Abstract
Coxiella burnetii is an obligate intracellular pathogen that replicates in an endolysosome-like compartment termed the Coxiella-containing vacuole (CCV). Formation of this unique replicative niche requires delivery of bacterial effector proteins into the host cytosol where they mediate crucial interactions with the host. We previously identified an essential Dot/Icm effector, CirA that is required for intracellular replication and CCV formation. Furthermore, CirA was shown to stimulate the GTPase activity of RhoA in vitro. In the current study, we used a bioinformatics-guided approach and identified three arginine finger-like motifs, often found in Rho GTPase-activating proteins (GAPs) and endosome–lysosome basolateral sorting signals associated with vesicle trafficking. When expressed in mammalian cells, mutation of either endosome-lysosome-basolateral sorting signals or the arginine finger-like motifs rescued stress phenotypes and decreased plasma membrane localization of ectopically expressed CirA. We further demonstrate that endosome–lysosome sorting signals are required for co-localization with Rab5 and Rab7. Collectively our data indicate that arginine finger-like motifs and endosome-lysosome-basolateral sorting signals within CirA are essential for interaction with the host cytoskeleton.
Keywords: Coxiella burnetii, Dot/Icm, CirA, Rho GTPase
1. Introduction
Coxiella burnetii is a Gram-negative, obligate intracellular pathogen of human and veterinary importance. Humans are typically infected through inhalation of contaminated aerosols generated by livestock such as cattle, sheep, and goats. In most cases, Q fever results in an acute disease characterized by fever and flu-like symptoms that resolves in 1–2 weeks. However a more severe, chronic disease manifesting as hepatitis, pneumonia, or endocarditis can occur with poor prognosis for resolution [1].
In a natural infection, C. burnetii preferentially targets alveolar macrophages and is internalized by actin-dependent endocytosis into a tight-fitting phagosome reminiscent of a phagolysosome [2]. The nascent CCV traffics through the default endocytic pathway and ultimately fuses with lysosomes and acquires Rab7 and Lamp1, markers of late endosomes and lysosomes respectively [2]. As the vacuolar pH drops, the bacteria transition from the metabolically inactive environmentally stable small-cell variant (SCV) to the metabolically active large-cell variant (LCV) [3]. Establishment of this replicative niche requires active bacterial protein synthesis [4] and involves manipulation of numerous host processes including modulation of the host transcriptome [5,6], manipulation of host kinases and phosphatases [7,8], recruitment of secretory components [9], inhibition of apoptosis [10], and induction of autophagy [11,12].
Intracellular replication, CCV formation, and virulence in a mouse model requires a functional Dot/Icm type IVb secretion system that can translocate bacterial virulence factors, termed effectors, into the host cytosol. This system is functionally analogous to the Dot/Icm secretion system of Legionella pneumophila [13–16], which is composed of 26 dot/icm genes. C. burnetii encodes 23/26 of these components, lacking homologs of DotJ, DotV, and IcmR [17]. While the secretion systems are highly similar, very few effectors are conserved owing to the differences in host range and intracellular replicative niches. However, similar features including the presence of eukaryotic-like domains [18–21], an acidic C-terminus [15,22], and a PmrA consensus sequence [15,18] have been used to identify T4SS candidate substrates. Using the β-lactamase or adenylate cyclase (CyaA) secretion assays, approximately 130 secretion system substrates have been identified [15–14,18–19], however only a few of these effectors have been functionally characterized to date.
Studies working toward identifying the function of C. burnetii effectors have emphasized their importance in formation and maintenance of its unique intracellular niche. While AnkG, CaeA, and CaeB inhibit apoptosis [23,24], IcaA inhibits non-conical inflammasome activation [25], highlighting the importance of promoting host cell viability. Cig2 engages autophagosomes and promotes homotypic fusion of CCVs [26]. Whereas CirA modulates the Rho GTPase RhoA to promote CCV development [16], CvpA coopts clathrin-mediated vesicular transport pathways to promote CCV maturation [27].
