Abstract
With the extirpation of tigers from the Indonesian island of Java in the 1980s, the endemic and Critically Endangered Javan leopard is the island’s last remaining large carnivore. Yet despite this, it has received little conservation attention and its population status and distribution remains poorly known. Using Maxent modeling, we predicted the locations of suitable leopard landscapes throughout the island of Java based on 228 verified Javan leopard samples and as a function of seven environmental variables. The identified landscapes covered over 1 million hectares, representing less than 9% of the island. Direct evidence of Javan leopard was confirmed from 22 of the 29 identified landscapes and included all national parks, which our analysis revealed as the single most important land type. Our study also emphasized the importance of maintaining connectivity between protected areas and human-modified landscapes because adjacent production forests and secondary forests were found to provide vital extensions for several Javan leopard subpopulations. Our predictive map greatly improves those previously produced by the Government of Indonesia’s Javan Leopard Action Plan and the IUCN global leopard distribution assessment. It shares only a 32% overlap with the IUCN range predictions, adds six new priority landscapes, all with confirmed presence of Javan leopard, and reveals an island-wide leopard population that occurs in several highly fragmented landscapes, which are far more isolated than previously thought. Our study provides reliable information on where conservation efforts must be prioritized both inside and outside of the protected area network to safeguard Java’s last remaining large carnivore.
Introduction
Carnivores are one of the most threatened groups of terrestrial mammals on earth [1,2]. Within this order, members of Felidae, have suffered the severest population declines and geographical range contractions [1,3,4]. The threats facing large felid species are widely shared. They include habitat loss and fragmentation caused by the expansion of small holder farmland, large-scale monoculture plantations and infrastructure, and also the killing of prey and the felid species itself, in retaliation to conflict or for trade [2,5].
The Endangered leopard (Panthera pardus) [6] has the widest geographic distribution of the wild felids [4,7]. It occurs in a broad range of habitats and continents, such as the savannah plains in Africa [4], temperate forests in Russia and China, and humid evergreen rainforests in Southeast Asia [4,8–10]. The species’ behavioral plasticity allows it to survive in areas where other big cats have been extirpated or isolated, such as those close to major human settlements or with an unnaturally low prey base [11–14]. Their high adaptability, however, does not make them tolerant of all threats and a recent assessment revealed that the leopard has lost between 63% and 75% of its historical range, for which a disproportionately high loss (83–87%) has occurred in Asia [4].
The Javan leopard P. p. melas (Cuvier 1809) is one of the most threatened subspecies of leopard. It is endemic to the Indonesian island of Java, which contains 141 million people and has one of the highest human population densities (1,115 people/km2) in the world, which greatly restricts the Javan leopard’s island-wide distribution [15]. From the nine recognized subspecies, the Javan leopard is among the three Critically Endangered, along with the Amur leopard and Arabian leopard [16].
Javan leopard is listed on CITES Appendix I [17] and a nationally protected species under Indonesian Government Regulation No. 7/1999. Yet to date, only a few scientific studies have been conducted on the Javan leopard [18–21] and there is a lack of reliable information on its population status across the island.
In 1990, a study recorded that Javan leopard occurred in 12 conservation areas, including national parks, nature reserves, game reserves and hunting reserves, and these supported an estimated population of 350–700 leopards [10]. Later studies, however, found that this subspecies also occurred outside of the conservation area network, such as in production forest and protected forest [22]. In 2013, this led a group of Indonesian scientists to estimated that there were 491–596 leopards in the remaining natural forests across Java [22]. The majority of these leopards existed in ten national parks, of which only two of them, Halimun Salak and Ujung Kulon, were considered to be able to support more than 100 individuals.
A recent global study on leopards found that the Javan leopard occurred in only 16% (20,600 km2) of its historical range that had once covered Java [4]. It also found that only 3% of the Javan leopard’s core area now remains and this yielded the lowest median patch size and core area index for any of the leopard subspecies; indicating that it is at a greater risk of extinction than any of the other leopard subspecies. Such spatial extent is much larger than that documented in the Javan Leopard National Action Plan (3% or 3,277 km2) [22]. This difference is most likely an artifact of the different spatial datasets used for deriving both estimates. The first estimate was based on 41 records, and restricted to protected areas, while the later on 34 localities with an unclear definition of whether a locality represented a distinct patch or not. This strongly suggests that a more comprehensive and finer-scale estimate is needed to produce a reliable baseline on Javan leopard distribution.
Species distribution modelling based on presence-only datasets is widely used to assist in the management of understudied and threatened species [23–25]. So, in this study we aim to: 1) collate all recent data from 2008–2014 on Javan leopard occurrence; 2) model suitable habitat of Javan leopard, 3) define the extent of suitable habitat inside and outside of the protected area network, 4) investigate land classes that have the potential to support Javan leopard outside of this network, and 5) identify priority actions for future Javan leopard conservation based on our key findings. The main purpose of this study is to provide decision makers and conservation planners alike with the first robust estimate of Javan leopard distribution using a species distribution modelling approach that is based on the most extensive Javan leopard occurrence dataset available.
Methods
Permits of several projects in this study were obtained from the national parks and the local natural resources conservation agencies, the Indonesian Ministry of Environment and Forestry.
Data preparation
We collated unpublished Javan leopard points of occurrences recorded by various field research projects and individuals between 2008 and 2014 (S1 Table). For security reasons, we present the approximate location of each Javan leopard locality within a 100 km2 rectangle (S1 Fig). We then selected points that had verifiable evidence, including camera trap videos and photographs, human-leopard conflict reports, pugmarks, scratches and feces. Given the absence of other large cats in Java, we believed that misidentification of these signs to be unlikely.
