Abstract
BACKGROUND
Chronic transfusion therapy (CTT) for sickle cell anemia (SCA) reduces disease complications by diluting sickle-erythrocytes with hemoglobin A (HbA)-containing erythrocytes and suppressing erythropoiesis. Minor antigen mismatches may result in alloimmunization, but it is unknown if antigen mismatches or recipient characteristics influence HbA clearance post-transfusion.
STUDY DESIGN AND METHODS
Children with SCA on CTT were followed prospectively for 12 months. All patients received units serologically matched for C/c, E/e, and K; patients with prior red blood cell (RBC) antibodies had additional matching for Fya, Jkb, and any previous alloantibodies. Patients’ RBC antigen genotypes, determined by PreciseType Human Erythrocyte Antigen (HEA), RHCE and RHD BeadChip assays, were compared to genotypes of transfused RBC units to assess for antigen mismatches. Decline in HbA (ΔHbA) from post-transfusion to the next transfusion was calculated for each transfusion episode.
RESULTS
Sixty patients received 789 transfusions, 740 with ∆HbA estimations, and 630 with donor HEA genotyping. In univariate mixed model analysis, ΔHbA was higher in patients with past RBC antibodies or splenomegaly and lower with in patients with splenectomy. RBC antigen mismatches were not associated with ∆HbA. In multivariate linear mixed effects modeling, ΔHbA was associated with RBC antibodies (2.70 vs. 2.45 g/dL/28 days, p=0.0028), splenomegaly (2.87 vs. 2.28 g/dL/28 days, p=0.019), and negatively associated with splenectomy (2.46 vs. 2.70 g/dL/28 days, p=0.011). CONCLUSIONS: HbA decline was increased among SCA patients with prior immunologic response to RBC antigens and decreased among those with prior splenectomy, demonstrating that recipient immunologic characteristics influenced the clearance of transfused RBC.
Keywords: Sickle cell anemia, chronic transfusion therapy, alloimmunization, splenectomy
INTRODUCTION
Chronic transfusion therapy (CTT) for sickle cell anemia (SCA) is an essential treatment for the prevention of severe sickle complications such as stroke.1–3 CTT is effective at preventing red blood cell (RBC) sickling by two mechanisms: 1) with frequent transfusions, non-sickle hemoglobin A (HbA)-containing erythrocytes predominate in the circulation compared to endogenous RBC, and 2) by maintaining higher hemoglobin (Hb) levels, endogenous erythropoiesis of sickle erythrocytes is suppressed. The effectiveness of CTT is monitored by measuring the percentage of sickle Hb (HbS), total Hb, and reticulocytes prior to each transfusion.4
HbS level is influenced by several factors, including erythopoietic drive, hemolysis of endogenous sickle erythrocytes, and the clearance of transfused RBCs. Individual variation in HbS suppression exists. Some patients achieve desired Hb and HbS goals with simple transfusions monthly, while others have poor suppression of HbS, necessitating either an increase in transfusion volume or frequency or implementation of exchange transfusion therapy.5,6 Better understanding of factors that influence the kinetics of transfused RBC clearance in CTT patients could facilitate development of transfusion practices that more effectively suppress HbS.
Patients with SCA have a high prevalence of RBC alloimmunization ranging from 18 to over 45%.7–10 Alloimmunization can result in hemolytic transfusion reactions (HTRs) and limits the supply of compatible RBC units for future transfusions.10–12 Although antigen matching for C/c, E/e, K, and potentially other antigens has been shown to reduce the rate of alloimmunization in SCA,13 both alloimmunization and HTRs remain prevalent problems among patients with SCA.14 RBC genotyping of recipients and donors offers several advantages over serologic antigen typing in attempts to minimize alloimmunization, including the identification of a greater number of antigens for which anti-sera are not available (e.g. Jsa/Jsb, Kpa/Kpb, V, VS), and the ability to obtain accurate antigen typing in patients with recent RBC transfusion or with RBC alloantibodies or autoantibodies.15
HTRs are an extreme example of rapid clearance of transfused RBCs, mediated by antibody production and complement activation. Although HTRs and alloimmunization events result in increased HbA clearance, it is not known if RBC antigen mismatches independently influence HbA clearance in the absence of serologically-detected RBC antibodies. Retrospective analysis of transfusion requirements during myeloablative bone marrow transplantation in SCD have suggested that increased antigen matching was associated with decreased transfusion volumes, suggesting improved RBC survival with antigen matching.16 The aims of this study were to: (1) determine the rate of clearance of donor HbA-containing erythrocytes following transfusions in children with SCA on CTT, and (2) determine the associations of HbA clearance with RBC minor antigen mismatches (determined by patient and donor RBC genotyping), recipient immunologic characteristics, and donor unit characteristics.
METHODS
A prospective observational study of children ages 3 – 20 years with SCA (HbSS) on CTT for at least 6 months was conducted at Children’s Healthcare of Atlanta (CHOA) and Children’s National Medical Center (CNMC). Written, informed consent and assent were obtained, and the study was approved by the Institutional Review Boards of CHOA and CNMC. Participants who were receiving monthly CTT were followed for a 12 – 17 month period to observe sequential hemoglobin (including HbA and HbS) and reticulocyte values as well as to observe characteristics of the transfused units, including RBC genotype and age of the blood units. Patients were included if they were receiving simple transfusions (ST) or partial manual exchange (PME), defined as removal of up to 10 mL/kg of whole blood immediately prior to a simple RBC transfusion. Patients were excluded if they received erythrocytapheresis (chronic or emergent) or had poor adherence to transfusion appointments (defined as delay in transfusion by ≥7 days on ≥3 occasions prior to or during study). Patients were also excluded if they received transfusion outside of the study institution during the study period, as determined by patient-reported history and pre-transfusion laboratory values compared to transfusion interval.
