Abstract
Background:
Platelets are considered an important source of prothrombotic agents associated with inflammation in cancer related diseases. We aimed to compare the diagnostic accuracy of the platelet distribution width (PDW) and CA19-9 in resectable pancreas cancer.
Method:
A total of 83 stage-1 and 2 pancreatic adenocarcinoma (PAC) patients, and 85 age and sex-matched healthy participants were included in the study. All preoperative patient data, including PDW and CA19-9 were analyzed in terms of sensitivity, specificity, positive and negative predictive values, likelihood ratios, and diagnostic accuracy.
Results:
Demographic features were not significantly different among the groups. Platelet distribution width and CA19-9 were significantly higher in PAC compared to control group (p= 0.0001). Diagnostically, the sensitivity and specificity were 79% and 85% for PDW, while 78% and 91% for CA19-9. Diagnostic accuracy was measured by the area under the ROC curve, and PDW differs significantly (p<0.001), with a value of 0.874 (95% CI: 0.804-0.929).
Conclusion:
Platelet distribution width indicated similar sensitivity and specificity with CA19-9 in patients with resectable PAC. This result strongly advice that PDW, which has more routine option and cost-effectivity than CA19-9, can be used for diagnosis of resectable PAC as a strong alternative.
Keywords: resectable pancreas cancer, platelet distribution width, CA19-9
1. INTRODUCTION
Pancreatic adenocarcinoma (PAC) is a destructive disease with an extremely poor prognosis (1). Resection through surgery represents unique cure option for these patients, which can provide 5-year-survival success to 20%, but is often not feasible because of its advanced-stage upon the diagnosis (2). Because it is not clinically normal, it almost becomes difficult to diagnose (3). For advanced disease, palliative chemotherapy is the treatment of choice although the regimens available to date are untargeted and have extensive side-effect profiles, making them unsuitable for patients with a low-performance status (4-6). For this reason, early diagnose of the resectable pancreatic cancer is essential in order to provide patients with an optimal therapeutic approach.
Accurate diagnosis with early diagnose this cancer is important because extension of the tumor growth makes PAC stage up (7). New early diagnostic biomarkers have been reported for PAC, but are not already available for using in the routine. This was because of the lack sensitivity and specificity (7, 8). Until now, serum cancer antigen 19-9 [CA19-9] is the most trusted and commonly used biomarker (9). Tumor burden, survival, and tumor resectability are correlated with CA19-9. But its cost is not efficient in repeated use and routine usage in today’s world where health spending has increased. A more cost-effective novel biomarker may facilitate this issue and may improve survival (10).
There are different type of studies investigating true more cost-effective biomarker. Hematological parameters related platelets constitute an important source of agents associated with inflammatory markers. Platelet-distribution-width (PDW) and mean platelet volume (MPV) are the most interesting and discussed in terms of the diagnostic values (11). These have been used by physicians not only in inflammatory diseases but also cancer-related diseases (12). Reports of those parameters did not deserve further investigation, due to their low specificity (5, 6). Platelet-distribution-width (PDW), a sign of platelet size changes, is an indicator of release of activated platelets (13). The platelet size is related to platelet function and newly used in variety of diseases (14).
PDW has not been studied in diagnosis and follow-up of resectable PAC The best of our knowledge, and this is the first case-controlled novel study to investigate PDW as early diagnostic and monitoring marker for resectable PAC, with comparison to CA19-9.
2. MATERIALS AND METHODS
We conducted the study in the dates covering December 2010 to January 2016 by medical records of patients with stage 1 and stage 2 PAC who examined at Istanbul Kanuni Sultan Suleyman Training Hospital and Ankara Dis Kapi Training Hospital. The study was performed retrospectively. The classification of TNM was used in proper to American Joint Committee on Cancer recommendations on the staging of PAC. According to the 7th edition of the Committee recommendations, unrespectable stage 3 and stage 4 pancreatic cancers excluded from this study. Written consent was obtained from the patients or patients’ carer. Patients who have any chronic infection, hematological or renal disease, coronary or cerebrovascular problem history, and any other cancer were out of the study, in addition to a history of receiving any preoperative chemoradiotherapy and those with postoperative infections. In total, 83 stage1 and stage 2 PAC patients and 85 sex and age match healthy individuals were enrolled in the study. All data including pre- and postoperative PDW and CA19-9 were recorded. No application or treatment used in routine procedures and any routine procedures were not needed in the study.