We used ten environmental variables that have been shown to be suitable predictors of large carnivore presence by other studies. These included distance from roads [23], distance from forest edges to the interior and exterior, and protected areas [26], elevation [23,26], distance from rivers [27], slope [23,28], land use classification, precipitation, and temperature [29]. The original spatial dataset included protected area land use classification layers [30], roads, rivers and Indonesia baseline maps [31], digital elevation map (30 m resolution) [32], and mean precipitation and temperature (1 km resolution) [33]. We converted the protected area layer into a binary raster containing '0' (non-protected area) and '1' (protected area).
The original land use classification layer contained 18 classes. For better interpretation, we reduced it to 15 classes based on similarities and converted them into raster layers. We generated the slope layer from the digital elevation map. We selected forest polygons from the land use classification and generated Euclidian distances to produce the distance from forest edge to the Javan leopard points recorded both inside and outside of the primary forest polygons. Thus, for distance from forest edge to the exterior, all Javan leopard points that fell within the forest were assigned a value of '0' and vice versa for the distance from forest edge to the interior. Similarly, we employed Euclidian distance to roads (vector) and rivers (vector) to generate the distance from roads and rivers (30 m resolution) layers, respectively.
The primary analysis tool used in this study required all datasets to have exactly overlapping cells and spatial extent [34], which in this study was confined to the island of Java. We generated a raster mask of 0.25 km2 cell size covering Java to provide a baseline environment setting for further resample processing of the background layers. We then re-sampled the Javan leopard points and all environmental variables with the application of a mask layer. This procedure removed any duplication of Javan leopard points within a cell for subsequent analysis. Because several environmental variables were re-sampled from finer to coarser resolution, we performed a bilinear interpolation technique to assign a new value to a cell by using a weighted distance average of four adjacent input cells. All spatial processes were performed using Raster Processing and Spatial Analyst tools in the software ArcGIS 10.2 (ESRI, Redlands).
The Javan leopard points were found to be biased toward the sampled areas, a situation common to presence-only datasets [35]. To control for this sampling bias, we converted the mask layer into a bias grid [36]. In the analysis, the value of a cell (c1) in the bias grid was used to assign greater weight to Javan leopard points (c2) with fewer spatial neighbors and vice versa. The value of c1 was a sum of the distances between c1 and c2 as calculated using the Gaussian Kernel function, w = exp(−d2/2s2), where w is the weight, d is the distance (in km) between c1 and c2, and s is the standard deviation. We used 4.2 km for s because it represents the known diameter of the largest Javan leopard home range size (13.6 km2) [18,19]. Following the Gaussian distribution, s, yielded points that are, for example, located 4.2 and 8.4 km away from a cell as having 60.6% and 13.5% as strong an influence, respectively. We used the Distance Among Points tool of the Geospatial Modeling Environment version 0.7.3.0 [37] and the Calculate Variable and Aggregate tools of the IBM SPSS Statistics software version 20 (IBM Corporation 2011) to calculate the distance between c1 and c2 and the Gaussian Kernel function, respectively.
Data analysis
We predicted suitable landscape of Javan leopard using Maxent version 3.4.1. Maxent has been widely used for presence only dataset over other techniques due to its robustness against autocorrelated environmental predictors [38,39], lower sensitivity to small sample sizes [40], and being less affected by spatial errors [41]. The final sample inputs consisted of 169 Javan leopard presence points. We performed a Pearson’s correlation analysis using the Hmsc package within R software (R Development Core Team 2010) to test for correlations between the ten environmental variables, from which a pair of variables was removed if the coefficient correlation was > 0.50 [42]. The final set of uncorrelated environmental variables used in the subsequent analysis included distance from forest edges to the interior and exterior, distance from roads, protected areas, elevation, distance from rivers, land classification and mean precipitation.
We set the protected areas and land classifications as categorical variables and the rest as continuous variables. We performed a Bootstrap procedure with 25% random tests, ten replicates, and 5,000 iterations, and kept the other settings at the default option. We also performed Jackknife tests to assess consistency in variable importance between the training and test gains [43]. The overall model performance was measured by the area under the curve (AUC) of the receiver operating characteristic (ROC) curve [39]. We estimated the relative importance of each predictor to the Maxent model using the percent contribution and permutation importance, averaged over ten replicates. We investigated the response curves to explore how the environmental predictors effected the Maxent prediction.
Identifying landscapes and defining its characteristics
We used the model prediction to determine suitable Javan leopard landscapes based on a ten percentile training presence logistic threshold. Model pixels with logistic probabilities smaller than the threshold were omitted from the final predictive model [24,34,44]. We converted the predicted suitable patch raster into a polygon format and defined the minimum suitable patch size for Javan leopard as being large enough to contain at least five mature individuals [28]. Subsequently, we retained all predicted suitable patches of at least 68 km2 or equal to five times the largest known Javan leopard home range [18,19]. For a Critically Endangered subspecies like Javan leopard, smaller suitable patches with confirmed evidence of Javan leopard may still be important if it supports connectivity between patches. We therefore retained all smaller suitable patches with and/or close to Javan leopard localities up to 4.2 km away or equal to the diameter of their home range. Predicted suitable patches that did not meet these criteria were removed from further analyses.
We assumed all protected areas that fully or partially overlapped with the predicted suitable patches as being important for Javan leopard. We updated these patches with protected areas using the Update tool of the ArcGIS 10.2. This step removed all stand-alone protected areas and combined them with associated suitable patches into one unique polygon. We calculated the area of the identified suitable patches in each protected area as a surrogate indicator of the ecological importance of each protected area to support Javan leopard.
