Abstract
BACKGROUND
With the global increase in life span expectancy, neurodegenerative disorders continue to affect an ever increasing number of individuals throughout the world. New treatment strategies for neurodegenerative diseases are desperately required given the lack of current treatment modalities.
METHODS
Here we examine novel strategies for neurodegenerative disorders that include circadian clock genes, non-coding ribonucleic acids (RNAs), and the mammalian forkhead transcription factors of the O class (FoxOs).
RESULTS
Circadian clock genes, non-coding RNAs, and FoxOs offer exciting prospects to potentially limit or remove the significant disability and death associated with neurodegenerative disorders. Each of these pathways have an intimate relationship with the programmed death pathways of autophagy and apoptosis and share a common link to the silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1) and the mechanistic target of rapamycin (mTOR). Circadian clock genes are necessary to modulate autophagy, limit cognitive loss, and prevent neuronal injury. Non-coding RNAs can control neuronal stem cell development and neuronal differentiation and offer protection against vascular disease such as atherosclerosis. FoxOs provide exciting prospects to block neuronal apoptotic death and to activate pathways of autophagy to remove toxic accumulations in neurons that can lead to neurodegenerative disorders.
CONCLUSIONS
Continued work with circadian clock genes, non-coding RNAs, and FoxOs can offer new prospects and hope for the development of vital strategies for the treatment of neurodegenerative diseases. These innovative investigative avenues have the potential to significantly limit disability and death from these devastating disorders.
Keywords: aging, aging-related disorders, Alzheimer’s disease, apoptosis, autophagy, BMAL1, cell longevity, circadian rhythm, circular RNA, CLOCK, clock genes, Cryptochrome, deoxyribonucleic acid, diabetes mellitus, erythropoietin, forkhead, FoxO, Huntington’s disease, metabolism, microRNA, mitochondria, mechanistic target of rapamycin (mTOR), non-coding RNA, oxidative stress, Parkinson’s disease, period (PER), programmed cell death, REV-ERBα, RORα, RORE, silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1), sirtuin, stem cells, transcription factors, vascular disease
1. Introduction
According to the World Health Organization, non-communicable diseases (NCDs) are on the rise and can be attributed to more than sixty percent of the annual fifty-seven million global deaths (1). The rise in NCDs parallels an observed increase in life expectancy of the world’s population. The age of the global population continues to increase with life expectancy approaching eighty years of age (2). In addition, the number of individuals over the age of sixty-five has doubled during the previous fifty years (3). It is expected that the number of elderly individuals in large developing countries such as India and China also will increase from five percent to ten percent over the next several decades (4, 5). There are a number of reasons that may account for the increased lifespan, but improvements in effective treatments for multiple disorders (6–10) and broader access to preventive care are believed to have contributed to the increased life span of the world’s population. For NCDs, greater than ten percent of the population under sixty years of age is affected in high-income countries (1). In contrast, NCDs affect a much larger proportion of the population in in low and middle-income countries with at least one-third of the population under the age of 60 suffering from NCDs.
Interestingly, neurodegenerative disorders form a significant component of NCDs (11). Neurodegenerative disorders include more than six hundred disease entities and progressively lead to nervous system dysfunction (12). Acute and chronic neurodegenerative disorders lead to disability and death for greater than thirty million individuals worldwide (13). Improvements in clinical care that have fostered an increased life span of the global population are also believed to have produced a continual rise in the presentation of neurodegenerative disorders. Neurodegenerative disorders also may be on the rise as a result of other disorders that can severely impair the peripheral and central nervous system (CNS) (14). One example is the contribution of other NCDs such as diabetes mellitus (DM) (15). DM affects the global population (16, 17) such that approximately three hundred and fifty million individuals suffer from DM (18–22). Another eight million individuals also have metabolic disorders but remain undiagnosed at present (23–25). Impaired glucose tolerance in the young (5, 26) and the presence of obesity increases the risk of developing DM in these individuals (19).
DM is a multi-system disease that results in progressive deterioration of the body (16, 24, 27, 28) and the nervous system (29). In the nervous system, it leads to visual impairment (24, 30–32), stroke (4, 33–37), peripheral nerve disease (28, 38), and cognitive loss that may be associated with Alzheimer’s disease (AD) (4, 39–43).
In addition to DM, vascular disease also contributes significantly to the onset and progression of disorders within the nervous system. Vascular disorders rank high among NCDs and fall within the five leading causes of death that include cardiac disease, cancer, chronic lower respiratory disease, stroke, and traumatic accidents (44). Within vascular disorders, hypertension and associated elevated serum lipids are significant risk factors for stroke. Ischemic and hemorrhagic disorders of the brain affect at least fifteen million individuals every year and lead to an annual cost of seventy-five billion dollars in the United States (2, 13, 45–47).
2. Novel Nervous System Strategies
As noted, it is estimated that the incidence of neurodegenerative disorders will continue to increase as a result of the advancing age of the global population and the progressive increase in life span. On example of a nervous system disorder that is expected to increase is AD. The incidence of sporadic cases of AD is expected to significantly increase throughout the globe (13, 48, 49). Healthcare resources will be impacted to a large extent (29, 50). In the United States (US) alone, more than five million individuals are diagnosed with sporadic AD and at least four million are under treatment at an annual cost of four billion US dollars. AD is not the result of a single etiology (42). Multiple mechanisms may lead to cognitive impairment and involve cellular injury from β-amyloid (Aβ), tau, excitotoxicity, mitochondrial damage, acetylcholine loss, astrocytic cell injury, oxidative stress, and cellular metabolic dysfunction with DM (14, 51–57). Yet, there is a growing arsenal of novel therapeutic strategies directed against AD and other neurodegenerative disorders that include circadian rhythm clock genes (9), non-coding ribonucleic acids (RNAs), and the mammalian forkhead transcription factors of the O class (FoxOs) (56, 58, 59). These pathways share common signal transduction mechanisms with the silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1) and the mechanistic target of rapamycin (mTOR). Each of these pathways can influence the onset and progression of neurodegenerative disorders and oversee critical pathways of programmed cell death that involve apoptosis and autophagy.
3. Autophagy and Apoptosis
Programmed cell death includes two vital pathways that involve apoptosis and autophagy (60–62) (Figure 1). Although apoptosis and autophagy are involved in programmed cell death, each pathway has unique characteristics that can differentiate apoptosis from autophagy (63). Apoptosis has two distinct phases that consist of an early phase that involves the loss of plasma membrane phosphatidylserine (PS) asymmetry and a subsequent later phase that leads to genomic DNA degradation (64–66). Apoptosis is the result of a series of cascade activation of nucleases and proteases that involve caspases (67, 68). These processes impact both the early phase of apoptosis with the loss of plasma membrane PS asymmetry and a later phase that leads to genomic DNA degradation. Loss of membrane PS asymmetry activates inflammatory cells to target, engulf, and remove injured cells (69–72). However, if the engulfment of inflammatory cells can be prevented, functional cells expressing membrane PS residues can be rescued and not be removed from the nervous system (24, 73–75). In contrast, once the destruction of cellular DNA occurs, it is usually not considered to be completely reversible (59). Apoptosis in the nervous system can be involved in retinal degeneration (24, 76), pain sensitivity and neuronal injury (77), Aβ injury (13, 78–82), epilepsy (83, 84), Parkinson’s disease (11, 41, 85–87), diabetic injury (14, 17, 26, 88–90), traumatic brain injury (41, 91–93), and autism (94).
Figure 1. Innovative Strategies for Neurodegenerative Disorders.
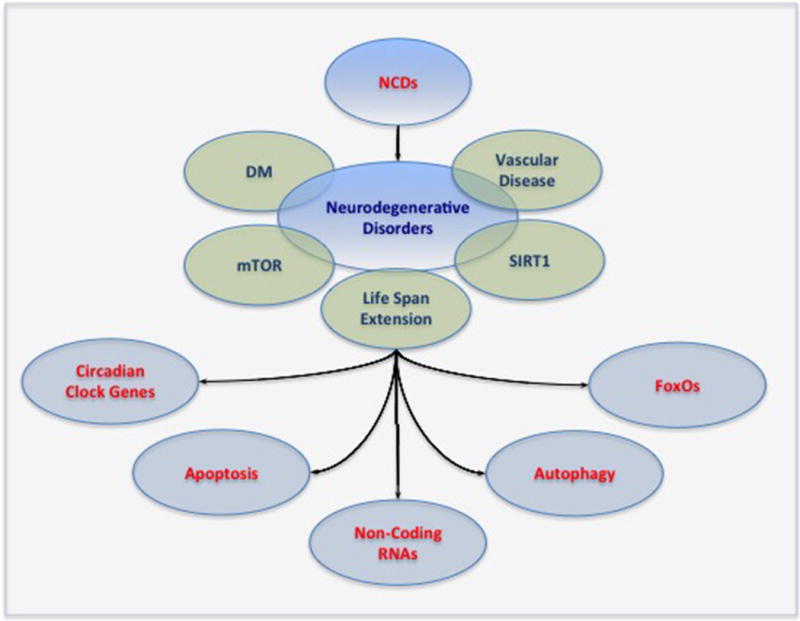
As global life span expectancy increases, non-communicable diseases (NCDs) and neurodegenerative disorders will impact a greater number of individuals throughout the world. New treatment strategies for neurodegenerative diseases are desperately warranted especially with the lack of current treatment modalities. Circadian clock genes, non-coding ribonucleic acids (RNAs), and the mammalian forkhead transcription factors of the O class (FoxOs) offer the potential to eliminate the disability and death associated with neurodegenerative disorders as well as address related disorders such as diabetes mellitus (DM) and vascular disease. These pathways have a close relationship with autophagy and apoptosis and share common links to the silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1) and the mechanistic target of rapamycin (mTOR).
Autophagy recycles components of the cytoplasm in cells for tissue remodeling and seeks to eliminate non-functional organelles (60, 62, 89, 95, 96). Macroautophagy is a classification of autophagy that recycles organelles and consists of the sequestration of cytoplasmic proteins and organelles into autophagosomes. Autophagosomes then combine with lysosomes for degradation and recycling (13, 97). Microautophagy is the invagination of the lysosomal membranes for the sequestration and digestion of cytoplasmic components (5). Chaperone-mediated autophagy (58) relies upon cytosolic chaperones to transport cytoplasmic components across lysosomal membranes (98). Autophagy can be linked to aging pathways. Studies with Drosophila demonstrate that neural aggregate accumulation observed with aging is linked to a reduction in the autophagy pathway. These neural aggregates lead to behavior impairments that can be resolved with the maintenance of autophagy pathways in neurons (99). In addition, autophagy is involved in a number of degenerative disorders such as cognitive decline (14, 56, 100), AD (40, 48, 83, 101, 102), Parkinson’s disease (11, 87, 98, 103), Huntington’s disease (59, 104, 105), DM (14, 17, 40, 89, 106, 107), and aging processes (8, 40, 85, 91, 108–111). Autophagy also may be required to preserve metabolic homeostasis with mTOR (112).
