD: None.
-
Contributions of Authors:
Jurij R. Bilyk, MD: A, B, D.
Ann P. Murchison, MD, MPH: A, B, C, D.
Benjamin T. Leiby, PhD: C, D.
Robert C. Sergott, MD: A, B, D.
Ralph C. Eagle, MD: B.
Laurence Needleman, MD: B
Peter J. Savino MD: A, B, D.
A=Design of study; B=Conduct of study; C=Statistical analysis; D=Manuscript preparation)
Other Acknowledgements: None
REFERENCES
- 1.Haugeberg G, Bie R, Bendvold A, Larsen AS, Johnsen V. Primary vasculitis in a Norwegian community hospital: a retrospective study. Clin Rheumatol. 1998;17(5):364–368. doi: 10.1007/BF01450893. [DOI] [PubMed] [Google Scholar]
- 2.Cotch MF, Rao JK. New insights into the epidemiology of systemic vasculitis. Curr Opin Rheumatol. 1996;8(1):19–25. doi: 10.1097/00002281-199601000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Niederkohr RD, Levin LA. Management of the patient with suspected temporal arteritis a decision-analytic approach. Ophthalmology. 2005;112(5):744–756. doi: 10.1016/j.ophtha.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 4.Devauchelle-Pensec V, Jousse S, Destombe C, Saraux A. Epidemiology, imaging, and treatment of giant cell arteritis. Joint Bone Spine. 2008;75(3):267–272. doi: 10.1016/j.jbspin.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Warrington KJ, Matteson EL. Management guidelines and outcome measures in giant cell arteritis (GCA) Clin Exp Rheumatol. 2007;25(6 Suppl 47):137–141. [PubMed] [Google Scholar]
- 6.Hayreh SS, Podhajsky PA, Zimmerman B. Ocular manifestations of giant cell arteritis. Am J Ophthalmol. 1998;125(4):509–520. doi: 10.1016/s0002-9394(99)80192-5. [DOI] [PubMed] [Google Scholar]
- 7.El-Dairi MA, Chang L, Proia AD, Cummings TJ, Stinnett SS, Bhatti MT. Diagnostic Algorithm for Patients With Suspected Giant Cell Arteritis. J Neuroophthalmol. 2015;35(3):246–253. doi: 10.1097/WNO.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 8.Huston KA, Hunder GG, Lie JT, Kennedy RH, Elveback LR. Temporal arteritis: a 25-year epidemiologic, clinical, and pathologic study. Ann Intern Med. 1978;88(2):162–167. doi: 10.7326/0003-4819-88-2-162. [DOI] [PubMed] [Google Scholar]
- 9.Caselli RJ, Hunder GG, Whisnant JP. Neurologic disease in biopsy-proven giant cell (temporal) arteritis. Neurology. 1988;38(3):352–359. doi: 10.1212/wnl.38.3.352. [DOI] [PubMed] [Google Scholar]
- 10.Cid MC, Font C, Oristrell J, et al. Association between strong inflammatory response and low risk of developing visual loss and other cranial ischemic complications in giant cell (temporal) arteritis. Arthritis Rheum. 1998;41(1):26–32. doi: 10.1002/1529-0131(199801)41:1<26::AID-ART4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Aiello PD, Trautmann JC, McPhee TJ, Kunselman AR, Hunder GG. Visual prognosis in giant cell arteritis. Ophthalmology. 1993;100(4):550–555. doi: 10.1016/s0161-6420(93)31608-8. [DOI] [PubMed] [Google Scholar]