We previously reported that theT4SS effector CirA is required for intracellular replication, CCV formation, and virulence in a mouse model [15,16]. We further demonstrated that CirA is necessary for recruitment of RhoA to the CCV and is capable of stimulating the GTPase-activity of RhoA in vitro [16]. In the current study, we identified several arginine finger-like motifs, typically found associated with GTPase-activating proteins (GAPs), and present evidence that these motifs contribute to toxicity in yeast and stress associated phenotypes in mammalian cells. Furthermore, we show that endosome-lysosome-basolateral sorting signals within ectopically expressed CirA are required for localization to the plasma membrane, co-localization with early and late endosomes, and that mutation of these motifs partially rescues stress associated phenotypes in mammalian cells. Taken together our results suggest that CirA contains functional motifs consistent with a potential role in modulating endolysosomal trafficking via interaction with the host-cell cytoskeleton.
2. Materials and methods
2.1. Cell culture
HeLa (ATCC) cells were cultured in DMEM with 10% FBS. All cell lines were maintained at 37 °C with 5% CO2.
2.2. Quikchange mutation of CirA
Bioinformatic analysis of CirA identified three potential arginine finger-like motifs, two Y-based sorting signals, and four endosome-lysosome-basolateral sorting signals. To generate mutants in the identified motifs, the primers in Table S1 were used with the QuikChange II site-directed mutagenesis kit® (Agilent). The integrity of the resulting constructs was verified by sequencing.
2.3. Yeast toxicity assay
To assess toxicity of arginine finger mutants in yeast, each ORF was cloned into pYesNTA, and the resulting constructs were transformed into S. cerevisiae W303 using a standard transformation protocol [28]. Transformants were spotted on uracil dropout media, supplemented with glucose or galactose, and incubated for 48 h at 30 °C to assess toxicity.
2.4. Immunofluorescence
HeLa cells were seeded in triplicate into 24-well glass bottom plates at 105/ml. Cells were transfected with Lipofectamine® per the manufactures instructions. For rounding and stress fiber experiments cells were fixed at 24 h post-transfection and for co-localization experiments cells were fixed at 15 h post-transfection [16]. Cells were fixed with 4% formaldehyde, permeabilized with 0.1% Triton-X 100, and nuclei were visualized with 1X Hoechst. To assess co-localization with Rab GTPases, cells were co-transfected with EGFP-CirA and Rab5, Rab7, and Rab11 (Table S2). Actin was visualized using Alexa Fluor® 555 conjugated Phalloidin (Life Technologies). As previously described [16], cells with at least two filaments present in the cytosol were considered positive for stress fibers, whereas cells with less than two were considered negative. Results were calculated from triplicate wells with at least 50 co-transfected cells per experiment. Similar results were obtained from at least three independent experiments. Images were captured on a Nikon A1 confocal microscope and images where processed using Nikon Elements software.
2.5. Data analysis
When required, statistical analysis was conducted using GraphPad Prism software. One-way ANOVA generated a minimum threshold of significance of p < 0.05. Dunnett was used as a post-test to compare all samples to the control.
3. Results
3.1. CirA encodes functional domains associated with Rho GTPase modification and endocytic trafficking
We previously demonstrated that CirA recruitments RhoA to the CCV in Coxiella infected mammalian cells and can stimulate the GTPase activity of RhoA in vitro [16]. Rho GTPases are targeted by many bacterial toxins and effector proteins. Rho GTPase-activating proteins (GAPs) possess an arginine finger motif (GXXR) with an invariant arginine that is necessary for efficient GTP catalysis [29,30]. Bioinformatic analysis of the amino acid sequence of CirA identified three potential arginine finger-like motifs (Fig. 1), similar to those described for Yersinia pseudotuberculosis YopE [30] and Salmonella typhimurium SptP [29]. The presence of arginine finger-like motifs within CirA is consistent with its ability to stimulate the GTPase activity of RhoA.