4. Circadian Clock Genes
Circadian rhythm clock genes have a significant role in the nervous system and with programmed cell death (9, 113) (Figure 1). The mammalian circadian clock resides in the suprachiasmatic nucleus (SCN) located above the optic chiasm and receives light input from photosensitive ganglion cells in the retina. The SCN depends upon the pineal gland, hypothalamic nuclei, and vasoactive intestinal peptide to control a number of processes that involve the release of hormones cortisol and melatonin, oxidative stress responses (114), and the regulation of body temperature (115).
In the clock gene family, members of the basic helix-loop-helix -PAS (Period-Arnt-Single-minded) transcription factor family, such as CLOCK and BMAL1 (116), oversee the expression of the genes Cryptochrome (Cry1 and Cry2) and Period (Per1, Per2, and Per3). Feedback is provided by PER:CRY heterodimers that can translocate to the nucleus to inhibit and block the transcription of CLOCK:BMAL1 complexes. Additional regulatory loops consist of retinoic acid-related orphan nuclear receptors REV-ERBα, also known as NR1D1 (nuclear receptor subfamily 1, group D, member 1), and RORα that are activated by CLOCK:BMAL1 heterodimers. The REV-ERBα and RORα receptors bind retinoic acid-related orphan receptor response elements (ROREs) present in the BMAL1 promoter to control transcription with RORs that can promote transcription and REV-ERBs that can repress transcription to result in circadian oscillation of BMAL1 (117, 118).
In the nervous system, rhythmic methylation of BMAL1 has been found to be changed in the brains of patients with AD, suggesting that alterations in the DNA methylation of clock genes may contribute to cognitive loss and behavior changes (53). Animal models of Parkinson’s disease with 6-hydroxydopamine (6-OHDA) have shown decreased BMAL1 and RORα persisted with levodopa treatment, indicating that long-term levodopa treatment may impair circadian rhythm function (119). Interestingly, clock genes also impact lifespan that is related to neurodegeneration. In studies with Drosophila melanogaster, lifespan was reduced in three arrhythmic mutants involving ClkAR, cyc0 and tim0. ClkAR mutants had significant faster age-related locomotor deficits. Restoring Clk function was able to rescue Drosophila from the locomotor deficits. An increase in oxidative stress was noted with the mutant phenotypes, but deficits appeared to correlate best with loss of dopaminergic neurons rather than directly to the presence of oxidative stress in this case (120).
Circadian rhythm dysfunction during cognitive loss and aging can be associated with autophagy induction (121). In animal models of AD, a basal circadian rhythm that controls macroautophagy may be necessary to limit cognitive decline and Aβ deposition (122). It has been noted that mild changes in the external environment that affect circadian rhythm may alter cognition. Chronic sleep fragmentation has been shown to affect autophagy proteins in the hippocampus (123) that may affect memory and cognition (48, 56, 102, 124, 125). Autophagy in the hippocampus also is depressed during the absence of the PER1 circadian clock protein that may worsen the pathology of cerebral ischemia (126).
Circadian pathways are intimately linked to not only autophagy, but also the mechanistic target of rapamycin (mTOR) (127, 128). mTOR also is known as the mammalian target of rapamycin and the FK506-binding protein 12-rapamycin complex-associated protein 1. mTOR controls multiple functions that determine the transcription of genes, proliferation and senescence of cells, protein formation, cellular metabolism, and cellular longevity (9, 57, 77, 129, 130). Melatonin, a pineal hormone that controls circadian rhythm, relies upon autophagy pathways and mTOR to control processes of aging and neurodegeneration (131). Loss of mTOR activation can be involved with altered circadian rhythm and cognitive decline during prolonged space flight (130). Furthermore, cerebral ischemic infarction may be influenced by an alteration in circadian rhythm genes and fluctuations in mTOR activity (126, 132).
Circadian rhythm and mTOR pathways are also dependent upon SIRT1 (14, 133, 134). SIRT1, a member of the sirtuin family, is a histone deacetylase (4, 56, 59, 67, 135–138) that can transfer acetyl groups from ε-N-acetyl lysine amino acids on the histones of DNA to control transcription. Seven identified mammalian homologues of Sir2 exist that include SIRT1 through SIRT7. These histone deacetylases control post-translational changes of proteins and oversee cellular proliferation, survival, and senescence. SIRT1 is dependent upon nicotinamide adenine dinucleotide (NAD+) as a substrate (138–142). SIRT1 is involved in neurodegenerative disorders (4, 143, 144) that require the modulation of autophagy and apoptosis (14, 58, 145, 146). SIRT1 can control stem cell survival by modulating autophagic flux (147). SIRT1 also can have an inverse relationship with mTOR in embryonic stem cells (20, 110) and block mTOR to promote autophagy and protect embryonic stem cells during oxidative stress (148). In regards to apoptotic pathways, SIRT1 activation can block external membrane PS exposure during the early phases of apoptosis in mature cells (70, 149–151). SIRT1 also can counteract apoptosis initiated by tumor necrosis factor-α (TNF-α) in endothelial progenitor cells (152). Loss of SIRT1 expression in endothelial progenitor cells leads to apoptotic cell death that can occur in smokers and chronic obstructive disease patients (153).
SIRT1 has been associated with altered circadian rhythm function that affects the development of disorders such as AD (113). SIRT1 control of circadian rhythm and melatonin also may affect glucose tolerance and DM (115) as well as inflammation during obesity (154). Increased SIRT1 activity with a disruption in circadian rhythm also leads to additional disorders such as increased susceptibility to mammary carcinogenesis (155). Yet, SIRT1 may be beneficial under specific circumstances to regulate circadian rhythm gene expression that can foster hepatocellular proliferation and liver regeneration following liver resection (156). More recent work also suggests an important role for SIRT1 targets with aging and circadian gene expression in the liver (157).
5. SIRT1 and Non-coding RNAs
Given the critical importance of vascular disease in affecting the nervous system, SIRT1 and its ability to oversee small non-coding ribonucleic acids (RNAs), termed microRNAs (miRNAs) (134, 158–160), have become exciting targets for vascular disease and the nervous system (Figure 1). SIRT1 pathways are involved in vascular survival and senescence (88, 152, 161), atherosclerosis (162–166), lifespan extension (4, 167–169), diabetic retinopathy (170), cellular metabolism and DM (14, 17, 20, 115, 145, 171, 172), oxidative stress pathways (58, 148, 173–179), and neuronal survival and cognition (11, 113, 180–183).
MiRNAs are composed of 19-25 nucleotides and can control gene expression by silencing targeted messenger RNAs (mRNAs) translated by specific genes. Non-coding ribonucleic acids play an important role with SIRT1 to control stem cell development and differentiated cell survival. Under some conditions, increased SIRT1 activity is beneficial. Silencing of miR-195 in old mesenchymal stem cells promotes stem cell proliferation by increasing SIRT1 activity to restore anti-aging factors expression that include telomerase reverse transcriptase, the forkhead transcription factor FOXO1 (59), and protein kinase B (Akt) (160). Stem cell proliferation also may require increased SIRT1 activity in combination with the inhibition or dysfunction of mTOR signaling that is controlled by miRNAs (184). Vascular cell maintenance during DM appears to need SIRT1 activity controlled by miRNAs. Diabetic endothelial vascular dysfunction that occurs during hyperglycemia with the release of elevated free fatty acids can occur during the up-regulation of miR-34a that depresses the expression of SIRT1. During periods of hyperglycemia, angiogenesis is impaired as a result of suppressed SIRT1 expression and the up-regulation of miR-34a expression (185). The retinal microvasculature also can be impacted by miRNAs. Studies in rats demonstrate that an up-regulation of senescence-associated markers that include miR-34a depress SIRT1 expression and accelerate aging and oxidative stress injury in the retinal vasculature (176). Despite these studies, it should be noted that a reduction in SIRT1 activity controlled by miRNAs may at times offer a benefit to neuronal stem cell populations. Neuronal differentiation can occur through miR-34a that leads to decreased SIRT1 expression and DNA-binding of p53 in mouse neural stem cells (186).
Other forms of non-coding RNAs also impact vascular disease. Circular ribonucleic acids (circRNAs) are non-coding RNAs of approximately 100 nucleotides in length that were initially identified as being circular in nature (187–189). CircRNAs have covalent bonds that maintain their circular structure, have both cis and trans regulation, regulate gene expression through the sponging of microRNAs (miRNAs) (190), function as biomarkers, and control apoptotic pathways (60, 62). Circular antisense non-coding RNA in the INK4 locus (circANRIL) in vascular smooth muscle cells and macrophages prevents exonuclease-mediated pre-ribosomal RNA processing, ribosome biogenesis, and proliferation of cells that may lead to atherosclerosis through the onset of apoptosis (191). However, circRNAs may not always be protective against programmed cell death and apoptosis. Up-regulation of specific circRNAs may foster apoptotic cell injury during cell models of ischemic-reperfusion injury (192).
6. FoxO Transcription Factors
Given the scope of the world’s population affected by neurodegenerative disorders, it is of particular concern that cognitive disorders such as AD can affect greater than 5 million individuals in the US alone (48, 193). Furthermore, approximately fifty million million people suffer from some form of dementia with approximately sixty percent of these cases resulting from AD (4, 12, 48, 194). Unfortunately, the availability of definitive treatments to resolve or prevent the onset of cognitive loss is limited and to the most extent such definitive treatments are non-existent (9, 50).
Mammalian forkhead transcription factors are a novel strategy to consider for neurodegenerative disorders, especially those that involve dementia and cognitive loss (59, 83, 195, 196) (Figure 1). Greater than one hundred forkhead genes and nineteen human subgroups that range from FOXA to FOXS have been identified since the original discovery of the Drosophila melanogaster gene forkhead (197). For neurodegenerative disorders, the mammalian FOXO proteins of the O class have significant relevance and have the members FOXO1, FOXO3, FOXO4, and FOXO6 (198). Previous terminology for forkhead proteins included forkhead in rhabdomyosarcoma (FKHR) (FOXO1), FKHRL1 (forkhead in rhabdomyosarcoma like protein 1) (FOXO3a), the Drosophila gene fork head (fkh), Forkhead RElated ACtivator (FREAC)-1 and -2, and the acute leukemia fusion gene located in chromosome X (AFX) (FOXO4) (199, 200). With the current nomenclature, an Arabic number is provided with the designation of “Fox”, then a subclass or subgroup letter is provided, and finally the member number is listed within the subclasses of the Fox proteins (201). All letters are capitalized for human Fox proteins. For the mouse, only the initial letter is listed as uppercase and for all other chordates the initial and subclass letters are in uppercase (200, 202, 203).
FoxO proteins are transcription factors that bind to deoxyribonucleic acid (DNA) through the FoxO-recognized element in the C-terminal basic region of the forkhead DNA binding domain (204, 205). Following forkhead binding to DNA, target gene expression is repressed or activated through fourteen protein-DNA contacts with the primary recognition site located at α-helix H3 (206). Phosphorylation or acetylation that can block FoxO activity may alter the binding of the C-terminal basic region to DNA to prevent transcriptional activity (207).
