Abstract
Although the kidney was initially thought to be the sole organ responsible for the production of 1,25(OH)2D via the enzyme CYP27b1, it is now appreciated that the expression of CYP27b1 in tissues other than the kidney is wide spread. However, the kidney is the major source for circulating 1,25(OH)2D. Only in certain granulomatous diseases such as sarcoidosis does the extra renal tissue produce sufficient 1,25(OH)2D to contribute to the circulating levels, generally associated with hypercalcemia, as illustrated by the case report preceding the review. Therefore the expression of CYP27b1 outside the kidney under normal circumstances begs the question why, and in particular whether the extra renal production of 1,25(OH)2D has physiologic importance. In this chapter this question will be discussed. First we discuss the sites for extra renal 1,25(OH)2D production. This is followed by a discussion of the regulation of CYP27b1 expression and activity in extra renal tissues, pointing out that such regulation is tissue specific and different from that of CYP27b1 in the kidney. Finally the physiologic significance of extra renal 1,25(OH)2D3 production is examined, with special focus on the role of CYP27b1 in regulation of cellular proliferation and differentiation, hormone secretion, and immune function. At this point the data do not clearly demonstrate an essential role for CYP27b1 expression in any tissue outside the kidney, but several examples pointing in this direction are provided. With the availability of the mouse enabling tissue specific deletion of CYP27b1, the role of extra renal CYP27b1 expression in normal and pathologic states can now be addressed definitively.
Keywords: CYP27b1, Immune function, Cancer, Keratinocytes, Macrophages
1. Introduction
The classic paradigm for vitamin D production and metabolism in which vitamin D is made in the skin, converted to its major circulating form 25hydroxy vitamin D (25OHD) in the liver, and to 1,25 dihydroxyvitamin D (1,25(OH)2D) in the kidney has undergone major revisions with the findings that cells other than epidermal keratinocytes have the capability of producing vitamin D following UVB exposure (Vantieghem et al., 2006), that several 25OHD hydroxylases exist and not only in the liver (Jones et al., 2014), and that numerous tissues other than the kidney produce 1,25(OH)2D, the subject of this review. When Fraser and Kodicek (Fraser and Kodicek, 1970) first identified the kidney as the source of 1,25(OH)2D in 1971, it was originally thought to be the sole source. Indeed two studies examining the conversion of radiolabeled 25OHD to 1,25(OH)2D in anephric rats failed to detect any formation of 1,25(OH)2D (Reeve et al., 1983; Shultz et al., 1983). However, when anephric rats are pregnant, 1,25(OH)2D is produced (Gray et al., 1979). Similarly a case report of a woman with chronic kidney disease who showed an increase in 1,25(OH)2D when pregnant was published (Turner et al., 1988), and the human placenta was shown to be capable of 1,25(OH)2D production (Weisman et al., 1979). Moreover, studies in anephric humans (Lambert et al., 1982; Dusso et al., 1988) and pigs (Littledike and Horst, 1982) that were not pregnant found detectable levels of 1,25(OH)2D at baseline that could be further increased with 25OHD or vitamin D administration. A report by Barbour et al. (Barbour et al., 1981) of an anephric patient with sarcoidosis with clearly detectable 1,25(OH)2D levels demonstrated a disease state in which extra renal 1,25(OH)2D3 production occurred. The source was soon discovered to be the activated pulmonary alveolar macrophages from the involved lungs (Adams et al., 1983). At about the same time, a number of investigators were finding 1,25(OH)2D production by bone cells (Turner et al., 1980), melanocytes (Frankel et al., 1983), and epidermal keratinocytes in vitro (Bikle et al., 1986a) and in vivo (Bikle et al., 1994). With the cloning of the 25OHD 1α hydroxylase (CYP27b1) in 1997 by 4 groups (Fu et al., 1997; Takeyama et al., 1997; St-Arnaud et al., 1997; Shinki et al., 1997) came the demonstration that the extrarenal CYP27b1 was the same as the renal CYP27b1 (Fu et al., 1997; Jones et al., 1999). Moreover, the mutations in CYP27b1 that led to pseudo vitamin D deficiency resulted in absent CYP27b1 activity in the skin (Fu et al., 1997) and placenta. The cloning also enabled the development of molecular probes and antibodies to CYP27b1 (Zehnder et al., 2001a; Zehnder et al., 2001b) facilitating the demonstration of its expression in many other tissues. It soon became apparent that the regulation of CYP27b1 activity in non-renal tissues differed from that in the kidney. This difference in regulation is clearly demonstrated in diseases such as sarcoidosis and other disorders that lead to unregulated increases in circulating 1,25(OH)2D and hypercalcemia as illustrated by the case report of a B cell lymphoma presenting with hypercalcemia that initiates this review. Thus the demonstration of CYP27b1 in tissues that were not obviously involved in bone mineral metabolism but which also expressed the vitamin D receptor raised the question of its physiologic role in these extrarenal sites. This question has started to be addressed with the development of a mouse model in which CYP27b1 has been deleted (Panda et al., 2001a; Dardenne et al., 2001), and more specifically a mouse model in which CYP27b1 can be deleted in a tissue specific fashion (St-Arnaud et al., 2003). This chapter will address the tissue distribution of CYP27b1, the regulation of CYP27b1 in these cells, and the potential significance of these extra renal locations in health and disease.
2. Case report
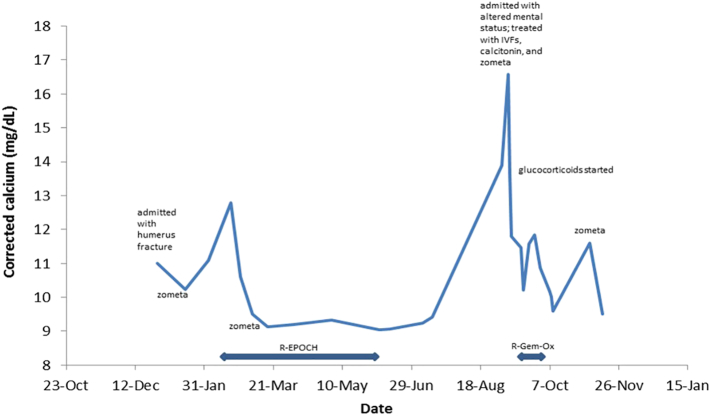
A 74 year old male with a past medical history of prostate cancer status-post radical prostatectomy presented to the Emergency Department with flank pain in 12/2015. Work up included a CT scan of the abdomen and pelvis, which showed multiple new lytic pelvic and spinal lesions as well as diffuse lymphadenopathy of unclear etiology. About two weeks later, he presented with acute right arm pain that started after he was getting out of a car. He was found to have a mid-shaft humeral fracture and was admitted to the hospital for further work-up and management. During this admission, a PET/CT (positron emission tomography/computed tomography) showed progression of disease also now involving the liver and spleen. About 1 week into this hospital admission, he developed acute bilateral lower extremity weakness. MRI of the spine showed T11 vertebral body compression and extension of tumor into the anterior epidural space causing cord compression. He underwent urgent decompressive laminectomy, and pathology from this resection revealed high grade B-cell lymphoma with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma. Chemotherapy was initiated, and he received 2 cycles of R-EPOCH (rituximab in combination with etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) and intrathecal methotrexate. On the first day of chemotherapy, his corrected calcium was 12.8, PTH 1.1 pg/ml (ref 15–88 pg/mL), PTH-related peptide 13 pg/ml (ref 14–27 pg/ml), and 25-hydroxyvitamin D 21.8 ng/ml (ref 25–50 ng/mL). 1,25-dihydroxyvitamin D was not measured at the time. Zometa was recommended by Oncology for treatment of pathological fractures. Over the next five months, he completed four additional cycles of R-EPOCH and one additional dose of Zometa. Repeat PET/CT showed continued progression of lymphoma. About three months following last cycle of chemotherapy (9/2016), he was admitted for altered mental status. He was residing in a skilled nursing facility and was noted to be more confused. Labs were notable for corrected calcium of 16.6 mg/dL. He received intravenous fluids, calcitonin and Zometa, both of which were given to reduce the contribution of bone resorption to the hypercalcemia. He was not taking vitamin D or calcium supplements. The following day his corrected calcium improved to 13.6 mg/dL. Other labs were notable for creatinine 0.8 mg/dL, phosphorus 2.6 mg/dL (ref 2.5–4.5 mg/dL), PTH 2.9 pg/ml (ref 15–88 pg/ml), PTH-related peptide 16 pg/ml (ref 14–27 pg/ml), alkaline phosphatase 84 U/L (ref 40–125 U/L), TSH 1.80 uU/mL (ref 0.4–6 uU/mL), 25-hydroxyvitamin D 25 ng/mL (ref 25–50 pg/ml). Subsequent immunohistochemical staining for 1 alpha hydroxylase (CYP27B1) in surgical tissue from the T11 mass resection was strongly positive both for the lymphoma and the adjacent osteoblasts and osteoclasts (Fig. 1). The etiology of the hypercalcemia was clearly related to the elevated 1,25-dihydroxyvitamin D production from the tumor, although the PTHrP was also not suppressed and was possibly playing a role. About 1.5 weeks into his hospitalization, the patient received palliative chemotherapy with rituximab, gemcitabine and oxaliplatin (R-Gem-Ox). He received a total of 2 cycles. He was also started on prednisone to reduce the burden of cells overproducing 1,25(OH)2D. A lower dose of 20 mg daily was chosen due to concerns of immunosuppression with concurrent chemotherapy. Two weeks into steroid therapy and after another dose of Zometa, his prednisone was increased to 40 mg daily given he continued to have hypercalcemia as well as elevated 1,25(OH)2D (82 pg/mL). One month into steroid therapy, he developed muscle weakness, concerning for steroid myopathy, and his prednisone was tapered back down to 20 mg daily with plans to completely taper off. However, his calcium was starting to increase again, so prednisone was increased back to 40 mg daily. A follow-up PET/CT confirmed no response to chemotherapy (Fig. 2). The patient was also experiencing functional and cognitive decline, prompting transition to hospice. He expired on December 14, 2016. His hospital course is shown in Fig. 3 with respect to serum calcium levels.
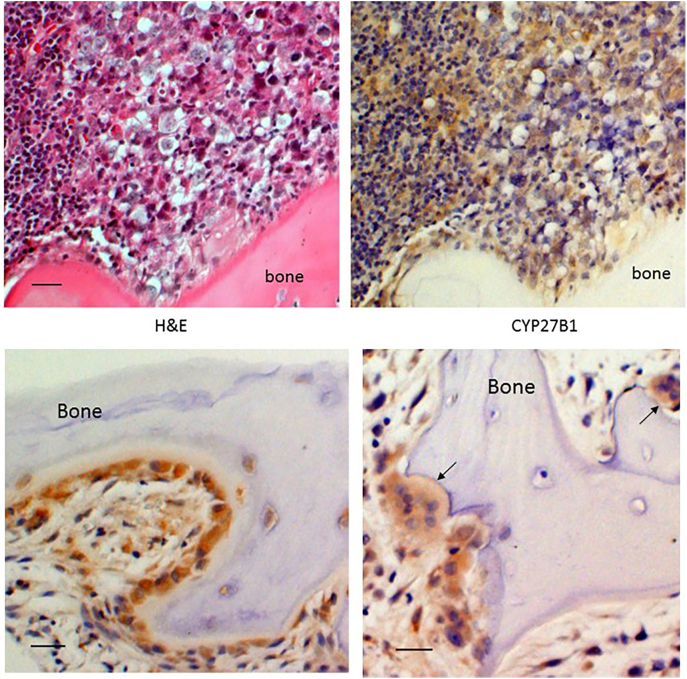
Fig. 1.
Increased expression of CYP27B1 in the infiltrating tumor and osteoblasts, osteocytes, and osteoclasts of adjacent bone. The upper panels show the tumor, with H&E staining on the left and a serial section immunostained for CYP27b1 on the right. The expression of CYP27b1 is depicted by the brown color. The lower panels demonstrate the strong staining for CYP27b1 in the osteoblasts lining the bone (lower left panel), osteocytes close to the surface of bone (lower left panel), and osteoclasts (arrow in lower right panel). The 20× bar = 50 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
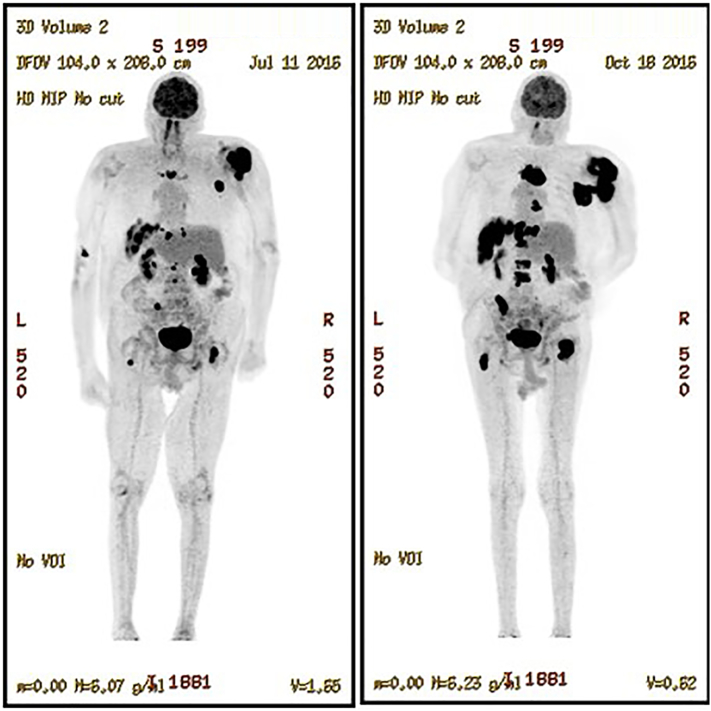
Fig. 2.
Whole body PET/CT. These show the results after 6 cycles of R-EPOCH on the left and 2 cycles of R-GEM-OX on the right. The black regions show the increased uptake of the fluordeoxyglucose used as the tracer for the PET/CT studies that signify the increased metabolic activity of the tumor.
Fig. 3.
Hospital course of the patient. The upper limits of calcium in this hospital are 10.5 mg/dl. Levels above 13 mg/dl are generally associated with altered mental status to coma and require emergency treatment as in this case.
Although this review will emphasize the physiologic role of extra renal CYP27b1, this case report emphasizes the pathologic role it can play. As indicated in this case report, efforts to control the pathologic expression of CYP27b1 include efforts to restrict bone resorption as with potent bisphosphonates such as Zometa (zoledronate), glucocorticoids such as prednisone that suppress cells often of the hematopoietic lineage such as lymphomas or macrophages that may produce 1,25(OH)2D in an uncontrolled fashion, and antifungal drugs such as ketoconazole that directly inhibit the CYP27b1.
3. Tissue distribution of CYP27b1
Table 1 lists the tissues, and the cells within those tissues, in which CYP27b1 expression has been identified. The list is growing, and is likely not complete. The biologic function of CYP27b1 in many of these tissues is not clear.
Table 1.
