Abstract
PROBLEM
To study the mechanisms of placenta function and the role of extracellular vesicles (EVs) in pregnancy, it is necessary to develop an ex vivo system that retains placental cytoarchitecture and the main metabolic aspects, in particular the release of EVs and soluble factors. Here, we developed such a system and investigated the pattern of secretion of cytokines, growth factors and extracellular vesicles by placental villous and amnion tissues ex vivo.
METHODS OF STUDY
Placental villous and amnion explants were cultured for two weeks at the air/liquid interface and their morphology and the released cytokines and EVs were analyzed. Cytokines were analyzed with multiplexed bead assays and individual EVs were analyzed with recently developed techniques that involved EV capture with magnetic nanoparticles coupled to anti-EV antibodies and flow cytometry.
RESULTS
Ex vivo tissues (i) remained viable and preserved their cytoarchitecture; (ii) maintained secretion of cytokines and growth factors; (iii) released EVs of syncytiotrophoblast and amnion epithelial cell origins that contain cytokines and growth factors.
CONCLUSION
A system of ex vivo placental villous and amnion tissues can be used as an adequate model to study placenta metabolic activity in normal and complicated pregnancies, in particular to characterize EVs by their surface markers and by encapsulated proteins. Establishment and bench-marking the placenta ex vivo system may provide new insight in the functional status of this organ in various placental disorders, particularly regarding the release of EVs and cytokines. Such EVs may have a prognostic value for pregnancy complications.
Keywords: Cytokine, pregnancy, 3D cultures, growth factors, syncytiotrophoblast, amnion, alarmins
Graphical abstract

Introduction
The placenta plays a critical role in fetal growth and development and orchestrates major maternal adaptations of pregnancy such as carbohydrate intolerance1–5 and immune adaptations6–30. Placental dysfunction has been implicated in major complications of pregnancy such as preeclampsia31–56, fetal growth restriction57–72, fetal death73–80, and preterm labor81–90. The placenta has also been considered at the center of the chronic disease universe91, 92.
The study of human placenta in vivo is challenging and has significant restrictions. Animal models have been useful, although there are fundamental differences in placentation among mammals93–97. Many studies of human placenta utilize isolated primary cells or placenta-derived cell lines98–101. While major discoveries have emerged from such studies102–104, isolated cells do not adequately recapitulate important aspects of tissue function related to cell-cell communications in vivo. This is the rationale to develop three-dimensional models which maintain the cellular relationships ex vivo. Such three-dimensional models have proven to be of major value in investigating cancer development105–109, viral pathogenesis110–113, and testing anti-cancer114 and antiviral compounds115 under controlled laboratory conditions.
It is now increasingly apparent that the maternal-fetal dialogue is more complex than previously recognized116–119. In addition to many soluble factors, such as hormones and cytokines implicated in this communication, it is now recognized that extracellular vesicles (EVs) can also mediate crosstalk between the feto-placental unit and the mother120–131. EVs carry lipids, proteins and miRNA that can convey information about the status of the fetus and placenta132–134. Moreover, EVs carry immune mediators (e.g. cytokines) that facilitate cell-to-cell communication, which are present on both the surface and inside the microvesicles135–145.
To study the mechanisms of placenta function and the role of EVs in pregnancy, it is necessary to develop an ex vivo system that retains placental cytoarchitecture and continues to release EVs and soluble factors under controlled laboratory conditions. Here, we report on such a system. Using nanotechnology, we analyzed individual EVs released by placental tissues ex vivo and assessed EV-bound and EV-encapsulated cytokines. Establishment and bench-marking this placenta ex vivo system provides a basis to study the nature of various placental disorders, and in particular the release of EVs and cytokines. Their release by the syncytiotrophoblast into the maternal circulation has been proposed as a placental liquid biopsy, which can provide insight into the functional status of the organ and may be a source of biomarkers to predict pregnancy complications146. Herein, we report a system of ex vivo placental villous and amnion tissues that can be used as an adequate model to study physiological and pathological processes during normal and complicated pregnancies.
Methods
Sample preparation and storage
Placental tissues (the placenta and fetal membranes) from women who delivered at term without labor (n=10) were obtained at the Detroit Medical Center, Wayne State University, and the Perinatology Research Branch, an intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services (NICHD/NIH/DHHS) (Detroit, MI, USA). The collection and utilization of biological materials for research purposes were approved by the Institutional Review Boards of these institutions. All participating women provided written informed consent. Immediately after delivery, three random samples from the placental villi were collected using a metal grid and the Random Position Generator DICE software (Perinatology Research Branch, Detroit, MI, USA). Amnion was gently separated from the chorion of the fetal membranes. Samples from the placental villi and amnion were placed in 50mL tubes containing DMEM and shipped overnight to NIH on cold packs. Upon receipt, villi were sectioned into 2 mm × 6 mm strips, washed thoroughly in 1X phosphate-buffered saline (PBS) and cultured on Gelfoam absorbable collagen sponges (Pfizer, New York, NY) at the air-liquid interface, as has been described for other tissues147 in 0.1 µm filtered phenol red free DMEM supplemented with 5% characterized, charcoal stripped FBS, 50 µg/ml gentamicin and 2.5 µg/ml Amphotericin B at 37°C, 5% CO2. Amniotic membrane was sectioned into 3 × 3 mm pieces, washed thoroughly with PBS, and cultured in same medium. Equivalent masses were cultured in triplicate for each donor. Tissues were collected at day 1, 7 and 14 and fixed in 10% formalin, sent for paraffin embedding, sectioning, and H&E staining. H&E sections were evaluated by perinatal and obstetric pathologists at Wayne State University School of Medicine. Medium was collected and changed at days 1, 4, 7, 10 and 14 after initiation. Medium samples were centrifuged at 400 × g for 5 minutes to remove cells and frozen at −80°C.
Preparation of EV fractions
Medium samples were split into multiple fractions. One aliquot was kept untreated, another portion was treated with Exoquick TC (System Biosciences, Palo Alto, CA), according to manufacturer’s protocols. Briefly, ExoQuick TC was added to supernatants at a ratio of 100 µl of ExoQuick TC to 500 µl of sample and refrigerated overnight at 4°C. ExoQuick/sample mixtures were centrifuged at 1500 × g for 30 minutes to pellet EVs. Supernatant was collected and saved for cytokine measurement of EV-free supernatant. The pellet was centrifuged again at 1500 × g for 5 minutes and all traces of fluid were removed resulting in an EV enriched preparation. The pellet was resuspended in 1X PBS in the original volume and cytokines were measured on intact and lysed EVs.
Cytokine measurement
We previously developed an in-house multiplexed bead-based assay for measurement of the following cytokines/growth factors: IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-13, IL-15, IL-16, IL-18, IL-33, Calgranulin A (S100A8), Calgranulin C (S100A12), C-reactive protein (CRP), CXCL6 (granulocyte chemotactic protein 2), CXCL13 (B lymphocyte chemoattractant), Eotaxin (CCL11), granulocyte-macrophage colony-stimulating factor (GM-CSF), growth-regulated alpha (GRO-α or CXCL1), HMGB1 (high mobility group box 1), interferon-β (IFN-β), interferon-γ (IFN-γ), interferon-γ-induced protein (IP-10 or CXCL10), interferon-inducible T-cell alpha chemoattractant (ITAC or CXCL11), lactoferrin, macrophage colony-stimulating factor (M-CSF), monocyte chemoattractant protein-1 (MCP-1 or CCL2), macrophage migration inhibitory factor (MIF), monokine induced by IFN-γ (MIG or CXCL9), macrophage inflammatory protein-1α (MIP-1α or CCL3), MIP-1β (CCL4), MIP-3α (CCL20), regulated on activation normally T-cell expressed and secreted (RANTES or CCL5), transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α), and TNF related apoptosis inducing ligand (TRAIL), as previously described with minor modifications148–150. All antibody pairs and protein standards were purchased from R&D Systems except those for IFN-β and lactoferrin (Abcam, Cambridge, MA). Additional in-house assays were designed for the following growth, angiogenic and anti-angiogenic factors and hormones: activin A, A disintegrin and metalloproteinase domain 12 (ADAM-12), adiponectin, angiogenin, CD40L, epidermal growth factor (EGF), endoglin, fasL, fibronectin, galectin-1, human chorionic gonadotropin (hCG), intercellular adhesion molecule 1 (ICAM-1), insulin-like growth factor-binding protein 1 (IGFBP1), interleukin-1 receptor antagonist (IL-1Ra), IL-27, leptin, matrix metalloproteinase-7 (MMP-7), MMP-9, pregnancy-associated plasma protein-A (PAPP-A), prostaglandin E2 (PGE2), placental growth factor (PIGF), resistin, serpin E1, tissue factor pathway inhibitor (TFPI), transforming growth factor beta 3 (TGFβ3), tyrosine-protein kinase receptor Tie-2, tissue inhibitor of matrix metalloproteinases 1 (TIMP-1), tissue factor, toll-like receptor 2 (TLR2), triggering receptor expressed on myeloid cells 1 (TREM-1), urokinase-type plasminogen activator (uPA), urokinase plasminogen activator receptor (uPAR), vascular endothelial growth factor (VEGF), vascular endothelial growth factor receptor 1 (VEGFR1 or Flt-1), and vascular endothelial growth factor receptor 2 (VEGFR2 or Flk-1). Antibody pairs and proteins were purchased from R&D Systems except those for hCG and PGE2 (Abcam).
Magnetic beads (Luminex, Austin, TX) with distinct spectral signatures (regions) were coupled to cytokine specific capture antibodies according to manufacturer’s recommendations and stored at 4°C. All antibody pairs were verified to be free of cross reactivity. Standards and samples were combined with bead mixtures and incubated overnight at 4°C. Intact EV samples and lysed EV samples, to which Triton X was added at final concentration of 1%, were run in separate wells. Plates were washed two times and incubated with mixtures of polyclonal biotinylated anti-cytokine antibodies for one hour at room temperature. Plates were washed two times and incubated for 25 minutes with 16 µg/ml streptavidin-phycoerythrin in PBS. Plates were washed two times and beads were resuspended in PBS and read on a Luminex 200 analyzer with acquisition of 100 beads for each region and analyzed using Bioplex Manager software (BioRad, Hercules, CA). Cytokine concentrations were determined using 5P regression algorithms.
EV labeling and capture
EVs were captured from culture supernatants via magnetic nanoparticles (MNPs) (Ocean NanoTech, San Diego, CA). MNPs were coupled to anti-PLAP (clone 8B6,Thermo Fisher, Waltham, MA and clone H17E2, BioRad), anti-CD90 (clone 5E10, Biolegend, San Diego, CA), anti-CD9 (clone H19a, Biolegend), anti-CD63 (H5C6 Biolegend), anti-HLA-ABC (W6/32 Biolegend) or mouse IgG (SouthernBiotech, Birmingham, AL) antibodies, per manufacturers’ protocol and as previously described151. Briefly, 200 µl of 15nm MNPs are activated and then coupled with 1mg antibody overnight. Coupled MNPs are washed twice on a magnet then resuspended in 2 ml of wash/storage buffer and stored at 4°C. EVs in 100 µl of culture supernatant were labeled with 1 µM Bodipy FL Maleimide [BODIPY™ FL N-(2-Aminoethyl) Maleimide, Thermo Fisher] for 15 minutes at RT, then captured with 20 µl of MNPs. MNPs are added in huge excess to EVs, and the ratio of MNPs to EVs was optimized to allow good capture efficiency and single particle detection, as previously described151. Fluorescent detection antibodies were added for 30 minutes at room temperature. Detection antibodies for placental villous cultures included mouse anti-human antibodies to CD51-PE (Sony Biotechnology, Champaign, IL), CD63-BV711 (BD Biosciences, San Jose, CA), CD105-PECy7 (Biolegend), CD200 BV650 (BD Biosciences), CD274 BV605 (Biolegend), syncytin-1 (Abnova, Walnut, CA) in-house labeled with AlexaFluor 647, and HLA-ABC APC/Cy7 (Biolegend). Detection antibodies for amnion explants included mouse anti-human antibodies to CD29 APC (Thermo Fisher), CD44 PE (Thermo Fisher), CD105 PECy7 (Biolegend), CD140b BV421 (BD Biosciences), CD324 PerCP/Cy5.5 (Biolegend), CD326 BV650 (Biolegend) and HLA-DR APC/Cy7 (Biolegend). Control staining was also performed with mouse anti-human CD31, CD41, and CD45 APC/Cy7 (Biolegend). The captured and stained complexes were separated from unbound EVs and antibodies using MS magnetic columns (Miltenyi Biotec) in a magnetic field using OctoMACS magnet (Miltenyi Biotec), washed four times with 500 µl of PBS and eluted from the column outside the magnet with 200 µl PBS and fixed with 1.5% paraformaldehyde. 123count ebeads (Thermo Fisher) were added to tubes for EV quantification. All antibodies were tested on EV/MNP complexes singly and in combination to verify that antibodies bound with the same efficiency and spectral overlap could be compensated.
EV flow cytometry analysis
Purified complexes were acquired on low speed on an LSRII (BD Biosciences) flow cytometer equipped with 355-, 407-, 488-, 532- and 638-nm lasers by triggering on Bopidy FL fluorescence to acquire only labeled EVs. Fluorescence minus one stainings and isotype controls were used were used for setting gates, compensations, and determining background staining. Megamix SSC beads (BioCytex, Parsippany, NJ) were used to set parameters for estimated EV size; in general EV size is overestimated due to the binding of MNPs to the EVs. Data were acquired with Diva 6.3 and analyzed with FlowJo software v10.4.1 (Treestar Software, Ashland, OR).
Measurement of EV-associated cytokines
EVs were captured as above using MNPs coupled to mouse anti-human antibodies to PLAP (8B6, Thermo Fisher), CD31 (WM59, Biolegend), CD90 (5E10, Biolegend) or HLA-G (87G, Biolegend) antibodies. 20 µl of MNPs were incubated with 100 µl of culture supernatants overnight at 4°C and purified using MS magnetic columns as above. EV/MNP complexes were eluted off columns, resuspended in their original volume, split in two and analyzed by multiplexed bead assays on intact fractions and lysed (1% Triton X) fractions. Total EVs from culture supernatants were collected using ExoQuick TC as above and analyzed the same way.
Statistical Analysis
We conducted statistical analysis using JMP10 (SAS Institute, Cary, NC). Results are represented as means ± standard error of the mean (SEM). The statistical differences were evaluated with paired Student’s t test. All hypothesis tests were two-tailed and a p value of ≤0.05 defined statistical significance.
Results
Ex vivo tissue viability and function
Histology
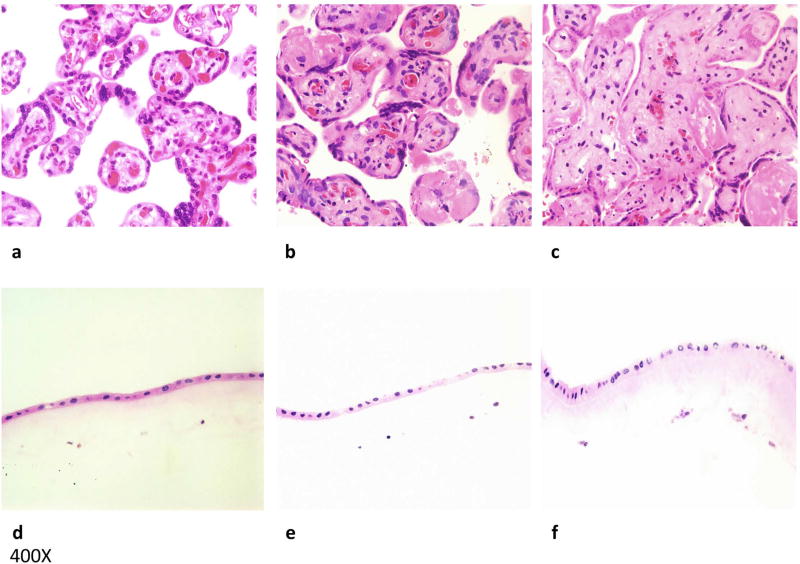
Samples of the villus tree and amnion were dissected and cultured as described in the methods section. Tissue samples were collected at day 1, 7 and 14 of culture, fixed, paraffin embedded, sectioned, and H&E stained (Fig. 1). At the start of culture, chorionic villi were viable and maintained normal morphology with well-preserved synctiotrophoblasts, intact blood vessels, and a lack of karyorrhexis; amnion tissue was well preserved as well. At day 7, much of the syncytiotrophoblast appeared viable and well preserved, with focal areas of early degenerative changes in the form of karyorrhectic debris in blood vessels and villous stromal-vascular karyorrhexis. Most of the amnion appeared well-preserved and viable at day 7. By day 14, placental villous tissue showed slightly more pronounced karyorrhexis and degeneration of syncytiotrophoblast than at day 7. Amnion tissue at day 14 also showed mild degenerative changes in the form of pyknosis.
