Abstract
Background
Allogeneic NK cell adoptive immunotherapy is a growing therapeutic option for patients. Clinical-scale production of NK cells using immunomagnetic selection complies with current good manufacturing practices (cGMPs) and allows for closed-system, automated purification. We report our experience with CD3/CD19 cell-depleted (CD3/CD19dep) NK cell production and compare to previous methods of CD3 cell-depletion and CD3 cell-depletion/CD56 cell-enrichment.
Study Design and Methods
Non-mobilized mononuclear cells collected by apheresis were incubated with anti-CD3/anti-CD19 microbeads and depleted in an automated cell selection system (CliniMACS, Miltenyi). The NK cell enriched products were incubated overnight in interleukin (IL)-2 or IL-15, washed, and resuspended prior to lot release testing and infusion.
Results
Since 2010, 94 freshly infusible CD3/CD19dep NK cell products were manufactured in support of 8 clinical trials. Sixty-six products were incubated in IL-2 and 28 products in IL-15. Processing resulted in a mean NK cell recovery of 74% and viability of 95.8%; NK cells, T cells, B cells, and monocytes accounted for 47%, 0.2%, 0.08%, and 49% of the final products, respectively. Seven products required dose adjustments to meet lot release. The specification for purity changed throughout the evolution of manufacturing. IL-2 or IL-15 activation enhanced in vitro cytotoxicity compared to pre-activated cells. There was no difference in final product composition or cytotoxicity between cytokine cohorts.
Conclusion
Clinical-scale/cGMP production of NK cells using CD3/CD19 cell-depletion effectively minimized T cell and B cell contamination in a single manipulation without compromise to NK cell recovery. Cytokine-activation increased in vitro cytotoxicity compared to column-depleted, pre-activated NK cells.
INTRODUCTION
Human natural killer (NK) cells are lymphocytes of the innate immune system with effector and regulatory functions that have been exploited for their antitumor and antiviral potential. Approximately 10 to 20 percent of circulating lymphocytes are NK cells that surveil for virally-infected and transformed cells and exert potent cytotoxic activity in the absence of prior antigenic sensitization.1 NK cells are a phenotypically and functionally heterogeneous population distinguished by surface expression of CD56 and lack of T-cell antigens such as the CD3 pan-T-cell marker or the T-cell receptor (CD56+CD3−). CD56 is an isoform of the neural cell adhesion molecule with unknown function, but CD56 density marks the maturation status of NK cells such that CD56bright NK cells differentiate into CD56dim NK cells supporting a linear model of development between these subsets.2 These subsets are functionally divided into a CD56bright immunoregulatory population that predominate in secondary lymphoid tissue and produce cytokines (e.g., IFN-γ, TNF-β, IL-10, IL-13, and GM-CSF), and a CD56dim cytotoxic population primarily in circulation.3,4 Subpopulations of the CD56dim cells expressing the low-affinity Fc receptor, CD16, participate in antibody-dependent cell-mediated cytotoxicity.5,6
NK cells lyse target cells in the absence of major histocompatibility complex class I (MHC-I) expression, which is unique from cytotoxic T-lymphocytes that require antigen presentation by MHC-I to become activated for killing.7 However, NK cells do not function independently of MHC-I. In a process known as licensing, naïve NK cells that engage MHC-I are “educated” to recognize self and remain quiescent while simultaneously becoming activated to lyse targets that lack or down-regulate this expression.8 Virally-infected and transformed cells commonly down-regulate MHC as a mechanism of immune evasion from cytotoxic T-lymphocytes, and this “loss of self” renders them sensitive to killing by NK cells.9
NK cell function is tightly regulated by a combination of signals received via inhibitory and stimulatory receptors that recognize MHC-I and other surface ligands.8,10,11 It is the net effect of complex signaling that determines whether NK cells become cytotoxic or remain quiescent. The killer immunoglobulin-like receptor (KIR), lectin NKG2 receptors, and natural cytotoxicity receptors (NCRs) are the main families of known NK cell receptors described.12 KIRs interact with classic HLA ligands (HLA-A, -B, -C) whereas NKG2 receptors interact with non-classic HLA ligands (HLA-E). KIR and NKG2 each have subsets of stimulatory and inhibitory receptors; under normal physiologic conditions the inhibitory signals prevail. It is not enough to remove the inhibitory signals to activate NK cell killing but rather, activating receptors must also interact with their cognate ligand, which are generally only upregulated under conditions that stress the target cell.1,8,12 With each NK cell expressing a different pattern of receptors, they can sense and react to subtle changes in the microenvironment.
NK cells have gained significant traction in adoptive immunotherapy clinical trials due to their graft-versus-tumor effect without apparent harm of graft-versus-host disease (GVHD).13,14 NK cells are cytotoxic against a variety of solid organ and hematologic malignancies in vitro and IL-2 or IL-15 exposure has been shown to augment cytotoxicity against NK-resistant cell lines.15 Early trials of autologous NK cells lacked clinically efficacy,16 presumably due to inhibition by self-MHC, and current immunotherapy is focused on allogeneic sources. Mismatch of KIR-HLA ligands, whereby the recipient is missing the cognate ligand to an inhibitory KIR on the donor cells, has been shown to enhance alloreactivity and graft-versus-leukemia in vitro and in vivo.17,18 The greatest clinical benefit for allogeneic NK cells has thus far been demonstrated in patients with acute myelogenous leukemia (AML), including as salvage therapy for relapsed or refractory disease,19–21 consolidation therapy following hematopoietic cell transplant (HCT),22,23 and NK donor lymphocyte infusions (NK-DLI) for relapse or graft rejection.24 Cytokine-induced memory-like NK cells have recently been described and show promising clinical efficacy in early trials.25
One challenge of adoptive transfer is the generation of enough alloreactive NK cells to overcome suppressive or competing host factors that prevent in vivo NK cell survival and expansion.20 We previously reported our 6-year manufacturing experience generating fresh allogeneic NK cells for human infusion in clinical trials using two methods, CD3 cell depletion (CD3dep) and CD3 cell depletion followed by CD56 cell enrichment (CD3dep/CD56enrich).26 Here we review clinical-scale allogeneic NK cell manufacturing under current good manufacturing practices (cGMP) at our institution. Our current standard of CD3/CD19 cell depletion (CD3/CD19dep) effectively minimizes T cell and B cell contamination in a single manipulation that enriches for NK cells and accessory monocytes, and this is the largest report of their manufacturing for human infusion.