Tissues and cells expressing CYP27b1.
| A. Epithelia |
|
|
|
|
|
|
|
|
|
| B. Placenta |
|
|
| C. Bone |
|
|
|
|
| D. Immune system |
|
|
|
|
|
| E. Endocrine glands |
|
|
|
|
|
|
| F. Other |
|
|
|
3.1. Epithelia
Epithelia form the largest group of cells that have been shown to produce 1,25(OH)2D3 and/or express CYP27b1. These cells provide the barrier between the inside environment of the body and the outside, the maintenance of which is likely one important function for CYP27b1 in these cells. The archetype cell is the epidermal keratinocyte, which we (Bikle et al., 1986a) showed over 30 years ago to be a very high expresser of CYP27b1, higher in fact than the kidney. Based on calculations from 1,25(OH)2D production by perfused pig skin we (Bikle et al., 1994) determined that epidermal production of 1,25(OH)2D could account for all the circulating 1,25(OH)2D found in anephric patients (or pigs) in the basal state or after supplementation with vitamin D or 25OHD. However, most if not all 1,25(OH)2D produced by the epidermis is used for autocrine or paracrine purposes with little contribution to the circulation even in hyperproliferative conditions such as psoriasis in which the expression of CYP27b1 is increased (Sumantran et al., 2016). This is likely because of the rapid and extensive induction of CYP24 in these cells (Sumantran et al., 2016), which limits the amount of secreted 1,25(OH)2D. However, in certain dermal conditions associated with inflammatory infiltrates such as slack skin disease, a T cell lymphoproliferative disease (Karakelides et al., 2006), and subcutaneous fat necrosis of the newborn (Farooque et al., 2009) the blood levels of calcium and 1,25(OH)2D increase in part due to the poorly regulated CYP27b1 activity of the inflammatory cells in the skin but not from the epidermal keratinocytes. In vitro, CYP27b1 activity as well as VDR levels decline as the keratinocytes differentiate (Pillai et al., 1988), results consistent with observations that the highest expression of both CYP27b1 (Zehnder et al., 2001a) and VDR (Milde et al., 1991; Stumpf et al., 1984) is found in the stratum basale, although both are found in the more differentiated layers as well. As will be discussed in the section dealing with function, 1,25(OH)2D plays a major role in regulating epidermal proliferation and differentiation (34)providing an effective barrier to the environment. Deletion of CYP27b1 disrupts this process (Bikle, 2004).
Subsequent to these initial observations in epidermal keratinocytes, CYP27b1 expression and activity have been found in most epithelia where it has been sought. These tissues include the prostate (Hsu et al., 2001; Chen, 2008), colonic mucosa (Zehnder et al., 2001a; Bareis et al., 2001; Tangpricha et al., 2001; Bises et al., 2004; Lechner et al., 2007), mammary epithelium (Segersten et al., 2005), cervical epithelium (Friedrich et al., 2002), endometrium (Agic et al., 2007; Vigano et al., 2006), various ocular barrier cells (Alsalem et al., 2014), and sinonasal epithelial cells (Sultan et al., 2013). A common feature of these cells is their barrier function, in particular to infectious organisms, which will be discussed when their role in innate immunity is described. As in the skin when these tissues are involved in inflammatory processes CYP27b1 expression may increase, and in some cases results in the entry of 1,25(OH)2D into the circulation. This was well demonstrated in patients with Crohns Disease, 42% of whom had elevated serum levels of 1,25(OH)2D associated with increased expression of CYP27b1 in their intestinal mucosa (Abreu et al., 2004) as well as in a mouse model of inflammatory bowel disease (Liu et al., 2008). Increased CYP27b1 expression relative to normal tissue is also found in endometriosis (Agic et al., 2007; Vigano et al., 2006). The increased expression of CYP27b1 in these tissues is likely due to stimulation of the CYP27b1 by inflammatory cytokines, regulation that will be discussed subsequently. Although the impact of the increased CYP27b1 expression is not clear, a recent publication in which monocytes overexpressing CYP27b1 were targeted to DSS induced colitis demonstrated improvement in this mouse model (Li et al., 2015), and deletion of CYP27b1 from these cells increased the susceptibility to DSS induced colitis (Liu et al., 2008).
Increased CYP27b1 expression is also found in the early stages of malignancy in many of these epithelia. Examples include both basal cell (Mitschele et al., 2004) and squamous cell (Reichrath et al., 2004) carcinoma, breast cancer (Segersten et al., 2005), colon cancer (Bareis et al., 2001; Tangpricha et al., 2001; Ogunkolade et al., 2002), prostate cancer (although malignancy appears to reduce CYP27b1 expression and activity in these cells) (Hsu et al., 2001; Chen, 2008), cervical cancer (Friedrich et al., 2002), lung cancer (Yokomura et al., 2003; Mawer et al., 1994), and endometrial cancer (Becker et al., 2007). This observation suggests a role for CYP27b1 in regulating proliferation and differentiation of these tissues, reducing tumor progression (prostate cancer being a possible exception). Support for this concept comes from a recent study in which CYP27b1 was deleted from cells of a breast cancer model, resulting in acceleration of tumorigenesis (Li et al., 2016). However, these cancers are also associated with alternatively spliced forms of CYP27b1, which may have less or no 1α hydroxylase activity and may block that of intact CYP27b1 as has been shown in proximal tubule cells (Wu et al., 2007). Such alternatively spliced forms have been demonstrated in breast (Cordes et al., 2007; Fischer et al., 2007a), endometrium (Becker et al., 2007), keratinocytes (Seifert et al., 2009), lung (Radermacher et al., 2006), and cervix (Radermacher et al., 2006; Friedrich et al., 2003). Moreover, the increase in CYP27b1 is often accompanied by an increase in CYP24A1, which would counter the increased endogenous production of 1,25(OH)2D as shown in breast cancer (Townsend et al., 2005), colon cancer (Bareis et al., 2001), lung cancer (Jones et al., 1999), and endometrial cancer (Agic et al., 2007). As tumors become less differentiated their CYP27b1 expression generally decreases (Bareis et al., 2001). This has been associated with increased promoter hypermethylation of CYP27b1 in a substantial number of breast cancers (Shi et al., 2002) and prostate tumors (Khorchide et al., 2005). Other studies suggest that the CYP27b1 promoter is down regulated by NFκB (Sakamoto et al., 2009) (Ebert et al., 2004), the expression of which is increased in many cancers surrounded by an inflammatory infiltrate (Townsend et al., 2005).
3.2. Placenta
As noted previously, the placenta was the first clearly identified extra renal source of 1,25(OH)2D production. CYP27b1 is expressed in both the maternal decidual stromal cells and the fetal trophoblasts (Weisman et al., 1979; Zehnder et al., 2001a; Delvin et al., 1985; Diaz et al., 2000; Zehnder et al., 2002a). During pregnancy the rise in maternal 1,25(OH)2D levels is due to production by the maternal portion of the placenta, as 1,25(OH)2D, unlike 25OHD, does not readily cross the placenta. This was well demonstrated in a study of Hannover pigs which lack a functional CYP27b1. During pregnancy there was no rise in serum 1,25(OH)2D despite heterozygote fetuses (Lachenmaier-Currle et al., 1989), which are capable of producing their own 1,25(OH)2D (Wieland et al., 1980). CYP27b1 expression increases during early pregnancy in both the trophoblasts and decidua, but not the endometrium (Vigano et al., 2006; Evans et al., 2004a). Unlike most tissues the rise in CYP27b1 expression is not accompanied by an increased expression of CYP24a1 (Evans et al., 2004a), which actually shows an increase in methylation of its promoter (Novakovic et al., 2009), presumably suppressing its expression. Deletion of CYP27b1 is associated with uterine hypoplasia and infertility in female mice (Panda et al., 2001a). Preeclampsia, a condition developing mid pregnancy and marked by hypertension is associated with a reduction in serum 1,25(OH)2D. Trophoblasts taken from preeclamptic placentae show decreased CYP27b1 activity and response to IGF1, which is also reduced in these patients (Diaz et al., 2002). However, other investigators observed an increase in CYP27b1 expression in preeclamptic placentae (Fischer et al., 2007b), suggesting a disparity between mRNA levels and function of the protein product as would happen if the increase in mRNA represented alternatively spliced forms of CYP27b1.
3.3. Bone
Bone cells were the next cell type to be identified as expressing CYP27b1 (Turner et al., 1980). The importance of this observation remained ignored for decades as the prevailing dogma was that vitamin D signaling in bone was minimal at best, in that the rickets of the CYP27b1 and Vdr knockouts could be corrected with a diet high in calcium and phosphate (Dardenne et al., 2003; Amling et al., 1999). However, a more extensive evaluation of the skeleton of these knockouts individually and in combination demonstrated that even when hypocalcemia and secondary hyperparathyroidism are prevented by the rescue diet not all changes in osteoblast number, mineral apposition rate and bone volume are rescued (Panda et al., 2004). In particular, the width of the growth plate remained increased in the Cyp27b1 knockout and double knockout of both Cyp27b1 and Vdr, the trabecular bone was markedly osteopenic with decreased mineral apposition rates in both cortical and trabecular bone, and alkaline phosphatase expression was reduced, all signifying decreased osteoblast number or activity. Osteoclast numbers in the trabeculae were likewise reduced as was expression of Rankl. In vitro, bone marrow stromal cell (BMSC) cultures from these animals demonstrated reductions in colony forming units and mineralized nodules. Such findings stimulated a reexamination of the role of Cyp27b1 in bone. Cyp27b1 is expressed in all bone cell types including chondrocytes (Pedrozo et al., 1999; Panda et al., 2001b), mesenchymal stem cells (MSC) (osteoprogenitors from bone) (Zhou et al., 2010; Geng et al., 2011a), osteoblasts (van Driel et al., 2006; Atkins et al., 2007), osteoclasts (Kogawa et al., 2010), and possibly osteocytes (shown in an osteocyte cell line MLO-A5) (Turner et al., 2014). The endogenously produced 1,25(OH)2D when these cells are treated with 25OHD stimulates their differentiation, and in the case of the MSC blocks their differentiation. Knockdown of Cyp27b1 with siRNA in MSC and osteoblasts blocked the ability of 25OHD to induce their differentiation (Geng et al., 2011a; Atkins et al., 2007). However, although 25OHD stimulated osteoclast differentiation from peripheral blood mononuclear cells (PBMC), it reduced the resorptive activity of the osteoclasts that formed. On the other hand, when splenocytes were used as the source of osteoclast progenitors, cells from CYP27b1 knockout animals were fewer and smaller but on a per cell basis had increased resorptive capacity (Reinke et al., 2016). Similar findings had been shown for Vdr knockout splenocytes (Kogawa et al., 2010). The cell specific deletion of Cyp27b1 from chondrocytes shows a reduction in osteoclastogenesis in the primary spongiosa associated with decreased Rankl expression, an increase in trabecular bone volume in the metaphysis of neonatal long bones, and a reduction in angiogenesis associated with a reduction in Vegf expression. The width of the hypertrophic zone of the growth plate was widened on embryonic day 15.5, but these changes in the growth plate did not persist (St-Arnaud et al., 2003; Naja et al., 2009). Some of these changes may have been secondary to the increase in circulating Fgf23 in these mice (St-Arnaud et al., 2003). When Cyp27b1 was overexpressed in chondrocytes the opposite effects to that of the knockout were observed (Naja et al., 2009).
3.4. Cells of the immune system
Although sarcoid tissue was clearly identified as the extrarenal source of 1,25(OH)2D in 1981 (Barbour et al., 1981), the pulmonary alveolar macrophage from patents with sarcoidosis 1983 was perhaps the clearest demonstration that the macrophage was responsible (Adams et al., 1983). Unlike the kidney, the production of 1,25(OH)2D by macrophages was substrate dependent (Adams et al., 1983) and not product limited (Adams and Gacad, 1985), and the CYP27b1 activity in macrophages was clearly not regulated by PTH and calcium (Insogna et al., 1988). Part of the lack of regulation is due to truncated versions of CYP24a1 which do not have enzymatic activity and so do not catabolize 1,25(OH)2D within the macrophage allowing it to enter the circulation (Ren et al., 2005). This truncated form of CYP24a1, which includes the substrate binding domain but not the mitochondrial targeting sequence, is postulated to act as a dominant negative form of CYP24a1, binding 1,25(OH)2D within the cytoplasm and preventing its catabolism (Ren et al., 2005). Both intact CYP24a1 and the truncated variant are induced in macrophage cell lines (HD-11 and THP-1), but CYP24a1 activity is not increased. As mentioned earlier, at least in the placenta the expression of CYP24a1 does not increase in parallel with the increase in CYP27b1 due to methylation (and inhibition) of its promoter, possibly also contributing to the decreased regulation of 1,25(OH)2D production by these cells (Novakovic et al., 2009).
Extrarenal CYP27b1 expression in macrophages is not limited to sarcoidosis. CYP27b1 expression has been found in pulmonary alveolar macrophages from patients with tuberculosis (Barnes et al., 1989), coccidiomycosis and cryptococcosis (Ali et al., 1999), the peritoneum from individuals undergoing peritoneal dialysis (Bacchetta et al., 2013) and from patients with peritonitis (Hayes et al., 1987), from the synovium of patients with inflammatory arthritis (Hayes et al., 1992), granulomata in the colon of patients with Crohn's disease (Abreu et al., 2004), granulomata in the skin of a patient with slack skin disease (a T cell lymphoproliferative disorder (Karakelides et al., 2006)), subcutaneous fat necrosis of the newborn (Farooque et al., 2009; Finne et al., 1988), granulomata within lymph nodes (Zehnder et al., 2001a), circulating monocytes from patients with chronic renal failure (Dusso et al., 1991), foreign body granulomata (Hindi et al., 2015) and normal monocytes activated in vitro (Gyetko et al., 1993). Kallas and Hewison provide a well referenced table of these infectious and inflammatory causes of elevated 1,25(OH)2D production (Kallas et al., 2010).
Dendritic cells (DC) require activation to express CYP27b1 (Fritsche et al., 2003). These are the principal antigen presenting cells to the T cells, a key feature of adaptive immunity. 1,25(OH)2D suppresses the maturation of DC and increases their expression of IL-10 that together increase tolerogenesis with reduction of the differentiation of Th1 and Th17 lymphocytes and increased differentiation of Th2 and Treg lymphocytes (Penna and Adorini, 2000). Th lymphocytes also express CYP27b1 as well as the VDR when activated (Sigmundsdottir et al., 2007; Bhalla et al., 1983). 1,25(OH)2D inhibits the proliferation of these cells as well as their production of cytokines such as IL-2, IFNγ, and TNF, while promoting the expression of Th2 and Treg cytokines such as IL-3, IL-4, IL-5, and IL-10 (Lemire et al., 1985; Boonstra et al., 2001). B lymphocytes also express CYP27b1 (Chen et al., 2007). 1,25(OH)2D reduced their proliferation and differentiation into plasma cells thus reducing antibody production (Chen et al., 2007). The role of endogenous 1,25(OH)2D production in T and B cells was recently tested by examining the response of these cells to OVA induced sensitization and intestinal infection with Heligmosomoides polygyrus, two means of inducing an IgE and IgG1 response (Lindner et al., 2017). In the global CYP27b1 knockout, IgE and IgG1 were increased more than controls in the OVA-sensitization model, but only IgE in the Heligmosomoides polygyrus model. When CYP27b1 was selectively deleted in either the T or B lymphocytes significant increases in IgE and antibody secreting cells after OVA challenge were seen only in the T cell specific knockout.