Figure 1. Placental villous and amnion tissue explants maintain their cytoarchitecture.
H&E sections of placental villous explants at (a) day 1, (b) 7, and (c) 14 of culture (one representative tissue out of 10). Villi maintained normal morphology with well-preserved syncytiotrophoblasts and blood vessels with some focal degenerative changes. H&E sections of amnion explants at (d) day 1, (e) 7, and (f) 14 of culture also show well-preserved tissue with focal degenerative changes at day 14.
Cytokine production
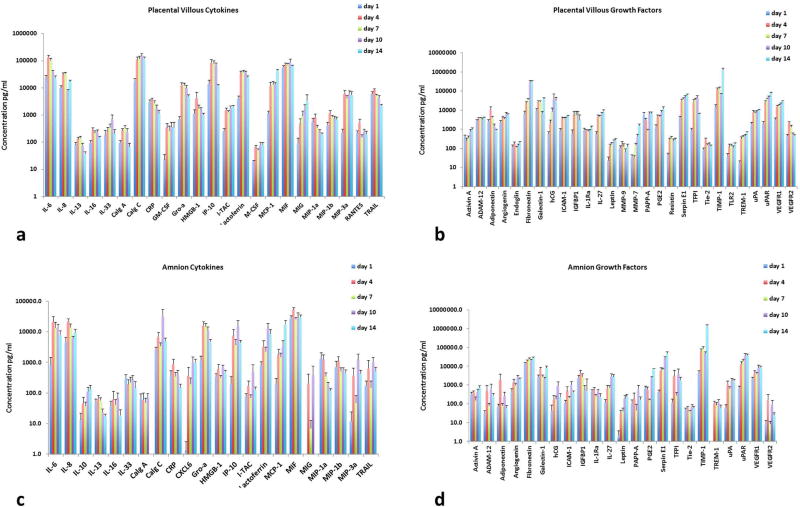
The release of cytokines by villi and amnion cultures over the entire culture period was determined using in-house designed multiplexed bead-based assays150. These assays revealed that cytokines are steadily produced in both placental villous and amnion cultures (Fig. 2a, c). Villous tissue produced large amounts of the pro-inflammatory cytokines IL-6, IL-8, GRO-α, IP-10 and MCP-1, as well as CRP and TRAIL (Fig. 2a). Cultures also released considerable amounts of the alarmins calgranulin A, calgranulin C, and HMGB1, and the antibacterial protein lactoferrin. IL-13, IL-16, and IL-33 were also released, as well as the chemokines ITAC, MIF, MIG, MIP-1α, MIP-1β, MIP-3α, and RANTES. Other cytokines were produced in smaller quantities (see Table S1).
Figure 2. Placental villous and amnion tissue explants maintain cytokine and growth factor production throughout culture period.
Soluble cytokines, growth factors, angiogenic and anti-angiogenic factors are produced by explants over the entire 14-day culture period (presented are average productions, mean ± SEM) as measured by multiplexed bead assays. Culture medium is replaced at each sampling time point.
(a) Placental villous explants: amounts of cytokines released at day 1, 4, 7, 10, and 14, n=10; (b) Placental villous explants: amounts of growth factors released at day 1, 4, 7, 10, and 14, n=10; (c) Amnion explants: amounts of cytokines released at day 1, 4, 7, 10, and 14, n=10; (d) Amnion explants: amounts of growth factors released at day 1, 4, 7, 10, and 14, n=10.
Amnion explants, similar to villi explants, produced cytokines constantly over the duration of the culture period (Fig. 2c). Amnion and villus explants also produced large amounts of the pro-inflammatory cytokines IL-6, IL-8, GRO-α, IP-10 and MCP-1 as well as CRP and TRAIL. Such explants also produced the antimicrobial proteins calgranulin C and lactoferrin as well as smaller amounts of calgranulin A. Moreover, the explants produced the prototypic alarmin HMGB1 as well as IL-10, IL-13, IL-16, IL-33, MIP-1α, MIP-1β, MIP-3α, MIF, CXCL6 and smaller amounts of ITAC, RANTES, and CXCL9 (see Table S1).
Production of growth factors, angiogenic and anti-angiogenic factors
The release of other growth factors, angiogenic factors, anti-angiogenic factors and hormones was determined by multiplexed bead assays. Both villi and amnion explants also continuously produced these factors over the duration of the culture period (Fig. 2b, d). Villi explants produced large amounts of ADAM-12, adiponectin, angiogenin, fibronectin, galectin-1, ICAM-1, IGFBP1, IL-1Ra, IL-27, PAPP-A, Serpin E1, TFPI, TIMP-1, uPA, uPAR, VEGFR1 and VEGFR2, as well as hCG and PGE2 (Fig. 2b). A complete list of factors produced is available in Table S2.
Amnion explants produced large amounts of many of the same growth and angiogenic factors as villi explants including adiponectin, angiogenin, fibronectin, galectin-1, IGFBP1, IL-1Ra, IL-27, Serpin E1, TFPI, TIMP-1, VEGFR1, uPA and uPAR, and the hormones hCG and PGE2 (Fig. 2d) (See Table S2 for complete list).
Analysis of Placental Villous EVs
To analyze EVs specifically from syncytiotrophoblasts (STB) of the explants, magnetic nanoparticles (MNPs) coupled to anti-PLAP antibody, an antigen specific to STB123, 152–155, were used. EVs were labeled with Bodipy FL as described in Methods. Among several commercially available anti-PLAP antibodies, we selected one (clone 8B6) that after coupling to MNPs was specific in capture of STB-generated EVs and captured EVs most efficiently. We analyzed the STB-generated EVs for other antigens that have been described on STBs or STB EVs.
Selection of PLAP antibodies for capture of syncytiotrophoblast EVs
We coupled two clones of PLAP antibodies to MNPs and captured EVs from placental villous culture supernatants. MNPs coupled to two PLAP clones captured similar amounts of EVs: With MNPs coupled to clone H17E2 we captured 108.8 ± 11.6% of EVs captured with MNPs coupled to clone 8B6. However, MNPs coupled to clone H17E2 captured 3.3 ± 0.3 (n=3) times more of non-specific EVs, expressing HLA-ABC. Therefore, we selected clone 8B6 for future experiments since MNPs coupled to the antibodies of this clone seemed to be more specific to capture PLAP-positive EVs.
Specificity of EV capture
We further verified the specificity of our anti-PLAP MNPs by incubation with amnion explant supernatants which should not contain PLAP+ EVs156 and found they captured on average 4.7 ± 0.5% of total EVs (n=3). That was not different from the amount captured with control mouse IgG isotype MNPs: With these MNPs we captured from the placental villous tissue supernatants 4.8 ± 1.1% of EVs that were captured by specific anti-PLAP MNPs (n=3).
The lack of non-STB antigens on anti-PLAP captured STB-generated EVs
To further confirm specificity of the PLAP-captured EVs, we captured EVs from villous samples pooled from multiple donors and stained for CD31, CD41, CD45, and HLA-ABC, all of which should be absent on STB EVs157, 158. All antibodies were labeled with the same fluorophore, and collected into a single “dump” gate. We found that they were present on only 1.6 ± 0.5% of captured EVs (n=3). For the remaining experiments, we included only HLA-ABC, and used the lack of this marker as an additional criterion for STB EVs. Single staining for HLA-ABC on EVs captured by anti-PLAP MNPs revealed 0.7 ± 0.3% of total EVs (n=3).
Syncytiotrophoblast markers on PLAP-positive EVs
Next, we evaluated the distribution of several “phenotypic” markers on the EVs captured by MNPs through PLAP. We chose markers which have been previously described in the literature as being surface markers either of STB themselves, or of the STB-generated EVs154, 159–162 namely, CD51, CD63, CD105, CD200, CD274, and Syncytin-1. Culture supernatants were stained with BoDipy-FL to label EVs, and then captured with anti-PLAP MNPs, stained with antibodies to the above-listed markers (as well as with antibodies to HLA-ABC). The MNP/labeled EV complexes were washed on magnetic columns, eluted and acquired on a flow cytometer set to threshold on the BoDipy-FL EV label. HLA-ABC+ EVs were excluded from the analysis and the rest quantified by expression of the markers and approximate size. (See Figure S1 for gating strategy).
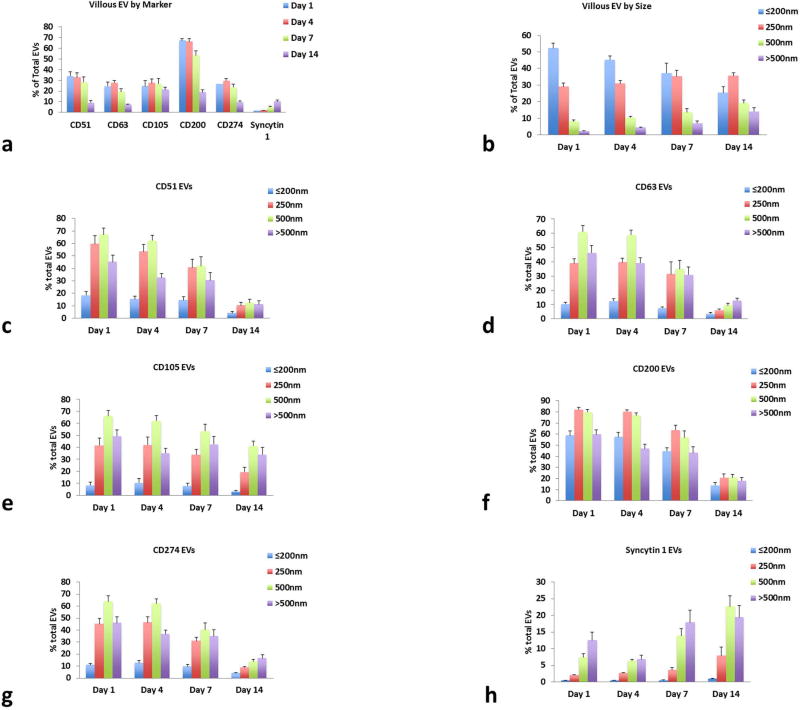
Flow-cytometry analysis revealed that CD200 had the highest expression on PLAP-MNPs captured EVs, being present on 67.3 ± 3.1% of vesicles at day 1, and Syncytin-1 was the lowest at 1.7 ± 0.2%. The other markers were expressed on 24 to 34% of vesicles (Fig. 3a). EV size was estimated using Megamix SSC beads. The vesicles formed a continuum rather than discreet populations, but small vesicles of the size 200nm or less were the most plentiful and over 500nm the least common (Fig. 3b).
Figure 3. Placental villous tissues release a variety of EVs carrying different surface markers.
Placental villous explants release EVs that (a) carry surface markers that are representative of syncytiotrophoblast cells throughout culture and (b) are of a variety of sizes (average % of total EVs for each time point ± SEM, n=10). EVs carrying (c) CD51, (d) CD63, (e) CD105, (f) CD200, (g) CD274, and (h) syncytin-1 maintain similar patterns of expression over time and some are preferentially on EVs of certain sizes (Average % of total EVs for each size range. Mean ± SEM, n=10).
The distribution of the markers varied with vesicles of different sizes (Fig. 3 c-h). Only CD200 was highly expressed (58.8 ± 4.4%) at day 1 on small vesicles (of the size of 200nm or less), while all other markers were present at lower levels on these small vesicles (0.5 – 18.4%). Levels of CD51, CD63, CD105, and CD274 were highest on vesicles of the size of 250–500nm, and syncytin-1 was highest on vesicles of the size above 500nm.
We analyzed co-expression of markers on individual vesicles and found CD51, CD63, CD105, and CD274 were most often co-expressed with CD200, the most highly expressed marker on the placental villous EVs (Fig. S2). Syncytin-1 was the least co-expressed molecule, but was most often co-expressed with CD105.
Assessment of PLAP-captured vesicles over time showed that the total number of vesicles decreased throughout the culture period. Total EVs at day 1 were 1.91 ± 3.3 × 106 EVs/ml and declined to 9.4 ± 1.7 × 104 EVs/ml by day 14 (see Table S3 for EV counts). The distribution of EVs in different size ranges shifted slightly over time (Fig. 3b). The amount of small vesicles (≤200nm) decreased over time, starting at 52.1 ± 3.0% at day 1 and dropping to 25.5 ± 3.6% at day 14, whereas vesicles of all other size ranges increased slightly in percentage with length of culture.
The amount of PLAP-captured vesicles expressing each marker were similar at day 1 and 4, but decreased slightly by day 7 and further by day 14, except for syncytin-1 expressing EVs, which increased in over time (Fig. 3c–h). Except for syncytin-1, all markers maintained over time a similar distribution between EVs of different size. The percentage of EVs double positive for markers was stable up to day 14, except EVs double positive for syncytin-1 and all other markers which increased slightly over time (Fig. S2a).
These results demonstrate that placental villous explants produce EVs carrying typical STB markers throughout the culture period. EVs expressing each marker maintained a similar size distribution over time, but the overall percent of vesicles carrying most of these markers decreased at later days of culture.
Analysis of Amnion EVs
In parallel to the analysis of the STB-released EVs, we analyzed the EVs released by amnion explants by identification of specific cellular antigens on these EVs. EVs were labeled with Bodipy FL as described in Methods. The main cells of interest in amnion explants were amnion epithelial cells (AECs) (since they are in contact with amniotic fluid, thus likely to be involved in fetal communication), as well as the underlying amnion mesenchymal stem cells (AMSCs). We used MNPs coupled to antibodies specific to antigens that these cells carry. Since CD90 is a marker expressed by both AECs and AMSCs163, we investigated this protein as a target for capture with MNPs using anti-CD90 antibodies.
Optimizing capture of amnion EVs
We incubated amnion explant culture supernatants with anti-CD90 MNPs to capture EVs and compared them to capture with anti-CD63, anti-CD9 and anti-HLA-ABC coupled MNPs. MNPs coupled to CD9, HLA-ABC and to CD63 captured 113 ± 5.3%, 75.8 ± 16.7%, and 93.7 ± 11.8% of that of coupled to CD90, respectively (n=3). Since CD90 is the most exclusive marker for our cells of interest, we used anti-CD90 MNPs for our further experiments.
Specificity of EV capture
Next, we verified whether anti-CD90 MNPs specifically capture only EVs carrying CD90. As a negative control, we used these MNPs to capture EVs from placental villous culture supernatants (which should release very few EVs carrying CD90, potentially from placental MSCs164). We found that these MNPs captured on average only 2.5 ± 0.8% of total EVs (n=3). We also confirmed MNP specificity by incubating amnion tissue supernatants with mouse IgG isotype MNPs, which captured 6.3 ± 1.4% of EVs compared to anti-CD90 MNPs (n=3).
Lack of irrelevant antigens on AEC-generated EVs
We captured EVs from amnion samples from multiple donors with anti-CD90 MNPs and stained captured EVs for CD31, CD41, CD45, and HLA-DR, which should not be present on EVs of this origin165. All antibodies were labeled with the same fluorophore, APC-Cy7, and collected into a single “dump” gate. Our staining revealed that these markers were present on only 4.8 ± 0.5% of captured EVs. Further analysis of amnion EVs included only antibodies against HLA-DR, which contributed 2.8 ± 0.3% of total EVs (n=3), and this population was excluded from flow cytometry analysis.
AEC and AMSC markers are revealed on amnion explant EVs
The distribution on EVs of several “phenotypic” markers expressed by AECs or AMSCs166, 167, namely CD29, CD44, CD105, CD140b, CD324, and CD326, were determined. EVs were labeled with BoDipy-FL, captured with anti-CD90 MNPs and stained with antibodies to the above markers (in addition to HLA-DR). The labeled EV-MNP complexes were washed on magnetic columns, eluted and then acquired on a flow cytometer set to threshold on the BoDipy-FL label. Any vesicles positive for HLA-DR were excluded and the remainder quantified by size, estimated by Megamix SSC beads, and expression of the markers of interest (see Fig. S1 for gating strategy).
