Abstract
Background
Oral immunotherapy (OIT) successfully desensitizes patients with food allergies, but the immune mechanisms mediating its efficacy remain obscure.
Objectives
We tested the hypothesis that allergen-specific regulatory T (Treg) cell function is impaired in food allergy and is restored by anti-IgE antibody (omalizumab)-supplemented OIT.
Methods
Peanut-specific T effector (Teff) and Treg cell proliferative responses, activation markers and cytokine expression were analyzed by flow cytometry in 13 peanut allergic subjects before the start of omalizumab-supplemented OIT and periodically in some subjects thereafter for up to two years. Peripheral blood Treg cells were analyzed for their peanut-specific suppressor function before and at one year following OIT. This study was registered on ClinicalTrials.gov (NCT01290913).
Results
Proliferation of allergen-specific Teff and Treg cells precipitously declined following the initiation of omalizumab therapy prior to OIT, followed by partial recovery after the initiation of OIT. At baseline, peanut-specific Treg cells exhibited a Th2 cell-like phenotype, characterized by increased IL-4 expression, which progressively reversed upon OIT. Peanut-specific Treg cell suppressor activity was absent at the start of omalizumab/OIT therapy but became robust following OIT. Absent peanut-specific Treg cell function could also be recovered by the acute blockade of IL-4/IL-4R receptor signaling in Treg cells, which inhibited their IL-4 production.
Conclusions & Clinical Relevance
OIT supplemented by omalizumab promotes allergen desensitization through an initial omalizumab-dependent step that acutely depletes allergen-reactive T cells, followed by an increase in allergen-specific Treg cell activity due to the reversal of their Th2 cell-like program. Improved Treg cell function may be a key mechanism by which OIT ameliorates food allergy.
Keywords: Food Allergy, FOXP3, Omalizumab, Oral Immunotherapy, Regulatory T Cells
INTRODUCTION
Food allergy is a major public health problem that is estimated to affect 6–8% of all children and up to 3% of adults in developed countries[1, 2]. Moreover, the prevalence of peanut allergy has markedly increased in the past decade for reasons that are unclear[3]. The current standard of care for food allergy is to avoid the offending foods and to pharmacologically treat food allergic reactions when they occur.
Oral immunotherapy (OIT) is currently being investigated as treatment modality to modify disease outcome by inducing unresponsiveness to food allergens[4]. OIT has been proven efficacious in desensitizing the majority of subjects with food allergy to their offending allergen, achieving 50–80% response rates[5]. However, induction of sustained unresponsiveness following cessation of OIT has remained problematic. For example, while up to half the subjects with peanut allergy remain unresponsive to peanut challenge one month following cessation of OIT [6], only 13% remained unresponsive 6 months following cessation of therapy [7].
The mechanism(s) by which OIT mediates allergen desensitization remain to be fully elucidated [8]. Of particular interest is the role in the process of FOXP3+ regulatory T (Treg) cells, given their cardinal function in actively maintaining oral tolerance in the gut [9, 10]. Jones and coworkers found that the number of FOXP3+ T cells observed in cultures of peripheral blood cells stimulated with peanut increased until 12 months of OIT and then decreased afterward[11]. Earlier studies showed that the ratio of FOXP3high to FOXP3intermediate Treg cells stimulated with peanut increased one year into peanut OIT[12]. More recently, Syed et al found that patients who were “immune tolerant” to peanut, or were able to pass an oral food challenge after interrupting OIT for 3–6 months, had higher numbers of allergen induced-Treg cells with greater suppressive function, and with higher levels of FOXP3 hypomethylation, compared to non-tolerant subjects [7]. Increased allergen-responsive Treg cells have also been noted upon naturally occurring tolerance acquisition to food in allergic subjects, indicative of common mechanisms operative in both natural and OIT-induced oral tolerance acquisition [13–15]. A critical observation explaining the failure of food allergen-specific Treg cells to control disease is the acquisition by these cells of Th2-cell like phenotype with increased IL-4 production, imparting a Th2-cell like phenotype, which impairs their regulatory function [16, 17]. The capacity of OIT to reverse this aberrant program is unknown.