3.5. Endocrine glands
Several endocrine glands, the parathyroid gland and pancreatic islets in particular, whose products are regulated by 1,25(OH)2D, also express CYP27b1 (Zehnder et al., 2001a; Bland et al., 2004; Correa et al., 2002; Ritter et al., 2012; Ritter et al., 2006). Expression of CYP27b1 appears to be greater in parathyroid adenomas, whether primary or secondary to renal failure, compared to normal glands (Correa et al., 2002; Segersten et al., 2002), and is preferentially expressed in oxyphil compared to chief cells (Ritter et al., 2012). When sequenced, none of these adenomas expressed inactivating mutations in CYP27b1 to suggest that CYP27b1 was functioning as a tumor suppressor gene (Lauter and Arnold, 2009). Malignant pancreatic tissue and cell lines derived from such tissue express CYP27b1 at levels comparable to normal tissue (Schwartz et al., 2004). Polymorphisms in CYP27b1 have been associated with increased risk of type 1 diabetes mellitus (Bailey et al., 2007), although it is unclear whether this link is due to regulation of insulin secretion or to the immune response leading to this form of diabetes. The thyroid has also been shown to express CYP27b1 (Clinckspoor et al., 2012), and this expression is increased in papillary carcinoma of this gland (Khadzkou et al., 2006), although CYP27b1 expression is decreased in metastases (Clinckspoor et al., 2012). However, unlike the parathyroid gland and pancreatic islet, it is unclear whether 1,25(OH)2D influences hormone secretion from the thyroid gland. CYP27b1 protein has been detected in the adrenal medulla by immunohistochemistry (Zehnder et al., 2001b), although no reports of 1,25(OH)2D3 regulated cathecholamine production or altered CYP27b1 expression in patients with pheochromocytoma have been published. The testes express CYP27b1 (Fu et al., 1997). Using the promoter of CYP27b1 to drive a luciferase promoter, Anderson et al. (Anderson et al., 2008) localized CYP27b1 expression in both Leydig and Sertoli cells of the testes, results subsequently confirmed (Blomberg Jensen et al., 2010). CYP27b1 expression has also been found in the ovary (Evans et al., 2004b) and ovarian cancer (Fischer et al., 2009; Brozyna et al., 2015) where its expression is negatively correlated with tumor growth. Dysgerminomas of the ovary have a much greater expression of CYP27b1 than normal ovarian tissue (Evans et al., 2004b). This has significance in that hypercalcemia is known to occur with dysgerminomas, and a recent case report attributed the hypercalcemia to increased 1,25(OH)2D levels in a patient with an ovarian dysgerminoma (Hibi et al., 2008).
3.6. Other tissues
The liver, well known to be the major site for 25OHD production, has also been shown to express CYP27b1 (Hollis, 1990), which at least in the rat appears to disappear postnatally (Takeuchi et al., 1994). This observation has received little further investigation following these initial and early publications.
Expression of CYP27b1 in the brain was also a surprise result of our initial tissue screen for CYP27b1 (Fu et al., 1997). Subsequent localization studies have demonstrated expression in the cerebellum and cerebral cortex (Zehnder et al., 2001a), and, in particular, in Purkinje cells (Anderson et al., 2008). Glioblastomas show increased CYP27b1 expression (Fischer et al., 2010) along with spliced variants of CYP27b1 (Maas et al., 2001), the significance of which is uncertain (Diesel et al., 2003). Zehnder et al. observed CYP27b1 expression in human endothelial cells and 1,25(OH)2D production by human umbilical vein endothelial cells in vitro (Zehnder et al., 2002b). 25OHD or 1,25(OH)2D decreased their proliferation and increased adhesion of the monocyte cell like U937 to these cells suggesting a role in endothelial cell adhesion.
4. Regulation of extrarenal CYP27b1 expression
An important feature of extrarenal CYP27b1 is that its regulation differs from that of the kidney, the regulation of CYP27b1 in the proximal convoluted tubule (PCT) of the kidney to be precise. CYP27b1 in the renal PCT is controlled principally by three hormones, parathyroid hormone (PTH), FGF23, and 1,25(OH)2D itself responding at least in part to changes in ambient calcium and phosphate levels (review in (Jones et al., 2014)). Calcitonin regulates CYP27b1 activity in the proximal straight tubule (Kawashima et al., 1981), but this has received little further study. PTH stimulates CYP27b1 expression principally by acting on its membrane receptor to promote cyclic AMP formation, PKA activation, and enhanced binding of CCAAT box binding protein to its proximal response element in the CYP27b1 promoter (Gao et al., 2002). FGF23 inhibits CYP27b1 expression by a mechanism involving its binding to one of several FGF receptors in association with Klotho, activating MAPK, but the molecular events leading to inhibition of CYP27b1 expression by this pathway are not well defined (Strom and Juppner, 2008). Calcium and phosphate, by regulating the secretion of PTH and FGF23 from the parathyroid glands and bone, respectively, indirectly regulate CYP27b1 activity in the kidney, although they also have direct effects (Omdahl et al., 1972; Tanaka and Deluca, 1973; Bikle, 1978). 1,25(OH)2D directly inhibits CYP27b1 expression in the kidney through a complex mechanism involving VDR that brings both histone deacetylases (HDAC) and DNA methyl transferases to the promoter of CYP27b1 inhibiting its transcription (Kim et al., 2007). These feedback loops provide very tight regulation of 1,25(OH)2D production by the PCT of the kidney, control that differs from that of CYP27b1 in other cell types including that of distal renal tubule cells where PTH has little effect (Bajwa et al., 2008). Meyer et al. (2017) recently identified a region in the CYP27b1 enhancer region in DNA from the kidney that was responsive to PTH, FGF23, and 1,25(OH)2D regulation. However, this region was not accessible to such regulation in the extrarenal tissues they tested including skin and bone, but was regulated by inflammatory factors, consistent with different regulatory mechanisms in extra renal tissues. However, this distinction is not so precise as the genomic data would suggest when tested directly in cells from non-renal tissues as we will now describe.
4.1. Keratinocytes
When we (Bikle et al., 1986b) first examined the ability of PTH to stimulate 1,25(OH)2D production by keratinocytes, we observed that their response to PTH differed from that of the kidney in that PTH binding to a receptor could not be demonstrated, and cyclic AMP did not appear to be involved, although the response was potentiated by inhibition of phosphodiesterase. Moreover, Flanagan et al. (Flanagan et al., 2003) using a CYP27b1 promoter/luciferase reporter assay demonstrated PTH stimulated expression in a kidney cell line but not in keratinocytes, suggesting that the effect of PTH may be post-transcriptional. However, this construct consisted of only 1500 bp 5′ of the promoter, and may not have contained the region of DNA that responded to PTH signaling in keratinocytes. PTH/PTHrP receptors have subsequently been identified in the epidermis and isolated keratinocytes (Errazahi et al., 2004), and Muehleisen et al. (Muehleisen et al., 2012) more recently made the surprising observation that incubation of keratinocytes with 25OHD could induce the expression of the PTH/PTHrP receptor. Moreover, epidermal keratinocytes produce PTHrP (Merendino Jr et al., 1986), so conceivably keratinocyte CYP27b1 is regulated by PTH/PTHrP, but this has not yet been demonstrated. Likewise, the effect of FGF23 on keratinocyte CYP27b1 expression or function has not been reported. Unlike the kidney, 1,25(OH)2D does not directly affect CYP27b1 expression in keratinocytes. Rather, 1,25(OH)2D regulates its own levels in the keratinocyte by inducing CYP24a1, the catabolic enzyme for 1,25(OH)2D (Xie et al., 2002). Tumor necrosis factor-α (TNF) (Bikle et al., 1991) and interferon-γ (IFN) (Bikle et al., 1989), on the other hand, are potent inducers of CYP27b1 activity in the keratinocyte as is TGFβ1 (Schauber et al., 2007). 1,25(OH)2D induces the expression TLR2 and CD14, and activation of TLR2 with a specific agonist, malp-2, but not with a TLR 4 agonist, lipopolysaccharide (LPS), induces CYP27b1 (Schauber et al., 2007). This feed forward mechanism plays a role in both wound healing and the response to infection as will be discussed subsequently.
4.2. Bone
CYP27b1 in human mesenchymal stem cells from bone marrow is stimulated by PTH through mechanisms involving both the phosphorylation of CREB (an acute response) and through the expression of IGF1 and the activation of its receptor (longer term response) (Geng et al., 2011b). 25OHD increases CYP27b1 expression in these cells, but that appears to be due to a combination of increased expression of the PTH/PTHrP receptor (Zhou et al., 2013) and IGF1 (Zhou et al., 2010) as 1,25(OH)2D decreases the expression of CYP27b1 in these cells (Zhou et al., 2010). Similarly, PTH stimulated osteoblasts from human bone explants (Somjen et al., 2007), although no mechanism was evaluated. In contrast to these results others have not found that PTH stimulated CYP27b1 in human osteoblasts (van Driel et al., 2006) or in ROS 17/2.8 osteoblasts transfected with a 1500 bp 5′ flanking region of CYP27b1 (Turner et al., 2007). Moreover, in the latter studies the authors observed a 50+ % inhibition by IGF1 and TGF-β with no inhibition by 1,25(OH)2D. However, as mentioned above, the enhancer regions regulating a gene may be at substantial distances from the promoter, so that such studies with short constructs may be misleading.
4.3. Macrophages and monocytes
The production of 1,25(OH)2D by pulmonary alveolar macrophages is activated by IFNγ and TNFα, but not by IFNα and IFNβ, and is inhibited by dexamethasone (Adams and Gacad, 1985; Pryke et al., 1990) (Adams and Gacad, 1985). The stimulation of CYP27b1 by IFNγ may involve increased NO production (Adams et al., 1986). Preincubation of these cells with 1,25(OH)2D did not alter enzymatic activity indicating no direct inhibition as in the kidney (Adams and Gacad, 1985). IL-1, IL-2 and IL-15 also stimulate CYP27b1 activity in peripheral blood mononuclear cells (PBMC), whereas IL-4 is suppressive (Gyetko et al., 1993; Edfeldt et al., 2010). In contrast to Th1 cells, which produce IFNγ and IL-2, Th2 cells produce not only IL-4 but IFNβ that increases IL-10 to decrease CYP27b1 activity (Teles et al., 2013). In other studies of PBMC, the combination of IFNγ and either phorbol ester (PMA) or LPS induced CYP27b1 (Stoffels et al., 2006). In these studies of PBMC the induction of CYP27b1 was blocked by inhibition of the JAK/STAT pathway, NFκB pathway, and p38 MAPK pathway (Stoffels et al., 2006). The same group then demonstrated in peritoneal macrophages (PM) that IFNγ stimulation of CYP27b1 required C/EBPβ and was inhibited by knocking out STAT1α or Interferon regulatory factor 1 (IRF1). LPS stimulates these pathways through TLR4 in association with the coreceptor CD14 (Stoffels et al., 2006), a point that will become important when the functional role of extra renal production of 1,25(OH)2D3 is discussed, and a difference from the role of TLR2 in keratinocytes (Vidya et al., 2017). Similar results were obtained with the macrophage cell line THP-1 (Overbergh et al., 2006). In a v myc transformed myelomonocytic cell line (HD-11), Adams et al. (1994) demonstrated stimulation of 1,25(OH)2D production by LPS and IFNγ, and failure of PTH or calcium and phosphate to regulate CYP27b1 activity. Both PBMC and PM express FGF receptors and Klotho, and respond to FGF23 with a reduction in CYP27b1 expression likely through the MAPK pathway similar to that in the kidney (Bacchetta et al., 2013).
4.4. Parathyroid gland
The parathyroid gland expresses both FGF receptors and Klotho (Silver and Naveh-Many, 2012). Unlike the kidney, FGF23 stimulates CYP27b1 expression in the parathyroid gland (Ritter et al., 2012; Krajisnik et al., 2007). Activation of the calcium sensing receptor in the parathyroid gland either by calcium or cinacalcet also increases CYP27b1 expression (112). Both FGF23 (Silver and Naveh-Many, 2012) and cincalcet (Ritter et al., 2012) reduce PTH secretion suggesting a link between PTH secretion and CYP27b1 expression. However, as yet unpublished observations by Chang et al. have shown that PTH stimulates CYP27b1 activity just as parathyroid gland specific knockout of CYP27b1 markedly increases PTH secretion (Chang et al., personal communication) indicating an intracrine feedback loop in the parathyroid gland between PTH and CYP27b1.
5. Function of extra renal CYP27b1
The kidney appears to be the major if not the sole source of circulating 1,25(OH)2D, so the role of extrarenal CYP27b1 needs to be addressed. Two considerations come to mind. First the substrate for CYP27b1, namely 25OHD, circulates at much higher concentrations than 1,25(OH)2D. Although essentially all of the 25OHD is protein bound (99.97%) (Bikle et al., 1986c), some cells express a mechanism involving megalin/cubilin that enables them to transport the 25OHD bound to its major carrier vitamin D binding protein (DBP) into the cell (Nykjaer et al., 1999). This was originally discovered in the kidney, enabling the kidney to recover DBP bound 25OHD from the glomerular filtrate, but megalin/cubilin expression has also been observed in a wide number of tissues including the placenta, parathyroid gland, choroid plexus, thyroid, intestinal epithelium, endometrium, epididymis (reviewed in (Christensen and Birn, 2002)), macrophages (Adams et al., 2007) and osteoblasts (Atkins et al., 2007). However, demonstration of uptake of DBP bound 25OHD by these cells has not been established in most cases. Second, circulating 1,25(OH)2D levels are tightly controlled, whereas the extrarenal CYP27b1 production operates under different controls and so may be more adaptable to the specific needs of that particular cell. That said the roles of extrarenal CYP27b1 can be considered to fall into three broad categories: the regulation of cellular proliferation and differentiation, the regulation of hormone secretion, and the regulation of immune function both innate and adaptive. These are not mutually exclusive. In all cases the role of endogenously produced 1,25(OH)2D is that of a ligand for the vitamin D receptor (VDR) expressed in nearly every cell in the body.
5.1. Regulation of proliferation and differentiation
Epidermal keratinocytes provide an excellent model to evaluate the ability of 1,25(OH)2D to regulate proliferation and differentiation in a normal cell. 1,25(OH)2D promotes the differentiation of keratinocytes while inhibiting their proliferation (Bikle and Pillai, 1993; Bikle, 2012). In the epidermis proliferation occurs in the basal layer, and as the keratinocytes move out of the basal layer differentiation is initiated. As the keratinocytes move from one layer of epidermis to the next, differentiation proceeds in a sequential fashion ultimately resulting in the enucleated corneocyte enmeshed in a lipid rich matrix that provides the barrier function. 1,25(OH)2D is involved in all steps of this process in that it limits proliferation in the basal layer while inducing in a sequential pattern the expression of genes whose products ultimately produce the permeability barrier. Included in the development of the permeability barrier is the production of antimicrobial peptides such as cathelicidin, important for the innate immune response in the epidermis (Schauber et al., 2007) and other epithelial and immune cells as will be discussed. CYP27b1 like VDR is found throughout the epidermis, although expression appears to be higher in the basal layer of the epidermis (Zehnder et al., 2001a; Milde et al., 1991; Stumpf et al., 1984). The CYP27b1 null mouse has increased keratinocyte proliferation, decreased epidermal differentiation, fails to form the calcium gradient within the epidermis important for the differentiation process, and is limited in the ability to reform the permeability barrier following disruption (Bikle et al., 2004). Moreover, these mice are less resistant to infection (Muehleisen et al., 2012) and have a blunted innate immune response to wounding (Schauber et al., 2007).