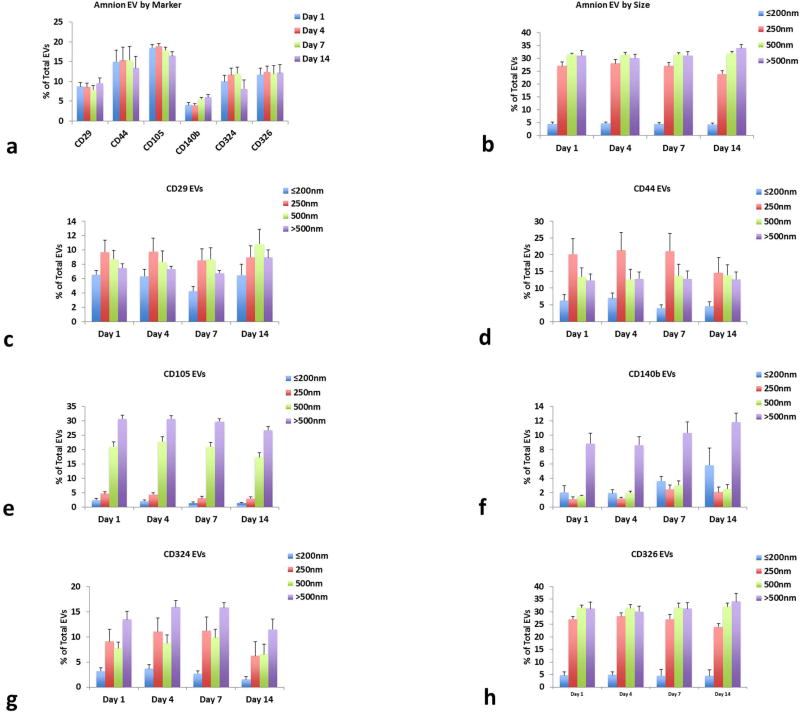
First, we evaluated EVs from amnion culture supernatants at day 1 of culture (Fig. 4a). We found that CD105 was the most highly expressed marker being present on 18.5 ± 0.7% of all captured EVs, and CD140b was the least expressed on 4.0 ±0.6% of EVs (n=10). CD44, CD326, CD324, and CD29 were on approximately on 15, 12, 10, and 9% of EVs respectively. EVs were equally distributed among most size ranges, except EVs of 200nm or less which were only 4.6 ± 0.6% of all EVs (Fig. 4b). Most markers were more likely to be on larger vesicles (Fig 4c–h). CD105 was highest on vesicles of the size of 500nm and over, CD140b was much higher on vesicles with the size over 500nm, and CD44, CD324, CD326 and CD29 were distributed more evenly between all size ranges except the smallest.
Figure 4. Amnion tissues release a variety of EVs carrying different surface markers.
Amnion explants release EVs that (a) carry numerous surface markers that are representative of amnion epithelial and mesenchymal cells throughout culture and (b) are of a variety of sizes (average % of total EVs for each time point ± SEM, n=10). EVs carrying (c) CD29, (d) CD44, (e) CD105, (f) CD140b, (g) CD324, and (h) CD326 maintain similar patterns of expression over time and some are preferentially on EVs of certain sizes (average % of total EVs for each size range. Mean ± SEM, n=10).
Evaluation of marker co-expression demonstrated that CD29 and CD44 were the most commonly found together (4.2 ± 0.7% of EVs at day 1), followed by CD140b and CD326 (3.1 ± 0.7% EVs at day 1) (Fig. S2b).
Next, we investigated how the number of CD90-captured vesicles changed over time. Unlike PLAP captured EVs from placental villous explants, the amount of amnion-generated vesicles captured with CD90-MNPs did not decrease over time. The total concentration of vesicles at day 1 was 9.5 ± 1.4 × 104/mL and at day 14 was 9.9 ± 1.8 × 104/mL (see Table S3 for all EV counts). The amount of amnion EVs remained constant over the entire culture period in all aspects: in size ranges of vesicles (Fig. 4b), in the fractions of total EVs for each (Fig. 4c–h), and for the fractions of double positive EVs (Fig. S2b).
These results confirm that amnion explants continually produce EVs representative of AECs and AMSCs over 14 days of culture.
Analysis of EV-associated cytokines
EVs from different cells carry different cytokines
We captured EVs from culture supernatants at day 4 with MNPs coupled with specific capture antibodies to investigate whether EVs with different surface markers (i.e. generated by different cells) carry different cytokines.
Placental villous EVs
Total EVs were isolated from placental villous culture supernatants using Exoquick TC™. From this isolate we captured several types of EVs using anti-PLAP coupled MNPs to capture STB-generated EVs, anti-CD31 MNPs to capture EVs generated by endothelial cells, and HLA-G to capture EVs released by cytotrophoblasts and placental MSCs. Following MNP capture, EVs were magnetically isolated as described in Methods, and the EV-associated cytokines and growth factors were evaluated. Surface associated proteins were measured directly with multiplexed bead assays, and total EV proteins were measured after EVs were lysed. We then subtracted the surface quantity from the total to determine the internal protein concentrations.
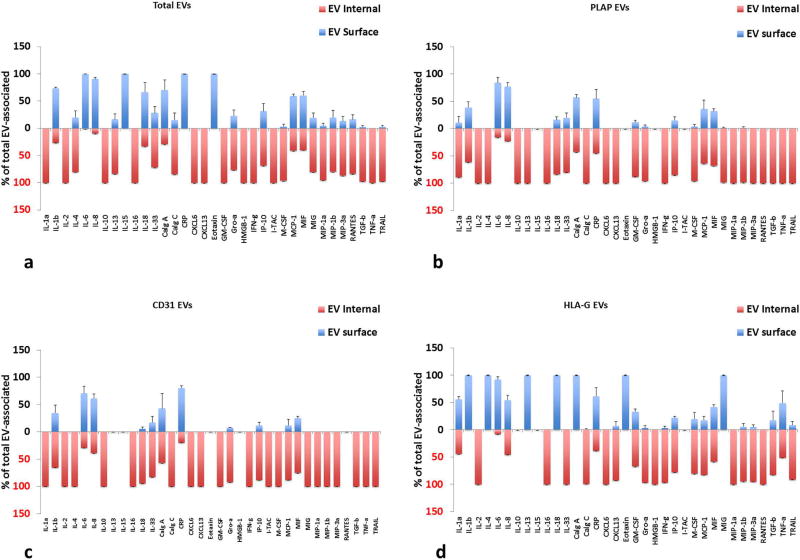
Most cytokines were found associated with EVs, and those in the greatest amounts were IL-4, IL-8, IL-10, IL-13, IL-33, Calgranulin C, CRP, IFNγ, IP-10, MIF, MIG, MIP-3α, and TRAIL. (See Table S4 for cytokine concentrations). Overall, cytokines tended to be EV-encapsulated rather than on their surface (Fig. 5). HLA-G captured EV had slightly more cytokines on their surface compared to anti-PLAP or anti-CD31 captured EVs. PLAP captured EVs carried significantly more IL-4, IL-16, MIG, and TGF-β compared to both other types of capture (p<0.05, n=5), and were located predominantly inside EVs (Fig. 5b). CD31 captured EVs were significantly higher in MIP3α and CXCL6 compared to HLA-G captured EVs only (p<0.05, n=5), and these were encapsulated (Fig. 5c). HLA-G captured EVs were higher than both other captures in GM-CSF, IP-10 and MIF (p<0.05, n=5) and these were both on the surface and encapsulated (Fig. 5d).
Figure 5. Distribution of cytokines between the surface and inner volume of EVs from placental villous tissues.
Distribution between encapsulated and surface cytokines is shown for placental villous cultures. (a) Total EVs isolated by Exoquick™(b) anti-PLAP MNP-captured EVs; (c) anti-CD31 MNP-captured EVs; (d) anti-HLA-G MNP-captured EVs. Free and EV-associated cytokines are expressed as percent of total (Mean ± SEM, n=5). Blue bars: surface-associated cytokines, red: EV-encapsulated. Multiplexed bead assay measurements on samples collected at day 4 (cumulative amount for days 1–4 of culture).
Amnion EVs
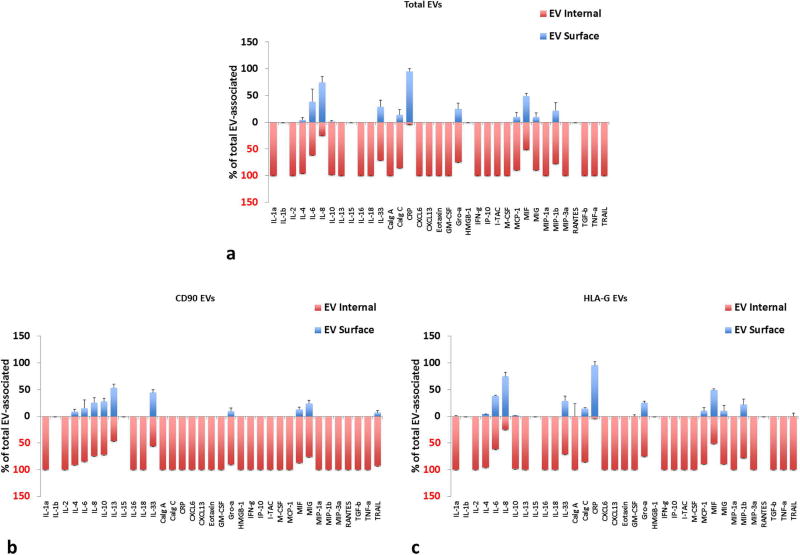
Total EVs were isolated from amnionic culture supernatants using Exoquick TC™. Amnion EVs were captured with anti-CD90 MNPs, to capture presumably EVs from both AECs and AMSCs, and HLA-G antibodies to capture EVs from selected cells, as HLA-G has been reported in various levels on AECs and only weakly on AMSCs. Also, many cytokines were associated with EV (see Table S5) especially IL-4, IL-8, IL-10, IL-13, IL-33, Calgranulin C, GRO-α, IFNγ, MIF, MIG, MIP-3α, and TRAIL. Similar to EVs from placental villous explants, for amnion explant EVs, most cytokines were predominantly inside EVs (Fig. 6). HLA-G captured EVs expressed slightly more cytokines on their surface compared to CD90 captured EVs (Fig. 6b–c). CD90 MNP-captured EVs had significantly higher amounts of IL-4, IL-10, IL-13, IL-33, CXCL6, Eotaxin, ITAC, MIG, MIP3α, and TGF-β than HLA-G captured EVs (p<0.05, n=5) and most were predominantly inside (Fig. 6b). HLA-G captured the highest levels of Calgranulin C, GM-CSF, MIF and MIP-1β compared to CD90 captured EVs (p<0.05, n=5), and most were internal to the EVs (Fig. 6c).
Figure 6. Distribution of cytokines between the surface and inner volume of EVs from amnion tissues.
Distribution between encapsulated and surface cytokines is shown for amnion cultures (a) Total EVs isolated by Exoquick™; (b) anti-CD90 MNP-captured EVs; (c) anti-HLA-G MNP-captured EVs. Free and EV-associated cytokines are expressed as percent of total (Mean ± SEM, n=5). Blue bars: surface-associated cytokines, red: EV-encapsulated. Multiplexed bead assay measurements on samples collected at day 4 (cumulative amount for days 1–4 of culture).
EVs from different cells carry different growth factors
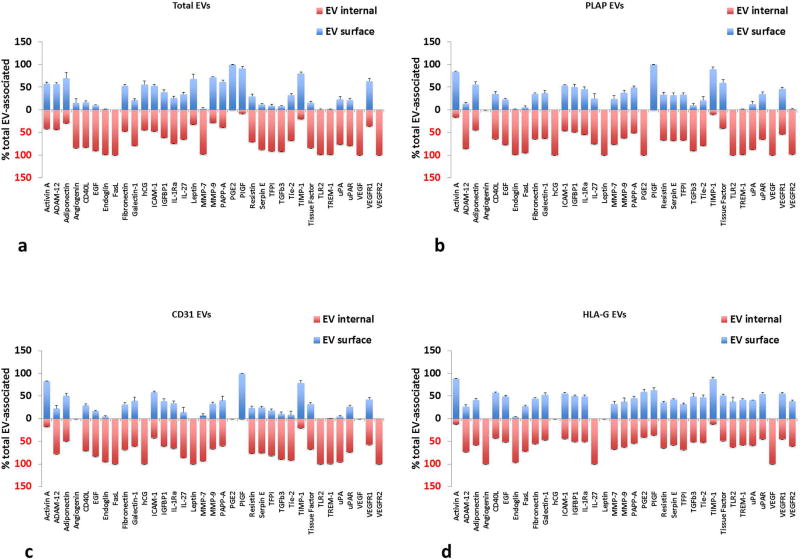
EVs from placental villous tissue also contained several growth factors and angiogenic related factors (see Table S6). Activin A, adiponectin, endoglin, fibronectin, galectin-1, ICAM-1, IL-1RA, IL-27, MMP-9, PAPP-A, serpin E1, TFPI, TIMP-1, TREM-1, uPA, uPAR, and VEGFR2 were found in the greatest quantities, as well as hCG and PGE2. Similar to cytokines, these growth factors were predominantly encapsulated within EVs rather than on their surface (Fig. 7), although HLA-G captured EVs had more surface-associated than the other two captures. PLAP captured EVs had significantly higher amounts of EV-associated ADAM12, endoglin, and PIGF than either CD31 or HLA-G captured EVs (p<0.05, n=5). PIGF was mostly on the surface of EVs, whereas ADAM12 and endoglin were predominantly encapsulated (Fig. 7b–d). CD31 captured EVs carried significantly higher amounts of internal IL-27 and TREM-1 than HLA-G EVs (p<0.05, n=5). HLA-G captured EVs contained significantly more adiponectin, CD40L, EGF, FasL, fibronectin, galectin-1, PGE2, Resistin, TFPI, TGF-β3, Tie-2, tissue factor, TREM-1, uPA, uPAR, VEGFR1, and VEGFR2 than both PLAP and CD31 captured EVs (p<0.05, n=5).
Figure 7. Distribution of growth factors between the surface and inner volume of EVs from placental villous tissues.
Distribution between encapsulated and surface growth factors is shown for placental villous cultures. (a) Total EVs isolated by Exoquick™; (b) anti-PLAP MNP-captured EVs; (c) anti-CD31 MNP-captured EVs; (d) anti-HLA-G MNP- captured EVs. Free and EV-associated growth factors are expressed as percent of total (Mean ± SEM, n=5). Blue bars: surface-associated growth factors, red: EV-encapsulated. Multiplexed bead assay measurements on samples collected at day 4 (cumulative amount for days 1–4 of culture).
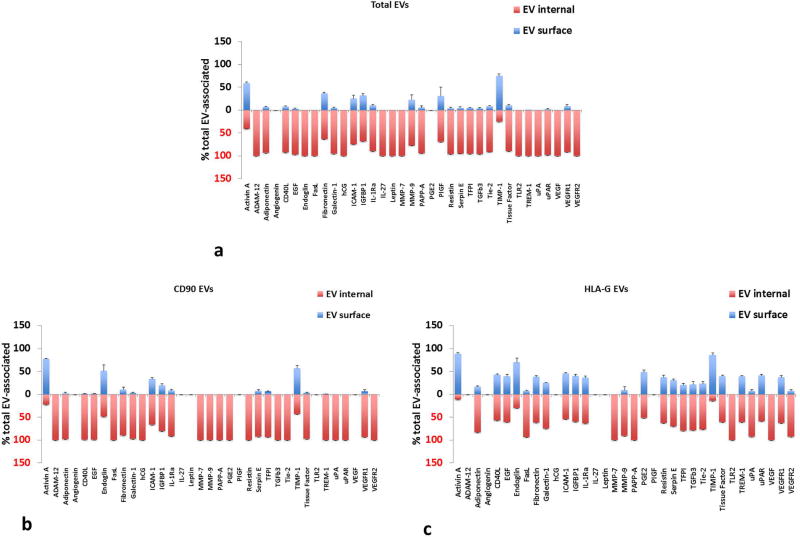
Growth and angiogenic factors were also found associated with amnion EVs (see Table S7), with activin A, adiponectin, fibronectin, galectin-1, ICAM-1, IL-1Ra, PAPP-A, serpin E1, TFPI, TIMP-1, TREM-1, uPA, uPAR, and VEGFR1, as well as hCG and PGE2, secreted in the highest amounts. Amnion EVs also carried most growth factors predominantly inside EVs, and HLA-G captured EVs had slightly more surface growth factors than CD90 captured (Fig. 8). CD90 MNPs captured EVs had significantly higher amounts of PAPP-A, and TREM-1 (p<0.05, n=5), with PAPP-A being predominantly inside and TREM-1 being both on the surface and inside (Fig. 8b). HLA-G captured significantly higher levels of adiponectin, CD40L, EGF, endoglin, FasL, galectin-1, ICAM-1, IGFBP1, IL-1Ra, PGE2, resistin, TFPI, TGF-β3, Tie-2, tissue factor, uPA, uPAR, VEGFR1 and VEGFR2 (p<0.05, n=5), much the same as HLA-G captured villi EVs (Fig. 8c).
Figure 8. Distribution of growth factors between the surface and inner volume of EVs from amnion tissues.