We included the protected area layer in the land use classification layer, so that it would be considered in the analysis. This step removed all land classes that fell within a protected area into one unique polygon coded as “protected area” and produced 16 land use classes. We defined suitable landscapes as patches that: 1) are predicted to be suitable for Javan leopard based on a ten percentile training presence logistic threshold, 2) are larger than 68 km2, and/or 3) have evidence of Javan leopard presence, and/or, 4) within 4.2 km of a Javan leopard locality. We then extracted the land use classes in each suitable landscape by intersecting the landscape layer with a land use classification layer using the ArcGIS 10.2 Intersect tool.
Results
We obtained 228 verified Javan leopard presence points, the majority of which came from a combined record of Javan leopard signs (n = 196) and conflict incidents (n = 32). The elevation range of these data points was 1 to 2,540 m asl, with a mean value of 714 m asl. Approximately half (50.9%) of the leopard records occurred inside the protected area network, with evidence of leopard from all nine national parks in Java. For those records occurring outside of the protected area network (51.6%), most (36.6%) points were located in secondary forest, followed by mixed agriculture (22.3%), production forest (20.5%) and other land use types (20.5%). For the conflict records, most (53.1%) leopard records were recorded from mixed agriculture areas (Table 1).
Table 1. Contribution of Javan leopard occurrence records in different land use types.
Javan leopard occurrence was identified based on direct and indirect signs, and conflict incidents.
| Land use | Conflict | Signs | Total | Percent |
|---|---|---|---|---|
| Protected area | 3 | 113 | 116 | 50.9% |
| Secondary forest | 1 | 40 | 41 | 18.0% |
| Production forest | 3 | 20 | 23 | 10.1% |
| Mixed agriculture | 17 | 8 | 25 | 11.0% |
| Plantation | 2 | 9 | 11 | 4.8% |
| Rice field | 3 | 4 | 7 | 3.1% |
| Settlement | 3 | 0 | 3 | 1.3% |
| Shrub | 0 | 2 | 2 | 0.9% |
| Total | 32 | 196 | 228 | 100% |
The Maxent analysis identified distance from forest edge and mean precipitation as providing the highest contribution to the predicted leopard distribution, respectively explaining 49.7% and 25.4% of the variation in the predicted suitable patches (Table 2). The Jackknife test for variable importance using the regularized training gain, test gain, and AUC on test data indicated that both distance from forest edge and mean precipitation were the two predictors with the highest gains when used in isolation, which decreased most when these variables were omitted from other candidate models.
Table 2. The relative contribution and permutation importance of each variable calculated by Maxent.
Values are averaged over the 10 replicates and normalized to give percentages. The permutation importance was used to assess variable importance.
| Variable | Percent contribution | Permutation importance |
|---|---|---|
| Distance from forest edge to the exterior | 49.7 | 60.1 |
| Precipitation | 25.4 | 21.5 |
| Protected Area | 9.8 | 3.6 |
| Land use type | 5.9 | 4.5 |
| Distance from river | 4.1 | 3.4 |
| Distance from forest edge to the interior | 2.9 | 3.3 |
| Elevation | 1.1 | 1.9 |
| Distance from road | 1 | 1.7 |
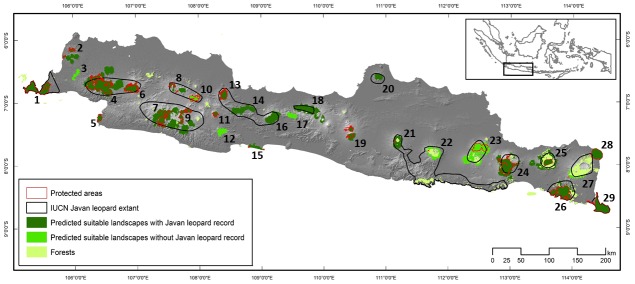
The response curves indicated that Javan leopard was more likely to occur in forest and in landscapes with higher precipitation (S2 Fig). The species distribution model performed well, with a mean AUC±SE of 0.95±0.007. Applying a ten percentile threshold, only model pixels with a logistic probability of 0.42 or greater were classified as being suitable for Javan leopard. The area of suitable landscape identified was 1,159,864 ha and covered 8.9% of the island of Java [15]. Protected area (40.5%), production forest (17.2%), and secondary forest (13.6%) contributed the largest area (62%) of suitable landscape (S2 Table). Evidence of Javan leopard was recorded from 22 of the 29 suitable landscapes, meaning that seven landscapes would require further field checks to confirm leopard presence (Fig 1).
Fig 1. Predictive map identifying suitable Javan leopard landscapes on the Indonesian island of Java.
The Maxent model outputs were defined to be suitable for Javan leopard if they had a logistic probability of 0.42 or greater. The numbers represent the 29 predicted suitable landscapes listed in S2 Table.
Discussion
The predictive map produced from our study not only refines maps produced from the Government of Indonesia’s Javan Leopard Action Plan [22] and the IUCN global leopard distribution assessment [4,16], but represents the first to be developed from a spatially-explicit modelling process for this subspecies. Our dataset, which is based on 228 occurrence records, is a marked improvement on the 2017 IUCN Javan leopard assessment that was based on significantly fewer (34) data points [16]. Our map shared only a 32% overlap with the IUCN range prediction, but adds six new landscapes (ID: 2, 5, 11, 15, 19, 28), all with confirmed evidence of Javan leopard, and reveals an island-wide leopard population that occurs in several highly fragmented landscapes, indicating that it is far more isolated than previously thought (Fig 2).
Fig 2. Protected areas in 24 out of 29 suitable landscapes.