5.1.1. Psoriasis
Psoriasis is an example of a hyperproliferative disease of the skin for which analogs of 1,25(OH)2D and 1,25(OH)2D itself have proven successful (Bruce et al., 1994). Psoriasis is a chronic, generalized, and scaly erythematous dermatosis thought to be due to a Th1 or Th17 mediated immune reaction to as yet unidentified antigens in the skin that may cause or at least is accompanied by increased proliferation and decreased differentiation of the keratinocytes in the epidermis. 1,25(OH)2D and its analogs likely work by inhibiting the inflammatory component of the disease via a direct action on the T cells (Bagot et al., 1994) as well as by reducing keratinocyte proliferation and enhancing their differentiation (Kragballe and Wildfang, 1990). Given the increased expression of CYP27b1 in these cells, topical treatment with 25OHD may also prove effective, but to my knowledge results with this form of therapy have not been reported.
5.1.2. Cancer
As noted in the section on tissue distribution CYP27b1 is expressed in a number of malignant tissues. Moreover, expression is often increased in the early stages of tumor development but is lost as the tumors dedifferentiate (Hobaus et al., 2013). Many animal studies have demonstrated a reduction in growth in such cancers following administration of 1,25(OH)2D or its analogs (Campbell and Trump, 2017), and epidemiologic evidence is consistent with a role for vitamin D in preventing many of these cancers in humans (Garland et al., 1985; Bostick et al., 1993; Kearney et al., 1996; Garland et al., 1990; Hanchette and Schwartz, 1992). Moreover, polymorphisms in CYP27b1 have been found in hepatocellular carcinoma (Lange et al., 2013), lung cancer (Kong et al., 2015), and prostate cancer (Gilbert et al., 2015). In a recent study Li et al. (Li et al., 2016) deleted CYP27b1 from the mammary epithelium in a mouse breast cancer model and demonstrated an acceleration of tumorigenesis. However, in the CYP27b1 null mouse we were unable to demonstrate an increase in UV induced tumor formation using a protocol comparable to that which induced tumors in VDR null mice (Bikle, 2013). Therefore, the role of extrarenal CYP27b1 in tumor development may be tissue specific.
5.2. Regulation of hormone secretion
5.2.1. Parathyroid hormone (PTH)
Circulating PTH levels are better correlated with 25OHD levels than with 1,25(OH)2D levels (Vieth et al., 2003) even though it is 1,25(OH)2D that inhibits the synthesis and secretion of PTH (Demay et al., 1992) and prevents the proliferation of the parathyroid gland (Demay et al., 1992; Martin and Gonzalez, 2004). The expression of CYP27b1 within the parathyroid gland could account for this observation. The parathyroid gene contains a negative VDRE through which 1,25(OH)2D exerts its suppression (Demay et al., 1992). 1,25(OH)2D also induces the calcium sensing receptor (CaSR) in the parathyroid gland (Canaff and Hendy, 2002), which sensitizes the parathyroid gland to calcium inhibition. As noted previously Cheng et al. in studies reported only in abstract form (ASBMR, 2011) have shown that deletion of CYP27b1 from the parathyroid gland markedly increased PTH secretion.
5.2.2. Insulin
A number of mostly case control and observational studies have suggested that vitamin D deficiency contributes to increased risk for types 1 and 2 diabetes mellitus (Pittas et al., 2007). In animal studies 1,25(OH)2D has been shown to stimulate insulin secretion, although the mechanism is not well defined (Kadowaki and Norman, 1985; Lee et al., 1994). Moreover, a recent study identified a polymorphism in CYP27b1 that predisposed to islet cell autoimmunity (Frederiksen et al., 2013). However, it is not clear what role the CYP27b1 in the pancreatic islet plays in this process as diabetes mellitus is not described in the Cyp27b1 knockout. This question will be best answered with tissue specific Cyp27b1 deletion.
5.2.3. Renin
The renin/angiotensin system is active in both heart and kidney. It is a major regulator of blood pressure. 1,25(OH)2D is a negative regulator of renin secretion (Li et al., 2004). The Cyp27b1 knockout mouse develops hypertension and cardiac hypertrophy with increased expression of the renin/angiotensin system in both heart and kidney. These changes persisted even when the mice were placed on the rescue diet to normalize their calcium, phosphate and PTH levels (Zhou et al., 2008). Although cardiac specific deletion of VDR has been reported showing similar cardiac changes (Gardner et al., 2013), comparable studies have not been reported with a cardiac specific Cyp27b1 knockout.
5.2.4. Fibroblast growth factor 23 (FGF23)
FGF23 is produced primarily in bone by osteoblasts and osteocytes. 1,25(OH)2D stimulates this process, but the mechanism is not clear (Kolek et al., 2005). Surprisingly, chondrocyte specific deletion of Cyp27b1 reduced circulating levels of Fgf23 presumably altering an as yet unidentified signal, possibly 1,25(OH)2D itself, from chondrocytes to the osteoblasts/osteocytes. At this point, osteoblast/osteocyte specific Cyp27b1 deletions have not been reported.
5.2.5. Estrogen and testosterone
1,25(OH)2D regulates the expression of aromatase involved in estrogen metabolism in mammary tumors potentially contributing to their antiproliferative actions (Swami et al., 2011). However, the impact of 1,25(OH)2D on ovarian production of estrogen has not been reported. 1,25(OH)2D does not alter testosterone production in the testes (Blomberg Jensen, 2014). Female mice lacking Cyp27b1 are infertile with hypoplastic uteri and loss of corpora lutea suggesting estrogen deficiency, but estrogen levels were not measured (Panda et al., 2001a). Male mice lacking Cyp27b1 remain fertile.
5.3. Regulation of immune function
5.3.1. Adaptive immunity
The adaptive immune response involves the ability of T and B lymphocytes to produce cytokines and immunoglobulins, respectively, to specifically combat the source of the antigen presented to them by cells such as macrophages and dendritic cells. As noted previously, CYP27b1 expression is increased when these cells are activated. 1,25(OH)2D exerts an inhibitory action on the adaptive immune system by suppressing the proliferation and differentiation of B-cell precursors into plasma cells (Chen et al., 2007), inhibiting T cell proliferation and function (Rigby et al., 1984), in particular Th1 (Lemire et al., 1995) and Th17 (Daniel et al., 2008) cells, and shifting the balance to favor Th2 cell (Boonstra et al., 2001) and regulatory T cell (Treg) function. At least in part these actions on T cell proliferation and differentiation stem from actions of 1,25(OH)2D on dendritic cells to reduce their antigen presenting capability. The ability of 1,25(OH)2D to suppress the adaptive immune system appears to be beneficial for a number of conditions in which the immune system is directed at self—i.e. autoimmunity. In a number of experimental models (Adorini, 2005; Deluca and Cantorna, 2001) including inflammatory arthritis, autoimmune diabetes, experimental allergic encephalitis (a model for multiple sclerosis), and inflammatory bowel disease 1,25(OH)2D administration has prevented and/or treated the disease process. Thus local production of 1,25(OH)2D in pulmonary alveolar macrophages, peritoneal macrophages, synovial macrophages, PBMC, pancreatic islets, neurons in the brain, lung, and intestinal mucosa, all tissues which express CYP27b1, may provide a mechanism to control the destructive immune process that is the etiology for these disease processes.
5.3.2. Innate immunity
Innate immune responses involve the activation of toll-like receptors (TLRs) in a number of epithelial cells including those of the epidermis, gingiva, intestine, vagina, placenta, bladder and lungs as well as cells of the immune system including PBMC and macrophages. TLRs are transmembrane pathogen-recognition receptors that interact with specific membrane patterns (PAMP) shed by infectious agents that trigger the innate immune response in the host (Medzhitov, 2007). Activation of TLRs leads to the induction of antimicrobial peptides and reactive oxygen species, which kill the organism. Among those antimicrobial peptides is cathelicidin. The expression of this antimicrobial peptide is induced by 1,25(OH)2D in both myeloid and epithelial cells (Gombart et al., 2005; Wang et al., 2004), cells which express both VDR and CYP27b1. The innate immune response of the macrophage, keratinocyte, and placenta provide the best examples of the role of local CYP27b1 activity to meet a local challenge. Vitamin D deficiency has long been associated with increased risk of tuberculosis (Ustianowski et al., 2005), and 1,25(OH)2D has long been recognized to potentiate the killing of mycobacteria by monocytes (Rook et al., 1986). Macrophages when activated by mycobacterial lipopeptides through TLR2/1 heterodimers express both CYP27b1 and VDR, leading to the production of 1,25(OH)2D from circulating 25OHD, which bound to VDR to induce cathelicidin that enhanced killing of the mycobacterium (Liu et al., 2006). Inadequate circulating 25OHD levels failed to support this process (Liu et al., 2006). In keratinocytes the expression of TLR2 and CD14, critical receptors for a number of pathogenic organisms activating the innate immune response, is increased by 1,25(OH)2D as is the expression of cathelicidin (Schauber et al., 2007). When the skin is wounded expression of CYP27b1 increases along with that of TLR2, CD14, and cathelicidin (Schauber et al., 2007). These changes do not occur in mice lacking Cyp27b1 in their epidermis (Schauber et al., 2007). In the placenta both the maternal decidua and fetal trophoblast produce cathelicidin in response to 1,25(OH)2D (Liu et al., 2009). When the placentas from Cyp27b1 knockout mice were evaluated, inflammatory cytokine production in response to LPS was amplified suggesting an important role for CYP27b1 in this tissue controlling the placental response to inflammatory stimuli (Liu et al., 2011). Other epithelia of tissues facing the outside environment must provide a protective barrier, and it is anticipated that the innate immune system in these tissues will likewise be regulated by local 1,25(OH)2D production. However, this needs to be demonstrated with tissue specific deletion of Cyp27b1.
6. Summary
CYP27b1 is expressed widely and in cells also expressing VDR. Although the data are limited with respect to tissue specific deletions of CYP27b1, in those tissues where this has been achieved local production of 1,25(OH)2D has been shown to exert effects on that tissue independent of circulating 1,25(OH)2D. However, such studies are the exception and not the rule, so that definitive data on this point will only come from additional studies with tissue specific deletion of CYP27b1. Should local expression of CYP27b1 be shown as critical for cell specific functions, the potential to manipulate 1,25(OH)2D production in the tissue of choice without incurring systemic changes in calcium homeostasis will be an important advance in the treatment of a variety of diseases. These include treatment of cancers with uncontrolled proliferation and disordered differentiation, better management of hyperparathyroidism and insulin secretion in diabetics, suppressing renin/angiotension in hypertension, improving wound repair, limiting tissue destruction in autoimmune diseases, and enhancing the resistance to infectious organisms such as streptococcus and tuberculosis.
Transparency document
Transparency document.
Footnotes
The Transparency document associated with this article can be found, in online version.
References
- Abreu M.T., Kantorovich V., Vasiliauskas E.A., Gruntmanis U., Matuk R., Daigle K., Chen S., Zehnder D., Lin Y.C., Yang H., Hewison M., Adams J.S. Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn's disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density. Gut. 2004;53(8):1129–1136. doi: 10.1136/gut.2003.036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J.S., Gacad M.A. Characterization of 1 alpha-hydroxylation of vitamin D3 sterols by cultured alveolar macrophages from patients with sarcoidosis. J. Exp. Med. 1985;161(4):755–765. doi: 10.1084/jem.161.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J.S., Sharma O.P., Gacad M.A., Singer F.R. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J. Clin. Invest. 1983;72(5):1856–1860. doi: 10.1172/JCI111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J.S., Gacad M.A., Singer F.R., Sharma O.P. Production of 1,25-dihydroxyvitamin D3 by pulmonary alveolar macrophages from patients with sarcoidosis. Ann. N. Y. Acad. Sci. 1986;465:587–594. doi: 10.1111/j.1749-6632.1986.tb18535.x. [DOI] [PubMed] [Google Scholar]
- Adams J.S., Ren S.Y., Arbelle J.E., Horiuchi N., Gray R.W., Clemens T.L., Shany S. Regulated production and intracrine action of 1,25-dihydroxyvitamin D3 in the chick myelomonocytic cell line HD-11. Endocrinology. 1994;134(6):2567–2573. doi: 10.1210/endo.134.6.8194484. [DOI] [PubMed] [Google Scholar]
- Adams J.S., Chen H., Chun R., Ren S., Wu S., Gacad M., Nguyen L., Ride J., Liu P., Modlin R., Hewison M. Substrate and enzyme trafficking as a means of regulating 1,25-dihydroxyvitamin D synthesis and action: the human innate immune response. J. Bone Miner. Res. 2007;22(Suppl. 2):V20–4. doi: 10.1359/jbmr.07s214. [DOI] [PubMed] [Google Scholar]
- Adorini L. Intervention in autoimmunity: the potential of vitamin D receptor agonists. Cell. Immunol. 2005;233(2):115–124. doi: 10.1016/j.cellimm.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Agic A., Xu H., Altgassen C., Noack F., Wolfler M.M., Diedrich K., Friedrich M., Taylor R.N., Hornung D. Relative expression of 1,25-dihydroxyvitamin D3 receptor, vitamin D 1 alpha-hydroxylase, vitamin D 24-hydroxylase, and vitamin D 25-hydroxylase in endometriosis and gynecologic cancers. Reprod. Sci. 2007;14(5):486–497. doi: 10.1177/1933719107304565. [DOI] [PubMed] [Google Scholar]