Distribution between encapsulated and surface growth factors is shown for amnion cultures. (a) Total EVs isolated by Exoquick™; (b) anti-CD90 MNP-captured EVs (c) anti-HLA-G MNP-captured EVs. Free and EV-associated growth factors are expressed as percent of total (Mean ± SEM, n=5). Blue bars: surface-associated growth factors, red: EV-encapsulated. Multiplexed bead assay measurements on samples collected at day 4 (cumulative amount for days 1–4 of culture).
Discussion
Previous studies of placental explants
Several techniques for maintaining placental explants have been described168–171, with different models being useful for different purposes. Typically, placental tissues are immersed in the culture medium either free floating or supported by Matrigel™ or Millicell inserts. In these experiments, tissues remain viable up to 9 days and produce human chorionic gonadotropin (hCG) and placental lactogen 172. Most of these models report that STB are lost in the first 1–2 days of culture but some regeneration was observed by 5–7 days173, 174.
Following the pioneer works of Hoffman et al175, 176, we developed cultures of ex vivo tissues maintained on collagen sponges at the medium/air interface to study HIV pathogenesis in human lymphoid177–181, cervico-vaginal182–184 and recto-sigmoid tissues185, and to investigate the physiology of atherosclerotic plaques 186, 187 ex vivo. A comparable culture method was used to study cytomegalovirus infection188. Here, we apply a similar technique to study placental tissue secretion of EVs, cytokines and growth factors ex vivo.
The establishment of a three-dimensional culture to study extracellular vesicles and cytokines
The purpose of the present study was to develop a laboratory model to study soluble factors and EVs generated by placental villous tissue. This is important since both EVs and soluble factors, in particular placental cytokines189, 190, are implicated in maternal-fetal communication. This especially concerns STB that are in direct contact with the maternal blood, and amnion epithelial cells that are surrounding the amniotic cavity containing the fetus. We found that under our protocol, explants of both placental villous tissue and amnion are viable for at least 14 days as evidenced by histological analysis. Both types of explants continue to secrete cytokines and growth factors over 14 days of culture providing further evidence of tissue viability and functionality.
Evaluating these secreted factors in tissue models allows the determination of tissue origin of these factors, which is not easily accomplished in vivo. Yet, we cannot rule out a minor contribution of factors derived from entrapped maternal or fetal cells in placental vessels.
A number of publications have addressed placental EVs and their potential role in pregnancy and its complications130, 132, 133, 146, 191–225. Several ex vivo (e.g. placental perfusion)214, 226–232 and in vivo233–236 systems have been used as a source of EVs. Placental perfusion is a useful method for obtaining large numbers of EVs directly from the placenta; however, this technique is suitable only for a short period of time (2–6 hours) after delivery237. In vivo studies on EVs obtained from maternal blood are difficult to interpret because of multiple potential cellular sources of these EVs. Focusing on the analysis of EVs generated by placental cells requires the ability to trace particular EVs to their cells of origin. Towards this goal, rather than “bulk” analysis of EVs, we employed a newly developed nanotechnology platform238, which allows capture of EVs with magnetic nanoparticles (MNPs) coupled to specific antibodies against EV surface antigens and analyzing these EVs individually. The captured EVs can then be stained with additional antibodies to reveal specific antigens of interest. Here, we applied this analysis to EVs generated by placental explants.
Analysis of placental villous extracellular vesicles
We found that STB-specific EVs can be captured from placental villous culture supernatants using anti-PLAP MNPs. PLAP is a sialoglycoprotein enzyme that is present almost exclusively on STB and has been used as a marker of STB-derived EVs123, 153, 155. We first demonstrated specificity of capture by demonstrating that anti-PLAP MNPs capture significant amounts of EVs from placental villous explants but very few EVs from culture supernatants of amnion explants. PLAP captured EVs also do not express non-STB markers including CD31, CD41, CD45, and HLA-ABC above the background level (EVs captured by isotype control MNPs). EVs were expressed throughout the entire 14 days of culture, though their quantities declined at day 14.
We assessed the PLAP-captured EVs for other surface proteins that have previously been described to be expressed on STB or on their EVs, CD51, CD63, CD105, CD200, CD274, and syncytin-1. All these proteins were found albeit in various quantities on PLAP-MNP-captured EVs239. CD51, or vitronectin receptor alpha chain, is an adhesion molecule239. CD63 is a tetraspanin known to associate with membranes of intracellular vesicles239. CD105, also known as endoglin, has a crucial role in the regulation of angiogenesis240. CD200, also named OX-2 membrane glycoprotein, may have a role in macrophage differentiation241. CD274 or programmed death-ligand 1(PD-L1) is an immune checkpoint molecule that may have a role in immune suppression during pregnancy242–244. Syncytin-1 mediates trophoblast fusion and may have a role in tolerance to fetal antigens245, 246. Herein, CD200 was the most widely expressed marker and syncytin-1 the least expressed. These markers demonstrated some differences in their expression on EVs of different size ranges, for instance most markers were expressed on only a small percentage of small EVs, except for CD200. These differences may reflect differential function of these EVs. Whatever are these functions, the overall the pattern of these antigens expression on the different sizes of EVs remained constant again demonstrating viability of the ex vivo tissues. Also, co-expression of the various markers remains fairly constant over time.
Analysis of extracellular vesicles produced by amnion
EVs were also produced by amnion and were captured with anti-CD90 MNPs. CD90 is a cell surface glycoprotein involved in cell adhesion that is expressed on both AECs and AMSCs, as well at varying levels on fibroblasts, neurons and activated endothelial cells163, 247–250. We confirmed specificity of capture by showing anti-CD90 MNPs captured very few EVs generated by placental villous tissue. Also, CD90 MNP-captured EVs lacked expression of markers that should not be present on amnion-generated EVs, including CD31, CD41, CD45, and HLA-DR. EVs were generated at constant levels throughout all the 14 days of culture, and maintained the same size distributions. These EVs carried other proteins on their surface that have previously been described on AECs and AMSCs. These included CD29, CD44, CD105, CD140b, CD324, and CD326, which are involved in cell-cell and cell-matrix interactions, cell adhesion, and migration251. CD29 (integrin beta-1) acts as a fibronectin receptor252. CD44 is a receptor for hyaluronic acid253. CD140b is a tyrosine kinase receptor for members of the platelet derived growth factor family and a marker for naive AMSCs254. CD324 or E-cadherin is a regulator of epithelial junction formation255. CD326, also known as Ep-CAM, is an epithelial cell surface antigen256. Herein, CD105 was the most widely expressed marker and CD140b was the least expressed on amnion-derived EVs. These markers demonstrated some differences in their expression on EVs of different size ranges, but all were least prevalent on the smallest vesicles. Overall the pattern of expression on the different sizes of EVs remained constant over time. Moreover, co-expression of the various markers remains fairly constant over time.
Cytokines and other factors in EVs of different phenotype
We previously reported that various cytokines are associated with EVs257. Here, we demonstrate that not only cytokines, but many other growth factors, angiogenic and anti-angiogenic factors are associated with EVs from placental villous and amnion tissues. These factors can be on the EV-surface or encapsulated within the vesicles. In this study, we took this analysis one step further from the analysis of association of these factors with general EVs to their association with EVs that carry particular membrane proteins. Specifically, we captured EVs using MNPs coupled to antibodies that select for certain EV populations, and analyzing the cytokine and growth factor content of these EV fractions.
We found that placental villous EVs captured via PLAP, CD31, and HLA-G not only carry different levels of these factors, but their distributions between the EV surface and internal space were different. Some cytokines segregated completely between different EVs. For example, Eotaxin and HMGB1 were present only in HLA-G MNP-captured EVs, and ITAC was observed exclusively in CD31 MNP-captured EVs. IL-13, RANTES, and PGE2 were not present in CD31 EVs but were found in both PLAP and HLA-G captured EVs, whereas hCG was absent in HLA-G EVs. Some cytokines were carried exclusively on the EV surface, for example IL-4, IL-13, and Eotaxin in HLA-G MNP-captured EVs, whereas IL-16, IL-33 and RANTES were exclusively inside HLA-G MNP-captured EVs. Other cytokines were found on the surface in EVs captured through one membrane protein, but internally in EVs captured through another protein. For example, IL-4 and MIG were found internally in EVs captured with PLAP MNP and CD31 MNP, but on the surface in HLA-G MNP-captured.
In amnion tissue, we specifically captured EVs using anti-CD90 and anti-HLA-G MNPs, analyzed their cytokine and growth factor content, and found differences in amounts and distributions of these EV-associated proteins. For example, only EVs captured via CD90 but not via HLA-G carried IL-4, Eotaxin and ITAC. CD40L, PGE2, and uPAR were encapsulated in CD90 MNP-captured EVs but were present both inside and on the surface HLA-G MNP captured EVs.
This complex differential distribution of cytokines between EVs of different origin and phenotype suggests a fine regulation of their biogenesis and indicates different biological functions of these EVs. To identify these functions EVs should be characterized individually rather than in bulk. The ability to characterize and distinguish individual EVs generated by different cell types and carrying various cytokines and growth factors is the major advantage of our methods. Also, we can identify EVs that co-express different membrane proteins. For instance, CD90 and HLA-G in amnion may be co-expressed on some EVs, and CD31 and HLA-G may be co-expressed in placental villous tissue. This distinction may be the reflection of their differential biological role.
The use of the placental tissue culture described herein coupled with the newly described nanotechnology provides a novel and powerful tool for probing maternal-fetal communication through EVs that can be now traced to their cellular/tissue origin, characterized by their surface-associated and encapsulated proteins. This multifactorial characterization of EVs in an ex vivo tissue system will enable us to narrow the search for possible placental biomarkers in maternal blood and amniotic fluid and identify their changes in various pathologies.
Supplementary Material
Acknowledgments
This research was supported, in part, by the Perinatology Research Branch (PRB), Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS), and, in part, with federal funds from the NICHD/NIH/DHHS under Contract No.HHSN275201300006C. N.G-L is also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health. We thank the physicians and nurses from the Center for Advanced Obstetrical Care and Research and the Intrapartum Unit, as well as the research assistants from the PRB Clinical Laboratory, for their help in collecting samples. In addition, we thank Suzanne M. Jacques, M.D. and Faisal Qureshi, M.D. for analysis of histological sections of tissue explants. This work was supported by NICHD Intramural Program.
Footnotes
Author Contributions:
W.F. conceived, designed and performed experiments, analyzed and discussed data, and contributed to writing of the manuscript. N.G-L. and O.E. analyzed and discussed data and contributed to writing of the manuscript. R.R. and L.M. conceived and designed experiments, analyzed and discussed the data and contributed to writing the manuscript.
Conflict of Interests
The authors declare no conflict of interests.
References
- 1.Desoye G, Shafrir E. Placental metabolism and its regulation in health and diabetes. Mol Aspects Med. 1994;15:505–682. doi: 10.1016/0098-2997(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 2.Hay WW., Jr Placental-fetal glucose exchange and fetal glucose metabolism. Trans Am Clin Climatol Assoc. 2006;117:321–339. discussion 339–340. [PMC free article] [PubMed] [Google Scholar]
- 3.Freemark M. Placental Hormones and the Control of Fetal Growth. The Journal of Clinical Endocrinology & Metabolism. 2010;95:2054–2057. doi: 10.1210/jc.2010-0517. [DOI] [PubMed] [Google Scholar]
- 4.Newbern D, Freemark M. Placental hormones and the control of maternal metabolism and fetal growth. Curr Opin Endocrinol Diabetes Obes. 2011;18:409–416. doi: 10.1097/MED.0b013e32834c800d. [DOI] [PubMed] [Google Scholar]
- 5.Martino J, Sebert S, Segura MT, Garcia-Valdes L, Florido J, Padilla MC, Marcos A, Rueda R, McArdle HJ, Budge H, Symonds ME, Campoy C. Maternal Body Weight and Gestational Diabetes Differentially Influence Placental and Pregnancy Outcomes. J Clin Endocrinol Metab. 2016;101:59–68. doi: 10.1210/jc.2015-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Croy BA, Wood W, King GJ. Evaluation of intrauterine immune suppression during pregnancy in a species with epitheliochorial placentation. J Immunol. 1987;139:1088–1095. [PubMed] [Google Scholar]
- 7.Chaouat G, Menu E, Athanassakis I, Wegmann TG. Maternal T cells regulate placental size and fetal survival. Reg Immunol. 1988;1:143–148. [PubMed] [Google Scholar]
- 8.Bulmer JN. Immune aspects of pathology of the placental bed contributing to pregnancy pathology. Baillieres Clin Obstet Gynaecol. 1992;6:461–488. doi: 10.1016/s0950-3552(05)80006-9. [DOI] [PubMed] [Google Scholar]
- 9.Redman CW. Immunological aspects of pre-eclampsia. Baillieres Clin Obstet Gynaecol. 1992;6:601–615. doi: 10.1016/s0950-3552(05)80012-4. [DOI] [PubMed] [Google Scholar]
- 10.Engelhardt H, King GJ. Uterine natural killer cells in species with epitheliochorial placentation. Nat Immun. 1996;15:53–69. [PubMed] [Google Scholar]
- 11.Guimond M, Wang B, Croy BA. Immune competence involving the natural killer cell lineage promotes placental growth. Placenta. 1999;20:441–450. doi: 10.1053/plac.1999.0398. [DOI] [PubMed] [Google Scholar]
- 12.Miles JR, Beetham PK, Segerson EC. Suppressor cell activity of ovine caruncular and intercaruncular tissues during the placentation period. Theriogenology. 2002;58:1097–1109. doi: 10.1016/s0093-691x(01)00681-1. [DOI] [PubMed] [Google Scholar]
- 13.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 14.Red-Horse K, Rivera J, Schanz A, Zhou Y, Winn V, Kapidzic M, Maltepe E, Okazaki K, Kochman R, Vo KC, Giudice L, Erlebacher A, McCune JM, Stoddart CA, Fisher SJ. Cytotrophoblast induction of arterial apoptosis and lymphangiogenesis in an in vivo model of human placentation. J Clin Invest. 2006;116:2643–2652. doi: 10.1172/JCI27306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Than NG, Romero R, Goodman M, Weckle A, Xing J, Dong Z, Xu Y, Tarquini F, Szilagyi A, Gal P, Hou Z, Tarca AL, Kim CJ, Kim JS, Haidarian S, Uddin M, Bohn H, Benirschke K, Santolaya-Forgas J, Grossman LI, Erez O, Hassan SS, Zavodszky P, Papp Z, Wildman DE. A primate subfamily of galectins expressed at the maternal-fetal interface that promote immune cell death. Proc Natl Acad Sci U S A. 2009;106:9731–9736. doi: 10.1073/pnas.0903568106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bulmer JN, Williams PJ, Lash GE. Immune cells in the placental bed. Int J Dev Biol. 2010;54:281–294. doi: 10.1387/ijdb.082763jb. [DOI] [PubMed] [Google Scholar]
