Abstract
A growing body of experimental data suggests that microbes in the gut influence behavior and can alter brain physiology and neurochemistry. Although promising, researchers are only starting to understand the potential of the gut microbiota for use in neurological disease. Recent evidence demonstrated that gastrointestinal activities are linked to mood disorders such as anxiety, depression, and most recently, cognitive functions in age-related neurodegenerative disorders. Studies from our group and others are uncovering new evidence suggesting that the gut microbiota plays a crucial role in the metabolism and bioavailability of certain dietary compounds and synthetic drugs. Based on this evidence, this review article will discuss the implications of the gut microbiota in mechanisms of bioavailability and biotransformation with an emphasis on dietary polyphenol compounds. This will be followed by a survey of ongoing innovative research identifying the ability of individual gut bacteria to enhance the bioavailability of gut-derived, brain-penetrating, bioactive polyphenol metabolites that ultimately influence mechanisms associated with the promotion of resilience against psychological and cognitive impairment in response to stress. Lastly, current research initiatives aimed at promoting the generation of brain bioactive polyphenol metabolites by specialized gut microbes will be discussed, specifically the use of gnotobiotic mice to develop bioengineered second generation probiotics. We propose that leveraging the gut microbial ecosystem to generate brain targeted bioactive metabolites from dietary polyphenols can attenuate lifestyle risk factors and promote resilience against age-related cognitive decline.
Keywords: Gnotobiotic mice, inflammation, microbiota, oxidative stress, polyphenol metabolism
INTRODUCTION
In recent years, the scientific community has begun to understand the intricate and dynamic interactions between the symbiotic bacteria in the gastrointestinal (GI) tract, the gut microbiota, and neurological function [1–8]. The bacteria of the gut have been show to affect high order cognition and physiological functions and they are crucial to physiological homeostasis [9–14]. Ongoing studies support the hypothesis that the gut microbiota’s influence on brain function is mediated through metabolic processing of certain dietary compounds such as polyphenols [15–18] and synthetic drugs [19–22] by the resident bacteria. Studies from our group and others suggest that the polyphenol metabolites produced by the microbiota modulate immune-inflammatory cascades [23–29], synaptic maladaptation [30–32], mood disorders [33, 34] as well as age-related neurodegenerative disorders [18, 31, 32, 35]. Given this ability of the microbiota to generate unique bioactive compounds, efforts have been made to manipulate the gut microbiota to improve the delivery and efficacy of orally consumed compounds, including phyto-drugs [36–38]. However, the role gut microbiota has on polyphenol metabolism remains elusive. Further efforts should explore how specific members of the gut microbiota promote polyphenol biotransformation in order to develop novel therapeutic strategies through probiotic bacteria.
WHAT IS THE GUT MICROBIOTA?
The gut microbiota is a community of diverse bacteria and microorganisms that form symbiotic relationships with their hosts to maintain host health [39, 40] The influence of the gut microbiota on the host is so complex that the gut microbiota is now considered to be an independent endocrine organ [41] with vast metabolic activities and the capacity to modulate several physiological states including inflammation, oxidative stress [42], and as this review will focus on, neurological processes [43]. The significant impact of the gut microbiota on host metabolism was amply illustrated when transplantaion of gut bacteria from twins discordant for obesity into mice resulted in identical metabolic phenotypes of the microbiota donor [44]. The compositon of the gut microbiota varies significantly between healthy individuals due to diet, antibiotic usage, exposure to pharmaceutical agents, interactions with the environment, physiological and psychological stress [45,46]. Although hundreds of various microbe families have been found in the GI tract [47], each individual harbors a unique combination of these microbes [48, 49]. Despite this diversity, a standard healthy gut microbiota profile has emerged as the composition of the microbiota is now recognized as an important factor in understanding individual nutritional needs [50,51]. Recent research has shown that a healthy gut microbiota is composed of a high proportion of metabolically-active butyrate-producing bacteria including the Ruminococcus spp. and Eubacterium spp., Bifidobacterium spp., which degrade long chain dietary fibers, a low ratio of the principle phyla Firmicutes to Bacteriodetes, and a reduced proportion of inflammatory pathogens including the Proteobacteria [52–54]. As a more accurate profile of the gut microbiota develops, efforts will look at the metabolic activity of specific bacteria and their role in generating biologically active compounds, such as compounds derived from dietary polyphenols
THE GUT MICROBIOTA AND ITS ROLE IN THE GENERATION OF BIOACTIVE POLYPHENOLIC METABOLITES
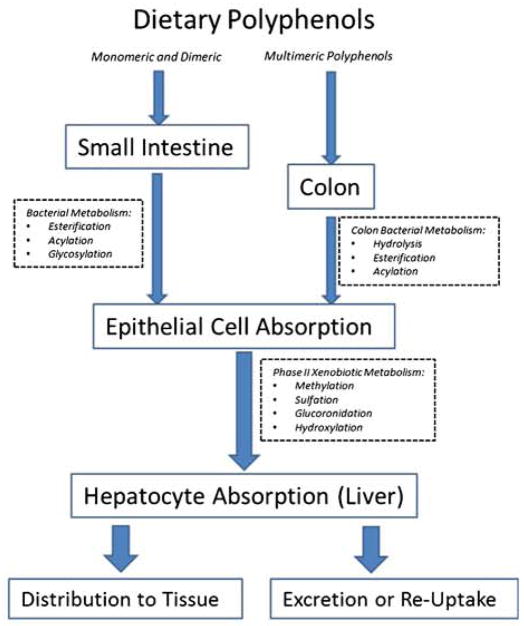
Research to date on the relationship between polyphenols and bacteria in the GI tract indicate that gut bacteria possess innate mechanisms that generate de novo and potentially bioactive compounds when provided with dietary polyphenols (Fig. 1). The gut microbiota is critical to enabling the bioavailability of ingested polyphenols as most parent polyphenols are not well absorbed by the small intestine [55]. Likewise, 90% of ingested polyphenols reach the large intestine and within the context of the resident microbiota, are transformed into bioavailable products. Following the ingestion of polyphenol compounds, usually in their glycosylated form, bacteria in the GI tract convert dietary polyphenols to low-molecular-weight phenolic compounds, such as phenolic acids [56, 57], that can be more efficiently absorbed by intestinal epithelial cells [58, 59] In some cases, biotransformation by the gut microbiota is essential to providing bioavailable polyphenolic acids. Polyphenols have been shown to undergo a variety of enzymatic processes by bacterial populations in the GI tract. These include the hydrolysis of glycosylaed flavonoids, acylation of flavanol-3-ols ad esterification of hydroxycinnamic acids [60]. Subsequent to enzymatic processing by bacteria, the polyphenol derivatives, usually the aglycons or hydroxyphenylacetic acids, are absorbed by epithelial cells of the small intestine. Once the polyphenol derivatives are in a form capable of being absorbed by the small intestine or colon, they undergo enterocyte phase II modifications, in which they are often converted to their O-Glucuronide or O-Sulfonate forms through methylation, sulfation, hydroxylation, and glucoronidation. Multimeric polyphenols that are not broken down in the small intestine move through the GI tract where their conjugating moieties are processed by colon specific bacteria and absorbed into the epithelial tissue for phase II metabolism [61] The modified polyphenols, from the enterocytes, then enter the blood Stream via the portal vein, are delivered to the liver where they can be further modified via conjugations, and finally are either secreted as bile components back into the intestine for enterohepatic recirculation, or into the blood stream to be delivered to the peripheral tissues including the brain [60].
Fig. 1.
The diversity of interpersonal bacteria differentially metabolizes polyphenols. After oral consumption, monomeric polyphenols undergo a variety of covalent modifications, such as acylation or esterfication, catalyzed by the bacterial enzymes unique to the upper gastrointestinal tract. The modified polyphenols then transit into the epithethial cells of the upper GI tract where they undergo enterocyte modifications by phase II metabolisim that involves the conjugation of glucuronide, methyl or hydroxyl groups. Multimeric polyphenol compounds pass through the small intestine because the resident bacteria do no transcribe hydrolytic enzymes required for the production of monomeric polyphenols, and their molecular weight prevents their uptake into upper GI epithethial cells. They pass into the colon where resident bacteria hydrolyze conjugating moieties of multimeric polyphenols allowing for their absorption and metabolic processing into simple phenols by colon epithelial cells. Another set of covalent modifications can occur when polyphenol metabolites pass through the portal vein and reach liver hepatocytes, whereupon they undergo additional phase II modifications. The polyphenol derivatives are then either distributed to the target tissue by circulation, or are excreted as bile for enterohepatic circulation or excretion. The interpersonal diversity of microbiota, represented in the scheme by the red bacteria, can substantially alter the potential bioavailability and bioactivity of a polyphenol’s derivatives, and subsequently their therapeutic efficacy in models of induced cognitive dysfunction.