- Ali M.Y., Gopal K.V., Llerena L.A., Taylor H.C. Hypercalcemia associated with infection by Cryptococcus neoformans and Coccidioides immitis. Am J Med Sci. 1999;318(6):419–423. doi: 10.1097/00000441-199912000-00010. [DOI] [PubMed] [Google Scholar]
- Alsalem J.A., Patel D., Susarla R., Coca-Prados M., Bland R., Walker E.A., Rauz S., Wallace G.R. Characterization of vitamin D production by human ocular barrier cells. Invest. Ophthalmol. Vis. Sci. 2014;55(4):2140–2147. doi: 10.1167/iovs.13-13019. [DOI] [PubMed] [Google Scholar]
- Amling M., Priemel M., Holzmann T., Chapin K., Rueger J.M., Baron R., Demay M.B. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology. 1999;140(11):4982–4987. doi: 10.1210/endo.140.11.7110. [DOI] [PubMed] [Google Scholar]
- Anderson P.H., Hendrix I., Sawyer R.K., Zarrinkalam R., Manavis J., Sarvestani G.T., May B.K., Morris H.A. Co-expression of CYP27B1 enzyme with the 1.5 kb CYP27B1 promoter-luciferase transgene in the mouse. Mol. Cell. Endocrinol. 2008;285(1–2):1–9. doi: 10.1016/j.mce.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Atkins G.J., Anderson P.H., Findlay D.M., Welldon K.J., Vincent C., Zannettino A.C., O'Loughlin P.D., Morris H.A. Metabolism of vitamin D3 in human osteoblasts: evidence for autocrine and paracrine activities of 1 alpha,25-dihydroxyvitamin D3. Bone. 2007;40(6):1517–1528. doi: 10.1016/j.bone.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Bacchetta J., Sea J.L., Chun R.F., Lisse T.S., Wesseling-Perry K., Gales B., Adams J.S., Salusky I.B., Hewison M. Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes. J. Bone Miner. Res. 2013;28(1):46–55. doi: 10.1002/jbmr.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot M., Charue D., Lescs M.C., Pamphile R.P., Revuz J. Immunosuppressive effects of 1,25-dihydroxyvitamin D3 and its analogue calcipotriol on epidermal cells. Br. J. Dermatol. 1994;130(4):424–431. doi: 10.1111/j.1365-2133.1994.tb03373.x. [DOI] [PubMed] [Google Scholar]
- Bailey R., Cooper J.D., Zeitels L., Smyth D.J., Yang J.H., Walker N.M., Hypponen E., Dunger D.B., Ramos-Lopez E., Badenhoop K., Nejentsev S., Todd J.A. Association of the vitamin D metabolism gene CYP27B1 with type 1 diabetes. Diabetes. 2007;56(10):2616–2621. doi: 10.2337/db07-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajwa A., Forster M.N., Maiti A., Woolbright B.L., Beckman M.J. Specific regulation of CYP27B1 and VDR in proximal versus distal renal cells. Arch. Biochem. Biophys. 2008;477(1):33–42. doi: 10.1016/j.abb.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Barbour G.L., Coburn J.W., Slatopolsky E., Norman A.W., Horst R.L. Hypercalcemia in an anephric patient with sarcoidosis: evidence for extrarenal generation of 1,25-dihydroxyvitamin D. N. Engl. J. Med. 1981;305(8):440–443. doi: 10.1056/NEJM198108203050807. [DOI] [PubMed] [Google Scholar]
- Bareis P., Bises G., Bischof M.G., Cross H.S., Peterlik M. 25-Hydroxy-vitamin d metabolism in human colon cancer cells during tumor progression. Biochem. Biophys. Res. Commun. 2001;285(4):1012–1017. doi: 10.1006/bbrc.2001.5289. [DOI] [PubMed] [Google Scholar]
- Barnes P.F., Modlin R.L., Bikle D.D., Adams J.S. Transpleural gradient of 1,25-dihydroxyvitamin D in tuberculous pleuritis. J. Clin. Invest. 1989;83(5):1527–1532. doi: 10.1172/JCI114048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S., Cordes T., Diesing D., Diedrich K., Friedrich M. Expression of 25 hydroxyvitamin D3-1alpha-hydroxylase in human endometrial tissue. J. Steroid Biochem. Mol. Biol. 2007;103(3–5):771–775. doi: 10.1016/j.jsbmb.2006.12.075. [DOI] [PubMed] [Google Scholar]
- Bhalla A.K., Amento E.P., Clemens T.L., Holick M.F., Krane S.M. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J. Clin. Endocrinol. Metab. 1983;57(6):1308–1310. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- Bikle D.D. A biochemical model for the ionic control of 25-hydroxyvitamin D3 1alpha-hydroxylase. J. Histochem. Cytochem. 1978;253(9):3042–3048. [PubMed] [Google Scholar]
- Bikle D.D. Vitamin D regulated keratinocyte differentiation. J. Cell. Biochem. 2004;92(3):436–444. doi: 10.1002/jcb.20095. [DOI] [PubMed] [Google Scholar]
- Bikle D.D. Vitamin D and the skin: physiology and pathophysiology. J. Invest. Dermatol. 2012;13(1):3–19. doi: 10.1007/s11154-011-9194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D.D. 2013. Sunlight, Vitamin D, and Skin Cancer. [Google Scholar]
- Bikle D.D., Pillai S. Vitamin D, calcium, and epidermal differentiation. Endocr. Rev. 1993;14:3–19. doi: 10.1210/edrv-14-1-3. [DOI] [PubMed] [Google Scholar]
- Bikle D.D., Nemanic M.K., Whitney J.O., Elias P.W. Neonatal human foreskin keratinocytes produce 1,25-dihydroxyvitamin D3. Biochemistry. 1986;25(7):1545–1548. doi: 10.1021/bi00355a013. [DOI] [PubMed] [Google Scholar]
- Bikle D.D., Nemanic M.K., Gee E., Elias P. 1,25-Dihydroxyvitamin D3 production by human keratinocytes. Kinetics and regulation. J. Clin. Invest. 1986;78(2):557–566. doi: 10.1172/JCI112609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D.D., Halloran B.P., Gee E., Ryzen E., Haddad J.G. Free 25-hydroxyvitamin D levels are normal in subjects with liver disease and reduced total 25-hydroxyvitamin D levels. J. Clin. Invest. 1986;78(3):748–752. doi: 10.1172/JCI112636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D.D., Pillai S., Gee E., Hincenbergs M. Regulation of 1,25-dihydroxyvitamin D production in human keratinocytes by interferon-gamma. Endocrinology. 1989;124(2):655–660. doi: 10.1210/endo-124-2-655. [DOI] [PubMed] [Google Scholar]
- Bikle D.D., Pillai S., Gee E., Hincenbergs M. Tumor necrosis factor-alpha regulation of 1,25-dihydroxyvitamin D production by human keratinocytes. Endocrinology. 1991;129(1):33–38. doi: 10.1210/endo-129-1-33. [DOI] [PubMed] [Google Scholar]
- Bikle D.D., Halloran B.P., Riviere J.E. Production of 1,25 dihydroxyvitamin D3 by perfused pig skin. J. Invest. Dermatol. 1994;102(5):796–798. doi: 10.1111/1523-1747.ep12378190. [DOI] [PubMed] [Google Scholar]
- Bikle D.D., Chang S., Crumrine D., Elalieh H., Man M.Q., Choi E.H., Dardenne O., Xie Z., Arnaud R.S., Feingold K., Elias P.M. 25 hydroxyvitamin D 1 alpha-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J. Invest. Dermatol. 2004;122(4):984–992. doi: 10.1111/j.0022-202X.2004.22424.x. [DOI] [PubMed] [Google Scholar]
- Bises G., Kallay E., Weiland T., Wrba F., Wenzl E., Bonner E., Kriwanek S., Obrist P., Cross H.S. 25-Hydroxyvitamin D3-1alpha-hydroxylase expression in normal and malignant human colon. J. Histochem. Cytochem. 2004;52(7):985–989. doi: 10.1369/jhc.4B6271.2004. [DOI] [PubMed] [Google Scholar]
- Bland R., Markovic D., Hills C.E., Hughes S.V., Chan S.L., Squires P.E., Hewison M. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in pancreatic islets. J. Steroid Biochem. Mol. Biol. 2004;89-90(1–5):121–125. doi: 10.1016/j.jsbmb.2004.03.115. [DOI] [PubMed] [Google Scholar]
- Blomberg Jensen M. Vitamin D and male reproduction. Nat. Rev. Endocrinol. 2014;10(3):175–186. doi: 10.1038/nrendo.2013.262. [DOI] [PubMed] [Google Scholar]
- Blomberg Jensen M., Nielsen J.E., Jorgensen A., Rajpert-De Meyts E., Kristensen D.M., Jorgensen N., Skakkebaek N.E., Juul A., Leffers H. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum. Reprod. 2010;25(5):1303–1311. doi: 10.1093/humrep/deq024. [DOI] [PubMed] [Google Scholar]
- Boonstra A., Barrat F.J., Crain C., Heath V.L., Savelkoul H.F., O'Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J. Immunol. 2001;167(9):4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- Bostick R.M., Potter J.D., Sellers T.A., McKenzie D.R., Kushi L.H., Folsom A.R. Relation of calcium, vitamin D, and dairy food intake to incidence of colon cancer among older women. The Iowa Women's Health Study. Am. J. Epidemiol. 1993;137(12):1302–1317. doi: 10.1093/oxfordjournals.aje.a116640. [DOI] [PubMed] [Google Scholar]
- Brozyna A.A., Jozwicki W., Jochymski C., Slominski A.T. Decreased expression of CYP27B1 correlates with the increased aggressiveness of ovarian carcinomas. Oncol. Rep. 2015;33(2):599–606. doi: 10.3892/or.2014.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce S., Epinette W.W., Funicella T., Ison A., Jones E.L., Loss R., Jr., McPhee M.E., Whitmore C. Comparative study of calcipotriene (MC 903) ointment and fluocinonide ointment in the treatment of psoriasis. J. Am. Acad. Dermatol. 1994;31(5 Pt 1):755–759. doi: 10.1016/s0190-9622(94)70237-3. [DOI] [PubMed] [Google Scholar]
- Campbell M.J., Trump D.L. Vitamin D receptor signaling and cancer. Endocrinol. Metab. Clin. N. Am. 2017;46(4):1009–1038. doi: 10.1016/j.ecl.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaff L., Hendy G.N. Human calcium-sensing receptor gene. Vitamin D response elements in promoters P1 and P2 confer transcriptional responsiveness to 1,25-dihydroxyvitamin D. J. Biol. Chem. 2002;277(33):30337–30350. doi: 10.1074/jbc.M201804200. [DOI] [PubMed] [Google Scholar]
- Chen T.C. 25-Hydroxyvitamin D-1 alpha-hydroxylase (CYP27B1) is a new class of tumor suppressor in the prostate. Anticancer Res. 2008;28(4A):2015–2017. [PubMed] [Google Scholar]
- Chen S., Sims G.P., Chen X.X., Gu Y.Y., Chen S., Lipsky P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007;179(3):1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- Christensen E.I., Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nat. Rev. Mol. Cell Biol. 2002;3(4):256–266. doi: 10.1038/nrm778. [DOI] [PubMed] [Google Scholar]
- Clinckspoor I., Hauben E., Verlinden L., Van den Bruel A., Vanwalleghem L., Vander Poorten V., Delaere P., Mathieu C., Verstuyf A., Decallonne B. Altered expression of key players in vitamin D metabolism and signaling in malignant and benign thyroid tumors. J. Histochem. Cytochem. 2012;60(7):502–511. doi: 10.1369/0022155412447296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes T., Diesing D., Becker S., Fischer D., Diedrich K., Friedrich M. Expression of splice variants of 1alpha-hydroxylase in mcf-7 breast cancer cells. J. Steroid Biochem. Mol. Biol. 2007;103(3–5):326–329. doi: 10.1016/j.jsbmb.2006.12.034. [DOI] [PubMed] [Google Scholar]
- Correa P., Segersten U., Hellman P., Akerstrom G., Westin G. Increased 25-hydroxyvitamin D3 1alpha-hydroxylase and reduced 25-hydroxyvitamin D3 24-hydroxylase expression in parathyroid tumors—new prospects for treatment of hyperparathyroidism with vitamin d. J. Clin. Endocrinol. Metab. 2002;87(12):5826–5829. doi: 10.1210/jc.2002-021356. [DOI] [PubMed] [Google Scholar]
- Daniel C., Sartory N.A., Zahn N., Radeke H.H., Stein J.M. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J. Pharmacol. Exp. Ther. 2008;324(1):23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- Dardenne O., Prud'homme J., Arabian A., Glorieux F.H., St-Arnaud R. Targeted inactivation of the 25-hydroxyvitamin D(3)-1(alpha)- hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D- deficiency rickets. Endocrinology. 2001;142(7):3135–3141. doi: 10.1210/endo.142.7.8281. [DOI] [PubMed] [Google Scholar]
- Dardenne O., Prud'homme J., Hacking S.A., Glorieux F.H., St-Arnaud R. Correction of the abnormal mineral ion homeostasis with a high-calcium, high-phosphorus, high-lactose diet rescues the PDDR phenotype of mice deficient for the 25-hydroxyvitamin D-1alpha-hydroxylase (CYP27B1) Bone. 2003;32(4):332–340. doi: 10.1016/s8756-3282(03)00023-1. [DOI] [PubMed] [Google Scholar]
- Deluca H.F., Cantorna M.T. Vitamin D: its role and uses in immunology. FASEB J. 2001;15(14):2579–2585. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- Delvin E.E., Arabian A., Glorieux F.H., Mamer O.A. In vitro metabolism of 25-hydroxycholecalciferol by isolated cells from human decidua. J. Clin. Endocrinol. Metab. 1985;60(5):880–885. doi: 10.1210/jcem-60-5-880. [DOI] [PubMed] [Google Scholar]
- Demay M.B., Kiernan M.S., DeLuca H.F., Kronenberg H.M. Sequences in the human parathyroid hormone gene that bind the 1,25- dihydroxyvitamin D3 receptor and mediate transcriptional repression in response to 1,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. U. S. A. 1992;89(17):8097–8101. doi: 10.1073/pnas.89.17.8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz L., Sanchez I., Avila E., Halhali A., Vilchis F., Larrea F. Identification of a 25-hydroxyvitamin D3 1alpha-hydroxylase gene transcription product in cultures of human syncytiotrophoblast cells. J. Clin. Endocrinol. Metab. 2000;85(7):2543–2549. doi: 10.1210/jcem.85.7.6693. [DOI] [PubMed] [Google Scholar]
- Diaz L., Arranz C., Avila E., Halhali A., Vilchis F., Larrea F. Expression and activity of 25-hydroxyvitamin D-1 alpha-hydroxylase are restricted in cultures of human syncytiotrophoblast cells from preeclamptic pregnancies. J. Clin. Endocrinol. Metab. 2002;87(8):3876–3882. doi: 10.1210/jcem.87.8.8730. [DOI] [PubMed] [Google Scholar]
- Diesel B., Fischer U., Meese E. Gene amplification and splice variants of 25-hydroxyvitamin D3 1,alpha-hydroxylase (CYP27B1) in glioblastoma multiforme—a possible role in tumor progression? Recent Results Cancer Res. 2003;164:151–155. doi: 10.1007/978-3-642-55580-0_11. [DOI] [PubMed] [Google Scholar]
- van Driel M., Koedam M., Buurman C.J., Hewison M., Chiba H., Uitterlinden A.G., Pols H.A., van Leeuwen J.P. Evidence for auto/paracrine actions of vitamin D in bone: 1alpha-hydroxylase expression and activity in human bone cells. FASEB J. 2006;20(13):2417–2419. doi: 10.1096/fj.06-6374fje. [DOI] [PubMed] [Google Scholar]