- 17.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiby SE, Apps R, Sharkey AM, Farrell LE, Gardner L, Mulder A, Claas FH, Walker JJ, Redman CW, Morgan L, Tower C, Regan L, Moore GE, Carrington M, Moffett A. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest. 2010;120:4102–4110. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munoz-Suano A, Hamilton AB, Betz AG. Gimme shelter: the immune system during pregnancy. Immunol Rev. 2011;241:20–38. doi: 10.1111/j.1600-065X.2011.01002.x. [DOI] [PubMed] [Google Scholar]
- 20.Colucci F, Boulenouar S, Kieckbusch J, Moffett A. How does variability of immune system genes affect placentation? Placenta. 2011;32:539–545. doi: 10.1016/j.placenta.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong S, Sharkey AM, Kennedy PR, Gardner L, Farrell LE, Chazara O, Bauer J, Hiby SE, Colucci F, Moffett A. Maternal uterine NK cell-activating receptor KIR2DS1 enhances placentation. J Clin Invest. 2013;123:4264–4272. doi: 10.1172/JCI68991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaouat G. Inflammation, NK cells and implantation: friend and foe (the good, the bad and the ugly?): replacing placental viviparity in an evolutionary perspective. J Reprod Immunol. 2013;97:2–13. doi: 10.1016/j.jri.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Mor G, Kwon JY. Trophoblast-microbiome interaction: a new paradigm on immune regulation. Am J Obstet Gynecol. 2015;213:S131–137. doi: 10.1016/j.ajog.2015.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.PrabhuDas M, Bonney E, Caron K, Dey S, Erlebacher A, Fazleabas A, Fisher S, Golos T, Matzuk M, McCune JM, Mor G, Schulz L, Soares M, Spencer T, Strominger J, Way SS, Yoshinaga K. Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol. 2015;16:328–334. doi: 10.1038/ni.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta SK, Malhotra SS, Malik A, Verma S, Chaudhary P. Cell Signaling Pathways Involved During Invasion and Syncytialization of Trophoblast Cells. Am J Reprod Immunol. 2016;75:361–371. doi: 10.1111/aji.12436. [DOI] [PubMed] [Google Scholar]
- 26.Wei J, Lau SY, Blenkiron C, Chen Q, James JL, Kleffmann T, Wise M, Stone PR, Chamley LW. Trophoblastic debris modifies endothelial cell transcriptome in vitro: a mechanism by which fetal cells might control maternal responses to pregnancy. Sci Rep. 2016;6:30632. doi: 10.1038/srep30632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conrad ML, Freitag N, Diessler ME, Hernandez R, Barrientos G, Rose M, Casas LA, Barbeito CG, Blois SM. Differential Spatiotemporal Patterns of Galectin Expression are a Hallmark of Endotheliochorial Placentation. Am J Reprod Immunol. 2016;75:317–325. doi: 10.1111/aji.12452. [DOI] [PubMed] [Google Scholar]
- 28.Bonney EA. Alternative theories: Pregnancy and immune tolerance. J Reprod Immunol. 2017;123:65–71. doi: 10.1016/j.jri.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Mor G, Aldo P, Alvero AB. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol. 2017;17:469–482. doi: 10.1038/nri.2017.64. [DOI] [PubMed] [Google Scholar]
- 30.Hackmon R, Pinnaduwage L, Zhang J, Lye SJ, Geraghty DE, Dunk CE. Definitive class I human leukocyte antigen expression in gestational placentation: HLA-F, HLA-E, HLA-C, and HLA-G in extravillous trophoblast invasion on placentation, pregnancy, and parturition. Am J Reprod Immunol. 2017:77. doi: 10.1111/aji.12643. [DOI] [PubMed] [Google Scholar]
- 31.Taylor RW, Metters J, Brush MG, Tye G. Placental function in pre-eclampsia. Proc R Soc Med. 1970;63:1102–1104. doi: 10.1177/003591577006311P111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sebire NJ, Goldin RD, Regan L. Term preeclampsia is associated with minimal histopathological placental features regardless of clinical severity. J Obstet Gynaecol. 2005;25:117–118. doi: 10.1080/014436105400041396. [DOI] [PubMed] [Google Scholar]
- 33.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D’Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 34.Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum Reprod Update. 2006;12:747–755. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol. 2010;5:173–192. doi: 10.1146/annurev-pathol-121808-102149. [DOI] [PubMed] [Google Scholar]
- 36.Sharp AN, Heazell AE, Crocker IP, Mor G. Placental apoptosis in health and disease. Am J Reprod Immunol. 2010;64:159–169. doi: 10.1111/j.1600-0897.2010.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geifman-Holtzman O, Xiong Y, Holtzman EJ, Hoffman B, Gaughan J, Liebermann DA. Increased placental telomerase mRNA in hypertensive disorders of pregnancy. Hypertens Pregnancy. 2010;29:434–445. doi: 10.3109/10641950903214625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204:193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts JM, Escudero C. The placenta in preeclampsia. Pregnancy Hypertens. 2012;2:72–83. doi: 10.1016/j.preghy.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naljayan MV, Karumanchi SA. New developments in the pathogenesis of preeclampsia. Adv Chronic Kidney Dis. 2013;20:265–270. doi: 10.1053/j.ackd.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Redman CW, Sargent IL, Staff AC. IFPA Senior Award Lecture: making sense of pre-eclampsia - two placental causes of preeclampsia? Placenta. 2014;(35 Suppl):S20–25. doi: 10.1016/j.placenta.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 42.Roberts JM. Pathophysiology of ischemic placental disease. Semin Perinatol. 2014;38:139–145. doi: 10.1053/j.semperi.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anton L, Brown AG, Bartolomei MS, Elovitz MA. Differential methylation of genes associated with cell adhesion in preeclamptic placentas. PLoS One. 2014;9:e100148. doi: 10.1371/journal.pone.0100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Redman CW, Staff AC. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. Am J Obstet Gynecol. 2015;213 doi: 10.1016/j.ajog.2015.08.003. S9 e1, S9–11. [DOI] [PubMed] [Google Scholar]
- 45.Anton L, Olarerin-George AO, Hogenesch JB, Elovitz MA. Placental expression of miR-517a/b and miR-517c contributes to trophoblast dysfunction and preeclampsia. PLoS One. 2015;10:e0122707. doi: 10.1371/journal.pone.0122707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Myatt L, Roberts JM. Preeclampsia: Syndrome or Disease? Curr Hypertens Rep. 2015;17:83. doi: 10.1007/s11906-015-0595-4. [DOI] [PubMed] [Google Scholar]
- 47.Alexander KL, Mejia CA, Jordan C, Nelson MB, Howell BM, Jones CM, Reynolds PR, Arroyo JA. Differential Receptor for Advanced Glycation End Products Expression in Preeclamptic, Intrauterine Growth Restricted, and Gestational Diabetic Placentas. Am J Reprod Immunol. 2016;75:172–180. doi: 10.1111/aji.12462. [DOI] [PubMed] [Google Scholar]
- 48.Daglar K, Kirbas A, Timur H, Ozturk Inal Z, Danisman N. Placental levels of total oxidative and anti-oxidative status, ADAMTS-12 and decorin in early- and late-onset severe preeclampsia. J Matern Fetal Neonatal Med. 2016;29:4059–4064. doi: 10.3109/14767058.2016.1154942. [DOI] [PubMed] [Google Scholar]
- 49.Andraweera PH, Bobek G, Bowen C, Burton GJ, Correa Frigerio P, Chaparro A, Dickinson H, Duncombe G, Hyett J, Illanes SE, Johnstone E, Kumar S, Morgan TK, Myers J, Orefice R, Roberts CT, Salafia CM, Thornburg KL, Whitehead CL, Bainbridge SA. IFPA meeting 2015 workshop report II: mechanistic role of the placenta in fetal programming; biomarkers of placental function and complications of pregnancy. Placenta. 2016;48(Suppl 1):S7–s11. doi: 10.1016/j.placenta.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 50.Nandi P, Siddiqui MF, Lala PK. Restraint of Trophoblast Invasion of the Uterus by Decorin: Role in Pre-eclampsia. Am J Reprod Immunol. 2016;75:351–360. doi: 10.1111/aji.12449. [DOI] [PubMed] [Google Scholar]
- 51.Resic Karara J, Zekic Tomas S, Marusic J, Roje D, Kuzmic Prusac I. Fas and FasL expression in placentas complicated with intrauterine growth retardation with and without preeclampsia. J Matern Fetal Neonatal Med. 2016;29:1154–1159. doi: 10.3109/14767058.2015.1038702. [DOI] [PubMed] [Google Scholar]
- 52.Labarrere CA, DiCarlo HL, Bammerlin E, Hardin JW, Kim YM, Chaemsaithong P, Haas DM, Kassab GS, Romero R. Failure of physiologic transformation of spiral arteries, endothelial and trophoblast cell activation, and acute atherosis in the basal plate of the placenta. Am J Obstet Gynecol. 2017;216:287 e281–287 e216. doi: 10.1016/j.ajog.2016.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ashar-Patel A, Kaymaz Y, Rajakumar A, Bailey JA, Karumanchi SA, Moore MJ. FLT1 and transcriptome-wide polyadenylation site (PAS) analysis in preeclampsia. Sci Rep. 2017;7:12139. doi: 10.1038/s41598-017-11639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nezu M, Souma T, Yu L, Sekine H, Takahashi N, Wei AZ, Ito S, Fukamizu A, Zsengeller ZK, Nakamura T, Hozawa A, Karumanchi SA, Suzuki N, Yamamoto M. Nrf2 inactivation enhances placental angiogenesis in a preeclampsia mouse model and improves maternal and fetal outcomes. Sci Signal. 2017:10. doi: 10.1126/scisignal.aam5711. [DOI] [PubMed] [Google Scholar]
- 55.Palmer KR, Kaitu’u-Lino TJ, Cannon P, Tuohey L, De Silva MS, Varas-Godoy M, Acuna S, Galaz J, Tong S, Illanes SE. Maternal plasma concentrations of the placental specific sFLT-1 variant, sFLT-1 e15a, in fetal growth restriction and preeclampsia. J Matern Fetal Neonatal Med. 2017;30:635–639. doi: 10.1080/14767058.2016.1182975. [DOI] [PubMed] [Google Scholar]
- 56.Ma Y, Kong LR, Ge Q, Lu YY, Hong MN, Zhang Y, Ruan CC, Gao PJ. Complement 5a–mediated trophoblasts dysfunction is involved in the development of pre-eclampsia. J Cell Mol Med. 2018;22:1034–1046. doi: 10.1111/jcmm.13466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krebs C, Macara LM, Leiser R, Bowman AW, Greer IA, Kingdom JC. Intrauterine growth restriction with absent end-diastolic flow velocity in the umbilical artery is associated with maldevelopment of the placental terminal villous tree. Am J Obstet Gynecol. 1996;175:1534–1542. doi: 10.1016/s0002-9378(96)70103-5. [DOI] [PubMed] [Google Scholar]
- 58.Kingdom J. Adriana and Luisa Castellucci Award Lecture 1997. Placental pathology in obstetrics: adaptation or failure of the villous tree? Placenta. 1998;19:347–351. doi: 10.1016/s0143-4004(98)90073-x. [DOI] [PubMed] [Google Scholar]
- 59.Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. 2000;92:35–43. doi: 10.1016/s0301-2115(00)00423-1. [DOI] [PubMed] [Google Scholar]
- 60.Gupta N, Sebire NJ, Miskry T, Rees HC. Massive perivillous fibrin deposition associated with discordant fetal growth in a dichorionic twin pregnancy. J Obstet Gynaecol. 2004;24:579–580. doi: 10.1080/01443610410001722752. [DOI] [PubMed] [Google Scholar]
- 61.Furness DL, Fenech MF, Khong YT, Romero R, Dekker GA. One-carbon metabolism enzyme polymorphisms and uteroplacental insufficiency. Am J Obstet Gynecol. 2008;199 doi: 10.1016/j.ajog.2008.06.020. 276.e271–278. [DOI] [PubMed] [Google Scholar]
- 62.Mifsud W, Sebire NJ. Placental pathology in early-onset and late-onset fetal growth restriction. Fetal Diagn Ther. 2014;36:117–128. doi: 10.1159/000359969. [DOI] [PubMed] [Google Scholar]
- 63.Kim YM, Chaemsaithong P, Romero R, Shaman M, Kim CJ, Kim JS, Qureshi F, Jacques SM, Ahmed AI, Chaiworapongsa T, Hassan SS, Yeo L, Korzeniewski SJ. The frequency of acute atherosis in normal pregnancy and preterm labor, preeclampsia, small-for-gestational age, fetal death and midtrimester spontaneous abortion. J Matern Fetal Neonatal Med. 2015:1–9. doi: 10.3109/14767058.2014.976198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang A, Zsengeller ZK, Hecht JL, Buccafusca R, Burke SD, Rajakumar A, Weingart E, Yu PB, Salahuddin S, Karumanchi SA. Excess placental secreted frizzled-related protein 1 in maternal smokers impairs fetal growth. J Clin Invest. 2015;125:4021–4025. doi: 10.1172/JCI80457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Mieghem T, Doherty A, Baczyk D, Drewlo S, Baud D, Carvalho J, Kingdom J. Apelin in Normal Pregnancy and Pregnancies Complicated by Placental Insufficiency. Reprod Sci. 2016;23:1037–1043. doi: 10.1177/1933719116630422. [DOI] [PubMed] [Google Scholar]
- 66.Wu F, Tian FJ, Lin Y, Xu WM. Oxidative Stress: Placenta Function and Dysfunction. Am J Reprod Immunol. 2016;76:258–271. doi: 10.1111/aji.12454. [DOI] [PubMed] [Google Scholar]
- 67.Gou C, Li M, Zhang X, Liu X, Huang X, Zhou Y, Fang Q. Placental characteristics in monochorionic twins with selective intrauterine growth restriction assessed by gradient angiography and three-dimensional reconstruction. J Matern Fetal Neonatal Med. 2017;30:2590–2595. doi: 10.1080/14767058.2016.1256995. [DOI] [PubMed] [Google Scholar]
- 68.Wu WB, Xu YY, Cheng WW, Yuan B, Zhao JR, Wang YL, Zhang HJ. Decreased PGF may contribute to trophoblast dysfunction in fetal growth restriction. Reproduction. 2017;154:219–229. doi: 10.1530/REP-17-0253. [DOI] [PubMed] [Google Scholar]
- 69.Ravikumar G, Crasta J, Prabhu JS, Thomas T, Dwarkanath P, Thomas A, Kurpad AV, Sridhar TS. CD15 as a marker of fetoplacental endothelial immaturity in IUGR placentas. J Matern Fetal Neonatal Med. 2017:1–8. doi: 10.1080/14767058.2017.1414179. [DOI] [PubMed] [Google Scholar]
- 70.Lean SC, Heazell AEP, Dilworth MR, Mills TA, Jones RL. Placental Dysfunction Underlies Increased Risk of Fetal Growth Restriction and Stillbirth in Advanced Maternal Age Women. Sci Rep. 2017;7:9677. doi: 10.1038/s41598-017-09814-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Joo JG, Rigo J, Jr, Borzsonyi B, Demendi C, Kornya L. Placental gene expression of the placental growth factor (PlGF) in intrauterine growth restriction. J Matern Fetal Neonatal Med. 2017;30:1471–1475. doi: 10.1080/14767058.2016.1219993. [DOI] [PubMed] [Google Scholar]
- 72.Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol. 2018;218:S745–s761. doi: 10.1016/j.ajog.2017.11.577. [DOI] [PubMed] [Google Scholar]
- 73.Smith GC, Yu CK, Papageorghiou AT, Cacho AM, Nicolaides KH Fetal Medicine Foundation Second Trimester Screening G. Maternal uterine artery Doppler flow velocimetry and the risk of stillbirth. Obstet Gynecol. 2007;109:144–151. doi: 10.1097/01.AOG.0000248536.94919.e3. [DOI] [PubMed] [Google Scholar]
- 74.Whitten AE, Romero R, Korzeniewski SJ, Tarca AL, Schwartz AG, Yeo L, Dong Z, Hassan SS, Chaiworapongsa T. Evidence of an imbalance of angiogenic/antiangiogenic factors in massive perivillous fibrin deposition (maternal floor infarction): a placental lesion associated with recurrent miscarriage and fetal death. Am J Obstet Gynecol. 2013;208:310 e311–310 e311. doi: 10.1016/j.ajog.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stanek J, Biesiada J. Relation of placental diagnosis in stillbirth to fetal maceration and gestational age at delivery. J Perinat Med. 2014;42:457–471. doi: 10.1515/jpm-2013-0219. [DOI] [PubMed] [Google Scholar]
- 76.McPherson E. Recurrence of stillbirth and second trimester pregnancy loss. Am J Med Genet A. 2016;170a:1174–1180. doi: 10.1002/ajmg.a.37606. [DOI] [PubMed] [Google Scholar]
- 77.Man J, Hutchinson JC, Heazell AE, Ashworth M, Jeffrey I, Sebire NJ. Stillbirth and intrauterine fetal death: role of routine histopathological placental findings to determine cause of death. Ultrasound Obstet Gynecol. 2016;48:579–584. doi: 10.1002/uog.16019. [DOI] [PubMed] [Google Scholar]