- 12.Font C, Cid MC, Coll-Vinent B, Lopez-Soto A, Grau JM. Clinical features in patients with permanent visual loss due to biopsy-proven giant cell arteritis. Br J Rheumatol. 1997;36(2):251–254. doi: 10.1093/rheumatology/36.2.251. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Gay MA, Garcia-Porrua C, Llorca J, et al. Visual manifestations of giant cell arteritis. Trends and clinical spectrum in 161 patients. Medicine (Baltimore) 2000;79(5):283–292. doi: 10.1097/00005792-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Liozon E, Herrmann F, Ly K, et al. Risk factors for visual loss in giant cell (temporal) arteritis: a prospective study of 174 patients. Am J Med. 2001;111(3):211–217. doi: 10.1016/s0002-9343(01)00770-7. [DOI] [PubMed] [Google Scholar]
- 15.Wagener HP, Hollenhorst RW. The ocular lesions of temporal arteritis. Trans Am Ophthalmol Soc. 1957;55:249–269. discussion 269–273. [PMC free article] [PubMed] [Google Scholar]
- 16.Kermani TA, Schmidt J, Crowson CS, et al. Utility of erythrocyte sedimentation rate and C-reactive protein for the diagnosis of giant cell arteritis. Semin Arthritis Rheum. 2012;41(6):866–871. doi: 10.1016/j.semarthrit.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen JJ, Leavitt JA, Fang C, Crowson CS, Matteson EL, Warrington KJ. Evaluating the Incidence of Arteritic Ischemic Optic Neuropathy and Other Causes of Vision Loss from Giant Cell Arteritis. Ophthalmology. 2016;123(9):1999–2003. doi: 10.1016/j.ophtha.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nesher G, Breuer GS. Giant Cell Arteritis and Polymyalgia Rheumatica: 2016 Update. Rambam Maimonides Med J. 2016;7(4) doi: 10.5041/RMMJ.10262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Proven A, Gabriel SE, Orces C, O’Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum. 2003;49(5):703–708. doi: 10.1002/art.11388. [DOI] [PubMed] [Google Scholar]
- 20.Hayreh SS. Ophthalmic features of giant cell arteritis. Baillieres Clin Rheumatol. 1991;5(3):431–459. doi: 10.1016/s0950-3579(05)80064-0. [DOI] [PubMed] [Google Scholar]
- 21.Nesher G, Sonnenblick M. Steroid-sparing medications in temporal arteritis--report of three cases and review of 174 reported patients. Clin Rheumatol. 1994;13(2):289–292. doi: 10.1007/BF02249029. [DOI] [PubMed] [Google Scholar]
- 22.Landau K, Savino PJ, Gruber P. Diagnosing giant cell arteritis: is ultrasound enough? J Neuroophthalmol. 2013;33(4):394–400. doi: 10.1097/WNO.0000000000000079. [DOI] [PubMed] [Google Scholar]
- 23.Hedges TR, 3rd, Gieger GL, Albert DM. The clinical value of negative temporal artery biopsy specimens. Arch Ophthalmol. 1983;101(8):1251–1254. doi: 10.1001/archopht.1983.01040020253019. [DOI] [PubMed] [Google Scholar]
- 24.Machado EB, Michet CJ, Ballard DJ, et al. Trends in incidence and clinical presentation of temporal arteritis in Olmsted County, Minnesota, 1950–1985. Arthritis Rheum. 1988;31(6):745–749. doi: 10.1002/art.1780310607. [DOI] [PubMed] [Google Scholar]
- 25.Hayreh SS, Podhajsky PA, Raman R, Zimmerman B. Giant cell arteritis: validity and reliability of various diagnostic criteria. Am J Ophthalmol. 1997;123(3):285–296. doi: 10.1016/s0002-9394(14)70123-0. [DOI] [PubMed] [Google Scholar]
- 26.Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33(8):1122–1128. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 27.Niederkohr RD, Levin LA. A Bayesian analysis of the true sensitivity of a temporal artery biopsy. Invest Ophthalmol Vis Sci. 2007;48(2):675–680. doi: 10.1167/iovs.06-1106. [DOI] [PubMed] [Google Scholar]