FoxO transcription factors may impact neurodegenerative disorders through apoptosis and autophagy (60, 62). Inhibition or loss of FoxO activity usually improves cell survival during apoptotic cell injury. Blockade of FoxO transcription factor activity can protect against microglial cell demise during oxidative stress (71) and Aβ exposure (208), foster the protective effects of metabotropic glutamate receptors (209), increase neuronal cell survival through nicotinamide adenine dinucleotide (NAD+) precursors (210), raise survival with growth factors (211), such as erythropoietin (EPO) (150, 212–214) and neurotrophins (215–217), and lessen metabolic and vascular disease (218). Antipsychotics, such as clozapine, may function through FoxO inhibition to protect against apoptotic neuronal cell loss (219). Pathways involving SIRT1 also can affect FoxO modulation of apoptotic cell death. SIRT1 can increase neuronal survival through modulation of FoxO activity (67, 140, 142, 220, 221). In addition, under some conditions, sirtuins and FoxO transcription factors may function synergistically to increase neuronal cell survival (4, 142). FoxO proteins in conjunction with SIRT1 pathways may offer protection against Aβ toxicity (181) and forkhead transcription factors, such as FoxO3a, may be dependent upon SIRT1 to reduce oxidative stress and cell injury during exposure to Aβ (222).
FoxO proteins may offer protection with neurons during autophagy induction. Increased FoxO activity, such as with FoxO1, can function to increase basal autophagy and reduce atherogenesis (56, 223). Ectopic expression of FoxO1 enhances autophagy and increases toxic Huntington’s disease Huntingtin (mHtt) protein clearance in neuronal cell cultures (224). In addition, loss of FoxO and SIRT1 activity with a reduction in autophagy activity in models with Drosophila can lead to neuronal accumulation of Aβ (225).
Given the close ties of mammalian forkhead transcription factors with the programmed death pathways of apoptosis and autophagy, FoxOs are considered as vital targets to treat neurodegenerative disorders. For example, FoxO transcription factors play a significant role in inflammation and can affect vascular inflammatory pathways (203) and cardiac injury (201). Calcineurin and FoxO3 can interact in astrocytes during Aβ exposure that results in pro-inflammatory cytokines and injury to neurons (226). Since nuclear translocation of FoxO3 is tied to apoptotic neuronal DNA damage (59, 227, 228), FoxO transcription factors could be considered a therapeutic strategy to prevent excessive Aβ production and suppress the onset of AD. Under some conditions, Aβ exposure can result in the dephosphorylation and mitochondrial translocation of FoxO3a that leads to mitochondrial dysfunction (196). Furthermore, increased FoxO activity can function in concert with tribbles pseudokinase 3 to result in apoptotic and autophagic Aβ induced neuronal cell death (79). Inhibition of FoxO activity under such conditions can protect against oxidative stress and Aβ toxicity (208, 229).
7. Considerations for the Future
NCDs are increasing in prevalence throughout the world that parallels a rise in life expectancy in the global population. As a result, the prevalence of neurodegenerative disorders also has been impacted by acute and chronic neurodegenerative disorders resulting in disability and death for greater than thirty million individuals worldwide. Neurodegenerative disorders also may be on the rise as a result of other disorders that can impair the peripheral and CNS that include DM and vascular disease. Present strategies to treat neurodegenerative disorders as well as associated disorders are extremely limited and warrant novel investigative pathways. In this respect, great enthusiasm is present for new therapies for neurodegenerative disorders that include circadian clock genes, non-coding RNAs, and FoxOs. Each of these pathways is able to modulate critical pathways of programmed cell death that involve autophagy and apoptosis. An intact circadian rhythm and expression of clock genes appear necessary to modulate autophagy, limit cognitive loss, and prevent neuronal injury. Non-coding RNAs can oversee neuronal stem cell development and differentiation, but also may be protective against vascular diseases, such as atherosclerosis. Mammalian forkhead transcription factors of the O class also offer exciting prospects for new therapies to prevent neuronal apoptotic death and to also employ pathways of autophagy to remove toxic accumulations in neurons that can lead to neurodegenerative disorders. Interestingly, the pathways of circadian clock genes, non-coding RNAs, and FoxOs are intimately dependent upon SIRT1 as well as mTOR pathways. Further investigation into each of these novel pathways should provide necessary insight into not only the treatment of neurodegenerative disorders, but also the ability to prevent the onset of disease in the nervous system.
Acknowledgments
This research was supported by the following grants to Kenneth Maiese: American Diabetes Association, American Heart Association, NIH NIEHS, NIH NIA, NIH NINDS, and NIH ARRA.
Footnotes
Competing Interests: There are no conflicts of interest to declare.
Author Contribution Statement: Kenneth Maiese solely conceived and designed the research, analyzed the results, and completed the writing of the manuscript.
References
- 1.World Health Organization. Description of the global burden of NCDs, their risk factors and determinants. Global status report on noncommunicable diseases. 2010;2011:1–176. April. [Google Scholar]
- 2.Maiese K. Cutting through the Complexities of mTOR for the Treatment of Stroke. Curr Neurovasc Res. 2014;11(2):177–86. doi: 10.2174/1567202611666140408104831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayutin A. Global demographic shifts create challenges and opportunities. PREA Quarterly. 2007 Fall;:46–53. [Google Scholar]
- 4.Maiese K. SIRT1 and stem cells: In the forefront with cardiovascular disease, neurodegeneration and cancer. World J Stem Cells. 2015;7(2):235–42. doi: 10.4252/wjsc.v7.i2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maiese K. Programming apoptosis and autophagy with novel approaches for diabetes mellitus. Curr Neurovasc Res. 2015;12(2):173–88. doi: 10.2174/1567202612666150305110929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamanti-Kandarakis E, Datillo M, Macut D, Duntas LH, Gonos E, Goulis D, et al. Mechanisms In Endocrinology: Aging and Anti-aging Endocrinology: A Combo Overview. Eur J Endocrinol. 2017 doi: 10.1530/EJE-16-1061. [DOI] [PubMed] [Google Scholar]
- 7.Fann DY, Ng GY, Poh L, Arumugam TV. Positive effects of intermittent fasting in ischemic stroke. Exp Gerontol. 2017;89:93–102. doi: 10.1016/j.exger.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Lushchak O, Strilbytska O, Piskovatska V, Storey KB, Koliada A, Vaiserman A. The role of the TOR pathway in mediating the link between nutrition and longevity. Mech Ageing Dev. 2017;164:127–38. doi: 10.1016/j.mad.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Maiese K. Moving to the Rhythm with Clock (Circadian) Genes, Autophagy, mTOR, and SIRT1 in Degenerative Disease and Cancer. Curr Neurovasc Res. 2017;14(3):299–304. doi: 10.2174/1567202614666170718092010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stefanatos R, Sanz A. The role of mitochondrial ROS in the aging brain. FEBS Lett. 2017 doi: 10.1002/1873-3468.12902. [DOI] [PubMed] [Google Scholar]
- 11.Maiese K. Targeting molecules to medicine with mTOR, autophagy and neurodegenerative disorders. Br J Clin Pharmacol. 2016;82(5):1245–66. doi: 10.1111/bcp.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75(3):207–46. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Maiese K. Driving neural regeneration through the mammalian target of rapamycin. Neural regeneration research. 2014;9(15):1413–7. doi: 10.4103/1673-5374.139453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maiese K. Novel nervous and multi-system regenerative therapeutic strategies for diabetes mellitus with mTOR. Neural regeneration research. 2016;11(3):372–85. doi: 10.4103/1673-5374.179032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou C, Na L, Shan R, Cheng Y, Li Y, Wu X, et al. Dietary Vitamin C Intake Reduces the Risk of Type 2 Diabetes in Chinese Adults: HOMA-IR and T-AOC as Potential Mediators. PLoS One. 2016;11(9):e0163571. doi: 10.1371/journal.pone.0163571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haldar SR, Chakrabarty A, Chowdhury S, Haldar A, Sengupta S, Bhattacharyya M. Oxidative stress-related genes in type 2 diabetes: association analysis and their clinical impact. Biochemical genetics. 2015;53(4-6):93–119. doi: 10.1007/s10528-015-9675-z. [DOI] [PubMed] [Google Scholar]
- 17.Maiese K. New Insights for Oxidative Stress and Diabetes Mellitus. Oxid Med Cell Longev. 2015;2015:2015:875961. doi: 10.1155/2015/875961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia G, Aroor AR, Martinez-Lemus LA, Sowers JR. Invited Review: Over-nutrition, mTOR Signaling and Cardiovascular Diseases. Am J Physiol Regul Integr Comp Physiol. 2014;307(10):R1198–206. doi: 10.1152/ajpregu.00262.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maiese K, Chong ZZ, Shang YC, Hou J. Novel Avenues of Drug Discovery and Biomarkers for Diabetes Mellitus. Journal of clinical pharmacology. 2011;51(2):128–52. doi: 10.1177/0091270010362904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maiese K, Chong ZZ, Shang YC, Wang S. Novel directions for diabetes mellitus drug discovery. Expert opinion on drug discovery. 2013;8(1):35–48. doi: 10.1517/17460441.2013.736485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutter MK, Massaro JM, Hoffmann U, O’Donnell CJ, Fox CS. Fasting Glucose, Obesity, and Coronary Artery Calcification in Community-Based People Without Diabetes. Diabetes Care. 2012;35(9):1944–50. doi: 10.2337/dc11-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu E, Schwab M, Marette A. Role of protein tyrosine phosphatases in the modulation of insulin signaling and their implication in the pathogenesis of obesity-linked insulin resistance. Rev Endocr Metab Disord. 2014;15(1):79–97. doi: 10.1007/s11154-013-9282-4. [DOI] [PubMed] [Google Scholar]
