Abstract
Purpose
Diets high in saturated fat acids (SFA) have been linked with cardio-metabolic disease risk. The purpose of this study was to determine whether only 1–2 weeks of a high SFA diet could impact disease risk factors in overweight adults who normally eat a relatively low proportion of SFA (i.e., <40% of dietary fat).
Methods
Twelve overweight (BMI: 27±1 kg/m2) young adults were studied before and after a 2-week diet that increased the proportion of SFA (<40% to 60% of dietary fat), while maintaining their daily intake of total fat, carbohydrate, protein, and calories. Insulin resistance, blood pressure, plasma markers of liver damage, total plasma cholesterol concentrations, and fatty acid profile within plasma and skeletal muscle lipid pools were assessed before and after the intervention.
Results
Total plasma cholesterol concentration increased (148±5 vs. 164±8 mg/dl; P<0.05) after only one week, due exclusively to an increase in LDL-cholesterol (78±4 vs. 95±7 mg/dl; P<0.05). After two weeks, plasma aspartate amino transferase (AST) concentration increased (P<0.05) but we found no change in insulin resistance, or resting blood pressure. The diet increase the proportion of SFA in plasma (35±1% vs. 39±2%; P<0.05) and the intramyocellular triglyceride pool (32±1% vs. 37±1%; P<0.05) suggesting the fatty acids in these pools may readily exchange.
Conclusions
Although blood lipids remain within normal clinical range, increasing saturated fat in diet for only 2 weeks raises plasma markers of cardiovascular risk (LDL-cholesterol) and liver damage (AST). In overweight, but healthy-young adults SFA accumulate in plasma and muscle after only 1–2 weeks of dietary increase.
Introduction
Dietary prescriptions aimed at reducing cardio-metabolic disease risk often recommend modifying the amount and “type” of dietary fat (i.e., saturated, monounsaturated, and polyunsaturated fat). Although the impact of the type of dietary fat on disease risk is debated [1], there is considerable evidence linking diets high in saturated fat with an atherogenic blood lipid profile and insulin resistance [2–7]. Importantly, the time-course for measurable changes in clinically relevant health outcomes in response to modifying the amount of dietary saturated and/or unsaturated fat remains unclear. Previously, we found that increasing the proportion of dietary saturated fats in subjects who typically ingest a diet relatively low in saturated fat, elevated plasma low-density lipoprotein cholesterol (LDL-C) concentration in just two weeks [8]. Although these subjects did not become hyperlipidemic, and their LDL-C did not reach the level linked to cardiovascular disease risk after the two-week diet, the fact that the increases in plasma LDL-C were statistically significant in such a short time is alarming.
The primary aim of the present study is to determine if this negative effect of a high saturated fat diet on blood lipid profile may be evident even earlier than 2 weeks, and to investigate if whole-body insulin action may also be affected by a very short exposure to a high saturated fat diet. Diets high in saturated fat may generate some negative health effects by increasing the incorporation of saturated fatty acids into various lipid pools. For example, increased saturated fatty acid incorporation in lipid pools within insulin responsive tissues (e.g., skeletal muscle) may influence insulin resistance [9]. However, the time course for changes in fatty acid profile within skeletal muscle lipids is not well described. Moreover, it is not known if changes in the plasma fatty acid profile readily reflect in a change in muscle lipids pools. Therefore, a secondary aim of this study was to characterize changes in the fatty acid profile of different lipid species within plasma and skeletal muscle in response to short-term exposure (two weeks) of a high saturated fat diet.
Materials and methods
Subjects
Twelve overweight, but otherwise healthy (age: 24±2 years; body mass index 27.2±0.8 kg/m2) men (n = 10) and women (n = 2) volunteered for this study. Participants were not taking any medications known to affect their metabolism. All participants were non-smokers, women were not pregnant or lactating, and all subjects were weight stable (i.e., ± 2 kg) for at least 6 months prior to the study. Participants were not involved in any type of exercise program. Written informed consent was obtained from all participants prior to participation. All procedures were approved by the University of Michigan Institutional Review Board and the Ethics Committee at the Hospital Virgen de la Salud in Toledo (Spain).
Study design
All participants completed an experimental trial before and again after a 2-week high saturated fat dietary intervention. The dietary intervention required subjects to exchange foods from their normal/habitual diet that were naturally high in unsaturated fat with foods high in saturated fats. The overall objective of the dietary intervention was to increase the proportion of saturated fat in their daily diet from <40% to 60% of their total fat intake, without changing total daily fat or energy intake. Before and after the 2-week diet period subjects arrived to the laboratory after an overnight fast. Nude body weight (Toledo, Metler, USA) was collected and 7-site skinfolds (Holtain, Tanner/Whitehouse, USA) measured to calculate body composition changes. Then, an intravenous catheter (BD Insyte, Becton Dickinson, Spain) was percutaneously inserted in an antecubital vein, and a 10 cc blood sample was collected for analysis of plasma concentrations of glucose, insulin, blood lipids, and liver enzymes (see details in “Plasma analytes” section, below). An OGTT started with the ingestion of 75 g of anhydrous glucose (Guinama Laboratorio, Spain) diluted into 250 mL of water. Immediately after all fluid was ingested, a timer was started and 5 mL blood samples were obtained every 15 min up to 120 min after glucose ingestion. The next day, biopsies samples were taken with suction from the vastus lateralis muscle under local anesthesia (2% lidocaine without epinephrine; Braun, Germany) using a modified Bergstrom needle. Muscle samples were rapidly cleaned of blood, fat and connective tissue, liquid N2 frozen and stored at -80°C until analysis. In addition to the OGTT and muscle biopsies collected before and after the 2-week dietary intervention, we also collected a fasting blood sample after just 1 week of the diet to assess whether the diet induced even more rapid changes in blood lipid profile. For both experimental trials, subjects abstained from any strenuous physical activity for at least 72 hours before the trials, otherwise they were asked to maintain their normal daily activities throughout the intervention.