One recent study demonstrated how a polyphenol-rich potato extract containing cholorogenic, caffeic, and ferulic acids was broken down in a colonic simulator containing human gut microbiota into several biotransformation products. A small proportion of the metabolites were transduced across a Caco-2/HepG2 co-culture; however, the absorption of the secondary metabolites following hepatic transformation resulted in significant increases of ferulic, dihydrocaffeic, 2-hydroxyphenylacetic acid, 3-hydroxyphenylpropionic acid, and coumaric acid [62]. In another study, a simulated gastrointestinal environment composed of rat gut microbiota metabolized three main anthocyanins from mulberry including cyanidin-3-glucoside, cyanidin-3-rutin, and delphinidin-3-rutinoside. Cyanidin-3-glucoside and cyaniding-3-ruitin resulted in protocatechuic, vanillic, and p-coumeric acids while deiphinidin-3-rutinoside into gallic acid, syringic acid, and 2,4,6-trihydroxybenzaldehyde: processes that were dependent on the presence of the gut microbiota [63]. In a cohort of elderly Japanese individuals, the composition of the gut microbiota was highly correlated with the rate of quercetin metabolism. The abundance of Surreellaceae and Oscillospiraceae were negatively correlated with quercetin metabolism, while Fusobacteria and Enterobacteriaceae showed a positive correlation [64], Furthermore, using 20 healthy volunteers who were administered green tea, one study found that colonic microbiota produced polyhydroxyphenyl-γ-valerolactones, which was the urinary catabolite produced in the greatest concentrations; it was shown to have a 10 times greater concentration than the other flavan-3-ol conjugates [65]. Recently phenyl-γ-valerolactones have been purported to provide protection to brown adipocytes against oxidative damage, which may provide an important observation for research into obesity [66].
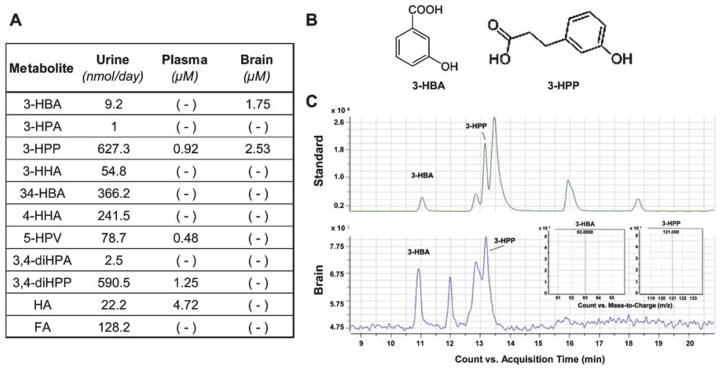
Our group has shown that mice administered with grape seed polyphenol extract (GSPE) resulted in the production of 11 unique polyphenol metabolites, as measured in urine, and 4 as measured in the plasma, while only of the 2 metabolites, 3-HBA and 3-HPP, were detected in the brain following perfusion (Fig. 2, adapted from Wang et al. [35]), Both 3-HBA and 3-HPP are likely derivatives of the flavonol quercetin and are produced following ring scission by Enterobacteria spp. in the gut and subsequent enterocyte phase II modification such as dehydration or reduction [67]. Another study using dietary supplementation with a red wine polyphenolic extract found that the addition of Lactobacillus plantarum increased the conversion of monomeric flavanols, such as those found in GSPE, and their intermediate metabolites into phenylpropionic acids, in particular 3-(3′-hydroxyphenyl) propionic acid (3-HPPA) [68]. In vivo studies using germ-free or antibiotic-treated mice also support the necessity of gut microbes for the conversion of polyphenol compounds into bioactive and bioavailable compounds whose concentration without bacteria metabolism would be subclinical 18, 68–72].
Fig. 2.
Polyphenol metabolites measured in urine, plasma and in the brain following the administration of GSPE. A) The administration of GSPE, at 250 mg/BW, to Sprague Dawley rats by gavage for 10 days resulted in the identification of eleven unique phenolic acids the urine, four of which were also found in the plasma, and two in the brain. B) Of the two quantified phenolic acids in the brain, 3-(3′-hydroxyphenyl) propionic acid (3-HPP) is a known derivative of the flavonol quercetin, the flavone apigenin, and of the flavan-3-ols catechin or epicatechin, which are processed by respective bacteria Enterococcus casseliflavus, Clostridium coccoides, and C. orbiscindens [60]. Meanwhile, the second brain quantified phenolic acid, 3-hydroxybenzoic acid (3-HBA), is known to be a derivative of the anthocyanin, cyanidin, which undergoes enzymatic processing by either Lactobacillus plantarum, Lactobacillus casei, Lactobacillus acidophilus, or Bifidobacterium lactis [60]. C) The concentration of each phenolic acid was determined via LC-MS/MS, using analytical grade molecular standards (top) and a sample brain specimen (top).
THE IDENTIFICATION OF BACTERIA CAPABLE OF CONVERTING POLYPHENOLS INTO BIOACTIVE COMPOUNDS
To best understand the role of gut bacteria in the breakdown of polyphenols into bioactive metabolites, it is critical to determine specific bacterial strains responsible for the enzymatic processing of polyphenols Understanding the catalytic abilites of each bacterial species will provide an opportunity to bio-engineer next-generation probiotics that most effectively transform polyphenols into the desired bioactive metabolites.
Several enzymes necessary for the biotransformation of dietary polyphenols have been identified. Eubacterium ramulus induces ring fission of the polyphenolic core generating several metabolites with altered health benefits [73]. For example, degradation of naringen by calchone isomerase and phloretin hydrolase converts the complex polyphenol into usable aromatic products [74] From the green tea polyphenols catechins, epicatechin, epicatechin galate, and epigallocatechin galate, 4-hydrobeenzoic acid and vanillic acid are produced [75] For example, ferulic acid, a hydroxycinnamic acid found in plant stems and various herbs, is broken down by the esterase activity of Lactobacillus spp. into 4-vinylguaiacol and hydroferulic acid [76]. Further breakdown converts these products into the caffeic and vanillic acid, which have potent therapeutic effects especially in Alzheimer’s disease (AD) [77]. Soya-ioflavone daidzein is converted exclusively in equol only by Adlercreutiza equolifaciens, giving it a greater pro-estrogenic effect [78] Furthermore, the conversion of ellagic acid into urolithin is conducted by Gordonibacter urolithinfaciens; however the accumulation of the variety of biotransformaion products was dependent on the presence of the complex microbiota [79]. Another important biotransformation elicited by the gut microbiota, notably Flavonifractor plautii, is the conversion proanthocyanidins into 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone (DHPV) [60, 80]. This is an important conversion as the anti-inflammatory action of DHPV is greater than its parent metabolites, notably the TNF-α-mediated induction of proinflammatory factors from monocytes. In addition to these examples, several other metabolites of gut bacterial biotransformation in the GI tract are outlined in a comprehensive review by Rowland et al. [81].