RBC genotyping of patients was performed with PreciseType Human Erythocyte Antigen (HEA) Molecular BeadChip assay and with RHCE and RHD BeadChip assays (Immucor, Norcross, GA) to identify single nucleotide polymorphisms (SNPs) associated with 35 minor antigens, >35 RHCE variants, and >80 RHD variants. For donor unit genotyping, genomic DNA was extracted from segments of leukoreduced (LR) RBC units and tested using the prototype HEA-LR BeadChip assay (Immucor, Norcross, GA) to detect the same profile of RBC antigens as the PreciseType HEA assay, as previously described.17,18 An antigen mismatch was defined as a recipient exposure to a RBC minor antigen that the recipient does not express, i.e. transfusion of an antigen-positive unit to an antigen-negative patient. RH genotypes were categorized as either conventional (homozygous or simple heterozygous) or variant (homozygous or compound heterozygous for variant alleles). Transfused units were assumed to have conventional Rh antigen expression unless denoted as homozygous for ce(733G) with/without 1006T by the HEA-LR assay.
Electronic medical records and the blood bank Laboratory Information Systems of CHOA and CNMC were reviewed to identify subjects’ CTT start date, history of splenectomy, splenomegaly present on physical exam at the time of transfusion, and RBC antibody histories including antibodies detected at either the current or previous institutions. At CHOA and CNMC, all RBC units for SCD patients were HbS negative, pre-storage leukoreduced, and serologically matched for C/c, E/e, and K antigens (category 1 matching). For patients with ≥1 clinically significant alloantibody or some cases of warm autoantibodies, RBC units were selected by extended serological matching for Fya and Jkb in addition to being antigen-negative for the putative antibodies (category 2 matching). Transfusion volume and frequency were determined by local institutional protocol, in which patients receive 10 – 15 mL/kg of packed RBC (1 – 3 RBC units) per transfusion, based on patient body weight and pre-transfusion Hb, for both ST and PME transfusions.
Post-Transfusion Donor Hemoglobin A calculation
Pre-transfusion Hb electrophoresis, including the percentage of HbA1 and any donor variant Hb (HbV) (e.g. HbC or others) was recorded for all transfusion episodes. The total amount of donor Hb was calculated as the sum of HbA1 and HbV; for simplicity, donor Hb was expressed as “HbA” where HbA (g/dL) = Hb (g/dL) × (HbA1% + HbV%). In a subset (approximately 20%) of the transfusion episodes, Hb electrophoresis was obtained at 15-30 minutes post-transfusion in order to directly measure the post-transfusion HbA. For transfusion episodes in which post-transfusion Hb electrophoresis was not measured, post-transfusion HbA was calculated based on the volume of transfusion (VolT, mL), estimated unit hematocrit (unit Hct, %), body weight (Wt, kg), phlebotomy volume (VolPh, mL, if applicable) and total blood volume (TBV, mL), using the following equation:
19,20HbA decline (ΔHbA, g/dL/28 days) was calculated as the difference between the estimated post-transfusion HbA and the pre-transfusion HbA of the subsequent transfusion episode, divided by the transfusion interval (number of days between HbA measurements), then multiplied by 28 to normalize all values to the change in HbA per 4 weeks. The unit Hct was estimated based on quality analysis data from each blood supplier for the unit’s preservative solution (citrate-phosphate-dextrose-adenine (CPDA), additive solution (AS), or frozen/deglycerized). If the blood supplier range of estimated unit Hct had a range of >10%, the post-transfusion HbA and thus ∆HbA were not estimated, and those units were excluded from analysis due to potentially high inaccuracy of the ∆HbA estimation.
Antigen Mismatches
For each transfusion episode, an antigen mismatch was defined as the number of units mismatched divided by the total number of units transfused (e.g. in a transfusion episode of 3 units, if an antigen was mismatched in 1 unit, the mismatch for that transfusion episode was 0.33 or 33% of the transfused RBC were mismatched for that antigen). In addition to individual antigens, mismatches were examined per blood group, in which the mismatch per group was the sum of the individual antigen mismatches (e.g. Rh mismatches were the sum of V, VS, D, C/c, E/e mismatches per transfusion). Antigen groups were excluded from analysis if the frequency of mismatch for all of antigens in that group was <2% (e.g. Diego, Landsteiner-Weiner, Sciana)
Statistical Analysis
For transfusions in which post-transfusion laboratory measurements were done, measured post-HbA was compared to estimated post-HbA by Bland-Altman limits of agreement and intraclass correlation coefficient (ICC). Bias was assessed as the median difference between measured and estimated HbA, and precision was assessed as the interquartile range (IQR) of the bias. Accuracy was assessed as the percentage of HbA estimates that were within 10% (P10) and 15% (P15) of the measured HbA. Intra-patient variance in ∆HbA was assessed as the IQR of ∆HbA per patient; due to non-normality of the data, the IQR of ∆HbA was compared to RBC antibody status by Wilcoxon-Rank-Sum test.
At the patient level, univariate comparisons of RBC antibody status were made by Student’s t-test for continuous variables or by chi-squared test for categorical variables. At the transfusion episode level, univariate comparisons of RBC antibody status with transfusion characteristics and pre-transfusion laboratory values were done by linear mixed modeling to account for repeated measures on identical patients. Mixed effects modeling was used in all analyses of transfusion episodes to account for both random effects (individual patients and transfusion episodes) and the fixed effects of interest (patient and transfusion characteristics). Thus, mixed effects modeling can account for repeated measures (i.e. multiple transfusions) per patient, allowing for generalizability of the analysis. Least-squares means (ls-means) were used to compare continuous variables with repeated measures.