Diets
Participants completed a thorough food frequency questionnaire [10] to assess the content and composition of their habitual diets (Table 1). Subjects also completed a detailed food diary for the three days leading up to the first experimental trial, and they maintained their detailed daily food journals and a physical activity journal (IPAQ [11]) throughout the 2-week dietary intervention. Computerized dietary analysis of subject’s records (CESNID, Barcelona, Spain) was used to calculate energy intake, macronutrient composition, and dietary fat composition (i.e., saturated, monounsaturated, and polyunsaturated fat). All subjects were Spanish citizens and their habitual dietary patterns before the intervention resembled those previously reported for Spanish adults [12] which included a relatively high proportion of unsaturated fat (Table 1). During the 2-week intervention, we provided subjects with 1.2 g of dietary fat/kg body weight each day, while they abstained from their habitual dietary fat sources. The foods provided were largely whole-fat dairy products that contained a very high proportion of saturated fat. The amount of dietary fat provided was calculated to match their habitual dietary fat intake. Subjects returned to the laboratory every 1–3 days to be weighed, inspect their dietary and physical activity journals, and to pick-up their diet rations.
Table 1. Subjects’ daily habitual diet and the “study diet” (high saturated fat diet).
| Habitual diet | Study diet | |
|---|---|---|
| Daily energy intake (kcals) | 2415±226 | 2379±114. |
| Fat (g) | 100±6. | 99±5. |
| Saturated fat (%total fat) | 37±2% | 60±1%* |
| Unsaturated fat (%total fat) | 63±2% | 40±1%* |
| Monounsaturated fat (%total fat) | 46±1% | 32±1%* |
| Polyunsaturated fat (%total fat). | 17±1% | 8±1%* |
| Carbohydrate (g) | 248±23 | 232±16. |
| Protein (g) | 119±12 | 120±9. |
Values are means±SE.
* Significantly different from Habitual diet, P<0.05
Analytical procedures
Plasma analytes
Plasma concentrations of glucose (glucose oxidase assay; Fisher Scientific), fatty acid (NEFA-HR assay kit; WAKO Chemicals USA), triglyceride (triglyceride reagent; Sigma Aldrich), total-cholesterol (Total-C) and high-density lipoprotein (HDL-C) (cholesterol oxidase reaction; BioSystems, Spain) were measured with commercially available colorimetric assay kits. Plasma insulin concentration was measured using a commercially available radioimmunoassay (RIA) kit (Human insulin RIA kit; Millipore). Plasma aspartate and alanine aminotransferase (AST and ALT) and C reactive protein (CRP) where analyzed using specific enzymatic kits (BioSystems, Spain) and a multichannel spectrometer plate reader (Versamax, Molecular Devices, USA).
Fatty acid species in muscle triglyceride, phospholipid, and plasma non-esterified fatty acid (NEFA)
Lipids were extracted from both plasma, and homogenized muscle samples using a single-phase mixture chloroform:methanol:saline (1:2:0.8) as previously described [13]. Internal lipid markers for triacylglycerol, diacylglycerol, monoacylglycerol, NEFA, phospholipid and cholesterol ester with fatty acid moieties of odd carbon number were added at the start of extraction, for subsequent purity and recovery determinations (NuChek; Avanti Polar Lipids). After chloroform separation, individual lipid species were eluted using specific solvent mixtures [14]. Fatty acid methyl esters (FAMES) were generated from purified glycerolipids in these samples while NEFA were converted to methyl esters [15]. FAMES were measured by gas chromatography and electron-impact mass spectrometry (Agilent 6890A GC and 5973N MSD), and quantified using FAME standards (NuChek).
Calculations
Insulin sensitivity index (ISI)
We estimated insulin sensitivity during the OGTT using the Matsuda Composite Index [16] (also referred to as the “Insulin sensitivity Index” (ISI)).
where FPG and FPI are fasting plasma glucose and insulin, respectively, and OGTT [glucose] and OGTT [insulin] represent the mean plasma glucose and insulin concentrations during the 2h OGTT procedure.
Homeostatic model assessment of insulin resistance (HOMA-IR)
Fasting plasma glucose and insulin were used to calculate HOMA-IR:
Low-density lipoprotein concentration
Low density lipoprotein cholesterol concentration (LDL-C) was calculated using the Friedewald equation [17].
Percent body fat
We calculated body density from the sum of skin folds using classic regression equations generated by Jackson and Pollock [18] and Durnin and Womersly [19] for both men and women. We used the mean body density calculated using both sets of equations and then percentage of body fat was calculated using the Siri equation.
Statistical analysis
We used a one-way ANOVA with repeated measures and Tukey post hoc analysis when appropriate, for Total-C, LDL-C, and HDL-C, which were measured at three different time points during the study (i.e., before, at 1 week and after 2 weeks of the intervention). All other outcomes were only measured before and after the intervention, and we used the student’s paired t-test to assess statistical significance, which was set at P < 0.05. All data are presented as means ± SE.
Results
Study diet
Energy intake, total fat, carbohydrate, and protein intake of the high saturated fat diet were all well matched with the subjects’ habitual diet (Table 1). Although the amount of daily fat ingestion during the study was identical to the subjects’ habitual diet, the subjects ate nearly 60% more saturated fat (and nearly 40% less unsaturated fat) during the study (Table 1).