- 78.Chaiworapongsa T, Romero R, Erez O, Tarca AL, Conde-Agudelo A, Chaemsaithong P, Kim CJ, Kim YM, Kim JS, Yoon BH, Hassan SS, Yeo L, Korzeniewski SJ. The prediction of fetal death with a simple maternal blood test at 20–24 weeks: a role for angiogenic index-1 (PlGF/sVEGFR-1 ratio) Am J Obstet Gynecol. 2017;217:13. doi: 10.1016/j.ajog.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith GC. Screening and prevention of stillbirth. Best Pract Res Clin Obstet Gynaecol. 2017;38:71–82. doi: 10.1016/j.bpobgyn.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 80.Chaiworapongsa T, Romero R, Korzeniewski SJ, Chaemsaithong P, Hernandez-Andrade E, Segars JH, DeCherney AH, McCoy MC, Kim CJ, Yeo L, Hassan SS. Pravastatin for the prevention of adverse pregnancy outcome: preeclampsia and more? J Matern Fetal Neonatal Med. 2017;30:3. doi: 10.3109/14767058.2015.1129779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arias F, Victoria A, Cho K, Kraus F. Placental histology and clinical characteristics of patients with preterm premature rupture of membranes. Obstet Gynecol. 1997;89:265–271. doi: 10.1016/S0029-7844(96)00451-6. [DOI] [PubMed] [Google Scholar]
- 82.Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, Rotmensch S, Romero R. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189:1063–1069. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 83.Srinivas SK, Ma Y, Sammel MD, Chou D, McGrath C, Parry S, Elovitz MA. Placental inflammation and viral infection are implicated in second trimester pregnancy loss. Am J Obstet Gynecol. 2006;195:797–802. doi: 10.1016/j.ajog.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 84.Romero R, Kusanovic JP, Chaiworapongsa T, Hassan SS. Placental bed disorders in preterm labor, preterm PROM, spontaneous abortion and abruptio placentae. Best Pract Res Clin Obstet Gynaecol. 2011;25:313–327. doi: 10.1016/j.bpobgyn.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bastek JA, Brown AG, Anton L, Srinivas SK, D’Addio A, Elovitz MA. Biomarkers of inflammation and placental dysfunction are associated with subsequent preterm birth. J Matern Fetal Neonatal Med. 2011;24:600–605. doi: 10.3109/14767058.2010.511340. [DOI] [PubMed] [Google Scholar]
- 86.Morgan TK. Placental Insufficiency Is a Leading Cause of Preterm Labor. NeoReviews. 2014;15:e518–e525. [Google Scholar]
- 87.Esplin MS, Manuck TA, Varner MW, Christensen B, Biggio J, Bukowski R, Parry S, Zhang H, Huang H, Andrews W, Saade G, Sadovsky Y, Reddy UM, Ilekis J. Cluster analysis of spontaneous preterm birth phenotypes identifies potential associations among preterm birth mechanisms. Am J Obstet Gynecol. 2015;213 doi: 10.1016/j.ajog.2015.06.011. 429.e421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Racicot K, Kwon JY, Aldo P, Abrahams V, El-Guindy A, Romero R, Mor G. Type I Interferon Regulates the Placental Inflammatory Response to Bacteria and is Targeted by Virus: Mechanism of Polymicrobial Infection-Induced Preterm Birth. Am J Reprod Immunol. 2016;75:451–460. doi: 10.1111/aji.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee H, Kwon JY, Lee S, Kim SJ, Shin JC, Park IY. Elevated placenta growth factor levels in the early second-trimester amniotic fluid are associated with preterm delivery. J Matern Fetal Neonatal Med. 2016;29:3374–3378. doi: 10.3109/14767058.2015.1127345. [DOI] [PubMed] [Google Scholar]
- 90.Sehgal S, Bhatnagar S, Pallavi SK. Provocative ideas on human placental biology: A prerequisite for prevention and treatment of neonatal health challenges. Am J Reprod Immunol. 2017:77. doi: 10.1111/aji.12656. [DOI] [PubMed] [Google Scholar]
- 91.Thornburg KL, Marshall N. The placenta is the center of the chronic disease universe. Am J Obstet Gynecol. 2015;213:S14–20. doi: 10.1016/j.ajog.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Burton GJ, Fowden AL, Thornburg KL. Placental Origins of Chronic Disease. Physiol Rev. 2016;96:1509–1565. doi: 10.1152/physrev.00029.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carter AM. Animal models of human placentation--a review. Placenta. 2007;28(Suppl A):S41–47. doi: 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 94.Chavatte-Palmer P, Tarrade A. Placentation in different mammalian species. Annales d’Endocrinologie. 2016;77:67–74. doi: 10.1016/j.ando.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 95.Armstrong DL, McGowen MR, Weckle A, Pantham P, Caravas J, Agnew D, Benirschke K, Savage-Rumbaugh S, Nevo E, Kim CJ, Wagner GP, Romero R, Wildman DE. The core transcriptome of mammalian placentas and the divergence of expression with placental shape. Placenta. 2017;57:71–78. doi: 10.1016/j.placenta.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Griffith OW, Chavan AR, Protopapas S, Maziarz J, Romero R, Wagner GP. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc Natl Acad Sci U S A. 2017;114:E6566–E6575. doi: 10.1073/pnas.1701129114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carter AM. Recent advances in understanding evolution of the placenta: insights from transcriptomics. F1000Res. 2018;7:89. doi: 10.12688/f1000research.13115.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cotte C, Easty GC, Neville AM, Monaghan P. Preparation of highly purified cytotrophoblast from human placenta with subsequent modulation to form syncytiotrophoblast in monolayer cultures. In Vitro. 1980;16:639–646. doi: 10.1007/BF02619191. [DOI] [PubMed] [Google Scholar]
- 99.Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF., 3rd Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- 100.Takao T, Asanoma K, Kato K, Fukushima K, Tsunematsu R, Hirakawa T, Matsumura S, Seki H, Takeda S, Wake N. Isolation and characterization of human trophoblast side-population (SP) cells in primary villous cytotrophoblasts and HTR-8/SVneo cell line. PLoS One. 2011;6:e21990. doi: 10.1371/journal.pone.0021990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee JS, Romero R, Han YM, Kim HC, Kim CJ, Hong JS, Huh D. Placenta-on-a-chip: a novel platform to study the biology of the human placenta. J Matern Fetal Neonatal Med. 2016;29:1046–1054. doi: 10.3109/14767058.2015.1038518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Winterhager E, Kaufmann P, Gruemmer R. Cell-cell-communication during placental development and possible implications for trophoblast proliferation and differentiation. Placenta. 2000;21(Suppl A):S61–68. doi: 10.1053/plac.1999.0516. [DOI] [PubMed] [Google Scholar]
- 103.Goeden N, Bonnin A. Ex vivo perfusion of mid-to-late-gestation mouse placenta for maternal-fetal interaction studies during pregnancy. Nat Protoc. 2013;8:66–74. doi: 10.1038/nprot.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McConkey CA, Delorme-Axford E, Nickerson CA, Kim KS, Sadovsky Y, Boyle JP, Coyne CB. A three-dimensional culture system recapitulates placental syncytiotrophoblast development and microbial resistance. Sci Adv. 2016;2:e1501462. doi: 10.1126/sciadv.1501462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weiswald LB, Richon S, Validire P, Briffod M, Lai-Kuen R, Cordelieres FP, Bertrand F, Dargere D, Massonnet G, Marangoni E, Gayet B, Pocard M, Bieche I, Poupon MF, Bellet D, Dangles-Marie V. Newly characterised ex vivo colospheres as a three-dimensional colon cancer cell model of tumour aggressiveness. Br J Cancer. 2009;101:473–482. doi: 10.1038/sj.bjc.6605173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Blanco TM, Mantalaris A, Bismarck A, Panoskaltsis N. The development of a three-dimensional scaffold for ex vivo biomimicry of human acute myeloid leukaemia. Biomaterials. 2010;31:2243–2251. doi: 10.1016/j.biomaterials.2009.11.094. [DOI] [PubMed] [Google Scholar]
- 107.Curtin P, Youm H, Salih E. Three-dimensional cancer-bone metastasis model using ex-vivo co-cultures of live calvarial bones and cancer cells. Biomaterials. 2012;33:1065–1078. doi: 10.1016/j.biomaterials.2011.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seano G, Chiaverina G, Gagliardi PA, di Blasio L, Sessa R, Bussolino F, Primo L. Modeling human tumor angiogenesis in a three-dimensional culture system. Blood. 2013;121:e129–137. doi: 10.1182/blood-2012-08-452292. [DOI] [PubMed] [Google Scholar]
- 109.Parikh MR, Belch AR, Pilarski LM, Kirshner J. A three-dimensional tissue culture model to study primary human bone marrow and its malignancies. J Vis Exp. 2014 doi: 10.3791/50947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gardner JK, Herbst-Kralovetz MM. Three-Dimensional Rotating Wall Vessel-Derived Cell Culture Models for Studying Virus-Host Interactions. Viruses. 2016:8. doi: 10.3390/v8110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hiller T, Rohrs V, Dehne EM, Wagner A, Fechner H, Lauster R, Kurreck J. Study of Viral Vectors in a Three-dimensional Liver Model Repopulated with the Human Hepatocellular Carcinoma Cell Line HepG2. J Vis Exp. 2016 doi: 10.3791/54633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Drummond CG, Nickerson CA, Coyne CB. A Three-Dimensional Cell Culture Model To Study Enterovirus Infection of Polarized Intestinal Epithelial Cells. mSphere. 2016:1. doi: 10.1128/mSphere.00030-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bramley JC, Drummond CG, Lennemann NJ, Good CA, Kim KS, Coyne CB. A Three-Dimensional Cell Culture System To Model RNA Virus Infections at the Blood-Brain Barrier. mSphere. 2017:2. doi: 10.1128/mSphere.00206-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kang A, Seo HI, Chung BG, Lee SH. Concave microwell array-mediated three-dimensional tumor model for screening anticancer drug-loaded nanoparticles. Nanomedicine. 2015;11:1153–1161. doi: 10.1016/j.nano.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 115.Koban R, Neumann M, Daugs A, Bloch O, Nitsche A, Langhammer S, Ellerbrok H. A novel three-dimensional cell culture method enhances antiviral drug screening in primary human cells. Antiviral Res. 2017;150:20–29. doi: 10.1016/j.antiviral.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 116.Lynge Nilsson L, Djurisic S, Hviid TV. Controlling the Immunological Crosstalk during Conception and Pregnancy: HLA-G in Reproduction. Front Immunol. 2014;5:198. doi: 10.3389/fimmu.2014.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nair RR, Verma P, Singh K. Immune-endocrine crosstalk during pregnancy. Gen Comp Endocrinol. 2017;242:18–23. doi: 10.1016/j.ygcen.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 118.Pavlicev M, Wagner GP, Chavan AR, Owens K, Maziarz J, Dunn-Fletcher C, Kallapur SG, Muglia L, Jones H. Single-cell transcriptomics of the human placenta: inferring the cell communication network of the maternalfetal interface. Genome Res. 2017;27:349–361. doi: 10.1101/gr.207597.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Anders AP, Gaddy JA, Doster RS, Aronoff DM. Current concepts in maternal-fetal immunology: Recognition and response to microbial pathogens by decidual stromal cells. Am J Reprod Immunol. 2017:77. doi: 10.1111/aji.12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smarason AK, Sargent IL, Starkey PM, Redman CW. The effect of placental syncytiotrophoblast microvillous membranes from normal and pre-eclamptic women on the growth of endothelial cells in vitro. Br J Obstet Gynaecol. 1993;100:943–949. doi: 10.1111/j.1471-0528.1993.tb15114.x. [DOI] [PubMed] [Google Scholar]
- 121.Cockell AP, Learmont JG, Smarason AK, Redman CW, Sargent IL, Poston L. Human placental syncytiotrophoblast microvillous membranes impair maternal vascular endothelial function. Br J Obstet Gynaecol. 1997;104:235–240. doi: 10.1111/j.1471-0528.1997.tb11052.x. [DOI] [PubMed] [Google Scholar]
- 122.Redman CW, Sargent IL. Placental debris, oxidative stress and pre-eclampsia. Placenta. 2000;21:597–602. doi: 10.1053/plac.2000.0560. [DOI] [PubMed] [Google Scholar]
- 123.Sabapatha A, Gercel-Taylor C, Taylor DD. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am J Reprod Immunol. 2006;56:345–355. doi: 10.1111/j.1600-0897.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 124.Jean-Pierre C, Perni SC, Bongiovanni AM, Kalish RB, Karasahan E, Ravich M, Ratushny V, Skupski DW, Witkin SS. Extracellular 70-kd heat shock protein in mid-trimester amniotic fluid and its effect on cytokine production by ex vivo-cultured amniotic fluid cells. Am J Obstet Gynecol. 2006;194:694–698. doi: 10.1016/j.ajog.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 125.Taylor DD, Akyol S, Gercel-Taylor C. Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol. 2006;176:1534–1542. doi: 10.4049/jimmunol.176.3.1534. [DOI] [PubMed] [Google Scholar]
- 126.Asea A, Jean-Pierre C, Kaur P, Rao P, Linhares IM, Skupski D, Witkin SS. Heat shock protein-containing exosomes in mid-trimester amniotic fluids. J Reprod Immunol. 2008;79:12–17. doi: 10.1016/j.jri.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 127.Pap E, Pallinger E, Falus A, Kiss AA, Kittel A, Kovacs P, Buzas EI. T lymphocytes are targets for platelet- and trophoblast-derived microvesicles during pregnancy. Placenta. 2008;29:826–832. doi: 10.1016/j.placenta.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 128.Gardiner C, Tannetta DS, Simms CA, Harrison P, Redman CW, Sargent IL. Syncytiotrophoblast microvesicles released from pre-eclampsia placentae exhibit increased tissue factor activity. PLoS One. 2011;6:e26313. doi: 10.1371/journal.pone.0026313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Delorme-Axford E, Donker RB, Mouillet JF, Chu T, Bayer A, Ouyang Y, Wang T, Stolz DB, Sarkar SN, Morelli AE, Sadovsky Y, Coyne CB. Human placental trophoblasts confer viral resistance to recipient cells. Proc Natl Acad Sci U S A. 2013;110:12048–12053. doi: 10.1073/pnas.1304718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ouyang Y, Mouillet JF, Coyne CB, Sadovsky Y. Review: placenta-specific microRNAs in exosomes - good things come in nano-packages. Placenta. 2014;(35 Suppl):S69–73. doi: 10.1016/j.placenta.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yáñez-Mó M, Siljander PRM, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E, Silva AC-d, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NHH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers E-M, Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Hoen ENMN-t, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pállinger É, del Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MHM, De Wever O. Biological properties of extracellular vesicles and their physiological functions. Journal of Extracellular Vesicles. 2015;4 doi: 10.3402/jev.v3404.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Toth B, Lok CA, Boing A, Diamant M, van der Post JA, Friese K, Nieuwland R. Microparticles and exosomes: impact on normal and complicated pregnancy. Am J Reprod Immunol. 2007;58:389–402. doi: 10.1111/j.1600-0897.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- 133.Mincheva-Nilsson L, Baranov V. Placenta-derived exosomes and syncytiotrophoblast microparticles and their role in human reproduction: immune modulation for pregnancy success. Am J Reprod Immunol. 2014;72:440–457. doi: 10.1111/aji.12311. [DOI] [PubMed] [Google Scholar]
- 134.Mouillet JF, Ouyang Y, Coyne CB, Sadovsky Y. MicroRNAs in placental health and disease. Am J Obstet Gynecol. 2015;213:S163–172. doi: 10.1016/j.ajog.2015.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Clayton A, Turkes A, Dewitt S, Steadman R, Mason MD, Hallett MB. Adhesion and signaling by B cell-derived exosomes: the role of integrins. FASEB J. 2004;18:977–979. doi: 10.1096/fj.03-1094fje. [DOI] [PubMed] [Google Scholar]
- 136.Cho JA, Yeo DJ, Son HY, Kim HW, Jung DS, Ko JK, Koh JS, Kim YN, Kim CW. Exosomes: a new delivery system for tumor antigens in cancer immunotherapy. Int J Cancer. 2005;114:613–622. doi: 10.1002/ijc.20757. [DOI] [PubMed] [Google Scholar]
- 137.Zhang Y, Wu XH, Luo CL, Zhang JM, He BC, Chen G. Interleukin-12-anchored exosomes increase cytotoxicity of T lymphocytes by reversing the JAK/STAT pathway impaired by tumor-derived exosomes. Int J Mol Med. 2010;25:695–700. doi: 10.3892/ijmm_00000393. [DOI] [PubMed] [Google Scholar]