Univariate associations of ∆HbA with categorical variables (splenectomy, splenomegaly, and prior RBC antibodies) and with continuous variables (age, unit age, transfusion volume, total antigen mismatches, and the Hb, HbS, and reticulocyte values prior to the next transfusion) were determined by linear mixed modeling. The effects of individual blood group mismatches on ∆HbA also were assessed by linear mixed modeling, stratifying by category 1 vs. 2 to account for the inherent differences in antigen matching between these 2 groups of transfusion populations. Variables with p≤0.20 in univariate analysis were introducted into multivariable linear mixed modeling to examine the effect of patient and transfusion characteristics on ∆HbA; additionally, variables with potential interaction were introduced into the model.
RESULTS
There were 60 SCA patients (24 with past RBC alloimmunization or RBC autoantibodies) who received 789 transfusions during the study. Of these, 49 transfusions were excluded for the inability to estimate ∆HbA (due to either lack of pre-transfusion HbA measurement or estimated unit Hct), and 110 transfusions in which ≥1 unit was missing RBC genotyping. Thus 630 transfusions (395 category 1 antigen matching, 235 category 2) were available to assess the relationships between ∆HbA and antigen mismatches (Figure 1).
Figure 1.
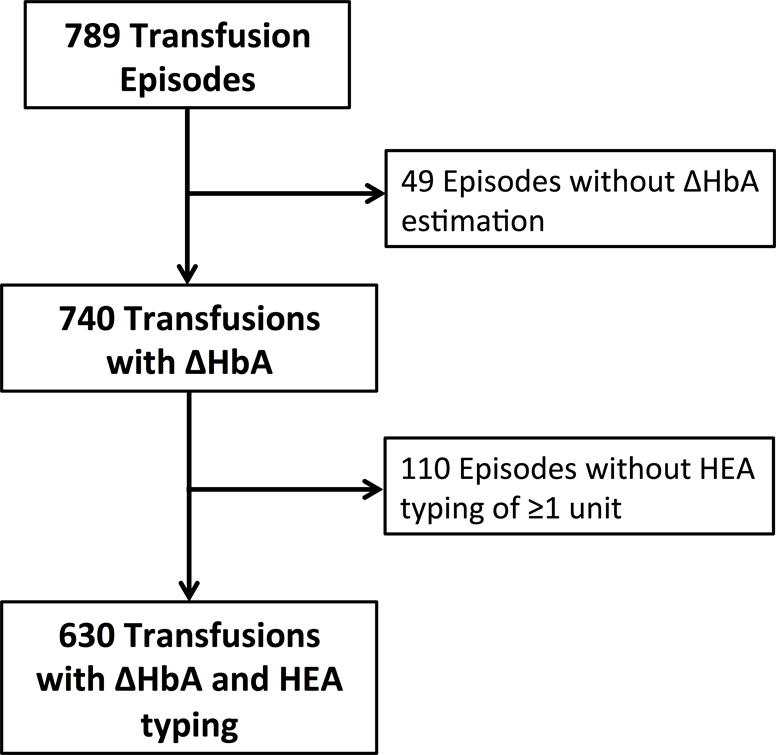
Flow Diagram of transfusion episode inclusion and exclusion.
Characteristics of the 60 patients and 789 transfusion episodes are shown in Table 1. Patients with past RBC antibody response (category 2 antigen matching) were significantly older (12.5 years vs. 10.2 years, p=0.017), and received units with a younger mean age (17.6 days vs. 20.5 days, p=0.0018), but did not have differences in splenectomy or splenomegaly frequencies. The median age of RBC units was 18 days (range 3 – 42 days), with 65% of units exceeding 14 days, 32% exceeding 21 days, and 13% exceeding 28 days.
Table 1.
Characteristics of SCA patients and 789 transfusion episodes.
Comparison of patients without past RBC antibodies vs. those with past RBC alloantibodies or autoantibodies.
| No RBC Antibodies (Category 1 Matching) | RBC Antibodies (Category 2 Matching) | p | |
|---|---|---|---|
| Patients | N=36 (36%) | N=24 (40%) | |
| Age (years), mean (SD) | 10.2 (3.8) | 12.5 (3.3) | 0.017 |
| Gender, male | 19 (53%) | 12 (50%) | 0.83 |
| CTT duration (years), mean (SD) | 4.7 (3.2) | 5.8 (3.5) | 0.22 |
| Splenectomy (%) | 7 (19.4%) | 5 (25.0%) | 0.61 |
| Splenomegaly (%) | 1 (2.8%) | 1 (4.2%) | 1.00 |
| Transfusions | N=488 | N=301 | |
| Antibody Screen positive (%) | 0 (0%) | 25 (8.3%) | – |
| Mean age of units per transfusion (days), LS-mean (SE) | 20.5 (0.59) | 17.6 (0.74) | 0.0018 |
| Volume of transfusion (mL/kg), LS-mean (SE) | 12.7 (0.25) | 13.1 (0.32) | 0.31 |
| Transfusion interval (days), LS-mean (SE) | 29.9 (0.55) | 29.2 (0.69) | 0.38 |
| Pre-transfusion Laboratory Values | |||
| Hb (g/dL), LS-mean (SE) | 9.35 (0.12) | 9.36 (0.14) | 0.97 |
| HbS (%), LS-mean (SE) | 23.5 (1.4) | 23.8 (1.7) | 0.84 |
| Reticulocytes (%), LS-mean (SE) | 9.9 (0.58) | 10.6 (0.70) | 0.35 |
| Reticulocytes, absolute (10^9/L), LS-mean (SE) | 309.8 (15.5) | 325.8 (18.9) | 0.47 |
CTT = chronic transfusion therapy; Hb = hemoglobin; HbS = sickle hemoglobin; LS-mean = least-squares mean; PME = partial manual exchange transfusion; SD = standard deviation; SE = standard error; ST = simple transfusion.