Body composition and physical activity
Body weight decreased very slightly (<1%), but because all subjects either maintained weight or experience a small reduction in body weight, this reductions in body mass was statistically significant after the two-week dietary intervention (Table 2). In contrast, percent body fat, fat mass, and fat free mass did not change significantly with the diet intervention (Table 2). Subjects’ habitual physical activity did not change during the 2 week study according to analysis of their activity journals (IPAQ; Table 2). In general, their IPAQ values (MET-min·week-1) were similar to those previously reported for Spanish adults in the same age range [20].
Table 2. Subject characteristics before and after the 2-week high saturated fat diet.
| BEFORE | AFTER | |
|---|---|---|
| Body weight (kg) | 82.3±2.9 | 81.5±2.9* |
| Body mass index (kg/m2) | 27.2±0.8 | 27.0±0.8* |
| Percent body fat (%) | 18.5±2.1 | 18.2±2.0 |
| Fat mass (kg) | 15.2±1.7 | 14.8±1.8 |
| Fat free mass (kg) | 67.1±3.0 | 66.8±3.0 |
| Physical activity IPAQ (MET-min·week-1) | 2141±211 | 2201±264 |
Values are means±SE.
* Significantly different from BEFORE, P<0.05
Clinical outcome measures
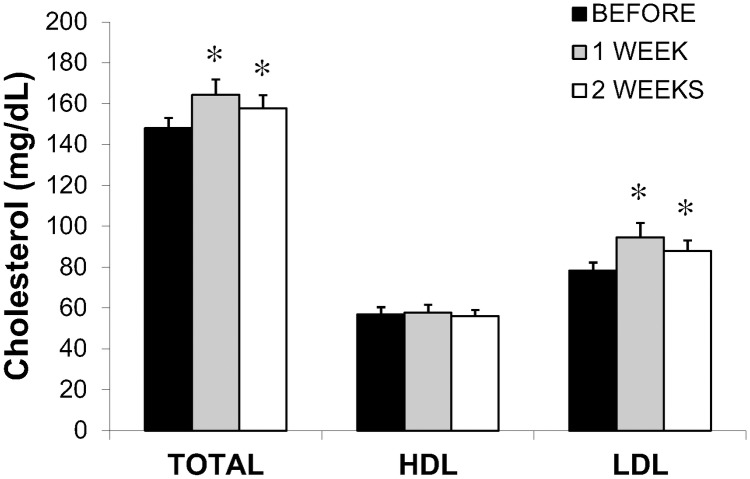
Plasma total cholesterol concentration was significantly elevated after only one week of the Study diet (P<0.05), and remained elevated at week 2 (P<0.05) (Fig 1). This increase in total cholesterol was due to an elevation in plasma LDL-C concentration, while HDL-C remained unchanged during the two weeks of the dietary intervention (Fig 1).
Fig 1. Plasma cholesterol concentrations before the dietary intervention and again after 1 week and 2 weeks of the high saturated fat diet intervention.
Values are means±SE. * Significant difference from BEFORE, P<0.05. Total cholesterol (Total-C), high density lipoprotein colesterol (HDL-C), and low density lipoprotein cholesterol (LDL-C).
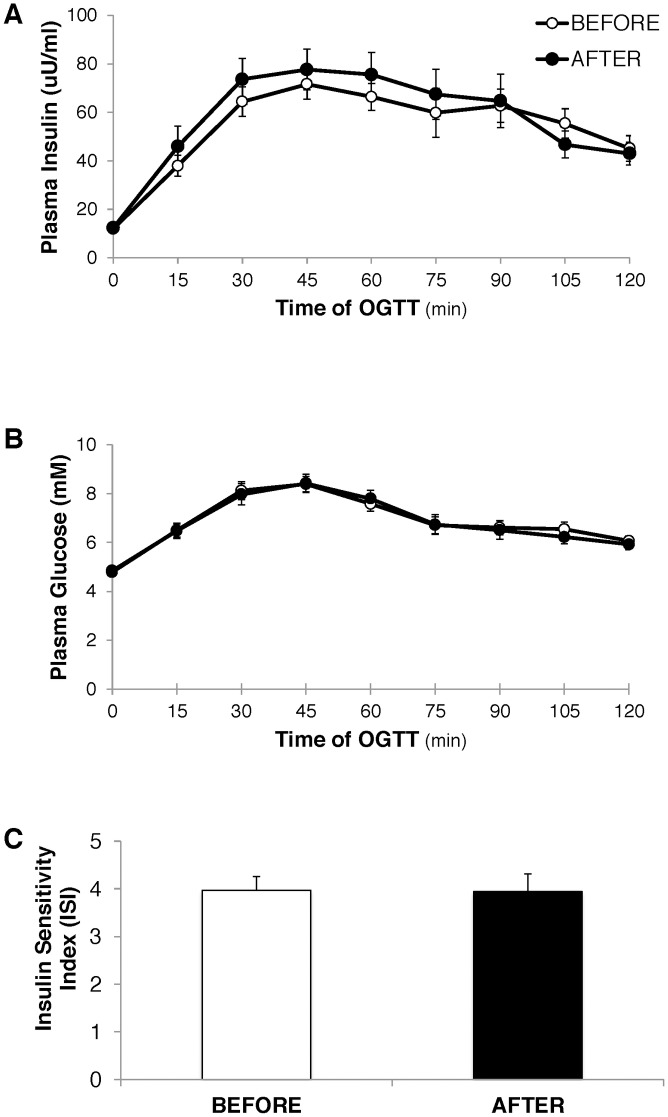
The relatively short-term exposure to the high saturated fat diet also increase plasma concentration of the liver enzyme, AST (Table 3), which provides a crude marker for hepatic steatosis. In contrast, the insulin and glucose responses during the OGTT were nearly identical before vs. after the diet (Fig 2A and 2B), and as a result, our assessment of insulin sensitivity (Insulin Sensitivity Index (ISI)) was also not affected by a 2-week exposure to the high saturated fat diet (Fig 2C).
