Abstract
Decreased motivation to seek rewards is a key feature of mood disorders that correlates with severity and treatment outcome. This anhedonia, or apathy, likely reflects impairment in reward circuitry, but the specific neuronal populations controlling motivation are unclear. Granule neurons generated in the adult hippocampus have been implicated in mood disorders, but are not generally considered as part of reward circuits. We investigated a possible role of these new neurons in motivation to work for food and sucrose rewards in operant conditioning tasks using GFAP-TK pharmacogenetic ablation of adult neurogenesis in both rats and mice. Rats and mice lacking adult neurogenesis showed normal lever press responding during fixed ratio training, reward devaluation, and Pavlovian Instrumental Transfer, suggesting no impairment in learning. However, on an exponentially progressive ratio schedule, or when regular chow was freely available in the testing chamber, TK rats and mice showed less effort to gain sucrose tablets. When working for balanced food tablets, which rats and mice of both genotypes strongly preferred over sucrose, the genotype effects on behavior were lost. This decrease in effort under conditions of low reward suggests that loss of adult neurogenesis decreases motivation to seek reward in a manner that may model behavioral apathy.
Keywords: granule cells, hippocampus, anhedonia, apathy, operant conditioning
INTRODUCTION
Anhedonia, or loss of interest or pleasure in previously favored activities, is a feature of many neuropsychiatric disorders including major depression. Roughly 40% of patients diagnosed with major depressive disorder exhibit clinically significant anhedonia (Pelizza and Ferrari, 2009), and this symptom is associated with more severe depression and poorer treatment outcome with standard antidepressants (Spijker et al., 2001; Uher et al., 2012). Anhedonia is generally defined broadly to encompass decreases in the amount of effort one is willing to expend to receive a reward as well as true hedonic deficits, i.e., decreased pleasure from rewards. However, several studies have suggested that anhedonia in depression is closely associated with decreased motivation to exert considerable effort to obtain a reward without a concomitant reduction in the subjective pleasure reported in response to rewarding stimuli (Dichter et al., 2010; Sherdell et al., 2012). This motivational aspect of anhedonia has also been called apathy or decisional anhedonia (Treadway and Zald, 2013; Bonnelle et al., 2015). The stronger effects of depression on motivational rather than hedonic processes may reflect differences in timing for these two processes, with intact hedonic response to currently available reward but impairment in memory for previously experienced reward and/or prediction of future reward, so-called affective forecasting (Treadway and Zald, 2013).
The key neural circuits responsible for specific motivational processes are not fully understood. The mesolimbic dopamine circuits, centered on the ventral tegmental area (VTA) projections to the nucleus accumbens (NAc), play a central role in reward meditated behaviors, with decreased NAc activity observed in depressed patients thought to reflect anhedonia (Russo and Nestler, 2013). However, the prefrontal cortex, amygdala, and hippocampus also receive dopaminergic inputs, and all of these regions have been implicated in depressive illness (Russo and Nestler, 2013; McNamara and Dupret, 2017). Although the hippocampus is not always considered to be a key part of the reward circuit, it receives dopaminergic signals from both the VTA and the locus coeruleus, and it sends projections to the NAc both directly and indirectly, via the medial prefrontal cortex (O’Donnell and Grace, 1995; McNamara and Dupret, 2017). Intriguingly, hippocampal functions in memory and prediction are consistent with the time traveling aspects of motivational impairment described above (Buckner, 2010), and recent work has shown that hippocampal activity is sensitive to reward context (Ambrose et al., 2016). Taken together, these findings suggest a possible role of the hippocampus in effort and reward-mediated behavior.
The granule neurons of the hippocampus continue to be generated throughout life in rodents and humans (Abrous et al., 2005; Snyder and Cameron, 2012; Spalding et al., 2013). Many studies have investigated the role of ongoing hippocampal neurogenesis in stressful, aversively motivated tasks, but few have examined the effects of inhibiting adult neurogenesis on reward-mediated behavior. Production of new neurons is sensitive to stress and, conversely, behavioral response to stress is affected by adult neurogenesis (Snyder et al., 2011; Lee et al., 2013; Tanti and Belzung, 2013; Schoenfeld and Cameron, 2015), suggesting an important function for new neurons in aversive situations. Intriguingly, decreased sucrose preference, which is commonly observed in chronic stress models (Willner, 1997), is seen in naïve, unstressed rats and mice following ablation of adult neurogenesis (Snyder et al., 2011; 2016), suggesting that motivational changes may be separable from stress response effects. The aim of the current study is to characterize the function of ongoing neurogenesis in reward-mediated behavior using operant conditioning tests of instrumental learning, reward palatability, and willingness to expend effort to obtain rewards in the absence of prior stress.
METHODS AND MATERIALS
Animals and General Procedures
Transgenic rats and mice expressing herpes-simplex virus-thymidine kinase under the GFAP promoter were generated as previously described (Snyder et al., 2011; 2016). Each experiment was performed on a different, naïve cohort of animals (8 cohorts of rats, 5 cohorts of mice). All animal procedures were performed in accordance with the Institute of Laboratory Animal Research guidelines and were approved by the Animal Care and Use Committee of the National Institute of Mental Health.
Heterozygous GFAP-TK rats were bred in-house and maintained on a Long-Evans background. Male pups were weaned into cages with siblings (2-6/cage) prior to genotyping by PCR, so the mixture of wild-type (WT) and heterozygous transgenic (TK) littermates in each cage was random. All rats were housed under a reversed light cycle (lights off at 9:00 a.m.) and meal fed 15-16g/rat/day of standard laboratory chow from weaning through the entire study. Beginning at 8 weeks of age (Figure 1A), and continuing throughout the study, WT and TK rats received 3.8 mg valganciclovir 2×/week, delivered in a 0.5g pellet of a 1:1 mixture of powdered chow and peanut butter. One cohort of rats was given chow/peanut butter mixture without valganciclovir to test for non-specific effects of the transgene (Figure 1G). Behavioral testing began 7 weeks after the start of valganciclovir treatment, unless otherwise indicated, and began at 10:00 am each day.
Figure 1.
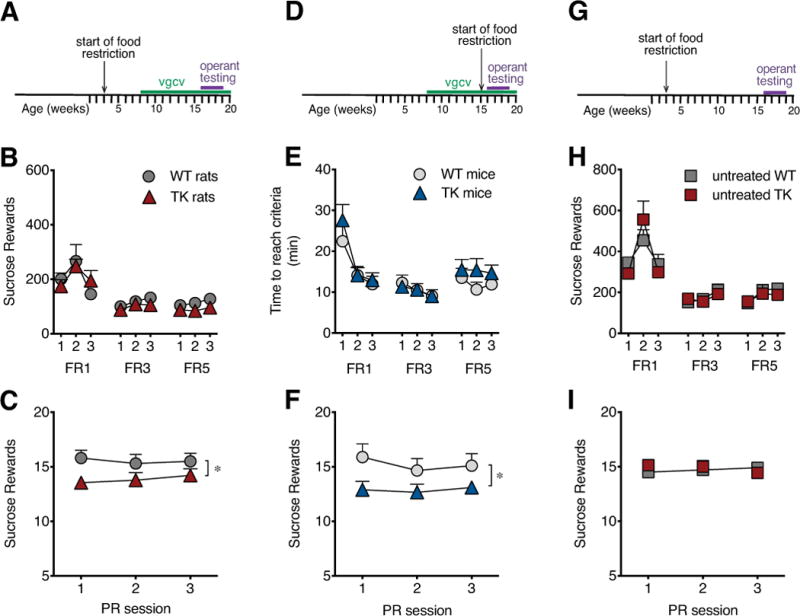
Sucrose reward operant training and progressive ratio testing in GFAP-TK rats and mice with and without valganciclovir treatment. Experimental timeline for rats showing valganciclovir (vgcv) treatment and testing (A). Rats lacking adult neurogenesis (TK rats) showed no difference relative to their wild type (WT) counterparts in the number of sucrose rewards earned during training on an increasing fixed ratio (FR) schedule (B), but they showed a significantly lower breakpoint for sucrose rewards under a progressive ratio (C). Experimental timeline showing vgcv treatment and testing in mice (D). TK mice showed no difference from WT mice in the time to earn 50 rewards under an increasing FR schedule (E) but, like rats, showed a lower breakpoint under a progressive ratio (F). Experimental timeline in a control experiment without vgcv (G). Untreated WT and TK rats showed normal learning on FR schedule (H) and a normal breakpoint (I). n=7-10/genotype. * P<0.05 main effect of genotype. Data are means ±SEM.