Post-Transfusion HbA Estimation
There were 217 (27.5%) transfusions (183 simple, 34 PME) in which post-transfusion HbA was both measured and estimated. The mean post-transfusion HbA was 9.64 g/dL (range 6.28 – 13.1 g/dL) by direct measurements vs. 9.32 g/dL (range 5.65 – 12.8 g/dL) by estimation. Estimated HbA had a negative bias of 0.32 g/dL (95% C.I. 0.23, 0.41), with a precision of 0.81 g/dL (95% C.I. 0.61, 1.09). The intraclass correlation coefficient between estimated and measured HbA was 0.75, indicating that the proportion of between-cluster (i.e. patient) variance to the total variance was high. Accuracy of the estimated HbA within 10% of measured HbA was 82.5% (P10) and within 15% of measured HbA was 93.6% (P15). Bland-Altman limit of agreement plot for measured vs. estimated post-transfusion HbA is shown in Figure 2.
Figure 2.
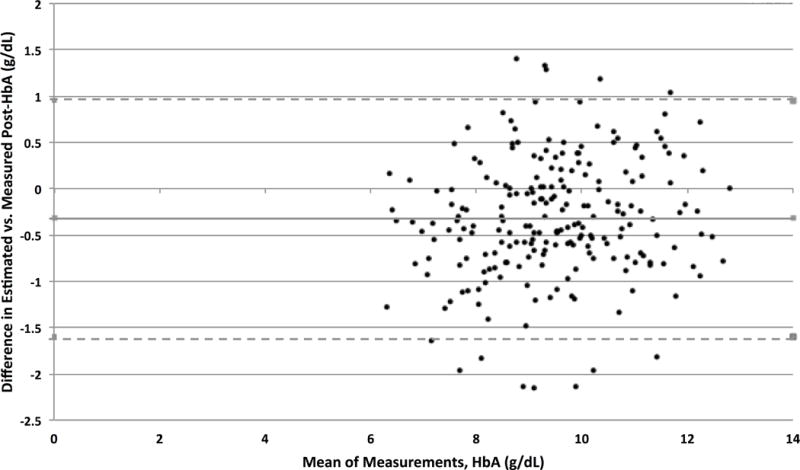
Bland-Altman limit of agreement plot for measured vs. estimated post-transfusion HbA.
HbA Decline (ΔHbA)
There were 740 transfusion episodes with ∆HbA estimations. Intra-patient variability in ∆HbA, demonstrated by the IQR of ∆HbA, is shown in figure 3. The ∆HbA IQR for all patients ranged 0.36 to 2.12 g/dL/28 days, with a median IQR of 0.80 g/dL/28 days. Intra-patient variability in ∆HbA was not different for patients with and without past antibody response (median IQR 0.86 vs. 0.73 g/dL/28 days, p=0.37).
Figure 3.
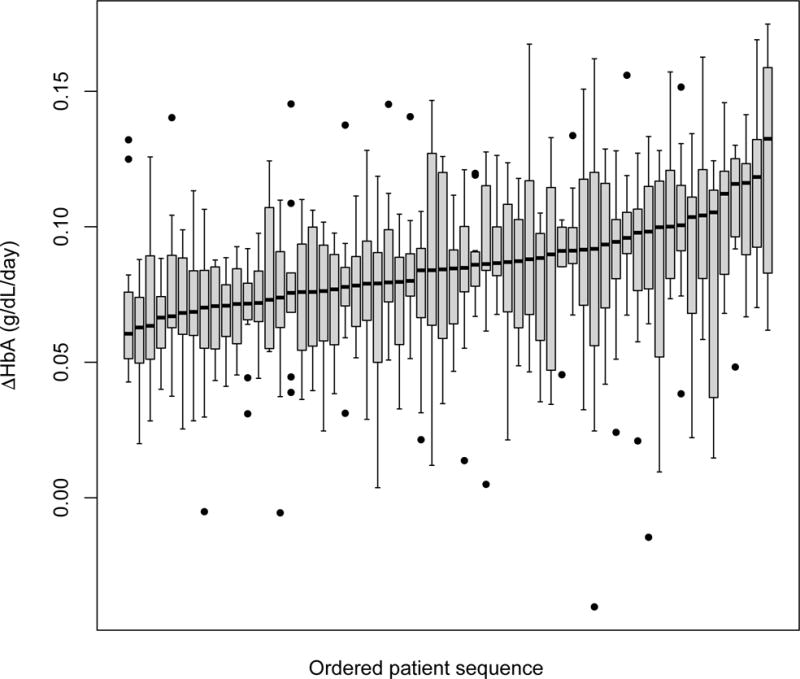
Intra-patient variability in ∆HbA. Box plots represent individual patients, demonstrating the interquartile range in ∆HbA following each transfusion episode.
In univariate mixed effects analyses, ∆HbA was significantly lower in patients with splenectomy than those without splenectomy (ls-means 2.14 vs. 2.40 g/dL/28 days, p=0.014) and was higher in those with splenomegaly (ls-means 3.01 vs. 2.33 g/dL/28 days, p=0.010). The ls-mean ∆HbA was 2.45 mg/dL/28 days for patients with past RBC antibodies vs. 2.28 g/dL/28 days for those without RBC antibodies (p=0.06). ∆HbA was not associated with the mean age of blood per transfusion (p=0.37) nor the maximum age of a unit in a transfusion (p=0.70).
Increased ∆HbA was associated with lower total Hb (p<0.0001), higher HbS percentage (p<0.0001), and higher reticulocyte count (p<0.0001) prior to the next transfusion episode, demonstrating the association of ΔHbA with suppression of erythropoiesis.
