Abstract
Cell-lineage tracing is used to study embryo development and stem-cell differentiation as well as to document tumor cell heterogeneity. Cre recombinase-mediated cell labeling is the preferred approach; however, its utility is restricted by when and where DNA recombination takes place. We generated a photoactivatable Cre recombinase by replacing a critical residue in its active site with a photocaged lysine derivative through genetic code expansion in zebrafish embryos. This allows high spatiotemporal control of DNA recombination by using 405 nm irradiation. Importantly, no background activity is seen before irradiation, and, after light-triggered removal of the caging group, Cre recombinase activity is restored. We demonstrate the utility of this tool as a cell-lineage tracer through its activation in different regions and at different time points in the early embryo. Direct control of Cre recombinase by light will allow more precise DNA recombination, thereby enabling more nuanced studies of metazoan development and disease.
Keywords: DNA recombination, lineage tracing, optogenetics, unnatural amino acids, zebrafish
Our understanding of embryogenesis and the patterning of adult tissues stems, to a large extent, from studies of cell lineages. Before the introduction of fluorescent protein markers, the fate of cells was traced by direct observation or by the injection of dyes into transparent embryos such as zebrafish embryos. Technology to trace cell progeny has evolved, and now it is being used to answer questions surrounding stem-cell differentiation and tumor cell heterogeneity, among other important biological questions.[1] Site-specific DNA recombination by using the Cre/loxP system allows exquisite control of genomic modification when studying biological systems.[2] By flanking a gene of interest with loxP sites, the gene can be excised or inverted depending on the orientation of the loxP sites.[3] It has been a reliable and indispensable tool in many areas, including cell-lineage tracing. The Cre/loxP methodology has also been used to generate conditional expression of channelrhodopsins for light-inducible silencing and activation of neural circuits,[4] for studying infectious disease,[5] carcinogenesis,[6] stem-cell dynamics,[7] and even to improve the development of induced pluripotent stem cells.[8] Stochastic induction of multiple fluorescent proteins with Cre/loxP in mice brain[9] and fish brain[10] yielded higher resolution fluorescence imaging of individual neural connections in a method coined “Brainbow”.
Cre/loxP is an important system for lineage tracing during embryogenesis[1] because it permanently labels progeny cells due to modification of the parent cells’ genome with fluorescent reporter proteins. However, one challenge in using Cre/ loxP for lineage tracing is controlling when and where recombination takes place, which is critical when studying rapidly developing biological systems such as the zebrafish embryo. Examples of spatial control include the use of tissue-specific promotors,[11] and examples of temporal control include using heat-shock-induced Cre recombinase expression[12] or the addition of 4-hydroxy tamoxifen to translocate a Cre–estrogen receptor fusion protein (Cre–ER) to the nucleus.[13] Both spatial and temporal control were achieved by using a photocaged 4-hydroxy tamoxifen analogue[14] with a Cre–ER-expressing fish line.[15] However, Cre–ER fusions have shown activation in the absence of 4-hydroxytamoxifen, and although this is reduced by certain mutants of ER, such as Cre–ERT2, some background activity is still possible.[16] Given the transparency of zebrafish embryos, light activation of protein function is a preferable approach.[17] Direct activation of Cre recombinase with light would allow facile, rapid, and high spatiotemporal control of DNA recombination for study in the developing zebrafish embryo. One approach towards direct, genetically encoded photocontrol of Cre recombinase is splitting the enzyme into two and attaching two photoresponsive proteins that dimerize upon light illumination.[18] This enabled recombination in the mouse brain, but required exposure to light for several hours for full recombination. Subsequent optimizations have shortened irradiation times, but complete recombination remained difficult to accomplish as dimer dissociation can occur after a period without photostimulation.[19] Optical activation of Cre recombinase in mammalian cells has also been achieved by replacing a critical lysine with a caged lysine, which would yield the wild-type enzyme upon light irradiation.[20] This approach resulted in complete recombination after just four minutes of exposure to light at 365 nm. Recently, an improved lysine analogue containing a photocaging group that can be photolysed with 405 nm light was introduced into proteins in zebrafish (Figure 1A).[21] This lysine analogue, hydroxycoumarin lysine (HCK), was expected also to block Cre recombinase activity if placed into the active site of Cre recombinase. HCK was used due to its previously demonstrated encoding in zebrafish embryos[21] and due to its ability to be decaged by 405 nm irradiation.[22] Upon exposure to light, enzyme activity would be restored, and DNA recombination would be triggered. K201 was selected for unnatural amino acid replacement because it plays a critical role in the cleavage reaction through hydrogen bonding to nucleotide bases (Figure 1B).[23]
Figure 1.