Heterozygous GFAP-TK mice generated on a C57Bl/6 background were backcrossed to a CD1 background for nine generations. Male pups were weaned into cages with siblings (3-4/cage) prior to genotyping by PCR, so the mixture of wild-type (WT) and heterozygous transgenic (TK) littermates in each cage was random. All mice were housed under a reversed light cycle (lights off at 8:00 a.m.). Beginning at 8 weeks of age (Figure 1D), and continuing throughout the experiment, mice of both genotypes were given valganciclovir mixed in powdered chow (227 mg/kg chow) 4 days of the week, alternating with standard pellet chow for 3 days. Mice were food restricted to reach 85-90% of their free feeding weight beginning six weeks after the start of VGCV treatment. After one week of food restriction, operant training began at 10:00 am each day.
Apparatus
Testing was done in species-specific operant chambers (Med Associates, St. Albans, VT, USA) housed within sound attenuating enclosures. Each chamber was equipped with two retractable levers with a reward magazine located in between the levers. The onset of the fan signaled the start of a training session. Active lever responses activated the pellet dispenser, which delivered one 14mg (for mice) or two 20mg (for rats) chocolate flavored sucrose reward pellets (Cat# 5TUT, TestDiet, MD, USA) for sucrose reward testing or 1×14mg (mouse)/2×20mg (rat) Dustless Precision Pellets (Cat# F05684 and F0071, BioServ, NJ, USA) for food reward testing. The operant chambers had 2 pellet dispensers installed in each chamber to enable training for both rewards simultaneously. Inactive lever presses were recorded but had no consequences. K-Limbic software (Conclusive, UK) or Med-PC IV (Med Associates, VT, USA) controlled and recorded lever presses and reward deliveries.
Progressive ratio testing
Training began at 15 weeks of age with a single 15 min session with 30 pellets made available in the magazine. The animals then underwent training on fixed ratios (FR) before advancing to progressive ratio (PR) testing to assess motivation. Mice were first trained to lever press, with active and inactive levers available, for rewards on an FR1 schedule with endpoint of 50 rewards or 50 min until they earned all of the rewards within the allotted time for 3 consecutive days. Mice then moved on to 3 sessions each of FR3 and FR5. Since rats are able to consume greater quantities of reward, they were trained on FR1 with unlimited rewards for 60 min, until they had reached a criterion of 100 lever presses for 3 consecutive days. This was followed by 3 daily 30 min sessions of FR3 and 3 of FR5. Following this training, rats and mice responded under a PR schedule where the reinforcement was contingent on the following ratio progression: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 603, etc. (Richardson and Roberts, 1996; Noonan et al., 2010) Breakpoints were defined as the number of ratios completed (i.e. number of rewards delivered), allowing up to 20 min to earn each reinforcement. PR was tested for 5 consecutive days; the first 2 were considered as habituation sessions, and the last 3 sessions were analyzed. Separate cohorts of animals were used to test motivation for sucrose or food rewards. Five rats (3/13 WT and 2/11 TK) were excluded from the sucrose study for failing to meet the FR1 criteria within 10 training sessions; no rats were excluded from the food reward experiment, and no mice were excluded from either experiment. A cohort of untreated WT and TK rats was tested for motivation to work for sucrose in order to determine whether there was a gene insertion effect on this behavior. In this cohort, 2/12WT and 3/10TK rats were excluded for not meeting the learning criteria described above.
Effort testing
Effort-related choice behavior was tested in a cohort of rats that had been trained in a pilot study for outcome specific Pavlovian Instrumental Transfer (osPIT) task (see below). After the osPIT test, at 24 weeks of age, rats were retrained on the Random Ratio (RR) 20 schedule (responses were reinforced on average every 20 lever presses, ranging from 2-38) and then tested in a concurrent RR/choice procedure that has been used previously to assess effort in instrumental responding (Salamone et al., 1991; 2007; Trifilieff et al., 2013). This consisted of having 16g of standard laboratory chow freely available in the operant chamber while the rat worked on an RR20 schedule for either sucrose or food pellets for 30 min. Rats were then retrained on RR20 in the absence of freely available laboratory chow for two days, followed the next day by a test for the other reward.
Reward preference testing
This experiment was conducted to determine whether the rats and mice had an intrinsic preference for either of the rewards used in previous studies. Naïve rats and mice were first trained to associate each lever (left or right) with a different reward (sucrose or food) on a FR1 schedule during 2 separate training sessions per day, each with an endpoint of 50 (mice) or 100 (rats) rewards or 30 min. After initial acquisition, the animals received 6 daily 30-min (mice) or 60-min (rat) sessions in which they had access to both levers and were able to press to deliver both rewards. The first 4 sessions were treated as habituation/stabilization sessions, and the last 2 sessions were analyzed and averaged.
Outcome devaluation
Outcome devaluation for sucrose was conducted in the same way for mice and rats. Mice started testing at the age of 14 weeks (6 weeks after start of valganciclovir treatment) and rats at 16 weeks old. Testing began with a single 15 min session with 30 pellets made available in the magazine. The animals were then trained for 3-4 sessions on FR1, 3 sessions on RR5, 3 sessions on RR10 and 2 sessions on RR20. On the following day, each animal was placed in a separate holding cage with either an empty glass jar (valued reward condition) or a glass jar containing 50 (mice) or 100 (rats) sucrose pellets (devalued reward condition) for a 1-hour period, and pellet consumption was recorded. Immediately following this devaluation treatment, the animals were given a 5-min devaluation test session during which responses were not reinforced with reward delivery. Because a trend toward a difference between WT and TK was observed in RR20 training before testing, we then continued RR20 training for an additional 8 sessions.
Outcome Specific Pavlovian Instrumental Transfer testing (osPIT)
Rats began training 4 weeks after the start of valganciclovir treatment (12 weeks of age), because a pilot study in valganciclovir-treated WT and TK rats started at 16 weeks old showed an unexpectedly high lever pressing rate in both genotypes. Rats were given an 80-min Pavlovian training session each day for 8 days. Each session consisted of 8 tone (2.9 kHz, 75 db) presentations and 8 white noise (75 db) presentations, each two min long. Each stimulus presentation was simultaneously paired with a reward delivery (two 20-mg sucrose pellets or two 20-mg food pellets) on a 30-s random interval. The order of stimulus-reward type presentations was pseudorandom with no more than two successive presentations of the same type. Each stimulus-reward pairing was preceded by a variable inter-trial interval of 2-4 min (average 3 min). Each stimulus-reward pairing was counterbalanced across genotype.
Next, rats were trained to respond on each of the 2 levers to earn the respective reward under a RR schedule. No Pavlovian conditioned stimuli were presented during this instrumental training. During each of two daily sessions, separated by 60 min, only one lever was available and was reinforced with one of the two outcomes received in Pavlovian training (sucrose pellets or food pellets). Each session ended when 30 rewards had been earned or when 30 min had elapsed. On day 1, each lever press was reinforced on an FR1 schedule, followed by two days each of RR2, RR5, and RR10, and four days of RR20.