Antigen Mismatches
For the 630 transfusions with donor antigen genotyping, the mean number of antigen mismatches was 3.3 (range 0 – 9 mismatches), with a mean of 3.7 mismatches for category 1 and 2.6 mismatches for category 2 transfusions (p<0.0001). The frequency of individual antigen mismatches per transfusion episode has been previously described.21 In univariate mixed effects analyses, stratified by antigen-matching category, the total number of antigen mismatches was not associated with ∆HbA for category 1 transfusions (p=0.097) or category 2 transfusions (p=0.39). There were no significant associations between specific antigen mismatches or blood group mismatches (Rh, Kell, Duffy, Kidd, M/N and S/s/U, Lutheran, Colton, and Dombrock groups) and ∆HbA while clustering on each patient, as assessed by linear mixed models and also accounting for patients’ RBC antibody status (Table 2).
Table 2.
Univariate Mixed Modeling Analysis of blood group mismatch effect on ∆HbA for category 1 and category 2 transfusions.
| Blood Group Mismatch | Category 1 Transfusions | Category 2 Transfusions | ||
|---|---|---|---|---|
| Parameter Estimate | p | Parameter Estimate | p | |
| RH | −0.039 | 0.48 | 0.0277 | 0.75 |
| KELL | −0.140 | 0.43 | −0.357 | 0.23 |
| DUFFY | −0.0316 | 0.75 | n/a | – |
| KIDD | −0.0083 | 0.94 | 0.354 | 0.18 |
| M,N | 0.0589 | 0.58 | 0.180 | 0.25 |
| S/s,U | 0.141 | 0.125 | 0.084 | 0.514 |
| LUTH | −0.141 | 0.50 | −0.460 | 0.30 |
| COLT | 0.128 | 0.57 | 0.178 | 0.59 |
| DOM | 0.0254 | 0.83 | −0.172 | 0.28 |
Blood group mismatches were calculated as the sum of individual antigen mismatches within each blood group. Individual antigen mismatches were calculated as the fraction of the transfusion episode mismatched (i.e. the number of units mismatched divided by the number of units in the transfusion episode).
New Alloimmunization Events
There were 5 new alloantibodies detected after 4 transfusion episodes during the study period: 1 with anti-Jsa and anti-Wra detected after the same transfusion, 1 with anti-Jsa, 1 with anti-Goa, and 1 with antibody of undetermined specificity (Table 3). There was no difference in median ΔHbA for transfusions with new RBC antibodies vs. transfusions without new alloimmunization (median 2.31 vs. 2.36 g/dL/28 days, p=0.91). Although there were no delayed hemolytic transfusion reactions identified during the study, the ΔHbA for the transfusion associated with 2 new alloantibodies was high, representing the 96th percentile for the range of ΔHbA for all transfusions in the study.
Table 3.
Transfusion episodes associated with new RBC alloimmunization.
| # Prior RBC antibodies | CTT Duration (years) | New RBC Antibody | ΔHbA (g/dL/28 days) | ΔHbA Percentile |
|---|---|---|---|---|
| 1 | 6.9 | Jsa | 1.45 | 13% |
| 3 | 1.8 | Goa | 1.88 | 26% |
| 5 | 10 | Jsa, Wra | 3.93 | 96% |
| 0 | 6.1 | AUS | 2.73 | 69% |
Multivariate Mixed-Effects Modeling
Since the age of blood was significantly lower in patients with past RBC antibodies, both RBC antibodies and age of blood were jointly assessed in a multivariable linear mixed model. There was a significant effect of RBC antibodies on ∆HbA (p=0.021) but no significant effects observed among either the mean unit age per transfusion (p=0.10) or the maximum age of any unit per transfusion (p=0.20). Thus, when controlling for age of the units, patients with past RBC antibody response had significantly higher ∆HbA. In multivariate linear mixed modeling (Table 4), past RBC antibody response (p=0.0028) and splenomegaly (p=0.019) were positively associated with ∆HbA, while splenectomy (p=0.011) was negatively associated with ∆HbA.
Table 4.
Multivariate Linear Mixed Modeling of ∆HbA.
| Variable | Parameter Estimate | Least−Squares Means ΔHbA (g/dL/28 days) |
p | |
|---|---|---|---|---|
| Age of patient (years) | −0.018 | 0.10 | ||
| Unit age (days) | 0.0037 | 0.38 | ||
| Past RBC Antibody Response (Category 2 Matching) | 0.248 | No Yes |
2.45 2.70 |
0.0028 |
| Splenectomy | −0.241 | No Yes |
2.70 2.46 |
0.011 |
| Splenomegaly | 0.585 | No Yes |
2.28 2.87 |
0.019 |
DISCUSSION
The ability of CTT to prevent complications of SCA is dependent upon maintaining a low percentage of circulating HbS-containing erythrocytes, which in turn is dependent on the rates of clearance of both endogenous and transfused RBC from circulation. As demonstrated within this study, the clearance of transfused RBC (reflected by ∆HbA) is highly variable, both among different patients and among sequential transfusions within the same patient. Elucidating the factors that influence clearance of transfused RBC is important to ultimately determine if certain characteristics of RBC units can be selected to minimize HbA decline and therefore optimize transfusion therapy to SCA patients.
In this study we showed significantly greater decline in HbA in patients with prior antibody response to RBC antigens, while controlling for the age of the transfused units. Although patients with past RBC antibodies received transfusions that were matched for both the existing antibodies and for a greater number of RBC antigens (to prevent induction of more RBC alloantibodies), neither the total number of antigen mismatches nor specific groups of antigen mismatches showed significant association with ∆HbA. Thus the increased ∆HbA in patients with prior RBC antibodies does not appear to be related to the specific antigen matches, but rather to the existence of a prior immune response to transfused erythrocytes. Patients with SCA with alloimmunization are known to have an increased risk for future alloimmunization,22 even with more stringent antigen matching.18 These findings strongly suggest that these “responder” patients have different immunologic responses to RBC antigens than “non-responder” patients who have not yet demonstrated RBC antibodies.