A) Structure of HCK. B) Active site of Cre recombinase showing replacement of K201 with HCK. Irradiation with 405 nm light releases the photocage to reveal the native lysine. C) Schematic of the DNA recombination reporter in the zebrafish line. Cre recombinase-catalyzed, site-specific DNA recombination switches cells from GFP expression to mCherry expression. D) Experimental design showing injection of RNA to express caged Cre recombinase, followed by localized irradiation with 405 nm light at the 6 or 14 hpf stage, and fluorescent imaging at 48 hpf.
The incorporation of HCK into Cre recombinase was performed through amber stop codon suppression with an engineered pyrrolysine aminoacyl tRNA-synthetase (HCKRS) that has been evolved to acylate its cognate tRNA (PylT) with HCK.[22, 24] In order to introduce caged Cre recombinase (Cre201HCK) into embryos, mRNA encoding Cre201TAG (400 pg), mRNA encoding HCKRS (600 pg), its cognate tRNA (80 ng), and HCK were injected into the embryo at the one-cell stage. Determination of the amounts of RNA injected was guided by recent genetic-code-expansion experiments in zebrafish.[21] The zebrafish line used in these experiments contains a gene cassette with GFP flanked by loxP sites and mCherry under a ubiquitin (Ub) promotor.[25] The stop codon of GFP prevents expression of mCherry, but when Cre recombinase is active, GFP is excised and mCherry is expressed (Figure 1C). At 6 hours post-fertilization (hpf), embryos were irradiated with light and then returned to the incubator. Fluorescence imaging was performed at 48 hpf to assess expression of mCherry as a marker for Cre recombinase activity (Figure 1D).
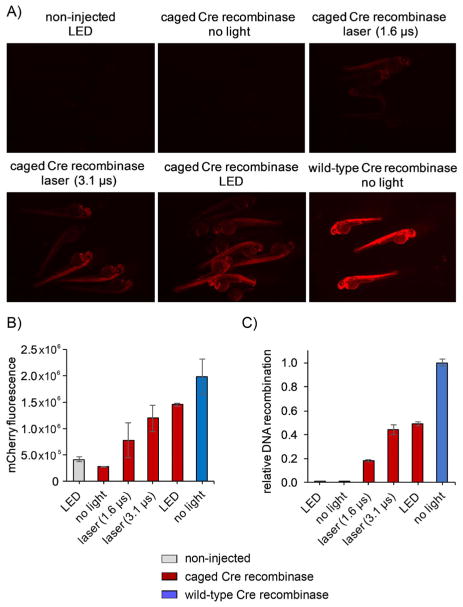
The first goal was to verify the optical response of the K201HCK substitution in Cre recombinase. Injection of mRNA encoding wild-type Cre recombinase at the one-cell stage and imaging at 48 hpf was used as a positive control, and different irradiation conditions were tested in the embryos expressing caged Cre recombinase at 6 hpf (Figure 2A). Three different irradiation conditions were tested; 5 min of irradiation with a 405 nm LED that allows many embryos to be irradiated at one time, and irradiation with a confocal 405 nm laser at ten z-planes at either of two different pixel dwell times (1.6 or 3.1 μs), which provides more localized exposure. No phototoxicity was observed in any of these experiments, based on numbers of normally developing embryos. No background mCherry signal was present in the nonirradiated embryos expressing caged Cre recombinase, thus demonstrating complete inactivity of the enzyme. With irradiation, the embryos expressing the caged Cre recombinase fluoresce with increasing intensity upon longer exposure times; the most fluorescence is present in the LED-irradiated embryos, as quantified by mCherry fluorescence intensity analysis (Figure 2B). To further quantify the activity of the caged and photoactivated Cre recombinase, quantitative PCR experiments were performed (Figure 2C). Three embryos were pooled at 48 hpf for each condition, and genomic DNA was extracted.