- Dusso A., Lopez-Hilker S., Rapp N., Slatopolsky E. Extra-renal production of calcitriol in chronic renal failure. Kidney Int. 1988;34(3):368–375. doi: 10.1038/ki.1988.190. [DOI] [PubMed] [Google Scholar]
- Dusso A.S., Finch J., Brown A., Ritter C., Delmez J., Schreiner G., Slatopolsky E. Extrarenal production of calcitriol in normal and uremic humans. J. Clin. Endocrinol. Metab. 1991;72(1):157–164. doi: 10.1210/jcem-72-1-157. [DOI] [PubMed] [Google Scholar]
- Ebert R., Jovanovic M., Ulmer M., Schneider D., Meissner-Weigl J., Adamski J., Jakob F. Down-regulation by nuclear factor kappaB of human 25-hydroxyvitamin D3 1alpha-hydroxylase promoter. Mol. Endocrinol. 2004;18(10):2440–2450. doi: 10.1210/me.2002-0441. [DOI] [PubMed] [Google Scholar]
- Edfeldt K., Liu P.T., Chun R., Fabri M., Schenk M., Wheelwright M., Keegan C., Krutzik S.R., Adams J.S., Hewison M., Modlin R.L. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc. Natl. Acad. Sci. U. S. A. 2010;107(52):22593–22598. doi: 10.1073/pnas.1011624108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errazahi A., Lieberherr M., Bouizar Z., Rizk-Rabin M. PTH-1R responses to PTHrP and regulation by vitamin D in keratinocytes and adjacent fibroblasts. J. Steroid Biochem. Mol. Biol. 2004;89-90(1–5):381–385. doi: 10.1016/j.jsbmb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Evans K.N., Bulmer J.N., Kilby M.D., Hewison M. Vitamin D and placental-decidual function. J. Soc. Gynecol. Investig. 2004;11(5):263–271. doi: 10.1016/j.jsgi.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Evans K.N., Taylor H., Zehnder D., Kilby M.D., Bulmer J.N., Shah F., Adams J.S., Hewison M. Increased expression of 25-hydroxyvitamin D-1alpha-hydroxylase in dysgerminomas: a novel form of humoral hypercalcemia of malignancy. Am. J. Pathol. 2004;165(3):807–813. doi: 10.1016/s0002-9440(10)63343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooque A., Moss C., Zehnder D., Hewison M., Shaw N.J. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in subcutaneous fat necrosis. Br. J. Dermatol. 2009;160(2):423–425. doi: 10.1111/j.1365-2133.2008.08844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finne P.H., Sanderud J., Aksnes L., Bratlid D., Aarskog D. Hypercalcemia with increased and unregulated 1,25-dihydroxyvitamin D production in a neonate with subcutaneous fat necrosis. J. Pediatr. 1988;112(5):792–794. doi: 10.1016/s0022-3476(88)80706-6. [DOI] [PubMed] [Google Scholar]
- Fischer D., Seifert M., Becker S., Ludders D., Cordes T., Reichrath J., Friedrich M. 25-Hydroxyvitamin D3 1alpha-hydroxylase splice variants in breast cell lines MCF-7 and MCF-10. Cancer Genomics Proteomics. 2007;4(4):295–300. [PubMed] [Google Scholar]
- Fischer D., Schroer A., Ludders D., Cordes T., Bucker B., Reichrath J., Friedrich M. Metabolism of vitamin D3 in the placental tissue of normal and preeclampsia complicated pregnancies and premature births. Clin. Exp. Obstet. Gynecol. 2007;34(2):80–84. [PubMed] [Google Scholar]
- Fischer D., Thome M., Becker S., Cordes T., Diedrich K., Friedrich M., Thill M. 25-Hydroxyvitamin D3 1alpha-hydroxylase splice variants in benign and malignant ovarian cell lines and tissue. Anticancer Res. 2009;29(9):3627–3633. [PubMed] [Google Scholar]
- Fischer U., Leidinger P., Keller A., Folarin A., Ketter R., Graf N., Lenhof H.P., Meese E. Amplicons on chromosome 12q13-21 in glioblastoma recurrences. Int. J. Cancer. 2010;126(11):2594–2602. doi: 10.1002/ijc.24971. [DOI] [PubMed] [Google Scholar]
- Flanagan J.N., Wang L., Tangpricha V., Reichrath J., Chen T.C., Holick M.F. Regulation of the 25-hydroxyvitamin D-1alpha-hydroxylase gene and its splice variant. Recent Results Cancer Res. 2003;164:157–167. doi: 10.1007/978-3-642-55580-0_12. [DOI] [PubMed] [Google Scholar]
- Frankel T.L., Mason R.S., Hersey P., Murray E., Posen S. The synthesis of vitamin D metabolites by human melanoma cells. J. Clin. Endocrinol. Metab. 1983;57(3):627–631. doi: 10.1210/jcem-57-3-627. [DOI] [PubMed] [Google Scholar]
- Fraser D.R., Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Frederiksen B.N., Kroehl M., Fingerlin T.E., Wong R., Steck A.K., Rewers M., Norris J.M. Association between vitamin D metabolism gene polymorphisms and risk of islet autoimmunity and progression to type 1 diabetes: the diabetes autoimmunity study in the young (DAISY) J. Clin. Endocrinol. Metab. 2013;98(11):E1845–51. doi: 10.1210/jc.2013-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich M., Villena-Heinsen C., Axt-Fliedner R., Meyberg R., Tilgen W., Schmidt W., Reichrath J. Analysis of 25-hydroxyvitamin D3-1alpha-hydroxylase in cervical tissue. Anticancer Res. 2002;22(1A):183–186. [PubMed] [Google Scholar]
- Friedrich M., Rafi L., Mitschele T., Tilgen W., Schmidt W., Reichrath J. Analysis of the vitamin D system in cervical carcinomas, breast cancer and ovarian cancer. Recent Results Cancer Res. 2003;164:239–246. doi: 10.1007/978-3-642-55580-0_17. [DOI] [PubMed] [Google Scholar]
- Fritsche J., Mondal K., Ehrnsperger A., Andreesen R., Kreutz M. Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood. 2003;102(9):3314–3316. doi: 10.1182/blood-2002-11-3521. [DOI] [PubMed] [Google Scholar]
- Fu G.K., Lin D., Zhang M.Y., Bikle D.D., Shackleton C.H., Miller W.L., Portale A.A. Cloning of human 25-hydroxyvitamin D-1 alpha-hydroxylase and mutations causing vitamin D-dependent rickets type 1. Mol. Endocrinol. 1997;11(13):1961–1970. doi: 10.1210/mend.11.13.0035. [DOI] [PubMed] [Google Scholar]
- Gao X.H., Dwivedi P.P., Choe S., Alba F., Morris H.A., Omdahl J.L., May B.K. Basal and parathyroid hormone induced expression of the human 25-hydroxyvitamin D 1alpha-hydroxylase gene promoter in kidney AOK-B50 cells: role of Sp1, Ets and CCAAT box protein binding sites. Int. J. Biochem. Cell Biol. 2002;34(8):921–930. doi: 10.1016/s1357-2725(01)00165-0. [DOI] [PubMed] [Google Scholar]
- Gardner D.G., Chen S., Glenn D.J. Vitamin D and the heart. Am. J. Phys. Regul. Integr. Comp. Phys. 2013;305(9):R969–77. doi: 10.1152/ajpregu.00322.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland C., Shekelle R.B., Barrett-Connor E., Criqui M.H., Rossof A.H., Paul O. Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet. 1985;1(8424):307–309. doi: 10.1016/s0140-6736(85)91082-7. [DOI] [PubMed] [Google Scholar]
- Garland F.C., Garland C.F., Gorham E.D., Young J.F. Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev. Med. 1990;19(6):614–622. doi: 10.1016/0091-7435(90)90058-r. [DOI] [PubMed] [Google Scholar]
- Geng S., Zhou S., Glowacki J. Effects of 25-hydroxyvitamin D(3) on proliferation and osteoblast differentiation of human marrow stromal cells require CYP27B1/1alpha-hydroxylase. J. Bone Miner. Res. 2011;26(5):1145–1153. doi: 10.1002/jbmr.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng S., Zhou S., Glowacki J. Age-related decline in osteoblastogenesis and 1alpha-hydroxylase/CYP27B1 in human mesenchymal stem cells: stimulation by parathyroid hormone. Aging Cell. 2011;10(6):962–971. doi: 10.1111/j.1474-9726.2011.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert R., Bonilla C., Metcalfe C., Lewis S., Evans D.M., Fraser W.D., Kemp J.P., Donovan J.L., Hamdy F.C., Neal D.E., Lane J.A., Smith G.D., Lathrop M., Martin R.M. Associations of vitamin D pathway genes with circulating 25-hydroxyvitamin-D, 1,25-dihydroxyvitamin-D, and prostate cancer: a nested case-control study. Cancer Causes Control. 2015;26(2):205–218. doi: 10.1007/s10552-014-0500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombart A.F., Borregaard N., Koeffler H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19(9):1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- Gray T.K., Lester G.E., Lorenc R.S. Evidence for extra-renal 1 alpha-hydroxylation of 25-hydroxyvitamin D3 in pregnancy. Science. 1979;204(4399):1311–1313. doi: 10.1126/science.451538. [DOI] [PubMed] [Google Scholar]
- Gyetko M.R., Hsu C.H., Wilkinson C.C., Patel S., Young E. Monocyte 1 alpha-hydroxylase regulation: induction by inflammatory cytokines and suppression by dexamethasone and uremia toxin. J. Leukoc. Biol. 1993;54(1):17–22. doi: 10.1002/jlb.54.1.17. [DOI] [PubMed] [Google Scholar]
- Hanchette C.L., Schwartz G.G. Geographic patterns of prostate cancer mortality. Evidence for a protective effect of ultraviolet radiation. Cancer. 1992;70(12):2861–2869. doi: 10.1002/1097-0142(19921215)70:12<2861::aid-cncr2820701224>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Hayes M.E., O'Donoghue D.J., Ballardie F.W., Mawer E.B. Peritonitis induces the synthesis of 1 alpha,25-dihydroxyvitamin D3 in macrophages from CAPD patients. FEBS Lett. 1987;220(2):307–310. doi: 10.1016/0014-5793(87)80836-0. [DOI] [PubMed] [Google Scholar]
- Hayes M.E., Bayley D., Still P., Palit J., Denton J., Freemont A.J., Cooper R.G., Mawer E.B. Differential metabolism of 25-hydroxyvitamin D3 by cultured synovial fluid macrophages and fibroblast-like cells from patients with arthritis. Ann. Rheum. Dis. 1992;51(2):220–226. doi: 10.1136/ard.51.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi M., Hara F., Tomishige H., Nishida Y., Kato T., Okumura N., Hashimoto T., Kato R. 1,25-Dihydroxyvitamin D-mediated hypercalcemia in ovarian dysgerminoma. Pediatr. Hematol. Oncol. 2008;25(1):73–78. doi: 10.1080/08880010701774033. [DOI] [PubMed] [Google Scholar]
- Hindi S.M., Wang Y., Jones K.D., Nussbaum J.C., Chang Y., Masharani U., Bikle D., Shoback D.M., Hsiao E.C. A case of hypercalcemia and overexpression of CYP27B1 in skeletal muscle lesions in a patient with HIV infection after cosmetic injections with Polymethylmethacrylate (PMMA) for wasting. Calcif. Tissue Int. 2015;97(6):634–639. doi: 10.1007/s00223-015-0048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobaus J., Thiem U., Hummel D.M., Kallay E. Role of calcium, vitamin D, and the extrarenal vitamin D hydroxylases in carcinogenesis. Anti Cancer Agents Med. Chem. 2013;13(1):20–35. [PMC free article] [PubMed] [Google Scholar]
- Hollis B.W. 25-Hydroxyvitamin D3-1 alpha-hydroxylase in porcine hepatic tissue: subcellular localization to both mitochondria and microsomes. Proc. Natl. Acad. Sci. U. S. A. 1990;87(16):6009–6013. doi: 10.1073/pnas.87.16.6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J.Y., Feldman D., McNeal J.E., Peehl D.M. Reduced 1alpha-hydroxylase activity in human prostate cancer cells correlates with decreased susceptibility to 25-hydroxyvitamin D3-induced growth inhibition. Cancer Res. 2001;61(7):2852–2856. [PubMed] [Google Scholar]
- Insogna K.L., Dreyer B.E., Mitnick M., Ellison A.F., Broadus A.E. Enhanced production rate of 1,25-dihydroxyvitamin D in sarcoidosis. J. Clin. Endocrinol. Metab. 1988;66(1):72–75. doi: 10.1210/jcem-66-1-72. [DOI] [PubMed] [Google Scholar]
- Jones G., Ramshaw H., Zhang A., Cook R., Byford V., White J., Petkovich M. Expression and activity of vitamin D-metabolizing cytochrome P450s (CYP1alpha and CYP24) in human nonsmall cell lung carcinomas. Endocrinology. 1999;140(7):3303–3310. doi: 10.1210/endo.140.7.6799. [DOI] [PubMed] [Google Scholar]
- Jones G., Prosser D.E., Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 2014;55(1):13–31. doi: 10.1194/jlr.R031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki S., Norman A.W. Demonstration that the vitamin D metabolite 1,25(OH)2-vitamin D3 and not 24R,25(OH)2-vitamin D3 is essential for normal insulin secretion in the perfused rat pancreas. Diabetes. 1985;34(4):315–320. doi: 10.2337/diab.34.4.315. [DOI] [PubMed] [Google Scholar]
- Kallas M., Green F., Hewison M., White C., Kline G. Rare causes of calcitriol-mediated hypercalcemia: a case report and literature review. J. Clin. Endocrinol. Metab. 2010;95(7):3111–3117. doi: 10.1210/jc.2009-2673. [DOI] [PubMed] [Google Scholar]
- Karakelides H., Geller J.L., Schroeter A.L., Chen H., Behn P.S., Adams J.S., Hewison M., Wermers R.A. Vitamin D-mediated hypercalcemia in slack skin disease: evidence for involvement of extrarenal 25-hydroxyvitamin D 1alpha-hydroxylase. J. Bone Miner. Res. 2006;21(9):1496–1499. doi: 10.1359/jbmr.060608. [DOI] [PubMed] [Google Scholar]
- Kawashima H., Torikai S., Kurokawa K. Calcitonin selectively stimulates 25-hydroxyvitamin D3-1 alpha-hydroxylase in proximal straight tubule of rat kidney. Nature. 1981;291(5813):327–329. doi: 10.1038/291327a0. [DOI] [PubMed] [Google Scholar]
- Kearney J., Giovannucci E., Rimm E.B., Ascherio A., Stampfer M.J., Colditz G.A., Wing A., Kampman E., Willett W.C. Calcium, vitamin D, and dairy foods and the occurrence of colon cancer in men. Am. J. Epidemiol. 1996;143(9):907–917. doi: 10.1093/oxfordjournals.aje.a008834. [DOI] [PubMed] [Google Scholar]
- Khadzkou K., Buchwald P., Westin G., Dralle H., Akerstrom G., Hellman P. 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D receptor expression in papillary thyroid carcinoma. J. Histochem. Cytochem. 2006;54(3):355–361. doi: 10.1369/jhc.5A6734.2005. [DOI] [PubMed] [Google Scholar]
- Khorchide M., Lechner D., Cross H.S. Epigenetic regulation of vitamin D hydroxylase expression and activity in normal and malignant human prostate cells. J. Steroid Biochem. Mol. Biol. 2005;93(2–5):167–172. doi: 10.1016/j.jsbmb.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Kim M.S., Fujiki R., Kitagawa H., Kato S. 1alpha,25(OH)2D3-induced DNA methylation suppresses the human CYP27B1 gene. Mol. Cell. Endocrinol. 2007;265-266:168–173. doi: 10.1016/j.mce.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Kogawa M., Findlay D.M., Anderson P.H., Ormsby R., Vincent C., Morris H.A., Atkins G.J. Osteoclastic metabolism of 25(OH)-vitamin D3: a potential mechanism for optimization of bone resorption. Endocrinology. 2010;151(10):4613–4625. doi: 10.1210/en.2010-0334. [DOI] [PubMed] [Google Scholar]
- Kolek O.I., Hines E.R., Jones M.D., LeSueur L.K., Lipko M.A., Kiela P.R., Collins J.F., Haussler M.R., Ghishan F.K. 1alpha,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289(6):G1036–42. doi: 10.1152/ajpgi.00243.2005. [DOI] [PubMed] [Google Scholar]