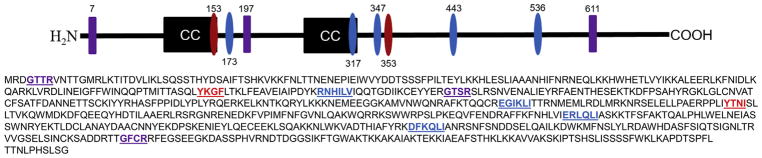
Fig. 1.
CirA possesses multiple function domains associated with modulation of Rho GTPases and endocytic trafficking. C. burnetii CirA possesses three arginine finger-like motifs (purple), and two coiled coils (black). Additionally, the Eukaryotic Linear Motif Resource (http://elm.eu.org/) predicted two Y- based sorting signals (red) and four endosome-lysosome-basolateral sorting signals (blue).
Intracellular trafficking of proteins requires short peptide motifs, termed sorting signals that determine which vesicular pathway will transport the cargo. These signals interact with adapter complexes, such as APs or GGAs at each stage of transport to reach their target membrane. Further analysis of CirA revealed the presence of putative Y-based and endosome-lysosome-basolateral sorting signals (Fig. 1) typically found in proteins that regulate aspects of vesicle trafficking [27]. The presence of putative endocytic sorting signals and three arginine finger -like motifs [16] suggest that CirA may engage Rho GTPases to modulate vesicle trafficking.
3.2. Arginine finger-like motifs are required for toxicity in yeast
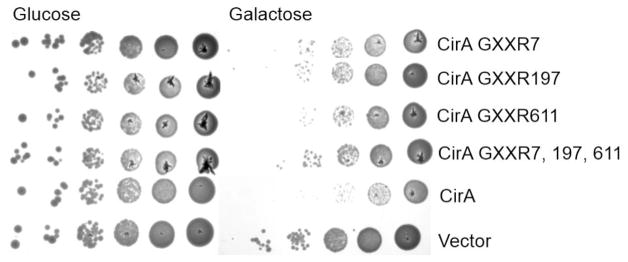
To determine whether arginine finger-like motifs are necessary for CirA-mediated toxicity, we made point mutations of the invariant arginine within each motif and transformed the constructs into yeast. Mutation of each individual motif resulted in a mild reduction of toxicity in yeast, however mutation of all three GXXR motifs resulted in a more pronounced rescue compared to the individual mutations (Fig. 2). No difference in protein expression was noted (Fig. S1A). These results indicate that arginine finger-like motifs contribute to toxicity induced by CirA expression in yeast.
Fig. 2.
Quikchange mutagenesis was employed to create arginine finger point mutations (Table S1) to determine if the bioinformatically predicted motifs are necessary for toxicity in yeast. Yeast were transformed with each constructed and spotted on drop-out media containing glucose or galactose. Data are representative of three independent experiments.
3.3. Arginine finger-like motifs are required for cell rounding and stress fiber disruption in mammalian cells
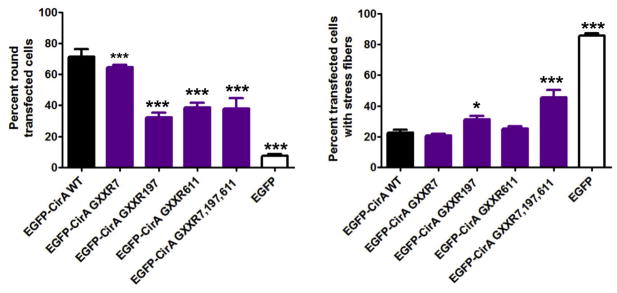
To determine whether arginine finger-like motifs are required for stress associated phenotypes in mammalian cells, we quantified two indicators of mammalian cell stress, cell rounding and stress fiber disruption in cells transfected with mutant CirA. HeLa cells were transfected with mutant CirA constructs carrying point mutations within the invariant arginine of each arginine finger-like motif and stress fiber formation and cell rounding were assessed at 24 h post-transfection. Interestingly, in mammalian cells, mutation of the individual arginine fingers at position 197 or 611 partially rescued cell rounding, however mutation of only GXXR197 significantly rescued stress fiber formation (Fig. 3, Fig. S2). Mutation of all arginine finger-like motifs significantly rescued both rounding and stress fiber formation, however stress fiber formation was still reduced when compared to EGFP vector transfected cells. No difference in protein expression was noted (Fig. S1B). Collectively, these data highlight the importance of CirA arginine finger-like domains for modulation of the host cytoskeleton and suggest that CirA may possess multiple motifs required to stimulate RhoA GTPase activity, however further study is needed to determine whether all three of these domains are biochemically functional.