Despite the large body of evidence supporting the role of the gut bacteria and their specific enzymes in the biotransformation of polyphenols, understanding the breadth of the microbiota’s effect on polyphenol metabolism remains in its infancy. Likewise, novel approaches are necessary in order to elucidate the specific activities of select bacteria and their role in polyphenolic biotransformation. A recent approach to characterize gut bacterial populations leveraged a method of metagenomic binning of bacterial DNA methylation to create bacterial barcodes for efficient identification of unique bacterial strains [82]. Other groups including ours, have used gnotobiotic and germ-free mice to manipulate bacterial populations to better understand host metabolism. With these new methods, we will be able to clarify the unique metabolism of gut microbiota, which differs between individuals, and how this may determine the bioavailbility and bioactivities of polyphenol metabolites [83, 84]
HUMANIZED GNOTOBIOTIC MICE AS A MODEL SYSTEM TO EXPLORE THE RELATIONSHIP BETWEEN THE HUMAN GUT MICROBIOTA AND THE BIOAVAILABILITY OF DIETARY POLYPHENOL PREPARATIONS
Mice raised in germ-free environments provide an excellent system for understanding the role of gut bacteria in various aspects of host physiology. Germ-free mice can be colonized at specific life stages with a complex or defined consortia of microbes to understand the role of the gut microbiota in host physiology using a highly controlled and easily manipulated system. One germ free mouse model found that consistent with in vitro evidence supporting the relevance of gut microbiota in the bioconversion and bioavailability of phenolic metabolites from botanical polyphenol components [85–87], germ-free mice or antibiotic-treated mice had decreased bioconversion of orally consumed polyphenol compounds to phenolic acid within their intestinal tract [88]. We recently developed and validated other methods for the transplantation of the human gut microbiota into gnotobiotic animals [89]. These humanized gnotobiotic mice have a high engraftment rate with 88% of genus-level taxa from the donor detected in the mice, while the genera that did not engraft were at very low abundance in the donor sample (0.008% on average). The applicability of these models to study human disease was evident when mice humanized with the microbiota of twins discordant for obesity developed phenotypes seen in the human host. Specifically, mice transplanted with gut microbiota of an obese twin had a higher increase in fat mass than mice transplanted with the corresponding lean twin microbiota [44]. The implications of these inter-individual variabilities on gut microbes and how they may influence the bioconversion of polyphenols in bioactive metabolites are further discussed below.
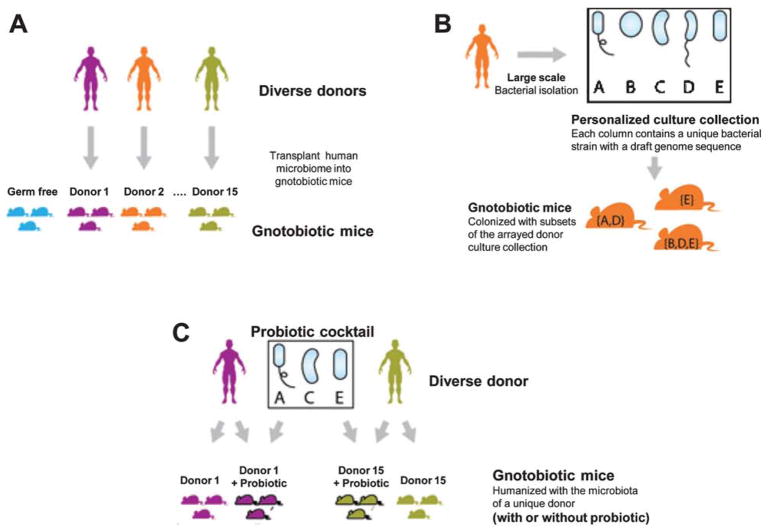
To the author’s knowledge, there are currently no published studies that directly explore the relationship between the human gut microbiota and the diversity and abundance of bioactive phenolic metabolites in vivo. Therefore, we currently are pursuing studies to characterize germ-free mice models to understand polyphenol metabolism. The microbiota of different human donors can be transplanted into separate groups of gnotobiotic mice to estimate variation in bioavailability of bioactive botanical polyphenols as a function of donor microbiota (Fig. 3A). High-throughput bacterial isolation can then be used to isolate a large proportion of the gut microbes from two donors of interest into personalized arrayed culture collections (one donor is shown) (Fig. 3B). These personal culture collections can be screened using a combinatorial gnotobiotic approach combined with statistical modeling to identify specific microbial strains that modulate the bioactive compounds of interest (target metabolites will be based on our prior research) (Fig. 3C). We believe our studies complement transplantation of human microbiota to gnotobiotic mice [84, 90]. With advancing genomic technologies allowing researchers to isolate, archive, and retrieve a large number of gut bacteria [48, 91], the necessary tools to identify and use a combination of gnotobiotic mice to possibly identify the microbial strains that modulate the metabolisin of bioactive polyphenols in the serum and the brain are now available [92].
Fig. 3.
The strategy used to identify interpersonal differences in the composition of the human GI microbiota. In order to understand the role individual bacteria play in the generation of polyphenol metabolites, a strategy was developed whereby (A) bacteria from a human gastrointestinal tract was transplanted to one of a gnotobiotic mouse; (B) the transplanted bacteria were analyzed through statistical methods to isolate unique strains and then transplanted into mice; (C) bacteria that were identified to produce specific metabolites were isolated, expanded, and transplanted into gnotobiotic mice to serve as a model system for next generation probiotics.
EMERGING EVIDENCE FOR THE ROLE OF THE GUT MICROBIOTA IN MOOD DISORDERS
The gut-brain axis (GBA) is a dynamic bidirectional system that is composed of the intestinal microbiota, the enteric nervous system, the peripheral and central nervous systems, humoral pathways, cytokines, neuropeptides and other signaling molecules [93]. The gut microbiota is critical not only for the production of polyphenol metabolites but also for functionality of the GBA and ultimately, neurological functions. The GI tract communicates with the brain through neuronal afferents, such as the vagus nerve, endocrine messages carried by the gut hormones and metabolic signaling, and immunological messages with cytokines [94]. With the emergence of evidence supporting a bi-directional relationship between the gut microbiota and the brain, the scientific community has begun to implicate the gut microbiota as a mediator of cognitive health [95]. The 2009 Walkerton Health Study that assessed participants with irritable bowel syndrome (IBS) over an eight-year period found that depression and anxiety were important risk factors for the persistence of IBS symptoms [96]. Studies such as the Walkerton Study, which revealed associations between gut microbes and the brain provoked an imperative to determine if psychiatric symptoms can be driven by gut dysbiosis [97–100]. One study supported this notion by showing that germ-free mice exhibited reduced anxious behavior as compared to specific pathogen free mice [101]. In spite of recent efforts and observations into a relationship between gut bacteria and mood disorders, explicit evidence indicating a mechanism of bidirectional communication between the gut and neuropsychiatric disorders remains elusive.
POLYPHENOLS: POTENTIAL THERAPEUTIC APPLICATIONS IN NEUROLOGICAL DISORDERS
Given the influence the gut-brain axis may have on the etiology of mood and behavioral disorders, our group set out to understand the capacity to which polyphenol metabolites produced by the gut microbiota can attenuate underlying physiological mechanisms of neurological disorders. Gut processed dietary polyphenols are plant-derived micronutrients found in high concentrations in certain diets such as the Mediterranean diet [86–88]. These compounds are associated with an array of health benefits and are a potential means to prevent a number of diseases [102–108]. Recent work from our group found that several polyphenol metabolites from grape seed extract (GSPE), concord grape juice (CGJ) and resveratrol (RSV), were able to protect against neuropathology and cognitive impairment in neurodegenerative disorders such as AD [35, 102, 103, 109–112] and related tau-mediated neurodegenerative disorders [113–116]. These studies led to the identification of 26 polyphenol metabolites from GSPE, CGJ, and RSV and each were found to accumulate in blood, and a subset in the brain, which had potential to modulate biological activitis in vivo [30, 31, 117–120] Moreover we found polyphenol metabolites that were present in the brain such as 3′-O-Me-epicatechin-5-glucuronide [32] and quercetin glucuronide [31]. 3-hydroxybenzoic acid and 3-(3′-hydroxyphenyl) propionic acid were primarily responsible for inhibiting the onset and progression of AD-type pathophysiology [18]. The compounds exerted their nuroprotective effects by interfering with the generation of neurotoxic AD-type amyloid-β peptides, which can interfere with neuroplasticity and memory consolidation. In addition to interfering with AD mechanisms, studies demonstrated that multiple biologically available microbiota-derived phenolic acids including 3,4-dihydroxypenylacetic acid, 3-(3′-hydroxyphenyl) propionic acid, 3,4-dihydroxyhydrocinnamic acid and homovanillic acid from a bioactive dietary polyphenol preparation (BDPP), composed of GSPR, CGJ, and RSV, were able to modulate biological mechanisms associated with inflammation and synaptic plasticity [18, 30, 121]. These processes are known to influence psychological and cognitive resilience in, for example, animal models of social defeat and sleep deprivation. The collective evidence from our group and from other investigators [83, 122–129] implicates gut-derived biologically available phenolic metabolites as primary facilitators of the observed health benefits associated with polyphenol rich diets.