The preparation for the test and the test itself required five consecutive days. Rats received RR20 instrumental retraining sessions on days 1-2 and a Pavlovian retraining session on day 3. On day 4, rats were given one 30-min extinction session in which both levers were available but were inactive (not reinforced), in order to decrease the responding rate. On day 5, rats were given one session of osPIT testing in which both levers were available but not reinforced. No Pavlovian conditioned stimuli were presented in the first 10 min to re-establish a low level of responding. After this baseline period, each Pavlovian conditioned stimulus was presented 4 times, each for 2 min, with 4-min inter-trial intervals, the last 2 min of which served as the pre-stimulus period for data analysis. osPIT performance was calculated as the rate of lever responses associated with the outcome predicted by the CS (Same) minus baseline responses (PreCS period), and the rate of responses made on the opposite lever, associated with the other CS (Different), minus baseline responding.
Reversal Learning
Rats and mice were trained to lever press on an FR1 schedule in daily sessions with endpoint of 30 rewards or 30 min. When they collected all of the rewards in the allotted time for 3 consecutive days, animals progressed to RR2 sessions lasting 25 min, during which they could earn unlimited rewards. After 3 training days for rats and 5 training days for mice, levers were reversed such that the reinforced lever became non-reinforced and vice versa. Rats and mice were given daily 15-minute reversal learning sessions for 5 days. The number of responses on each lever and the number of rewards earned in each session were collected and analyzed. A total of 2/11 WT and 2/14 TK mice were excluded from the study as they did not meet the endpoint of 30 rewards during the training session; no rats were excluded in this experiment.
Histology
The WT/TK rats that did not receive valganciclovir were transcardially perfused with 4% paraformaldehyde in phosphate buffered saline solution (pH 7.4). Brains were post-fixed in the same fixative for 24 hrs then cryoprotected in 20% sucrose. Brains were coronally sectioned (40 μm) on a sliding microtome, and 1:12 series of sections through the entire hippocampal dentate gyrus were immunostained for doublecortin (DCX) as follows: Free-floating sections were blocked in Normal Donkey Serum + Tween-20 solution and incubated with goat anti-DCX antibody (1:200; Santa Cruz Biotechnology, TX, USA) at 4°C for 4 days, followed by incubation in biotinylated donkey anti-goat IgG (1:200; Jackson Immunolabs), avidin-biotin-horseradish peroxidase ABC kit (Vector Laboratories, CA, USA), and cobalt-enhanced DAB (Sigma Fast tablets). Slides were counterstained with cresyl violet and coverslipped under Permount (Fisher Scientific). DCX+ cells in the hippocampal granule cell layer were counted using a 60× objective throughout the entire rostral-caudal extent of the hippocampus.
For all other experiments, which used animals treated with valganciclovir, several hippocampal sections from each postfixed brain were immunostained for DCX as above, but with fluorescent detection, and inspected for the presence or absence of DCX to verify genotype and treatment effectiveness. Blinded examination of DCX staining confirmed virtually complete loss of young neurons throughout the hippocampus in all valganciclovir-treated mice and rats, as expected based on previous work from this lab in these transgenic lines (Snyder et al., 2011; 2016; Soumier et al., 2016).
Analysis
Data was analyzed using STATISTICA 9.0 (Dell Software, Aliso Viejo, CA). All comparisons were run as two-way or three-way ANOVA with between-subject and within subject factors, as appropriate. All post hoc comparisons were made using Tukey’s honest significant difference test to correct for multiple comparisons.
RESULTS
Motivation testing for sucrose rewards
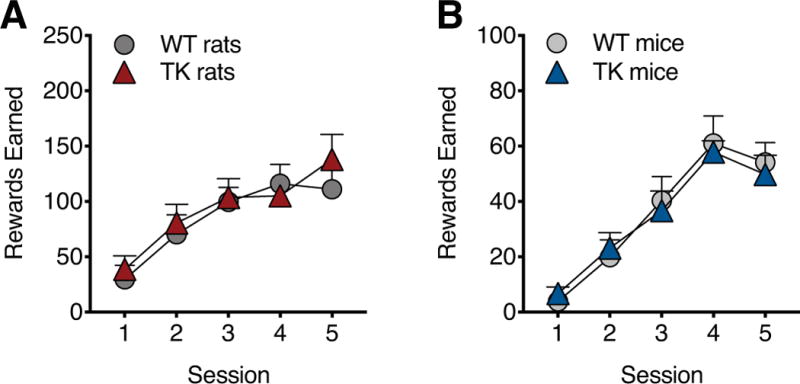
To assess motivation to work for sucrose rewards, WT and TK rats and mice were tested on FR1, FR3, and FR5 schedules followed by a PR schedule. WT and TK rats showed no difference in number of sessions to reach the FR1 learning criterion (WT: 4.1 ±0.3 sessions, TK: 5.1 ±0.4, P=0.18) or active lever pressing during the fixed ratio (FR) training for sucrose rewards (Figure 1B). Both groups showed similarly low levels of inactive lever pressing during the FR training (WT: 6.26 ±1.06 lever presses, TK: 5.16 ±0.96, P=0.46). However, TK rats showed a significantly lower breakpoint than WT controls during PR testing with sucrose rewards (F1,17=7.48, P<0.05, Figure 1C).
Mice also showed normal learning during the FR training for sucrose, with no significant difference in reaching the learning criterion (WT: 3.7 ±0.3 sessions, TK: 4.5 ±0.3, P=0.61), sucrose rewards earned (Figure 1E) or inactive lever presses (WT: 6.69 ±0.97 lever presses, TK: 5.21 ±1.03, P=0.31) between WT and TK animals. However, TK mice, like TK rats (above), showed reduced responding relative to WT controls on the PR schedule with sucrose rewards (effect of genotype: F1,16=7.75, P<0.05, Figure 1F).
Untreated WT and TK rats were not significantly different in either FR (Figure 1H) or PR testing (Figure 1I). DCX staining showed no difference between untreated WT and TK rats in the number of labeled immature neurons in the dentate gyrus (WT: 15033 ±1373, TK: 14072 ±1463, P=0.64), together indicating that gene insertion had no effect on neurogenesis or PR behavior in the absence of antiviral drug treatment (loss of neurogenesis).
Motivation testing for food rewards
When trained for food rewards, TK rats showed normal acquisition of lever press behavior on a fixed ratio schedule (no genotype x session interaction) but earned fewer rewards (main effect of genotype: F1,22=8.00, P<0.01; ratio: F2,44=92.69, P<0.001; session: F2,44=18.52, P<0.001, ratio x session: F4,88=15.08, P<0.001; no significant genotype interactions; Figure 2A). WT and TK rats showed no significant difference in reaching the learning criteria (WT: 4.6 ±0.5 sessions, TK: 4.4 ±0.4, P=0.67) or in inactive lever presses during FR training (WT: 6.70 ±1.25 lever presses, TK: 6.61 ±0.89, P=0.96). In the PR test, TK rats were not significantly different from WT rats (Figure 2B), in contrast to the findings from sucrose reward testing.
Figure 2.