- 28.Karassa FB, Matsagas MI, Schmidt WA, Ioannidis JP. Meta-analysis: test performance of ultrasonography for giant-cell arteritis. Ann Intern Med. 2005;142(5):359–369. doi: 10.7326/0003-4819-142-5-200503010-00011. [DOI] [PubMed] [Google Scholar]
- 29.Salvarani C, Pipitone N, Versari A, Hunder GG. Clinical features of polymyalgia rheumatica and giant cell arteritis. Nat Rev Rheumatol. 2012;8(9):509–521. doi: 10.1038/nrrheum.2012.97. [DOI] [PubMed] [Google Scholar]
- 30.Tyndall A. Is the halo always holy? Glucocorticoid impact on detecting cranial large-vessel arteritis. Rheumatology (Oxford) 2012;51(11):1927–1928. doi: 10.1093/rheumatology/kes203. [DOI] [PubMed] [Google Scholar]
- 31.Foroozan R, Danesh-Meyer H, Savino PJ, Gamble G, Mekari-Sabbagh ON, Sergott RC. Thrombocytosis in patients with biopsy-proven giant cell arteritis. Ophthalmology. 2002;109(7):1267–1271. doi: 10.1016/s0161-6420(02)01076-x. [DOI] [PubMed] [Google Scholar]
- 32.Parikh M, Miller NR, Lee AG, et al. Prevalence of a normal C-reactive protein with an elevated erythrocyte sedimentation rate in biopsy-proven giant cell arteritis. Ophthalmology. 2006;113(10):1842–1845. doi: 10.1016/j.ophtha.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 33.Breuer GS, Nesher R, Nesher G. Effect of biopsy length on the rate of positive temporal artery biopsies. Clin Exp Rheumatol. 2009;27(1 Suppl 52):S10–13. [PubMed] [Google Scholar]
- 34.Ikard RW. Clinical efficacy of temporal artery biopsy in Nashville, Tennessee. South Med J. 1988;81(10):1222–1224. doi: 10.1097/00007611-198810000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Yoon MK, McCulley TJ, Horton JC. Brow ptosis after temporal artery biopsy. Ophthalmology. 2013;120(6):e34. doi: 10.1016/j.ophtha.2013.01.047. [DOI] [PubMed] [Google Scholar]
- 36.Yoon MK, Horton JC, McCulley TJ. Facial nerve injury: a complication of superficial temporal artery biopsy. Am J Ophthalmol. 2011;152(2):251–255 e251. doi: 10.1016/j.ajo.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurence in a population-based study. Arthritis Rheum. 2001;45(2):140–145. doi: 10.1002/1529-0131(200104)45:2<140::AID-ANR166>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 38.Murchison AP, Bilyk JR. Brow ptosis after temporal artery biopsy: incidence and associations. Ophthalmology. 2012;119(12):2637–2642. doi: 10.1016/j.ophtha.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt WA, Kraft HE, Volker L, Vorpahl K, Gromnica-Ihle EJ. Colour Doppler sonography to diagnose temporal arteritis. Lancet. 1995;345(8953):866. doi: 10.1016/s0140-6736(95)93005-1. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt WA, Kraft HE, Vorpahl K, Volker L, Gromnica-Ihle EJ. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med. 1997;337(19):1336–1342. doi: 10.1056/NEJM199711063371902. [DOI] [PubMed] [Google Scholar]
- 41.Murgatroyd H, Nimmo M, Evans A, MacEwen C. The use of ultrasound as an aid in the diagnosis of giant cell arteritis: a pilot study comparing histological features with ultrasound findings. Eye (Lond) 2003;17(3):415–419. doi: 10.1038/sj.eye.6700350. [DOI] [PubMed] [Google Scholar]