Depression and depression-like disorder are major contributors to the expanding health costs in the United States [100]. The treatment options currently available, which target neurochemical or neurobiological mechanisms identified retrospectively following discovery of the drug’s initial antidepressant efficacy, produce temporary remission for approximately 50% of patients [99,100,130]. Such discouraging evidence highlights the need for novel therapeutics that can target genetic or proteomic mechanisms underlying depression. Accumulating evidence suggests that immunological abnormalities, particularly imbalances in select pro-inflammatory mediators, play important roles in the expression and continuity of depressive symptoms in vulnerable individuals [131]. These inflammatory mediators are now recognized as important biological signatures as well as key mechanistic contributory factors of depression. As such, these inflammatory mediators are also considered important novel therapeutic targets for depression [132].
Polyphenols can potentially modulate depressive disorders through their potent anti-inflammatory effects [133–139]. It has been suggested that polyphenols attenuate inflammatory conditions via its inherent anti-oxidative [134] capabilities; oxidative stress directly facilities the production of numerous proinflammatory cytokines and has been shown to contribute to the progression of cancer [140]. Futhermore, oxidative stress can either directly decrease neuronal plasticity through covalent interactions with protein involved in axonal transport or synaptic integrity, which results in synaptic degeneration and neuronal apoptosis or indirectly through the aforementioned production of cytokines [141]. This preclinical evidence gave encouragement to our group that the administration of select bioactive polyphenol-rich preparation may intercede with the deleterious mechanisms of radical oxygen species and could attenuate depressive phenotypes in clinical conditions that often persist in spite of treatment with a serotonin selective reuptake inhibitor, such as major depressive disorder provoked anhedonia, characterized by a markedly diminished response to pleasure [131–133, 142–144] that is linked to impaired psychosocial functioning, poor treatment outcome, and suicidal behavior. Moreover, the current evidence emphasized in the review supports the potential therapeutic application of a combination of polyphenols, such as BDPP, largely reported by our group, in promoting resilience against the underlying heightened inflammatory mechanisms in stress induced depression [145]. As we expand our preclinical studies, we will look in to whether it is warranted to support further clinical development of BDPP for treating depression.
CONCLUSION
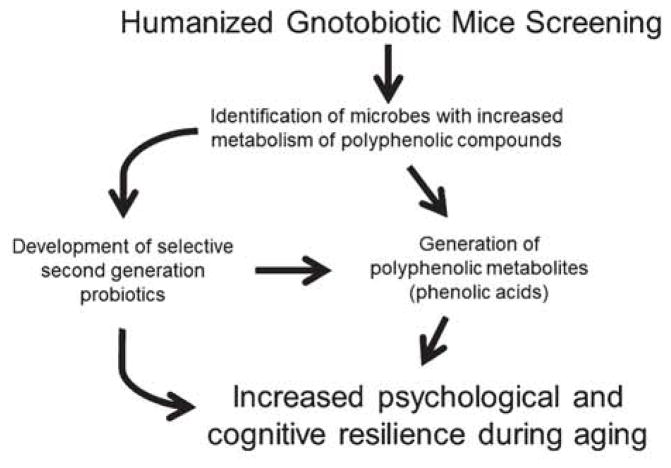
Of the numerous ways symbiotic bacteria in the GI tract influence homeostasis and affect the health of its host, a novel perspective looks at their ability to supply their host with bioactive and bioavailable compounds. As polyphenols have been shown to promote cognitive resilience against a variety of neurological disorders, work from our group, as well as others, has shown that these effects are mediated through microbiota metabolism and the subsequent generation of bioactive and bioavailable polyphenol metabolites. Further investigations should expand into understanding the specific bacteria responsible for the generation of these polyphenol metabolites. As summarized in Fig. 4, one could conjecture a strategy to develop next generation probiotic therapeutics that supplement bacteria known to promote psychological and cognitive resilience via its generation of bioactive compounds.
Fig. 4.
Development strategy for the generation of bioactive polyphenol metabolites using probiotics. As the metabolism of polyphenols by the microbiome becomes clearer it will be possible to identify the unique bacterial populations responsible for the generation of bioactive polyphenols. Gnotobiotic mice constitute an important tool to achieve this goal; they will allow future research groups the ability to selective transplant bacterial strains into the gastrointestinal tract and to determine their unique metabolic function on polyphenols. Ultimately, the goal will be to develop therapeutic approaches to increase the bioavailability of polyphenol derivatives that have properties capable of promoting resilience against age or stress induced cognitive dysfunction, and its potential mediator inflammation.
Acknowledgments
This study was supported by Grant Number P50AT008661-01 from the NCCIH and the ODS. Dr. Pasinetti holds a Senior VA Career Scientist Award. We acknowledge that the contents of this study do not represent the views of the NCCIH, the ODS, the NIH, the U.S. Department of Veterans Affairs, or the United States Government.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/17-1151r1).
References
- 1.Schachter J, Martel J, Lin CS, Chang CJ, Wu TR, Lu CC, Ko YF, Lai HC, Ojcius DM, Young JD. Effects of obesity on depression: A role for inflammation and the gut microbiota. Brain Behav Immun. 2018;69:1–8. doi: 10.1016/j.bbi.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 2.Malan-Muller S, Valles-Colomer M, Raes J, Lowry CA, Seedat S, Hemmings SM. The gut microbiome and mental health: Implications for anxiety-and trauma-related disorders. OMICS. 2017;22:90–107. doi: 10.1089/omi.2017.0077. [DOI] [PubMed] [Google Scholar]
- 3.Schroeder BO, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22:1079–1089. doi: 10.1038/nm.4185. [DOI] [PubMed] [Google Scholar]
- 4.Torres-Fuentes C, Schellekens H, Dinan TG, Cryan JF. The microbiota–gut–brain axis in obesity. Lancet Gastroenterol Hepatol. 2017;2:747–756. doi: 10.1016/S2468-1253(17)30147-4. [DOI] [PubMed] [Google Scholar]
- 5.Rea K, Dinan TG, Cryan JF. The microbiome: A key regulator of stress and neuroinflammation. Neurobiol Stress. 2016;4:23–33. doi: 10.1016/j.ynstr.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee HU, McPhersun ZE, Tan B, Korecka A, Pettersson S. Host-microbiome interactions: The aryl hydrocarbon receptor and the central nervous system. J Mol Med. 2017;95:1–11. doi: 10.1007/s00109-016-1486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yarandi SS, Peterson DA, Treisman GJ, Moran TH, Pasricha PJ. Modulatory effects of gut microbiota on the central nervous system: How gut could play a role in neuropsychiatric health and diseases. J Neurogastroenterol Motil. 2016;22:201. doi: 10.5056/jnm15146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167:915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manderino L, Carroll I, Azcarate-Peril MA, Rochette A, Heinberg L, Peat C, Steffen K, Mitchell J, Gunstad J. Preliminary evidence for an association between the composition of the gut microbiome and cognitive function in neurologically healthy older adults. J Int Neuropsychol Soc. 2017;23:700–705. doi: 10.1017/S1355617717000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrence K, Hyde J. Microbiome restoration diet improves digestion, cognition and physical and emotional wellbeing. PLoS One. 2017;12:e0179017. doi: 10.1371/journal.pone.0179017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Jiang C, Li G, Huang P, Liu Z, Zhao B. The gut micobiota and Alzheimer’s disease. J Alzheimers Dis. 2017;58:1–15. doi: 10.3233/JAD-161141. [DOI] [PubMed] [Google Scholar]
- 12.Kopman M, El Aidy S. Depressed gut? The micobiota-diet-inflammation tralogue in depression. Curr Opin Psychiatry. 2017;30:369–377. doi: 10.1097/YCO.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 13.Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017;62:46–52. doi: 10.1016/j.bbi.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Palma G, Blennerhassett P, Lu J, Deng Y, Park AJ, Green W, Denou E, Silva MA, Santacruz A, Sanz Y, Surette MG. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat Comm. 2015;6:7735. doi: 10.1038/ncomms8735. [DOI] [PubMed] [Google Scholar]