We have developed an OIT protocol combined with the anti-IgE monoclonal antibody therapy omalizumab (Xolair, Genentech) that increased the rapidity of food allergy desensitization [18–20]. In patients who were desensitized to milk after receiving omalizumab, there was an acute and significant reduction of milk-specific T cell responses, without increased FOXP3+ Treg cell development, suggesting the induction of anergy or deletion of T effector (Teff) cells [21]. The initially reduced Teff cell response was later replaced by a CD4+ T cell response characterized by reduced IL-4 production, accompanied by decreased milk-specific IgE responses, attenuated milk-specific basophil degranulation, and increased milk-specific serum IgG4 concentrations [21].
Here we investigated the immunological mechanisms by which OIT supplemented by omalizumab mediates clinical improvement. We describe the dynamic peanut-specific immunological changes in a pilot cohort of children who received open label peanut OIT with omalizumab[19]. We show that OIT supplemented with omalizumab suppressed the Th2 cell-like phenotype of peanut-specific Treg cells and restored their function, an effect that could be recapitulated in vitro by the blockade of IL-4R signaling in Treg cells. Our findings support a major role for the functional restoration of allergen-specific Treg cells in mediating long-term beneficial effects of omalizumab-OIT therapy. They also provide mechanistic insights into how such an effect is achieved, which can be harnessed to further improve the efficacy of future OIT protocols.
METHODS
Study Population
We evaluated blood samples from 13 peanut allergic patients (8 boys and 5 girls) originally reported by Schneider et al who underwent OIT supplemented by omalizumab[19]. All patients had a history of significant IgE-mediated peanut allergy (defined as having a significant immediate reaction with peanut ingestion, including generalized urticaria, vomiting and/or anaphylaxis)[19]. These patients failed an initial double-blind placebo-controlled food challenge (DBPCFC) at peanut protein doses of 50 mg or less. At enrollment the median age was 10 years, the median peanut-specific IgE level was 229kU/L and the median total serum IgE level was 621 kU/L. The peanut-specific allergic responses in the course of the study are detailed in Table E1 in the Online Repository. An additional five untreated children who met clinical and laboratory criteria for IgE-mediated peanut allergy were recruited for studies on the impact of IL-4R neutralization on peanut-specific Treg cell function (Table E2 in the Online Repository). The Institutional Review Board of Boston Children’s Hospital approved the protocol and all participants and/or their parents provided written informed consent. This study was registered on ClinicalTrials.gov (NCT01290913).
Proliferative labeling and cell culture
Frozen PBMC from different time-points were thawed and labeled with Cell Trace Violet (CTV) dye by incubating PBMC (2 × 106 cells/ml) in RPMI with CTV at a final concentration of 10 μM for 15 minutes at 37 °C with gentle shaking, then washing excess dye away. CTV-labeled PBMC were then cultured in the presence of peanut protein (200 μg/mL), anti-CD3 (0.5 μg/mL) or left unstimulated in RPMI-1640 containing gentamycin and supplemented with 10% fetal calf serum and glutamine. Cells were distributed in 48-well flat-bottom plates, at a concentration of 1 × 106/400 μl medium per well. Some of the cultures were also treated with rhIL2 (50 IU/mL) or with/without anti-IL10 (JES3-9D7, 5μg/ml). After 7 days of culture, the cells were collected and stained with eFluor-450-conjugated anti-CD4, APC-Cy7-conjugated anti-CD3, Alexa Fluor 488-conjugated anti-CD25, Alexa Fluor 700-conjugated anti-FOXP3, PerCpCy5.5-conjugated CCR7, Alexa Fluor 488-conjugated anti-CD45RA, Pe-Cy7-conjugated anti-CD45RO, and Pe-Cy7-conjugated CD127 mAbs.
Detection of cytokine production by flow cytometry
After 7 days of culture with 200μg/mL peanut protein, PBMC were re-stimulated with phorbol myristate acetate (PMA) (25 ng/ml), ionomycin (0.5 μg/ml) and monensin for 4½ hours, then fixed and permeabilized with BD Cytofix/Cytoperm Fixation/Permeabilization Kit (BD Biosciences) and stained with PerCpCy5.5-conjugated anti-CD4, APC-Cy7-conjugated anti-CD3, Alexa Fluor 488-conjugated anti-CD25, eFluor-450-conjugated anti-FOXP3, APC-conjugated anti-IL-4 and Alexa Fluor 700-conjugated anti-IFN-γ mAbs. Some cultures were also stained with APC-conjugated anti-IL-13 mAb.
