Abstract
Objective
Cut homeodomain transcription factor CUX2 plays an important role in dendrite branching, spine development, and synapse formation in layer II–III neurons of the cerebral cortex. We identify a recurrent de novo CUX2 p.Glu590Lys as a novel genetic cause for developmental and epileptic encephalopathy (DEE).
Methods
The de novo p.Glu590Lys variant was identified by whole-exome sequencing (n=5) or targeted gene panel (n=4). We performed electroclinical and imaging phenotyping on all patients.
Results
The cohort comprised 7 males and 2 females. Mean age at study was 13 years [0.5–21]. Median age at seizure onset was 6 months [2 months to 9 years]. Seizure types at onset were myoclonic, atypical absence with myoclonic components, and focal seizures. Epileptiform activity on EEG was seen in 8 cases: generalized polyspike-wave (6) or multifocal discharges (2). Seizures were drug-resistant in 7 or controlled with valproate (2). Six patients had a DEE: myoclonic DEE (3), Lennox-Gastaut syndrome (2) and West Syndrome (1). Two had a static encephalopathy and genetic generalized epilepsy, including absence epilepsy in one. One infant had multifocal epilepsy. Eight had severe cognitive impairment, with autistic features in six. The p.Glu590Lys variant affects a highly-conserved glutamine residue in the CUT domain predicted to interfere with CUX2 binding to DNA targets during neuronal development.
Interpretation
Patients with CUX2 p.Glu590Lys display a distinctive phenotypic spectrum which is predominantly generalized epilepsy, with infantile-onset myoclonic DEE at the severe end and generalized epilepsy with severe static developmental encephalopathy at the milder end of the spectrum.
Keywords: intellectual disability, developmental and epileptic encephalopathy, myoclonic seizures, CUX2, CUT domain, de novo variant
INTRODUCTION
The developmental and epileptic encephalopathies (DEEs) are a group of severe infantile and childhood onset epilepsies characterized by developmental slowing or regression in the context of recurrent seizures and frequent interictal epileptiform discharges, often on a background of developmental delay1. The DEEs have a wide range of etiologies, including both acquired and genetic causes. Considerable genetic heterogeneity has been demonstrated by recent advances in molecular genetics and next-generation sequencing, with more than 100 genes implicated, with various modes of inheritance. Identification of the pathogenic variant ends the diagnostic odyssey for patients and their families, provides information regarding prognosis and co-morbidities, and informs accurate genetic counselling. In addition, the identification of patients with a genetically defined epilepsy can allow therapeutic management to be optimized and pave the way to novel targeted approaches2. Pathogenic variants in a subset of genes account for a significant proportion of patients with DEEs, such as SCN1A, SCN2A, SCN8A, KCNQ2, STXBP1 and CDKL5, whereas many other genetic causes are rare3. For these rare genetic disorders, the small number of patients with pathogenic variants in single genes often requires international collaborations to identify multiple cases to facilitate careful delineation of the phenotype4.
The de novo c.1768G>A (p.Glu590Lys) missense variant in CUX2 (NM_015267.3) was first reported in single cases from two large-scale whole-exome sequencing (WES) studies: one from a study of intellectual disability and the other a cohort of two specific DEEs, infantile spasms and Lennox-Gastaut syndrome 5, 6. In this paper, we describe seven additional patients with the same recurrent p.Glu590Lys missense variant in CUX2, shown to be de novo in all cases. We present the clinical data for these nine patients and describe the phenotypic spectrum associated with this new recurrent genetic disorder which most frequently presents as a severe developmental and epileptic encephalopathy.
PATIENTS AND METHODS
This study was approved by the institutional review boards of the participating institutions. Informed consent was provided by the parent or the patient’s legal guardian as all patients were minors or had intellectual disability. CUX2 variants were identified by whole exome sequencing (n=2) or targeted gene panel sequencing (n=5) using previously described methods7, 8 and were collated through the Matchmaker Exchange network4 or personal communication with colleagues.
We analyzed the clinical phenotype for the seven new patients as well as the two individuals previously reported5, 6. Seizure onset age, seizure types, EEG and neuroimaging findings were obtained, together with a history of each patient’s developmental course.
3D modeling of structural effects was performed using either the mutate_model script in MODELLER9 or using the available solution structure for residues 544–631 (PDB 1X2L) for the CUT1 domain of CUX2. The resulting models were visualized using the SWISS-PdbViewer10. The same 1X2L PDB file was also used as input for protein stability prediction applications: CUPSAT11, I-mutant3.012, SNPs&GO3d13. The web servers HOPE14, or PHYRE215 were also used for in silico prediction of pathogenic variant effects on protein function. Multiple protein sequence alignments were done on Clustal Omega at EMBL-EBI server16.