- 15.Cassidy A, Minihane AM. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietry flavonoids. Am J Clin Nutr. 2016;105:10–22. doi: 10.3945/ajcn.116.136051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Espín JC, González-Sarrías A, Tomás-Barberán FA. The gut microbiota: A key factor in the therapeutic effects of (poly) phenols. Biochem Pharmacol. 2017;139:82–93. doi: 10.1016/j.bcp.2017.04.033. [DOI] [PubMed] [Google Scholar]
- 17.Ozdal T, Sela DA, Xiao J, Boyacioglu D, Chen F, Capanoglu E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients. 2016;8:78. doi: 10.3390/nu8020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D, Ho L, Faith J, Ono K, Janle EM, Lachcik PJ, Cooper BR, Jannasch AH, D’arcy BR, Williams BA, Ferruzzi MG. Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease β-amyloid oligomerization. Mol Nutr Food Res. 2015;59:1025–1040. doi: 10.1002/mnfr.201400544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jourova L, Anzenbacher P, Anzenbacherova E. Human gut microbiota plays a role in the metabolism of drugs. Biomed Pap. 2016;160:317–326. doi: 10.5507/bp.2016.039. [DOI] [PubMed] [Google Scholar]
- 20.Stojančevič M, Bojić G, Al-Salami H, Mikov M. The influence of intestinal tract and probiotics on the fate orally administered drugs. Curr Issues Mol Biol. 2013;16:55–68. [PubMed] [Google Scholar]
- 21.Li CY, Suzuki K, Hung YL, Yang MS, Yu CP, Lin SP, Hou YC, Fang SH. Aloe metabolites prevent LPS-induced sepsis and inflammatory response by inhibiting mitogen-activated protein kinase activation. Am J Chin Med. 2017;45:1–15. doi: 10.1142/S0192415X17500458. [DOI] [PubMed] [Google Scholar]
- 22.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klaassen CD, Cui JY. Mechanisms of how the intestinal microbiota alters the effects of drugs and bile acids. Drug Metab Dispos. 2015;43:1505–1521. doi: 10.1124/dmd.115.065698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia G, Nanni S, Figueira I, Ivanov I, McDougall GJ, Stewart D, Ferreira RB, Pinto P, Silva RF, Brites D, Santos CN. Bioaccessible (poly) phenol metabolites from raspberry protect neural cells from oxidative stress and attenuate microglia activation. Food Chem. 2017;215:274–283. doi: 10.1016/j.foodchem.2016.07.128. [DOI] [PubMed] [Google Scholar]
- 25.di Gesso JL, Kerr JS, Zhang Q, Raheem S, Yalamanchili SK, O’hagan D, Kay CD, O’connell MA. Flavonoid metabolites reduce tumor necrosis factor-α secretion to a greater extent than their precursor compounds in human THP-1 monocytes. Mol Nutr Food Res. 2015;59:1143–1154. doi: 10.1002/mnfr.201400799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piwowarki JP, Granica S, Kiss AK. Influence of gut microbiota-derived ellagitannins’ metabolites urolithins on pro-inflammatory activities of human neutrophils. Planta Med. 2014;80:887–895. doi: 10.1055/s-0034-1368615. [DOI] [PubMed] [Google Scholar]
- 27.Liu Q, Chen Y, Shen C, Xiao Y, Wang Y, Li Z, Liu X. Chicoric acid supplementation prevents systemic inflammation-induced memory impairment and amyloidogenesis via inhibition of NF-κB. FASEB J. 2017;31:1494–1507. doi: 10.1096/fj.201601071R. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Huo Y, Zhao L, Lu F, Wang O, Yang X, Zhou F. Cyanidin-3-glucoside and its phenolic acid metabolites attenuate visible light-induced retinal degeneration in vivo via activation of Nrf2/HO-1 pathway and NF-κB suppression. Mol Nutr Food Res. 2016;60:1564–1577. doi: 10.1002/mnfr.201501048. [DOI] [PubMed] [Google Scholar]
- 29.Choi KC, Son YO, Hang JM, Kim BT, Chae M, Lee JC. Antioxidant, anti-inflammatory and anti-septic potential of phenolc acids and flavonoid fractions isolated from Lolium multiflorum. Pharm Biol. 2017;55:611–619. doi: 10.1080/13880209.2016.1266673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao W, Wang J, Bi W, Ferruzzi M, Yemul S, Freire D, Mazzola P, Ho L, Dubner L, Pasinetti GM. Novel application of brain-targeting polyphenol compounds in sleep deprivation-induced cognitive dysfunction. Neurochem lnt. 2015;89:191–197. doi: 10.1016/j.neuint.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho L, Ferruzzi MG, Janle EM, Wang J, Gong B, Chen TY, Lobo J, Cooper B, Wu QL, Talcott ST, Percival SS. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. FASEB J. 2013;27:769–781. doi: 10.1096/fj.12-212118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Ferruzzi MG, Ho L, Blount J, Janle EM, Gong B, Pan Y, Gowda GN, Raftery D, Arrieta-Cruz I, Sharma V. Brain-targeted proanthocyanidin metabolites Alzheimer’s disease treatment. J Neurosci. 2012;32:5144–5150. doi: 10.1523/JNEUROSCI.6437-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward L, Pasinetti GM. Recommendations for development of botanical polyphenols as “natural drugs” for promotion of resilience against stress-induced depression and cognitive impairment. Neuromolecular Med. 2016;18:487–495. doi: 10.1007/s12017-016-8418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bahramsoltani R, Farzaei MH, Farahani MS, Rahimi R. Phytochemical constituents as future antidepressants: A comprehensive review. Rev Neurosci. 2015;26:699–719. doi: 10.1515/revneuro-2015-0009. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Bi W, Cheng A, Freire D, Vempati P, Zhao W, Gong B, Janle EM, Chen TY, Ferruzzi MG, Schmeidler J. Targeting multiple pathogenic mechanisms with polyphenols for the treatment of Alzheimer’s disease-experimental approach and therapeutic implications. Front Aging Neurosci. 2014;6:42. doi: 10.3389/fnagi.2014.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan H, Lu J, Wang Y, Gu W, Yang X, Yu J. Intake of total saponins and polysaccharides from Polygonatum kingianum affects the gut microbiota in diabetic rats. Phytomedicine. 2017;26:45–54. doi: 10.1016/j.phymed.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Urbanska AM, Zhang X, Prakash S. Bio-engineered colorectal cancer drugs: Orally delivered anti-inflammatory agents. Cell Biochem Biophys. 2015;72:757–769. doi: 10.1007/s12013-015-0528-5. [DOI] [PubMed] [Google Scholar]
- 38.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, Chang EB. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tilg H, Moschen A. Microbiota and diabetes: An evolving relationship. Gut. 2014;63:151–521. doi: 10.1136/gutjnl-2014-306928. [DOI] [PubMed] [Google Scholar]
- 40.Huttenhower C, Gevers D, Knight R, Abubucker S, Badger JH, Chinwalla AT, Creasy HH, Earl AM, FitzGerald MG, Fulton RS, Giglio MG. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: Gut microbiota: The neglected endocrine organ. Mol Endocrin. 2014;28:1221–1238. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomasello G, Mazzola M, Leone A, Sinagra E, Zummo G, Farina F, Damiani P, Cappello F, Gerges Geagea A, Jurjus A, Bou Assi T. Nutrition, oxidative stress and intesstinal dysbiosis: Influence of diet on gut microbiota in inflammatory bowel diseases. Biomed Pap. 2016;160:461–466. doi: 10.5507/bp.2016.052. [DOI] [PubMed] [Google Scholar]