MATERIALS AND METHODS
Cell processing was performed between 2010 and 2015 at the Clinical Cell Therapy Laboratory housed within the Molecular and Cellular Therapeutics (MCT) cGMP facility at the University of Minnesota.27 Institutional Review Board approval was obtained for all clinical trials using NK cell products supported by our facility as well as FDA approval for investigational new drug status required for novel cellular therapies.
NK cell processing
One day prior to infusion, mononuclear cells (MNC) were collected from nonmobilized donor peripheral blood using a 15L separation process on the COBE Spectra Apheresis System (TerumoBCT, Lakewood, CO). All processing was performed in a closed system. T-cell and B-cell depletion was performed using the Miltenyi Biotec CliniMACS cell selection system and reagents (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacture’s specification. In the depletion process, up to 2×1010 total nucleated cells (TNCs) were incubated with cGMP-grade anti-CD3- and anti-CD19-conjugated ferromagnetic microbeads. The cells were washed and loaded onto an LS column where the labeled cells were separated in a magnetic field within the automated CliniMACS using Depletion 2.1 software.
The cells were resuspended at 2×106 cells/mL in serum-free X-VIVO 15 medium, without gentamicin and phenol red (Cambrex BioScience, Walkersville, MD), and 10% human AB serum (Valley Biomedical Products and Services, Inc., Winchester, VA). The medium was supplemented with either 1000 U/mL IL-2 (Proleukin, Chiron Corp., Emeryville, CA) or 10 ng/mL IL-15 (NCI at Frederick, Frederick, MD ), depending on the trial protocol for which the cells were prepared. They were incubated overnight in VueLife Teflon (FEP) bags (American Fluoroseal, Gaithersburg, MD) at 37°C and 5% CO2. The cells were washed twice and re-suspended in 5% human serum albumin (Baxter Healthcare) at a concentration of approximately 25×106 NCs/mL. Samples were sent for lot release testing prior to product release for infusion.
Product characterization
Samples sent for flow cytometry were processed in our Translational Therapy Lab where the cells were stained with a 4-color or 10-color panel, acquired on a FACSCalibur (BD Biosciences, San Jose, CA) or LSR-II flow cytometer (BD Biosciences), respectively, and analyzed using FlowJo (FlowJo LLC). The cells were characterized using antibodies against CD3, CD14, CD16, CD19, CD20, CD45, CD56, KIR, and NKG2A (BD Biosciences).
Lot release testing
Lot release testing included cell counts, purity, viability, Gram stain, and endotoxin. Patient dosages were based on cell counts obtained using a hematology analyzer (Coulter MD II, Beckman Coulter, Fullerton, CA; Sysmex XS1000i, Sysmex America, Inc., Lincolnshire, IL) for NC dose (<8×107/kg) and flow cytometry for T cell dose (<3×105 CD3+ cells/kg). NK cell purity standards required adjustments throughout our manufacturing process. Purity was initially set at ≥20% (CD56+CD3−) NK cells and increased to ≥30% upon recommendation by the FDA that the addition of CD19 cell depletion called for a higher threshold than was previously established for CD3dep products. The specification was adjusted to a 3-fold increase in NK cell percentage between the final product and the apheresis MNCs after 2 products did not meet 30% NK cell purity due to low starting NK cell content. Another product failure led to speculation of interference from nonspecific antibody binding and lymphocyte gating was added without further issue. Specification for B cells was set at <3% CD20+ cells.
Viability testing was performed using flow cytometric analysis of 7-aminoactinomycin D (BD Biosciences, Franklin Lakes, NJ) with a lot release specification of ≥70%. The Clinical Microbiology Laboratory carried out Gram staining by standard methods and product release specification was no organisms identified. In-house endotoxin testing was performed using a validated chromogenic limulus amebocyte lysate-based endotoxin assay (Kinetic-QCL, Lonza, Walkersville, MD) with release criteria of ≤5 EU/kg per total product. Mycoplasma testing was not required by the FDA due to closed system processing and a brief culture time that did not include animal serum products. Sterility testing using a 14 day culture in an automated BacT/ALERT microbial detection system (bioMérieux, Inc., Durham, NC) or BACTEC blood culture system (BD Biosciences, Franklin Lakes, NJ) was performed as post-release testing but was not required for lot release.
Cytotoxicity testing
In vitro cytotoxicity using a 4-hour chromium release assay was tested against the NK cell-sensitive K562 line as previously described.28 Effector-to-target ratios were based on NC counts and ranged from 20:1 to 0.08:1.
Statistical analysis
The percentages of NK cells and other continuous variables were presented with mean ± standard deviation (SD) for different groups. T-test was used for comparisons between two groups. Linear regression model was used to compare cytotoxicity between the CD3/CD19dep IL-2 activated and IL-15 activated groups, adjusting for their baseline level. Bar charts with mean and standard error (SE) of the mean were presented for NK cell subsets and receptor profiles. Mean cytotoxicity was presented in line charts for different unstimulated and cytokine-activated NK cell products, at different effector-to-target ratio levels. All tests were 2-sided. P-values < 0.05 were considered statistically significant. All analyses were performed with SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Since 2010, the MCT facility has manufactured over 100 CD3/CD19dep NK cell products from non-mobilized peripheral blood MNCs of related haploidentical donors in support of 8 clinical trials at the University of Minnesota (www.clinicaltrials.gov, NCT01105650, NCT01106950, NCT01181258, NCT01370213, NCT01385423, NCT01593670, NCT02118285, NCT02395822). Reported here are 94 freshly infused NK cells products that, following depletion, were incubated overnight in either IL-2 (5 clinical trials, n=66) or IL-15 (2 clinical trials, n=28) to promote NK cell activation. Data for a trial involving cryopreservation of the NK cells and those shipped to outside institutions is excluded. Previously published data from 2 processing methods (CD3dep and CD3dep/CD56enrich) performed at our facility between 2000 and 2005 is also summarized here for comparison.26
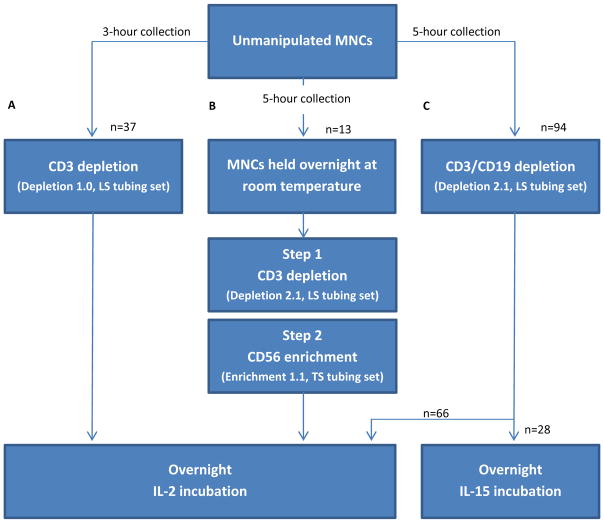
Briefly, we manufactured 57 CD3dep NK cell products and reported on 37 products that were not part of a dose escalation trial. Likewise, 13 products underwent a 2-step manipulation of CD3dep/CD56enrich. Cell processing was similar between all 3 manufacturing methods with the following exceptions (see Figure 1): 1) apheresis collection time was 3-hours for CD3dep and 5-hours for subsequent processing, 2) Depletion 1.1 software (Miltenyi Biotec) used for CD3dep was upgraded to Depletion 2.1 software for subsequent processing, 3) the unmanipulated MNCs from the CD3dep/CD56enrich products were held overnight at room temperature and processed the day after collection due to the lengthier 2-step process, whereas all other products were processed on the day of MNC collection, 4) Enrichment 1.1 software was used for the CD56 positive selection, and 5) all products were incubated overnight in IL-2 except for the most recently manufactured CD3/CD19dep NK cells, which were incubated in IL-15.