[26] This was followed by qPCR analysis with primers designed to efficiently amplify only the recombined genomic region. The PCR amplified the sequence from the 3′-end of the Ub promotor to the 5′-end of the mCherry gene, an amplicon that is too long for the selected qPCR parameters for efficient amplification from the non-recombined genome, but is much shorter and is amplified much more efficiently in the case of the recombined DNA.[27] Nonirradiated Cre201HCK displayed no DNA recombination (identical to the untreated control group) and LED photolysis of the caged Cre recombinase led to 50 % DNA recombination relative to embryos expressing wild-type enzyme. The lower level of recombination relative to wild-type Cre recombinase is likely due to inadequate activation and/or inadequate DNA recombination in all embryonic cells. Although the wild-type Cre recombinase immediately induces recombination at the one-to two-cell stage, which leads to all progeny cells containing the recombined genome, the photoactivated Cre recombinase begins recombination at a stage with about 16 000 cells (6 hpf);[28] this increases the likelihood that not all cells respond. LED irradiation resulted in more recombination than irradiation with the confocal laser due to the highly localized nature of its activation, thus it is a better candidate for irradiating small populations of cells. As demonstrated below, the obtained recombination rates are suitable for cell-lineage tracing and temporal control of Cre recombination in small populations of cells in the zebrafish embryo.
Figure 2.
Initial optimization of caged Cre recombinase photoactivation. A) Micrographs of mCherry fluorescence after irradiation with 405 nm light at 6 hpf. Confocal irradiation consisted of irradiation at ten z-planes with 1.6 or 3.1 μs pixel dwell times, whereas the LED irradiation lasted 5 min. Images were taken at 48 hpf. The three embryos with the highest level of mCherry expression were selected from each condition and pooled for fluorescence quantification and genomic DNA isolation for qPCR experiments. B) Quantification of mCherry fluorescence intensity by using ImageJ software. Error bars represent standard deviation of the three selected embryos. C) qPCR quantification of the amplified recombined gene relative to wild-type Cre recombinase. Error bars represent standard error of the mean from technical qPCR triplicates.
Imaging of the irradiated embryos injected with Cre recombinase demonstrated strong mCherry expression with little or no background as observed in the non-injected embryos (Figure 3). We noted that embryos expressing wild-type Cre recombinase showed no GFP at 48 hpf, but GFP fluorescence was still visible in light-triggered Cre recombinase embryos due to inadequate recombination in all cells of the developing embryo (Figure 3). Another reason for observing residual GFP in the photoactivated embryos is its expression before optically triggered recombination in conjunction with a long GFP half-life of ≈26 hours in cells.[29]
Figure 3.
From left to right: micrographs of 48 hpf embryos that were not injected but irradiated with 405 nm LED for 5 min at 6 hpf (N =9), embryos expressing wild-type Cre recombinase (N =3), embryos expressing caged Cre recombinase in the absence of irradiation (N =5), and embryos expressing caged Cre recombinase after 405 nm LED irradiation for 5 min at 6 hpf (N =9).