Table 3. Fasting plasma substrate and hormone concentrations and other clinical measures before and after the high saturated fat diet intervention.
| BEFORE | AFTER | |
|---|---|---|
| Glucose concentration (mM) | 4.8±0.1 | 4.8±0.1 |
| Insulin concentration (μIU/ml) | 13±1 | 12±1 |
| HOMA-IR | 2.62±0.19 | 2.75±0.34 |
| Fatty acid concentration (mM) | 0.43±0.07 | 0.45±0.07 |
| Triacylglycerol concentration (mM) | 0.74±0.11 | 0.79±0.09 |
| C-reactive protein (μg/ml) | 0.5±0.1 | 0.6±0.2 |
| AST (U/L) | 28.2±2.8 | 35.4±3.5* |
| ALT (U/L) | 29.7±2.8 | 32.7±3.7 |
| Systolic BP (mmHg) | 119±3 | 125±4. |
| Diastolic BP (mmHg) | 72±2 | 75±2 |
Values are means±SE. AST: aspartate transaminase; ALT: alanine transaminase; HOMA-IR: Homeostatic Model Assessment of Insulin Resistance.
* Significantly different from BEFORE, P<0.05.
Fig 2. Responses during the oral glucose tolerance (OGTT) before at after the 2 week high saturated fat diet intervention.
A. Plasma insulin concentration, B. Plasma glucose concentration, and C. “Insulin sensitivity index” (Matsuda Composite Index) calculated from plasma insulin and glucose concentrations during the OGTT.
Similarly, resting blood pressure, as well as fasting plasma concentrations of glucose, insulin, fatty acid, triacylglycerol, and C-reactive protein (a crude index of systemic inflammation) were also not affected by the 2-week high saturated fat diet (Table 3).
Plasma and muscle fatty acid profiles
The two weeks high saturated fatty acid diet did not affect concentrations of triacylglycerol or phospholipids in skeletal muscle (i.e., intramuscular phospholipids; IMPL), and both remained remarkably stable throughout the intervention (IMTG: 10.2±1.8 vs. 10.2±2.8 and IMPL: 6.3±0.2 vs. 6.3±0.1 nmol/mg wet weight before vs. after the 2 week high saturated fat diet, respectively). However, the high saturated fat diet did significantly increase the proportion of saturated fatty acids in the plasma fatty acid pool, with a similar increase in the proportion of saturated fatty acids in the IMTG pool (Table 4). Conversely, the proportion of unsaturated fatty acids decreased in these fractions, almost exclusively due to a reduction in the proportion of polyunsaturated fatty acids (Table 4). Interestingly, the percentage of saturated fatty acids within IMPL pool did not increase with the high saturated fat diet. In fact, we observed a very slight, yet significant reduction (P<0.05) in the proportion of saturated fatty acids in IMPL, along with a compensatory increase (P<0.05) in the proportion of polyunsaturated fatty acids in IMPL after the high saturated fat diet intervention (Table 4). Details of the specific fatty acid profiles within these fractions is available in supplemental table 1 (S1 Table).
Table 4. Fatty acid composition in diet, plasma pool, muscle triacylglycerol and phospholipid fractions before and after 2 weeks of a diet high in saturated fat.
| Dietary fat | Plasma Fatty acid | Muscle triacylglycerol | Muscle phospholipid | ||
|---|---|---|---|---|---|
| Saturated Fatty acids | BEFORE | 37±2% | 35±1% | 32±1% | 44±1% |
| AFTER | 66±1%* | 39±2%* | 37±1%* | 43±1%* | |
| Unsaturated Fatty acids | BEFORE | 63±2% | 65±1% | 68±1% | 56±1% |
| AFTER | 34±1%* | 61±2%* | 63±1%* | 57±1%* | |
| Polyunsaturated | BEFORE | 17±1% | 18±1% | 18±1% | 46±1% |
| AFTER | 9±1%* | 15±1%* | 15±1%* | 48±1%* | |
| Monounsaturated | BEFORE | 46±1% | 47±1% | 50±1% | 10±1% |
| AFTER | 25±1%* | 46±1% | 48±1% | 9±1%* |
Values are means±SE.
* Significantly different from BEFORE, P<0.05.
Discussion
A key clinically relevant finding from the present study was that plasma total cholesterol and LDL-C increased after only 1 week of the isocaloric high saturated fat diet. Additionally, we found that a clinical marker of liver damage was measurably elevated after our 2-week high saturated fat diet intervention. Together these findings suggest that even a very short-term exposure of a high saturated fat diet can significantly increase markers of disease risk factors in overweight young adults. It is important to note that these rapid changes in disease risk in response to the high saturated fat diet were observed despite a very slight but significant weight loss. Interestingly, we also found that the increased proportion of saturated fatty acids within the plasma fatty acid pool was nearly identical to the increase found in the intramyocellular triacylglycerol pool. In contrast, the fatty acid profile within the intramyocellular phospholipid pool was “protected” from an increase in saturated fatty acids. Our data reveals that two weeks increase in dietary saturated fat raises plasma proportions of saturated fat and triacylglycerol composition in skeletal muscle which could potentially affect insulin actions in this tissue.