Figure 1. Comparative manufacturing of clinical-scale, cGMP-grade NK cell products.
Three methods have been used to purify NK cells from apheresed MNCs of nonmobilized peripheral blood donors using the CliniMACS cell separation system: (A) 3-hour apheresis collection, CD3 cell depletion using Depletion 1.0 software and an LS tubing set, and IL-2 incubation, (B) 5-hour apheresis collection, a two-step process of CD3 cell depletion using Depletion 2.1 software and an LS tubing set followed by CD56 cell enrichment using Enrichment 1.1 software and a TS tubing set, and IL-2 incubation, and (C) 5-hour apheresis collection, a single-step CD3/CD19 cell depletion using Depletion 2.1 software and an LS tubing set, and IL-2 or IL-15 incubation. CD3dep and CD3/CD19dep products were processed on the day of MNC collection, whereas the more time consuming 2-step processing of the CD3dep/CD56enrich products took place the day after MNC collection.
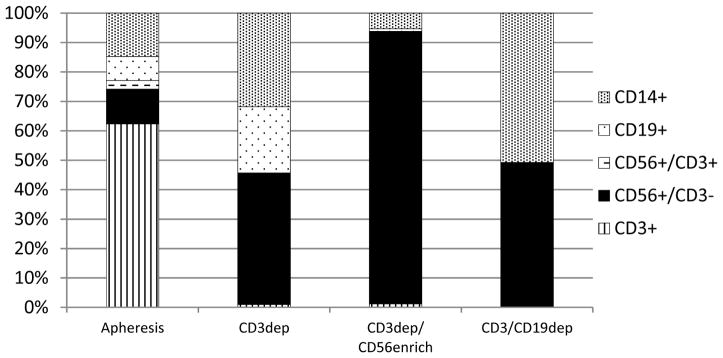
Comparative processing results are summarized in Table 1. Five-hour apheresis collections generated greater numbers of MNCs compared to 3-hour collections. The mean MNC collection for CD3/CD19dep contained 2.3×1010 TNCs. CD56+CD3− NK cells increased from 12 ± 5% (mean ± SD) in the unmanipulated MNCs to 39 ± 15% following column depletion to 47 ± 18% in the cytokine-activated product. CD3/CD19dep resulted in a greater log reduction of T cells (based on 25 products that underwent interim analysis prior to cytokine exposure) compared to CD3dep (3.5 versus 2.7, P<0.001) and comparable NK cell recoveries (74% versus 79%, P>0.05). The CD3+ T cell and CD19+ B cell content was reduced from 63% and 10% in the unmanipulated MNCs to 0.2% and 0.07%, respectively, in the cytokine-activated CD3/CD19dep products (see Figure 2). There was a relative enrichment of CD14+ monocytes from 16% to 49%. In comparison, CD3dep NK cell products contained 38 ± 13% NK cells, 26% B cells, 1% T cells, and 31% monocytes. The mean viability of CD3/CD19dep products was 95.8%. No difference was observed in the mean composition or viability between the IL-2 and IL-15 activated CD3/CD19dep products (data not shown).
Table 1.
Comparative summary of processing results for NK cell manufacturing methods
| CD3dep (n=37) | CD3dep/CD56enrich (n=13) | CD3/CD19dep (n=94) | ||
|---|---|---|---|---|
|
|
||||
| Apheresis MNCs | TNC count (x1010)* | 2.0 ± 0.8 | 2.4 ± 0.7 | 2.3 ± 0.7 |
| % NK cells | 11 ± 4 | 12 ± 6 | 12 ± 5 | |
| Post-column processing | TNC (109) | 4.7 ± 2.4 | 0.6 ± 0.4 | 4.9 ± 1.4 |
| % NK cells | 38 ± 13 | 90 ± 7 | 39 ± 15 (n=25)† | |
| % NK cell recovery | 79 ± 15 | 19 ± 11 | 74 ± 15 (n=25)† | |
| Log T-cell depletion | 2.7 ± 0.4 | 4.3 ± 0.5 | 3.5 ± 0.5 (n=25)† | |
| Cytokine activated product | % NK cells | 39 ± 9 | 75 ± 6 | 47 ± 18 |
| Total NK cells infused (x107)‡ | 68.7 ± 26.5 | 25.3 ± 14.0 | 158.1 ± 109.9 | |
|
| ||||
| Processing time (hours)ˠ | 12 | 17 | 12 | |
| Processing cost (US dollars)ˠ | 9,757 | 14,607 | 12,742 | |
Data is reported as the mean ± SD
Apheresis collection times were 3-hours for CD3dep products and 5-hours for all other products
Based on 25 of the 94 products that had post-depletion data collected prior to cytokine exposure
Lot release specification for NC dose increased from 3×107/kg for CD3dep and CD3dep/CD56enrich products to 8×107/kg for CD3/CD19dep products, thus increasing the total number of NK cells available for infusion
The cell processing time difference is attributed to concurrent cell depletion versus sequential depletion and enrichment. Cost includes cell processing supplies, lot release testing, and quality assurance review, with differences based on the number of columns and immunomagnetic bead reagents and technician time
Fig. 2. Comparative characterization of NK cell products.