Peanut-specific Treg cells suppression assays
APCs, Treg cells, and CD4+ Teff cells were purified by fluorescence activated cell sorting (FACS) using a BD cell sorter (ARIA), from PBMC taken from the same patient at week 0 and week 52 of the study. CD4+ Teff cells were labeled with CTV dye prior to co-culture with unlabeled APCs and regulatory T cells at a ratio of 2:2:1 in a flat-bottom 96 well plate with a total cell number of 2 × 105 cells per well and stimulated for 6 days with peanut protein to gauge antigen-specific suppression. At Day 6, PBMC were restimulated with phorbol myristate acetate (PMA) (25 ng/ml), ionomycin (0.5 μg/ml) and monensin for 4½ hours, then fixed and permeabilized with BD Cytofix/Cytoperm Fixation/Permeabilization Kit (BD Biosciences) and stained with PerCpCy5.5-conjugated anti-CD4, APC-Cy7-conjugated anti-CD3, Alexa Fluor 488-conjugated anti-CD25, Alexa Fluor-700-conjugated anti-FOXP3, and PeCy7-conjugated anti-IFN-γ mAbs. Neutralizing mouse IgG2a mAb to human IL4 receptor alpha chain (IL4Rα) or mouse IgG2a isotype control mAb (R&D Systems) were added to cell cultures at a final concentration of 50ng/ml.
Methylation of CNS2 CpG in the FOXP3 gene of sorted T cells
Genomic DNA of Teff and Treg cells isolated by FACS was prepared with the DNeasy Blood & Tissue Kit (Qiagen) according to the manufacturer’s instructions. FOXP3 CNS2 methylation analysis by Bisulfite sequencing was done as previously described [22]. Following bisulfite treatment of DNA, the CNS2 sequence was amplified by nested PCR using the following primer sets: Outer primer-1: 5′-TGGTTTGGTTTATGTGGTTGG-3′; Outer primer 2: 5′-TCCTCTCCACAACCCAAAAA-3′; Inner primer 1: 5′-GGTTGGTTTGTGGTTATTTTT GA-3′; Inner primer 2: 5′-TCACCTACC ACATCCACCAA-3′. The PCR products were purified and subjected to Sanger sequencing.
Statistics
Analysis was carried out using paired t-test, nonparametric Mann-Whitney test, 1 way or 2 way ANOVA for matched values, as indicated (GraphPad Prism Software 7.0; GraphPad Software, La Jolla, Calif). A p-value less that 0.05 was considered statistically significant.
Other Methods
Additional information on the OIT supplemented with omalizumab clinical protocol and patient outcome, the preparation of patient PBMC samples and the preparation of peanut protein for in vitro T cell stimulation is provided in the Methods section in this article’s Online Repository.
RESULTS
Patient OIT and clinical outcomes
Patient demographics, omalizumab-enabled OIT protocol and clinical outcomes at one-year post initiation of OIT have been previously reported [19]. Details of clinical outcomes between weeks 52 and 104 are described in Table EI in the Online Repository. Of the 13 initially enrolled subjects, two dropped out of the study at weeks 16 (Patient 04) and 45 (patient 07), and a third subject dropped out at week 90 (patient 08), all due to OIT-related side effects.