Fig. 3.
Quikchange mutagenesis was employed to create arginine finger point mutants to determine if the predicted motifs are required for the cell rounding and stress fiber disruption observed in mammalian cells HeLa cells were transfected with each construct and 15 h post-transfection cells were fixed in formaldehyde and actin was stained with phalloidin 555. Statistical analysis was tabulated using one-way ANOVA and resulted in a statistical difference of ***p < 0.0001 or *p < 0.05 compared to full-length CirA. Data are representative of three independent experiments with at least 50 transfected cells per experiment.
3.4. CirA localization to the plasma membrane and stress fiber disruption requires trafficking through the endocytic pathway
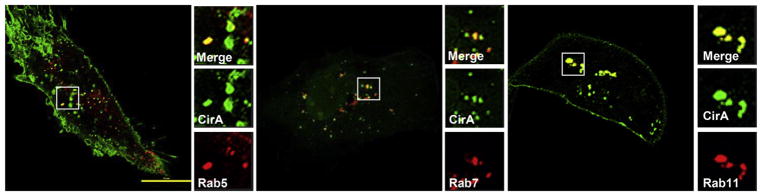
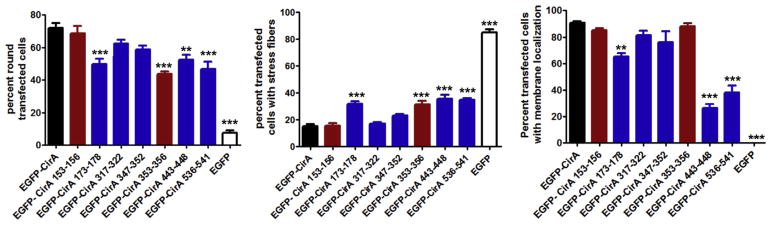
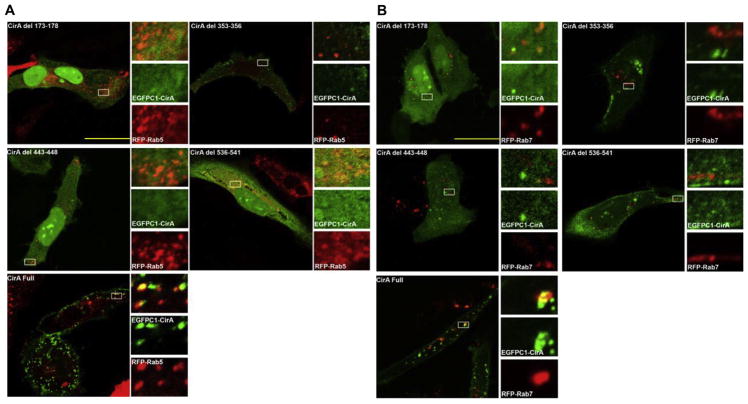
To assess CirA’s potential involvement in vesicle trafficking, we assessed co-localization of CirA positive vesicles with markers of early, late, and recycling endosomes. Because rounded cells lacked predominant puncta we assessed co-localization at 15 h post-transfection, a time prior to cell rounding, and only focused on cells with visible puncta. As shown in Fig. 4, CirA vesicles co-localized with Rab5, Rab7, and Rab11. To assess the importance of CirA trafficking, sorting signal deletion mutants were generated and used to transfect HeLa cells. In contrast to the wild-type CirA, loss of the endosome-lysosome-basolateral sorting signals (residues 173–178, 443–448, or 536–541) significantly reduced localization to the plasma membrane and instead resulted in a predominantly cytoplasmic signal (Fig. 5 and Fig. S3). Furthermore, loss of these signals significantly reduced CirA’s ability to disrupt stress fiber formation and induce cell rounding (Fig. 5). In contrast, loss of the Y-based signal 353–356 reduced stress fiber disruption and cell rounding (Fig. 5), but did not significantly affect plasma membrane localization. Deletion of any of these four aforementioned trafficking motifs reduced co-localization with Rab5 and Rab7 (Fig. 6). These results suggest that CirA trafficking through the endocytic and secretory pathways is essential for localization to the plasma membrane and for interaction with the host cell cytoskeleton.