- 43.Dinan TG, Cryan JF. Gut instincts: Microbiota a key regulator of brain development, ageing and neuroegeneration. J Physiol. 2017;595:489–503. doi: 10.1113/JP273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rojo D, Méndez-García C, Raczkowska BA, Bargiela R, Moya A, Ferrer M, Barbas C. Exploring the human microbiome from multiple perspectives: Factors altering its composition and function. FEMS Microbiol Lett. 2017;43:453–478. doi: 10.1093/femsre/fuw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M. Enterotypes of the human gut microbiome. Nature. 2011;473:174. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sears CL. A dynamic partnership: Celebrating our gut flora. Anaerobe. 2005;11:247–251. doi: 10.1016/j.anaerobe.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, Rosenbaum M. The long-term stability of the human gut microbiota. Science. 2013;341:1237439. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bäckhed F, Fraser CM, Ringel Y, Sanders ME, Sartor RB, Sherman PM, Versalovic J, Young V, Finlay BB. Defining a healthy human gut microbiome: Current concepts, future directions, and clinical applications. Cell Host Microbe. 2012;12:611–622. doi: 10.1016/j.chom.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 52.Biagi E, Franceschi C, Rampelli S, Severgnini M, Ostan R, Turroni S, Consolandi C, Quercia S, Scurti M, Monti D, Capri M. Gut microbiota and extreme longevity. Curr Biol. 2016;26:1480–1485. doi: 10.1016/j.cub.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 53.Kong F, Hua Y, Zeng B, Ning R, Li Y, Zhao J. Gut microbiota signatures of longevity. Curr Biol. 2016;26:R832–R833. doi: 10.1016/j.cub.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 54.O’Mahony L. Host-microbiome interactions in health and disease. Clin Liver Dis. 2015;5:142–144. doi: 10.1002/cld.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 56.Bode LM, Bunzel D, Huch M, Cho GS, Ruhland D, Bunzel M, Bub A, Franz CM, Kulling SE. In vivo and in vitro metabolism of trans-resveratrol by human gut micobiota. Am J Clin Nutr. 2013;97:295–309. doi: 10.3945/ajcn.112.049379. [DOI] [PubMed] [Google Scholar]
- 57.Cueva C, Sánchez-Patán F, Monagas M, Walton GE, Gibson GR, Martín-Álvarez PJ, Bartolomé B, Moreno-Arribas MV. In vitro fermentation of grape seed flavan-3-ol fractions by human faecal microbiota: Changes in microbial groups and phenolic metabolites. FEMS Microbiol Ecol. 2013;83:792–805. doi: 10.1111/1574-6941.12037. [DOI] [PubMed] [Google Scholar]
- 58.Monagas M, Urpi-Sarda M, Sánchez-Patán F, Llorach R, Garrido I, Gómez-Cordovés C, Andres-Lacueva C, Bartolomé B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010;1:233–253. doi: 10.1039/c0fo00132e. [DOI] [PubMed] [Google Scholar]
- 59.Aura AM, Mattila I, Hyötyläinen T, Gopalacharyulu P, Cheynier V, Souquet JM, Bes M, Le Bourvellec C, Guyot S, Orešič M. Characterization of microbial metabolism of Syrah grape products in an in vitro colon model using targeted and non-targeted analytical approaches. Eur J Nutr. 2013;52:833–846. doi: 10.1007/s00394-012-0391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marín L, Miguélez EM, Villar CJ, Lombó F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. Biomed Res Int. 2015;2015:905215. doi: 10.1155/2015/905215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Calani L, Dall’Asta M, Derlindati E, Scazzina F, Bruni R, Del Rio D. Colonic metabolism of polyphenols from coffee, green tea, and hazelnut skins. Clin J Gastroenterol. 2012;46:S95–S99. doi: 10.1097/MCG.0b013e318264e82b. [DOI] [PubMed] [Google Scholar]
- 62.Sadeghi Ekbtan S, Iskandar MM, Sleno L, Sabally K, Khairallah J, Prakash S, Kubow S. Absorption and metabolism phenolics from digests of polyphenol-rich potato extracts using the Caco-2/HepG2 co-culture system. Foods. 2018;7:8. doi: 10.3390/foods7010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Y, Wang M, Rosen RT, Ho CT. 2, 2-Diphenyl-1-picrylhydrazyl radical-scavenging active components from Polygonum multiflorum Thunb. J Agric Food Chem. 1999;47:2226–2228. doi: 10.1021/jf990092f. [DOI] [PubMed] [Google Scholar]
- 64.Tamura M, Kageyama D, Honda N, Fujimoto H, Kato A. Enzymatic activity necessary to restore the lethality due to Escherichia coli RNase E deficiency is distributed among bacteria lacking RNase E homologues. PLoS One. 2017;12:e0177915. doi: 10.1371/journal.pone.0177915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Del Rio D, Calani L, Cordero C, Salvatore S, Pellegrini N, Brighenti F. Bioavailability and catabolism of green tea flavan-3-ols in humans. Nutrition. 2010;26:1110–1116. doi: 10.1016/j.nut.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 66.Mele L, Carobbio S, Brindani N, Curti C, Rodriguez-Cuenca S, Bidault G, Mena P, Zanotti I, Vacca M, Vidal-Puig A, Del Rio D. Phenyl-γ-valerolactones, flavan-3-ol colonic metabolites, protect brown adipocytes from oxidative stress without affecting their differentiation or function. Mol Nutr Food Res. 2017;61:9. doi: 10.1002/mnfr.201700074. [DOI] [PubMed] [Google Scholar]
- 67.Terao J. Flavonols: Metabolism, bioavailability, and health impacts. In: Fraga CG, editor. Plant Polyphenolics and Human Health: Biochemistry, Nutrition, and Pharmacology. John Wiley & Sons; Hoboken: 2009. pp. 185–196. [Google Scholar]
- 68.Chen H, Hayek S, Guzman JR, Gillitt ND, Ibrahim SA, Jobin C, Sang S. The microbiota is essential for the generation of black tea theaflavins-derived metabolites. PLoS One. 2012;7:e51001. doi: 10.1371/journal.pone.0051001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gross G, Jacobs DM, Peters S, Possemiers S, van Duynhoven J, Vaughan EE, Van de Wiele T. In vitro bioconversion of polyphenols from black tea and red wine/grape juice by human intestinal microbiota displays strong interindividual variability. J Agric Food Chem. 2010;58:10236–10246. doi: 10.1021/jf101475m. [DOI] [PubMed] [Google Scholar]
- 70.Dall’ Asta M, Calani L, Tedeschi M, Jechiu L, Brighenti F, Del Rio D. Identification of microbial metabolites derived from in vitro fecal fermentation of different polyphenolic food sources. Nutrition. 2012;28:197–203. doi: 10.1016/j.nut.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 71.Sánchez-Patán F, Cueva C, Monagas M, Walton GE, Gibson MGR, Quitanilla-López JE, Lebrón-Aguilar R, Martín-Álvarez PJ, Moreno-Arribas MV, Bartolomé B. In vitro fermentation of a red wine extract by human gut microbiota: Changes in microbial groups and formation of phenolic metabolites. J Agric Food Chem. 2012;6:2136–2147. doi: 10.1021/jf2040115. [DOI] [PubMed] [Google Scholar]
- 72.Stalmach A, Edwards CA, Wightman JD, Crozier A. Colonic catabolism of dietary phenolic and polyphenolic compounds from Concord grape juice. Food Funct. 2013;4:52–62. doi: 10.1039/c2fo30151b. [DOI] [PubMed] [Google Scholar]
- 73.Clavel T, Henderson G, Engst W, Doré J, Blaut M. Phylogeny of human intestinal bacteria that activate the dietary lignan secoisolariciresinol diglucoside. FEMS Microbiol Ecol. 2006;55:471–478. doi: 10.1111/j.1574-6941.2005.00057.x. [DOI] [PubMed] [Google Scholar]
- 74.Schoefer L, Braune A, Blaut M. Cloning and expression of a phloretin hydrolase gene from Eubacterium ramulus and characterization of the recombinant enzyme. J Appl Environ Microbiol. 2004;70:6131–6137. doi: 10.1128/AEM.70.10.6131-6137.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, Merion RM. Characteristics associated with liver graft failure: The concept of a donor risk index. Am J Transplant. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 76.Knockaert G, Pulissery SK, Lemmens L, Van Buggenhout S, Hendrickx M, Van Loey A. Carrot β-carotene degradation and isomerization kinetics during thermal processing in the presence of oil. J Agric Food Chem. 2012;60:10312–10319. doi: 10.1021/jf3025776. [DOI] [PubMed] [Google Scholar]
- 77.Sgarbossa A, Giacomazza D, Di Carlo M. Ferulic acid: A hope for Alzheimer’s disease therapy from plants. Nutrients. 2015;7:5764–5782. doi: 10.3390/nu7075246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van Vliet MJ, Tissing WJ, Dun CA, Meessen NE, Kamps WA, de Bont ES, Harmsen HJ. Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially patogenic bacteria in the gut. Clin Res Infect Dis. 2009;49:262–270. doi: 10.1086/599346. [DOI] [PubMed] [Google Scholar]
- 79.Selma MV, Beltrán D, García-Villalba R, Espín JC, Tomás-Barberán FA. Description of urolithin production capacity from ellagic acid of two human intestinal Gordonibacter species. Food Funct. 2014;5:1779–1784. doi: 10.1039/c4fo00092g. [DOI] [PubMed] [Google Scholar]