Study limitations
A limitation of this study was that it was unable to incorporate a data layer on prey availability due to the absence of such data from the island of Java [22]. It was not, therefore, possible to investigate the potential of the predicted suitable landscapes, especially those are outside the protected areas and forest habitats, to support viable populations of Javan leopard over the long-term. Future studies should therefore aim to address this question, especially in the priority landscapes. The Javan leopard occurrence data used in our analysis might have certain limitations that are associated with the use of a presence-only dataset. First, data were collated from multiple sources and not based on surveys that used the same sampling method. However, because such a dataset does not exist, we therefore aimed to collect as much data as possible and then filter these to remove redundancy whilst maintaining a comprehensive dataset. Second, although Maxent is less sensitive to small sample sizes [40] and less affected by spatial errors [41], it requires that the samples (occurrence data points) be unbiased and therefore independent of the distribution of the target species [43]. The high AUC value recorded in our model might be an artifact of the AUC statistic, which tends to be higher for species with small home range sizes relative to the study area [43]. However, the potential sampling bias here might have been overcome by our study using a relatively large dataset recorded from all possible habitat types across Java. Furthermore, we controlled for potential sampling bias by using bias grids as a function of their Gaussian Kernel distribution and overcame the dependency issue by resampling the original Javan leopard records into a density of one point per 0.25 km2.
Characterizing Javan leopard landscapes
Our study provides fresh thinking into the role of modified landscapes surrounding protected areas for improving the carrying capacity and, ultimately, long-term survival of the Javan leopard. The Maxent model shows higher association between leopards and more productive landscapes in western Java than the drier forests in eastern Java. This was revealed by the sharp increase in the probability of Javan leopard occurrence in landscapes with higher precipitation. Thus, higher precipitation should support higher plant productivity for the ungulate prey base [45]. However, with the absence of prey data, we were not able to further evaluate the relationship between precipitation and prey base availability. The low contribution of the protected area variable to the overall model output might be explained by the fact that most protected area boundaries were located within larger forest areas, therefore outperformed by the distance from the forest habitats variable.
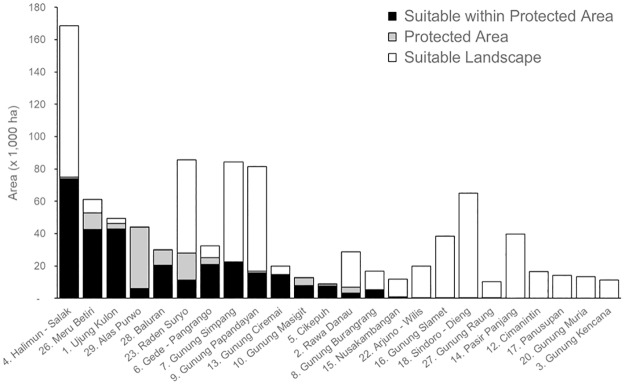
We identified 70% of the protected area network in Java as providing suitable leopard habitat. However, this network contributed only 40% of the total area of the suitable landscapes. A population viability analysis on ten leopard populations in South Africa predicted that no population with fewer than 50 individuals would survive over 100 years without dispersal and connectivity to a neighboring population [46]. Thus, based on a maximum estimated Javan leopard density, 16 leopards/100 km2 [19], 12 suitable landscape patches (#1, 4, 6, 7, 9, 14, 16, 18, 23, 24, 25, 26) were considered large enough to support at least 50 individuals. Here, Javan leopard recovery efforts should focus on strengthening protected area management to reduce poaching and maintain landscape integrity, before moving on to options such as connecting landscapes and their respective leopard populations.
From the landscapes identified with good recovery potential, it is important to stress that, besides the national parks, all other protected area types were found to be too small on their own to hold viable populations of Javan leopard. This situation was also found in several studies in West Africa and North America where many wildlife reserves were to be too small or had inadequate natural resources for achieving large carnivore conservation objectives [47,48]. For the Javan leopard, this highlights the importance of managing suitable landscapes that adjoin these protected areas so as to support viable populations. However, these buffer zone habitats may represent poorer quality habitat and have a lower prey base and therefore support a lower density and number of leopards. Determining leopard densities in these areas and, therefore, how large these areas should be is a topic for future research.
National Park is listed as a Category II protected area of the World Conservation Union [49] and is the strongest protected area type afforded by the Government of Indonesia, in terms of infrastructure, management, financial support and human resources allocated. Thus, strengthening national park management should be considered as a top priority for the long-term survival of the Javan leopard. We identified only three national parks with sufficient habitat to potentially support more than 50 individuals: Gunung Halimun Salak (4, 74,018 ha); Ujung Kulon (1, 42,910 ha); and, Meru Betiri (#26, 42,465 ha). Among these, only Gunung Halimun Salak could potentially support more than 100 individuals, which underlines its importance as a flagship protected area. Two other national parks, Gunung Gede Pangrango (#6) and Bromo Tengger Semeru (#24) could potentially support 50 leopards or more if suitable habitat in the adjacent areas is included.
Understanding the wider landscape characteristics is vital to increasing the population size of large carnivores that are threatened with extinction [50,51]. This not only includes the identification of suitable landscape adjacent to the protected areas, but the key stakeholder groups who should be engaged by conservation managers. Our study found that more than half of the suitable landscape outside of the protected areas is in production forest and secondary forest. Further, with less than 5% of primary forest occurring outside of protected areas, production and secondary forests should therefore play an important role in providing additional habitat for the remaining Javan leopard populations. Here, Government Regulation No. 72/2010 mandates the management of non-conservation state forest in Java to Perum Perhutani, a company under the Ministry of State-owned Enterprises, as the main investor, and the Ministry of Environment and Forestry, as an advisor for its technical and operational activities.