- 23.Harris MI, Eastman RC. Early detection of undiagnosed diabetes mellitus: a US perspective. Diabetes Metab Res Rev. 2000;16(4):230–6. doi: 10.1002/1520-7560(2000)9999:9999<::aid-dmrr122>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 24.Maiese K. Novel applications of trophic factors, Wnt and WISP for neuronal repair and regeneration in metabolic disease. Neural regeneration research. 2015;10(4):518–28. doi: 10.4103/1673-5374.155427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maiese K, Chong ZZ, Shang YC. Mechanistic insights into diabetes mellitus and oxidative stress. Curr Med Chem. 2007;14(16):1729–38. doi: 10.2174/092986707781058968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tulsulkar J, Nada SE, Slotterbeck BD, McInerney MF, Shah ZA. Obesity and hyperglycemia lead to impaired post-ischemic recovery after permanent ischemia in mice. Obesity (Silver Spring, Md) 2015;24(2):417–23. doi: 10.1002/oby.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esser N, Paquot N, Scheen AJ. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert opinion on investigational drugs. 2015;24(3):283–307. doi: 10.1517/13543784.2015.974804. [DOI] [PubMed] [Google Scholar]
- 28.Gomez-Brouchet A, Blaes N, Mouledous L, Fourcade O, Tack I, Frances B, et al. Beneficial effects of levobupivacaine regional anaesthesia on postoperative opioid induced hyperalgesia in diabetic mice. Journal of translational medicine. 2015;13(1):208. doi: 10.1186/s12967-015-0575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maiese K. Late Onset Alzheimer’s Disease: Novel Clinical Prospects for the Future. Curr Neurovasc Res. 2017;14(2):89. doi: 10.2174/1567202614999170313155128. [DOI] [PubMed] [Google Scholar]
- 30.Busch S, Kannt A, Kolibabka M, Schlotterer A, Wang Q, Lin J, et al. Systemic treatment with erythropoietin protects the neurovascular unit in a rat model of retinal neurodegeneration. PLoS One. 2014;9(7):e102013. doi: 10.1371/journal.pone.0102013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu D, Wu M, Zhang J, Du M, Yang S, Hammad SM, et al. Mechanisms of modified LDL-induced pericyte loss and retinal injury in diabetic retinopathy. Diabetologia. 2012;55(11):3128–40. doi: 10.1007/s00125-012-2692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee K, Hu Y, Ding L, Chen Y, Takahashi Y, Mott R, et al. Therapeutic potential of a monoclonal antibody blocking the Wnt pathway in diabetic retinopathy. Diabetes. 2012;61(11):2948–57. doi: 10.2337/db11-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexandru N, Popov D, Georgescu A. Platelet dysfunction in vascular pathologies and how can it be treated. Thromb Res. 2012;129(2):116–26. doi: 10.1016/j.thromres.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 34.Jiang T, Yu JT, Zhu XC, Wang HF, Tan MS, Cao L, et al. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol. 2014;171(13):3146–57. doi: 10.1111/bph.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maiese K, Chong ZZ, Hou J, Shang YC. Erythropoietin and oxidative stress. Curr Neurovasc Res. 2008;5(2):125–42. doi: 10.2174/156720208784310231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao FH, He YH, Li QG, Wu H, Luo LH, Kong QP. A genome-wide scan reveals important roles of DNA methylation in human longevity by regulating age-related disease genes. PLoS One. 2015;10(3):e0120388. doi: 10.1371/journal.pone.0120388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu YJ, Tappia PS, Neki NS, Dhalla NS. Prevention of diabetes-induced cardiovascular complications upon treatment with antioxidants. Heart failure reviews. 2014;19(1):113–21. doi: 10.1007/s10741-013-9379-6. [DOI] [PubMed] [Google Scholar]
- 38.Gomes MB, Negrato CA. Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetology & metabolic syndrome. 2014;6(1):80. doi: 10.1186/1758-5996-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crespo MC, Tome-Carneiro J, Pintado C, Davalos A, Visioli F, Burgos-Ramos E. Hydroxytyrosol restores proper insulin signaling in an astrocytic model of Alzheimer’s disease. BioFactors (Oxford, England) 2017 doi: 10.1002/biof.1356. [DOI] [PubMed] [Google Scholar]
- 40.Li L. The Molecular Mechanism of Glucagon-Like Peptide-1 Therapy in Alzheimer’s Disease, Based on a Mechanistic Target of Rapamycin Pathway. CNS drugs. 2017;31(7):535–49. doi: 10.1007/s40263-017-0431-2. [DOI] [PubMed] [Google Scholar]
- 41.Maiese K, Chong ZZ, Wang S, Shang YC. Oxidant Stress and Signal Transduction in the Nervous System with the PI 3-K, Akt, and mTOR Cascade. International journal of molecular sciences. 2013;13(11):13830–66. doi: 10.3390/ijms131113830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ong WY, Wu YJ, Farooqui T, Farooqui AA. Qi Fu Yin-a Ming Dynasty Prescription for the Treatment of Dementia. Mol Neurobiol. 2018 doi: 10.1007/s12035-018-0908-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu W, Liu J, Ma D, Yuan G, Lu Y, Yang Y. Capsaicin reduces Alzheimer-associated tau changes in the hippocampus of type 2 diabetes rats. PLoS One. 2017;12(2):e0172477. doi: 10.1371/journal.pone.0172477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minino AM, Murphy SL. Death in the United States, 2010. NCHS data brief. 2012(99):1–8. [PubMed] [Google Scholar]
- 45.Kim JY, Park J, Chang JY, Kim SH, Lee JE. Inflammation after Ischemic Stroke: The Role of Leukocytes and Glial Cells. Experimental neurobiology. 2016;25(5):241–51. doi: 10.5607/en.2016.25.5.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shahjouei S, Ansari S, Pourmotabbed T, Zand R. Potential Roles of Adropin in Central Nervous System: Review of Current Literature. Frontiers in molecular biosciences. 2016;3:25. doi: 10.3389/fmolb.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao EY, Efendizade A, Cai L, Ding Y. The role of Akt (protein kinase B) and protein kinase C in ischemia-reperfusion injury. Neurol Res. 2016:1–8. doi: 10.1080/01616412.2015.1133024. [DOI] [PubMed] [Google Scholar]
- 48.Maiese K. Taking aim at Alzheimer’s disease through the mammalian target of rapamycin. Ann Med. 2014;46(8):587–96. doi: 10.3109/07853890.2014.941921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schluesener JK, Zhu X, Schluesener HJ, Wang GW, Ao P. Key network approach reveals new insight into Alzheimer’s disease. IET systems biology. 2014;8(4):169–75. doi: 10.1049/iet-syb.2013.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mravec B, Horvathova L, Padova A. Brain Under Stress and Alzheimer’s Disease. Cell Mol Neurobiol. 2017 Jul 11; doi: 10.1007/s10571-017-0521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahshin-Majd S, Zamani S, Kiamari T, Kiasalari Z, Baluchnejadmojarad T, Roghani M. Carnosine ameliorates cognitive deficits in streptozotocin-induced diabetic rats: Possible involved mechanisms. Peptides. 2016;86:102–11. doi: 10.1016/j.peptides.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 52.Bellozi PM, Lima IV, Doria JG, Vieira EL, Campos AC, Candelario-Jalil E, et al. Neuroprotective effects of the anticancer drug NVP-BEZ235 (dactolisib) on amyloid-beta 1-42 induced neurotoxicity and memory impairment. Scientific reports. 2016;6:25226. doi: 10.1038/srep25226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cronin P, McCarthy MJ, Lim ASP, Salmon DP, Galasko D, Masliah E, et al. Circadian alterations during early stages of Alzheimer’s disease are associated with aberrant cycles of DNA methylation in BMAL1. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2017;13(6):689–700. doi: 10.1016/j.jalz.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Rosa M, Malaguarnera L. Chitotriosidase: A New Inflammatory Marker in Diabetic Complications. Pathobiology. 2016;83(4):211–9. doi: 10.1159/000443932. [DOI] [PubMed] [Google Scholar]
- 55.Hu M, Liu Z, Lv P, Wang H, Zhu Y, Qi Q, et al. Nimodipine activates neuroprotective signaling events and inactivates autophages in the VCID rat hippocampus. Neurol Res. 2017:1–6. doi: 10.1080/01616412.2017.1356157. [DOI] [PubMed] [Google Scholar]
- 56.Maiese K. Forkhead transcription factors: new considerations for alzheimer’s disease and dementia. J Transl Sci. 2016;2(4):241–7. doi: 10.15761/JTS.1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park JA, Lee CH. Temporal changes in mammalian target of rapamycin (mTOR) and phosphorylated-mTOR expressions in the hippocampal CA1 region of rat with vascular dementia. Journal of veterinary science. 2017;18(1):11–6. doi: 10.4142/jvs.2017.18.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maiese K. FoxO Transcription Factors and Regenerative Pathways in Diabetes Mellitus. Curr Neurovasc Res. 2015;12(4):404–13. doi: 10.2174/1567202612666150807112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maiese K. FoxO Proteins in the Nervous System. Anal Cell Pathol (Amst) 2015;2015:569392. doi: 10.1155/2015/569392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. (3rd) 2016;12(1):1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maiese K. Autophagy to the Rescue. Curr Neurovasc Res. 2017;14(3):199. doi: 10.2174/1567202614666170724160119. [DOI] [PubMed] [Google Scholar]
- 62.Maiese K, Chong ZZ, Shang YC, Wang S. Targeting disease through novel pathways of apoptosis and autophagy. Expert opinion on therapeutic targets. 2012;16(12):1203–14. doi: 10.1517/14728222.2012.719499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maiese K, Chong ZZ, Hou J, Shang YC. Oxidative stress: Biomarkers and novel therapeutic pathways. Exp Gerontol. 2010;45(3):217–34. doi: 10.1016/j.exger.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shang YC, Chong ZZ, Hou J, Maiese K. Wnt1, FoxO3a, and NF-kappaB oversee microglial integrity and activation during oxidant stress. Cell Signal. 2010;22(9):1317–29. doi: 10.1016/j.cellsig.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Viola G, Bortolozzi R, Hamel E, Moro S, Brun P, Castagliuolo I, et al. MG-2477, a new tubulin inhibitor, induces autophagy through inhibition of the Akt/mTOR pathway and delayed apoptosis in A549 cells. Biochem Pharmacol. 2012;83(1):16–26. doi: 10.1016/j.bcp.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wong DZ, Kadir HA, Lee CL, Goh BH. Neuroprotective properties of Loranthus parasiticus aqueous fraction against oxidative stress-induced damage in NG108-15 cells. J Nat Med. 2012;66(3):544–51. doi: 10.1007/s11418-011-0622-y. [DOI] [PubMed] [Google Scholar]