Flow cytometry analysis of the unmanipulated apheresis MNCs (combined from all 3 manufacturing processes; n=144) and the NK cell products from each manufacturing process: CD3dep (n=37), CD3dep/CD56enrich (n=13), and CD3/CD19dep (n=94). The mean of the cell compositions did not equal 100 percent and data is adjusted to a relative proportion of 100 percent.
As expected, the addition of CD56 enrichment resulted in highly purified NK cells (90 ± 7% NK cells, 5 % monocytes, 0.6% B cells, 0.2% T cells) with the greatest log reduction of T-cells (4.3, P<0.001 when compared to CD3/CD19dep). However, there was considerable cell loss in the 2-step manipulation and NK cell recovery was significantly compromised (19% versus 74%, P<0.001). Furthermore, the CD56+CD3− NK cell purity decreased to 75% following overnight IL-2 incubation and the T cell content increased to 1% (data not shown). This was not observed with CD3dep or CD3/CD19dep NK cell products and we speculate that the additional overnight hold of the apheresis MNCs may have led to CD56 downregulation in senescent NK cells. Paradoxically, CD3/CD19dep NK cells increased from 39% post-processing to 47% following cytokine activation, but only one-quarter of the CD3/CD19dep NK cell products underwent interim post-processing flow cytometric immunophenotyping and this apparent increase in CD56 expression may not be entirely representative. Since NK cell expansion does not occur with overnight incubation, this finding may reflect the rapid CD56 upregulation known to occur with IL-2 or IL-15.
Lot release specifications for NC and T cell dosages have increased since our earlier products from 3×107 to 8×107 NCs/kg and from 3×104 to 3×105 T cells/kg. In addition to the extended 5-hour apheresis collection time, these factors have resulted in the greatest number of total NK cells in the final products (mean 1.6×109). Cell processing from a single apheresis yielded a mean NK cell dosage of 2 (range 1.0–6.2) ×107 cells/kg with only 7 products requiring dose adjustments to be within lot release limits for infusion (1 for NC dose and 6 for T cell dose). All products have passed NK cell purity testing since modification of the lot release specification as described in the lot release testing above. There was a mean 9.7-fold increase in CD56+CD3− NK cells between lymphoid-gated apheresis MNCs and final CD3/CD19dep NK cell products.
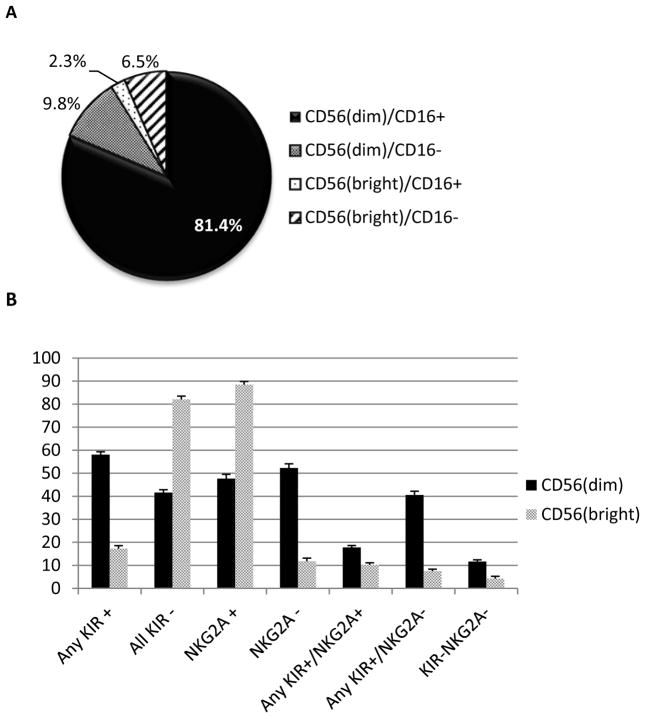
The predominating phenotype of the cytokine-activated CD3/CD19dep NK cells was CD56dimCD16+ (mean 81.4%, see Figure 3a), followed by CD56dimCD16− (9.8%), CD56brightCD16− (6.5%), and CD56brightCD16+ (2.3%). Of the CD56dim cells, 58% expressed at least 1 inhibitory KIR (see Fig. 3b), including KIR2DL1, KIR2DL2/3, or KIR3DL1, which are cognate ligands for HLA-C2, HLA-C1, HLA-BW4, respectively; 48% expressed inhibitory NKG2A, the cognate ligand for HLA-E. CD56dim co-expression of at least 1 KIR and NKG2A occurred in 18%. In contrast, the majority of CD56bright cells lacked any KIR expression (mean 82%) but highly expressed NKG2A (89%). Neither inhibitory ligand was expressed in 12% of CD56dim and 4% CD56bright NK cells. There was no difference in the mean KIR and NKG2A expression levels between IL-2- and IL-15-activated groups (data not shown).
Fig 3. NK cell subsets and receptor profiles.
Flow cytometry profiling of CD3/CD19dep, cytokine-activated CD56(dim) and CD56(bright) NK cell subsets with respect to CD16 expression (A) and KIR and NKG2A expression (B).
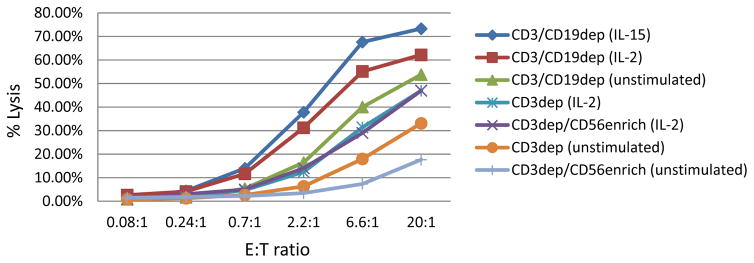
Comparative cytotoxicity results summarized in Figure 4 show that cytokine exposure enhanced the cytotoxicity of CD3dep, CD3dep/CD56enrich, and CD3/CD19dep NK cells products over their unstimulated counterparts. The unstimulated CD3/CD19dep cells had different baseline cytotoxicity between the IL-15 group (58% prior to cytokine activation) and the IL-2 group (47%) at 20:1 effector-to-target ratio (data not shown). After adjusting for the difference in baseline, the cytotoxicity of the IL-15- and IL-2-activated groups (73% and 62%, respectively, at 20:1 effector-to-target ratio) were not significantly different(P=0.77). The unstimulated CD3/CD19dep NK cells in the IL-2 group had a limited data set for analysis (n=19 of the 66 products) and the true baseline cytotoxicity may not be representative.