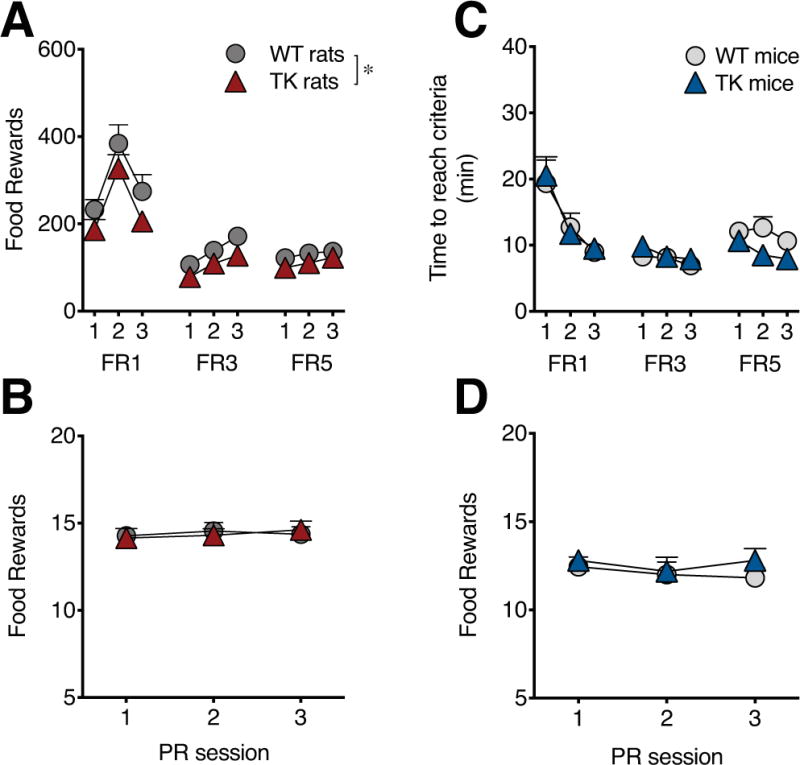
Food reward operant training and progressive ratio testing in rats and mice lacking adult hippocampal neurogenesis. There was a significant decrease in food reward intake during the fixed ratio (FR) training in TK rats relative to WT rats (A) but no difference in breakpoint during progressive ratio testing (B). TK mice showed no difference in time to earn 50 rewards during FR training (C) or in breakpoint during progressive ratio testing (D). n=10-13/genotype. * P<0.01. Data are means ±SEM.
TK mice showed normal learning (Figure 2C; WT: 4.6 ±0.3 sessions, TK: 5.3 ±0.7, P=0.39) and inactive lever pressing (WT: 3.52 ±0.65 lever presses, TK: 3.76 ±0.73, P=0.81) during FR1 training. They also showed normal motivation to work for food rewards in PR testing (Figure 2D), consistent with the PR data from rats but, again, contrasting with the results observed using sucrose as a reward.
Effort and preference testing
To determine whether decreased motivation to work for sucrose but not food rewards could be replicated in a different test, we asked whether WT and TK rats differ in the amount of effort they expend for rewards when laboratory chow is freely available in the chamber. Rats were first trained and tested on a RR20 schedule and showed no significant genotype difference in lever press rate for sucrose (WT: 67.86 ±4.01 lever presses/min, TK: 61.21 ±3.47, P=0.23) or food (WT: 68.41 ±5.15 lever presses/min, TK: 67.39 ±4.51, P=0.89). During the effort/choice testing, there was a significant effect of reward type on the number of rewards earned (F1,16=21.76, P<0.001) and a trend for genotype x reward interaction (F1,16=3.54, P=0.078, Figure 3A). Post-hoc testing indicated that TK rats earned fewer sucrose rewards compared to WT controls (Figure 3A). There was a significant difference in the amount of regular laboratory chow that was consumed depending on the reward type (F1,16=35.83, P<0.001) but no main effect of genotype or genotype x reward type interaction (Figure 3B).
Figure 3.
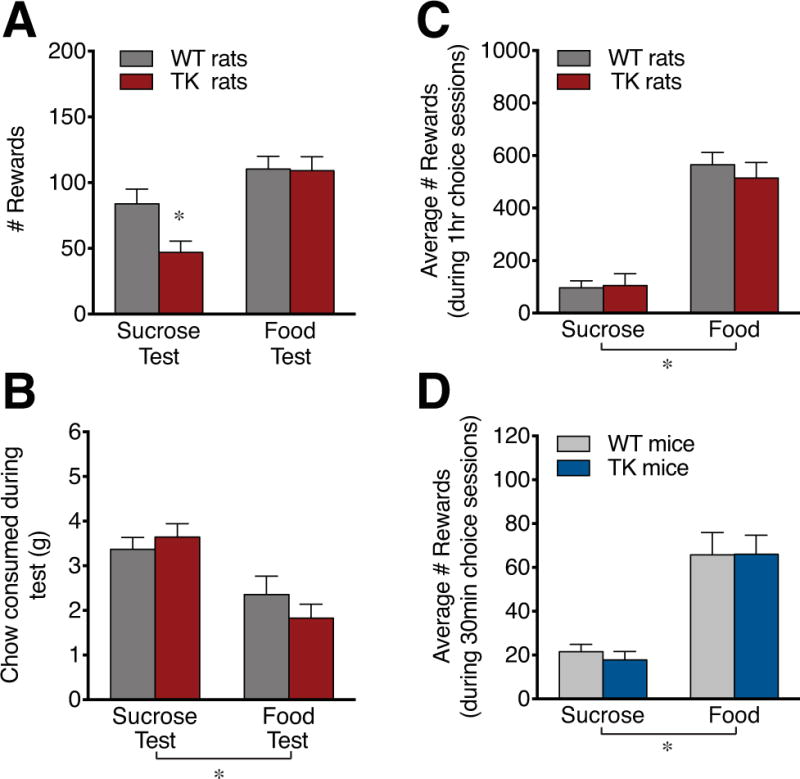
The effect of adult hippocampal neurogenesis on effort testing and reward preference. TK rats earned fewer rewards than WT rats when given the option to lever press on an RR20 schedule to receive sucrose rewards or freely consume standard laboratory chow, whilst they showed normal effort when lever pressing for the food reward (A). Both genotypes ate similar amounts of standard pellet chow relative to each other but ate more of this chow during the sucrose test than the food test (B). When given the choice to lever press (FR1) for either sucrose or food rewards, both WT and TK rats (C) and mice (D) showed greater preference for the food rewards compared with sucrose rewards during 60 min and 30 min choice sessions, respectively. n=7-12/genotype. * P<0.05 in post hoc relative to WT rats (in A) or main effect of reward type (B-D). Data are means ±SEM.
To better understand the difference in effects seen with sucrose and food rewards, reward preference was tested by training rats and mice to associate a particular lever (left or right) with a particular reward (sucrose or food) and testing consumption when both rewards were available simultaneously on an FR1 schedule. Preference testing showed no effect of genotype or genotype x reward interaction but showed a strong and significant effect of reward type (F1,22=68.16, P<0.001) indicating a >5-fold preference for food rewards relative to sucrose rewards in rats, independent of adult neurogenesis status (Figure 3C).
A preference test in mice showed a significant effect of reward type (F1,17 =40.00, P<0.001) with no effect of genotype or genotype x reward interaction, reflecting a >3-fold adult neurogenesis-independent preference for food rewards over sucrose rewards in mice (Figure 3D).
Outcome devaluation for sucrose rewards
To assess sensitivity to the sucrose reward in TK rats, we performed a reward devaluation study in which animals were trained on a RR20 schedule and tested in a 5 min extinction session following 1hr of free consumption of the sucrose reward. During random ratio reward schedule training, rats showed significant main effects of session (F15,690=58.11, P<0.001) but no genotype or genotype x session interaction effect (Figure 4A). TK rats showed a trend toward greater lever press rate during the early RR20 sessions (P=0.09), but when training was continued after testing TK rats matched the performance of WT rats. There was no genotype difference in reward consumption during free feeding for devaluation (WT: 94.60 ±5.40 rewards, TK 96.43±2.43, P=0.73). Testing following consumption of freely available sucrose pellets showed that devaluation reduced responding rates in both genotypes (F1,44=14.63, P<0.001) and showed no effect of genotype or genotype x valuation interaction (Figure 4B).
Figure 4.