The final step was to demonstrate spatiotemporal control of Cre recombinase function by tracing certain early cell populations. For the 6 hpf embryo, cell populations that would develop into the telencephalon (forebrain) and the cardiac muscle were irradiated (Figure 4B–C).[30] The tail region was also irradiated at a later developmental stage (14 hpf), and embryos were imaged at 48 hpf (Figure 4A). Cell-lineage traces were recapitulated in three distinct tissues at two different times of development. In more differentiated embryos at 14 hpf, it was easier to identify the target cell population for irradiation, as reflected in the consistent labeling of the tail region with mCherry (n =4, all embryos irradiated displayed mCherry in the expected tail region). The heart and telencephalon irradiations were less consistent between embryos due to difficulties in labeling populations of cells at an early developmental stage (heart: 1/5 showed cardiac mCherry expression; telencephalon: 3/5 irradiated embryos showed mCherry expression in the expected CNS region). Because these irradiations happened at the onset of the gastrula stage, the irradiated cells would undergo significant migration and mixing while the embryo involuted and extended its axis.[28]
Figure 4.
Localized activation of Cre recombinase. Embryos expressing caged Cre recombinase were irradiated at 6 or 14 hpf in the areas highlighted in purple with the region expected to express mCherry highlighted in red on the 48 hpf embryo cartoon. Fluorescence images at 48 hpf show regional expression of mCherry that matches the zebrafish developmental plan, thereby demonstrating selective (spatially and temporally controlled) activation of Cre recombinase. mCherry expression was merged with bright-field images to show the localized region of Cre recombinase activation.
In summary, direct optical activation of Cre recombinase in the developing zebrafish embryo has been demonstrated. Lineage tracing of the heart, telencephalon, and tail was successful. This method stands out for its high spatiotemporal control, allowing activation of Cre recombinase in small populations of cells and, possibly, single-cell activation. Recombination activity is restored immediately after irradiation, thus allowing rapid activation at any embryonic stage. Compared to tissue promotor-driven control of Cre recombinase, which can be difficult to define for some subpopulations of cells, the method presented here has the advantage of restricting active Cre recombinase to irradiated cells. This approach to DNA recombination could be extended beyond fate mapping to, for example, studying stem-cell differentiation or tumor heterogeneity[1] in the zebrafish embryo. Genetic-code expansion with the pyrrolysyl tRNA/ tRNA synthetase system can also allow the installation of other photocaged amino acids,[31] and because of their amenability to photocontrolling cellular processes, zebrafish embryos provide an excellent vertebrate model for studying signaling pathways and protein expression. A photoactivatable Cre recombinase will find further applications in establishing gene knock-ins and knock-outs with high spatiotemporal resolution in individual cells to generate cellular heterogeneity in a developing organ or tissue. The recent development and expansion of single-cell mRNA sequencing[32] has shown that significant heterogeneity in cell gene expression exists in organs, tissues, and tumors.[33] A caged Cre recombinase approach could contribute to understanding the role of transcript expression heterogeneity in organogenesis due to the ability to alter the expression profiles of select cells in a tissue with precise light activation.
Experimental Section
Materials
The zebrafish experiments were performed according to a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pittsburgh. The Tg(ubi:loxP-EGFP-loxP-mCherry)[25] strain was maintained under standard conditions at the University of Pittsburgh School of Medicine in accordance with institutional and federal guidelines. HCK was synthesized as described previously.[22]
Plasmid construction
The pCS2-Cre-WT plasmid was generated by Jie Zhang in our laboratory. The K201TAG mutation was introduced on pCS2-Cre-WT by using site-directed mutagenesis (fwd: 5′-gttaa tccat attgg cagaa cgtag acgct ggtta gcacc gcagg-3′, rev: 5′-cctgc ggtgc taacc agcgt ctacg ttctg ccaat atgga ttaac-3′, TAG mutation underlined).