ADVIA 2120i Siemens Healthcare Diagnostic Item (Malvern Siemens, USA) analyzed the hematological parameters including PDW in the Ethylene diamine tetraacetic acid treated (EDTA) blood within 1 h after vein puncture. Available enzyme immunoassay was used to measure CA19-9 levels, with a commercial ELISA kit (Roche Diagnostics CA 19-9 Enzymun-Test, Germany). According to value of the manufacturer, the cutoff was 22 U1-1.
Statistical analysis
Student’s T-Test and Oneway analysis of variance were used on parametric values, while Mann Whitney-U, χ2, and Kruskal Wallis tests were used on an-parametric. Statistical data were expressed as mean±SD. Receiver operating characteristic curve analysis (ROC) was performed to find out exact PDW cut-off, with its diagnostic sensitivity and specificity. Likelihood ratios, diagnostic accuracy, negative and positive predictive values were all calculated according to the results. SPSS was used as a statistical analysis software (SPSSv24, Illinois, USA) was used for the statistics. All outcomes were evaluated within the 95% Confidence Interval (CI). P-value < 0.05 was accepted as statistically significant.
3. RESULTS
This study included 83 PAC and 85 healthy individuals. The mean ages of PAC patients were 54±11 (range: 42-71 years) and 51±13 (range: 39-73 years) for control. Pancreatic cancer patients was comprised of 51.8% (43/83) male and 48.2% (40/83) female; Control was comprised of 49.4% (42/85) male and 50.6% (43/85) female. There was no significant difference in terms of demographics including age and sex. Comparing PDW in preoperative results of the PAC and control patients, there were significant differences (p=0.0001), as given in Table1. Additionally, ratio of standard deviation to means was lower for PDW compared to CA19-9, as a trusted biomarker.
Table 1. Preoperative results of the PAC and control patients. Abbreviations: PDW: Platelet distribution width, PAC: Pancreatic Cancer, P.C: Pearson Correlation.
| PAC | Control | p value | P.C. | |
|---|---|---|---|---|
| CA19.9 (U/mL) | 498±1100 | 11.7±6.1 | 0,004 | 0,985 |
| PDW (fL) | 29.8±16.8 | 15.2±2.1 | 0,00001 |
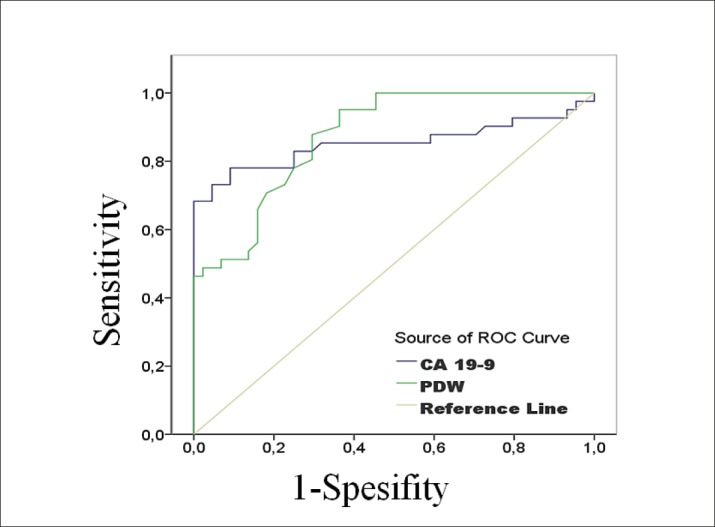
There was no correlation between CA19-9 and PDW. The diagnostic analyze of PDW and CA19-9 values are shared in Table 2 as detailed. Diagnostically, the sensitivity and specificity were 79% and 85% for PDW, while 78% and 91% for CA19-9. We used the area under ROC curve to find diagnostic accuracy of PDW. It showed significant difference with a value of 0.874 (p:0.001; 95%CI:0.804-0.929). PDW and CA19-9 showed a similar diagnostic accuracy. Figure 1 indicates ROC curve for PDW and CA19-9.
Table 2. Diagnostic comparisons of blood parameters. Abbreviations: AUC: Area under curve; DA: Diagnostic accuracy; NLR: Negative likelihood ratio; NPV: Negative predictive value; PLR: Positive likelihood ratio; PPV: Positive predictive value.
| Cut-off | AUC | Sensitivity | Specifity | PPV | NPV | PLR | NLR | |
|---|---|---|---|---|---|---|---|---|
| CA19.9 (U/mL) | 20,5 | 0,852 | 78% | 91% | 88,8% | 81,6% | 8,66 | 0,28 |
| PDW (fL) | 16,4 | 0,874 | 79% | 85% | 75,7% | 80,1% | 5,26 | 0,24 |
Figure 1. Receiver operating characteristic curves (ROC).