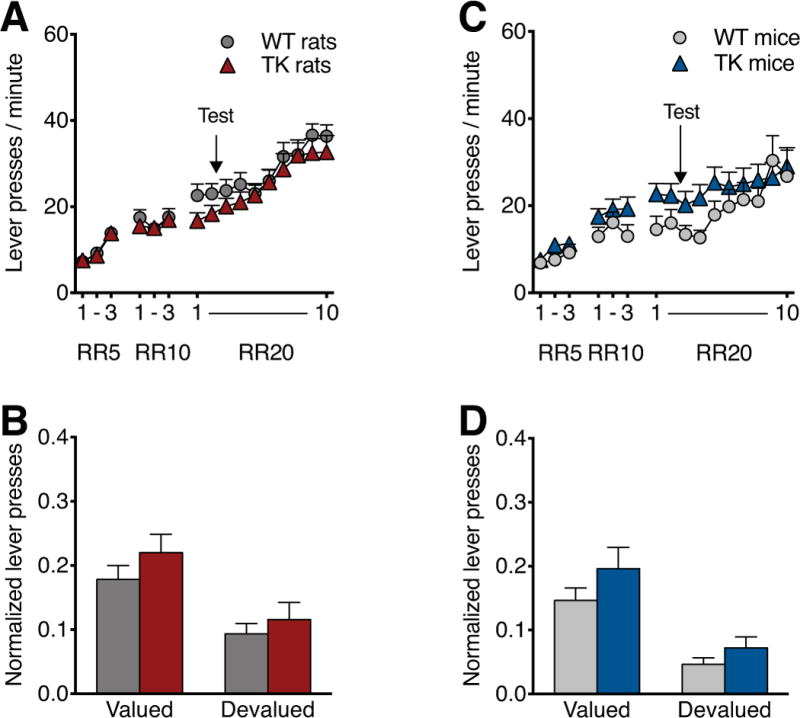
Random ratio (RR) training and devaluation testing for sucrose reward in TK rats and mice. TK rats showed a trend toward decreased lever pressing, relative to WT controls, limited to the first two sessions of RR training prior to testing (A). In contrast, TK mice showed a trend toward increased responding during the initial sessions of RR20 acquisition (C). For devaluation testing, lever pressing during was normalized to the rate of the previous training day, and both TK rats (B) and TK mice (D) showed normal devaluation to the sucrose reward, similar to that seen in WT animals. n=9-13/genotype/condition. Data are means ±SEM.
RR testing in mice showed a significant effect of session (F15,420=15.83, P<0.001, Figure 4C) during the RR training. There was a trend for a difference between WT and TK mice prior to testing (P=0.061), though with continued training on the RR20 schedule WT mice caught up with the TK mice. Reward consumption during free feeding for devaluation showed no effect of genotype (WT: 42.50 ±4.76 rewards, TK: 40.66 ±3.80, P=0.76). During devaluation testing, free consumption of sucrose pellets reduced the responding rates in both WT and TK mice (F1,25=22.48, P<0.001, Figure 4D) with no effect of genotype or genotype x valuation interaction.
Pavlovian Instrumental Transfer testing
To determine whether reward-associated cues alter the motivation to respond to a reward normally in TK rats, we performed a osPIT test. Rats were trained on Pavlovian conditioning followed by instrumental conditioning and then the transfer test. During Pavlovian conditioning, both WT and TK rats learned the CS-reward association, as indicated by the difference between preCS and CS head entries (F1,44=61.27, P<0.01, Figure 5A). Neither the main effect of genotype nor genotype x period interaction was significant. During the instrumental training phase, there was a genotype x session interaction (F10,220=3.87, P<0.001) and a significant effect of session (F10,220=99.62, P<0.001) but no main effect of genotype. Post-hoc analysis showed trends toward greater responding in TK rats on the second (P=0.073) and fourth (P=0.054) session on the RR20, suggesting that TK rats increased their lever press rate with continued training and/or higher ratios (Figure 5B). During the outcome-specific PIT test, there was a main effect of CS type (F1,44=13.71, P<0.001), but there was no main effect of genotype or genotype x CS type interaction, suggesting that TK rats have normal stimulus-evoked reward-seeking behaviors (Figure 5C).
Figure 5.
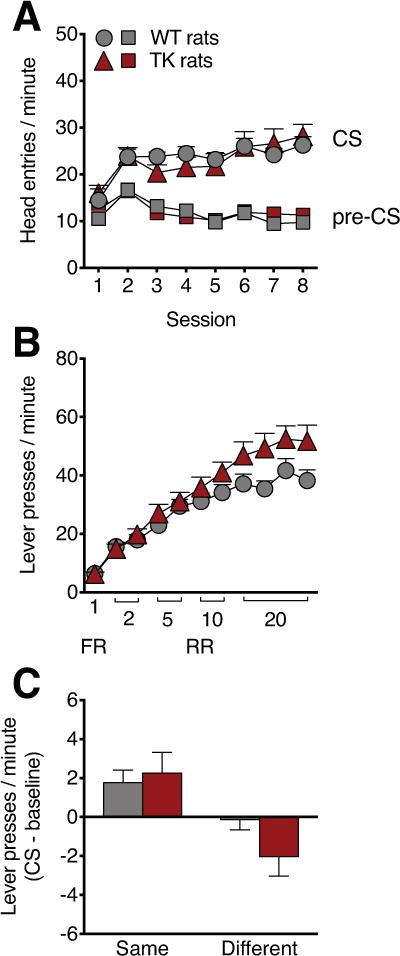
Outcome specific Pavlovian Instrumental Transfer (osPIT) in TK rats. Both WT and TK rats showed increased responding to the conditioned stimulus (CS) over the 8 Pavlovian training sessions (A). There was a significant genotype x session interaction, with increased lever pressing by TK rats in later sessions (B). Both WT and TK rats showed increased lever pressing rates on the lever associated with the reward that was previously associated with the CS (same) relative to the lever associated with the other reward (different) (D). n=12/genotype. Data are means ±SEM.
Reversal Learning
Previous reports have found normal acquisition but impaired reversal learning in an operant task after dorsal hippocampal lesions (Gourley et al., 2010). To assess whether ablation of adult neurogenesis also impairs instrumental reversal learning, we trained TK rats and mice to lever press on an RR2 schedule and then reversed the active and inactive levers. Rats showed no significant genotype differences in rewards earned during the RR2 training (data not shown) or in reversal responses over 5 sessions (Figure 6A). TK mice similarly showed normal RR2 training (data not shown) and reversal learning (Figure 6B).
Figure 6.
Reversal learning in TK rats and mice. There was no significant difference in behavioral performance across reversal learning sessions in TK rats (A) or mice (B) compared to WT controls. n=6-12/genotype. Data are means ±SEM.
DISCUSSION
We investigated the role of adult hippocampal neurogenesis in appetitive motivation using sucrose- and food-rewarded operant conditioning tasks. Complete inhibition of adult neurogenesis produced no detectable effect on fixed ratio responding, acquisition of Pavlovian conditioning, Pavlovian Instrumental Transfer, active lever reversal, or reward devaluation, suggesting that animals lacking adult neurogenesis are unaffected in their ability to associate cues and actions with rewards and to use reward-associated cues to guide actions. However, both rats and mice showed blunted motivation for sucrose rewards following ablation of adult neurogenesis, as demonstrated by a lower breakpoint on the progressive ratio task and decreased effort to earn sucrose rewards when plain laboratory chow was available. Motivation was unaffected in transgenic animals that did not receive valganciclovir, indicating that the behavioral change reflects the loss of adult neurogenesis and not the transgene alone or a random gene insertion effect (Groves et al., 2013). Intriguingly, the effects of inhibiting adult neurogenesis were also not apparent when balanced food rewards were used in place of sucrose rewards. A free choice test showed that rats and mice both strongly prefer the balanced food rewards over the sucrose rewards and that this preference is unaffected by the presence or absence of new neurons. These findings provide clear evidence that loss of adult neurogenesis affects behavior under non-threatening, reward situations. In addition, the results suggest that new neurons affect effort-based decision making, enhancing effort when the work requirement is high and reward is relatively weak.