Fig 4. Comparative cytotoxicity for unstimulated (pre-cytokine) and cytokine-activated NK cell products against K-562 target cells.
Effector-to-target (E:T) ratios were based on nucleated cell count. CD3dep (n=37) and CD3dep/CD56enrich (n=13) NK cells were cytokine-activated with IL-2; CD3/CD19dep NK cells were cytokine-activated with IL-2 (n=66) or IL-15 (n=28). The unstimulated CD3/CD19dep (n=47) NK cells include all unstimulated products in the IL-15 cohort (n=28) and 19 of the 66 unstimulated products in the IL-2 cohort (limited data set). The difference in cytotoxicity between the IL-2- and IL-15-activated CD3/CD19dep NK cells was not statistically significant after adjusting for the difference in baseline cytotoxicity between these groups (P=0.77 at 20:1 E:T ratio).
It appears that baseline cytotoxicity of unstimulated products is enhanced with increasing absolute or relative monocyte content, with CD3dep/CD56enrich having the fewest monocytes (5%) and lowest baseline cytotoxicity (18% at 20:1 effector-to-target ratio), CD3dep NK cell products with31% monocytes having intermediate baseline cytotoxicity (33%), and CD3/CD19dep having the greatest number of monocytes (49%) and highest baseline cytotoxicity (54%). This may be a real phenomenon; however, caution should be exercised in comparative observation between manufacturing methods. The overnight hold of unmanipulated MNCs for the CD3dep/CD56enrich cohort prior to processing may have compromised their cytotoxic potential. In addition, effector-to-target ratios are based on NC count and the NK cell content between manufacturing methods differ.
DISCUSSION
Adoptive immunotherapy using allogeneic NK cells for the treatment of malignancy with or without concurrent HCT is increasing as growing evidence supports a graft-versus-tumor effect and protection against GVHD, graft rejection, and disease relapse.13,17,29–32 Over the past 15 years, the MCT facility has manufactured approximately 260 cGMP-grade NK cell products from non-mobilized peripheral blood of related haploidentical donors in support of human phase I and phase II clinical trials. These cells have been used for the treatment of advanced solid organ and hematologic malignancies, including breast and ovarian cancer, melanoma, renal cell carcinoma, Hodgkin’s lymphoma, and AML.19,20,33,34 We have demonstrated exportability with overnight shipping of processed NK cells and in-transit cytokine activation for infusion within 48 hours of apheresis collection.35,36
The first successful NK cell adoptive immunotherapy trial at the University of Minnesota used CD3dep ex vivo IL-2 activated NK cells as salvage therapy for poor prognostic cancers.20 Remarkably, 5 of 19 patients with refractory and relapsed AML who received preconditioning with high-dose lymphodepleting chemotherapy achieved complete hematologic remission. Reduction in competing host lymphocytes inversely correlated with a rise in endogenous IL-15 and appeared to be prerequisite for donor NK cell persistence and expansion. Dose escalation showed that infusion of 2×107 MNCs/kg was safe and a maximal tolerated dose was not reached. Transfusion-associated (TA)-GVHD was not observed at a mean T cell dose of 2.1 (range 0.5–6.5) ×105 cells/kg. However, 2 cases of passenger lymphocyte syndrome (PLS)-induced hemolytic anemia37 and 1 fatal case of lymphoproliferative disease secondary to reactivation of Epstein-Barr virus were observed.20 To our knowledge these entities, caused by mature B cell contamination and well-known in the setting of solid organ and HCT,38 had previously not been described in relation to NK cell infusions.
It was speculated that high-level purification with CD3dep/CD56enrich may reduce B cell-related complications, improve T cell depletion, and potentially enhance the immunotherapeutic efficacy of alloreactive NK cells. However, an improvement in purity proved to be at the expense of NK cell recovery and was dually affected by cell loss from the 2-step manipulation and procedural time logistics with an additional overnight hold of the apheresis MNCs prior to processing. These highly enriched products contained approximately one-third the NK cell dose of CD3dep products and failed to induce hematologic remissions.19 Next day processing may have had deleterious effects on NK cell viability and functionality (cytotoxicity). Koehl et al described significant NK cell loss using the same CD56 enrichment method, albeit with better NK cell recovery (41%, range 14–76%) than our experience (19%, range 3–38%).39 In most cases they performed CD3 depletion on the day of MNC collection, thus enriching for NK cells, monocytes, and B cells, followed by CD56 selection on day 2. In some cases, two CD3 depleted products were pooled prior to CD56 selection. They noted that NK cell recovery was more compromised in the exceptional instances where the MNCs were held overnight at 4°C on a waver and processed on day 2. They improved NK recovery to 68% (range 40–85%) in a cohort that underwent a faster method of CD3 depletion using newer Depletion 3.1 software (as opposed to Depletion 2.1 software), which was considerably less effective at T cell reduction.
Schaffer et al evaluated haploidentical CD3dep/CD56enrich NK cell infusions following lymphodepleting chemotherapy in patients with relapsed or progressive AML (n=6) or myelodysplastic syndrome (MDS, n=2) following HCT.21 One adult patient with MDS and one pediatric patient with AML achieved a complete response, and a third adult patient with MDS had resolution of dysplastic features with persistence of the abnormal karyotype. The NK cells were completely processed on the day of MNC collection and infused the following day without in vitro cytokine incubation. The mean NK cell purity and recovery were 98.9% and 57.2% (range 41.3–86.8%), respectively. NK cell dosages were 0.62× 107/kg in adult patients (n=4) and 1.86 ×107/kg in pediatric patients (n=4). These results demonstrate the feasibility of this method and suggest that refinement of our protocol to minimize time from apheresis to processing may lead to better clinical outcomes. In another phase I trial by Romee et al, adoptive transfer of memory-like NK cells that were induced from CD3dep/CD56enrich NK cells using IL-12, IL-15, and IL-18 for relapsed and refractory AML has shown promising results.25 In nine evaluable patients the overall response rate and the complete remission rate (including incomplete blood count recovery) were 55% and 45%, respectively, with demonstration of NK cell persistence and expansion in vivo.