RESULTS
Identification of patients with the p.Glu590Lys variant
Next generation sequencing identified a heterozygous de novo variant: Chr12:111748354G>A (hg19), c.1768G>A transition in CUX2 in all seven patients. The c.1768G>A is predicted to lead to a substitution of the glutamate residue p. Glu590Lys. This variant has not been reported in the ExAC/gnomAD databases. Taken together with the two previously published cases, 2 individuals were identified through clinical diagnostic testing, 3/1222 through research studies in patients with DEE, and 4/2207 individuals were identified by screening of patient cohorts with intellectual disability with/without epilepsy.
Phenotype of patients with the CUX2 Glu590Lys variant
Seven patients were male and 2 were female. Median age at study inclusion was 13 years [0.5–21](Table 1). Six of the nine patients had a DEE; whereas, two patients presented with a severe, static, developmental encephalopathy with genetic generalized epilepsy (GGE). Their GGE comprised absence seizures in one (patient 9) and early-onset absence epilepsy and myoclonic seizures in the other (patient 5). Patient 8 had focal epilepsy but, as he is only 6 months old, he is too young to predict his developmental outcome (Table 1). The pregnancy and perinatal period were unremarkable in all patients.
Table 1.
Clinical features of the nine patients with the recurrent de novo p.Glu590Lys variant of CUX2
| Patient n° | Sex | Age at study (years) | Age at first seizures (months) | Type(s) of seizures at onset | Evolution of seizures |
|---|---|---|---|---|---|
| Patient 1 5 | M | 8 | 6 | Focal spasms | Seizure free |
| Patient 2 | M | 19 | 7 | Myoclonic seizures and right occipital seizures with apnea | 30 months: 1 febrile tonic seizure. 3 years: 2 focal seizures. 4 years:Myoclonic seizures. 7 years: GTCS and myoclonic seizures |
| Patient 3 | M | 21 | 6 | Atypical absences with myoclonus | Atypical absences, tonic seizures with myoclonic component, atonic seizures, GTCS |
| Patient 4 | F | 9 | 6 | Myoclonic | Myoclonic seizures, tonic seizures, GTCS |
| Patient 5 | F | 14 | 12 | Absence seizures | Myoclonic seizures. NCSE |
| Patient 6 6 | M | 12 | 2 | Myoclonic seizures | Atonic seizures, myoclonic and absence seizures with eye fluttering, GTCS |
| Patient 7 | M | 16 | 5 | Myoclonic seizures | Myoclonic seizures, especially in the mornings, myoclonic drop attacks |
| Patient 8 | M | 0.5 | 2 | GTCS, atypical absences with apnea and myoclonus, right hemiclonic seizures | GTCS, Focal impaired awareness seizures, 1 febrile seizure after vaccination |
| Patient 9 26 | M | 14 | 108 (9 years) | Absence seizures | Seizure free from age 12 |
F = female; GTCS = generalized tonic-clonic seizures; M = male; NCSE = Nonconvulsive status epilepticus.
Median age at seizure onset was 6 months [range 2 months −9 years]. Seizure types at onset were myoclonic (n=4), absence (n=2), atypical absence with myoclonic components (2) and focal (n=3) including occipital (1), hemiclonic (1) and focal epileptic spasms (1). Generalized tonic-clonic seizures only occurred in one child at presentation. Apnea occurred with occipital seizures in one infant and with atypical absence seizures in another. Seizures were frequent at onset with multiple seizures per day. EEG was abnormal at seizure onset in all individuals; most frequently showing generalized spike-wave or polyspike-wave (n=5) (Table 2). Focal features were also noted including temporal discharges (n=1), occipital discharges (n=1), multifocal discharges (n=1), hypsarrhythmia (n=1) and focal slowing (n=1).
Table 2.