Learning and Sensorimotor Abilities
The changes in behavior observed here do not appear to reflect learning impairments. Previous studies have found normal behavior in animals with decreased neurogenesis in a wide variety of learning tasks, including the ability to form conditioned associations between stimuli (Meshi et al., 2006; Noonan et al., 2010; Groves et al., 2013; Cameron and Glover, 2015; Seo et al., 2015). Consistent with this, we found no effect of new neuron loss on the acquisition of lever pressing, simultaneous learning of two different lever-reward contingencies (with inactive levers or multiple rewards in the free choice task), reversal of active and inactive levers, or memory for the spatial location of rewarded levers over days. In addition, we found no effects of adult neurogenesis in a Pavlovian learning task or in an outcome-specific Pavlovian Instrumental Transfer task. The ability to transfer the cue association to the instrumental condition in this task suggests that animals lacking adult neurogenesis are not only able to learn cue-stimulus associations but to flexibly use this information like control animals.
Impairments in physical abilities or sensitivity to reward are also unlikely to explain the behavioral changes observed in TK animals. Weakness or rapid tiring could decrease breakpoints in progressive ratio testing, but such differences should have affected behavior in tests using food reward as well as sucrose reward. Decreased lever pressing in the TK animals could result from general hypoactivity, but neither hypoactivity nor hyperactivity was evident in active or inactive lever pressing during FR testing, consistent with previous findings that activity in exploratory tests is unaffected by inhibition of adult neurogenesis (Saxe et al., 2006; Snyder et al., 2011; Kheirbek et al., 2012; Snyder et al., 2016; Glover et al., 2017). Damage to the hippocampus can affect activity levels, but generally produces a hyperactive, rather than hypoactive, state (Wilkinson et al., 1993; Whishaw and Jarrard, 1995; Bannerman et al., 1999). Finally, changes in sensitivity to the value of the sucrose reward are unlikely to underlie the observed behavioral changes, because WTs and TKs showed similar reduction in instrumental responding (in both species) in the devaluation test, a sensory-specific satiety task (Cartoni et al., 2016). In the absence of physical or learning impairments, the decreased breakpoint in progressive responding and decreased instrumental responding in the presence of freely available chow most likely reflect decreased willingness to work for reward – decreased effort, motivation, or “wanting” – in animals lacking adult neurogenesis.
The GFAP-TK model inhibits adult neurogenesis in the olfactory bulb as well as the dentate gyrus, so behavioral changes could potentially result from changes in olfaction. However, loss of neurogenesis had no effect on reward-mediated lever pressing on a fixed ration schedule or reward preference, suggesting that any olfactory sensory abilities used in this task are unimpaired and unlikely to the underlie observed behavioral changes. Complete loss of the both olfactory bulbs produces a depression-like phenotype in rats (Song and Leonard, 2005), which could alter motivation if it was mimicked by loss of olfactory bulb neurogenesis. However, the widespread cellular dysfunction in the dentate gyrus and CA1 that results from bulbectomy suggests that its depressive-like effects stem from hippocampal damage rather than olfactory changes (Morales-Medina et al., 2017), pointing back to the hippocampus as the most likely source of the new neurons responsible for the observed motivational changes.
Reward and Anhedonia Circuits
The pattern of effects on PR and effort choice observed here after inhibition of adult neurogenesis differs from that seen with manipulations in neocortical areas, which generally affect either PR or effort choice but not both (Bailey et al., 2016). However, manipulations of the nucleus accumbens core, ventral tegmental area, and amygdala, like adult neurogenesis inhibition, alter behavior in both of these tests (de Jong et al., 2015; Bailey et al., 2016). Glutamatergic projections from the hippocampus to the VTA and NAc (O’Donnell and Grace, 1995), a direct projection from the VTA to the dentate gyrus co-releasing glutamate and GABA (Gasbarri et al., 1994; Ntamati and Lüscher, 2016), and dopamine release from locus coeruleus projections into the dorsal hippocampus (Kempadoo et al., 2016; Takeuchi et al., 2016) all suggest that the dentate gyrus is an integral part of the circuitry mediating reward-related behavior (Russo and Nestler, 2013). Despite these anatomical connections, few studies have addressed the role of the hippocampus in effort. No study, to our knowledge, has previously investigated the effects of hippocampal manipulations in the effort-based choice test (Bailey et al., 2016). The only study to examine the effect of hippocampal lesions on progressive ratios found that lesions of dorsal and ventral hippocampus both increased breakpoints (Gourley et al., 2010), suggesting that damage to the hippocampus proper has an effect opposite to that of removing only the adult-born granule neurons, as seen in the current study. One previous study tested progressive ratio responding for sucrose in rats irradiated to inhibit adult neurogenesis and found no effect (Noonan et al., 2010). Two possible explanations for this difference seem most likely. First, adult neurogenesis was completely ablated throughout the entire dentate gyrus in the current study but was only completely ablated in the dorsal hippocampus in the previous study, suggesting that new neurons remaining in the ventral region may support progressive ratio responding. Alternatively, the plain sucrose tablets used in the earlier study may be more strongly rewarding than our chocolate sucrose tablets, like the food rewards in the current study. A third difference between the studies is that inhibition of neurogenesis began at 8 weeks of age in the current study and 5 weeks of age, prior to sexual maturity (Lewis et al., 2002), in the earlier study. However, earlier loss of neurogenesis is normally associated with greater rather than smaller effects (Bayer et al., 1973; Wei et al., 2011; Cushman et al., 2012).
Random Ratio Training
Genotype effects were observed early in RR20 training in some experiments. In the PIT test, TK rats showed greater responding than WT rats at RR20. However, in the devaluation experiment, TK rats showed a trend toward lower baseline RR20 response rates than WT rats early in training. Mice in this test showed the opposite effect, with a trend toward increased responding in the TK animals during the first two RR20 sessions. In the effort testing experiment, rats showed no genotype effect at any RR schedule. Test data in all of these experiments were normalized to the prior session response rate, which appears to have been effective as similar devaluation effects were observed in both species despite opposing trends in early RR20. However, it is unclear why TK rats showed increased responding, decreased responding, and identical responding on the RR20 reward schedule in the three experiments. One possibility is that loss of new neurons disrupts effort calculations made by the animals under conditions of high ambiguity (Glover et al., 2017), including random ratio reward schedules, allowing other factors to push effort in one direction or the other. These other factors could include minor differences in age or training prior to RR20 or random cohort effects.
Motivation and Apathy
While decreased pleasure is commonly viewed as a feature of depression, increasing evidence suggests that depression affects anticipation of rewards and motivation to seek rewards more than the actual experience of rewards (Rizvi et al., 2016). The sucrose preference test is commonly used as a test of depressive-like anhedonia in rodents based on the observation that chronic mild stress decreases sucrose consumption (Willner et al., 1992). However, the stress effect on sucrose consumption has sometimes proven unreliable (Willner, 1997). We have previously found that inhibition of adult neurogenesis can alter sucrose preference but that the effect requires specific testing conditions and is not always apparent across days even within the same cohort (Snyder et al., 2011; 2016). Although decreased sucrose preference is generally thought to reflect a hedonic change, motivation may play a role as well. Small changes in the testing protocols may differentially target hedonic or motivational components of the behavior, potentially explaining apparent inconsistencies. For example, concurrent choices (in 2-bottle tests) and successive choices (in 1-bottle tests) may show differential sensitivity to the effects of motivation or satiety. Similarly, switching bottle locations over days may test motivation to seek sucrose when it is not found in the expected location. Finally, differences in the concentration of sucrose used and the length of water or food deprivation prior to testing may affect relative reward value. Operant conditioning-based effort testing may allow dissection of hedonic and motivational components of reward-seeking behavior that is difficult to address in standard preference tests.