mRNA synthesis
Cre-WT, Cre201TAG, and HCKRS mRNA were synthesized according to the following procedure. The corresponding pCS2 + plasmid (10 μg) was linearized through NotI digestion (2 μL of NotI in 40 μL reaction mixture, 37 °C, overnight). The linearized product (40 μL) was mixed with water (10 μL) and phenol/chloroform/isoamyl alcohol (PCIA, 50 μL), and centrifuged at maximum speed (21130 g) for 5 min. The top layer ( ≈50 μL) was collected, and further mixed with NaOAc (5 μL, 3 M, pH 5.2) and 100 % ethanol (125 μL). The mixture was centrifuged at maximum speed (21130 g) for 5 min. The pellet was washed with 70 % ethanol (600 μL), and dissolved in water (10 μL). The concentration of linear DNA was obtained on a nanodrop. Linear DNA (1 μg) was used to generate mRNA in a 20 μL reaction mixture with the mMESSAGE mMACHINE SP6 Transcription Kit (Ambion; 37 °C, 4 h). DNase (1 μL, provided with the kit) was then added to remove the linear DNA template (37 °C, 30 min). The reaction mixture (20 μL) was mixed with water (30 μL) and PCIA (50 μL), and was centrifuged at maximum speed (21130 g) for 5 min. The top layer ( ≈50 μL) was purified through a G-50 Sephadex spin column (Roche, #11274015001) according to the product manual. The RNA solution (50 μL) was mixed with water (50 μL), NaOAc (10 μL, 3 M, pH 5.2), and 100 % ethanol (300 μL). The mixture was placed in the freezer overnight (−20 °C), and was then centrifuged at maximum speed (21130 g) for 15 min. The RNA pellet was washed with 70 % ethanol and dissolved in water (15 μL). The quality was verified by 1 % agarose gel (run at 80 V for 45 min).
PylT synthesis
The PylT sequence contained a U25C mutation in order to increase incorporation efficiency.[34] The PylT DNA with a truncated T7 promoter was amplified by PCR from a PylT oligonucleotide (Integrated DNA Technologies, Coralville, Iowa, USA), (fwd: 5′-taata cgact cacta tagga, rev: 5′-cggaa acccc gggaa tctaa). The PCR was performed with PylT template (10 μM, 4 μL), dNTP (10 mM, 4 μL), forward and reverse primers (100 μM, 2 μL each), 10× Taq PCR buffer (20 μL), Taq polymerase (2 μL), and water (166 μL). The PCR product (100 μL) was mixed with PCIA (100 μL) and centrifuged at maximum speed (21130 g) for 5 min. The top layer (≈100 μL) was collected and further mixed with NaOAc (3 M, 10 μL, pH 5.2) and 100 % ethanol (250 μL). The mixture was centrifuged at maximum speed (21130 g) for 5 min. The pellet was washed with 70 % ethanol (600 μL) and dissolved in water (10 μL). The concentration of PylT DNA was obtained on a nanodrop. The pylT DNA (1.5 μg) was used as a template to generate PylT RNA in a 20 μL reaction mixture by using the MEGAscript T7 Transcription Kit (Ambion; 37 °C, 4 h). DNase (1 μL, provided with the kit) was then added to remove the linear DNA template (37 °C, 30 min). The reaction mixture (20 μL) was mixed with water (30 μL) and PCIA (50 μL), and was centrifuged at maximum speed (21130 g) for 5 min. The top layer ( ≈50 μL) was mixed with water (50 μL), NaOAc (3 M, 10 μL, pH 5.2), and 100 % ethanol (300 μL). The mixture was placed in the freezer overnight (−20 °C), and was then centrifuged at maximum speed (21130 g) for 15 min. The RNA pellet was washed with 70 % ethanol (600 μL) and dissolved in water (10 μL), and the quality was verified by 1.5% agarose gel.
Microinjection of embryos
We used the transgenic zebrafish line Tg(ubi:loxP-EGFP-loxP-mCherry).[25] To express wild-type Cre recombinase, Cre-WT mRNA (2 nL, 25 ngμL−1) was injected into each embryo. To express photocaged Cre recombinase, the injection mixture was prepared with Cre-K201TAG mRNA (200 ngμL−1), HCKRS mRNA (300 ngμL−1), PylT (40 μgμL−1), and HCK (10 mM from a 100 mM stock in 100 % DMSO) in a total volume of 1.5 μL. Embryos from natural mating were obtained and microinjected at the one-cell stage with the injection mixture (2 nL) by using a World Precision Instruments Pneumatic PicoPump injector.