EEG features of the nine patients with the recurrent de novo p.Glu590Lys variant of CUX2
| Patient n° | EEG at onset | Later EEG studies | AEDs trialled | Current AEDs | Epilepsy syndrome |
|---|---|---|---|---|---|
| Patient 1 5 | Hypsarrhythmia | Background slowing, but no epileptiform discharges | STM, VPA, Synacthen for 3 months with improvement | VPA | DEE: West syndrome |
| Patient 2 | GSW, sometimes with myoclonic seizures. Right occipital seizure recorded | 3 Hz GSW, absence seizures with myoclonic components | VPA, CLB | VPA | Myoclonic DEE |
| Patient 3 | 3–4 Hz GSW, GPSW | Background slowing, frequent trains of 1.5–2 Hz and 3–4 Hz GSW; myoclonic seizures with GSW | VPA, OXC, CBZ, LTG, VGB, ZNS, PHT, NZP | LEV, CLB, VPA, ZNS VNS (beneficial) |
DEE: Lennox-Gastaut syndrome |
| Patient 4 | GSW, GPSW | Myoclonic seizures with GPSW. Tonic seizures with generalized polyspike | LEV, CZP, ESX, CBZ, LCM, RUF | ESX, CLB, LCM, RUF, ketogenic diet | DEE: Lennox-Gastaut syndrome |
| Patient 5 | 3 Hz GSW | Slow background, Absence seizure with 3 Hz GSW, fast irregular GSW, GPSW | VPA | VPA, LTG | Severe Developmental Encephalopathy with Genetic Generalised Epilepsy (early onset absence epilepsy and myoclonic seizures) |
| Patient 6 6 | Left temporal spikes/sharp waves | Slow background with multifocal epileptiform discharges | CBZ, VPA, CLB, ACZ, LTG, FBM, DZP, LEV, CZP, TPM, prednisolone, VNS, ketogenic diet | VPA, CZP, TPM | Myoclonic DEE |
| Patient 7 | GSW, GPSW | GSW with myoclonic seizure. During sleep, continuous 1–2 Hz GSW | CBZ, VPA, ACZ, LTG, TPM, CZP, LEV, RUF, LCM, CLB, PER | VPA, CLB, STP | Myoclonic DEE |
| Patient 8 | Multifocal discharges, mainly independent bi-parieto-temporal | Slowing and sharp waves in the left temporal region | VPA, DZP, PB, LEV | VPA | Multifocal epilepsy, too early to diagnose DEE |
| Patient 9 26 | Left fronto-central slowing | Normal EEG | VPA | VPA | Severe Developmental Encephalopathy with absence epilepsy |
ACZ = Acetazolamide; AED = anti-epileptic drug; CBZ = carbamazepine; CLB = Clobazam; CZP = Clonazepam; DEE = Developmental and Epileptic Encephalopathy; DZP = Diazepam; ESX = Ethosuximide; FBM = Felbamate; GSW = generalized spike-wave; GPSW = generalized polyspike-wave; LCM lacosamide; LEV = Levetiracetam; LTG = lamotrigine; MDZ = Midazolam; NZP = nitrazepam; OXC = Oxcarbazepine; PB = Phenobarbitone; PHT = phenytoin; PER = perampanel; RUF = Rufinamide; STM = Sulthiame; STP = Stiripentol; TPM = Topiramate; VGB = Vigabatrin; VNS = vagal nerve stimulation; VPA = valproate; ZNS = Zonisamide.
Valproate fully controlled seizures in two patients (Table 3). In patient 5, absence seizures were initially well controlled on valproate until an episode of non-convulsive status epilepticus at age three years, with rare episodes occurring every few years. The remaining five patients were refractory to multiple anti-epileptic drugs, including valproate. With the evolution of their epilepsy, multiple seizure types often emerged including myoclonic seizures (n=5), absence (n=5) and generalized tonic-clonic seizures (n=5).
Table 3.
Other characteristics of the nine patients with the recurrent de novo p.Glu590Lys variant of CUX2
| Patient n° | Brain MRI (age) | Head circumference (age) | Intellectual disability | Other neurological features |
|---|---|---|---|---|
| Patient 1 5 | Normal (8 mo) | 50 cm (7 years, 50th centile) | Severe | Non verbal, Movement disorder: stereotypies (hand flapping) |
| Patient 2 | Normal (8 mo) | 51 cm (13 years, 25th centile) | Never normal, Regression at 7 months and at 12 years. Profound | Non-verbal Movement disorder: athetosis, dystonia, stereotypies. Hypotonic at 1 year, then spastic tetraparesis from 12 years with loss of ambulation. |
| Patient 3 | Cerebellar atrophy (6 mo) | 56 cm (21 years, 73rd percentile) | Severe | Non-verbal. No eye contact, inappropriate laughter episodes |
| Patient 4 | Hippocampal asymmetry (6yr 3mo) | 52 cm (9 years, 44th percentile) | Never normal, severe regression at 8 years. Severe | Non-verbal. Decreased reflexes, ataxic gait |
| Patient 5 | Normal (5 yr) | 54 cm (14 years, 50th centile) | Severe | Movement disorder: stereotypies (hand flapping), obsessional features |
| Patient 6 6 | Normal (6mo) | 51 cm (7 years, 50th centile) | Severe | Ataxic gait, Movement disorder: stereotypies (hand flapping), inappropriate laughter episodes |
| Patient 7 | Normal (16mo, 15 yr) | 49,5 cm (3,5 years, 25th centile) | Severe | Non-verbal, autistic features |
| Patient 8 | Normal (15 yr) | 41 cm (0.5 years, 15th centile) | Too young to assess | Movement disorder: dyskinesia, inappropriate laughter spells, mild hypotonia |
| Patient 9 26 | Thin posterior corpus callosum (5yr 8mo) | 54 cm (14 years, 50th centile) | Severe | Non-verbal, Autistic features |
Mo = months, yr =years
EEG studies at follow-up showed variable features including generalized spike-wave (GSW) (5), focal or multifocal discharges (2) and background slowing (4) (Table 2). The EEG was normal in patient 9 who was seizure free. Myoclonic seizures were recorded with GSW or GPSW.