- 67.Chong ZZ, Shang YC, Wang S, Maiese K. SIRT1: New avenues of discovery for disorders of oxidative stress. Expert opinion on therapeutic targets. 2012;16(2):167–78. doi: 10.1517/14728222.2012.648926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Troy CM, Akpan N, Jean YY. Regulation of caspases in the nervous system implications for functions in health and disease. Prog Mol Biol Transl Sci. 2011;99:265–305. doi: 10.1016/B978-0-12-385504-6.00007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bailey TJ, Fossum SL, Fimbel SM, Montgomery JE, Hyde DR. The inhibitor of phagocytosis, O-phospho-L-serine, suppresses Muller glia proliferation and cone cell regeneration in the light-damaged zebrafish retina. Exp Eye Res. 2010;91(5):601–12. doi: 10.1016/j.exer.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hou J, Chong ZZ, Shang YC, Maiese K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr Neurovasc Res. 2010;7(2):95–112. doi: 10.2174/156720210791184899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shang YC, Chong ZZ, Hou J, Maiese K. FoxO3a governs early microglial proliferation and employs mitochondrial depolarization with caspase 3, 8, and 9 cleavage during oxidant induced apoptosis. Curr Neurovasc Res. 2009;6(4):223–38. doi: 10.2174/156720209789630302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wei L, Sun C, Lei M, Li G, Yi L, Luo F, et al. Activation of Wnt/beta-catenin Pathway by Exogenous Wnt1 Protects SH-SY5Y Cells Against 6-Hydroxydopamine Toxicity. J Mol Neurosci. 2013;49(1):105–15. doi: 10.1007/s12031-012-9900-8. [DOI] [PubMed] [Google Scholar]
- 73.Kim S, Kang IH, Nam JB, Cho Y, Chung DY, Kim SH, et al. Ameliorating the Effect of Astragaloside IV on Learning and Memory Deficit after Chronic Cerebral Hypoperfusion in Rats. Molecules. 2015;20(2):1904–21. doi: 10.3390/molecules20021904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xin YJ, Yuan B, Yu B, Wang YQ, Wu JJ, Zhou WH, et al. Tet1-mediated DNA demethylation regulates neuronal cell death induced by oxidative stress. Scientific reports. 2015;5:7645. doi: 10.1038/srep07645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu T, Li L, Chen T, Liu Z, Liu H, Li Z. Erythropoietin attenuates advanced glycation endproducts-induced toxicity of schwann cells in vitro. Neurochem Res. 2015;40(4):698–712. doi: 10.1007/s11064-015-1516-2. [DOI] [PubMed] [Google Scholar]
- 76.Almasieh M, Catrinescu MM, Binan L, Costantino S, Levin LA. Axonal Degeneration in Retinal Ganglion Cells is Associated with a Membrane Polarity-Sensitive Redox Process. J Neurosci. 2017 doi: 10.1523/JNEUROSCI.3882-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maiese K. Warming Up to New Possibilities with the Capsaicin Receptor TRPV1: mTOR, AMPK, and Erythropoietin. Curr Neurovasc Res. 2017;14(2):184–9. doi: 10.2174/1567202614666170313105337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin N, Xiong LL, Zhang RP, Zheng H, Wang L, Qian ZY, et al. Injection of Abeta 1–40 into hippocampus induced cognitive lesion associated with neuronal apoptosis and multiple gene expressions in the tree shrew. Apoptosis. 2016 doi: 10.1007/s10495-016-1227-4. [DOI] [PubMed] [Google Scholar]
- 79.Saleem S, Biswas SC. Tribbles Pseudokinase 3 Induces Both Apoptosis and Autophagy in Amyloid-beta-induced Neuronal Death. J Biol Chem. 2017;292(7):2571–85. doi: 10.1074/jbc.M116.744730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shang YC, Chong ZZ, Wang S, Maiese K. WNT1 Inducible Signaling Pathway Protein 1 (WISP1) Targets PRAS40 to Govern beta-Amyloid Apoptotic Injury of Microglia. Curr Neurovasc Res. 2012;9(4):239–49. doi: 10.2174/156720212803530618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shang YC, Chong ZZ, Wang S, Maiese K. Prevention of beta-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging (Albany NY) 2012;4(3):187–201. doi: 10.18632/aging.100440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shang YC, Chong ZZ, Wang S, Maiese K. Tuberous sclerosis protein 2 (TSC2) modulates CCN4 cytoprotection during apoptotic amyloid toxicity in microglia. Curr Neurovasc Res. 2013;10(1):29–38. doi: 10.2174/156720213804806007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chong ZZ, Shang YC, Wang S, Maiese K. Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Prog Neurobiol. 2012;99(2):128–48. doi: 10.1016/j.pneurobio.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang BH, Hou Q, Lu YQ, Jia MM, Qiu T, Wang XH, et al. Ketogenic diet attenuates neuronal injury via autophagy and mitochondrial pathways in pentylenetetrazol-kindled seizures. Brain Res. 2017 doi: 10.1016/j.brainres.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 85.Wen Z, Zhang J, Tang P, Tu N, Wang K, Wu G. Overexpression of miR185 inhibits autophagy and apoptosis of dopaminergic neurons by regulating the AMPK/mTOR signaling pathway in Parkinson’s disease. Molecular medicine reports. 2017 doi: 10.3892/mmr.2017.7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang L, Cen L, Qu S, Wei L, Mo M, Feng J, et al. Enhancing Beta-Catenin Activity via GSK3beta Inhibition Protects PC12 Cells against Rotenone Toxicity through Nurr1 Induction. PLoS One. 2016;11(4):e0152931. doi: 10.1371/journal.pone.0152931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou Q, Chen B, Wang X, Wu L, Yang Y, Cheng X, et al. Sulforaphane protects against rotenone-induced neurotoxicity in vivo: Involvement of the mTOR, Nrf2, and autophagy pathways. Scientific reports. 2016;6:32206. doi: 10.1038/srep32206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arunachalam G, Samuel SM, Marei I, Ding H, Triggle CR. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. Br J Pharmacol. 2014;171(2):523–35. doi: 10.1111/bph.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Di Rosa M, Distefano G, Gagliano C, Rusciano D, Malaguarnera L. AUTOPHAGY IN DIABETIC RETINOPATHY. Current neuropharmacology. 2016;14(8):810–25. doi: 10.2174/1570159X14666160321122900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang X, Huo F, Liu B, Liu J, Chen T, Li J, et al. Crocin Inhibits Oxidative Stress and Pro-inflammatory Response of Microglial Cells Associated with Diabetic Retinopathy Through the Activation of PI3K/Akt Signaling Pathway. J Mol Neurosci. 2017 doi: 10.1007/s12031-017-0899-8. [DOI] [PubMed] [Google Scholar]
- 91.Maiese K. Charting a course for erythropoietin in traumatic brain injury. J Transl Sci. 2016;2(2):140–4. doi: 10.15761/jts.1000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ye Y, Zhang P, Qian Y, Yin B, Yan M. The Effect of Pyrroloquinoline Quinone on the Expression of WISP1 in Traumatic Brain Injury. Stem cells international. 2017;2017:4782820. doi: 10.1155/2017/4782820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang P, Ye Y, Qian Y, Yin B, Zhao J, Zhu S, et al. The effect of pyrroloquinoline quinone on apoptosis and autophagy in traumatic brain injury. CNS Neurol Disord Drug Targets. 2017 doi: 10.2174/1871527316666170124164306. [DOI] [PubMed] [Google Scholar]
- 94.Zhang J, Liu LM, Ni JF. Rapamycin modulated brain-derived neurotrophic factor and B-cell lymphoma 2 to mitigate autism spectrum disorder in rats. Neuropsychiatric disease and treatment. 2017;13:835–42. doi: 10.2147/NDT.S125088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen C, Lu Y, Siu HM, Guan J, Zhu L, Zhang S, et al. Identification of Novel Vacuolin-1 Analogues as Autophagy Inhibitors by Virtual Drug Screening and Chemical Synthesis. Molecules. 2017;22(6) doi: 10.3390/molecules22060891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maiese K. mTOR: Driving apoptosis and autophagy for neurocardiac complications of diabetes mellitus. World J Diabetes. 2015;6(2):217–24. doi: 10.4239/wjd.v6.i2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.White CR, Datta G, Giordano S. High-Density Lipoprotein Regulation of Mitochondrial Function. Adv Exp Med Biol. 2017;982:407–29. doi: 10.1007/978-3-319-55330-6_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moors TE, Hoozemans JJ, Ingrassia A, Beccari T, Parnetti L, Chartier-Harlin MC, et al. Therapeutic potential of autophagy-enhancing agents in Parkinson’s disease. Molecular neurodegeneration. 2017;12(1):11. doi: 10.1186/s13024-017-0154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ratliff EP, Mauntz RE, Kotzebue RW, Gonzalez A, Achal M, Barekat A, et al. Aging and Autophagic Function Influences the Progressive Decline of Adult Drosophila Behaviors. PLoS One. 2015;10(7):e0132768. doi: 10.1371/journal.pone.0132768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Crino PB. The mTOR signalling cascade: paving new roads to cure neurological disease. Nature reviews Neurology. 2016;12(7):379–92. doi: 10.1038/nrneurol.2016.81. [DOI] [PubMed] [Google Scholar]
- 101.Murphy KE, Park JJ. Can Co-Activation of Nrf2 and Neurotrophic Signaling Pathway Slow Alzheimer’s Disease? . International journal of molecular sciences. 2017;18(6) doi: 10.3390/ijms18061168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang ZH, Wu QY, Zheng R, Chen C, Chen Y, Liu Q, et al. Selenomethionine mitigates cognitive decline by targeting both tau hyperphosphorylation and autophagic clearance in an Alzheimer’s disease mouse model. J Neurosci. 2017;37(9):2449–62. doi: 10.1523/JNEUROSCI.3229-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jang W, Kim HJ, Li H, Jo KD, Lee MK, Yang HO. The Neuroprotective Effect of Erythropoietin on Rotenone-Induced Neurotoxicity in SH-SY5Y Cells Through the Induction of Autophagy. Mol Neurobiol. 2015;53(6):3812–21. doi: 10.1007/s12035-015-9316-x. [DOI] [PubMed] [Google Scholar]