Light activation and microscopy
All embryos were irradiated at 6 hpf with the exception of the tail-irradiated embryos, which were irradiated at 14 hpf. For confocal 405 nm irradiation, a Zeiss LSM 700 laser scanning confocal microscope was used. The embryo was embedded in 1.5 % low-melting-point agarose. For global irradiation, the Z-stack function was used for irradiation at ten z-planes at 100 % laser intensity (405 nm laser 5 mW, 1.6 or 3.1 μs dwell time). To irradiate small populations of cells, the bleach function was used with laser power set at 30 % for 20 iterations (1.6 μs dwell time). For global LED 405 nm irradiation, the light source (Luxeonstar 405 nm LED) was placed 3 cm above the petri dish containing the embryos for 5 min (power output was measured at 350 mW). At 48 hpf, the embryos were imaged with a Leica M205 FA microscope with EGFP, mCherry, and bright-field channels. mCherry fluorescence was quantified by using ImageJ software.
qPCR validation of Cre recombination
Embryos were injected as mentioned above. At 6 hpf, animals expressing Cre201HCK were globally irradiated. At 48 hpf, three embryos were pooled for each condition, and genomic DNA was extracted[26] and purified (Gene-JET Genomic DNA Purification Kit, Thermo Scientific). qPCR was performed in triplicate for each sample by using the following primers: for: 5′-gggag aagtg caaaa catac attat tggct ag-3′, rev: 5′-cttga tgatg gccat gttgt cctc-3′. EF1α was used as a reference gene with previously reported primers.[35] A SYBR green assay was used on a CFX96 Touch Real-Time PCR detection system (Bio-Rad). The following protocol was used: 95° C for 5 min, 70 cycles of 95 °C for 5 s, then 56.6 °C for 60 s. The data were analyzed by using the ΔΔCt method[36] to obtain relative quantities of DNA amplification, then normalized to the wild-type Cre recombinase control.
Acknowledgments
This work was supported by the Charles E. Kaufman Foundation (to A.D. and M.T.), the National Science Foundation (CBET-1603930 to A.D.), and the National Institutes of Health (R01GM112728 to A.D.). We thank Dr. Leonard Zon for the Tg(ubi:loxP- EGFP-loxP-mCherry) zebrafish, and Dr. Donghun Shin for critical discussions.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
The ORCID identification numbers for the authors of this article can be found under https://doi.org/10.1002/cbic.201800040.
This article is part of a Special Issue on the Optical Control of Biological Processes.
References
- 1.Kretzschmar K, Watt M. Cell. 2012;148:33–45. doi: 10.1016/j.cell.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Meinke G, Bohm A, Hauber J, Pisabarro MT, Buchholz F. Chem Rev. 2016;116:12785–12820. doi: 10.1021/acs.chemrev.6b00077. [DOI] [PubMed] [Google Scholar]
- 3.Sauer B. Methods. 1998;14:381–392. doi: 10.1006/meth.1998.0593. [DOI] [PubMed] [Google Scholar]