Beside state forest, the Government of Indonesia recognizes the legal right of local communities to manage private forest, which is commonly known as hutan rakyat (Community Forest). The role of local communities in managing hutan rakyat adjacent to state forest is legalized by Perum Perhutani through the establishment of a Community Village Forest Institution (Lembaga Masyarakat Desa Hutan). Under this scheme, local communities are permitted to develop agroforestry for commodities, such as teak, acacia, silk trees and mahogany. In 2009, 2.7 million ha of hutan rakyat in Java was managed by 690,895 households through agroforestry schemes [52,53]. Most hutan rakyat is situated adjacent to state forests and may therefore contribute significant Javan leopard habitat that is located around protected areas.
This study confirms the resilience of leopard outside of its main forest habitat type [54]. We found that nearly half of the Javan leopard data points were recorded outside of protected areas and primary forest, which concurs with other studies that found a high level of leopard adaptability in modified habitats [4,7,11,12,24]. This indicates the ability of leopards to subsist in and move through modified habitat [13], as has been found in India [55], South Africa [56] and Russia [57]. These modified habitat may therefore serve as structural corridors that facilitate dispersal to enable source-sink connectivity between viable and otherwise non-viable leopard populations [46]. Balme et al [58] recorded leopards moving far beyond the productive natural habitats and into areas where they were then killed, either deliberately or accidentally, by people. A successful strategy for conserving a wide-ranging large carnivore will therefore rely on protecting the source population and providing dispersal opportunities with sink populations through maintaining connectivity [24,29,59,60]. This should be conducted with a reduction in leopard and prey offtake from hunting, retaliatory killing and problem animal removal [46]. Further studies on Javan leopard movement and home range size as a function of prey availability and potential threats in different habitat types, especially human-modified ones, will allow conservation managers to better understand the species’ response to available resources and different threats, and thus their survival and reproduction potential [61].
More than a quarter of our Javan leopard occurrences were outside of the protected area network and from conflicts with local communities. For a Critically Endangered subspecies, this is of great concern because of the risk posed by retaliatory killings to conflict incidents can have a disproportionately large impact on small population sizes. These types of attacks are well documented in causing negative local community perceptions and attitudes towards the conservation of large carnivores [62–64]. For Java, the history of the tiger’s extirpation from the island approximately 30 years’ ago provides a salutary lesson for the future of the Javan leopard because ultimately the loss of habitat and ensuing competition with people for space and resources, particularly its ungulate prey base, was to the tiger’s great detriment [65]. The Javan leopard, despite being more adaptable than the tiger, now occupies an island that whilst only accounting for 6.9% of Indonesia’s land mass is home to 60% of its human population. This restricts core forest habitat to volcanic peaks and coastal corners. Despite this, our study identifies that many of the occupied landscapes may still contain viable leopard populations, making their protection a top priority. It also identifies smaller landscapes that should be connected to achieve the same aim. Thus, our spatially-explicit modelling provides time-critical information for implementing the 2016–2026 Javan leopard action plan to better effect. This approach could also be adopted for other Critically Endangered species for which there is sparse data, such as the saola.
Supporting information
For security reasons, Javan leopard localities were approximated in 100 km2 rectangles.
(TIF)
The curves show the mean response of the 10 replicates (red) and associated one standard deviation (grey area, error bar for categorical variables).
(DOCX)
(DOCX)
Suitable landscapes were defined based on Maxent model outputs with logistic probabilities of 0.42 or greater.
(PDF)
Acknowledgments
We would like to thank the Indonesian Ministry of Environment and Forestry, civil society partners, and individual contributors for supporting the project as listed in the S1 Table.
Data Availability
Due to the sensitive nature and ownership of the study data, it has not been made publicly available. Data deposition could present some other threat, such as revealing the locations of Javan leopard that are listed as Critically Endangered by the IUCN Red Databook and protected by the Indonesian Law No. 5/1990 regarding the Biodiversity Conservation and Ecosystem, Government Regulation No. 7/1999 regarding the Preservation of Animals and Plants. Part of the dataset are owned by third parties who limit the use of their data for the purpose of this study. Others can access these datasets by directly contacting the individual contributors listed in S1 Table and we confirm that others would be able to access these data in the same manner as the authors. Additionally, the corresponding author may assist others in obtaining contacts of the individual contributors. We also confirm that we did not have any special access privileges that others would not have.
Funding Statement
Projects in this study were partly funded by the Mohammed Bin Zayed Species Conservation Fund 13256220 (URL: https://www.speciesconservation.org/case-studies-projects/javan-leopard/6220) (EW), IdeaWild for a project titled "Initiating the protection of the last big carnivore of Java at Mount Slamet, Central Java" (HAW), and PT Holchim Indonesia and Fauna & Flora International for a biodiversity conservation planning in Nusakambangan Island (IP, MP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ceballos G, Ehrlich PR, Soberon J, Salazar I, Fay JP. Global mammal conservation: What must we manage? Science (80-). 2005;309: 603–607. doi: 10.1126/science.1114015 [DOI] [PubMed] [Google Scholar]
- 2.Karanth KU, Chellam R. Carnivore conservation at the crossroads. Oryx. 2009;43: 1 doi: 10.1017/S003060530843106X [Google Scholar]
- 3.Schipper J, Chanson JS, Chiozza F, Cox NA, Hoffmann M, Katariya V, et al. The status of the world’s and and marine mammals: Diversity, threat, and knowledge. Science (80-). 2008;322: 225–230. doi: 10.1126/science.1165115 [DOI] [PubMed] [Google Scholar]
- 4.Jacobson AP, Gerngross P, Lemeris JR Jr, Schoonover RF, Anco C, Breitenmoser-Würsten C, et al. Leopard (Panthera pardus) status, distribution, and the research efforts across its range. PeerJ. 2016;4: e1974 doi: 10.7717/peerj.1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nowell K, Jackson P. Wild cats: Status survey and conservation action plan. Gland, Switzerland; 1996. [Google Scholar]
- 6.Kitchener A, Breitenmoser-Würsten C, Eizirik E, Gentry A, Werdelin L, Wilting A, et al. A revised taxonomy of the Felidae. Cat News. 2017; 80. [Google Scholar]
- 7.Swanepoel LH, Somers MJ, van Hoven W, Schiess-Meier M, Owen C, Snyman A, et al. Survival rates and causes of mortality of leopards Panthera pardus in southern Africa. Oryx. 2015;49: 595–603. doi: 10.1017/S0030605313001282 [Google Scholar]
- 8.Al-Johany AMH. Distribution and conservation of the Arabian leopard Panthera pardus nimr in Saudi Arabia. J Arid Environ. 2007;68: 20–30. doi: 10.1016/j.jaridenv.2006.04.002 [Google Scholar]
- 9.Chapron G, McCarthy TM. Snow leopard survival strategy. 4649 Sunnyside Ave. N. Suite 325, Seattle, WA 98103; 2003.