Seven of the 9 patients had severe intellectual disability and were non-verbal; one had profound impairment (Table 3). The remaining patient is currently 6 months old so his outcome is unclear. Seven patients could walk. Patients 2 and 4 experienced marked developmental regression during childhood, not related to frequent seizures or epileptiform activity. Two patients had autistic features. Movement disorders were seen in five cases, often comprising stereotypies such as hand-flapping, dyskinesia or athetosis. In three patients inappropriate episodes of laughter were observed, these episodes did not have an EEG correlate in two individuals and were not thought to be epileptic in origin. Brain MRI was normal in 6/9 individuals; findings in the remaining three patients showed cerebellar atrophy (1), hippocampal asymmetry (n=1) and a thin corpus callosum (n=1).
In silico analysis of the p.Glu590Lys pathogenic variant
The p.Glu590Lys missense variant is located within the third alpha helix of the first DNA-binding CUT domain (IPR003350) (Fig 1A). This residue is highly conserved among orthologues (Fig 1B). Moreover, the negative charge at this residue seems particularly important, as it is conserved among all CUT domains, and up to the homologous domain Cro/C1-type helix-turn-helix domain (IPR001387) from Repressor protein cI of bacteriophages lambda (Fig 1C). This variant was predicted to be damaging by several computational tools (SIFT=0.001, Polyphen-2=1, fathmm-MKL_coding_score=0.985, CADD=33.0). Additionally, the SNPs&GO3d tool, which combines structural information from the 1X2L crystal structure and Gene Ontology terms, classified the variant as disease associated.
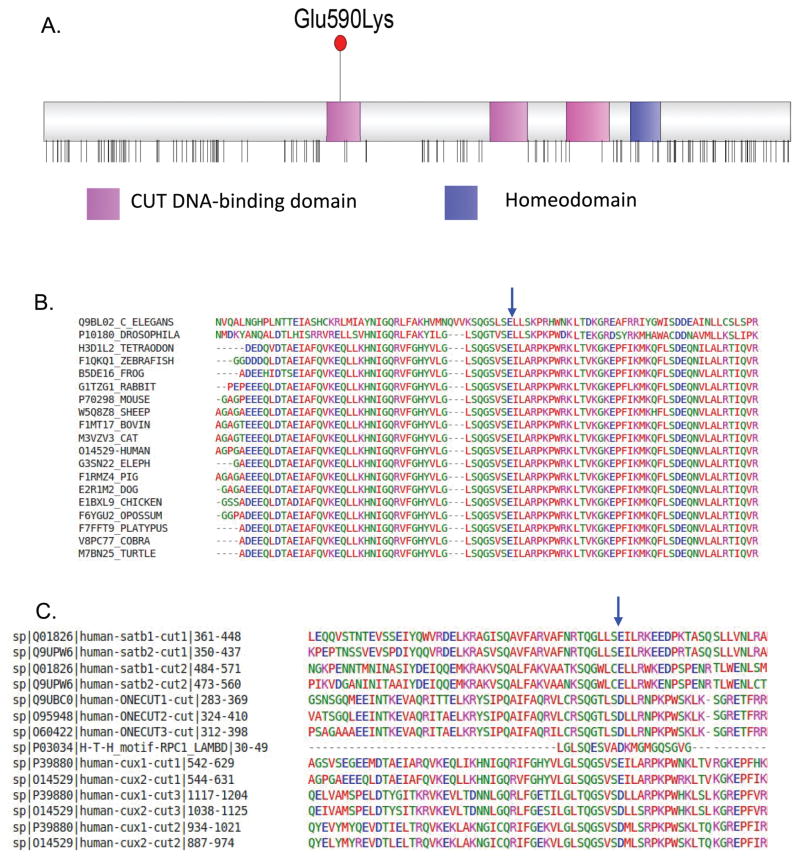
Figure 1. The recurrent Glu590Lys pathogenic missense variant and CUX2 structure.
A. The CUX2 protein consists of three DNA-binding CUT domains (pink) and a single homeodomain (blue). Vertical lines below the protein show the location of missense variants that are reported more than twice in the Gnomad dataset. The CUX2 p.Glu590Lys pathogenic variant lies in the first CUT domain, these CUT domains are largely devoid of missense variants in the general population (data from gnomAD).