It has been well-described that diets high in saturated fatty acids augment plasma total cholesterol and LDL-C cholesterol [2, 4, 6, 7, 21]. We have reported that under similar conditions, plasma LDL-C increased after just 2 weeks of a high saturated fat diet [8] and others find similar results after 3 weeks [22]. The present finding that LDL-C increased after only one week of the high saturated fat diet is even more alarming. It appears that the increase in LDL synthesis may not be responsible for the increased LDL-C concentration found after exposure to high dietary saturated fatty acids [23, 24]. Evidence suggests that LDL receptor activity can be suppressed by diets high in saturated fat [25], reducing LDL-C clearance and thereby increasing LDL-C accumulation in the circulation. Impaired LDL receptor function may be due to reduced cell membrane fluidity stemming from high saturated fatty acid availability [25, 26]. Therefore, daily exposure to a high abundance of saturated fatty acids may underlie acute modifications in receptor function, which may help explain the rapid increase in LDL-C found with our dietary intervention.
Our finding that plasma AST concentration increased after 2 weeks of the high saturated fat diet, provides a crude indication of elevated hepatic stress, perhaps in consequence to increased accumulation of liver fat. Because of limitations in the ability to directly test liver fat content and liver function in human subjects, controlled experiments in animal models may be best suited to assess the impact of saturated fat on liver dysfunction. It has been found that increasing dietary saturated fat in rats (without increasing total energy intake) augmented hepatic triacylglycerol content after only one week, while plasma AST concentration was elevated by the fourth week of the diet. The authors also reported a concomitant increase in saturated fats into the endoplasmic reticulum (ER) membrane, which results in ER stress [27, 28]. Although our intervention did not yield profound alterations in either blood lipid profile or marker of liver damage, the fact the we did find measurable increases in plasma LDL-C and AST in only 1–2 week exposure to a high saturated fat diet without increasing total fat content suggests that the type of dietary fat may be an important contributor to long-term hepatic health.
Much epidemiological evidence suggests that diets high in saturated fatty acids are associated with a greater degree of insulin resistance [2, 29] but this finding is not universal [30, 31]. We acknowledge our finding that the high saturated fat diet did not impair our measurement of insulin sensitivity could certainly be a consequence of the dietary intervention being short, like in other investigations [32]. In addition, the use of OGTT to assess insulin sensitivity may not provide the resolution required to detect impairments in insulin action [33]. However, available evidence from intervention studies that provided a longer dietary intervention period and used more accurate measures of assessing insulin action are still equivocal [9, 34, 35]. Overall, it appears that many well-controlled intervention studies do not all support the notion that diets high in saturated fats induce insulin resistance [30]. Importantly, Vessby, et al., [9] reported impaired insulin sensitivity after 3 months of a diet high in saturated fat, but this effect was not observed in their participant with a total dietary fat content greater than 37% of total daily energy intake. Similarly, Jebb et al., [36] reported that in subjects ingesting more than 38% of their daily energy intake from fat, replacing dietary saturated fats with unsaturated fats for 6 months did not improve insulin sensitivity. In our subjects, fat represented 39% of their energy intake, which may help to explain why dietary substitution of unsaturated by saturated fats did not impair our assessment of insulin action. Although daily dietary fat intake approaching 40% of total energy is relatively high, this is often found to be close to the norm in many countries, including the United States [37, 38] and Spain [39]. Therefore, with a relatively high, yet normal daily fat intake, consuming a relatively high proportion of saturated fat during 2 weeks does not appear to have much/any impact on insulin sensitivity.
Our high saturated fat diet increased the proportion saturated fatty acids in plasma. However, the ~10% increase in the proportion of saturated plasma fatty acids was very small when compared with the nearly 80% increase in dietary saturated fat we implemented with the diet. Importantly, because we measured fatty acid profile in the overnight fasted condition, the fatty acids measured in the circulation were largely derived from endogenous triacylglycerol stored in adipose tissue. As such, the disparity between the fatty acid profiles within the diet and the plasma fatty acid pool is likely a consequence of dilution of the dietary fat within the fatty acids stored in adipose tissue, which are very large and very slow to turnover. In fact, it has been estimated that the lipid within adipocyte turn over approximately every 1.6 years [40].
Despite the very slow absolute rate of turnover of triacylglycerol stored in adipose tissue, other endogenous stores of triacylglycerol can turnover at a relatively high rate. Our finding that the proportion of saturated fatty acids within the plasma fatty acid pool were remarkably similar to that found in the intramyocellular triacylglycerol pool suggests that these lipid pools may exchange fatty acids quite readily [41]. Indeed, it was been suggested that all (or nearly all) fatty acids entering the muscle cell are initially stored as triacylglycerol, and then differentially metabolized within the cell [42, 43]. Our finding supports the idea that these two lipid pools are closely coupled. We did not measure muscle diacylglycerol concentration in this study, but it has been reported that similar to IMTG, the abundance of saturated fatty acids in diacylglycerides also increase in response to diets high in saturated fat [44]. In contrast to our findings in the IMTG pool, the fatty acid profile within the IMPL pool appeared to be largely protected from the dietary increases in saturated fat. This may reflect a relatively slow turnover of fatty acids within the muscle phospholipid pool or alternatively, the fatty acids within plasma and IMTG may not exchange readily with the IMPL pool. Phosphosolipids are the most abundant lipids found in cell membranes, and a relatively high proportion of saturated fatty acids within the phospholipid pool can reduce membrane fluidity, which in turn can impair cellular signaling [45–47]. Along these lines, elevated abundance of saturated fatty acids within skeletal muscle phospholipids have been linked with insulin resistance [48–50]. Therefore, our finding that 2 weeks of a high saturated fat diet did not rapidly increase the saturated fatty acid abundance in the IMPL pool may have contributed to our observation of unchanged insulin action after the diet intervention.