The optimal manufacturing method to maximize NK cell alloreactivity against tumor cell is ultimately not known and crosstalk between various hematopoietic cells and cytokines may be an important factor that is eliminated with high-level purification. In the setting of haploidentical HCT, graft manipulation with CD3/CD19 cell depletion is an alternative to highly purified CD34 selected grafts, which require “megadoses” of CD34+ cells to overcome HLA-mismatch barriers. The cocktail of CD34+ cells, CD34− cells, NK cells, monocytes, dendritic cells, and other potentially supporting factors appear to facilitate faster engraftment and immune reconstitution, and augment the NK cell graft-versus-leukemia effect.40,41 Thus, removal of accessory monocytes, which have a supporting role in NK cell survival and function through direct cell-cell contact and humoral factors,28 may be detrimental to optimal NK cell processing. In addition, high-level purification with the 2-step depletion and selection process increases production cost and manufacturing time compared to single-step depletion.
CD3/CD19dep is a single-step manipulation that sufficiently reduces T and B cell contamination without compromising NK cell recovery or depleting accessory monocytes. Clinical remissions have been demonstrated in patients with relapsed and refractory AML who received CD3/CD19dep NK cell infusions following lymphodepleting chemotherapy, and the remission rate was enhanced by targeted host T regulatory cell (Treg) depletion.19 Host Tregs can suppress donor NK cells and have shown resistance to high-dose lymphodepleting chemotherapy with rapid proliferation in response to subcutaneous IL-2 administered after donor NK cell infusion.33,34 IL-15 is important, if not required, for in vivo survival and expansion of mature NK cells,20,42 and IL-15 activation may render NK cells less susceptible to host Treg suppression.19 Two trials are ongoing at the University of Minnesota to evaluate the effects of intravenous and subcutaneous IL-15 on in vivo NK cell survival and expansion (NCT01385423 and NCT02395822). In support of these trials we began in vitro IL-15 activation of CD3/CD19dep NK cells. In our experience there was no difference between overnight IL-2 and IL-15 exposure on NK cell purity, viability, or immunophenotype; in vitro cytotoxicity of IL-15 activated NK cells was as effective as IL-2 activation.
GVHD has not been observed with NK cells produced by any of our three manufacturing processes. T cell depletion is standard practice to minimize the risk of TA-GVHD, a rare and fatal complication whereby mature donor T cells are alloreactive against host antigen presenting tissues, particularly in the setting of immune suppression and haploidentical MHC mismatch.43 Unlike HCT-associated GVHD where engraftment protects the bone marrow compartment, TA-GVHD leads to bone marrow aplasia. Despite effective T cell depletion with each of our manufacturing methods, a significant T cell load is nonetheless infused in the final NK cell product. Our lot release criteria for T cell dose is a higher threshold than other reports showing GVHD occurrence at lower T cell doses in the HCT setting,44,45 and significantly higher than what the FDA allots for transfusion of blood components.46 Ablation of host antigen presenting cells by alloreactive NK cells such that T cell priming is prevented has been proposed as a mechanism of protection against GVHD by adoptively transferred NK cells.8,18
NK cell receptors are under intense investigation for their graft-versus-tumor mediating effects. NK cells acquire KIR ligand expression as they go through education and licensing, and greater expression of inhibitory receptors leads to greater cytotoxic potential in the absence of their cognate ligand and in the presence of activating signals.47 Thus, KIR-positive subsets are thought to be more functionally active than KIR-negative subsets. Ex vivo cytokine incubation has been shown to induce activating NK cell receptors, although we have not evaluated for upregulation of these receptors in our NK cells with overnight cytokine incubation.39 The majority of our NK cells had a CD56dimCD16+ phenotype, which is consistent with the major subset of circulating NK cells known for their cytolytic function, and expressed at least 1 inhibitory KIR or inhibitory NKG2A, or both. Given the high expression of CD16 on CD56dim NK cells, antibodies such as rituximab may be synergistic with NK cell therapy through ADCC.48 Likewise, antibody blockage of NKG2A or other inhibitory KIRs may augment cytolytic effects by blocking interaction with the cognate ligand.49
CONCLUSION
Optimization and standardization of NK cell manufacturing is a clinical necessity to maximize reproducible antitumor effectiveness while minimizing toxicity. We are consistent in producing clinical-scale, cGMP-grade allogeneic NK cells from peripheral blood MNCs without the use of ex vivo expansion or feeder cells. Advantages of a peripheral blood source include collection and processing in a closed system to minimize contamination and the ability to return to the donor should subsequent collection be needed. Our current practice standard of T-cell and B-cell removal through CD3 and CD19 depletion improves NK cell recovery and conserves monocytes compared to our previous method of CD3 depletion followed by CD56 enrichment, which is more effective at T-cell removal. Ultimately, the optimal processing method may depend on the setting for which the cells are prepared. Further development of our process will depend on the continuing elucidation of basic NK cell biology as well as ongoing clinical trial experience and outcomes.
