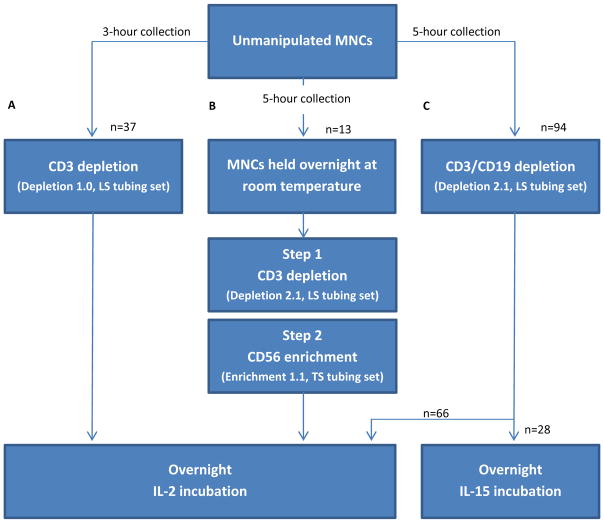
Figure 1. Comparative manufacturing of clinical-scale, cGMP-grade NK cell products.
Three methods have been used to purify NK cells from apheresed MNCs of nonmobilized peripheral blood donors using the CliniMACS cell separation system: (A) 3-hour apheresis collection, CD3 cell depletion using Depletion 1.0 software and an LS tubing set, and IL-2 incubation, (B) 5-hour apheresis collection, a two-step process of CD3 cell depletion using Depletion 2.1 software and an LS tubing set followed by CD56 cell enrichment using Enrichment 1.1 software and a TS tubing set, and IL-2 incubation, and (C) 5-hour apheresis collection, a single-step CD3/CD19 cell depletion using Depletion 2.1 software and an LS tubing set, and IL-2 or IL-15 incubation. CD3dep and CD3/CD19dep products were processed on the day of MNC collection, whereas the more time consuming 2-step processing of the CD3dep/CD56enrich products took place the day after MNC collection.