4. DISCUSSION
Diagnose of pancreatic cancer needs a lot of spending money for both healthcare workers and patients, and also limitations (15). The best biomarker used up to this day has been CA19-9. As well known by the literature, novel studies investigating inflammatory biomarkers in PAC, as an alternative to CA19-9 (16). Platelets constitute an important source of agents associated with inflammatory markers. Especially, hematological parameters that triggered by inflammation have been concentrated on by physicians, in cancer-related diseases (17-19). But, the missing point of the recent researches has been PDW. Our findings in the present study will illuminate this point and change the angle of view to CA19-9 used in diagnose and follow-up of the resectable PAC.
According to the novel studies, inflammatory markers means platelets as an important source of agents associated with them (20). Large-sized platelets play a role in the initiation and spread of vascular-mediated inflammatory events, as they have many granules that effect hemostatic and proinflammatory effects more effectively (21). Precisely for these reasons, hematological parameters including MPV and PDW can indicate functions and activation of platelets (22). Although MPV has a longer history than PDW especially in cancer research, PDW has been increasing its popularity with its plus diagnostic aspects like a higher specificity (23). Digestive system cancers are one of the areas where diagnostic competition is experienced between MPV and PDW.
Novel diagnostic studies for digestive cancers began via gastric cancer with MPV. Kilincalp et al reported early diagnosis and monitoring for gastric cancer (24). According to the results of this 31 gastric-cancer-patients study, with hepatocellular adenocarcinoma. Pre-operative gastric cancer patients had higher MPV level than healthy individuals. They observed a significant decrease following the surgical tumor resection. Karaman et al retrospectively analyzed and reported that hematological markers such as mean platelet volume could have a predictive potential for differing pancreatic adenocarcinoma from a non-functional pancreatic neuroendocrine tumor (9). Patients with PNET had lower MPV levels than patients with pancreatic adenocarcinoma, in preoperative period. CA19-9 and MPV had diagnostic value to distinguish PNETs from PAC. On the contrary, a study by Aliustaoglu et al, significant difference was not seen for hematological variables in pancreas cancer (7). In similar. Afsar et al was also reported that a hematological variable such as MPV had no diagnostic role and no relationship with survival of PAC (1). Hirahara et al reported MPV as a non-specific predictor parameter to diagnose esophagus cancers and pointed other hematological parameters out for further studies (25). As seen in the studies, MPV was reported as a potential biomarker to diagnose gastric and pancreas cancer, despite of its low sensitivity and specificity. This was because MPV had the closest diagnostic biomarker values to CA19-9 so far. But the results of our current study changed this acceptance in favor of the PDW. Our results showed that PDW had a significant potential as a diagnostic biomarker as CA19-9. While MPV is present in most hematology analyzers and describes the mean trombosite volume, PDW is a measurement of the platelet volume heterogeneity, defined as distribution width at the 20% frequency level. PDW had advantages in different directions on diagnose the gastrointestinal cancers. For instance, Zhang et al retrospectively analyzed 294 gastric cancer cases (14). Tumor stage, age, carcinoembryonic-antigen, nodule stage, and metastases stage were associated with the reduced PDW. They reported that reduced levels of PDW could be an unfavorable prognostic value for gastric cancers. PDW and MPV have a direct relationship both usually change in the same direction under physiological conditions. PDW directly indicates changes with platelet activation, variability in size of platelet and projects the heterogeneity in platelet morphology (21). Additionally, conflicting reports have been presented in the literature that show that they are affected by different mechanisms about their relationship (20, 26, 27).
A study supporting diagnostic potential of PDW was performed by Song et al., with inflammation-based hematological indices related to various malignancies, including colorectal cancer (3). They reported that lymph node involvement, CA19-9, PDW, and combination of NLR-PDW were correlated with both overall and recurrence-free-survive. According to the Gunaldi et al, PDW might open a consideration path for treatment planning of gastric cancers in the surgical and chemotherapeutic ways (13). Although its sensitivity and specificity were low for gastric cancers unlike their hopes, they offered PDW as trustable marker instead. Our results showed that PDW had a significant potential as a diagnostic biomarker as CA19-9. Diagnostically, the sensitivity and specificity were 79% and 85% for PDW, while 78% and 91% for CA19-9. Under ROC curve showed significant difference with a value of 0.874 (p:0.001; 95%CI:0.804-0.929). As a result, diagnostic accuracy is similar for PDW and CA19.9. The present study proves that the PDW has very similar diagnostic values such as sensitivity and specificity to CA19-9.