Patients with a history of RBC alloimmunization are at risk for HTR, an immune-mediated event in which transfused RBC (and sometimes bystander, autologous RBC) are rapidly cleared. While the consequences of antibody interactions with RBCs are beginning to be characterized,23–27 less is known about possible other mediators of donor RBC clearance in the absence of detectable HTR. In patients with decreased survival of transfused RBC, it is possible that non-humoral immune responses or evanescent antibodies that are undetected by antibody-screening techniques have a role in facilitating RBC clearance, as evidenced by high proportions of delayed HTR with undetectable antibodies.28–31 We found that ∆HbA was higher in the patients with splenomegaly while lower in patients with splenectomy, demonstrating variability in the rate of RBC clearance by the reticuloendothelial system during CTT.32–34 Further, we demonstrated that ∆HbA does directly impact CTT outcomes, as increased HbA clearance leads to lower pre-transfusion Hb, higher HbS, and higher reticulocytes. Although the factors that dictate RBC alloimmunization continue to be an area of active investigation35–40, as patients with RBC antibodies have increased HbA clearance, it is possible that increased removal of transfused RBC may reflect increased RBC uptake by immune cells that are uniquely poised to facilitate RBC alloimmunization.41 Conversely, in a murine model of alloimmunization, donor RBC that were subjected to pre-transfusion oxidant stress to cause rapid post-transfusion clearance by the RES were significantly more immunogenic than donor RBC with longer post-transfusion survival, suggesting that clearance rate by the RES influence alloimmunization respones.42 Additional studies are certainly needed to determine if accelerated RBC removal predicts RBC alloimmunization, if it is a consequence of RBC alloimmunization, or if it is only correlative and not directly related to RBC alloimmunization.
A striking finding of the study was the intra-patient variability in ∆HbA, which suggests that characteristics of the donor and/or stored RBC unit may have significant influence on the clearance of transfused RBC apart from recipient immunologic characteristics. Antigen mismatches between donor and recipient (whether analyzed as individual antigens or aggregated blood group mismatches) showed no association with ∆HbA. The storage age of the transfused units was not associated with HbA decline following transfusion, suggesting that in vivo survival of RBC was not influenced by storage for the range of unit ages in this study. This analysis was not designed to assess the effect of the oldest units on HbA decline and included a full spectrum of ages from 3 – 42 days (median 18 days), with few units reaching the maximum allowable storage time of 42 days. Category 2 matched units were significantly younger than category 1 units in this study. This may be related to the need to identify repeat donors who meet the specific antigen-matching requirements of the category 2 patients. As antigen mismatches and unit age were not significant predictors of ∆HbA, further donor characteristics should be investigated to determine the impact of in vivo survival of transfused RBC, including metabolomics profiles of donor RBC that may be associated with RBC survival and response to oxidative stress.
A limitation of this study was the lack of direct measurement of post-transfusion HbA for the majority of transfusions and the lack of direct measurement of transfused RBC survival by labeling techniques such as biotin or chromium-51 labeling of transfused RBC. While the post-transfusion HbA estimates showed high accuracy and low bias, the use of estimated (rather than directly measured) unit Hct to estimate post-transfusion HbA did introduce error, with some post-HbA estimates demonstrating low precision. The need to estimate post-transfusion HbA thus does introduce some error and bias into ∆HbA, the primary outcome of interest. To account for this bias, estimated post-HbA (not measured post-HbA) was used to calculate ∆HbA in all transfusion episodes, in order to provide a uniform approach. While ∆HbA correlates appropriately with subsequent Hb and reticulocyte counts, it is a surrogate for the true outcome of interest which is the clearance of transfused RBC. Transfused RBCs remain detectable in vivo for up to 4 months post transfusion, thus the decline in HbA in a given time interval reflects the clearance of RBC from multiple recent transfusions, not solely the most recent one. Direct measurement of RBC survival, with labeling of RBC units prior to transfusion, would provide a more accurate reflection of transfusion clearance. Another limitation is the lack of exact spleen measurements by radiologic imaging, which would provide a more sensitive measure of spleen presence and enlargement than physical exam. Lastly, this study focuses on children and adolescents with SCA, who potentially could have different kinetics of RBC clearance than adults, given possible differences in splenic function and erythropoietic drive in children as compared to adults; therefore the aims of this study bear replication in adult SCA populations.
In summary, HbA decline following chronic transfusion episodes in children with SCA had inter-patient and intra-patient variability over 12 months of transfusion episodes. This variability suggests that HbA decline is influenced by multiple donor and recipient characteristics. While the overall number of RBC antigen mismatches did not have a significant effect on HbA decline, patients who had previously developed RBC antibodies and received transfusions with extended antigen matching had higher HbA decline than patients without past RBC antibodies. Decline in HbA was lower in patients with splenectomy, while 2 patients with splenomegaly demonstrated higher HbA decline. Immunologic and RES characteristics of SCA patients thus appear to influence the clearance of transfused RBC, even in the absence of detectable RBC antibody.
Acknowledgments
This work was supported by an unrestricted grant from Immucor and by Cooperative Agreement U58 DD001138, funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or the Department of Health and Human Services. Data management through REDCap was supported by grant UL1 TR000424. The authors acknowledge the Children’s Healthcare of Atlanta and Emory University Pediatric Biostatistics Core for support in data analysis. The authors thank Christopher Lough, MD of LifeSouth Community Blood Centers and Jose Lima, MD of American Red Cross for their help in providing donor RBC genotype results.
Support: This work was supported by an unrestricted grant from Immucor, and by Cooperative Agreement U58 DD001138, funded by the Centers for Disease Control and Prevention. Data management through REDCap was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award UL1 TR000424.