- 42.Lauwerys BR, Puttemans T, Houssiau FA, Devogelaer JP. Color Doppler sonography of the temporal arteries in giant cell arteritis and polymyalgia rheumatica. J Rheumatol. 1997;24(8):1570–1574. [PubMed] [Google Scholar]
- 43.Ball EL, Walsh SR, Tang TY, Gohil R, Clarke JM. Role of ultrasonography in the diagnosis of temporal arteritis. Br J Surg. 2010;97(12):1765–1771. doi: 10.1002/bjs.7252. [DOI] [PubMed] [Google Scholar]
- 44.Karahaliou M, Vaiopoulos G, Papaspyrou S, Kanakis MA, Revenas K, Sfikakis PP. Colour duplex sonography of temporal arteries before decision for biopsy: a prospective study in 55 patients with suspected giant cell arteritis. Arthritis Res Ther. 2006;8(4):R116. doi: 10.1186/ar2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maldini C, Depinay-Dhellemmes C, Tra TT, et al. Limited value of temporal artery ultrasonography examinations for diagnosis of giant cell arteritis: analysis of 77 subjects. J Rheumatol. 2010;37(11):2326–2330. doi: 10.3899/jrheum.100353. [DOI] [PubMed] [Google Scholar]
- 46.Black R, Roach D, Rischmueller M, Lester SL, Hill CL. The use of temporal artery ultrasound in the diagnosis of giant cell arteritis in routine practice. Int J Rheum Dis. 2013;16(3):352–357. doi: 10.1111/1756-185X.12108. [DOI] [PubMed] [Google Scholar]
- 47.Dadoniene J, Jatuzis D, Laurinaviciuus A. The diagnostic value of untrasound examination in temporal arteritis. Acta Medica Lituanica. 2010;17:71–76. [Google Scholar]
- 48.Romera-Villegas A, Vila-Coll R, Poca-Dias V, Cairols-Castellote MA. The role of color duplex sonography in the diagnosis of giant cell arteritis. J Ultrasound Med. 2004;23(11):1493–1498. doi: 10.7863/jum.2004.23.11.1493. [DOI] [PubMed] [Google Scholar]
- 49.Muratore F, Boiardi L, Restuccia G, et al. Comparison between colour duplex sonography findings and different histological patterns of temporal artery. Rheumatology (Oxford) 2013;52(12):2268–2274. doi: 10.1093/rheumatology/ket258. [DOI] [PubMed] [Google Scholar]
- 50.Suelves AM, Espana-Gregori E, Tembl J, Rohrweck S, Millan JM, Diaz-Llopis M. Doppler ultrasound and giant cell arteritis. Clin Ophthalmol. 2010;4:1383–1384. doi: 10.2147/OPTH.S13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Habib HM, Essa AA, Hassan AA. Color duplex ultrasonography of temporal arteries: role in diagnosis and follow-up of suspected cases of temporal arteritis. Clin Rheumatol. 2012;31(2):231–237. doi: 10.1007/s10067-011-1808-0. [DOI] [PubMed] [Google Scholar]
- 52.LeSar CJ, Meier GH, DeMasi RJ, et al. The utility of color duplex ultrasonography in the diagnosis of temporal arteritis. J Vasc Surg. 2002;36(6):1154–1160. doi: 10.1067/mva.2002.129648. [DOI] [PubMed] [Google Scholar]
- 53.Salvarani C, Silingardi M, Ghirarduzzi A, et al. Is duplex ultrasonography useful for the diagnosis of giant-cell arteritis? Ann Intern Med. 2002;137(4):232–238. doi: 10.7326/0003-4819-137-4-200208200-00006. [DOI] [PubMed] [Google Scholar]
- 54.Murchison AP, Bilyk JR, Eagle RC, Jr, Savino PJ. Shrinkage revisited: how long is long enough? Ophthal Plast Reconstr Surg. 2012;28(4):261–263. doi: 10.1097/IOP.0b013e31824ee720. [DOI] [PubMed] [Google Scholar]