Mice and rats lacking adult neurogenesis showed decreased effort to obtain sucrose rewards, but not food rewards. Only one genotype effect was observed in tests using food rewards: a small, but significant, decrease in food rewards earned by TK rats during fixed ratio responding with no limit on rewards earned. This effect may reflect a difference in satiety observed only in this test due to the very large numbers of rewards consumed in these sessions. Although weight did not differ by genotype in the meal fed rats used here, slightly lower weights have been observed in TK and irradiated rats housed under ad lib feeding conditions (Snyder et al., 2005; 2016), consistent with the possibility that rats lacking new neurons may devalue food more rapidly, resulting in less overeating (Martin et al., 2010). Contrary to our expectations, both mice and rats showed a strong preference for food rewards over sucrose rewards, indicating that it was the weaker reward that was more susceptible to motivational effects of new neuron ablation. This finding, taken together with the normal motivation to work for sucrose when the workload was low (as in low ratio FR testing), suggests that ablation of adult neurogenesis affects motivation only in situations with low reward and high effort requirement. A recent human study testing emotional, cognitive, and behavioral subcomponents of apathy (Bonnelle et al., 2015) found that individuals with high behavioral apathy scores show less effort than control individuals under low reward conditions while exerting similar effort when rewards are high or little effort is required. Inhibition of adult neurogenesis in rodents may therefore model human behavioral apathy.
Increased behavioral apathy in TK animals suggests an impairment in the cost-benefit valuation system (Bonnelle et al., 2015). Weak rewards, or greater potential for failure, may result in more closely matched effort-reward options, making response choices more difficult – and more reliant on the hippocampus (Redish, 2016). An impairment of this type is consistent with previously observed effects of adult neurogenesis in novelty-suppressed feeding and social preference, two tests that also involve rewards with potential costs (Snyder et al., 2011; Opendak et al., 2016; Yun et al., 2016; Glover et al., 2017). Notably, these effects, like those in the current study, are seen in naïve animals, not previously exposed to stress. However, stress could affect the neurogenesis dependence in other behavioral situations by changing the weighting of costs or benefits, making options more evenly matched. Differential weighting of the cost term, specifically, is likely to be associated with apathy, because reward sensitivity is seemingly unaffected in both animals lacking adult neurogenesis and patients suffering from depression. This possibility fits with Gray and McNaughton’s (2000) suggested hippocampal function in resolving conflicts between concurrent goals, in part through weighting of potential negative consequences. Taken together, these findings suggest that new neurons may function to adjust the weight assigned to potential costs of possible actions, balancing the likelihood and impact of negative consequences with required effort and potential gains. This possibility may provide a link between the role of adult neurogenesis in stress-related anxiety-and depressive-like behavior (Snyder et al., 2011; Glover et al., 2017) and anhedonia-related features of depression such as fatigue, lack of energy, and lack of motivation (Demyttenaere et al., 2005; Treadway and Zald, 2011; Fava et al., 2014).
Acknowledgments
This work was supported by the Intramural Program of the NIH, National Institute of Mental Health, ZIAMH002784 (H.A.C).
Grant Sponsor: Intramural Research Program of the National Institute of Mental Health, National Institutes of Health; Grant number: ZIAMH002784
Footnotes
FINANCIAL DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
References
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Ambrose RE, Pfeiffer BE, Foster DJ. Reverse Replay of Hippocampal Place Cells Is Uniquely Modulated by Changing Reward. Neuron. 2016;91:1124–1136. doi: 10.1016/j.neuron.2016.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MR, Simpson EH, Balsam PD. Neural substrates underlying effort, time, and risk-based decision making in motivated behavior. Neurobiol Learn Mem. 2016;133:233–256. doi: 10.1016/j.nlm.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Yee BK, Good MA, Heupel MJ, Iversen SD, Rawlins JN. Double dissociation of function within the hippocampus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behav Neurosci. 1999;113:1170–1188. doi: 10.1037//0735-7044.113.6.1170. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Brunner RL, Hine R, Altman J. Behavioural effects of interference with the postnatal acquisition of hippocampal granule cells. Nature New Biol. 1973;242:222–224. doi: 10.1038/newbio242222a0. [DOI] [PubMed] [Google Scholar]
- Bonnelle V, Veromann K-R, Burnett Heyes S, Sterzo Lo E, Manohar S, Husain M. Characterization of reward and effort mechanisms in apathy. J Physiol Paris. 2015;109:16–26. doi: 10.1016/j.jphysparis.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. The role of the hippocampus in prediction and imagination. Annual review of psychology. 2010;61:27–48. C1–8. doi: 10.1146/annurev.psych.60.110707.163508. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Glover LR. Adult neurogenesis: beyond learning and memory. Annual review of psychology. 2015;66:53–81. doi: 10.1146/annurev-psych-010814-015006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartoni E, Balleine B, Baldassarre G. Appetitive Pavlovian-instrumental Transfer: A review. Neurosci Biobehav Rev. 2016;71:829–848. doi: 10.1016/j.neubiorev.2016.09.020. [DOI] [PubMed] [Google Scholar]
- Cushman JD, Maldonado J, Kwon EE, Garcia AD, Fan G, Imura T, Sofroniew MV, Fanselow MS. Juvenile neurogenesis makes essential contributions to adult brain structure and plays a sex-dependent role in fear memories. Front Behav Neurosci. 2012;6:3. doi: 10.3389/fnbeh.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong JW, Roelofs TJM, Mol FMU, Hillen AEJ, Meijboom KE, Luijendijk MCM, van der Eerden HAM, Garner KM, Vanderschuren LJMJ, Adan RAH. Reducing Ventral Tegmental Dopamine D2 Receptor Expression Selectively Boosts Incentive Motivation. Neuropsychopharmacology. 2015;40:2085–2095. doi: 10.1038/npp.2015.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyttenaere K, De Fruyt J, Stahl SM. The many faces of fatigue in major depressive disorder. Int J Neuropsychopharm. 2005;8:93–105. doi: 10.1017/S1461145704004729. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Smoski MJ, Kampov-Polevoy AB, Gallop R, Garbutt JC. Unipolar depression does not moderate responses to the Sweet Taste Test. Depress Anxiety. 2010;27:859–863. doi: 10.1002/da.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Ball S, Nelson JC, Sparks J, Konechnik T, Classi P, Dube S, Thase ME. Clinical relevance of fatigue as a residual symptom in major depressive disorder. Depress Anxiety. 2014;31:250–257. doi: 10.1002/da.22199. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Verney C, Innocenzi R, Campana E, Pacitti C. Mesolimbic dopaminergic neurons innervating the hippocampal formation in the rat: a combined retrograde tracing and immunohistochemical study. Brain Res. 1994;668:71–79. doi: 10.1016/0006-8993(94)90512-6. [DOI] [PubMed] [Google Scholar]
- Glover LR, Schoenfeld TJ, Karlsson R-M, Bannerman DM, Cameron HA. Ongoing neurogenesis in the adult dentate gyrus mediates behavioral responses to ambiguous threat cues. PLoS Biol. 2017;15:e2001154. doi: 10.1371/journal.pbio.2001154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Lee AS, Howell JL, Pittenger C, Taylor JR. Dissociable regulation of instrumental action within mouse prefrontal cortex. Eur J Neurosci. 2010;32:1726–1734. doi: 10.1111/j.1460-9568.2010.07438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety: An enquiry into the function of the septo-hippocampal system Second. Oxford University Press; 2000. [Google Scholar]