- 104.Hyrskyluoto A, Reijonen S, Kivinen J, Lindholm D, Korhonen L. GADD34 mediates cytoprotective autophagy in mutant huntingtin expressing cells via the mTOR pathway. Exp Cell Res. 2012;318(1):33–42. doi: 10.1016/j.yexcr.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 105.Lee JH, Tecedor L, Chen YH, Monteys AM, Sowada MJ, Thompson LM, et al. Reinstating aberrant mTORC1 activity in Huntington’s disease mice improves disease phenotypes. Neuron. 2015;85(2):303–15. doi: 10.1016/j.neuron.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gurlo T, Rivera JF, Butler AE, Cory M, Hoang J, Costes S, et al. CHOP Contributes to, But Is Not the Only Mediator of, IAPP Induced beta-Cell Apoptosis. Mol Endocrinol. 2016;30(4):446–54. doi: 10.1210/me.2015-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qi Z, Xia J, Xue X, He Q, Ji L, Ding S. Long-term treatment with nicotinamide induces glucose intolerance and skeletal muscle lipotoxicity in normal chow-fed mice: compared to diet-induced obesity. The Journal of nutritional biochemistry. 2016;36:31–41. doi: 10.1016/j.jnutbio.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 108.Kochetkova EY, Blinova GI, Bystrova OA, Martynova MG, Pospelov VA, Pospelova TV. Targeted elimination of senescent Ras-transformed cells by suppression of MEK/ERK pathway. Aging (Albany NY) 2017;9(11):2352–75. doi: 10.18632/aging.101325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maiese K. The bright side of reactive oxygen species: lifespan extension without cellular demise. J Transl Sci. 2016;2(3):185–7. doi: 10.15761/JTS.1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maiese K. Erythropoietin and mTOR: A “One-Two Punch” for Aging-Related Disorders Accompanied by Enhanced Life Expectancy. Curr Neurovasc Res. 2016;13(4):329–40. doi: 10.2174/1567202613666160729164900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Milisav I, Poljsak B, Ribaric S. Reduced risk of apoptosis: mechanisms of stress responses. Apoptosis. 2017;22(2):265–83. doi: 10.1007/s10495-016-1317-3. [DOI] [PubMed] [Google Scholar]
- 112.Esterline RL, Vaag A, Oscarsson J, Vora J. MECHANISMS IN ENDOCRINOLOGY: SGLT2 inhibitors; clinical benefits by restoration of normal diurnal metabolism? . Eur J Endocrinol. 2018 doi: 10.1530/EJE-17-0832. [DOI] [PubMed] [Google Scholar]
- 113.Bellanti F, Iannelli G, Blonda M, Tamborra R, Villani R, Romano A, et al. Alterations of Clock Gene RNA Expression in Brain Regions of a Triple Transgenic Model of Alzheimer’s Disease. J Alzheimers Dis. 2017 doi: 10.3233/JAD-160942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Patel SA, Velingkaar NS, Kondratov RV. Transcriptional Control of Antioxidant Defense by the Circadian Clock. Antioxid Redox Signal. 2014;20(18):2997–3006. doi: 10.1089/ars.2013.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hardeland R. Melatonin and the pathologies of weakened or dysregulated circadian oscillators. J Pineal Res. 2016 doi: 10.1111/jpi.12377. [DOI] [PubMed] [Google Scholar]
- 116.Lin F, Chen Y, Li X, Zhao Q, Tan Z. Over-expression of circadian clock gene Bmal1 affects proliferation and the canonical Wnt pathway in NIH-3T3 cells. Cell Biochem Funct. 2013;31(2):166–72. doi: 10.1002/cbf.2871. [DOI] [PubMed] [Google Scholar]
- 117.Bunney BG, Li JZ, Walsh DM, Stein R, Vawter MP, Cartagena P, et al. Circadian dysregulation of clock genes: clues to rapid treatments in major depressive disorder. Mol Psychiatry. 2015;20(1):48–55. doi: 10.1038/mp.2014.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hood S, Amir S. Neurodegeneration and the Circadian Clock. Frontiers in aging neuroscience. 2017;9:170. doi: 10.3389/fnagi.2017.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li SY, Wang YL, Liu WW, Lyu DJ, Wang F, Mao CJ, et al. Long-term Levodopa Treatment Accelerates the Circadian Rhythm Dysfunction in a 6-hydroxydopamine Rat Model of Parkinson’s Disease. Chin Med J (Engl) 2017;130(9):1085–92. doi: 10.4103/0366-6999.204920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vaccaro A, Issa AR, Seugnet L, Birman S, Klarsfeld A. Drosophila Clock Is Required in Brain Pacemaker Neurons to Prevent Premature Locomotor Aging Independently of Its Circadian Function. PLoS Genet. 2017;13(1):e1006507. doi: 10.1371/journal.pgen.1006507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bondy S, Maiese K. Aging and Age-Related Disorders. Springer Science; 2010. [Google Scholar]
- 122.Chen X, Kondo K, Motoki K, Homma H, Okazawa H. Fasting activates macroautophagy in neurons of Alzheimer’s disease mouse model but is insufficient to degrade amyloid-beta. Scientific reports. 2015;5:12115. doi: 10.1038/srep12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.He Y, Cornelissen-Guillaume GG, He J, Kastin AJ, Harrison LM, Pan W. Circadian rhythm of autophagy proteins in hippocampus is blunted by sleep fragmentation. Chronobiology international. 2016;33(5):553–60. doi: 10.3109/07420528.2015.1137581. [DOI] [PubMed] [Google Scholar]
- 124.Dong W, Wang R, Ma LN, Xu BL, Zhang JS, Zhao ZW, et al. Influence of age-related learning and memory capacity of mice: different effects of a high and low caloric diet. Aging Clin Exp Res. 2016;28(2):303–11. doi: 10.1007/s40520-015-0398-0. [DOI] [PubMed] [Google Scholar]
- 125.Min JJ, Huo XL, Xiang LY, Qin YQ, Chai KQ, Wu B, et al. Protective effect of Dl-3n-butylphthalide on learning and memory impairment induced by chronic intermittent hypoxia-hypercapnia exposure. Scientific reports. 2014;4:5555. doi: 10.1038/srep05555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rami A, Rawashdeh O. The hippocampal autophagic machinery is depressed in the absence of the circadian clock protein PER1 that may lead to vulnerability during cerebral ischemia. Curr Neurovasc Res. 2017;14(3):207–14. doi: 10.2174/1567202614666170619083239. [DOI] [PubMed] [Google Scholar]
- 127.Johnson SC, Sangesland M, Kaeberlein M, Rabinovitch PS. Modulating mTOR in aging and health. Interdisciplinary topics in gerontology. 2015;40:107–27. doi: 10.1159/000364974. [DOI] [PubMed] [Google Scholar]
- 128.Maiese K. Stem cell guidance through the mechanistic target of rapamycin. World J Stem Cells. 2015;7(7):999–1009. doi: 10.4252/wjsc.v7.i7.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ka M, Smith AL, Kim WY. MTOR controls genesis and autophagy of GABAergic interneurons during brain development. Autophagy. 2017;0 doi: 10.1080/15548627.2017.1327927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wu X, Li D, Liu J, Diao L, Ling S, Li Y, et al. Dammarane Sapogenins Ameliorates Neurocognitive Functional Impairment Induced by Simulated Long-Duration Spaceflight. Frontiers in pharmacology. 2017;8:315. doi: 10.3389/fphar.2017.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jenwitheesuk A, Nopparat C, Mukda S, Wongchitrat P, Govitrapong P. Melatonin regulates aging and neurodegeneration through energy metabolism, epigenetics, autophagy and circadian rhythm pathways. International journal of molecular sciences. 2014;15(9):16848–84. doi: 10.3390/ijms150916848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Beker MC, Caglayan B, Yalcin E, Caglayan AB, Turkseven S, Gurel B, et al. Time-of-Day Dependent Neuronal Injury After Ischemic Stroke: Implication of Circadian Clock Transcriptional Factor Bmal1 and Survival Kinase AKT. Mol Neurobiol. 2017 Apr 18; doi: 10.1007/s12035-017-0524-4. [DOI] [PubMed] [Google Scholar]
- 133.Ma L, Dong W, Wang R, Li Y, Xu B, Zhang J, et al. Effect of caloric restriction on the SIRT1/mTOR signaling pathways in senile mice. Brain Res Bull. 2015;116:67–72. doi: 10.1016/j.brainresbull.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 134.Maiese K. MicroRNAs and SIRT1: A Strategy for Stem Cell Renewal and Clinical Development? J Transl Sci. 2015;1(3):55–7. [PMC free article] [PubMed] [Google Scholar]
- 135.Charles S, Raj V, Arokiaraj J, Mala K. Caveolin1/protein arginine methyltransferase1/sirtuin1 axis as a potential target against endothelial dysfunction. Pharmacol Res. 2017;119:1–11. doi: 10.1016/j.phrs.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 136.Cui L, Guo J, Zhang Q, Yin J, Li J, Zhou W, et al. Erythropoietin activates SIRT1 to protect human cardiomyocytes against doxorubicin-induced mitochondrial dysfunction and toxicity. Toxicol Lett. 2017;275:28–38. doi: 10.1016/j.toxlet.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 137.Geng C, Xu H, Zhang Y, Gao Y, Li M, Liu X, et al. Retinoic acid ameliorates high-fat diet-induced liver steatosis through sirt1. Science China Life sciences. 2017 doi: 10.1007/s11427-016-9027-6. [DOI] [PubMed] [Google Scholar]
- 138.Hwang ES, Song SB. Nicotinamide is an inhibitor of SIRT1 in vitro, but can be a stimulator in cells. Cell Mol Life Sci. 2017 doi: 10.1007/s00018-017-2527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bruckbauer A, Banerjee J, Cao Q, Cui X, Jing J, Zha L, et al. Leucine-nicotinic acid synergy stimulates AMPK/Sirt1 signaling and regulates lipid metabolism and lifespan in Caenorhabditis elegans, and hyperlipidemia and atherosclerosis in mice. American journal of cardiovascular disease. 2017;7(2):33–47. [PMC free article] [PubMed] [Google Scholar]
- 140.Chong ZZ, Wang S, Shang YC, Maiese K. Targeting cardiovascular disease with novel SIRT1 pathways. Future Cardiol. 2012;8(1):89–100. doi: 10.2217/fca.11.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li WY, Ren JH, Tao NN, Ran LK, Chen X, Zhou HZ, et al. The SIRT1 inhibitor, nicotinamide, inhibits hepatitis B virus replication in vitro and in vivo. Archives of virology. 2015 doi: 10.1007/s00705-015-2712-8. [DOI] [PubMed] [Google Scholar]
- 142.Maiese K, Chong ZZ, Shang YC, Wang S. Translating cell survival and cell longevity into treatment strategies with SIRT1. Rom J Morphol Embryol. 2011;52(4):1173–85. [PMC free article] [PubMed] [Google Scholar]
- 143.Duan W. Sirtuins: from metabolic regulation to brain aging. Frontiers in aging neuroscience. 2013;5:36. doi: 10.3389/fnagi.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Martin A, Tegla CA, Cudrici CD, Kruszewski AM, Azimzadeh P, Boodhoo D, et al. Role of SIRT1 in autoimmune demyelination and neurodegeneration. Immunologic research. 2015;61(3):187–97. doi: 10.1007/s12026-014-8557-5. [DOI] [PubMed] [Google Scholar]
- 145.Ma L, Fu R, Duan Z, Lu J, Gao J, Tian L, et al. Sirt1 is essential for resveratrol enhancement of hypoxia-induced autophagy in the type 2 diabetic nephropathy rat. Pathology, research and practice. 2016 doi: 10.1016/j.prp.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 146.Ramalinga M, Roy A, Srivastava A, Bhattarai A, Harish V, Suy S, et al. MicroRNA-212 negatively regulates starvation induced autophagy in prostate cancer cells by inhibiting SIRT1 and is a modulator of angiogenesis and cellular senescence. Oncotarget. 2015 doi: 10.18632/oncotarget.5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mazzoccoli G, Tevy MF, Borghesan M, Delle Vergini MR, Vinciguerra M. Caloric restriction and aging stem cells: the stick and the carrot? Exp Gerontol. 2014;50:137–48. doi: 10.1016/j.exger.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 148.Ou X, Lee MR, Huang X, Messina-Graham S, Broxmeyer HE. SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem Cells. 2014;32(5):1183–94. doi: 10.1002/stem.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fong Y, Lin YC, Wu CY, Wang HM, Lin LL, Chou HL, et al. The antiproliferative and apoptotic effects of sirtinol, a sirtuin inhibitor on human lung cancer cells by modulating Akt/beta-catenin-Foxo3a axis. ScientificWorldJournal. 2014;2014:937051. doi: 10.1155/2014/937051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hou J, Wang S, Shang YC, Chong ZZ, Maiese K. Erythropoietin Employs Cell Longevity Pathways of SIRT1 to Foster Endothelial Vascular Integrity During Oxidant Stress. Curr Neurovasc Res. 2011;8(3):220–35. doi: 10.2174/156720211796558069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang T, Cui H, Ma N, Jiang Y. Nicotinamide-mediated inhibition of SIRT1 deacetylase is associated with the viability of cancer cells exposed to antitumor agents and apoptosis. Oncology letters. 2013;6(2):600–4. doi: 10.3892/ol.2013.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Du G, Song Y, Zhang T, Ma L, Bian N, Chen X, et al. Simvastatin attenuates TNFalphainduced apoptosis in endothelial progenitor cells via the upregulation of SIRT1. Int J Mol Med. 2014;34(1):177–82. doi: 10.3892/ijmm.2014.1740. [DOI] [PubMed] [Google Scholar]