Fig. 4.
CirA co-localizes with early, late, and recycling endosomes. Representative immunofluorescence images of HeLa cells co-transfected with EGFP-CirA (green) and dsRed-tagged ectopically expressed Rab5, Rab7, or Rab11 (red). White boxes denote the area used for insets. Scale bars in merged images are 10 μm. Data are representative of three independent experiments with at least 100 co-transfected cells scored per experiment.
Fig. 5.
CirA localization to the membrane, host cell rounding, and disruption of stress fibers requires endocytic sorting signals. QuikChange mutagenesis was employed to individually delete Y-based (red) and Endosome-Lysosome-Basolateral (blue) sorting signals. Rounding and stress fiber disruption were determined 24 h post-transfection and membrane localization was determined 15 h post-transfection by counting at least 50 transfected cells from triplicate wells. Data are representative of at least three independent experiments. Statistical analysis was tabulated using one-way ANOVA and generated a statistical difference of ***p < 0.0001 or **p < 0.01 compared to CirA.
Fig. 6.
CirA sorting signals are necessary for localization with endosomes. HeLa cells were transfected with CirA deletion mutants and at 15 h post-transfection co-localization with ectopically expressed Rab5 and Rab7 was accessed. Data are representative of at least three independent experiments with at least 100 co-transfected cells scored per experiment.
4. Discussion
The T4SS effector CirA, is essential for intracellular replication, CCV formation, and virulence in a SCID mouse model [15,16]. When ectopically expressed in eukaryotic cells, CirA can inhibit the growth of yeast and induce a phenotype reminiscent of cytoskeletal stress in mammalian cells. CirA toxicity in yeast and stress induced in mammalian cells can be suppressed through overexpression of RhoA, suggesting that it is a probable target of CirA during Coxiella burnetti infection. Suppression of toxicity requires the GTP-bound state of RhoA, suggesting that CirA toxicity is due to its ability to stimulate RhoA GTPase activity [16]. RhoA is normally recruited to the CCV during infection but C. burnetii mutants lacking CirA fail to recruit RhoA suggesting that this process is essential for normal replication and growth.
In the current study we used a bioinformatics approach to analyze the CirA coding sequence for motifs associated with GTPase regulation. We found that CirA possesses multiple arginine finger-like motifs, often found in GTPase-activating proteins (GAPs), and sorting signal motifs associated with vesicle trafficking. Mutations of arginine finger-like motifs or sorting signals diminished cell rounding, affected plasma membrane localization, and rescued stress fiber formation, suggesting that CirA modulates endolysosomal trafficking through interaction with the host-cell cytoskeleton.
Since suppression of CirA cytoskeletal stress phenotypes in mammalian cells required the GTP-bound form of the GTPase [16], we hypothesized that CirA acts to antagonize the function of Rho GTPases. Sequence analysis revealed that CirA possesses multiple arginine finger-like motifs, associated with Rho GAP proteins. Both eukaryotic and prokaryotic Rho GAPs possess an invariant arginine and a conserved glutamine residue that stimulate GTP hydrolysis through coordination of the GTP molecule as well as participating in the nucleophilic attack by water [31]. Interestingly point mutation of the invariant arginine did not completely abolish CirA toxicity in yeast or cell rounding and stress fiber disruption in mammalian cells, suggesting it might possess additional functional motifs associated with manipulation of Rho GTPases. Furthermore, disruption of stress fibers and rounding are all phenotypes associated with bacterial proteins that act as GAPs [30]. In agreement with our present study, elimination of arginine finger-like motifs of bacterial GAPs has been shown to disrupt stress fibers and induce cell rounding [29,30].