There were a number of limitations in our study that may confound our interpretation. Although we attempted to tightly control energy intake and energy expenditure subjects did lose on average 0.8 kg although no subject lost more than 2% of their initial body weight. Thus, we cannot rule out the possibility that this small weight loss may have counteracted some effects of the high saturated fat diet. Another limitation of our study was our assessment of insulin sensitivity. Although the Matsuda composite index matches tightly with clamp assessments [16], it is certainly possible that this method may not have been sensitive enough to detect relative small impairments in insulin action. Also, other than being overweight, our research participants were physically active and generally in good health. Our selective recruitment of this healthy subject population was a strategic decision for the study design, because this generally healthy group of subjects may be more susceptible to the negative effects of a high saturated fat diet. However, it is possible the degree of adiposity and physical fitness level of our participants may have masked some of the potential negative impact of the high saturated fat diet. In addition, the high saturated fat diet used in our study was largely derived from dairy products, which has been suggested may be more protective against insulin resistance compared with other sources of fat [51], however, this finding is not universal [52]. Finally, although we did not observe differences in any outcome between our male and female subjects, and the interpretation of our findings do not change if we remove the female subjects from our analyses, we still cannot rule out sex differences in the responses to a high saturated fat diet.
Overall, our findings highlight that diets high in saturated fat can induce rapid disarrangements in blood lipid profile and markers of hepatic health in as little as 1–2 weeks. Our finding that the high saturated fat diet changed fatty acid profile nearly identically within the plasma fatty acid pool and the intramyocellular lipid pool suggests that these lipid pools may readily and rapidly exchange fatty acids. In contrast, because the abundance of saturated fatty acids did not increase within the intramyocellular phospholipid pool, it appears that the muscle phospholipids may be somewhat protected from an increase in saturated fatty acids, which may in turn be helpful for the maintenance of membrane fluidity delaying the appearance of insulin resistance. In conclusion, although some important clinical markers (i.e., insulin sensitivity, blood pressure, fasting plasma glucose, and triacylglycerol concentrations) were not affected by a short-term increase in dietary saturated fat, our data reveals rapid increases in blood lipids (i.e., total cholesterol and LDL-C), liver damage markers, and the proportion of saturated fatty acids within plasma and muscle lipids, which over the long term may contribute to the development of metabolic and/or cardiovascular disease.
Supporting information
(PDF)
Acknowledgments
The technical assistance of Drs. Valentin E Fernandez-Elias, Emma Estevez and Juan del Coso is greatly appreciated.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from National Institutes of Health (JFH, grant numbers R01 DK077966, P30-DK089503) and the Spanish Ministry of Economy, Industry y Competitivity (RMR grant numbers DEP-2017-83244-R). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, et al. Association of Dietary, Circulating, and Supplement Fatty Acids With Coronary Risk: A Systematic Review and Meta-analysis. Annals of Internal Medicine. 2014;160(6):398–406. doi: 10.7326/M13-1788 [DOI] [PubMed] [Google Scholar]
- 2.Hu FB, van Dam RM, Liu S. Diet and risk of Type II diabetes: the role of types of fat and carbohydrate. Diabetologia. 2001;44(7):805–17. doi: 10.1007/s001250100547 . [DOI] [PubMed] [Google Scholar]
- 3.Lottenberg AM, Afonso MS, Lavrador MSF, Machado RM, Nakandakare ER. The role of dietary fatty acids in the pathology of metabolic syndrome. The Journal of Nutritional Biochemistry. 2012;23(9):1027–40. doi: 10.1016/j.jnutbio.2012.03.004 . [DOI] [PubMed] [Google Scholar]
- 4.Mensink RP. Effects of saturated fatty acids on serum lipids and lipoproteins: a systematic review and regression analysis. World Health Organization, 2016 Apr 06. Report No.