- Groves JO, Leslie I, Huang G-J, Mchugh SB, Taylor A, Mott R, Munafo M, Bannerman DM, Flint J. Ablating adult neurogenesis in the rat has no effect on spatial processing: evidence from a novel pharmacogenetic model. PLoS Genet. 2013;9:e1003718. doi: 10.1371/journal.pgen.1003718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci USA. 2016;113:14835–14840. doi: 10.1073/pnas.1616515114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Tannenholz L, Hen R. NR2B-Dependent Plasticity of Adult-Born Granule Cells is Necessary for Context Discrimination. J Neurosci. 2012;32:8696–8702. doi: 10.1523/JNEUROSCI.1692-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MM, Reif A, Schmitt AG. Major depression: a role for hippocampal neurogenesis? Curr Top Behav Neurosci. 2013;14:153–179. doi: 10.1007/7854_2012_226. [DOI] [PubMed] [Google Scholar]
- Lewis EM, Barnett JF, Freshwater L, Hoberman AM, Christian MS. Sexual maturation data for Crl Sprague-Dawley rats: criteria and confounding factors. Drug Chem Toxicol. 2002;25:437–458. doi: 10.1081/dct-120014794. [DOI] [PubMed] [Google Scholar]
- Martin B, Ji S, Maudsley S, Mattson MP. “Control” laboratory rodents are metabolically morbid: why it matters. Proc Natl Acad Sci USA. 2010;107:6127–6133. doi: 10.1073/pnas.0912955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara CG, Dupret D. Two sources of dopamine for the hippocampus. Trends Neurosci. 2017;40:383–384. doi: 10.1016/j.tins.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- Morales-Medina JC, Iannitti T, Freeman A, Caldwell HK. The olfactory bulbectomized rat as a model of depression: The hippocampal pathway. Behav Brain Res. 2017;317:562–575. doi: 10.1016/j.bbr.2016.09.029. [DOI] [PubMed] [Google Scholar]
- Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci. 2010;30:304–315. doi: 10.1523/JNEUROSCI.4256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntamati NR, Lüscher C. VTA Projection Neurons Releasing GABA and Glutamate in the Dentate Gyrus. Eneuro. 2016;3 doi: 10.1523/ENEURO.0137-16.2016. ENEURO.0137-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opendak M, Offit L, Monari P, Schoenfeld TJ, Sonti AN, Cameron HA, Gould E. Lasting Adaptations in Social Behavior Produced by Social Disruption and Inhibition of Adult Neurogenesis. J Neurosci. 2016;36:7027–7038. doi: 10.1523/JNEUROSCI.4435-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelizza L, Ferrari A. Anhedonia in schizophrenia and major depression: state or trait? Ann Gen Psychiatry. 2009;8:22. doi: 10.1186/1744-859X-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD. Vicarious trial and error. Nat Rev Neurosci. 2016;17:147–159. doi: 10.1038/nrn.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rizvi SJ, Pizzagalli DA, Sproule BA, Kennedy SH. Assessing anhedonia in depression: Potentials and pitfalls. Neurosci Biobehav Rev. 2016;65:21–35. doi: 10.1016/j.neubiorev.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology (Berl) 1991;104:515–521. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang J-W, Malleret G, David DJ, Monckton JE, Garcia ADR, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld TJ, Cameron HA. Adult neurogenesis and mental illness. Neuropsychopharmacology. 2015;40:113–128. doi: 10.1038/npp.2014.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D-O, Carillo MA, Chih-Hsiung Lim S, Tanaka KF, Drew MR. Adult Hippocampal Neurogenesis Modulates Fear Learning through Associative and Nonassociative Mechanisms. J Neurosci. 2015;35:11330–11345. doi: 10.1523/JNEUROSCI.0483-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherdell L, Waugh CE, Gotlib IH. Anticipatory pleasure predicts motivation for reward in major depression. Journal of abnormal psychology. 2012;121:51–60. doi: 10.1037/a0024945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Cameron HA. Could adult hippocampal neurogenesis be relevant for human behavior? Behav Brain Res. 2012;227:384–390. doi: 10.1016/j.bbr.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Grigereit L, Russo A, Seib D, Brewer M, Pickel J, Cameron HA. A transgenic rat for specifically inhibiting adult neurogenesis. Eneuro. 2016:1–13. doi: 10.1523/ENEURO.0064-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Leonard BE. The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev. 2005;29:627–647. doi: 10.1016/j.neubiorev.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Soumier A, Carter RM, Schoenfeld TJ, Cameron HA. New Hippocampal Neurons Mature Rapidly in Response to Ketamine But Are Not Required for Its Acute Antidepressant Effects on Neophagia in Rats. Eneuro. 2016;3 doi: 10.1523/ENEURO.0116-15.2016. ENEURO.0116-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Boström E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisén J. Dynamics of Hippocampal Neurogenesis in Adult Humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spijker J, Bijl RV, de Graaf R, Nolen WA. Determinants of poor 1-year outcome of DSM-III-R major depression in the general population: results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS) Acta Psychiatr Scand. 2001;103:122–130. doi: 10.1034/j.1600-0447.2001.103002122.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Duszkiewicz AJ, Sonneborn A, Spooner PA, Yamasaki M, Watanabe M, Smith CC, Fernandez G, Deisseroth K, Greene RW, Morris RGM. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature. 2016;537:357–362. doi: 10.1038/nature19325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanti A, Belzung C. Hippocampal neurogenesis: a biomarker for depression or antidepressant effects? Methodological considerations and perspectives for future research. Cell Tissue Res. 2013;354:203–219. doi: 10.1007/s00441-013-1612-z. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Parsing Anhedonia: Translational Models of Reward-Processing Deficits in Psychopathology. Curr Dir Psychol Sci. 2013;22:244–249. doi: 10.1177/0963721412474460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P, Feng B, Urizar E, Winiger V, Ward RD, Taylor KM, Martinez D, Moore H, Balsam PD, Simpson EH, Javitch JA. Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation. Mol Psychiatry. 2013;18:1025–1033. doi: 10.1038/mp.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, Perlis RH, Placentino A, Dernovšek MZ, Henigsberg N, Mors O, Maier W, McGuffin P, Farmer A. Self-report and clinician-rated measures of depression severity: can one replace the other? Depress Anxiety. 2012;29:1043–1049. doi: 10.1002/da.21993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Meaney MJ, Duman RS, Kaffman A. Affiliative behavior requires juvenile, but not adult neurogenesis. J Neurosci. 2011;31:14335–14345. doi: 10.1523/JNEUROSCI.1333-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Jarrard LE. Similarities vs. differences in place learning and circadian activity in rats after fimbria-fornix section or ibotenate removal of hippocampal cells. Hippocampus. 1995;5:595–604. doi: 10.1002/hipo.450050610. [DOI] [PubMed] [Google Scholar]
- Wilkinson LS, Mittleman G, Torres E, Humby T, Hall FS, Robbins TW. Enhancement of amphetamine-induced locomotor activity and dopamine release in nucleus accumbens following excitotoxic lesions of the hippocampus. Behav Brain Res. 1993;55:143–150. doi: 10.1016/0166-4328(93)90110-c. [DOI] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Yun S, Donovan MH, Ross MN, Richardson DR, Reister R, Farnbauch LA, Fischer SJ, Riethmacher D, Gershenfeld HK, Lagace DC, Eisch AJ. Stress-induced anxiety- and depressive-like phenotype associated with transient reduction in neurogenesis in adult nestin-CreERT2/diphtheria toxin fragment A transgenic mice. PLoS ONE. 2016;11:e0147256. doi: 10.1371/journal.pone.0147256. [DOI] [PMC free article] [PubMed] [Google Scholar]