- 4.Madisen L, Mao T, Koch H, Zhuo J-m, Berenyi A, Fujisawa S, Hsu Y-WA, Garcia AJ, III, Gu X, Zanella S, Kidney J, Gu H, Mao Y, Hooks BM, Boyden ES, Buzsáki G, Ramirez JM, Jones AR, Svoboda K, Han X, Turner EE, Zeng H. Nat Neurosci. 2012;15:793. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koshy AA, Fouts AE, Lodoen MB, Alkan O, Blau HM, Boothroyd JC. Nat Methods. 2010;7:307. doi: 10.1038/nmeth.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schönhuber N, Seidler B, Schuck K, Veltkamp C, Schachtler C, Zukowska M, Eser S, Feyerabend TB, Paul MC, Eser P, Klein S, Lowy AM, Banerjee R, Yang F, Lee CL, Moding EJ, Kirsch DG, Scheideler A, Alessi DR, Varela I, Bradley A, Kind A, Schnieke AE, Rodewald HR, Rad R, Schmid RM, Schneider G, Saur D. Nat Med. 2014;20:1340. doi: 10.1038/nm.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, KroonVeenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, Clevers H. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Haenebalcke L, Goossens S, Dierickx P, Bartunkova S, D’Hont J, Haigh K, Hochepied T, Wirth D, Nagy A, Haigh JJ. Cell Rep. 2013;3:335–341. doi: 10.1016/j.celrep.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW. Nature. 2007;450:56. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- 10.Pan YA, Freundlich T, Weissman TA, Schoppik D, Wang XC, Zimmerman S, Ciruna B, Sanes JR, Lichtman JW, Schier AF. Development. 2013;140:2835–2846. doi: 10.1242/dev.094631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan X, Wan H, Chia W, Tong Y, Gong Z. Transgenic Res. 2005;14:217–223. doi: 10.1007/s11248-004-5790-z. [DOI] [PubMed] [Google Scholar]
- 12.Le X, Langenau DM, Keefe MD, Kutok JL, Neuberg DS, Zon LI. Proc Natl Acad Sci USA. 2007;104:9410–9415. doi: 10.1073/pnas.0611302104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hans S, Kaslin J, Freudenreich D, Brand M. PLoS One. 2009;4:e4640. doi: 10.1371/journal.pone.0004640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.a) Link KH, Shi Y, Koh JT. J Am Chem Soc. 2005;127:13088–13089. doi: 10.1021/ja0531226. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Feng Z, Nam S, Hamouri F, Aujard I, Ducos B, Vriz S, Volovitch M, Jullien L, Lin S, Weiss S, Bensimon D. Sci Rep. 2017;7:9195. doi: 10.1038/s41598-017-09697-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.a) Sinha DK, Neveu P, Gagey N, Aujard I, Le Saux T, Rampon C, Gauron C, Kawakami K, Leucht C, Bally-Cuif L, Volovitch M, Bensimon D, Jullien L, Vriz S. Zebrafish. 2010;7:199–204. doi: 10.1089/zeb.2009.0632. [DOI] [PubMed] [Google Scholar]; b) Zhang W, Hamouri F, Feng Z, Aujard I, Ducos B, Ye S, Weiss S, Volovitch M, Vriz S, Jullien L, Bensimon D. ChemBioChem. 2018 doi: 10.1002/cbic.201700630. https://doi.org/10.1002/cbic.201700630 (this issue) [DOI] [PubMed]
- 16.Feil R, Wagner J, Metzger D, Chambon P. Biochem Biophys Res Commun. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- 17.a) Ankenbruck N, Courtney T, Naro Y, Deiters A. Angew Chem Int Ed. 2018;57:2768–2798;. doi: 10.1002/anie.201700171. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2018;130:2816–2848. [Google Scholar]; b) Baker AS, Deiters A. ACS Chem Biol. 2014;9:1398–1407. doi: 10.1021/cb500176x. [DOI] [PubMed] [Google Scholar]; c) Kowalik L, Chen JK. Nat Chem Biol. 2017;13:587–598. doi: 10.1038/nchembio.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.a) Kennedy MJ, Hughes RM, Peteya LA, Schwartz JW, Ehlers MD, Tucker CL. Nat Methods. 2010;7:973. doi: 10.1038/nmeth.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Schindler SE, McCall JG, Yan P, Hyrc KL, Li M, Tucker CL, Lee J-M, Bruchas MR, Diamond MI. Sci Rep. 2015;5:13627. doi: 10.1038/srep13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawano F, Okazaki R, Yazawa M, Sato M. Nat Chem Biol. 2016;12:1059. doi: 10.1038/nchembio.2205. [DOI] [PubMed] [Google Scholar]