- 153.Paschalaki KE, Starke RD, Hu Y, Mercado N, Margariti A, Gorgoulis VG, et al. Dysfunction of endothelial progenitor cells from smokers and chronic obstructive pulmonary disease patients due to increased DNA damage and senescence. Stem Cells. 2013;31(12):2813–26. doi: 10.1002/stem.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu Z, Gan L, Zhang T, Ren Q, Sun C. Melatonin alleviates adipose inflammation through elevating alpha-ketoglutarate and diverting adipose-derived exosomes to macrophages in mice. J Pineal Res. 2017 doi: 10.1111/jpi.12455. [DOI] [PubMed] [Google Scholar]
- 155.Fang M, Ohman Strickland PA, Kang HG, Zarbl H. Uncoupling genotoxic stress responses from circadian control increases susceptibility to mammary carcinogenesis. Oncotarget. 2017;8(20):32752–68. doi: 10.18632/oncotarget.15678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bellet MM, Masri S, Astarita G, Sassone-Corsi P, Della Fazia MA, Servillo G. Histone Deacetylase SIRT1 Controls Proliferation, Circadian Rhythm, and Lipid Metabolism during Liver Regeneration in Mice. J Biol Chem. 2016;291(44):23318–29. doi: 10.1074/jbc.M116.737114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sato S, Solanas G, Peixoto FO, Bee L, Symeonidi A, Schmidt MS, et al. Circadian Reprogramming in the Liver Identifies Metabolic Pathways of Aging. Cell. 2017;170(4):664–77 e11. doi: 10.1016/j.cell.2017.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen H, Lu Q, Fei X, Shen L, Jiang D, Dai D. miR-22 inhibits the proliferation, motility, and invasion of human glioblastoma cells by directly targeting SIRT1. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015 doi: 10.1007/s13277-015-4575-8. [DOI] [PubMed] [Google Scholar]
- 159.Gabay O, Clouse KA. Epigenetics of cartilage diseases. Joint, bone, spine : revue du rhumatisme. 2015 doi: 10.1016/j.jbspin.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 160.Okada M, Kim HW, Matsu-Ura K, Wang YG, Xu M, Ashraf M. Abrogation of Age-Induced MicroRNA-195 Rejuvenates the Senescent Mesenchymal Stem Cells by Reactivating Telomerase. Stem Cells. 2015 doi: 10.1002/stem.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chiara B, Ilaria C, Antonietta C, Francesca C, Marco M, Lucia A, et al. SIRT1 Inhibition Affects Angiogenic Properties of Human MSCs. BioMed research international. 2014;2014:783459. doi: 10.1155/2014/783459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hung CH, Chan SH, Chu PM, Tsai KL. Quercetin is a potent anti-atherosclerotic compound by activation of SIRT1 signaling under oxLDL stimulation. Molecular nutrition & food research. 2015 doi: 10.1002/mnfr.201500144. [DOI] [PubMed] [Google Scholar]
- 163.Jin X, Chen M, Yi L, Chang H, Zhang T, Wang L, et al. Delphinidin-3-glucoside protects human umbilical vein endothelial cells against oxidized low-density lipoprotein-induced injury by autophagy upregulation via the AMPK/SIRT1 signaling pathway. Molecular nutrition & food research. 2014;58(10):1941–51. doi: 10.1002/mnfr.201400161. [DOI] [PubMed] [Google Scholar]
- 164.Kedenko L, Lamina C, Kedenko I, Kollerits B, Kiesslich T, Iglseder B, et al. Genetic polymorphisms at SIRT1 and FOXO1 are associated with carotid atherosclerosis in the SAPHIR cohort. BMC Med Genet. 2014;15(1):112. doi: 10.1186/s12881-014-0112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Luo XY, Qu SL, Tang ZH, Zhang Y, Liu MH, Peng J, et al. SIRT1 in cardiovascular aging. Clin Chim Acta. 2014;437:106–14. doi: 10.1016/j.cca.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 166.Xu S, Bai P, Little PJ, Liu P. Poly(ADP-ribose) polymerase 1 (PARP1) in atherosclerosis: from molecular mechanisms to therapeutic implications. Med Res Rev. 2014;34(3):644–75. doi: 10.1002/med.21300. [DOI] [PubMed] [Google Scholar]
- 167.Balan V, Miller GS, Kaplun L, Balan K, Chong ZZ, Li F, et al. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008;283(41):27810–9. doi: 10.1074/jbc.M804681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Moroz N, Carmona JJ, Anderson E, Hart AC, Sinclair DA, Blackwell TK. Dietary restriction involves NAD-dependent mechanisms and a shift toward oxidative metabolism. Aging Cell. 2014;13(6):1075–85. doi: 10.1111/acel.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Poljsak B, Milisav I. The NAD(+)-depletion theory of ageing: NAD(+) as the link between oxidative stress, inflammation, caloric restriction, exercise, DNA repair, longevity and health span. Rejuvenation Res. 2016 doi: 10.1089/rej.2015.1767. [DOI] [PubMed] [Google Scholar]
- 170.Mishra M, Duraisamy AJ, Kowluru RA. Sirt1-A Guardian of the Development of Diabetic Retinopathy. Diabetes. 2018 doi: 10.2337/db17-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shao Y, Lv C, Wu C, Zhou Y, Wang Q. Mir-217 promotes inflammation and fibrosis in high glucose cultured rat glomerular mesangial cells via Sirt1/HIF-1alpha signaling pathway. Diabetes Metab Res Rev. 2016;32(6):534–43. doi: 10.1002/dmrr.2788. [DOI] [PubMed] [Google Scholar]
- 172.Vikram A, Kim YR, Kumar S, Li Q, Kassan M, Jacobs JS, et al. Vascular microRNA-204 is remotely governed by the microbiome and impairs endothelium-dependent vasorelaxation by downregulating Sirtuin1. Nature communications. 2016;7:12565. doi: 10.1038/ncomms12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Akasaki Y, Alvarez-Garcia O, Saito M, Carames B, Iwamoto Y, Lotz MK. FOXO transcription factors support oxidative stress resistance in human chondrocytes. Arthritis & rheumatology (Hoboken, NJ) 2014;66(12):3349–58. doi: 10.1002/art.38868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chong ZZ, Maiese K. Enhanced Tolerance against Early and Late Apoptotic Oxidative Stress in Mammalian Neurons through Nicotinamidase and Sirtuin Mediated Pathways. Curr Neurovasc Res. 2008;5(3):159–70. doi: 10.2174/156720208785425666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Colak Y, Yesil A, Mutlu HH, Caklili OT, Ulasoglu C, Senates E, et al. A potential treatment of non-alcoholic fatty liver disease with SIRT1 activators. Journal of gastrointestinal and liver diseases : JGLD. 2014;23(3):311–9. doi: 10.15403/jgld.2014.1121.233.yck. [DOI] [PubMed] [Google Scholar]
- 176.Lamoke F, Shaw S, Yuan J, Ananth S, Duncan M, Martin P, et al. Increased Oxidative and Nitrative Stress Accelerates Aging of the Retinal Vasculature in the Diabetic Retina. PLoS One. 2015;10(10):e0139664. doi: 10.1371/journal.pone.0139664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Saboori S, Koohdani F, Nematipour E, Yousefi Rad E, Saboor-Yaraghi AA, Javanbakht MH, et al. Beneficial effects of omega-3 and vitamin E coadministration on gene expression of SIRT1 and PGC1alpha and serum antioxidant enzymes in patients with coronary artery disease. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2016;26(6):489–94. doi: 10.1016/j.numecd.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 178.Zhang F, Hu Y, Xu X, Zhai X, Wang G, Ning S, et al. Icariin protects against intestinal ischemia-reperfusion injury. J Surg Res. 2015;194(1):127–38. doi: 10.1016/j.jss.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 179.Zhang XS, Wu Q, Wu LY, Ye ZN, Jiang TW, Li W, et al. Sirtuin 1 activation protects against early brain injury after experimental subarachnoid hemorrhage in rats. Cell death & disease. 2016;7(10):e2416. doi: 10.1038/cddis.2016.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Du LL, Chai DM, Zhao LN, Li XH, Zhang FC, Zhang HB, et al. AMPK Activation Ameliorates Alzheimer’s Disease-Like Pathology and Spatial Memory Impairment in a Streptozotocin-Induced Alzheimer’s Disease Model in Rats. J Alzheimers Dis. 2015;43(3):775–84. doi: 10.3233/JAD-140564. [DOI] [PubMed] [Google Scholar]
- 181.Guo P, Wang D, Wang X, Feng H, Tang Y, Sun R, et al. Effect and mechanism of fuzhisan and donepezil on the sirtuin 1 pathway and amyloid precursor protein metabolism in PC12 cells. Molecular medicine reports. 2016;13(4):3539–46. doi: 10.3892/mmr.2016.4957. [DOI] [PubMed] [Google Scholar]
- 182.Maiese K. Forkhead Transcription Factors: Formulating a FOXO Target for Cognitive Loss. Curr Neurovasc Res. 2017 doi: 10.2174/1567202614666171116102911. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sun Q, Jia N, Wang W, Jin H, Xu J, Hu H. Activation of SIRT1 by curcumin blocks the neurotoxicity of amyloid-beta25-35 in rat cortical neurons. Biochem Biophys Res Commun. 2014;448(1):89–94. doi: 10.1016/j.bbrc.2014.04.066. [DOI] [PubMed] [Google Scholar]
- 184.Jung CJ, Iyengar S, Blahnik KR, Jiang JX, Tahimic C, Torok NJ, et al. Human ESC self-renewal promoting microRNAs induce epithelial-mesenchymal transition in hepatocytes by controlling the PTEN and TGFbeta tumor suppressor signaling pathways. Mol Cancer Res. 2012;10(7):979–91. doi: 10.1158/1541-7786.MCR-11-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Arunachalam G, Lakshmanan AP, Samuel SM, Triggle CR, Ding H. Molecular Interplay between microRNA-34a and Sirtuin1 in Hyperglycemia-Mediated Impaired Angiogenesis in Endothelial Cells: Effects of Metformin. J Pharmacol Exp Ther. 2016;356(2):314–23. doi: 10.1124/jpet.115.226894. [DOI] [PubMed] [Google Scholar]
- 186.Aranha MM, Santos DM, Sola S, Steer CJ, Rodrigues CM. miR-34a regulates mouse neural stem cell differentiation. PLoS One. 2011;6(8):e21396. doi: 10.1371/journal.pone.0021396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cocquerelle C, Mascrez B, Hetuin D, Bailleul B. Mis-splicing yields circular RNA molecules. FASEB J. 1993;7(1):155–60. doi: 10.1096/fasebj.7.1.7678559. [DOI] [PubMed] [Google Scholar]