B. The glutamine residue at amino acid position 590 (blue arrow) is fully conserved among cut-like Homeobox proteins across 19 species using Clustal alignment. GERP score GERP++=4.770, phyloP100way_vertebrate= 9.998, phastCons100way_vertebrate =1.000).
C. High conservation of a negatively charge amino acid in third helix of all human CUT domains (IPR003350), as well as in related Cro/C1-type helix-turn-helix domain (IPR001387) (blue arrow). Clustal alignment of CUT domains of human ONECUT, SATB and CUX proteins, along with the H-L-H domain of bacteriophage lambda RPC1.
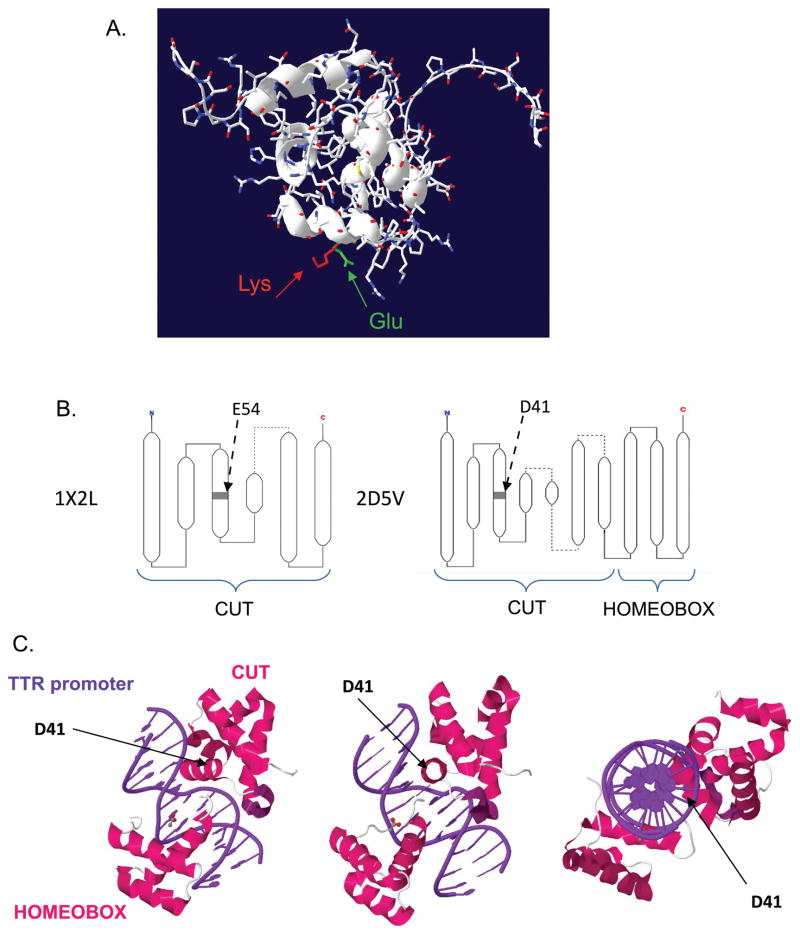
Computational modeling using the available crystal structure for the first CUT domain (1X2L) suggested the substitution causes no major change in the alpha helix (Fig 2A). The conformation of the CUT1 domain of CUX2 relative to the DNA is not yet known. However, the CUT1 domain of human homeobox protein CUX2 (1X2L) has been crystalized. Also the rat HNF6α CUT and Homeobox domains (2D5V) have been crystalized along with the DNA sequence of a specific promoter. The E54 residue (1X2L) and D41 residue (2D5V), homologues of the Glu590 are located in the middle of the third helix (Fig 2B), directly facing the major groove of the DNA helix (Fig 2C).
Figure 2. In silico predictions of the consequences of the p.Glu590Lys.
A. Glu590 in CUT1 domain lays in the 3rd helix of the CUT domain. Sequence Chain View of 1X2L Solution structure of the first CUT domain of human homeobox protein CUX2 (Cut-like 2). Alpha helix is not obviously modified by the Glu to Lys mutation. pdb-viewer superposition of 1X2L and the structure generated by MODELLER mutate_model.py script.
B. Similar position in third helix of E54 in 1X2L structure (CUT domain of human homeobox protein CUX2) and D41 in 2D5V structure (rat HNF-6alpha DNA-binding domain in complex with the TTR promoter), as reported on PDB (http://www.ebi.ac.uk/pdbe/))
C. Three 3D views of 2D5V structure, from http://www.rcsb.org/pdb, showing the position of D41 within the major groove of DNA.