Peanut-specific CD4 T cell proliferation is reduced during rush desensitization
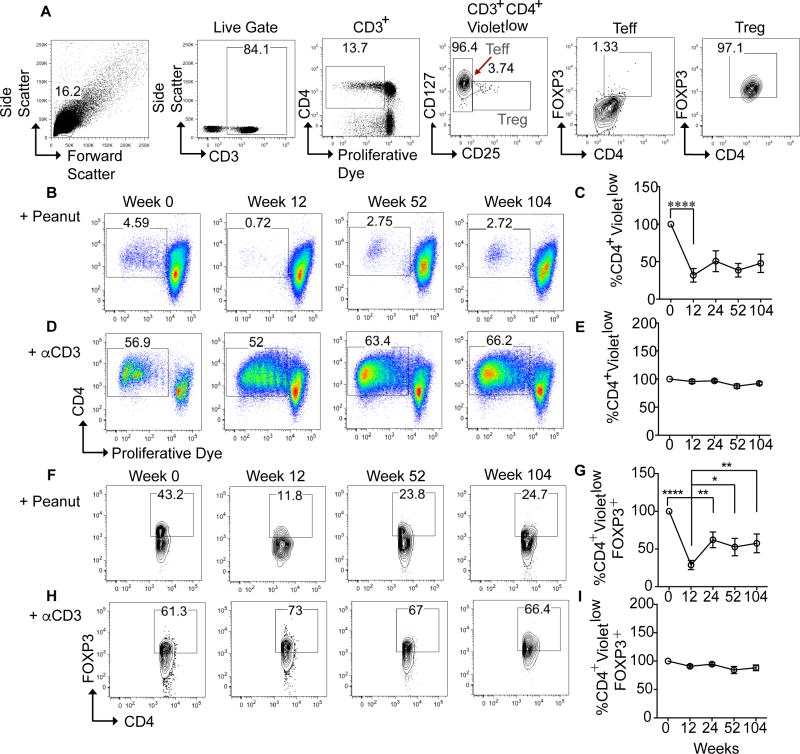
Peripheral blood mononuclear cells (PBMC) were isolated at multiple time points during the desensitization protocol (Fig E1 in the Online Repository), and frozen in aliquots in liquid nitrogen for later examination. Blood from patient 04 was collected and analyzed through week 52, patient 07 through week 45 and patient 08 through week 90 of the study. To characterize the response to peanut, PBMC from multiple time points for each patient were thawed simultaneously, labeled with CTV proliferative dye and cultured in the presence of peanut protein, (Fig. 1C, 1G) for 7 days. Cells were also cultured separately with anti-CD3 beads at every time-point to confirm that the functional integrity of the cells was unaffected by the freeze-thaw process. Cell viability was assessed after thawing by Trypan Blue dye staining and confirmed at over 90%. The gating strategy for identifying proliferating Treg and Teff cells is shown in Fig 1, A. Proliferating CD4+ T cells to peanut (and similarly to anti-CD3 beads) were identified as VioletlowCD4+ T cells, representing cells that had undergone multiple cell divisions and accordingly diluted their CTV dye. Within that population, Treg cells were defined as CD4+CD25+CD127low, and were revealed to be ≥95% positive for high-level expression of FOXP3. Teff cells were defined as CD4+CD25−CD127high, and were ≤3% positive for low level expression of FOXP3 (Fig 1, A).
Fig 1. Peanut-specific CD4 T cell proliferation is reduced during rush desensitization.
A, Gating strategy for identifying proliferating VioletlowCD4+CD3+ Treg cells in PBMC cultures stimulated with anti-CD3+anti-CD28 mAb beads. Treg cells were first identified by gating on CD25highCD127low cells among VioletlowCD4+CD3+ T cells, followed by gating on CD4+FOXP3+ cells. The same gating strategy was used for peanut protein-stimulated cultures. B–E, Flow cytometric analysis and enumeration of proliferating Violetlow total CD3+CD4+ cells loaded with CTV proliferation dye and cultured with peanut protein (Fig 1, B and C) or with anti-CD3+anti-CD28 mAb beads (Fig 1, D and E). Graph represents average data for all patients normalized to day 0 ±SEM over the course of the study. ****p<0.0001 versus baseline determined using paired t-test. F–I, Representative plots at 4 weeks 0, 12, 52, 104 of % peanut-specific Violetlow CD4+ CD3+ FOXP3+ cells. G and I, Graphs representing the average data for all patients normalized to day 0 ±SEM over the course of the study. *p<0.05, **p<0.01, ****p<0.0001 by one way ANOVA with post test analysis with comparisons made to week 12 values.
In all patients examined, there was a vigorous proliferative response to peanut at week 0, as demonstrated by flow cytometry, which showed a significant number of VioletlowCD4+ T cells (Fig 1, A; Fig 1, B and C). However, a sharp decrease in peanut-specific CD4+ T cell proliferation was observed when the patients were tested just prior to the onset of peanut OIT, which took place 12 weeks after the initiation of omalizumab therapy (Fig 1, C). This drop, which was seen in all 13 patients (Fig 1, C, Fig E2, A in the Online Repository) was partially reversed upon the initiation of peanut OIT. As the daily dose of peanut increased, so did the percentages of CD4+ T cell proliferation (Fig 1, C), although for most patients they did not reach their pre-treatment levels. These changes in peanut-stimulated proliferative responses were specific in that they were not observed with the anti-CD3 mAb treatment or upon stimulation with tetanus toxoid (Fig 1, D and E, Fig E3, A and B in the Online Repository). Furthermore, in vitro addition of IL-2 or anti-IL-10 mAb failed to rescue the decreased proliferation of peanut-specific CD4+ Teff or Treg cells (Fig E4, A and B in the Online Repository). These results suggested that decreased IL-2 availability (anergy) or increased IL-10-mediated suppression may not account for the observed decline in peanut reactivity.