- 80.Kutschera M, Engst W, Blaut M, Braune A. Isolation of catechin-converting human intestinal bacteria. J Appl Microbiol. 2011;111:165–175. doi: 10.1111/j.1365-2672.2011.05025.x. [DOI] [PubMed] [Google Scholar]
- 81.Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, Tuohy K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur J Nutr. 2017;9:1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beaulaurier J, Zhu S, Deikus G, Mogno I, Zhang XS, Davis-Richardson A, Canepa R, Triplett EW, Faith JJ, Sebra R, Schadt EE. Metagenomic binning and association of plasmids with bacterial host genomes using DNA methylation. Nat Biotechnol. 2017;36:61–69. doi: 10.1038/nbt.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal. 2013;18:1818–1892. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Godman AL, Kallstrom G, Faith JJ, Reyes A, Moore A, Dantas G, Gordon JI. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. Proc Nat Acad Sci USA. 2011;108:6252–6257. doi: 10.1073/pnas.1102938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin Y, Yan Y. Biosynthesis of caffeic acid in Escherichia coli using its endogenous hydroxylase complex. Microb Cell Fact. 2012;11:42. doi: 10.1186/1475-2859-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parkar SG, Trower TM, Stevenson DE. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe. 2013;23:12–19. doi: 10.1016/j.anaerobe.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 87.Stoupi S, Williamson G, Drynan JW, Barron D, Clifford MN. A comparison of the in vitro biotransformation of (−)-epicatechin and procyanidin B2 by human faecal microbiota. Mol Nutr Food Res. 2010;54:747–759. doi: 10.1002/mnfr.200900123. [DOI] [PubMed] [Google Scholar]
- 88.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci Trans Med. 2009;1:6ra14–6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Faith JJ, Ahern PP, Ridaura VK, Cheng J, Gordon JI. Identifying gut microbe–host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci Trans Med. 2014;6:220ra11–220ra11. doi: 10.1126/scitranslmed.3008051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen H, Parks T, Chen X, Gillitt ND, Jobin C, Sang S. Structural identification of mouse fecal metabolites of theaflavin 3, 3′-digallate using liquid chromatography tandem mass spectrometry. J Chromatogr A. 2011;1218:7297–7306. doi: 10.1016/j.chroma.2011.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ho L, Cheng H, Wang J, Simon JE, Wu QL, Zhao D, Carry E, Ferruzzi MG, Faith J, Valcarcel B, Pasinetti GM. A comprehensive database and analysis framework incorporate multiscale data types and enable integrated analysis of bioactive polyphenols. Mol Pharm. 2017;15:840–850. doi: 10.1021/acs.molpharmaceut.7b00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Westfall S, Lomis N, Kahouli I, Dia S, Singh SP, Prakash S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell Mol Life Sci. 2017;74:3769–3787. doi: 10.1007/s00018-017-2550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sherwin E, Dinan TG, Cryan JF. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann NY Acad Sci. 2017 doi: 10.1111/nyas.13416. [DOI] [PubMed] [Google Scholar]
- 95.Smith PA. Brain, meet gut. Nature. 2015;526:312. doi: 10.1038/526312a. [DOI] [PubMed] [Google Scholar]
- 96.Marshall JK, Thabane M, Garg AX, Clark WF, Moayyedi P, Collins SM Walkerton Health Study Investigators. Eight year prognosis of postinfectious irritable bowel syndrome following waterborne bacterial dysentery. Gut. 2010;59:605–611. doi: 10.1136/gut.2009.202234. [DOI] [PubMed] [Google Scholar]
- 97.Cenit MC, Nuevo IC, Codoñer-Franch P, Dinan TG, Sanz Y. Gut microbiota and attention deficit hyperactivity disorder: New perspectives for a challenging condition. Eur Child Adolesc. 2017;26:1–12. doi: 10.1007/s00787-017-0969-z. [DOI] [PubMed] [Google Scholar]
- 98.Ding HT, Taur Y, Walkup JT. Gut microbiota and autism: Key concepts and findings. J Autism Dev Disord. 2017;47:480–489. doi: 10.1007/s10803-016-2960-9. [DOI] [PubMed] [Google Scholar]
- 99.Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: New clinical, neurobiological, and treatment perspectives. Lancet. 2012;379:1045–1055. doi: 10.1016/S0140-6736(11)60602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Casacalenda N, Perry JC, Looper K. Remission in major depressive disorder: A comparison of pharmacotherapy, psychotherapy, and control conditions. Am J Psychiatry. 2002;159:1354–1360. doi: 10.1176/appi.ajp.159.8.1354. [DOI] [PubMed] [Google Scholar]
- 101.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23:255. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 102.Scarmeas N, Stem Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. 2006;59:912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Panza F, Solfrizzi V, Colacicco AM, D’introno A, Capurso C, Torres F, Del Parigi A, Capurso S, Capurso A. Mediterranean diet and cognitive decline. Public Health Nutr. 2004;7:959–963. doi: 10.1079/phn2004561. [DOI] [PubMed] [Google Scholar]
- 104.Dohadwala MM, Vita JA. Grapes and cardiovascular disease. J Nutr. 2009;139:1788S–1793S. doi: 10.3945/jn.109.107474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tussing-Humphreys L, Lamar M, Blumenthal JA, Babyak M, Fantuzzi G, Blumstein L, Schiffer L, Fitzgibbon ML. Building research in diet and cognition: The BRIDGE randomized controlled trial. Contemp Clin Trials. 2017;59:87–89. doi: 10.1016/j.cct.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Keyserling TC, Samuel-Hodge CD, Pitts SJ, Garcia BA, Johnston LF, Gizlice Z, Miller CL, Braxton DF, Evenson KR, Smith JC, Davis GB. A community-based lifestyle and weight loss intervention promoting a Mediterranean-style diet pattern evaluated in the stroke belt of North Carolina: The Heart Healthy Lenoir Project. BMC Public Health. 2016;16:732. doi: 10.1186/s12889-016-3370-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mattioli AV, Palmiero P, Manfrini O, Puddu PE, Nodari S, Dei Cas A, Mercuro G, Scrutinio D, Palermo P, Sciomer S, Di Francesco S. Mediterranean diet impact on cardiovascular diseases: A narrative review. J Cardiovasc Med. 2017;18:925–935. doi: 10.2459/JCM.0000000000000573. [DOI] [PubMed] [Google Scholar]
- 108.Mirmiran P, Amirhamidi Z, Ejtahed HS, Bahadoran Z, Azizi F. Relationship between diet and non-alcoholic fatty liver disease: A review article. Iran J Public Health. 2017;46:1007. [PMC free article] [PubMed] [Google Scholar]
- 109.D’Cunha NM, McKune AJ, Panagiotakos DB, Georgousopoulou EN, Thomas J, Mellor DD, Naumovski N. Evaluation of dietary and lifestyle changes as modifiers of S100β levels in Alzheimer’s disease. Nutr Neurosci. 2017;11:1–18. doi: 10.1080/1028415X.2017.1349032. [DOI] [PubMed] [Google Scholar]
- 110.Ono K, Condron MM, Ho L, Wang J, Zhao W, Pasinetti GM, Teplow DB. Effects of grape seed-derived polyphenols on amyloid β-protein self-assembly and cytotoxicity. J Biol Chem. 2008;283:32176–32187. doi: 10.1074/jbc.M806154200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang J, Ho L, Zhao W, Ono K, Rosensweig C, Chen Humala N, Teplow DB, Pasinetti GM. Grape-derived polyphenolics prevent Aβ oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J Neurosci. 2008;28:6388–6392. doi: 10.1523/JNEUROSCI.0364-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang J, Varghese M, Ono K, Yamada M, Levine S, Tzavaras N, Gong B, Hurst WJ, Blitzer RD, Pasinetti GM. Cocoa extracts reduce oligomerization of amyloid-β Implications for cognitive improvement in Alzheimer’s disease. J Alzheimers Dis. 2014;41:643–650. doi: 10.3233/JAD-132231. [DOI] [PubMed] [Google Scholar]