Acknowledgments
Supported in part by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R25HL128372; Production Assistance for Cellular Therapies (PACT) program from NIH/NHLBI at University of Minnesota Molecular and Cellular Therapeutics Facility (PACT Contract # HHSN268201000008C; NIH P30 CA77598 utilizing the Translational Therapy Laboratory Shared Resource of the Masonic Cancer Center, University of Minnesota; Children’s Cancer Research Fund, Leukemia Research Fund, and American Cancer Society, NIH P01 CA111412 and P01 CA65493. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–9. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan A, Hong DL, Atzberger A, Kollnberger S, Filer AD, Buckley CD, McMichael A, Enver T, Bowness P. CD56bright human NK cells differentiate into CD56dim cells: role of contact with peripheral fibroblasts. J Immunol. 2007;179:89–94. doi: 10.4049/jimmunol.179.1.89. [DOI] [PubMed] [Google Scholar]
- 3.Carrega P, Bonaccorsi I, Di Carlo E, Morandi B, Paul P, Rizzello V, Cipollone G, Navarra G, Mingari MC, Moretta L, Ferlazzo G. CD56(bright)perforin(low) noncytotoxic human NK cells are abundant in both healthy and neoplastic solid tissues and recirculate to secondary lymphoid organs via afferent lymph. J Immunol. 2014;192:3805–15. doi: 10.4049/jimmunol.1301889. [DOI] [PubMed] [Google Scholar]
- 4.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–51. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 5.Alderson KL, Sondel PM. Clinical cancer therapy by NK cells via antibody-dependent cell-mediated cytotoxicity. J Biomed Biotechnol. 2011;2011:379123. doi: 10.1155/2011/379123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trinchieri G, Valiante N. Receptors for the Fc fragment of IgG on natural killer cells. Nat Immun. 1993;12:218–34. [PubMed] [Google Scholar]
- 7.Raulet DH, Held W. Natural killer cell receptors: the offs and ons of NK cell recognition. Cell. 1995;82:697–700. doi: 10.1016/0092-8674(95)90466-2. [DOI] [PubMed] [Google Scholar]
- 8.Moretta A, Pende D, Locatelli F, Moretta L. Activating and inhibitory killer immunoglobulin-like receptors (KIR) in haploidentical haemopoietic stem cell transplantation to cure high-risk leukaemias. Clin Exp Immunol. 2009;157:325–31. doi: 10.1111/j.1365-2249.2009.03983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–44. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 10.Godal R, Bachanova V, Gleason M, McCullar V, Yun GH, Cooley S, Verneris MR, McGlave PB, Miller JS. Natural killer cell killing of acute myelogenous leukemia and acute lymphoblastic leukemia blasts by killer cell immunoglobulin-like receptor-negative natural killer cells after NKG2A and LIR-1 blockade. Biol Blood Marrow Transplant. 2010;16:612–21. doi: 10.1016/j.bbmt.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varbanova V, Naumova E, Mihaylova A. Killer-cell immunoglobulin-like receptor genes and ligands and their role in hematologic malignancies. Cancer Immunol Immunother. 2016;65:427–40. doi: 10.1007/s00262-016-1806-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farag SS, Fehniger TA, Ruggeri L, Velardi A, Caligiuri MA. Natural killer cell receptors: new biology and insights into the graft-versus-leukemia effect. Blood. 2002;100:1935–47. doi: 10.1182/blood-2002-02-0350. [DOI] [PubMed] [Google Scholar]
- 14.Olson JA, Leveson-Gower DB, Gill S, Baker J, Beilhack A, Negrin RS. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood. 2010;115:4293–301. doi: 10.1182/blood-2009-05-222190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachanova V, Miller JS. NK cells in therapy of cancer. Crit Rev Oncog. 2014;19:133–41. doi: 10.1615/critrevoncog.2014011091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns LJ, Weisdorf DJ, DeFor TE, Vesole DH, Repka TL, Blazar BR, Burger SR, Panoskaltsis-Mortari A, Keever-Taylor CA, Zhang MJ, Miller JS. IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: a phase I/II trial. Bone Marrow Transplant. 2003;32:177–86. doi: 10.1038/sj.bmt.1704086. [DOI] [PubMed] [Google Scholar]
- 17.Ruggeri L, Capanni M, Casucci M, Volpi I, Tosti A, Perruccio K, Urbani E, Negrin RS, Martelli MF, Velardi A. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94:333–9. [PubMed] [Google Scholar]
- 18.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 19.Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lewis D, Hippen K, McGlave P, Weisdorf DJ, Blazar BR, Miller JS. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123:3855–63. doi: 10.1182/blood-2013-10-532531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 21.Shaffer BC, Le Luduec JB, Forlenza C, Jakubowski AA, Perales MA, Young JW, Hsu KC. Phase II Study of Haploidentical Natural Killer Cell Infusion for Treatment of Relapsed or Persistent Myeloid Malignancies Following Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2016;22:705–9. doi: 10.1016/j.bbmt.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curti A, Ruggeri L, Parisi S, Bontadini A, Dan E, Motta MR, Rizzi S, Trabanelli S, Ocadlikova D, Lecciso M, Giudice V, Fruet F, Urbani E, Papayannidis C, Martinelli G, Bandini G, Bonifazi F, Lewis RE, Cavo M, Velardi A, Lemoli RM. Larger Size of Donor Alloreactive NK Cell Repertoire Correlates with Better Response to NK Cell Immunotherapy in Elderly Acute Myeloid Leukemia Patients. Clin Cancer Res. 2016;22:1914–21. doi: 10.1158/1078-0432.CCR-15-1604. [DOI] [PubMed] [Google Scholar]
- 23.Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, Pui CH, Leung W. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28:955–9. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Passweg JR, Tichelli A, Meyer-Monard S, Heim D, Stern M, Kuhne T, Favre G, Gratwohl A. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia. 2004;18:1835–8. doi: 10.1038/sj.leu.2403524. [DOI] [PubMed] [Google Scholar]
- 25.Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, Leong JW, Abdel-Latif S, Schneider SE, Willey S, Neal CC, Yu L, Oh ST, Lee YS, Mulder A, Claas F, Cooper MA, Fehniger TA. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med. 2016;8:357ra123. doi: 10.1126/scitranslmed.aaf2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKenna DH, Jr, Sumstad D, Bostrom N, Kadidlo DM, Fautsch S, McNearney S, Dewaard R, McGlave PB, Weisdorf DJ, Wagner JE, McCullough J, Miller JS. Good manufacturing practices production of natural killer cells for immunotherapy: a six-year single-institution experience. Transfusion. 2007;47:520–8. doi: 10.1111/j.1537-2995.2006.01145.x. [DOI] [PubMed] [Google Scholar]