- 55.Genders TS, Spronk S, Stijnen T, Steyerberg EW, Lesaffre E, Hunink MG. Methods for calculating sensitivity and specificity of clustered data: a tutorial. Radiology. 2012;265(3):910–916. doi: 10.1148/radiol.12120509. [DOI] [PubMed] [Google Scholar]
- 56.Miller A, Green M, Robinson D. Simple rule for calculating normal erythrocyte sedimentation rate. Br Med J (Clin Res Ed) 1983;286(6361):266. doi: 10.1136/bmj.286.6361.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Danesh-Meyer HV. Temporal artery biopsy: skip it at your patient’s peril. Am J Ophthalmol. 2012;154(4):617–619 e611. doi: 10.1016/j.ajo.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 58.Frohman L, Wong AB, Matheos K, Leon-Alvarado LG, Danesh-Meyer HV. New developments in giant cell arteritis. Surv Ophthalmol. 2016;61(4):400–421. doi: 10.1016/j.survophthal.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 59.Alberts MS, Mosen DM. Diagnosing temporal arteritis: duplex vs. biopsy. QJM. 2007;100(12):785–789. doi: 10.1093/qjmed/hcm103. [DOI] [PubMed] [Google Scholar]
- 60.Pfenninger L, Horst A, Stuckmann G, Flury R, Sturmer J. Comparison of histopathological findings with duplex sonography of the temporal arteries in suspected giant cell arteritis. Klin Monbl Augenheilkd. 2012;229(4):369–373. doi: 10.1055/s-0031-1299232. [DOI] [PubMed] [Google Scholar]
- 61.Mallett S, Halligan S, Thompson M, Collins GS, Altman DG. Interpreting diagnostic accuracy studies for patient care. BMJ. 2012;345:e3999. doi: 10.1136/bmj.e3999. [DOI] [PubMed] [Google Scholar]
- 62.Albert DM, Ruchman MC, Keltner JL. Skip areas in temporal arteritis. Arch Ophthalmol. 1976;94(12):2072–2077. doi: 10.1001/archopht.1976.03910040732006. [DOI] [PubMed] [Google Scholar]
- 63.Breuer GS, Nesher G, Nesher R. Rate of discordant findings in bilateral temporal artery biopsy to diagnose giant cell arteritis. J Rheumatol. 2009;36(4):794–796. doi: 10.3899/jrheum.080792. [DOI] [PubMed] [Google Scholar]
- 64.Breuer GS, Nesher R, Nesher G. Negative temporal artery biopsies: eventual diagnoses and features of patients with biopsy-negative giant cell arteritis compared to patients without arteritis. Clin Exp Rheumatol. 2008;26(6):1103–1106. [PubMed] [Google Scholar]
- 65.Saleh M, Turesson C, Englund M, Merkel PA, Mohammad AJ. Visual Complications in Patients with Biopsy-proven Giant Cell Arteritis: A Population-based Study. J Rheumatol. 2016;43(8):1559–1565. doi: 10.3899/jrheum.151033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nesher G, Berkun Y, Mates M, et al. Risk factors for cranial ischemic complications in giant cell arteritis. Medicine (Baltimore) 2004;83(2):114–122. doi: 10.1097/01.md.0000119761.27564.c9. [DOI] [PubMed] [Google Scholar]
- 67.Gonzalez-Gay MA, Castaneda S, Llorca J. Giant Cell Arteritis: Visual Loss Is Our Major Concern. J Rheumatol. 2016;43(8):1458–1461. doi: 10.3899/jrheum.160466. [DOI] [PubMed] [Google Scholar]
- 68.Younge BR, Cook BE, Jr, Bartley GB, Hodge DO, Hunder GG. Initiation of glucocorticoid therapy: before or after temporal artery biopsy? Mayo Clin Proc. 2004;79(4):483–491. doi: 10.4065/79.4.483. [DOI] [PubMed] [Google Scholar]