- Kong J., Xu F., Qu J., Wang Y., Gao M., Yu H., Qian B. Genetic polymorphisms in the vitamin D pathway in relation to lung cancer risk and survival. Oncotarget. 2015;6(4):2573–2582. doi: 10.18632/oncotarget.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragballe K., Wildfang I.L. Calcipotriol (MC 903), a novel vitamin D3 analogue stimulates terminal differentiation and inhibits proliferation of cultured human keratinocytes. Arch. Dermatol. Res. 1990;282(3):164–167. doi: 10.1007/BF00372616. [DOI] [PubMed] [Google Scholar]
- Krajisnik T., Bjorklund P., Marsell R., Ljunggren O., Akerstrom G., Jonsson K.B., Westin G., Larsson T.E. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J. Endocrinol. 2007;195(1):125–131. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- Lachenmaier-Currle U., Breves G., Harmeyer J. Role of 1,25-(OH)2D3 during pregnancy; studies with pigs suffering from pseudo-vitamin D-deficiency rickets, type I. Q. J. Exp. Physiol. 1989;74(6):875–881. doi: 10.1113/expphysiol.1989.sp003358. [DOI] [PubMed] [Google Scholar]
- Lambert P.W., Stern P.H., Avioli R.C., Brackett N.C., Turner R.T., Greene A., Fu I.Y., Bell N.H. Evidence for extrarenal production of 1 alpha,25-dihydroxyvitamin D in man. J. Clin. Invest. 1982;69(3):722–725. doi: 10.1172/JCI110501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C.M., Miki D., Ochi H., Nischalke H.D., Bojunga J., Bibert S., Morikawa K., Gouttenoire J., Cerny A., Dufour J.F., Gorgievski-Hrisoho M., Heim M.H., Malinverni R., Mullhaupt B., Negro F., Semela D., Kutalik Z., Muller T., Spengler U., Berg T., Chayama K., Moradpour D., Bochud P.Y., Hiroshima Liver Study G, Swiss Hepatitis CCSG Genetic analyses reveal a role for vitamin D insufficiency in HCV-associated hepatocellular carcinoma development. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0064053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauter K., Arnold A. Analysis of CYP27B1, encoding 25-hydroxyvitamin D-1alpha-hydroxylase, as a candidate tumor suppressor gene in primary and severe secondary/tertiary hyperparathyroidism. J. Bone Miner. Res. 2009;24:102–104. doi: 10.1359/JBMR.080903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner D., Kallay E., Cross H.S. 1alpha,25-Dihydroxyvitamin D3 downregulates CYP27B1 and induces CYP24A1 in colon cells. Mol. Cell. Endocrinol. 2007;263(1–2):55–64. doi: 10.1016/j.mce.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Lee S., Clark S.A., Gill R.K., Christakos S. 1,25-Dihydroxyvitamin D3 and pancreatic beta-cell function: vitamin D receptors, gene expression, and insulin secretion. Endocrinology. 1994;134(4):1602–1610. doi: 10.1210/endo.134.4.8137721. [DOI] [PubMed] [Google Scholar]
- Lemire J.M., Adams J.S., Kermani-Arab V., Bakke A.C., Sakai R., Jordan S.C. 1,25-Dihydroxyvitamin D3 suppresses human T helper/inducer lymphocyte activity in vitro. J. Immunol. 1985;134(5):3032–3035. [PubMed] [Google Scholar]
- Lemire J.M., Archer D.C., Beck L., Spiegelberg H.L. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J. Nutr. 1995;125(6 Suppl):1704S–1708S. doi: 10.1093/jn/125.suppl_6.1704S. [DOI] [PubMed] [Google Scholar]
- Li Y.C., Qiao G., Uskokovic M., Xiang W., Zheng W., Kong J. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J. Steroid Biochem. Mol. Biol. 2004;89-90(1–5):387–392. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Li B., Baylink D.J., Walter M.H., Lau K.H., Meng X., Wang J., Cherkas A., Tang X., Qin X. Targeted 25-hydroxyvitamin D3 1alpha-hydroxylase adoptive gene therapy ameliorates dss-induced colitis without causing hypercalcemia in mice. Mol. Ther. 2015;23(2):339–351. doi: 10.1038/mt.2014.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Luco A.L., Ochietti B., Fadhil I., Camirand A., Reinhardt T.A., St-Arnaud R., Muller W., Kremer R. Tumoral vitamin D synthesis by CYP27B1 1-alpha-hydroxylase delays mammary tumor progression in the PyMT-MMTV mouse model and its action involves NF-kappaB modulation. Endocrinology. 2016;157(6):2204–2216. doi: 10.1210/en.2015-1824. [DOI] [PubMed] [Google Scholar]
- Lindner J., Rausch S., Treptow S., Geldmeyer-Hilt K., Krause T., St-Arnaud R., Arabian A., Radbruch A., Hartmann S., Worm M., Heine G. Endogenous calcitriol synthesis controls the humoral IgE response in mice. J. Immunol. 2017;199:3952–3958. doi: 10.4049/jimmunol.1602080. [DOI] [PubMed] [Google Scholar]
- Littledike E.T., Horst R.L. Metabolism of vitamin D3 in nephrectomized pigs given pharmacological amounts of vitamin D3. Endocrinology. 1982;111(6):2008–2013. doi: 10.1210/endo-111-6-2008. [DOI] [PubMed] [Google Scholar]
- Liu P.T., Stenger S., Li H., Wenzel L., Tan B.H., Krutzik S.R., Ochoa M.T., Schauber J., Wu K., Meinken C., Kamen D.L., Wagner M., Bals R., Steinmeyer A., Zugel U., Gallo R.L., Eisenberg D., Hewison M., Hollis B.W., Adams J.S., Bloom B.R., Modlin R.L. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Liu N., Nguyen L., Chun R.F., Lagishetty V., Ren S., Wu S., Hollis B., DeLuca H.F., Adams J.S., Hewison M. Altered endocrine and autocrine metabolism of vitamin D in a mouse model of gastrointestinal inflammation. Endocrinology. 2008;149(10):4799–4808. doi: 10.1210/en.2008-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Kaplan A.T., Low J., Nguyen L., Liu G.Y., Equils O., Hewison M. Vitamin D induces innate antibacterial responses in human trophoblasts via an intracrine pathway. Biol. Reprod. 2009;80(3):398–406. doi: 10.1095/biolreprod.108.073577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N.Q., Kaplan A.T., Lagishetty V., Ouyang Y.B., Ouyang Y., Simmons C.F., Equils O., Hewison M. Vitamin D and the regulation of placental inflammation. J. Immunol. 2011;186(10):5968–5974. doi: 10.4049/jimmunol.1003332. [DOI] [PubMed] [Google Scholar]
- Maas R.M., Reus K., Diesel B., Steudel W.I., Feiden W., Fischer U., Meese E. Amplification and expression of splice variants of the gene encoding the P450 cytochrome 25-hydroxyvitamin D(3) 1,alpha-hydroxylase (CYP 27B1) in human malignant glioma. Clin. Cancer Res. 2001;7(4):868–875. [PubMed] [Google Scholar]
- Martin K.J., Gonzalez E.A. Vitamin D analogs: actions and role in the treatment of secondary hyperparathyroidism. Semin. Nephrol. 2004;24(5):456–459. doi: 10.1016/j.semnephrol.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Mawer E.B., Hayes M.E., Heys S.E., Davies M., White A., Stewart M.F., Smith G.N. Constitutive synthesis of 1,25-dihydroxyvitamin D3 by a human small cell lung cancer cell line. J. Clin. Endocrinol. Metab. 1994;79(2):554–560. doi: 10.1210/jcem.79.2.8045976. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449(7164):819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- Merendino J.J., Jr., Insogna K.L., Milstone L.M., Broadus A.E., Stewart A.F. A parathyroid hormone-like protein from cultured human keratinocytes. Science. 1986;231(4736):388–390. doi: 10.1126/science.2417317. [DOI] [PubMed] [Google Scholar]
- Meyer M.B., Benkusky N.A., Kaufmann M., Lee S.M., Onal M., Jones G., Pike J.W. A kidney-specific genetic control module in mice governs endocrine regulation of the cytochrome P450 gene Cyp27b1 essential for vitamin D3 activation. J. Biol. Chem. 2017;292(42):17541–17558. doi: 10.1074/jbc.M117.806901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milde P., Hauser U., Simon T., Mall G., Ernst V., Haussler M.R., Frosch P., Rauterberg E.W. Expression of 1,25-dihydroxyvitamin D3 receptors in normal and psoriatic skin. J. Invest. Dermatol. 1991;97(2):230–239. doi: 10.1111/1523-1747.ep12480255. [DOI] [PubMed] [Google Scholar]
- Mitschele T., Diesel B., Friedrich M., Meineke V., Maas R.M., Gartner B.C., Kamradt J., Meese E., Tilgen W., Reichrath J. Analysis of the vitamin D system in basal cell carcinomas (BCCs) Lab. Investig. 2004;84(6):693–702. doi: 10.1038/labinvest.3700096. [DOI] [PubMed] [Google Scholar]
- Muehleisen B., Bikle D.D., Aguilera C., Burton D.W., Sen G.L., Deftos L.J., Gallo R.L. PTH/PTHrP and vitamin D control antimicrobial peptide expression and susceptibility to bacterial skin infection. Sci. Transl. Med. 2012;4(135) doi: 10.1126/scitranslmed.3003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naja R.P., Dardenne O., Arabian A., St Arnaud R. Chondrocyte-specific modulation of Cyp27b1 expression supports a role for local synthesis of 1,25-dihydroxyvitamin D3 in growth plate development. Endocrinology. 2009;150(9):4024–4032. doi: 10.1210/en.2008-1410. [DOI] [PubMed] [Google Scholar]
- Novakovic B., Sibson M., Ng H.K., Manuelpillai U., Rakyan V., Down T., Beck S., Fournier T., Evain-Brion D., Dimitriadis E., Craig J.M., Morley R., Saffery R. Placenta-specific methylation of the vitamin D 24-hydroxylase gene: implications for feedback autoregulation of active vitamin D levels at the fetomaternal interface. J. Biol. Chem. 2009;284(22):14838–14848. doi: 10.1074/jbc.M809542200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer A., Dragun D., Walther D., Vorum H., Jacobsen C., Herz J., Melsen F., Christensen E.I., Willnow T.E. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96(4):507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- Ogunkolade B.W., Boucher B.J., Fairclough P.D., Hitman G.A., Dorudi S., Jenkins P.J., Bustin S.A. Expression of 25-hydroxyvitamin D-1-alpha-hydroxylase mRNA in individuals with colorectal cancer. Lancet. 2002;359(9320):1831–1832. doi: 10.1016/s0140-6736(02)08680-4. [DOI] [PubMed] [Google Scholar]
- Omdahl J.L., Gray R.W., Boyle I.T., Knutson J., DeLuca H.F. Regulation of metabolism of 25-hydroxycholecalciferol by kidney tissue in vitro by dietary calcium. Nat. New Biol. 1972;237(71):63–64. doi: 10.1038/newbio237063a0. [DOI] [PubMed] [Google Scholar]
- Overbergh L., Stoffels K., Waer M., Verstuyf A., Bouillon R., Mathieu C. Immune regulation of 25-hydroxyvitamin D-1alpha-hydroxylase in human monocytic THP1 cells: mechanisms of interferon-gamma-mediated induction. J. Clin. Endocrinol. Metab. 2006;91(9):3566–3574. doi: 10.1210/jc.2006-0678. [DOI] [PubMed] [Google Scholar]
- Panda D.K., Miao D., Tremblay M.L., Sirois J., Farookhi R., Hendy G.N., Goltzman D. Targeted ablation of the 25-hydroxyvitamin D 1alpha -hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc. Natl. Acad. Sci. U. S. A. 2001;98(13):7498–7503. doi: 10.1073/pnas.131029498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda D.K., Al Kawas S., Seldin M.F., Hendy G.N., Goltzman D. 25-Hydroxyvitamin D 1alpha-hydroxylase: structure of the mouse gene, chromosomal assignment, and developmental expression. J. Bone Miner. Res. 2001;16(1):46–56. doi: 10.1359/jbmr.2001.16.1.46. [DOI] [PubMed] [Google Scholar]
- Panda D.K., Miao D., Bolivar I., Li J., Huo R., Hendy G.N., Goltzman D. Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J. Biol. Chem. 2004;279(16):16754–16766. doi: 10.1074/jbc.M310271200. [DOI] [PubMed] [Google Scholar]
- Pedrozo H.A., Boyan B.D., Mazock J., Dean D.D., Gomez R., Schwartz Z. TGFbeta1 regulates 25-hydroxyvitamin D3 1alpha- and 24-hydroxylase activity in cultured growth plate chondrocytes in a maturation-dependent manner. Calcif. Tissue Int. 1999;64(1):50–56. doi: 10.1007/s002239900578. [DOI] [PubMed] [Google Scholar]
- Penna G., Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 2000;164(5):2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- Pillai S., Bikle D.D., Elias P.M. 1,25-Dihydroxyvitamin D production and receptor binding in human keratinocytes varies with differentiation. J. Biol. Chem. 1988;263(11):5390–5395. [PubMed] [Google Scholar]
- Pittas A.G., Lau J., Hu F.B., Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2007;92(6):2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryke A.M., Duggan C., White C.P., Posen S., Mason R.S. Tumor necrosis factor-alpha induces vitamin D-1-hydroxylase activity in normal human alveolar macrophages. J. Cell. Physiol. 1990;142(3):652–656. doi: 10.1002/jcp.1041420327. [DOI] [PubMed] [Google Scholar]
- Radermacher J., Diesel B., Seifert M., Tilgen W., Reichrath J., Fischer U., Meese E. Expression analysis of CYP27B1 in tumor biopsies and cell cultures. Anticancer Res. 2006;26(4A):2683–2686. [PubMed] [Google Scholar]
- Reeve L., Tanaka Y., DeLuca H.F. Studies on the site of 1,25-dihydroxyvitamin D3 synthesis in vivo. J. Biol. Chem. 1983;258(6):3615–3617. [PubMed] [Google Scholar]
- Reichrath J., Rafi L., Rech M., Mitschele T., Meineke V., Gartner B.C., Tilgen W., Holick M.F. Analysis of the vitamin D system in cutaneous squamous cell carcinomas. J. Cutan. Pathol. 2004;31(3):224–231. doi: 10.1111/j.0303-6987.2003.00183.x. [DOI] [PubMed] [Google Scholar]
- Reinke D.C., Kogawa M., Barratt K.R., Morris H.A., Anderson P.H., Atkins G.J. Evidence for altered osteoclastogenesis in splenocyte cultures from Cyp27b1 knockout mice. J. Steroid Biochem. Mol. Biol. 2016;164:353–360. doi: 10.1016/j.jsbmb.2015.11.015. [DOI] [PubMed] [Google Scholar]
- Ren S., Nguyen L., Wu S., Encinas C., Adams J.S., Hewison M. Alternative splicing of vitamin D-24-hydroxylase: a novel mechanism for the regulation of extrarenal 1,25-dihydroxyvitamin D synthesis. J. Biol. Chem. 2005;280(21):20604–20611. doi: 10.1074/jbc.M414522200. [DOI] [PubMed] [Google Scholar]
- Rigby W.F., Stacy T., Fanger M.W. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol) J. Clin. Invest. 1984;74(4):1451–1455. doi: 10.1172/JCI111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter C.S., Armbrecht H.J., Slatopolsky E., Brown A.J. 25-Hydroxyvitamin D(3) suppresses PTH synthesis and secretion by bovine parathyroid cells. Kidney Int. 2006;70(4):654–659. doi: 10.1038/sj.ki.5000394. [DOI] [PubMed] [Google Scholar]