- 10.Santiapillai C, Ramono WS. Status of the leopard (Panthera pardus) in Java, Indonesia. Tiger Paper. 1992. pp. 1–5.
- 11.Athreya V, Odden M, Linnell JDC, Krishnaswamy J, Karanth KU. A cat among the dogs: Leopard Panthera pardus diet in a human-dominated landscape in western Maharashtra, India. Oryx. 2014;50: 1–7. doi: 10.1017/S0030605314000106 [Google Scholar]
- 12.Athreya V, Odden M, Linnell JDC, Krishnaswamy J, Karanth U. Big cats in our backyards: Persistence of large carnivores in a human dominated landscape in India. PLoS One. 2013;8: 2–9. doi: 10.1371/journal.pone.0057872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harihar A, Pandav B, Goyal SP. Responses of leopard Panthera pardus to the recovery of a tiger Panthera tigris population. J Appl Ecol. 2011;48: 806–814. doi: 10.1111/j.1365-2664.2011.01981.x [Google Scholar]
- 14.Rayan DM, Linkie M. Managing conservation flagship species in competition: Tiger, leopard and dhole in Malaysia. Biol Conserv. 2016;204: 360–366. doi: 10.1016/j.biocon.2016.11.009 [Google Scholar]
- 15.Biro Pusat Statistik. Kepadatan Penduduk Menurut Propinsi, 2000–2015 [Internet]. 2017 [cited 2 Sep 2017]. https://www.bps.go.id/linkTableDinamis/view/id/842
- 16.Stein AB, Athreya V, Gerngross P, Balme GA, Henschel P, Karanth UK, et al. Panthera pardus. In: The IUCN Red List of Threatened Species [Internet]. 2016 [cited 7 Mar 2017].
- 17.UNEP, WCMC. Checklist of CITES Species [Internet]. Geneva, Switzerland and UNEP-WCMC, Cambridge, United Kingdom; 2014. http://checklist.cites.org/#/en
- 18.Ario A. Javan leopard (Panthera pardus melas) among human activities: Preliminary assessment on the carrying capacity of Mount Salak Forest Area, Mount Halimun-Salak National Park. Jakarta, Indonesia; 2007.
- 19.Ario A, Hidayat E, Supian. Protection and monitoring of the endangered species of Javan leopard (Panthera pardus melas) in Gunung Gede Pangrango National Park, West Java, Indonesia. Jakarta, Indonesia; 2009.
- 20.Gunawan H, Prasetyo LB, Mardiastuti A, Kartono AP. Habitat of Javan leopard (Panthera pardus melas Cuvier 1809) in pine plantation forest landscape. Penelit Hutan dan Konserv Alam. 2012;9: 49–67. [Google Scholar]
- 21.Gunawan H, Prasetyo LB, Mardiastuti A, Kartono AP, Belakang AL. Habitat macan tutul jawa (Panthera pardus melas Cuvier 1809) di lanskap hutan produksi yang terfragmentasi. Penelit Hutan dan Konserv Alam. 2009;6: 95–114. [Google Scholar]
- 22.KLHK. Strategi dan rencana aksi konservasi macan tutul jawa (Panthera pardus melas): 2016–2026. Jakarta, Indonesia: Direktorat Jenderal Konservasi Sumberdaya Alam dan Ekosistem, Kementerian Lingkungan Hidup dan Kehutanan Republik Indonesia; 2016.