- 5.Mensink RP, Zock PL, Kester ADM, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. American Journal of Clinical Nutrition. 2003;77(5):1146–55. doi: 10.1093/ajcn/77.5.1146 . [DOI] [PubMed] [Google Scholar]
- 6.Phillips CM. Nutrigenetics and metabolic disease: current status and implications for personalised nutrition. Nutrients. 2013;5(1):32–57. doi: 10.3390/nu5010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zong G, Li Y, Wanders AJ, Alssema M, Zock PL, Willett WC, et al. Intake of individual saturated fatty acids and risk of coronary heart disease in US men and women: two prospective longitudinal cohort studies. BMJ. 2016:i5796–11. doi: 10.1136/bmj.i5796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ortega JF, Fernández-Elías VE, Hamouti N, Mora-Rodriguez R. Increased blood cholesterol after a high saturated fat diet is prevented by aerobic exercise training. Applied Physiology, Nutrition, and Metabolism 2013;38(1):42–8. doi: 10.1139/apnm-2012-0123 . [DOI] [PubMed] [Google Scholar]
- 9.Vessby B, Uusitupa M, Hermansen K, Riccardi G, Rivellese AA, Tapsell LC, et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU Study. Diabetologia. 2001;44(3):312–9. . [DOI] [PubMed] [Google Scholar]
- 10.De la Fuente-Arrillaga C, Ruiz ZV, Bes-Rastrollo M, Sampson L, Martínez-González MA. Reproducibility of an FFQ validated in Spain. Public Health Nutrition. 2010;13(9):1364–72. doi: 10.1017/S1368980009993065 . [DOI] [PubMed] [Google Scholar]
- 11.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Medicine Science Sports Exercise. 2003;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB . [DOI] [PubMed] [Google Scholar]
- 12.Ruiz E, Ávila J, Valero T, del Pozo S, Rodriguez P, Aranceta-Bartrina J, et al. Macronutrient Distribution and Dietary Sources in the Spanish Population: Findings from the ANIBES Study. Nutrients. 2016;8(3):177–25. doi: 10.3390/nu8030177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology. 1959;37(8):911–7. doi: 10.1139/o59-099 . [DOI] [PubMed] [Google Scholar]
- 14.Bodennec J, Famy C, Brichon G, Zwingelstein G, Portoukalian J. Purification of free sphingoid bases by solid-phase extraction on weak cation exchanger cartridges. Analytical Biochemistry. 2000;279(2):245–8. doi: 10.1006/abio.2000.4454 . [DOI] [PubMed] [Google Scholar]
- 15.Patterson BW, Zhao G, Elias N, Hachey D, Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. Journal of Lipid Research. 1999;40(11):2118–24. [PubMed] [Google Scholar]
- 16.Matsuda M, DeForonzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–70. [DOI] [PubMed] [Google Scholar]
- 17.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry. 1972;18(6):499–502. . [PubMed] [Google Scholar]
- 18.Jackson AS, Pollock ML, Ward A. Generalized equations for predicting body density of women. Medicine and Science in Sports and Exercise. 1980;12(3):175–81. . [PubMed] [Google Scholar]
- 19.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. The British Journal of Nutrition. 1974;32(1):77–97. . [DOI] [PubMed] [Google Scholar]
- 20.Mora-Rodriguez R, Ortega JF, Fernandez-Elias VE, Kapsokefalou M, Malisova O, Athanasatou A, et al. Influence of Physical Activity and Ambient Temperature on Hydration: The European Hydration Research Study (EHRS). Nutrients. 2016;8(5). doi: 10.3390/nu8050252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishida C, Uauy R, Kumanyika S, Shetty P. The joint WHO/FAO expert consultation on diet, nutrition and the prevention of chronic diseases: process, product and policy implications. Public Health Nutrition; February 2004. p. 245–50. [DOI] [PubMed] [Google Scholar]
- 22.Chiu S, Williams PT, Krauss RM. Effects of a very high saturated fat diet on LDL particles in adults with atherogenic dyslipidemia: A randomized controlled trial. PLoS One. 2017;12(2):e0170664 doi: 10.1371/journal.pone.0170664 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones PJ, Ausman LM, Croll DH, Feng JY, Schaefer EA, Lichtenstein AH. Validation of deuterium incorporation against sterol balance for measurement of human cholesterol biosynthesis. Journal of Lipid Research. 1998;39(5):1111–7. . [PubMed] [Google Scholar]
- 24.Mattson FH, Grundy SM. Comparison of effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on plasma lipids and lipoproteins in man. Journal of Lipid Research. 1985;26(2):194–202. . [PubMed] [Google Scholar]
- 25.Tripodi A, Loria P, Dilengite MA, Carulli N. Effect of fish oil and coconut oil diet on the LDL receptor activity of rat liver plasma membranes. Biochimica et Biophysica Acta. 1991;1083(3):298–304. . [DOI] [PubMed] [Google Scholar]
- 26.Kuo P, Weinfeld M, Loscalzo J. Effect of membrane fatty acyl composition on LDL metabolism in Hep G2 hepatocytes. Biochemistry. 1990;29(28):6626–32. . [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147(2):943–51. doi: 10.1210/en.2005-0570 . [DOI] [PubMed] [Google Scholar]
- 28.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. American Journal of Physiology Endocrinology and Metabolism. 2006;291(2):E275–81. doi: 10.1152/ajpendo.00644.2005 . [DOI] [PubMed] [Google Scholar]
- 29.Rivellese AA, Lilli S. Quality of dietary fatty acids, insulin sensitivity and type 2 diabetes. Biomedicine and Pharmacotherapy. 2003;57(2):84–7. . [DOI] [PubMed] [Google Scholar]
- 30.Galgani JE, Uauy RD, Aguirre CA, Díaz EO. Effect of the dietary fat quality on insulin sensitivity. The British Journal of Nutrition. 2008;100(3):471–9. doi: 10.1017/S0007114508894408 . [DOI] [PubMed] [Google Scholar]