- 113.Santa-Maria I, Diaz-Ruiz C, Ksiezak-Reding H, Chen A, Ho L, Wang J, Pasinetti GM. GSPE interferes with tau aggregation in vivo: Implication for treating tauopathy. Neurobiol Aging. 2012;33:2072–2081. doi: 10.1016/j.neurobiolaging.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang J, Santa-Maria I, Ho L, Ksiezak-Reding H, Ono K, Teplow DB, Pasinetti GM. Grape derived polyphenols attenuate tau neuropathology in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2010;22:653–661. doi: 10.3233/JAD-2010-101074. [DOI] [PubMed] [Google Scholar]
- 115.Ho L, Pasinetti GM. Polyphenolic compounds for treating neurodegenerative disorders involving protein misfolding. Expert Rev Proteomics. 2010;7:579–589. doi: 10.1586/epr.10.69. [DOI] [PubMed] [Google Scholar]
- 116.Ho L, Yemul S, Wang J, Pasinetti GM. Grape seed polyphenolic extract as a potential novel therapeutic agent in tauopathies. J Alzheimers Dis. 2009;16:433–439. doi: 10.3233/JAD-2009-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang J, Tang C, Ferruzzi MG, Gong B, Song BJ, Janle EM, Chen TY, Cooper B, Varghese M, Cheng A, Freire D. Role o standardized grape polyphenol preparation as a novel treatment to improve synaptic plasticity through attenuation of features of metabolic syndrome in a mouse model. Mol Nutr Food Res. 2013;57:2091–2102. doi: 10.1002/mnfr.201300230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Novotny JA, Chen TY, Terekhov AI, Gebauer SK, Baer DJ, Ho L, Pasinetti GM, Ferruzzi MG. The effect of obesity and repeated exposure on pharmacokinetic response to grape polyphenols in humans. Mol Nutr Food Res. 2017;61:11. doi: 10.1002/mnfr.201700043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tabasco R, Sánchez-Patán F, Monagas M, Bartolomé B, Moreno-Arribas MV, Peláez C, Requena T. Effect of grape polyphenols on lactic acid bacteria and bifidobacteria growth: Resistance and metabolism. Food Microbiol. 2011;28:1345–1352. doi: 10.1016/j.fm.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 120.Pasinetti GM, Wang J, Marambaud P, Feruzzi M, Gregor P, Knable LA, Ho L. Neuroprotective and metabolic effects of resveratrol: Therapeutic implications for Huntington’s disease and other neurodegenerative disorders. Exp Neurol. 2011;232:1–6. doi: 10.1016/j.expneurol.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ho L, Faith J, Ono K, Pasinetti GM. Protective roles of intestinal microbiota in Alzheimer’s disease through mechanisms involving short chain fatty acids and phenolic acids. Alzheimers Dement. 2016;12:224–225. [Google Scholar]
- 122.Teixeira LL, Costa GR, Dörr FA, Ong TP, Pinto E, Lajolo FM, Hassimotto NMA. Potential antiproliferative activity of polyphenol metabolites against human breast cancer cells and their urine excretion pattern in healthy subjects following acute intake of a polyphenol-rich juice of grumixama (Eugenia brasiliensis Lam.) Food Funct. 2017;8:2266–2274. doi: 10.1039/c7fo00076f. [DOI] [PubMed] [Google Scholar]
- 123.Tang XL, Liu JX, Dong W, Li P, Li L, Lin CR, Hou JC. The cardioprotective effect of protocatechuic acid on myocardial ischemia/reperfusion injury. J Pharmacol Sci. 2014;125:176–183. doi: 10.1254/jphs.13247fp. [DOI] [PubMed] [Google Scholar]
- 124.Guo X, Shen L, Tong Y, Zhang J, Wu G, He Q, Lian XY. Antitumor activity of caffeic acid 3, 4-dihydroxyphenethyl ester and its pharmacokinetic and metabolic properties. Phytomedicine. 2013;20:904–912. doi: 10.1016/j.phymed.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 125.Winter AN, Brenner MC, Punessen N, Snodgrass M, Byars C, Arora Y, Linseman DA. Comparison of the neuroprotective and anti-inflammatory effects of he anthocyanin metabolites, protocatechuic acid and 4-hydroxybenzoic acid. Oxid Med Cell Longev. 2017;2017:6297080. doi: 10.1155/2017/6297080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rey FE, Gonzalez MD, Cheng J, Wu M, Ahern PP, Gordon JI. Metabolic niche of a prominent sulfate-reducing human gut bacterium. Proc Natl Acad Sci USA. 2013;110:13582–13587. doi: 10.1073/pnas.1312524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Alam MA, Sernia C, Brown L. Ferulic acid improves cardiovascular and kidney structure and function in hypertensive rats. J Cardiovas Pharmacol. 2013;61:240–249. doi: 10.1097/FJC.0b013e31827cb600. [DOI] [PubMed] [Google Scholar]
- 128.Côté J, Caillet S, Doyon G, Sylvain JF, Lacroix M. Bioactive compounds in cranberries and their biological properties. Crit Rev Food Sci Nutr. 2010;50:666–679. doi: 10.1080/10408390903044107. [DOI] [PubMed] [Google Scholar]
- 129.Liu P, Kemper LJ, Wang J, Zahs KR, Ashe KH, Pasinetti GM. Grape seed polyphenolic extract specifically decreases Aβ * 56 in the brains of Tg2576 mice. J Alzheimers Dis. 2011;26:657–666. doi: 10.3233/JAD-2011-110383. [DOI] [PubMed] [Google Scholar]
- 130.Rush AJ, Warden D, Wisniewski SR, Fava M, Trivedi MH, Gaynes BN, Nierenberg AA. STAR* D. CNS Drugs. 2009;23:627–647. doi: 10.2165/00023210-200923080-00001. [DOI] [PubMed] [Google Scholar]
- 131.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 132.Jeon SW, Kim YK. Neuroinflammation and cytokine abnormality in major depression: Cause or consequence in that illness. World J Psychiatry. 2016;6:283. doi: 10.5498/wjp.v6.i3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gothai S, Ganesan P, Park SY, Fakurazi S, Choi DK, Arulselvan P. Natural phyto-bioactive compounds for the treatment of type 2 diabetes: Inflammation as a target. Nutrients. 2016;8:461. doi: 10.3390/nu8080461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid Med Cell Longev. 2016;2016:7432797. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Entsuah AR, Huang H, Thase ME. Response and remission rates in different subpopulations with major depressive disorder administered venlafaxine, selective serotonin reuptake inhibitors, or placebo. J Clin Psychiatry. 2001;62:869–876. doi: 10.4088/jcp.v62n1106. [DOI] [PubMed] [Google Scholar]
- 136.Joseph SV, Edirisinghe I, Burton-Freeman BM. Fruit polyphenols: A review of anti-inflammatory effects in humans. Crit Rev Food Sci Nutr. 2016;56:419–444. doi: 10.1080/10408398.2013.767221. [DOI] [PubMed] [Google Scholar]
- 137.Kumar GP, Khanum F. Neuroprotective potential of phytochemicals. Pharmacogn Rev. 2012;6:81. doi: 10.4103/0973-7847.99898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sureda A, Tejada S, del Mar Bibiloni M, Antoni Tur J, Pons A. Polyhenols: Well beyond the antioxidant capacity: Poyphenol supplementation and exercise-induced oxidative stress and inflammation. Curr Pharm Biotechnol. 2014;15:373–379. doi: 10.2174/1389201015666140813123843. [DOI] [PubMed] [Google Scholar]
- 139.Vendrame S, Klimis-Zacas D. Anti-inflammatory effect of anthocyanins via modulation of nuclear factor-κB and mitogen-activated protein kinase signaling cascades. Nutr Rev. 2015;73:348–358. doi: 10.1093/nutrit/nuu066. [DOI] [PubMed] [Google Scholar]
- 140.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mattson MP, Liu D. Energetics and oxidative stress in synaptic plasticity and neurodegenerative disorders. Neuromolecular Med. 2002;2:215–231. doi: 10.1385/NMM:2:2:215. [DOI] [PubMed] [Google Scholar]
- 142.McCabe C, Cowen PJ, Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology. 2009;205:667–677. doi: 10.1007/s00213-009-1573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Meagher MW, Johnson RR, Young EE, Vichaya EG, Lunt S, Hardin EA, Connor MA, Welsh CJR. Interleukin-6 as a mechanism for the adverse effects of social stress on acute Theiler’s virus infection. Brain Behav Immun. 2007;21:1083–1095. doi: 10.1016/j.bbi.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Treadway MT, Zald DH. Reconsidering anhedonia in depression: Lessons from transnational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang J. Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress disorders and depression. Nat Comm. 2017;9:477. doi: 10.1038/s41467-017-02794-5. [DOI] [PMC free article] [PubMed] [Google Scholar]