Footnotes
Conflict of Interest: Marianne E. M. Yee has served as a speaker for Immucor; Ross M. Fasano has served as an advisory board member for Immucor; Cassandra D. Josephson has served as a consultant for Immucor. All other authors have no conflicts of interest.
References
- 1.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. The New England journal of medicine. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 2.Lee MT, Piomelli S, Granger S, et al. Stroke Prevention Trial in Sickle Cell Anemia (STOP): extended follow-up and final results. Blood. 2006;108:847–52. doi: 10.1182/blood-2005-10-009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pegelow CH, Adams RJ, McKie V, et al. Risk of recurrent stroke in patients with sickle cell disease treated with erythrocyte transfusions. The Journal of pediatrics. 1995;126:896–9. doi: 10.1016/s0022-3476(95)70204-0. [DOI] [PubMed] [Google Scholar]
- 4.Chou ST, Fasano RM. Management of Patients with Sickle Cell Disease Using Transfusion Therapy: Guidelines and Complications. Hematol Oncol Clin North Am. 2016;30:591–608. doi: 10.1016/j.hoc.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Fasano RM, Leong T, Kaushal M, Sagiv E, Luban NL, Meier ER. Effectiveness of red blood cell exchange, partial manual exchange, and simple transfusion concurrently with iron chelation therapy in reducing iron overload in chronically transfused sickle cell anemia patients. Transfusion. 2016;56:1707–15. doi: 10.1111/trf.13558. [DOI] [PubMed] [Google Scholar]
- 6.Koehl B, Sommet J, Holvoet L, et al. Comparison of automated erythrocytapheresis versus manual exchange transfusion to treat cerebral macrovasculopathy in sickle cell anemia. Transfusion. 2016;56:1121–8. doi: 10.1111/trf.13548. [DOI] [PubMed] [Google Scholar]
- 7.Rosse WF, Gallagher D, Kinney TR, et al. Transfusion and alloimmunization in sickle cell disease. The Cooperative Study of Sickle Cell Disease. Blood. 1990;76:1431–7. [PubMed] [Google Scholar]
- 8.Vichinsky EP, Earles A, Johnson RA, Hoag MS, Williams A, Lubin B. Alloimmunization in sickle cell anemia and transfusion of racially unmatched blood. The New England journal of medicine. 1990;322:1617–21. doi: 10.1056/NEJM199006073222301. [DOI] [PubMed] [Google Scholar]
- 9.Chou ST, Liem RI, Thompson AA. Challenges of alloimmunization in patients with haemoglobinopathies. Br J Haematol. 2012;159:394–404. doi: 10.1111/bjh.12061. [DOI] [PubMed] [Google Scholar]
- 10.Chou ST, Jackson T, Vege S, et al. High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh-matched minority donors. Blood. 2013;122:1062–71. doi: 10.1182/blood-2013-03-490623. [DOI] [PubMed] [Google Scholar]
- 11.Cox JV, Steane E, Cunningham G, Frenkel EP. Risk of alloimmunization and delayed hemolytic transfusion reactions in patients with sickle cell disease. Arch Intern Med. 1988;148:2485–9. [PubMed] [Google Scholar]
- 12.Petz LD, Calhoun L, Shulman IA, Johnson C, Herron RM. The sickle cell hemolytic transfusion reaction syndrome. Transfusion. 1997;37:382–92. doi: 10.1046/j.1537-2995.1997.37497265338.x. [DOI] [PubMed] [Google Scholar]
- 13.Castro O, Sandler SG, Houston-Yu P, Rana S. Predicting the effect of transfusing only phenotype-matched RBCs to patients with sickle cell disease: theoretical and practical implications. Transfusion. 2002;42:684–90. doi: 10.1046/j.1537-2995.2002.00126.x. [DOI] [PubMed] [Google Scholar]
- 14.Nickel RS, Hendrickson JE, Fasano RM, et al. Impact of red blood cell alloimmunization on sickle cell disease mortality: a case series. Transfusion. 2016;56:107–14. doi: 10.1111/trf.13379. [DOI] [PubMed] [Google Scholar]
- 15.Fasano RM, Chou ST. Red Blood Cell Antigen Genotyping for Sickle Cell Disease, Thalassemia, and Other Transfusion Complications. Transfus Med Rev. 2016 doi: 10.1016/j.tmrv.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 16.McPherson ME, Anderson AR, Haight AE, et al. Transfusion management of sickle cell patients during bone marrow transplantation with matched sibling donor. Transfusion. 2009;49:1977–86. doi: 10.1111/j.1537-2995.2009.02213.x. [DOI] [PubMed] [Google Scholar]
- 17.Dang JP, Maurice CB, Orengo L, Stack G. Blood Group Genotype Testing on Leukoreduced RBC Segments: Establishing the Feasibility of Hospital-Based Donor Genotyping. AABB; Anaheim, CA: 2015. p. 142A. Transfusion. [Google Scholar]
- 18.Yee MEM, Josephson CD, Winkler AM, et al. Red blood cell minor antigen mismatches during chronic transfusion therapy for sickle cell anemia. Transfusion. 2017 doi: 10.1111/trf.14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies P, Robertson S, Hegde S, Greenwood R, Massey E, Davis P. Calculating the required transfusion volume in children. Transfusion. 2007;47:212–6. doi: 10.1111/j.1537-2995.2007.01091.x. [DOI] [PubMed] [Google Scholar]
- 20.Neyrinck MM, Vrielink H, Joint Task Force for E, Certification Calculations in apheresis. J Clin Apher. 2015;30:38–42. doi: 10.1002/jca.21347. [DOI] [PubMed] [Google Scholar]
- 21.Yee MEM, Josephson CD, Winkler AM, et al. Red blood cell minor antigen mismatches during chronic transfusion therapy for sickle cell anemia. Transfusion. 2017;57:2738–46. doi: 10.1111/trf.14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JM, Sloan SR. Stochastic modeling of human RBC alloimmunization: evidence for a distinct population of immunologic responders. Blood. 2008;112:2546–53. doi: 10.1182/blood-2008-03-146415. [DOI] [PubMed] [Google Scholar]