- 23.Erfanian B, Mirkarimi SH, Mahini AS, Rezaei HR. A presence-only habitat suitability model for Persian leopard Panthera pardus saxicolor in Golestan National Park, Iran. Wildlife Biol. 2013;19: 170–178. doi: 10.2981/12-045 [Google Scholar]
- 24.Swanepoel LH, Lindsey P, Somers MJ, van Hoven W, Dalerum F. Extent and fragmentation of suitable leopard habitat in South Africa. Anim Conserv. 2013;16: 41–50. doi: 10.1111/j.1469-1795.2012.00566.x [Google Scholar]
- 25.Hemery LG, Marion SR, Romsos CG, Kurapov AL, Henkel SK. Ecological niche and species distribution modelling of sea stars along the Pacific Northwest continental shelf. Divers Distrib. 2016;22: 1314–1327. doi: 10.1111/ddi.12490 [Google Scholar]
- 26.Wibisono HT, Linkie M, Guillera-Arroita G, Smith JA, Sunarto, Pusparini W, et al. Population status of a cryptic top predator: An island-wide assessment of tigers in Sumatran rainforests. PLoS One. 2011;6: e25931 doi: 10.1371/journal.pone.0025931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ngoprasert D, Lynam AJ, Gale GA. Human disturbance affects habitat use and behaviour of Asiatic leopard Panthera pardus in Kaeng Krachan National Park, Thailand. Oryx. 2007;41: 343–351. doi: 10.1017/S0030605307001102 [Google Scholar]
- 28.Hebblewhite M, Miquelle DG, Murzin AA, Aramilev VV, Pikunov DG. Predicting potential habitat and population size for reintroduction of the Far Eastern leopards in the Russian Far East. Biol Conserv. Elsevier Ltd; 2011;144: 2403–2413. doi: 10.1016/j.biocon.2011.03.020 [Google Scholar]
- 29.Gavashelishvili A, Lukarevskiy V. Modelling the habitat requirements of leopard Panthera pardus in West and Central Asia. J Appl Ecol. 2008;45: 579–588. [Google Scholar]
- 30.KLHK. Land Cover Classification [Internet]. 2011 [cited 25 Aug 2016]. http://webgis.dephut.go.id:8080/kemenhut/index.php/id/
- 31.Geospatial Untuk Negeri. Peta Rupa Bumi Indonesia [Internet]. 2016 [cited 10 Nov 2016]. http://tanahair.indonesia.go.id/home/
- 32.United State Geological Survey. Earth Explorer. In: United State Department of the Interior [Internet]. 2017 [cited 30 Aug 2016]. https://earthexplorer.usgs.gov/
- 33.Hijmans RJ, Cameron S, Parra J, Jones P, Jarvis A, Richardson K. WorldClim—Global Climate Data: WorldClim Version 1. In: Museum of Vertebrate Zoology, University of California, Berkeley [Internet]. 2005 [cited 30 Aug 2015]. http://www.worldclim.org/version1
- 34.Young N, Carter L, Evangelista P. A Maxent model v3.3.3e Tutorial (ArcGIS v7). Colorado State University; 2011.
- 35.Phillips SJ, Dudik M, Elith J, Graham CH, Leathwick J, Ferrier S, et al. Sample selection bias and presence-only distribution models: Implications for background and pseudo-absence data. Ecol Appl. 2009;19: 181–197. [DOI] [PubMed] [Google Scholar]
- 36.Clements GR, Rayan DM, Aziz SA, Traeholt C, Magintan D, Fadlli M, et al. Predicting the distribution of the Asian tapir in Peninsular Malaysia using maximum entropy modeling. Integr Zool. 2012;7: 400–406. doi: 10.1111/j.1749-4877.2012.00314.x [DOI] [PubMed] [Google Scholar]
- 37.Beyer HL. Geospatial Modelling Environment [Internet]. 2012. http://www.spatialecology.com/gme
- 38.Elith J, Kearney M, Phillips S. The art of modelling range-shifting species. Methods Ecol Evol. 2010;1: 330–342. doi: 10.1111/j.2041-210X.2010.00036.x [Google Scholar]
- 39.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Modell. 2006;190: 231–259. doi: 10.1016/j.ecolmodel.2005.03.026 [Google Scholar]
- 40.Elith J, Graham CH, Anderson RP, Dudı M, Ferrier S, Guisan A, et al. Novel methods improve prediction of species’ distributions from occurrence data. 2006;2: 129–151. [Google Scholar]
- 41.Graham CH, Elith J, Hijmans RJ, Guisan A, Peterson AT, Loiselle BA, et al. The influence of spatial errors in species occurrence data used in distribution models. J Appl Ecol. 2008;45: 239–247. doi: 10.1111/j.1365-2664.2007.01408.x [Google Scholar]
- 42.McCarthy JL, Wibisono HT, McCarthy KP, Fuller TK, Andayani N. Assessing the distribution and habitat use of four felid species in Bukit Barisan Selatan National Park, Sumatra, Indonesia. Glob Ecol Conserv. 2015;3: 210–221. doi: 10.1016/j.gecco.2014.11.009 [Google Scholar]
- 43.Phillips S. A brief tutorial on Maxent. AT&T Res. 2008; 1–38. [Google Scholar]
- 44.Raes N, Roos MC, Slik JWF, Van Loon EE, Ter Steege H. Botanical richness and endemicity patterns of Borneo derived from species distribution models. Ecography (Cop). 2009;32: 180–192. doi: 10.1111/j.1600-0587.2009.05800.x [Google Scholar]
- 45.Sunquist ME, Karanth UK, Sunquist F. Ecology, behavior, and resilient of the tiger and its conservation needs In: Seidensticker J, Christie S, Jackson P, editors. Riding the tiger. 1st ed Cambridge University Press; 1999. pp. 5–18. [Google Scholar]
- 46.Daly B, Power J, Camacho G, Traylor-Holzer K, Barber S, Catterall S, et al. Leopard (Panthera pardus) population and habitat viability assessment. SSC/IUCN CBSG South Africa, Endangered Wildlife Trust; 2015. p. 105 doi: 10.13140/RG.2.1.1148.4649 [Google Scholar]
- 47.Linnell JDC, Swenson JE, Andersen R, Al ET. Predators and people: Conservation of large carnivores is possible at high human densities if management policy is favourable. Anim Conserv. 2001;4: 345–349. [Google Scholar]
- 48.Brashares JS, Arcese P, Sam MK. Human demography and reserve size predict wildlife extinction in West Africa. Proc R Soc B Biol Sci. 2001;268: 2473–2478. doi: 10.1098/rspb.2001.1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.IUCN. Protected Areas Categories | IUCN [Internet]. 2017 [cited 23 Aug 2017]. https://www.iucn.org/theme/protected-areas/about/protected-areas-categories
- 50.Kautz R, Kawula R, Hoctor T, Comiskey J, Jansen D, Jennings D, et al. How much is enough? Landscape-scale conservation for the Florida panther. Biol Conserv. 2006;130: 118–133. doi: 10.1016/j.biocon.2005.12.007 [Google Scholar]
- 51.Hayward MW, O’Brien J, Kerley GIH. Carrying capacity of large African predators: Predictions and tests. Biol Conserv. 2007;139: 219–229. doi: 10.1016/j.biocon.2007.06.018 [Google Scholar]
- 52.Mugiyono. Penyusunan database hutan rakyat di Pulau Jawa: Sebagai pra kondisi implementasi sistem legalisasi kayu dan rencana proyek karbon. Yogyakarta; 2009.