- 138.Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res. 2010;107:1047–1057. doi: 10.1161/CIRCRESAHA.110.226456. [DOI] [PubMed] [Google Scholar]
- 139.Gutierrez-Vazquez C, Villarroya-Beltri C, Mittelbrunn M, Sanchez-Madrid F. Transfer of extracellular vesicles during immune cell-cell interactions. Immunol Rev. 2013;251:125–142. doi: 10.1111/imr.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kaur S, Singh SP, Elkahloun AG, Wu W, Abu-Asab MS, Roberts DD. CD47-dependent immunomodulatory and angiogenic activities of extracellular vesicles produced by T cells. Matrix Biol. 2014;37:49–59. doi: 10.1016/j.matbio.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Knickelbein JE, Liu B, Arakelyan A, Zicari S, Hannes S, Chen P, Li Z, Grivel JC, Chaigne-Delalande B, Sen HN, Margolis L, Nussenblatt RB. Modulation of Immune Responses by Extracellular Vesicles From Retinal Pigment Epithelium. Invest Ophthalmol Vis Sci. 2016;57:4101–4107. doi: 10.1167/iovs.15-18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hansen HP, Trad A, Dams M, Zigrino P, Moss M, Tator M, Schon G, Grenzi PC, Bachurski D, Aquino B, Durkop H, Reiners KS, von Bergwelt-Baildon M, Hallek M, Grotzinger J, Engert A, Paes Leme AF, Pogge von Strandmann E. CD30 on extracellular vesicles from malignant Hodgkin cells supports damaging of CD30 ligand-expressing bystander cells with Brentuximab-Vedotin, in vitro. Oncotarget. 2016;7:30523–30535. doi: 10.18632/oncotarget.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nolte-’t Hoen E, Cremer T, Gallo RC, Margolis LB. Extracellular vesicles and viruses: Are they close relatives? Proc Natl Acad Sci U S A. 2016;113:9155–9161. doi: 10.1073/pnas.1605146113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Arakelyan A, Fitzgerald W, Zicari S, Vanpouille C, Margolis L. Extracellular Vesicles Carry HIV Env and Facilitate Hiv Infection of Human Lymphoid Tissue. Sci Rep. 2017;7:1695. doi: 10.1038/s41598-017-01739-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Truong G, Guanzon D, Kinhal V, Elfeky O, Lai A, Longo S, Nuzhat Z, Palma C, Scholz-Romero K, Menon R, Mol BW, Rice GE, Salomon C. Oxygen tension regulates the miRNA profile and bioactivity of exosomes released from extravillous trophoblast cells - Liquid biopsies for monitoring complications of pregnancy. PLoS One. 2017;12:e0174514. doi: 10.1371/journal.pone.0174514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Grivel JC, Margolis L. Use of human tissue explants to study human infectious agents. Nat Protoc. 2009;4:256–269. doi: 10.1038/nprot.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Biancotto A, Grivel JC, Iglehart SJ, Vanpouille C, Lisco A, Sieg SF, Debernardo R, Garate K, Rodriguez B, Margolis LB, Lederman MM. Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1. Blood. 2007;109:4272–4279. doi: 10.1182/blood-2006-11-055764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lisco A, Introini A, Munawwar A, Vanpouille C, Grivel JC, Blank P, Singh S, Margolis L. HIV-1 imposes rigidity on blood and semen cytokine networks. Am J Reprod Immunol. 2012;68:515–521. doi: 10.1111/aji.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Romero R, Grivel JC, Tarca AL, Chaemsaithong P, Xu Z, Fitzgerald W, Hassan SS, Chaiworapongsa T, Margolis L. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol. 2015;213:836.e831–836.e818. doi: 10.1016/j.ajog.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Arakelyan A, Fitzgerald W, Margolis L, Grivel JC. Nanoparticle-based flow virometry for the analysis of individual virions. J Clin Invest. 2013;123:3716–3727. doi: 10.1172/JCI67042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Leitner K, Szlauer R, Ellinger I, Ellinger A, Zimmer KP, Fuchs R. Placental alkaline phosphatase expression at the apical and basal plasma membrane in term villous trophoblasts. J Histochem Cytochem. 2001;49:1155–1164. doi: 10.1177/002215540104900909. [DOI] [PubMed] [Google Scholar]
- 153.Salomon C, Torres MJ, Kobayashi M, Scholz-Romero K, Sobrevia L, Dobierzewska A, Illanes SE, Mitchell MD, Rice GE. A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLoS One. 2014;9:e98667. doi: 10.1371/journal.pone.0098667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dragovic RA, Collett GP, Hole P, Ferguson DJ, Redman CW, Sargent IL, Tannetta DS. Isolation of syncytiotrophoblast microvesicles and exosomes and their characterisation by multicolour flow cytometry and fluorescence Nanoparticle Tracking Analysis. Methods. 2015;87:64–74. doi: 10.1016/j.ymeth.2015.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jin J, Menon R. Placental exosomes: A proxy to understand pregnancy complications. Am J Reprod Immunol. 2017 doi: 10.1111/aji.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Park S, Koh SE, Hur CY, Lee WD, Lim J, Lee YJ. Comparison of human first and third trimester placental mesenchymal stem cell. Cell Biol Int. 2013;37:242–249. doi: 10.1002/cbin.10032. [DOI] [PubMed] [Google Scholar]
- 157.Hedlund M, Stenqvist AC, Nagaeva O, Kjellberg L, Wulff M, Baranov V, Mincheva-Nilsson L. Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: evidence for immunosuppressive function. J Immunol. 2009;183:340–351. doi: 10.4049/jimmunol.0803477. [DOI] [PubMed] [Google Scholar]
- 158.Apps R, Murphy SP, Fernando R, Gardner L, Ahad T, Moffett A. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology. 2009;127:26–39. doi: 10.1111/j.1365-2567.2008.03019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kertesz Z, Linton EA, Redman CW. Adhesion molecules of syncytiotrophoblast microvillous membranes inhibit proliferation of human umbilical vein endothelial cells. Placenta. 2000;21:150–159. doi: 10.1053/plac.1999.0476. [DOI] [PubMed] [Google Scholar]
- 160.Tolosa JM, Schjenken JE, Clifton VL, Vargas A, Barbeau B, Lowry P, Maiti K, Smith R. The endogenous retroviral envelope protein syncytin-1 inhibits LPS/PHA-stimulated cytokine responses in human blood and is sorted into placental exosomes. Placenta. 2012;33:933–941. doi: 10.1016/j.placenta.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 161.Sargent I. Microvesicles and pre-eclampsia. Pregnancy Hypertens. 2013;3:58. doi: 10.1016/j.preghy.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 162.Dragovic RA, Southcombe JH, Tannetta DS, Redman CW, Sargent IL. Multicolor flow cytometry and nanoparticle tracking analysis of extracellular vesicles in the plasma of normal pregnant and pre-eclamptic women. Biol Reprod. 2013;89:151. doi: 10.1095/biolreprod.113.113266. [DOI] [PubMed] [Google Scholar]
- 163.Bilic G, Zeisberger SM, Mallik AS, Zimmermann R, Zisch AH. Comparative characterization of cultured human term amnion epithelial and mesenchymal stromal cells for application in cell therapy. Cell Transplant. 2008;17:955–968. doi: 10.3727/096368908786576507. [DOI] [PubMed] [Google Scholar]
- 164.Raynaud CM, Maleki M, Lis R, Ahmed B, Al-Azwani I, Malek J, Safadi FF, Rafii A. Comprehensive characterization of mesenchymal stem cells from human placenta and fetal membrane and their response to osteoactivin stimulation. Stem Cells Int. 2012;2012:658356. doi: 10.1155/2012/658356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Murphy S, Rosli S, Acharya R, Mathias L, Lim R, Wallace E, Jenkin G. Amnion epithelial cell isolation and characterization for clinical use. Curr Protoc Stem Cell Biol. 2010 doi: 10.1002/9780470151808.sc01e06s13. Chapter 1:Unit 1E 6. [DOI] [PubMed] [Google Scholar]
- 166.Sheller S, Papaconstantinou J, Urrabaz-Garza R, Richardson L, Saade G, Salomon C, Menon R. Amnion-Epithelial-Cell-Derived Exosomes Demonstrate Physiologic State of Cell under Oxidative Stress. PLoS One. 2016;11:e0157614. doi: 10.1371/journal.pone.0157614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Garcia-Lopez G, Garcia-Castro IL, Avila-Gonzalez D, Molina-Hernandez A, Flores-Herrera H, Merchant-Larios H, Diaz-Martinez F. [Human amniotic epithelium (HAE) as a possible source of stem cells (SC)] Gac Med Mex. 2015;151:66–74. [PubMed] [Google Scholar]
- 168.Miller RK, Genbacev O, Turner MA, Aplin JD, Caniggia I, Huppertz B. Human placental explants in culture: approaches and assessments. Placenta. 2005;26:439–448. doi: 10.1016/j.placenta.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 169.Huckle WR. Cell- and Tissue-Based Models for Study of Placental Development. Prog Mol Biol Transl Sci. 2017;145:29–37. doi: 10.1016/bs.pmbts.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 170.Tong M, Chamley LW. Isolation and Characterization of Extracellular Vesicles from Ex Vivo Cultured Human Placental Explants. Methods Mol Biol. 2018;1710:117–129. doi: 10.1007/978-1-4939-7498-6_9. [DOI] [PubMed] [Google Scholar]
- 171.Pantham P, Chamley LW. Harvesting and Characterization of Syncytial Nuclear Aggregates Following Culture of First Trimester Human Placental Explants. Methods Mol Biol. 2018;1710:155–163. doi: 10.1007/978-1-4939-7498-6_12. [DOI] [PubMed] [Google Scholar]
- 172.Polliotti BM, Abramowsky C, Schwartz DA, Keesling SS, Lee GR, Caba J, Zhang W, Panigel M, Nahmias AJ. Culture of first-trimester and full-term human chorionic villus explants: role of human chorionic gonadotropin and human placental lactogen as a viability index. Early Pregnancy. 1995;1:270–280. [PubMed] [Google Scholar]
- 173.Palmer ME, Watson AL, Burton GJ. Morphological analysis of degeneration and regeneration of syncytiotrophoblast in first trimester placental villi during organ culture. Hum Reprod. 1997;12:379–382. doi: 10.1093/humrep/12.2.379. [DOI] [PubMed] [Google Scholar]
- 174.Siman CM, Sibley CP, Jones CJ, Turner MA, Greenwood SL. The functional regeneration of syncytiotrophoblast in cultured explants of term placenta. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1116–1122. doi: 10.1152/ajpregu.2001.280.4.R1116. [DOI] [PubMed] [Google Scholar]
- 175.Freeman AE, Hoffman RM. In vivo-like growth of human tumors in vitro. Proc Natl Acad Sci U S A. 1986;83:2694–2698. doi: 10.1073/pnas.83.8.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Vescio RA, Redfern CH, Nelson TJ, Ugoretz S, Stern PH, Hoffman RM. In vivo-like drug responses of human tumors growing in three-dimensional gel-supported primary culture. Proc Natl Acad Sci U S A. 1987;84:5029–5033. doi: 10.1073/pnas.84.14.5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Glushakova S, Grivel JC, Fitzgerald W, Sylwester A, Zimmerberg J, Margolis LB. Evidence for the HIV-1 phenotype switch as a causal factor in acquired immunodeficiency. Nat Med. 1998;4:346–349. doi: 10.1038/nm0398-346. [DOI] [PubMed] [Google Scholar]
- 178.Glushakova S, Yi Y, Grivel JC, Singh A, Schols D, De Clercq E, Collman RG, Margolis L. Preferential coreceptor utilization and cytopathicity by dual-tropic HIV-1 in human lymphoid tissue ex vivo. J Clin Invest. 1999;104:R7–r11. doi: 10.1172/JCI7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Grivel JC, Margolis LB. CCR5- and CXCR4-tropic HIV-1 are equally cytopathic for their T-cell targets in human lymphoid tissue. Nat Med. 1999;5:344–346. doi: 10.1038/6565. [DOI] [PubMed] [Google Scholar]
- 180.Grivel JC, Penn ML, Eckstein DA, Schramm B, Speck RF, Abbey NW, Herndier B, Margolis L, Goldsmith MA. Human immunodeficiency virus type 1 coreceptor preferences determine target T-cell depletion and cellular tropism in human lymphoid tissue. J Virol. 2000;74:5347–5351. doi: 10.1128/jvi.74.11.5347-5351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Biancotto A, Iglehart SJ, Vanpouille C, Condack CE, Lisco A, Ruecker E, Hirsch I, Margolis LB, Grivel JC. HIV-1 induced activation of CD4+ T cells creates new targets for HIV-1 infection in human lymphoid tissue ex vivo. Blood. 2008;111:699–704. doi: 10.1182/blood-2007-05-088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Saba E, Grivel JC, Vanpouille C, Brichacek B, Fitzgerald W, Margolis L, Lisco A. HIV-1 sexual transmission: early events of HIV-1 infection of human cervico-vaginal tissue in an optimized ex vivo model. Mucosal Immunol. 2010;3:280–290. doi: 10.1038/mi.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Merbah M, Arakelyan A, Edmonds T, Ochsenbauer C, Kappes JC, Shattock RJ, Grivel JC, Margolis LB. HIV-1 expressing the envelopes of transmitted/founder or control/reference viruses have similar infection patterns of CD4 T-cells in human cervical tissue ex vivo. PLoS One. 2012;7:e50839. doi: 10.1371/journal.pone.0050839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Introini A, Vanpouille C, Lisco A, Grivel JC, Margolis L. Interleukin-7 facilitates HIV-1 transmission to cervico-vaginal tissue ex vivo. PLoS Pathog. 2013;9:e1003148. doi: 10.1371/journal.ppat.1003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Grivel JC, Elliott J, Lisco A, Biancotto A, Condack C, Shattock RJ, McGowan I, Margolis L, Anton P. HIV-1 pathogenesis differs in rectosigmoid and tonsillar tissues infected ex vivo with CCR5- and CXCR4-tropic HIV-1. Aids. 2007;21:1263–1272. doi: 10.1097/QAD.0b013e3281864667. [DOI] [PubMed] [Google Scholar]
- 186.Vorobyova DA, Lebedev AM, Vagida MS, Ivanova OI, Felker EI, Gontarenko VN, Shpektor AV, Margolis LB, Vasilieva EY. [Immunological Analysis of Human Atherosclerotic Plaques in ex vivo Culture System] Kardiologiia. 2016;56:78–85. doi: 10.18565/cardio.2016.11.78-85. [DOI] [PubMed] [Google Scholar]
- 187.Lebedeva A, Vorobyeva D, Vagida M, Ivanova O, Felker E, Fitzgerald W, Danilova N, Gontarenko V, Shpektor A, Vasilieva E, Margolis L. Ex vivo culture of human atherosclerotic plaques: A model to study immune cells in atherogenesis. Atherosclerosis. 2017;267:90–98. doi: 10.1016/j.atherosclerosis.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hamilton ST, Scott G, Naing Z, Iwasenko J, Hall B, Graf N, Arbuckle S, Craig ME, Rawlinson WD. Human cytomegalovirus-induces cytokine changes in the placenta with implications for adverse pregnancy outcomes. PLoS One. 2012;7:e52899. doi: 10.1371/journal.pone.0052899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wegmann TG, Guilbert LJ. Immune signalling at the maternal-fetal interface and trophoblast differentiation. Dev Comp Immunol. 1992;16:425–430. doi: 10.1016/0145-305x(92)90026-9. [DOI] [PubMed] [Google Scholar]
- 190.Bowen JM, Chamley L, Mitchell MD, Keelan JA. Cytokines of the placenta and extra-placental membranes: biosynthesis, secretion and roles in establishment of pregnancy in women. Placenta. 2002;23:239–256. doi: 10.1053/plac.2001.0781. [DOI] [PubMed] [Google Scholar]
- 191.Biro E, Lok CA, Hack CE, van der Post JA, Schaap MC, Sturk A, Nieuwland R. Cell-derived microparticles and complement activation in preeclampsia versus normal pregnancy. Placenta. 2007;28:928–935. doi: 10.1016/j.placenta.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 192.Germain SJ, Sacks GP, Sooranna SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol. 2007;178:5949–5956. doi: 10.4049/jimmunol.178.9.5949. [DOI] [PubMed] [Google Scholar]
- 193.Luo SS, Ishibashi O, Ishikawa G, Ishikawa T, Katayama A, Mishima T, Takizawa T, Shigihara T, Goto T, Izumi A, Ohkuchi A, Matsubara S, Takeshita T, Takizawa T. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol Reprod. 2009;81:717–729. doi: 10.1095/biolreprod.108.075481. [DOI] [PubMed] [Google Scholar]