- 188.Hsu MT, Coca-Prados M. Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature. 1979;280(5720):339–40. doi: 10.1038/280339a0. [DOI] [PubMed] [Google Scholar]
- 189.Maiese K. Disease onset and aging in the world of circular RNAs. J Transl Sci. 2016;2(6):327–9. doi: 10.15761/jts.1000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zheng Q, Bao C, Guo W, Li S, Chen J, Chen B, et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nature communications. 2016;7:11215. doi: 10.1038/ncomms11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Holdt LM, Stahringer A, Sass K, Pichler G, Kulak NA, Wilfert W, et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nature communications. 2016;7:12429. doi: 10.1038/ncomms12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lin SP, Ye S, Long Y, Fan Y, Mao HF, Chen MT, et al. Circular RNA expression alterations are involved in OGD/R-induced neuron injury. Biochem Biophys Res Commun. 2016;471(1):52–6. doi: 10.1016/j.bbrc.2016.01.183. [DOI] [PubMed] [Google Scholar]
- 193.Filley CM, Rollins YD, Anderson CA, Arciniegas DB, Howard KL, Murrell JR, et al. The genetics of very early onset Alzheimer disease. Cognitive and behavioral neurology : official journal of the Society for Behavioral and Cognitive Neurology. 2007;20(3):149–56. doi: 10.1097/WNN.0b013e318145a8c8. [DOI] [PubMed] [Google Scholar]
- 194.Maiese K, Chong ZZ, Shang YC, Wang S. mTOR: on target for novel therapeutic strategies in the nervous system. Trends Mol Med. 2013;19(1):51–60. doi: 10.1016/j.molmed.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Saha P, Biswas SC. Amyloid-beta induced astrocytosis and astrocyte death: Implication of FoxO3a-Bim-caspase3 death signaling. Mol Cell Neurosci. 2015;68:203–11. doi: 10.1016/j.mcn.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 196.Shi C, Zhu J, Leng S, Long D, Luo X. Mitochondrial FOXO3a is involved in amyloid beta peptide-induced mitochondrial dysfunction. Journal of bioenergetics and biomembranes. 2016;48(3):189–96. doi: 10.1007/s10863-016-9645-0. [DOI] [PubMed] [Google Scholar]
- 197.Weigel D, Jurgens G, Kuttner F, Seifert E, Jackle H. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell. 1989;57(4):645–58. doi: 10.1016/0092-8674(89)90133-5. [DOI] [PubMed] [Google Scholar]
- 198.Maiese K. Advances in Experimental Medicine and Biology. Springer Science and Business Media; 2010. Forkhead Transcription Factors: Vital Elements in Biology and Medicine; p. 665. [PubMed] [Google Scholar]
- 199.Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med. 2008;14(5):219–27. doi: 10.1016/j.molmed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Maiese K, Chong ZZ, Shang YC, Hou J. FoxO proteins: cunning concepts and considerations for the cardiovascular system. Clin Sci (Lond) 2009;116(3):191–203. doi: 10.1042/CS20080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Maiese K, Hou J, Chong ZZ, Shang YC. A fork in the path: Developing therapeutic inroads with FoxO proteins. Oxid Med Cell Longev. 2009;2(3):119–29. doi: 10.4161/oxim.2.3.8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Farhan M, Wang H, Gaur U, Little PJ, Xu J, Zheng W. FOXO Signaling Pathways as Therapeutic Targets in Cancer. Int J Biol Sci. 2017;13(7):815–27. doi: 10.7150/ijbs.20052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Maiese K, Chong ZZ, Shang YC, Hou J. A “FOXO” in sight: targeting Foxo proteins from conception to cancer. Med Res Rev. 2009;29(3):395–418. doi: 10.1002/med.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Biggs WH, 3rd, Cavenee WK, Arden KC. Identification and characterization of members of the FKHR (FOX O) subclass of winged-helix transcription factors in the mouse. Mamm Genome. 2001;12(6):416–25. doi: 10.1007/s003350020002. [DOI] [PubMed] [Google Scholar]
- 205.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120(Pt 15):2479–87. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 206.Clark KL, Halay ED, Lai E, Burley SK. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature. 1993;364(6436):412–20. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]
- 207.Tsai KL, Sun YJ, Huang CY, Yang JY, Hung MC, Hsiao CD. Crystal structure of the human FOXO3a-DBD/DNA complex suggests the effects of post-translational modification. Nucleic Acids Res. 2007;35(20):6984–94. doi: 10.1093/nar/gkm703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shang YC, Chong ZZ, Hou J, Maiese K. The forkhead transcription factor FoxO3a controls microglial inflammatory activation and eventual apoptotic injury through caspase 3. Curr Neurovasc Res. 2009;6(1):20–31. doi: 10.2174/156720209787466064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chong ZZ, Li F, Maiese K. Group I Metabotropic Receptor Neuroprotection Requires Akt and Its Substrates that Govern FOXO3a, Bim, and beta-Catenin During Oxidative Stress. Curr Neurovasc Res. 2006;3(2):107–17. doi: 10.2174/156720206776875830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chong ZZ, Lin SH, Maiese K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J Cereb Blood Flow Metab. 2004;24(7):728–43. doi: 10.1097/01.WCB.0000122746.72175.0E. [DOI] [PubMed] [Google Scholar]
- 211.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. Jama. 2005;293(1):90–5. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chamorro ME, Wenker SD, Vota DM, Vittori DC, Nesse AB. Signaling pathways of cell proliferation are involved in the differential effect of erythropoietin and its carbamylated derivative. Biochim Biophys Acta. 2013;1833(8):1960–8. doi: 10.1016/j.bbamcr.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 213.Chong ZZ, Hou J, Shang YC, Wang S, Maiese K. EPO Relies upon Novel Signaling of Wnt1 that Requires Akt1, FoxO3a, GSK-3beta, and beta-Catenin to Foster Vascular Integrity During Experimental Diabetes. Curr Neurovasc Res. 2011;8(2):103–20. doi: 10.2174/156720211795495402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chong ZZ, Maiese K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br J Pharmacol. 2007;150(7):839–50. doi: 10.1038/sj.bjp.0707161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Anitha M, Gondha C, Sutliff R, Parsadanian A, Mwangi S, Sitaraman SV, et al. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116(2):344–56. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zheng WH, Kar S, Quirion R. FKHRL1 and its homologs are new targets of nerve growth factor Trk receptor signaling. J Neurochem. 2002;80(6):1049–61. doi: 10.1046/j.0022-3042.2002.00783.x. [DOI] [PubMed] [Google Scholar]
- 217.Zhu W, Bijur GN, Styles NA, Li X. Regulation of FOXO3a by brain-derived neurotrophic factor in differentiated human SH-SY5Y neuroblastoma cells. Brain Res Mol Brain Res. 2004;126(1):45–56. doi: 10.1016/j.molbrainres.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 218.Kandula V, Kosuru R, Li H, Yan D, Zhu Q, Lian Q, et al. Forkhead box transcription factor 1: role in the pathogenesis of diabetic cardiomyopathy. Cardiovasc Diabetol. 2016;15(1):44. doi: 10.1186/s12933-016-0361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zeng Z, Wang X, Bhardwaj SK, Zhou X, Little PJ, Quirion R, et al. The Atypical Antipsychotic Agent, Clozapine, Protects Against Corticosterone-Induced Death of PC12 Cells by Regulating the Akt/FoxO3a Signaling Pathway. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-9904-4. [DOI] [PubMed] [Google Scholar]
- 220.Paraiso AF, Mendes KL, Santos SH. Brain activation of SIRT1: role in neuropathology. Mol Neurobiol. 2013;48(3):681–9. doi: 10.1007/s12035-013-8459-x. [DOI] [PubMed] [Google Scholar]
- 221.Wang W, Yan C, Zhang J, Lin R, Lin Q, Yang L, et al. SIRT1 inhibits TNF-alpha-induced apoptosis of vascular adventitial fibroblasts partly through the deacetylation of FoxO1. Apoptosis. 2013;18(6):689–701. doi: 10.1007/s10495-013-0833-7. [DOI] [PubMed] [Google Scholar]
- 222.Lin CL, Huang WN, Li HH, Huang CN, Hsieh S, Lai C, et al. Hydrogen-rich water attenuates amyloid beta-induced cytotoxicity through upregulation of Sirt1-FoxO3a by stimulation of AMP-activated protein kinase in SK-N-MC cells. Chem Biol Interact. 2015;240:12–21. doi: 10.1016/j.cbi.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 223.Weikel KA, Cacicedo JM, Ruderman NB, Ido Y. Knockdown of GSK3beta Increases Basal Autophagy and AMPK Signaling in Nutrient-laden Human Aortic Endothelial Cells. Bioscience reports. 2016 doi: 10.1042/BSR20160174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Vidal RL, Figueroa A, Court FA, Thielen P, Molina C, Wirth C, et al. Targeting the UPR transcription factor XBP1 protects against Huntington’s disease through the regulation of FoxO1 and autophagy. Hum Mol Genet. 2012;21(10):2245–62. doi: 10.1093/hmg/dds040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Omata Y, Lim YM, Akao Y, Tsuda L. Age-induced reduction of autophagy-related gene expression is associated with onset of Alzheimer’s disease. American journal of neurodegenerative disease. 2014;3(3):134–42. [PMC free article] [PubMed] [Google Scholar]
- 226.Fernandez AM, Hervas R, Dominguez-Fraile M, Garrido VN, Gomez-Gutierrez P, Vega M, et al. Blockade of the Interaction of Calcineurin with FOXO in Astrocytes Protects Against Amyloid-beta-Induced Neuronal Death. J Alzheimers Dis. 2016;52(4):1471–8. doi: 10.3233/JAD-160149. [DOI] [PubMed] [Google Scholar]
- 227.Xu G, Liu J, Yoshimoto K, Chen G, Iwata T, Mizusawa N, et al. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces expression of p27(kip(1)) and FoxO3a in female rat cerebral cortex and PC12 cells. Toxicol Lett. 2014;226(3):294–302. doi: 10.1016/j.toxlet.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 228.Zeldich E, Chen CD, Colvin TA, Bove-Fenderson EA, Liang J, Tucker Zhou TB, et al. The neuroprotective effect of Klotho is mediated via regulation of members of the redox system. J Biol Chem. 2014;289(35):24700–15. doi: 10.1074/jbc.M114.567321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hong YK, Lee S, Park SH, Lee JH, Han SY, Kim ST, et al. Inhibition of JNK/dFOXO pathway and caspases rescues neurological impairments in Drosophila Alzheimer’s disease model. Biochem Biophys Res Commun. 2012;419(1):49–53. doi: 10.1016/j.bbrc.2012.01.122. [DOI] [PubMed] [Google Scholar]