- 53.Suprapto E. Hutan rakyat: Aspek produksi, ekologi, dan kelembagaan. Seminar nasional kontribusi pengurangan emisi karbon dari kawasan hutan yang dikelola masyarakat secara lestari dan berkelanjutan. Jakarta, Indonesia: Forest Watch Indonesia; 2010. pp. 1–8.
- 54.Wang SW, Macdonald DW. The use of camera traps for estimating tiger and leopard populations in the high altitude mountains of Bhutan. Biol Conserv. 2009;142: 606–613. doi: 10.1016/j.biocon.2008.11.023 [Google Scholar]
- 55.Dutta T, Sharma S, Maldonado JE, Wood TC, Panwar HS, Seidensticker J. Fine-scale population genetic structure in a wide-ranging carnivore, the leopard (Panthera pardus fusca) in central India. Divers Distrib. 2013;19: 760–771. doi: 10.1111/ddi.12024 [Google Scholar]
- 56.Bailey TN. The African Leopard: Ecology and behavior of a solitary felid. New York, USA: Columbia University Press; 1993. [Google Scholar]
- 57.Miquelle DG, Rozhnov VV, Ermoshin V, Murzin AA, Nikolaev IG, Hernandez-Blanco JA, et al. Identifying ecological corridors for Amur tigers (Panthera tigris altaica) and Amur leopards (Panthera pardus orientalis). Integr Zool. 2015;10: 389–402. doi: 10.1111/1749-4877.12146 [DOI] [PubMed] [Google Scholar]
- 58.Balme GA, Slotow R, Hunter LTB. Impact of conservation interventions on the dynamics and persistence of a persecuted leopard (Panthera pardus) population. Biol Conserv. 2009;142: 2681–2690. doi: 10.1016/j.biocon.2009.06.020 [Google Scholar]
- 59.Walston J, Karanth U, Stokes E. Avoiding the unthinkable: What will it cost to prevent tigers becoming extinct in the wild? New York, USA: Wildlife Conservation Society; 2010. [Google Scholar]
- 60.Wibisono HT, Martyr DJ, Haidir IA. Managing tiger conservation landscapes habitat connectivity in sumatra: threats and possible solutions. In: Global Tiger Initiative Secretariat, editor. Managing tiger conservation landscapes and habitat connectivity: Threats and possible solutions: Experiences from Bangladesh, India, Indonesia, Malaysia, Myanmar, Nepal, Thailand, and Vietnam. Washington, D.C.; 2012. pp. 14–29.
- 61.Simcharoen S, Barlow ACD, Simcharoen A, Smith JLD. Home range size and daytime habitat selection of leopards in Huai Kha Khaeng Wildlife Sanctuary, Thailand. Biol Conserv. 2008;141: 2242–2250. doi: 10.1016/j.biocon.2008.06.015 [Google Scholar]
- 62.Wang SW, Lassoie JP, Curtis PD. Farmer attitudes towards conservation in Jigme Singye Wangchuck National Park, Bhutan. Environ Conserv. 2006;33: 148–156. doi: 10.1017/S0376892906002931 [Google Scholar]
- 63.Wang SW, Curtis PD, Lassoie JP. Farmer perceptions of crop damage by wildlife in Jigme Singye Wangchuck National Park, Bhutan. Source Wildl Soc Bull. 2006;34: 359–365. doi: 10.2193/0091-7648(2006)34[359:fpocdb]2.0.co;2 [Google Scholar]
- 64.Dar NI, Minhas RA, Zaman Q, Linkie M. Predicting the patterns, perceptions and causes of human-carnivore conflict in and around Machiara National Park, Pakistan. Biol Conserv. 2009;142: 2076–2082. doi: 10.1016/j.biocon.2009.04.003 [Google Scholar]
- 65.Seidensticker J. Bearing witness: observations on the extinction of Panthera tigris balica and P. t. sondaica In: Tilson RL, Seal US, editors. Tigers of the world: The biology, biopolotics, management, and conservation of an endangered species. Park Ridges: Noyes Publications; 1987. pp. 1–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For security reasons, Javan leopard localities were approximated in 100 km2 rectangles.
(TIF)
The curves show the mean response of the 10 replicates (red) and associated one standard deviation (grey area, error bar for categorical variables).
(DOCX)
(DOCX)
Suitable landscapes were defined based on Maxent model outputs with logistic probabilities of 0.42 or greater.
(PDF)
Data Availability Statement
Due to the sensitive nature and ownership of the study data, it has not been made publicly available. Data deposition could present some other threat, such as revealing the locations of Javan leopard that are listed as Critically Endangered by the IUCN Red Databook and protected by the Indonesian Law No. 5/1990 regarding the Biodiversity Conservation and Ecosystem, Government Regulation No. 7/1999 regarding the Preservation of Animals and Plants. Part of the dataset are owned by third parties who limit the use of their data for the purpose of this study. Others can access these datasets by directly contacting the individual contributors listed in S1 Table and we confirm that others would be able to access these data in the same manner as the authors. Additionally, the corresponding author may assist others in obtaining contacts of the individual contributors. We also confirm that we did not have any special access privileges that others would not have.