- 23.Stowell SR, Liepkalns JS, Hendrickson JE, et al. Antigen modulation confers protection to red blood cells from antibody through Fcgamma receptor ligation. J Immunol. 2013;191:5013–25. doi: 10.4049/jimmunol.1300885. [DOI] [PubMed] [Google Scholar]
- 24.Girard-Pierce KR, Stowell SR, Smith NH, et al. A novel role for C3 in antibody-induced red blood cell clearance and antigen modulation. Blood. 2013;122:1793–801. doi: 10.1182/blood-2013-06-508952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liepkalns JS, Cadwell CM, Stowell SR, Hod EA, Spitalnik SL, Zimring JC. Resistance of a subset of red blood cells to clearance by antibodies in a mouse model of incompatible transfusion. Transfusion. 2013;53:1319–27. doi: 10.1111/j.1537-2995.2012.03910.x. [DOI] [PubMed] [Google Scholar]
- 26.Liepkalns JS, Hod EA, Stowell SR, Cadwell CM, Spitalnik SL, Zimring JC. Biphasic clearance of incompatible red blood cells through a novel mechanism requiring neither complement nor Fcgamma receptors in a murine model. Transfusion. 2012;52:2631–45. doi: 10.1111/j.1537-2995.2012.03647.x. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan HC, Gerner-Smidt C, Nooka AK, et al. Daratumumab (anti-CD38) induces loss of CD38 on red blood cells. Blood. 2017;129:3033–7. doi: 10.1182/blood-2016-11-749432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bao W, Yu J, Heck S, Yazdanbakhsh K. Regulatory T-cell status in red cell alloimmunized responder and nonresponder mice. Blood. 2009;113:5624–7. doi: 10.1182/blood-2008-12-193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu H, Stowell SR, Bernardo L, et al. Antibody-mediated immune suppression of erythrocyte alloimmunization can occur independently from red cell clearance or epitope masking in a murine model. J Immunol. 2014;193:2902–10. doi: 10.4049/jimmunol.1302287. [DOI] [PubMed] [Google Scholar]
- 30.Yu J, Heck S, Yazdanbakhsh K. Prevention of red cell alloimmunization by CD25 regulatory T cells in mouse models. Am J Hematol. 2007;82:691–6. doi: 10.1002/ajh.20959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Habibi A, Mekontso-Dessap A, Guillaud C, et al. Delayed hemolytic transfusion reaction in adult sickle-cell disease: presentations, outcomes, and treatments of 99 referral center episodes. Am J Hematol. 2016;91:989–94. doi: 10.1002/ajh.24460. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Santhanakrishnan M, Natarajan P, et al. Antigen modulation as a potential mechanism of anti-KEL immunoprophylaxis in mice. Blood. 2016;128:3159–68. doi: 10.1182/blood-2016-06-724732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larsson A, Hult A, Nilsson A, Olsson M, Oldenborg PA. Red blood cells with elevated cytoplasmic Ca(2+) are primarily taken up by splenic marginal zone macrophages and CD207+ dendritic cells. Transfusion. 2016;56:1834–44. doi: 10.1111/trf.13612. [DOI] [PubMed] [Google Scholar]
- 34.Gottlieb Y, Topaz O, Cohen LA, et al. Physiologically aged red blood cells undergo erythrophagocytosis in vivo but not in vitro. Haematologica. 2012;97:994–1002. doi: 10.3324/haematol.2011.057620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fasano RM, Booth GS, Miles M, et al. Red blood cell alloimmunization is influenced by recipient inflammatory state at time of transfusion in patients with sickle cell disease. Br J Haematol. 2015;168:291–300. doi: 10.1111/bjh.13123. [DOI] [PubMed] [Google Scholar]
- 36.Arthur CM, Patel SR, Smith NH, et al. Antigen Density Dictates Immune Responsiveness following Red Blood Cell Transfusion. J Immunol. 2017;198:2671–80. doi: 10.4049/jimmunol.1601736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evers D, van der Bom JG, Tijmensen J, et al. Absence of the spleen and the occurrence of primary red cell alloimmunization in humans. Haematologica. 2017;102:e289–e92. doi: 10.3324/haematol.2016.162685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hendrickson JE, Saakadze N, Cadwell CM, et al. The spleen plays a central role in primary humoral alloimmunization to transfused mHEL red blood cells. Transfusion. 2009 doi: 10.1111/j.1537-2995.2009.02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evers D, van der Bom JG, Tijmensen J, et al. Red cell alloimmunisation in patients with different types of infections. Br J Haematol. 2016;175:956–66. doi: 10.1111/bjh.14307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stowell SR, Arthur CM, Girard-Pierce KR, et al. Anti-KEL sera prevents alloimmunization to transfused KEL RBCs in a murine model. Haematologica. 2015;100:e394–7. doi: 10.3324/haematol.2015.128603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calabro S, Gallman A, Gowthaman U, et al. Bridging channel dendritic cells induce immunity to transfused red blood cells. J Exp Med. 2016;213:887–96. doi: 10.1084/jem.20151720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hendrickson JE, Hod EA, Cadwell CM, et al. Rapid clearance of transfused murine red blood cells is associated with recipient cytokine storm and enhanced alloimmunogenicity. Transfusion. 2011;51:2445–54. doi: 10.1111/j.1537-2995.2011.03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