Analysis using the HOPE prediction tool showed that salt bridges are normally formed between amino acid positions 594 and 596 that will be disrupted by the pathogenic variant. The lysine residue is also larger and is positively charged, while wildtype glutamine has a negative charge. Calculations of protein stability using various prediction tools, suggested this substitution could lead to destabilization of CUX2 (CUPSAT: ΔΔG=−0.17 kcal/mol, I-mutant3.0: ΔΔG=−0.85 kcal/mol).
DISCUSSION
In this study, we describe a recurrent de novo missense variant p.Glu590Lys in CUX2 as a novel cause for DEE in the majority of patients. All patients carry the same recurrent missense variant, suggesting an important role for this residue in CUX2 function. Based on the ExAC dataset, CUX2 is generally intolerant to variation; the probability of Loss-of-function intolerance score is very high (pLI= 1), as it is for missense variants (Z-score= 3.61) in CUX2.17 Since the same pathogenic variant has occurred independently in nine patients, and that CUX2 is intolerant to genetic variation, we hypothesize that this amino acid position plays a specific role in the pathophysiologic process. The predominant phenotype was an infantile-onset myoclonic DEE with a phenotypic DEE spectrum that extended to Lennox-Gastaut syndrome and infantile spasms, as well as a static developmental encephalopathy with genetic generalized epilepsy.
Infantile onset of seizures was usual with a median onset of six months. Myoclonic seizures or absence seizures with a myoclonic component were the most frequent type of seizures at onset (5/9). Absence seizures were also prominent, especially in the two cases with developmental encephalopathy, who did not have evidence for an epileptic encephalopathy. This is distinguished by developmental delay leading to intellectual disability, without plateauing or regression of development, and associated with epilepsy1. One had episodes of non-convulsive status epilepticus but these were rare and not associated with developmental slowing; however, it does suggest a more severe form of absence epilepsy, as can be observed in early-onset absence epilepsy (defined as onset under four years)17, 18.
In general, seizures were refractory to multiple AEDs, although two cases were seizure free on valproate. Additional seizure types often evolved and included atypical absences, tonic, and generalized tonic-clonic seizures. Focal seizures were rare.
The majority of patients with the p.Glu590Lys recurrent de novo variant had severe to profound intellectual disability, with developmental regression observed in two cases. The majority were non-verbal but could walk. Additional features included movement disorders with stereotypies such as hand-flapping and dyskinesia, autistic features and inappropriate non-epileptic laughing episodes. This severe cognitive impairment is due to a developmental component, together with a superimposed epileptic encephalopathy in those with a DEE. This developmental component is illustrated by early developmental delay in some cases, prior to seizure onset, and by the two individuals with severe ID without a DEE.
Cux2 and its orthologue Cux1 encode the vertebrate homologs of the Drosophila homeobox DNA-binding transcription factor Cut19, 20. Despite their divergent evolution, Cux1 and Cux2 exhibit high sequence conservation within specific protein regions, notably the Cut repeat domains. Whereas Cux1 is expressed in most tissues, Cux2 is expressed primarily in neuronal tissues during development and continues to be expressed in post-mitotic neurons, where it is a marker of the upper layer II–III neurons21. Cux2−/− knock-out mice had normal cortical organization. Similar to these findings, there were no obvious structural or organizational abnormalities in the majority of patients as brain MRI was normal or showed mild nonspecific features (n=3). However, Cux2−/− knock-out mice displayed decreased dendritic length as well as reduction in the number of branches, density of spines, and synaptic function in the layer II–III neurons22, 23. These abnormal dendrites and synapses in Cux2−/− mice correlate with reduced synaptic function and defects in working memory, though seizures were not reported24. The intellectual disability phenotypes observed in patients may be related to putative abnormal dendritic branching or function, especially in layer II–III neurons of brain cortex, as seen in Cux2 knockout mice.
The CUX2 p.Glu590Lys pathogenic variant is located in the first alpha helix of the CUT domain 1 (CR1). This protein domain plays an important role in the binding of CUX2 to its DNA targets, where it controls gene expression as a transcription factor. Our in-silico analyses point toward a destabilization effect in the presence of the mutant lysine residue. This change to a highly conserved positive charge (lysine) could therefore alter the interaction with the negatively charged DNA’s phosphate backbone. Interestingly no los-of-function (truncating) variants have been observed in patients nor controls, suggesting complete loss of CUX2 function may be lethal. Collectively, these results suggest that the p.Glu590Lys pathogenic variant alters either the stability or specificity of the interaction of CUX2 with the DNA, which is known to be transient25. This may confer a partial loss of function effect for the p.Glu590Lys variant. Alternativeley, another possibility is that this variant confer gain of function or dominant negative properties, in which the mutant protein has an inhibitory interaction on the normal allele, or binds ectopic target DNA regions. In order to determine the functional effect of this missense variant functional studies in CUX2 knockout and the CUX2 p.Glu590Lys variant in vitro and in vivo need to be performed.