Ectopically expressed CirA predominately localized to the plasma membrane, however small puncta were observed that appeared to bud from the membrane and traffic throughout the cytoplasm [16]. In the current work we sought to address the importance of this phenotype by assessing co-localization of CirA puncta with various markers of vesicle trafficking predicted to be involved in CCV development. Rab GTPases are master regulators of intracellular membrane traffic and specific Rabs are associated with various stages of vesicle trafficking, making them ideal markers [32]. We observed co-localization of CirA puncta and Rab5, Rab7, and Rab11, markers of early, late, and recycling endosomes respectfully. The early endosome serves as a sorting station for cargo derived from the plasma membrane and the trans-Golgi network (TGN) [32]. From the endosome, the cargo is targeted for degradation by fusion with the lysosomes or is recycled back to the plasma membrane or TGN [32]. Proteins are targeted to the endocytic and secretory pathway by sorting signals such as the tyrosine-based or endosome-lysosome-basolateral sorting signals that interact with the adapter protein (AP) complex. Bioinformatic analysis of CirA identified several endocytic sorting signals, suggesting that it associates with the endocytic pathway. Mutation of specific motifs reduced co-localization with Rab5 and Rab7, confirming the importance of these motifs for CirA trafficking. Of note, we found no evidence for a physical interaction between CirA and the Rab GTPases used as endosomal markers in this study.
Movement of vesicles around the cell is mediated by changes in the cytoskeleton. During endocytosis, actin polymerization facilities elongation of the growing vesicle by exerting force on the plasma membrane [32]. Additionally, actin plays a key role in generating sorting intermediates along the endocytic pathway. WASP family members recruit the Arp2/3 complex to generate actin-rich patches on the endosomes to promote endosome maturation and fusion with lysosomes [33] while branched actin polymers help to separate out recycling endosomes [34]. In yeast, rerouting of vesicles requires the presence of the GTPases and its regulators in the vesicle which enables dynamic reorganization of the cytoskeleton [34]. Bem3, a yeast Rho GAP, traffics in recycling endosomes to promote delivery of secretory vesicles by an undefined mechanism. Here we demonstrate that similar to Bem3, CirA localizes to several membranes including plasma and endosomal membranes. Association with these compartments and Rho GTPase mediated cytoskeletal arrangements required CirA sorting signals. In the absence of these signals, CirA was unable to disrupt stress fiber formation or associate with plasma or endosomal membrane. These results suggest CirA associates with these compartments to engage Rho GTPases to promote dynamic disassembly of stress fibers to modulate endocytic trafficking.
Collectively our results fit a model in which CirA is secreted post-uptake and localizes to endosomal and plasma membranes. We propose that CirA engages Rho GTPases, potentially as a Rho GAP to promote rapid and dynamic disassembly of actin stress fibers to redirect membrane traffic to the growing CCV. In the absence of CirA, endosomal targeting is perturbed, resulting in the inability to form a spacious replicative compartment.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health- National Institute of Allergy and Infectious Disease Grants AI088430 (JES) and AI090142 (JES) Defense Threat Reduction Agency HDTRA1-13-1-0003 (JES).
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.micinf.2017.12.013.
Footnotes
Conflict of interest
None.