The present study had some limitations. This study was a retrospective and a single institution design study. The study size can be accepted as small compared to its necessary. Additionally, we could not evaluate the survival of the patients in resectable PAC. Larger-sized prospective studies are required to elucidate the precise mechanisms that relate PDW to survival in resectable pancreas cancer patients. But, this limitation does not lessen the clinical relevance and gains of our results.
5. CONLUSION
Our study showed that the PDW has very similar diagnostic values such as sensitivity and specificity to CA19-9. It will be a reliable biomarker for physicians like CA19-9 because it is cost effective if it is widely used in PDW’s routine. Although hematology parameters have been widely discussed in the literature and are cautious in terms of their diagnostic value, PDW has shown a different line in this respect than hematological parameters such as MPV. We suggest that PDW should be considered up with CA19-9 in diagnose and follow-up of the resectable pancreas adenocarcinoma.
Acknowledgement:
We thank to Dr. Barış YILMAZ because of his kind supports on evaluating patient data and excel graphic preparations. No specific grant with a funding agency in the public, commercial, or not-for-profit sectors were received or used for this research article.
Conflict of Interest:
All authors declared that there is no conflict of interest.
Author Contribution:
Each author participated in each step of research. Article was revised and final approval for publishing was given.
REFERENCES
- 1.Afsar CU, Gunaldi M, Kum P, Sahin B, Erkisi M, Kara IO, et al. Pancreatic carcinoma, thrombosis and mean platelet volume: single center experience from the southeast region of Turkey. Asian Pacific Journal of Cancer Prevention. 2014;15(21):9143–9146. doi: 10.7314/apjcp.2014.15.21.9143. [DOI] [PubMed] [Google Scholar]
- 2.La Torre M, Nigri G, Cavallini M, Mercantini P, Ziparo V, Ramacciato G. The glasgow prognostic score as a predictor of survival in patients with potentially resectable pancreatic adenocarcinoma. Annals of surgical oncology. 2012;19(9):2917–2923. doi: 10.1245/s10434-012-2348-9. [DOI] [PubMed] [Google Scholar]
- 3.Song X, Zhu H, Pei Q, Tan F, Li C, Zhou Z, et al. Significance of inflammation-based indices in the prognosis of patients with non-metastatic colorectal cancer. Oncotarget. 2017;8(28):45178–45189. doi: 10.18632/oncotarget.16774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang JC, Kundranda M. Novel Diagnostic and Predictive Biomarkers in Pancreatic Adenocarcinoma. International Journal of Molecular Sciences. 2017;18(3) doi: 10.3390/ijms18030667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Distler M, Pilarsky E, Kersting S, Grutzmann R. Preoperative CEA and CA 19-9 are prognostic markers for survival after curative resection for ductal adenocarcinoma of the pancreas - a retrospective tumor marker prognostic study. International journal of surgery (London, England) 2013;11(10):1067–1072. doi: 10.1016/j.ijsu.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Poruk KE, Gay DZ, Brown K, Mulvihill JD, Boucher KM, Scaife CL, et al. The clinical utility of CA 19-9 in pancreatic adenocarcinoma: diagnostic and prognostic updates. Current molecular medicine. 2013;13(3):340–351. doi: 10.2174/1566524011313030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aliustaoglu M, Bilici A, Seker M, Dane F, Gocun M, Konya V, et al. The association of pre-treatment peripheral blood markers with survival in patients with pancreatic cancer. Hepato-gastroenterology. 2010;57(99-100):640–645. [PubMed] [Google Scholar]
- 8.Asari S, Matsumoto I, Toyama H, Shinzeki M, Goto T, Ishida J, et al. Preoperative independent prognostic factors in patients with borderline resectable pancreatic ductal adenocarcinoma following curative resection: the neutrophil-lymphocyte and platelet-lymphocyte ratios. Surgery today. 2016;46(5):583–592. doi: 10.1007/s00595-015-1206-3. [DOI] [PubMed] [Google Scholar]
- 9.Karaman K, Bostanci EB, Aksoy E, Kurt M, Celep B, Ulas M, et al. The predictive value of mean platelet volume in differential diagnosis of non-functional pancreatic neuroendocrine tumors from pancreatic adenocarcinomas. European journal of internal medicine. 2011;22(6):e95–e98. doi: 10.1016/j.ejim.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Agrawal S. Potential prognostic biomarkers in pancreatic juice of resectable pancreatic ductal adenocarcinoma. World journal of clinical oncology. 2017;8(3):255–260. doi: 10.5306/wjco.v8.i3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurt H, Demirkiran D. Changing of Hemoglobin A1c Affects Mean Platelet Volume in Type-2 Diabetes Mellitus. Ulutas Med J. 2016;2(1):27–35. [Google Scholar]