- 194.Redman CW, Sargent IL. Circulating microparticles in normal pregnancy and pre-eclampsia. Placenta. 2008;29(Suppl A):S73–77. doi: 10.1016/j.placenta.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 195.Chen DB, Wang W. Human placental microRNAs and preeclampsia. Biol Reprod. 2013;88:130. doi: 10.1095/biolreprod.113.107805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tannetta DS, Dragovic RA, Gardiner C, Redman CW, Sargent IL. Characterisation of syncytiotrophoblast vesicles in normal pregnancy and pre-eclampsia: expression of Flt-1 and endoglin. PLoS One. 2013;8:e56754. doi: 10.1371/journal.pone.0056754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Salomon C, Yee SW, Mitchell MD, Rice GE. The possible role of extravillous trophoblast-derived exosomes on the uterine spiral arterial remodeling under both normal and pathological conditions. Biomed Res Int. 2014;2014:693157. doi: 10.1155/2014/693157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sarker S, Scholz-Romero K, Perez A, Illanes SE, Mitchell MD, Rice GE, Salomon C. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J Transl Med. 2014;12:204. doi: 10.1186/1479-5876-12-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Vargas A, Zhou S, Ethier-Chiasson M, Flipo D, Lafond J, Gilbert C, Barbeau B. Syncytin proteins incorporated in placenta exosomes are important for cell uptake and show variation in abundance in serum exosomes from patients with preeclampsia. Faseb j. 2014;28:3703–3719. doi: 10.1096/fj.13-239053. [DOI] [PubMed] [Google Scholar]
- 200.Tan KH, Tan SS, Sze SK, Lee WK, Ng MJ, Lim SK. Plasma biomarker discovery in preeclampsia using a novel differential isolation technology for circulating extracellular vesicles. Am J Obstet Gynecol. 2014;211 doi: 10.1016/j.ajog.2014.03.038. 380.e381–313. [DOI] [PubMed] [Google Scholar]
- 201.Sadovsky Y, Mouillet JF, Ouyang Y, Bayer A, Coyne CB. The Function of TrophomiRs and Other MicroRNAs in the Human Placenta. Cold Spring Harb Perspect Med. 2015;5:a023036. doi: 10.1101/cshperspect.a023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rice GE, Scholz-Romero K, Sweeney E, Peiris H, Kobayashi M, Duncombe G, Mitchell MD, Salomon C. The Effect of Glucose on the Release and Bioactivity of Exosomes From First Trimester Trophoblast Cells. J Clin Endocrinol Metab. 2015;100:E1280–1288. doi: 10.1210/jc.2015-2270. [DOI] [PubMed] [Google Scholar]
- 203.Mitchell MD, Peiris HN, Kobayashi M, Koh YQ, Duncombe G, Illanes SE, Rice GE, Salomon C. Placental exosomes in normal and complicated pregnancy. Am J Obstet Gynecol. 2015;213:S173–181. doi: 10.1016/j.ajog.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 204.Aharon A. The role of extracellular vesicles in placental vascular complications. Thromb Res. 2015;135(Suppl 1):S23–25. doi: 10.1016/S0049-3848(15)50435-0. [DOI] [PubMed] [Google Scholar]
- 205.Patil R, Ghosh K, Shetty S. Could procoagulant cell-derived microparticles have a more crucial role in pregnancy complications rather than exosomes? Am J Obstet Gynecol. 2016;214:765–766. doi: 10.1016/j.ajog.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 206.Tong M, Kleffmann T, Pradhan S, Johansson CL, DeSousa J, Stone PR, James JL, Chen Q, Chamley LW. Proteomic characterization of macro-, micro- and nano-extracellular vesicles derived from the same first trimester placenta: relevance for feto-maternal communication. Hum Reprod. 2016;31:687–699. doi: 10.1093/humrep/dew004. [DOI] [PubMed] [Google Scholar]
- 207.Ilekis JV, Tsilou E, Fisher S, Abrahams VM, Soares MJ, Cross JC, Zamudio S, Illsley NP, Myatt L, Colvis C, Costantine MM, Haas DM, Sadovsky Y, Weiner C, Rytting E, Bidwell G. Placental origins of adverse pregnancy outcomes: potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am J Obstet Gynecol. 2016;215:S1–s46. doi: 10.1016/j.ajog.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gilani SI, Weissgerber TL, Garovic VD, Jayachandran M. Preeclampsia and Extracellular Vesicles. Curr Hypertens Rep. 2016;18:68. doi: 10.1007/s11906-016-0678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cantonwine DE, Zhang Z, Rosenblatt K, Goudy KS, Doss RC, Ezrin AM, Page G, Brohman B, McElrath TF. Evaluation of proteomic biomarkers associated with circulating microparticles as an effective means to stratify the risk of spontaneous preterm birth. Am J Obstet Gynecol. 2016;214:631.e631–631.e611. doi: 10.1016/j.ajog.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Escudero CA, Herlitz K, Troncoso F, Acurio J, Aguayo C, Roberts JM, Truong G, Duncombe G, Rice G, Salomon C. Role of Extracellular Vesicles and microRNAs on Dysfunctional Angiogenesis during Preeclamptic Pregnancies. Front Physiol. 2016;7:98. doi: 10.3389/fphys.2016.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chaparro A, Gaedechens D, Ramirez V, Zuniga E, Kusanovic JP, Inostroza C, Varas-Godoy M, Silva K, Salomon C, Rice G, Illanes SE. Placental biomarkers and angiogenic factors in oral fluids of patients with preeclampsia. Prenat Diagn. 2016;36:476–482. doi: 10.1002/pd.4811. [DOI] [PubMed] [Google Scholar]
- 212.Sheller S, Urrabaz-Garza R, Saade G, Menon R. Packaging of alarmin, HMGB1, in oxidative stress induced amnion cell exosomes: a signal from senescent fetal cells at term. Am J Obstet Gynecol. 2016;214:S418. [Google Scholar]
- 213.Rodosthenous RS, Burris HH, Sanders AP, Just AC, Dereix AE, Svensson K, Solano M, Tellez-Rojo MM, Wright RO, Baccarelli AA. Second trimester extracellular microRNAs in maternal blood and fetal growth: An exploratory study. Epigenetics. 2017;12:804–810. doi: 10.1080/15592294.2017.1358345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Motta-Mejia C, Kandzija N, Zhang W, Mhlomi V, Cerdeira AS, Burdujan A, Tannetta D, Dragovic R, Sargent IL, Redman CW, Kishore U, Vatish M. Placental Vesicles Carry Active Endothelial Nitric Oxide Synthase and Their Activity is Reduced in Preeclampsia. Hypertension. 2017;70:372–381. doi: 10.1161/HYPERTENSIONAHA.117.09321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Salomon C, Guanzon D, Scholz-Romero K, Longo S, Correa P, Illanes SE, Rice GE. Placental Exosomes as Early Biomarker of Preeclampsia: Potential Role of Exosomal MicroRNAs Across Gestation. J Clin Endocrinol Metab. 2017;102:3182–3194. doi: 10.1210/jc.2017-00672. [DOI] [PubMed] [Google Scholar]
- 216.Tannetta D, Masliukaite I, Vatish M, Redman C, Sargent I. Update of syncytiotrophoblast derived extracellular vesicles in normal pregnancy and preeclampsia. J Reprod Immunol. 2017;119:98–106. doi: 10.1016/j.jri.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 217.Parchem J, Papanna R, Yang S, Mann LM, Patel A, Moise KJ, Johnson A, Kalluri R. Exploring the diagnostic and prognostic potential of amniotic fluid exosomes in twin-twin transfusion syndrome (TTTS) Am J Obstet Gynecol. 2017;216:S63–S64. [Google Scholar]
- 218.Salomon C, Rice GE. Role of Exosomes in Placental Homeostasis and Pregnancy Disorders. Prog Mol Biol Transl Sci. 2017;145:163–179. doi: 10.1016/bs.pmbts.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 219.Familari M, Cronqvist T, Masoumi Z, Hansson SR. Placenta-derived extracellular vesicles: their cargo and possible functions. Reprod Fertil Dev. 2017;29:433–447. doi: 10.1071/RD15143. [DOI] [PubMed] [Google Scholar]
- 220.Chiarello DI, Salsoso R, Toledo F, Mate A, Vazquez CM, Sobrevia L. Foetoplacental communication via extracellular vesicles in normal pregnancy and preeclampsia. Mol Aspects Med. 2017 doi: 10.1016/j.mam.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 221.Pillay P, Moodley K, Moodley J, Mackraj I. Placenta-derived exosomes: potential biomarkers of preeclampsia. Int J Nanomedicine. 2017;12:8009–8023. doi: 10.2147/IJN.S142732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Biro O, Alasztics B, Molvarec A, Joo J, Nagy B, Rigo J., Jr Various levels of circulating exosomal total-miRNA and miR-210 hypoxamiR in different forms of pregnancy hypertension. Pregnancy Hypertens. 2017;10:207–212. doi: 10.1016/j.preghy.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 223.Jayabalan N, Nair S, Nuzhat Z, Rice GE, Zuniga FA, Sobrevia L, Leiva A, Sanhueza C, Gutierrez JA, Lappas M, Freeman DJ, Salomon C. Cross Talk between Adipose Tissue and Placenta in Obese and Gestational Diabetes Mellitus Pregnancies via Exosomes. Front Endocrinol (Lausanne) 2017;8:239. doi: 10.3389/fendo.2017.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Salomon C, Nuzhat Z, Dixon CL, Menon R. Placental exosomes during gestation: liquid biopsies carrying signals for the regulation of human parturition. Curr Pharm Des. 2018 doi: 10.2174/1381612824666180125164429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Shen L, Li Y, Li R, Diao Z, Yany M, Wu M, Sun H, Yan G, Hu Y. Placentaassociated serum exosomal miR155 derived from patients with preeclampsia inhibits eNOS expression in human umbilical vein endothelial cells. Int J Mol Med. 2018;41:1731–1739. doi: 10.3892/ijmm.2018.3367. [DOI] [PubMed] [Google Scholar]
- 226.Cronqvist T, Salje K, Familari M, Guller S, Schneider H, Gardiner C, Sargent IL, Redman CW, Morgelin M, Akerstrom B, Gram M, Hansson SR. Syncytiotrophoblast vesicles show altered micro-RNA and haemoglobin content after ex-vivo perfusion of placentas with haemoglobin to mimic preeclampsia. PLoS One. 2014;9:e90020. doi: 10.1371/journal.pone.0090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Gohner C, Weber M, Tannetta DS, Groten T, Plosch T, Faas MM, Scherjon SA, Schleussner E, Markert UR, Fitzgerald JS. A New Enzyme-linked Sorbent Assay (ELSA) to Quantify Syncytiotrophoblast Extracellular Vesicles in Biological Fluids. Am J Reprod Immunol. 2015;73:582–588. doi: 10.1111/aji.12367. [DOI] [PubMed] [Google Scholar]
- 228.Tannetta DS, Hunt K, Jones CI, Davidson N, Coxon CH, Ferguson D, Redman CW, Gibbins JM, Sargent IL, Tucker KL. Syncytiotrophoblast Extracellular Vesicles from Pre-Eclampsia Placentas Differentially Affect Platelet Function. PLoS One. 2015;10:e0142538. doi: 10.1371/journal.pone.0142538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sheller S, Urrabaz-Garza R, Kechichian T, Saade G, Menon R. Contractile gene activation of myometrial cells treated with amnion epithelial cell-derived exosomes. Am J Obstet Gynecol. 2016;214:S198. [Google Scholar]
- 230.Tong M, Chen Q, James JL, Stone PR, Chamley LW. Micro- and Nano-vesicles from First Trimester Human Placentae Carry Flt-1 and Levels Are Increased in Severe Preeclampsia. Front Endocrinol (Lausanne) 2017;8:174. doi: 10.3389/fendo.2017.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hadley EE, Sheller S, Urrabaz-Garza R, Kechichian T, Saade G, Menon R. Effect of amnion derived exosomes on feto-maternal gestational cells: new signalers in the labor cascade? Am J Obstet Gynecol. 2017;216:S431–S432. [Google Scholar]
- 232.Brownbill P, Sebire N, McGillick EV, Ellery S, Murthi P. Ex Vivo Dual Perfusion of the Human Placenta: Disease Simulation, Therapeutic Pharmacokinetics and Analysis of Off-Target Effects. Methods Mol Biol. 2018;1710:173–189. doi: 10.1007/978-1-4939-7498-6_14. [DOI] [PubMed] [Google Scholar]
- 233.Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J, Nolte-’t Hoen EN, Piper MG, Sivaraman S, Skog J, Thery C, Wauben MH, Hochberg F. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013:2. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Xu R, Greening DW, Zhu HJ, Takahashi N, Simpson RJ. Extracellular vesicle isolation and characterization: toward clinical application. J Clin Invest. 2016;126:1152–1162. doi: 10.1172/JCI81129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Chandler WL. Measurement of microvesicle levels in human blood using flow cytometry. Cytometry B Clin Cytom. 2016;90:326–336. doi: 10.1002/cyto.b.21343. [DOI] [PubMed] [Google Scholar]
- 236.Sheller S, Urrabaz-Garza R, Kechichian T, Saade G, Menon R. Isolation and characterization of amnion epithelial cell-derived exosomes. Am J Obstet Gynecol. 2016;214:S417–S418. [Google Scholar]
- 237.Partanen H, Vahakangas K, Woo CS, Auriola S, Veid J, Chen Y, Myllynen P, El Nezami H. Transplacental transfer of melamine. Placenta. 2012;33:60–66. doi: 10.1016/j.placenta.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 238.Arakelyan A, Ivanova O, Vasilieva E, Grivel JC, Margolis L. Antigenic composition of single nano-sized extracellular blood vesicles. Nanomedicine. 2015;11:489–498. doi: 10.1016/j.nano.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhang HG, Grizzle WE. Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am J Pathol. 2014;184:28–41. doi: 10.1016/j.ajpath.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 241.Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 242.Gu YZ, Xue Q, Chen YJ, Yu GH, Qing MD, Shen Y, Wang MY, Shi Q, Zhang XG. Different roles of PD-L1 and FasL in immunomodulation mediated by human placenta-derived mesenchymal stem cells. Hum Immunol. 2013;74:267–276. doi: 10.1016/j.humimm.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 243.Li G, Lu C, Gao J, Wang X, Wu H, Lee C, Xing B, Zhang Q. Association between PD-1/PD-L1 and T regulate cells in early recurrent miscarriage. Int J Clin Exp Pathol. 2015;8:6512–6518. [PMC free article] [PubMed] [Google Scholar]
- 244.Enninga EAL, Harrington SM, Creedon DJ, Ruano R, Markovic SN, Dong H, Dronca RS. Immune checkpoint molecules soluble program death ligand 1 and galectin-9 are increased in pregnancy. Am J Reprod Immunol. 2018:79. doi: 10.1111/aji.12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Noorali S, Rotar IC, Lewis C, Pestaner JP, Pace DG, Sison A, Bagasra O. Role of HERV-W syncytin-1 in placentation and maintenance of human pregnancy. Appl Immunohistochem Mol Morphol. 2009;17:319–328. doi: 10.1097/PAI.0b013e31819640f9. [DOI] [PubMed] [Google Scholar]
- 246.Bolze PA, Mommert M, Mallet F. Contribution of Syncytins and Other Endogenous Retroviral Envelopes to Human Placenta Pathologies. Prog Mol Biol Transl Sci. 2017;145:111–162. doi: 10.1016/bs.pmbts.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 247.Clark R, Springer T. Protein reviews on the Web. CD901999. [Google Scholar]
- 248.Nakamura Y, Muguruma Y, Yahata T, Miyatake H, Sakai D, Mochida J, Hotta T, Ando K. Expression of CD90 on keratinocyte stem/progenitor cells. Br J Dermatol. 2006;154:1062–1070. doi: 10.1111/j.1365-2133.2006.07209.x. [DOI] [PubMed] [Google Scholar]
- 249.Araki H, Yoshinaga K, Boccuni P, Zhao Y, Hoffman R, Mahmud N. Chromatin-modifying agents permit human hematopoietic stem cells to undergo multiple cell divisions while retaining their repopulating potential. Blood. 2007;109:3570–3578. doi: 10.1182/blood-2006-07-035287. [DOI] [PubMed] [Google Scholar]
- 250.Haack-Sorensen M, Friis T, Bindslev L, Mortensen S, Johnsen HE, Kastrup J. Comparison of different culture conditions for human mesenchymal stromal cells for clinical stem cell therapy. Scand J Clin Lab Invest. 2008;68:192–203. doi: 10.1080/00365510701601681. [DOI] [PubMed] [Google Scholar]
- 251.Battula VL, Evans KW, Hollier BG, Shi Y, Marini FC, Ayyanan A, Wang RY, Brisken C, Guerra R, Andreeff M, Mani SA. Epithelial-mesenchymal transition-derived cells exhibit multilineage differentiation potential similar to mesenchymal stem cells. Stem Cells. 2010;28:1435–1445. doi: 10.1002/stem.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Yang JT, Hynes RO. Fibronectin receptor functions in embryonic cells deficient in alpha 5 beta 1 integrin can be replaced by alpha V integrins. Mol Biol Cell. 1996;7:1737–1748. doi: 10.1091/mbc.7.11.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lesley J, Hyman R. CD44 can be activated to function as an hyaluronic acid receptor in normal murine T cells. Eur J Immunol. 1992;22:2719–2723. doi: 10.1002/eji.1830221036. [DOI] [PubMed] [Google Scholar]
- 254.Lindenmair A, Hatlapatka T, Kollwig G, Hennerbichler S, Gabriel C, Wolbank S, Redl H, Kasper C. Mesenchymal stem or stromal cells from amnion and umbilical cord tissue and their potential for clinical applications. Cells. 2012;1:1061–1088. doi: 10.3390/cells1041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Ni J, Cozzi PJ, Duan W, Shigdar S, Graham PH, John KH, Li Y. Role of the EpCAM (CD326) in prostate cancer metastasis and progression. Cancer Metastasis Rev. 2012;31:779–791. doi: 10.1007/s10555-012-9389-1. [DOI] [PubMed] [Google Scholar]
- 257.Fitzgerald W, Lederman MM, Vasilieva E, Romero R, Margolis L. Soluble cytokines are not soluble: Biologically regulated system of cytokines encapsulated in extra-cellular vesicles. Sci Rep. 2018 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.