References
- 1.van Schaik EJ, Chen C, Mertens K, Weber MM, Samuel JE. Molecular pathogenesis of the obligate intracellular bacterium Coxiella burnetii. Nat Rev Microbiol. 2013;11:561–73. doi: 10.1038/nrmicro3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howe D, Shannon JG, Winfree S, Dorward DW, Heinzen RA. Coxiella burnetii phase I and II variants replicate with similar kinetics in degradative phagolysosome-like compartments of human macrophages. Infect Immun. 2010;78(8):3465–74. doi: 10.1128/IAI.00406-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman SA, Fischer ER, Howe D, Mead DJ, Heinzen RA. Temporal analysis of Coxiella burnetii morphological differentiation. J Bacteriol. 2004;186:7344–52. doi: 10.1128/JB.186.21.7344-7352.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howe D, Melnicakova J, Barak I, Heinzen RA. Maturation of the Coxiella burnetii parasitophorous vacuole requires bacterial protein synthesis but not replication. Cell Microbiol. 2003;5:469–80. doi: 10.1046/j.1462-5822.2003.00293.x. [DOI] [PubMed] [Google Scholar]
- 5.Mahapatra S, Ayoubi P, Shaw EI. Coxiella burnetii Nine Mile II proteins modulate gene expression of monocytic host cells during infection. BMC Microbiol. 2010;10:244–57. doi: 10.1186/1471-2180-10-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber MM, Faris R, Tellez A, Wright WU, Galvan G, Luo ZQ, et al. Modulation of the host transcriptome by Coxiella burnetii nuclear effector, Cbu1314. Microb Infect. 2016;18:336–45. doi: 10.1016/j.micinf.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Voth DE, Heinzen RA. Sustained activation of Akt and Erk1/2 is required for Coxiella burnetii antiapoptotic activity. Infect Immun. 2009;77:205–13. doi: 10.1128/IAI.01124-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussain SK, Broederdorf LJ, Sharma UM, Voth DE. Host kinase activity is required for Coxiella burnetii parasitophorous vacuole formation. Front Microbiol. 2010;1:137. doi: 10.3389/fmicb.2010.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campoy EM, Zoppino FCM, Colombo MI. The early secretory pathway contributes to the growth of the Coxiella-replicative niche. Infect Immun. 2011;79:402–13. doi: 10.1128/IAI.00688-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voth DE, Howe D, Heinzen RA. Coxiella burnetii inhibits apoptosis in human THP-1 cells and monkey primary alveolar macrophages. Infect Immun. 2007;75:4263–71. doi: 10.1128/IAI.00594-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romano PS, Gutierrez MG, Berón W, Rabinovitch M, Colombo MI. The auto-phagic pathway is actively modulated by phase II Coxiella burnetii to efficiently replicate in the host cell. Cell Microbiol. 2007;9:891–909. doi: 10.1111/j.1462-5822.2006.00838.x. [DOI] [PubMed] [Google Scholar]
- 12.Winchell CG, Graham JG, Kurten RC, Voth DE. Coxiella burnetii type IV secretion-dependent recruitment of macrophage autophagosomes. Infect Immun. 2014;82:2229–38. doi: 10.1128/IAI.01236-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beare PA, Gilk SD, Larson CL, Hill J, Stead CM, Omsland A, et al. Dot/Icm type IVb secretion system requirements for Coxiella burnetii growth in human macrophages. mBio. 2011;2(4):e00175–11. doi: 10.1128/mBio.00175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carey KL, Newton HJ, Lührmann A, Roy CR. The Coxiella burnetii Dot/Icm system delivers a unique repertoire of type IV effectors into host cells and is required for intracellular replication. PLoS Pathog. 2011;7:e1002056. doi: 10.1371/journal.ppat.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber MM, Chen C, Rowin K, Mertens K, Galvan G, Zhi H, et al. Identification of Coxiella burnetii type four secretion substrates required for intracellular replication and Coxiella-containing vacuole formation. J Bacteriol. 2013;195(17):3914–24. doi: 10.1128/JB.00071-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber MM, Faris R, van Schaik EJ, Roman VA, McLachlan J, Wright WU, et al. The type IV secreted effector protein CirA stimulates the GTPase activity of RhoA and is required for virulence in a mouse model of Coxiella burnetii infection. Infect Immun. 