- Ritter C.S., Haughey B.H., Armbrecht H.J., Brown A.J. Distribution and regulation of the 25-hydroxyvitamin D3 1alpha-hydroxylase in human parathyroid glands. J. Steroid Biochem. Mol. Biol. 2012;130(1–2):73–80. doi: 10.1016/j.jsbmb.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Rook G.A., Steele J., Fraher L., Barker S., Karmali R., O'Riordan J., Stanford J. Vitamin D3, gamma interferon, and control of proliferation of mycobacterium tuberculosis by human monocytes. Immunology. 1986;57(1):159–163. [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K., Maeda S., Hikiba Y., Nakagawa H., Hayakawa Y., Shibata W., Yanai A., Ogura K., Omata M. Constitutive NF-kappaB activation in colorectal carcinoma plays a key role in angiogenesis, promoting tumor growth. Clin. Cancer Res. 2009;15(7):2248–2258. doi: 10.1158/1078-0432.CCR-08-1383. [DOI] [PubMed] [Google Scholar]
- Schauber J., Dorschner R.A., Coda A.B., Buchau A.S., Liu P.T., Kiken D., Helfrich Y.R., Kang S., Elalieh H.Z., Steinmeyer A., Zugel U., Bikle D.D., Modlin R.L., Gallo R.L. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J. Clin. Invest. 2007;117(3):803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G.G., Eads D., Rao A., Cramer S.D., Willingham M.C., Chen T.C., Jamieson D.P., Wang L., Burnstein K.L., Holick M.F., Koumenis C. Pancreatic cancer cells express 25-hydroxyvitamin D-1 alpha-hydroxylase and their proliferation is inhibited by the prohormone 25-hydroxyvitamin D3. Carcinogenesis. 2004;25(6):1015–1026. doi: 10.1093/carcin/bgh086. [DOI] [PubMed] [Google Scholar]
- Segersten U., Correa P., Hewison M., Hellman P., Dralle H., Carling T., Akerstrom G., Westin G. 25-Hydroxyvitamin D(3)-1alpha-hydroxylase expression in normal and pathological parathyroid glands. J. Clin. Endocrinol. Metab. 2002;87(6):2967–2972. doi: 10.1210/jcem.87.6.8604. [DOI] [PubMed] [Google Scholar]
- Segersten U., Holm P.K., Bjorklund P., Hessman O., Nordgren H., Binderup L., Akerstrom G., Hellman P., Westin G. 25-Hydroxyvitamin D3 1alpha-hydroxylase expression in breast cancer and use of non-1alpha-hydroxylated vitamin D analogue. Breast Cancer Res. 2005;7(6):R980–6. doi: 10.1186/bcr1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert M., Tilgen W., Reichrath J. Expression of 25-hydroxyvitamin D-1alpha-hydroxylase (1alphaOHase, CYP27B1) splice variants in HaCaT keratinocytes and other skin cells: modulation by culture conditions and UV-B treatment in vitro. Anticancer Res. 2009;29(9):3659–3667. [PubMed] [Google Scholar]
- Shi H., Yan P.S., Chen C.M., Rahmatpanah F., Lofton-Day C., Caldwell C.W., Huang T.H. Expressed CpG island sequence tag microarray for dual screening of DNA hypermethylation and gene silencing in cancer cells. Cancer Res. 2002;62(11):3214–3220. [PubMed] [Google Scholar]
- Shinki T., Shimada H., Wakino S., Anazawa H., Hayashi M., Saruta T., DeLuca H.F., Suda T. Cloning and expression of rat 25-hydroxyvitamin D3-1alpha-hydroxylase cDNA. Proc. Natl. Acad. Sci. U. S. A. 1997;94(24):12920–12925. doi: 10.1073/pnas.94.24.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz T.D., Fox J., Heath H., 3rd, Kumar R. Do tissues other than the kidney produce 1,25-dihydroxyvitamin D3 in vivo? A reexamination. Proc. Natl. Acad. Sci. U. S. A. 1983;80(6):1746–1750. doi: 10.1073/pnas.80.6.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmundsdottir H., Pan J., Debes G.F., Alt C., Habtezion A., Soler D., Butcher E.C. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat. Immunol. 2007;8(3):285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- Silver J., Naveh-Many T. FGF23 and the parathyroid. Adv. Exp. Med. Biol. 2012;728:92–99. doi: 10.1007/978-1-4614-0887-1_6. [DOI] [PubMed] [Google Scholar]
- Somjen D., Katzburg S., Stern N., Kohen F., Sharon O., Limor R., Jaccard N., Hendel D., Weisman Y. 25 Hydroxy-vitamin D(3)-1alpha hydroxylase expression and activity in cultured human osteoblasts and their modulation by parathyroid hormone, estrogenic compounds and dihydrotestosterone. J. Steroid Biochem. Mol. Biol. 2007;107(3–5):238–244. doi: 10.1016/j.jsbmb.2007.03.048. [DOI] [PubMed] [Google Scholar]
- St-Arnaud R., Messerlian S., Moir J.M., Omdahl J.L., Glorieux F.H. The 25-hydroxyvitamin D 1-alpha-hydroxylase gene maps to the pseudovitamin D-deficiency rickets (PDDR) disease locus. J. Bone Miner. Res. 1997;12(10):1552–1559. doi: 10.1359/jbmr.1997.12.10.1552. [DOI] [PubMed] [Google Scholar]
- St-Arnaud R., Dardenne O., Prud'homme J., Hacking S.A., Glorieux F.H. Conventional and tissue-specific inactivation of the 25-hydroxyvitamin D-1alpha-hydroxylase (CYP27B1) J. Cell. Biochem. 2003;88(2):245–251. doi: 10.1002/jcb.10348. [DOI] [PubMed] [Google Scholar]
- Stoffels K., Overbergh L., Giulietti A., Verlinden L., Bouillon R., Mathieu C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J. Bone Miner. Res. 2006;21(1):37–47. doi: 10.1359/JBMR.050908. [DOI] [PubMed] [Google Scholar]
- Strom T.M., Juppner H. PHEX, FGF23, DMP1 and beyond. Curr. Opin. Nephrol. Hypertens. 2008;17(4):357–362. doi: 10.1097/MNH.0b013e3282fd6e5b. [DOI] [PubMed] [Google Scholar]
- Stumpf W.E., Clark S.A., Sar M., DeLuca H.F. Topographical and developmental studies on target sites of 1,25 (OH)2 vitamin D3 in skin. Cell Tissue Res. 1984;238(3):489–496. doi: 10.1007/BF00219863. [DOI] [PubMed] [Google Scholar]
- Sultan B., Ramanathan M., Jr., Lee J., May L., Lane A.P. Sinonasal epithelial cells synthesize active vitamin D, augmenting host innate immune function. Int. Forum Allergy Rhinol. 2013;3(1):26–30. doi: 10.1002/alr.21087. [DOI] [PubMed] [Google Scholar]
- Sumantran V.N., Mishra P., Bera R., Sudhakar N. Microarray analysis of differentially-expressed genes encoding CYP450 and phase II drug metabolizing enzymes in psoriasis and melanoma. Pharmaceutics. 2016;8(1) doi: 10.3390/pharmaceutics8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swami S., Krishnan A.V., Wang J.Y., Jensen K., Peng L., Albertelli M.A., Feldman D. Inhibitory effects of calcitriol on the growth of MCF-7 breast cancer xenografts in nude mice: selective modulation of aromatase expression in vivo. Horm Cancer. 2011;2(3):190–202. doi: 10.1007/s12672-011-0073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Okano T., Sekimoto H., Kobayashi T. The enzymatic formation of 1 alpha,25-dihydroxyvitamin D3 from 25-hydroxyvitamin D3 in the liver of fetal rats. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1994;109(1):1–7. doi: 10.1016/0742-8413(94)00046-d. [DOI] [PubMed] [Google Scholar]
- Takeyama K., Kitanaka S., Sato T., Kobori M., Yanagisawa J., Kato S. 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis. Science. 1997;277(5333):1827–1830. doi: 10.1126/science.277.5333.1827. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Deluca H.F. The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch. Biochem. Biophys. 1973;154(2):566–574. doi: 10.1016/0003-9861(73)90010-6. [DOI] [PubMed] [Google Scholar]
- Tangpricha V., Flanagan J.N., Whitlatch L.W., Tseng C.C., Chen T.C., Holt P.R., Lipkin M.S., Holick M.F. 25-Hydroxyvitamin D-1alpha-hydroxylase in normal and malignant colon tissue. Lancet. 2001;357(9269):1673–1674. doi: 10.1016/S0140-6736(00)04831-5. [DOI] [PubMed] [Google Scholar]
- Teles R.M., Graeber T.G., Krutzik S.R., Montoya D., Schenk M., Lee D.J., Komisopoulou E., Kelly-Scumpia K., Chun R., Iyer S.S., Sarno E.N., Rea T.H., Hewison M., Adams J.S., Popper S.J., Relman D.A., Stenger S., Bloom B.R., Cheng G., Modlin R.L. Type I interferon suppresses type II interferon-triggered human anti-mycobacterial responses. Science. 2013;339(6126):1448–1453. doi: 10.1126/science.1233665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend K., Banwell C.M., Guy M., Colston K.W., Mansi J.L., Stewart P.M., Campbell M.J., Hewison M. Autocrine metabolism of vitamin D in normal and malignant breast tissue. Clin. Cancer Res. 2005;11(9):3579–3586. doi: 10.1158/1078-0432.CCR-04-2359. [DOI] [PubMed] [Google Scholar]
- Turner R.T., Puzas J.E., Forte M.D., Lester G.E., Gray T.K., Howard G.A., Baylink D.J. In vitro synthesis of 1 alpha,25-dihydroxycholecalciferol and 24,25-dihydroxycholecalciferol by isolated calvarial cells. Proc. Natl. Acad. Sci. U. S. A. 1980;77(10):5720–5724. doi: 10.1073/pnas.77.10.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M., Barre P.E., Benjamin A., Goltzman D., Gascon-Barre M. Does the maternal kidney contribute to the increased circulating 1,25-dihydroxyvitamin D concentrations during pregnancy? Miner. Electrolyte Metab. 1988;14(4):246–252. [PubMed] [Google Scholar]
- Turner A.G., Dwivedi P.P., May B.K., Morris H.A. Regulation of the CYP27B1 5′-flanking region by transforming growth factor-beta in ROS 17/2.8 osteoblast-like cells. J. Steroid Biochem. Mol. Biol. 2007;103(3–5):322–325. doi: 10.1016/j.jsbmb.2006.12.041. [DOI] [PubMed] [Google Scholar]
- Turner A.G., Hanrath M.A., Morris H.A., Atkins G.J., Anderson P.H. The local production of 1,25(OH)2D3 promotes osteoblast and osteocyte maturation. J. Steroid Biochem. Mol. Biol. 2014;144(Pt A):114–118. doi: 10.1016/j.jsbmb.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Ustianowski A., Shaffer R., Collin S., Wilkinson R.J., Davidson R.N. Prevalence and associations of vitamin D deficiency in foreign-born persons with tuberculosis in London. J. Inf. Secur. 2005;50(5):432–437. doi: 10.1016/j.jinf.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Vantieghem K., Overbergh L., Carmeliet G., De Haes P., Bouillon R., Segaert S. UVB-induced 1,25(OH)2D3 production and vitamin D activity in intestinal CaCo-2 cells and in THP-1 macrophages pretreated with a sterol Delta7-reductase inhibitor. J. Cell. Biochem. 2006;99(1):229–240. doi: 10.1002/jcb.20910. [DOI] [PubMed] [Google Scholar]
- Vidya M.K., Kumar V.G., Sejian V., Bagath M., Krishnan G., Bhatta R. Toll-like receptors: significance, ligands, signaling pathways, and functions in mammals. Int. Rev. Immunol. 2017:1–17. doi: 10.1080/08830185.2017.1380200. [DOI] [PubMed] [Google Scholar]
- Vieth R., Ladak Y., Walfish P.G. Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J. Clin. Endocrinol. Metab. 2003;88(1):185–191. doi: 10.1210/jc.2002-021064. [DOI] [PubMed] [Google Scholar]
- Vigano P., Lattuada D., Mangioni S., Ermellino L., Vignali M., Caporizzo E., Panina-Bordignon P., Besozzi M., Di Blasio A.M. Cycling and early pregnant endometrium as a site of regulated expression of the vitamin D system. J. Mol. Endocrinol. 2006;36(3):415–424. doi: 10.1677/jme.1.01946. [DOI] [PubMed] [Google Scholar]
- Wang T.T., Nestel F.P., Bourdeau V., Nagai Y., Wang Q., Liao J., Tavera-Mendoza L., Lin R., Hanrahan J.W., Mader S., White J.H. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004;173(5):2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- Weisman Y., Harell A., Edelstein S., David M., Spirer Z., Golander A. 1 alpha, 25-Dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 in vitro synthesis by human decidua and placenta. Nature. 1979;281(5729):317–319. doi: 10.1038/281317a0. [DOI] [PubMed] [Google Scholar]
- Wieland P., Fischer J.A., Trechsel U., Roth H.R., Vetter K., Schneider H., Huch A. Perinatal parathyroid hormone, vitamin D metabolites, and calcitonin in man. Am. J. Phys. 1980;239(5):E385–90. doi: 10.1152/ajpendo.1980.239.5.E385. [DOI] [PubMed] [Google Scholar]
- Wu S., Ren S., Nguyen L., Adams J.S., Hewison M. Splice variants of the CYP27b1 gene and the regulation of 1,25-dihydroxyvitamin D3 production. Endocrinology. 2007;148(7):3410–3418. doi: 10.1210/en.2006-1388. [DOI] [PubMed] [Google Scholar]
- Xie Z., Munson S.J., Huang N., Portale A.A., Miller W.L., Bikle D.D. The mechanism of 1,25-dihydroxyvitamin D(3) autoregulation in keratinocytes. J. Biol. Chem. 2002;277(40):36987–36990. doi: 10.1074/jbc.M201404200. [DOI] [PubMed] [Google Scholar]
- Yokomura K., Suda T., Sasaki S., Inui N., Chida K., Nakamura H. Increased expression of the 25-hydroxyvitamin D(3)-1alpha-hydroxylase gene in alveolar macrophages of patients with lung cancer. J. Clin. Endocrinol. Metab. 2003;88(12):5704–5709. doi: 10.1210/jc.2003-030537. [DOI] [PubMed] [Google Scholar]
- Zehnder D., Bland R., Williams M.C., McNinch R.W., Howie A.J., Stewart P.M., Hewison M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J. Clin. Endocrinol. Metab. 2001;86(2):888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- Zehnder D., Bland R., Williams M.C., McNinch R.W., Howie A.J., Stewart P.M., Hewison M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J. Clin. Endocrinol. Metab. 2001;86(2):888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- Zehnder D., Evans K.N., Kilby M.D., Bulmer J.N., Innes B.A., Stewart P.M., Hewison M. The ontogeny of 25-hydroxyvitamin D(3) 1alpha-hydroxylase expression in human placenta and decidua. Am. J. Pathol. 2002;161(1):105–114. doi: 10.1016/s0002-9440(10)64162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder D., Bland R., Chana R.S., Wheeler D.C., Howie A.J., Williams M.C., Stewart P.M., Hewison M. Synthesis of 1,25-dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J. Am. Soc. Nephrol. 2002;13(3):621–629. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- Zhou C., Lu F., Cao K., Xu D., Goltzman D., Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int. 2008;74(2):170–179. doi: 10.1038/ki.2008.101. [DOI] [PubMed] [Google Scholar]
- Zhou S., LeBoff M.S., Glowacki J. Vitamin D metabolism and action in human bone marrow stromal cells. Endocrinology. 2010;151(1):14–22. doi: 10.1210/en.2009-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Geng S., Glowacki J. Histone deacetylation mediates the rejuvenation of osteoblastogenesis by the combination of 25(OH)D3 and parathyroid hormone in MSCs from elders. J. Steroid Biochem. Mol. Biol. 2013;136:156–159. doi: 10.1016/j.jsbmb.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.