- 31.Mayer-Davis EJ, Monaco JH, Hoen HM, Carmichael S, Vitolins MZ, Rewers MJ, et al. Dietary fat and insulin sensitivity in a triethnic population: the role of obesity. The Insulin Resistance Atherosclerosis Study (IRAS). American Journal of Clinical Nutrition. 1997;65(1):79–87. doi: 10.1093/ajcn/65.1.79 . [DOI] [PubMed] [Google Scholar]
- 32.Anderson AS, Haynie KR, McMillan RP, Osterberg KL, Boutagy NE, Frisard MI, et al. Early skeletal muscle adaptations to short-term high-fat diet in humans before changes in insulin sensitivity. Obesity (Silver Spring). 2015;23(4):720–4. doi: 10.1002/oby.21031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ortega JF, Hamouti N, Fernandez-Elias VE, Mora-Rodriguez R. Comparison of glucose tolerance tests to detect the insulin sensitizing effects of a bout of continuous exercise. Applied Physiology Nutrition Metabolism. 2014;39(7):787–92. doi: 10.1139/apnm-2013-0507 . [DOI] [PubMed] [Google Scholar]
- 34.Fasching P, Ratheiser K, Schneeweiss B, Rohac M, Nowotny P, Waldhausl W. No effect of short-term dietary supplementation of saturated and poly- and monounsaturated fatty acids on insulin secretion and sensitivity in healthy men. Annals of Nutrition and Metabolism. 1996;40(2):116–22. doi: 10.1159/000177904 . [DOI] [PubMed] [Google Scholar]
- 35.Moon JH, Lee JY, Kang SB, Park JS, Lee BW, Kang ES, et al. Dietary monounsaturated fatty acids but not saturated fatty acids preserve the insulin signaling pathway via IRS-1/PI3K in rat skeletal muscle. Lipids. 2010;45(12):1109–16. doi: 10.1007/s11745-010-3475-3 . [DOI] [PubMed] [Google Scholar]
- 36.Jebb SA, Lovegrove JA, Griffin BA, Frost GS, Moore CS, Chatfield MD, et al. Effect of changing the amount and type of fat and carbohydrate on insulin sensitivity and cardiovascular risk: the RISCK (Reading, Imperial, Surrey, Cambridge, and Kings) trial. The American Journal of Clinical Nutrition. 2010;92(4):748–58. doi: 10.3945/ajcn.2009.29096 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Annual Review of Nutrition. 2004;24:401–31. doi: 10.1146/annurev.nutr.23.011702.073349 . [DOI] [PubMed] [Google Scholar]
- 38.Wright JD, Wang C-Y. Trends in intake of energy and macronutrients in adults from 1999–2000 through 2007–2008. NCHS data brief. 2010;(49):1–8. . [PubMed] [Google Scholar]
- 39.Valdés J, Grau M, Subirana I, Marrugat J, Covas M-I, Schröder H. Secular trends in energy intake and diet quality in a Mediterranean population. Annals of Nutrition and Metabolism. 2009;54(3):177–83. doi: 10.1159/000217814 . [DOI] [PubMed] [Google Scholar]
- 40.Arner P, Bernard S, Salehpour M, Possnert G, Liebl J, Steier P, et al. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature. 2011;478(7367):110–3. doi: 10.1038/nature10426 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andersson A, Nälsén C, Tengblad S, Vessby B. Fatty acid composition of skeletal muscle reflects dietary fat composition in humans. American Journal of Clinical Nutrition. 2002;76(6):1222–9. doi: 10.1093/ajcn/76.6.1222 . [DOI] [PubMed] [Google Scholar]
- 42.Holloway GP, Benton CR, Mullen KL, Yoshida Y, Snook LA, Han X-X, et al. In obese rat muscle transport of palmitate is increased and is channeled to triacylglycerol storage despite an increase in mitochondrial palmitate oxidation. American Journal of Physiology Endocrinology and Metabolism. 2009;296(4):E738–47. doi: 10.1152/ajpendo.90896.2008 . [DOI] [PubMed] [Google Scholar]
- 43.Kanaley JA, Shadid S, Sheehan MT, Guo Z, Jensen MD. Relationship between plasma free fatty acid, intramyocellular triglycerides and long-chain acylcarnitines in resting humans. The Journal of Physiology. 2009;587(Pt 24):5939–50. doi: 10.1113/jphysiol.2009.180695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kien CL, Everingham KI, D Stevens R, Fukagawa NK, Muoio DM. Short-term effects of dietary fatty acids on muscle lipid composition and serum acylcarnitine profile in human subjects. Obesity. 2011;19(2):305–11. doi: 10.1038/oby.2010.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ginsberg BH, Jabour J, Spector AA. Effect of alterations in membrane lipid unsaturation on the properties of the insulin receptor of Ehrlich ascites cells. Biochimica et Biophysica Acta. 1982;690(2):157–64. . [DOI] [PubMed] [Google Scholar]
- 46.Grunfeld C, Baird KL, Kahn CR. Maintenance of 3T3-L1 cells in culture media containing saturated fatty acids decreases insulin binding and insulin action. Biochemical and Biophysical Research Communications. 1981;103(1):219–26. . [DOI] [PubMed] [Google Scholar]
- 47.Pilon M. Revisiting the membrane-centric view of diabetes. Lipids in Health and Disease. 2016;15(1):1–6. doi: 10.1186/s12944-016-0342-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andersson A, Sjödin A, Olsson R, Vessby B. Effects of physical exercise on phospholipid fatty acid composition in skeletal muscle. The American Journal of Physiology. 1998;274(3 Pt 1):E432–8. . [DOI] [PubMed] [Google Scholar]
- 49.Storlien LH, Baur LA, Kriketos AD, Pan DA, Cooney GJ, Jenkins AB, et al. Dietary fats and insulin action. Diabetologia. 1996;39(6):621–31. . [DOI] [PubMed] [Google Scholar]
- 50.Storlien LH, Jenkins AB, Chisholm DJ, Pascoe WS, Khouri S, Kraegen EW. Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes. 1991;40(2):280–9. . [DOI] [PubMed] [Google Scholar]
- 51.Petyaev IM, Bashmakov YK. Could cheese be the missing piece in the French paradox puzzle? Medical Hypotheses. 2012;79(6):746–9. doi: 10.1016/j.mehy.2012.08.018 . [DOI] [PubMed] [Google Scholar]
- 52.Drouin-Chartier J-P, Côté JA, Labonté M-È, Brassard D, Tessier-Grenier M, Desroches S, et al. Comprehensive Review of the Impact of Dairy Foods and Dairy Fat on Cardiometabolic Risk. Advances in Nutrition. 2016;7(6):1041–51. doi: 10.3945/an.115.011619 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.