- 27.McKenna DH, Kadidlo DM, Miller JS, Orchard PJ, Wagner JE, McCullough J. The Minnesota Molecular and Cellular Therapeutics Facility: a state-of-the-art biotherapeutics engineering laboratory. Transfus Med Rev. 2005;19:217–28. doi: 10.1016/j.tmrv.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Miller JS, Oelkers S, Verfaillie C, McGlave P. Role of monocytes in the expansion of human activated natural killer cells. Blood. 1992;80:2221–9. [PubMed] [Google Scholar]
- 29.Cooley S, Trachtenberg E, Bergemann TL, Saeteurn K, Klein J, Le CT, Marsh SG, Guethlein LA, Parham P, Miller JS, Weisdorf DJ. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113:726–32. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu KC, Keever-Taylor CA, Wilton A, Pinto C, Heller G, Arkun K, O’Reilly RJ, Horowitz MM, Dupont B. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105:4878–84. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leung W, Iyengar R, Turner V, Lang P, Bader P, Conn P, Niethammer D, Handgretinger R. Determinants of antileukemia effects of allogeneic NK cells. J Immunol. 2004;172:644–50. doi: 10.4049/jimmunol.172.1.644. [DOI] [PubMed] [Google Scholar]
- 32.Lundqvist A, McCoy JP, Samsel L, Childs R. Reduction of GVHD and enhanced antitumor effects after adoptive infusion of alloreactive Ly49-mismatched NK cells from MHC-matched donors. Blood. 2007;109:3603–6. doi: 10.1182/blood-2006-05-024315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bachanova V, Burns LJ, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lindgren BR, Cooley S, Weisdorf D, Miller JS. Allogeneic natural killer cells for refractory lymphoma. Cancer Immunol Immunother. 2010;59:1739–44. doi: 10.1007/s00262-010-0896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, Jonson AL, Panoskaltsis-Mortari A, Curtsinger J, McKenna D, Dusenbery K, Bliss R, Downs LS, Miller JS. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13:98–107. doi: 10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klingemann H, Grodman C, Cutler E, Duque M, Kadidlo D, Klein AK, Sprague KA, Miller KB, Comenzo RL, Kewalramani T, Yu N, Van Etten RA, McKenna DH. Autologous stem cell transplant recipients tolerate haploidentical related-donor natural killer cell-enriched infusions. Transfusion. 2013;53:412–8. doi: 10.1111/j.1537-2995.2012.03764.x. quiz 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koepsell SA, Kadidlo DM, Fautsch S, McCullough J, Klingemann H, Wagner JE, Miller JS, McKenna DH., Jr Successful “in-flight” activation of natural killer cells during long-distance shipping. Transfusion. 2013;53:398–403. doi: 10.1111/j.1537-2995.2012.03695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skeate R, Singh C, Cooley S, Geller M, Northouse J, Welbig J, Slungaard A, Miller J, McKenna D. Hemolytic anemia due to passenger lymphocyte syndrome in solid malignancy patients treated with allogeneic natural killer cell products. Transfusion. 2013;53:419–23. doi: 10.1111/j.1537-2995.2012.03942.x. [DOI] [PubMed] [Google Scholar]
- 38.Curtis RE, Travis LB, Rowlings PA, Socie G, Kingma DW, Banks PM, Jaffe ES, Sale GE, Horowitz MM, Witherspoon RP, Shriner DA, Weisdorf DJ, Kolb HJ, Sullivan KM, Sobocinski KA, Gale RP, Hoover RN, Fraumeni JF, Jr, Deeg HJ. Risk of lymphoproliferative disorders after bone marrow transplantation: a multi-institutional study. Blood. 1999;94:2208–16. [PubMed] [Google Scholar]
- 39.Koehl U, Brehm C, Huenecke S, Zimmermann SY, Kloess S, Bremm M, Ullrich E, Soerensen J, Quaiser A, Erben S, Wunram C, Gardlowski T, Auth E, Tonn T, Seidl C, Meyer-Monard S, Stern M, Passweg J, Klingebiel T, Bader P, Schwabe D, Esser R. Clinical grade purification and expansion of NK cell products for an optimized manufacturing protocol. Front Oncol. 2013;3:118. doi: 10.3389/fonc.2013.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eissens DN, Schaap NP, Preijers FW, Dolstra H, van Cranenbroek B, Schattenberg AV, Joosten I, van der Meer A. CD3+/CD19+-depleted grafts in HLA-matched allogeneic peripheral blood stem cell transplantation lead to early NK cell cytolytic responses and reduced inhibitory activity of NKG2A. Leukemia. 2010;24:583–91. doi: 10.1038/leu.2009.269. [DOI] [PubMed] [Google Scholar]
- 41.Perez-Martinez A, Gonzalez-Vicent M, Valentin J, Aleo E, Lassaletta A, Sevilla J, Vicario JL, Ramirez M, Diaz MA. Early evaluation of immune reconstitution following allogeneic CD3/CD19-depleted grafts from alternative donors in childhood acute leukemia. Bone Marrow Transplant. 2012;47:1419–27. doi: 10.1038/bmt.2012.43. [DOI] [PubMed] [Google Scholar]
- 42.Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, Aguila HL, Caligiuri MA. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–8. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 43.Schroeder ML. Transfusion-associated graft-versus-host disease. Br J Haematol. 2002;117:275–87. doi: 10.1046/j.1365-2141.2002.03450.x. [DOI] [PubMed] [Google Scholar]
- 44.Lewalle P, Triffet A, Delforge A, Crombez P, Selleslag D, De Muynck H, Bron D, Martiat P. Donor lymphocyte infusions in adult haploidentical transplant: a dose finding study. Bone Marrow Transplant. 2003;31:39–44. doi: 10.1038/sj.bmt.1703779. [DOI] [PubMed] [Google Scholar]
- 45.Muller S, Schulz A, Reiss U, Schwarz K, Schreiner T, Wiesneth M, Debatin KM, Friedrich W. Definition of a critical T cell threshold for prevention of GVHD after HLA non-identical PBPC transplantation in children. Bone Marrow Transplant. 1999;24:575–81. doi: 10.1038/sj.bmt.1701970. [DOI] [PubMed] [Google Scholar]
- 46.Hummon D, Zantek ND, Sumstad D, Miller JS, McKenna DH. Transfusion-associated graft-versus-host disease: a perspective from a cell therapy laboratory. Transfusion. 2009;49:1018–9. doi: 10.1111/j.1537-2995.2009.02121.x. [DOI] [PubMed] [Google Scholar]
- 47.Joncker NT, Fernandez NC, Treiner E, Vivier E, Raulet DH. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: the rheostat model. J Immunol. 2009;182:4572–80. doi: 10.4049/jimmunol.0803900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Battella S, Cox MC, Santoni A, Palmieri G. Natural killer (NK) cells and anti-tumor therapeutic mAb: unexplored interactions. J Leukoc Biol. 2016;99:87–96. doi: 10.1189/jlb.5VMR0415-141R. [DOI] [PubMed] [Google Scholar]
- 49.Ruggeri L, Urbani E, Andre P, Mancusi A, Tosti A, Topini F, Blery M, Animobono L, Romagne F, Wagtmann N, Velardi A. Effects of anti-NKG2A antibody administration on leukemia and normal hematopoietic cells. Haematologica. 2016;101:626–33. doi: 10.3324/haematol.2015.135301. [DOI] [PMC free article] [PubMed] [Google Scholar]