2016;84(9):2524–33. doi: 10.1128/IAI.01554-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seshadri R, Paulsen IT, Eisen JA, Read TD, Nelson KE, Ward NL, et al. Complete genome sequence of the Q-fever pathogen Coxiella burnetii. Proc Natl Acad Sci USA. 2003;100:5455–60. doi: 10.1073/pnas.0931379100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C, Banga S, Mertens K, Weber MM, Gorbaslieva I, Tan Y, et al. Large-scale identification and translocation of type IV secretion substrates by Coxiella burnetii. Proc Natl Acad Sci USA. 2010;107:21755–60. doi: 10.1073/pnas.1010485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larson CL, Beare PA, Voth DE, Howe D, Cockrell DC, Bastidas RJ, et al. Coxiella burnetii effector proteins that localize to the parasitophorous vacuole membrane promote intracellular replication. Infect Immun. 2014;83:661–70. doi: 10.1128/IAI.02763-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan X, Luhrmann A, Satoh A, Laskowski-Arce MA, Roy CR. Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors. Science. 2008;320:1651–4. doi: 10.1126/science.1158160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voth DE, Howe D, Beare PA, Vogel JP, Unsworth N, Samuel JE, et al. The Coxiella burnetii ankyrin repeat domain containing protein family is heterogeneous, with C-terminal truncations that influence Dot/Icm-mediated secretion. J Bacteriol. 2009;191:4232–42. doi: 10.1128/JB.01656-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang L, Boyd D, Amyot WM, Hempstead AD, Luo ZQ, O’Connor TJ, et al. The E block motif is associated with Legionella pneumophila translocated substrates. Cell Microbiol. 2011;13:227–45. doi: 10.1111/j.1462-5822.2010.01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lührmann A, Nogueira CV, Carey KL, Roy CR. Inhibition of pathogen-induced apoptosis by a Coxiella burnetii type IV effector protein. Proc Natl Acad Sci USA. 2010;107:18997–001. doi: 10.1073/pnas.1004380107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klingenbeck L, Eckart RA, Berens C, Lührmann A. The Coxiella burnetii type IV secretion system substrate CaeB inhibits intrinsic apoptosis at the mitochondrial level. Cell Microbiol. 2012;32:675–87. doi: 10.1111/cmi.12066. [DOI] [PubMed] [Google Scholar]
- 25.Cunha LD, Ribeiro JM, Fernandes TD, Massis LM, Khoo CA, Moffatt JH, et al. Inhibition of inflammasome activation by Coxiella burnetii type IV secretion system effector IcaA. Nat Commun. 2015;6:10205. doi: 10.1038/ncomms10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newton HJ, Kohler LJ, McDonough JA, Temoche-Diaz M, Crabill E, Hartland EL, et al. A Screen of Coxiella burnetii mutants reveals important roles for Dot/Icm effectors and host autophagy in vacuole biogenesis. PLoS Pathog. 2014;10:e1004286. doi: 10.1371/journal.ppat.1004286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larson CL, Beare PA, Howe D, Heinzen RA. Coxiella burnetii effector protein subverts clathrin-mediated vesicular trafficking for pathogen vacuole biogenesis. Proc Natl Acad Sci USA. 2013;110:e4770–9. doi: 10.1073/pnas.1309195110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gietz D, St Jean A, Woods RA, Schiestl RH. Improved method for high efficiency transformation of intact yeast cells. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu Y, Galán JE. A Salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature. 1999;401:293–7. doi: 10.1038/45829. [DOI] [PubMed] [Google Scholar]
- 30.Black DS, Bliska JB. The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol Microbiol. 2000;37:515–27. doi: 10.1046/j.1365-2958.2000.02021.x. [DOI] [PubMed] [Google Scholar]
- 31.Aktories K. Bacterial protein toxins that modify host regulatory GTPases. Nat Rev Microbiol. 2011;9:487–98. doi: 10.1038/nrmicro2592. [DOI] [PubMed] [Google Scholar]
- 32.Granger E, McNee G, Allan V, Woodman P. The role of the cytoskeleton and molecular motors in endosomal dynamics. 2014;31:20–9. doi: 10.1016/j.semcdb.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seaman MN, Gautreau A, Billadeau DD. Retromer-mediated endosomal protein sorting: all WASHed up! 2013;23:522–8. doi: 10.1016/j.tcb.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukherjee D, Sen A, Aguilar RC. RhoGTPase-binding proteins, the exocyst complex and polarized vesicle trafficking. Small GTPases. 2014;5:e28453–7. doi: 10.4161/sgtp.28453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.