- 12.Ulukent SC, Sarici IS, Ulutas KT. All CBC parameters in diagnosis of acute appendicitis. International journal of clinical and experimental medicine. 2016;9(6):11871–11876. [Google Scholar]
- 13.Gunaldi M, Erdem D, Goksu S, Gunduz S, Okuturlar Y, Tiken E, et al. Platelet Distribution Width as a Predictor of Metastasis in Gastric Cancer Patients. Journal of gastrointestinal cancer. 2016. [DOI] [PubMed]
- 14.Zhang X, Cui MM, Fu S, Li LL, Liu YS, Liu ZP, et al. Platelet distribution width correlates with prognosis of gastric cancer. Oncotarget. 2017;8(12):20213–20219. doi: 10.18632/oncotarget.15561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freelove R, Walling AD. Pancreatic cancer: diagnosis and management. American family physician. 2006;73(3):485–92. [PubMed] [Google Scholar]
- 16.Shiratori K. Early diagnosis and staging of pancreatic cancer. Nihon Geka Gakkai zasshi. 2006;107(4):164–167. [PubMed] [Google Scholar]
- 17.Kilincalp S, Coban S, Akinci H, Hamamci M, Karaahmet F, Coskun Y, et al. Neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and mean platelet volume as potential biomarkers for early detection and monitoring of colorectal adenocarcinoma. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation (ECP) 2015;24(4):328–333. doi: 10.1097/CEJ.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 18.Yavuzcan A, Caglar M, Ustun Y, Dilbaz S, Ozdemir I, Yildiz E, et al. Mean platelet volume, neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in severe preeclampsia. Ginekologia polska. 2014;85(3):197–203. [PubMed] [Google Scholar]
- 19.Yucel B, Ustun B. Neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, mean platelet volume, red cell distribution width and plateletcrit in preeclampsia. Pregnancy hypertension. 2017;7:29–32. doi: 10.1016/j.preghy.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Huczek Z, Kochman J, Filipiak KJ, Horszczaruk GJ, Grabowski M, Piatkowski R, et al. Mean Platelet Volume on Admission Predicts Impaired Reperfusion and Long-Term Mortality in Acute Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention. Journal of the American College of Cardiology. 2005;46(2):284–290. doi: 10.1016/j.jacc.2005.03.065. [DOI] [PubMed] [Google Scholar]
- 21.Budak YU, Polat M, Huysal K. The use of platelet indices, plateletcrit, mean platelet volume and platelet distribution width in emergency non-traumatic abdominal surgery: a systematic review. Biochemia Medica. 2016;26(2):178–193. doi: 10.11613/BM.2016.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vagdatli E, Gounari E, Lazaridou E, Katsibourlia E, Tsikopoulou F, Labrianou I. Platelet distribution width: a simple, practical and specific marker of activation of coagulation. Hippokratia. 2010;14(1):28–32. [PMC free article] [PubMed] [Google Scholar]
- 23.Ulutas KT, Dokuyucu R, Sefil F, Yengil E, Sumbul AT, Rizaoglu H, et al. Evaluation of mean platelet volume in patients with type 2 diabetes mellitus and blood glucose regulation: a marker for atherosclerosis? Int J Clin Exp Med. 2014;7(4):955–961. [PMC free article] [PubMed] [Google Scholar]
- 24.Kilincalp S, Ekiz F, Basar O, Ayte MR, Coban S, Yilmaz B, et al. Mean platelet volume could be possible biomarker in early diagnosis and monitoring of gastric cancer. Platelets. 2014;25(8):592–594. doi: 10.3109/09537104.2013.783689. [DOI] [PubMed] [Google Scholar]
- 25.Hirahara N, Matsubara T, Kawahara D, Mizota Y, Ishibashi S, Tajima Y. Prognostic value of hematological parameters in patients undergoing esophagectomy for esophageal squamous cell carcinoma. International journal of clinical oncology. 2016;21(5):909–919. doi: 10.1007/s10147-016-0986-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ates I, Bulut M, Ozkayar N, Dede F. Association Between High Platelet Indices and Proteinuria in Patients With Hypertension. Annals of Laboratory Medicine. 2015;35(6):630. doi: 10.3343/alm.2015.35.6.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.David Bessman J, Williams LJ, Ridgway Gilmer P. Mean Platelet Volume. The Inverse Relation of Platelet Size and Count in Normal Subjects, and an Artifact of Other Particles. American Journal of Clinical Pathology. 1981;76(3):289–293. doi: 10.1093/ajcp/76.3.289. [DOI] [PubMed] [Google Scholar]