Our study highlights the importance of international collaborative efforts to identify novel genes implicated in epilepsy, which are likely to be rare overall and thus require large cohorts of patients to identify recurrent mutations in genes. Moreover, until now, novel gene discovery in epilepsy has largely focused on truncating variants, partly because these variants are easier to interpret in the context of a haploinsufficient model. However, in accordance with a recent study that included molecular data from patients 6 and 9, the present study emphasizes the importance of recurrent missense variation in neurodevelopmental disorders, including intellectual disability and epilepsy26. This observation has also been illustrated in the epilepsies in a recent study that identified a recurrent missense mutation in a more common epilepsy gene, SCN1A, that produced a profound phenotype, far more severe than the well-established Dravet syndrome presentation of this gene7. In addition, the role of missense mutations in DEE, and especially recurrent ones, has been emphysized in another recent paper27. The study of recurrent pathogenic missense variation provides a unique avenue to understand the role of a protein (and its domains) in neuronal development and function. Future functional studies will be important to understand the pathophysiological process of this specific CUX2 pathogenic variant, but also the function of CUX2.
Acknowledgments
The authors would like to thank the families for their participation. We thank the NGS platform of the University Hospital of Lyon (Hospices Civils de Lyon). GLC is supported by NIH NINDS NS089858, and a CURE Taking Flight Award. Work on patient 9 was supported by the Italian Ministry of Health and ‘5 per mille’ funding”. HCM is supported by National Institutes of Health (NINDS grant NS069605). IES is supported by the National Health and Medical Research Council of Australia.
Footnotes
URLS
Clustal-Omega multiple sequence alignment: http://www.ebi.ac.uk/Tools/msa/clustalo/
SIFT: http://sift.jcvi.org/
Polyphen-2: http://genetics.bwh.harvard.edu/pph2/
CADD: http://cadd.gs.washington.edu/
Fathmm: http://fathmm.biocompute.org.uk/
GERP: http://mendel.stanford.edu/SidowLab/downloads/gerp/
HOPE: http://www.cmbi.ru.nl/hope/about
CUPSAT: http://cupsat.tu-bs.de/
denovo-db: http://denovo-db.gs.washington.edu/denovo-db/
AUTHORS CONTRIBUTIONS
NC, GLC and GL contributed to the conception and design of the study. NC, RS, NLC, ALS, AK, AL, TS, LB, CB, EJK, RP, CR, JA, AA, MV, MJM, DM, VdP, PE, DW, EG, IES, DS, GLC and GL contributed to the acquisition and analysis of data. NC, ALS, EG, IES, HM, GLC, GL contributed to drafting the text and preparing the figures.
POTENTIAL CONFLICTS OF INTEREST
Nothing to report.
References
- 1.Scheffer IE. A new classification and class 1 evidence transform clinical practice in epilepsy. Lancet Neurol. 2017 Jan;17(1):7–8. doi: 10.1016/S1474-4422(17)30432-5. [DOI] [PubMed] [Google Scholar]
- 2.Consortium E. A roadmap for precision medicine in the epilepsies. Lancet Neurol. 2015 Dec;14(12):1219–28. doi: 10.1016/S1474-4422(15)00199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lesca G, Rudolf G, Labalme A, et al. Epileptic encephalopathies of the Landau-Kleffner and continuous spike and waves during slow-wave sleep types: genomic dissection makes the link with autism. Epilepsia. 2012;53(9):1526–38. doi: 10.1111/j.1528-1167.2012.03559.x. [DOI] [PubMed] [Google Scholar]
- 4.Philippakis AA, Azzariti DR, Beltran S, et al. The Matchmaker Exchange: a platform for rare disease gene discovery. Hum Mutat. 2015 Oct;36(10):915–21. doi: 10.1002/humu.22858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rauch A, Wieczorek D, Graf E, et al. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet. 2012 doi: 10.1016/S0140-6736(12)61480-9. [DOI] [PubMed] [Google Scholar]
- 6.Allen AS, Berkovic SF, Cossette P, et al. De novo mutations in epileptic encephalopathies. Nature. 2013 Sep 12;501(7466):217–21. doi: 10.1038/nature12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carvill GL, Heavin SB, Yendle SC, et al. Targeted resequencing in epileptic encephalopathies identifies de novo mutations in CHD2 and SYNGAP1. Nat Genet. 2013 Jul;45(7):825–30. doi: 10.1038/ng.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berryer MH, Hamdan FF, Klitten LL, et al. Mutations in SYNGAP1 Cause Intellectual Disability, Autism and a Specific form of Epilepsy by Inducing Haploinsufficiency. Human mutation. 2012 doi: 10.1002/humu.22248. [DOI] [PubMed] [Google Scholar]