- 69.McGee S. Simplifying likelihood ratios. J Gen Intern Med. 2002;17(8):646–649. doi: 10.1046/j.1525-1497.2002.10750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hauenstein C, Reinhard M, Geiger J, et al. Effects of early corticosteroid treatment on magnetic resonance imaging and ultrasonography findings in giant cell arteritis. Rheumatology (Oxford) 2012;51(11):1999–2003. doi: 10.1093/rheumatology/kes153. [DOI] [PubMed] [Google Scholar]
- 71.Schmidt WA, Blockmans D. Use of ultrasonography and positron emission tomography in the diagnosis and assessment of large-vessel vasculitis. Curr Opin Rheumatol. 2005;17(1):9–15. doi: 10.1097/01.bor.0000147282.02411.c6. [DOI] [PubMed] [Google Scholar]
- 72.Rao JK, Allen NB, Pincus T. Limitations of the 1990 American College of Rheumatology classification criteria in the diagnosis of vasculitis. Ann Intern Med. 1998;129(5):345–352. doi: 10.7326/0003-4819-129-5-199809010-00001. [DOI] [PubMed] [Google Scholar]
- 73.Hunder GG. The use and misuse of classification and diagnostic criteria for complex diseases. Ann Intern Med. 1998;129(5):417–418. doi: 10.7326/0003-4819-129-5-199809010-00013. [DOI] [PubMed] [Google Scholar]
- 74.Murchison AP, Gilbert ME, Bilyk JR, et al. Validity of the American College of Rheumatology criteria for the diagnosis of giant cell arteritis. Am J Ophthalmol. 2012;154(4):722–729. doi: 10.1016/j.ajo.2012.03.045. [DOI] [PubMed] [Google Scholar]
- 75.Lopez-Diaz MJ, Llorca J, Gonzalez-Juanatey C, Pena-Sagredo JL, Martin J, Gonzalez-Gay MA. The erythrocyte sedimentation rate is associated with the development of visual complications in biopsy-proven giant cell arteritis. Semin Arthritis Rheum. 2008;38(2):116–123. doi: 10.1016/j.semarthrit.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 76.Walvick MD, Walvick MP. Giant cell arteritis: laboratory predictors of a positive temporal artery biopsy. Ophthalmology. 2011;118(6):1201–1204. doi: 10.1016/j.ophtha.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 77.Gonzalez-Gay MA, Lopez-Diaz MJ, Barros S, et al. Giant cell arteritis: laboratory tests at the time of diagnosis in a series of 240 patients. Medicine (Baltimore) 2005;84(5):277–290. doi: 10.1097/01.md.0000180043.19285.54. [DOI] [PubMed] [Google Scholar]
- 78.Fabry TL. Mechanism of erythrocyte aggregation and sedimentation. Blood. 1987;70(5):1572–1576. [PubMed] [Google Scholar]
- 79.Pawlotsky Y, Goasguen J, Guggenbuhl P, et al. Sigma ESR: an erythrocyte sedimentation rate adjusted for the hematocrit and hemoglobin concentration. Am J Clin Pathol. 2004;122(5):802–810. doi: 10.1309/8H4L-45F5-G0G1-VKY1. [DOI] [PubMed] [Google Scholar]
- 80.Hegg R, Lee AG, Tagg NT, Zimmerman MB. Statin or nonsteroidal anti-inflammatory drug use is associated with lower erythrocyte sedimentation rate in patients with giant cell arteritis. J Neuroophthalmol. 2011;31(2):135–138. doi: 10.1097/WNO.0b013e31820c4421. [DOI] [PubMed] [Google Scholar]
- 81.Alberg AJ, Park JW, Hager BW, Brock MV, Diener-West M. The use of “overall accuracy” to evaluate the validity of screening or diagnostic tests. J Gen Intern Med. 2004;19(5 Pt 1):460–465. doi: 10.1111/j.1525-1497.2004.30091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]