- 20.Luo J, Arbely E, Zhang J, Chou C, Uprety R, Chin JW, Deiters A. Chem Commun. 2016;52:8529–8532. doi: 10.1039/c6cc03934k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Hemphill J, Samanta S, Tsang M, Deiters A. J Am Chem Soc. 2017;139:9100–9103. doi: 10.1021/jacs.7b02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo J, Uprety R, Naro Y, Chou C, Nguyen DP, Chin JW, Deiters A. J Am Chem Soc. 2014;136:15551–15558. doi: 10.1021/ja5055862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibb B, Gupta K, Ghosh K, Sharp R, Chen J, Van Duyne GD. Nucleic Acids Res. 2010;38:5817–5832. doi: 10.1093/nar/gkq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.a) Wan W, Tharp JM, Liu WR. Biochim Biophys Acta Proteins Proteomics. 2014;1844:1059–1070. doi: 10.1016/j.bbapap.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chin JW. Annu Rev Biochem. 2014;83:379–408. doi: 10.1146/annurev-biochem-060713-035737. [DOI] [PubMed] [Google Scholar]; c) Liu CC, Schultz PG. Annu Rev Biochem. 2010;79:413–444. doi: 10.1146/annurev.biochem.052308.105824. [DOI] [PubMed] [Google Scholar]
- 25.Mosimann C, Kaufman CK, Li P, Pugach EK, Tamplin OJ, Zon LI. Development. 2011;138:169–177. doi: 10.1242/dev.059345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meeker ND, Hutchinson SA, Ho L, Trede NS. BioTechniques. 2007;43:610–614. doi: 10.2144/000112619. [DOI] [PubMed] [Google Scholar]
- 27.Weis B, Schmidt J, Lyko F, Linhart HG. BMC Biotechnol. 2010;10:75–75. doi: 10.1186/1472-6750-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 29.Corish P, Tyler-Smith C. Protein Eng. 1999;12:1035–1040. doi: 10.1093/protein/12.12.1035. [DOI] [PubMed] [Google Scholar]
- 30.a) Stainier DY, Lee RK, Fishman MC. Development. 1993;119:31–40. doi: 10.1242/dev.119.1.31. [DOI] [PubMed] [Google Scholar]; b) Kimelman D, Martin BL. Wiley Interdiscip Rev Dev Biol. 2012;1:253–266. doi: 10.1002/wdev.25. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Keegan BR, Meyer D, Yelon D. Development. 2004;131:3081–3091. doi: 10.1242/dev.01185. [DOI] [PubMed] [Google Scholar]
- 31.a) Luo J, Torres-Kolbus J, Liu J, Deiters A. ChemBioChem. 2017;18:1442–1447. doi: 10.1002/cbic.201700147. [DOI] [PubMed] [Google Scholar]; b) Uprety R, Luo J, Liu J, Naro Y, Samanta S, Deiters A. ChemBioChem. 2014;15:1793–1799. doi: 10.1002/cbic.201400073. [DOI] [PubMed] [Google Scholar]
- 32.Tang F, Barbacioru C, Wang Y, Nordman E, Lee C, Xu N, Wang X, Bodeau J, Tuch BB, Siddiqui A, Lao K, Surani MA. Nat Methods. 2009;6:377. doi: 10.1038/nmeth.1315. [DOI] [PubMed] [Google Scholar]
- 33.a) Deng Q, Ramsköld D, Reinius B, Sandberg R. Science. 2014;343:193–196. doi: 10.1126/science.1245316. [DOI] [PubMed] [Google Scholar]; b) Zhu S, Qing T, Zheng Y, Jin L, Shi L. Oncotarget. 2017;8:53763–53779. doi: 10.18632/oncotarget.17893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chatterjee A, Sun SB, Furman JL, Xiao H, Schultz PG. Biochemistry. 2013;52:1828–1837. doi: 10.1021/bi4000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dodd A, Tang R, Lai D, McNabb WC, Love DR. Acta Biochim Biophys Sin (Shanghai) 2007;39:384–390. doi: 10.1111/j.1745-7270.2007.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]