- 9.Webb B, Sali A. Protein Structure Modeling with MODELLER. Methods Mol Biol. 2017;1654:39–54. doi: 10.1007/978-1-4939-7231-9_4. [DOI] [PubMed] [Google Scholar]
- 10.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1998 Dec;18(15):2714–23. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 11.Parthiban V, Gromiha MM, Schomburg D. CUPSAT: prediction of protein stability upon point mutations. Nucleic Acids Res. 2006 Jul 01;34(Web Server issue):W239–42. doi: 10.1093/nar/gkl190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capriotti E, Fariselli P, Casadio R. I-Mutant2.0: predicting stability changes upon mutation from the protein sequence or structure. Nucleic Acids Res. 2005 Jul 01;33(Web Server issue):W306–10. doi: 10.1093/nar/gki375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capriotti E, Calabrese R, Fariselli P, Martelli PL, Altman RB, Casadio R. WS-SNPs&GO: a web server for predicting the deleterious effect of human protein variants using functional annotation. BMC genomics. 2013;14( Suppl 3):S6. doi: 10.1186/1471-2164-14-S3-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venselaar H, Te Beek TA, Kuipers RK, Hekkelman ML, Vriend G. Protein structure analysis of mutations causing inheritable diseases. An e-Science approach with life scientist friendly interfaces. BMC Bioinformatics. 2010;11:548. doi: 10.1186/1471-2105-11-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. The Phyre2 web portal for protein modeling, prediction and analysis. Nature protocols. 2015 Jun;10(6):845–58. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sievers F, Wilm A, Dineen D, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molecular systems biology. 2011 Oct 11;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suls A, Mullen SA, Weber YG, et al. Early-onset absence epilepsy caused by mutations in the glucose transporter GLUT1. Ann Neurol. 2009;66(3):415–9. doi: 10.1002/ana.21724. [DOI] [PubMed] [Google Scholar]
- 18.Taylor I, Berkovic SF, Scheffer IE. Genetics of epilepsy syndromes in families with photosensitivity. Neurology. 2013 Apr 2;80(14):1322–9. doi: 10.1212/WNL.0b013e31828ab349. [DOI] [PubMed] [Google Scholar]
- 19.Quaggin SE, Heuvel GB, Golden K, Bodmer R, Igarashi P. Primary structure, neural-specific expression, and chromosomal localization of Cux-2, a second murine homeobox gene related to Drosophila cut. J Biol Chem. 1996 Sep 13;271(37):22624–34. doi: 10.1074/jbc.271.37.22624. [DOI] [PubMed] [Google Scholar]
- 20.Sansregret L, Nepveu A. The multiple roles of CUX1: insights from mouse models and cell-based assays. Gene. 2008 Apr 15;412(1–2):84–94. doi: 10.1016/j.gene.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Yamada M, Clark J, McClelland C, Capaldo E, Ray A, Iulianella A. Cux2 activity defines a subpopulation of perinatal neurogenic progenitors in the hippocampus. Hippocampus. 2014 Feb;25(2):253–67. doi: 10.1002/hipo.22370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cubelos B, Sebastian-Serrano A, Kim S, et al. Cux-2 controls the proliferation of neuronal intermediate precursors of the cortical subventricular zone. Cerebral cortex (New York, NY: 1991) 2008 Aug;18(8):1758–70. doi: 10.1093/cercor/bhm199. [DOI] [PubMed] [Google Scholar]
- 23.Cubelos B, Sebastian-Serrano A, Kim S, Redondo JM, Walsh C, Nieto M. Cux-1 and Cux-2 control the development of Reelin expressing cortical interneurons. Developmental neurobiology. 2008 Jun;68(7):917–25. doi: 10.1002/dneu.20626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cubelos B, Sebastian-Serrano A, Beccari L, et al. Cux1 and Cux2 regulate dendritic branching, spine morphology, and synapses of the upper layer neurons of the cortex. Neuron. 2010 May 27;66(4):523–35. doi: 10.1016/j.neuron.2010.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gingras H, Cases O, Krasilnikova M, Berube G, Nepveu A. Biochemical characterization of the mammalian Cux2 protein. Gene. 2005 Jan 3;344:273–85. doi: 10.1016/j.gene.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Geisheker MR, Heymann G, Wang T, et al. Hotspots of missense mutation identify neurodevelopmental disorder genes and functional domains. Nat Neurosci. 2017 Aug;20(8):1043–51. doi: 10.1038/nn.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamdan FF, Myers CT, Cossette P, et al. High Rate of Recurrent De Novo Mutations in Developmental and Epileptic Encephalopathies. Am J Hum Genet. 2017 Nov 2;101(5):664–85. doi: 10.1016/j.ajhg.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]