We further examined the impact of OIT on peanut-specific CD4+ Treg cell populations. There was a decrease in the frequencies of peanut-specific Treg cells (VioletlowCD4+CD127lowCD25+FOXP3+) in cultures stimulated with peanut protein during the rush desensitization phase (Fig 1, F and G). The same pattern was also observed when the absolute numbers of Treg cells were analyzed, with decreased in parallel to those of Teff cells (Fig E5, A and B). The frequencies and numbers of FOXP3+ Treg cells eventually increased over the ensuing 3–4 months while the subjects remained on daily oral peanut, but remained lower than at baseline (Fig 1, I).
We also analyzed the peanut-specific T cell proliferative responses in the 3 patients who did not respond to OIT treatment. Although there was an initial drop in the number of peanut-specific CD4+ Teff and Treg cells, the frequency of Treg cells did not decrease in the 2 patients that dropped out of the study at weeks 45 and 90, respectively (Fig E2, A).
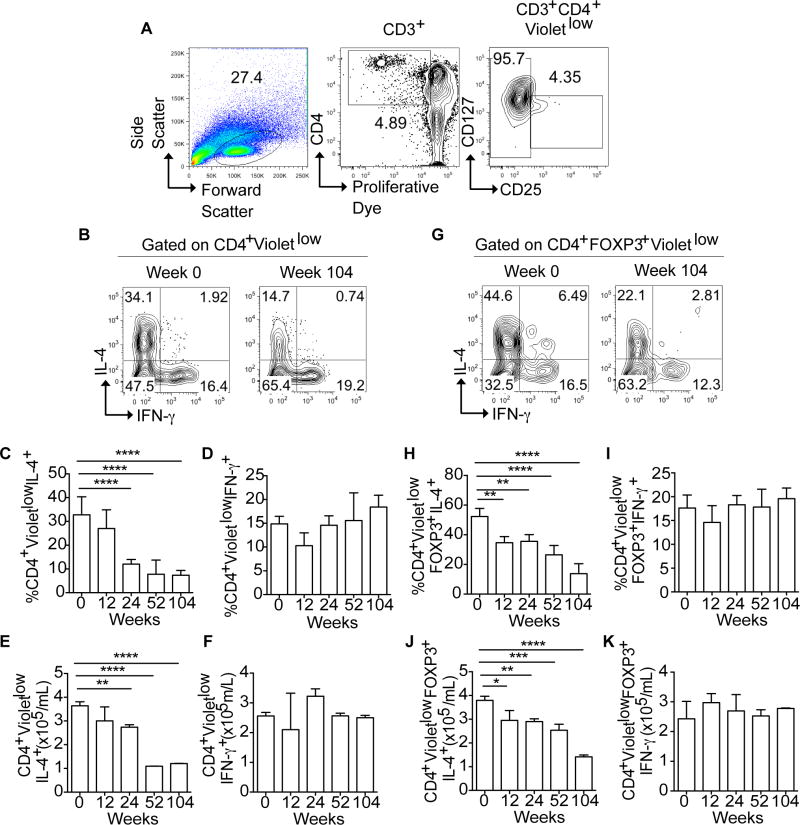
OIT facilitated by omalizumab reduces disease-associated Th2 cell skewing
To determine the impact of OIT-omalizumab treatment on the peanut-specific Th2 cell response, we analyzed Th2 and Th1 cell cytokine expression in Teff and Treg cells at baseline and in the course of treatment. The gating strategy for these studies is shown in Fig 2, A. At baseline, a sizable fraction of peanut-specific Violetlow CD4+ Teff cells expressed IL-4, consistent with Th2 cell bias characteristic of food allergy (Fig 2, B and C). However, the percentage and number of IL-4+ peanut-specific CD4+ Teff cells progressively decreased in the course of treatment (Fig 2, C and E), as we have previously reported [21]. In contrast, expression of IFN-γ did not significantly change (Fig 2, D and F).
Figure 2. Reversal of Th2 Cytokine skewing during peanut desensitization.
A, Gating strategy for analysis of Treg and Teff cells. VioletlowCD3+CD4+ T cells were further gated on CD4+CD25+CD127low (Treg cells) and CD4+CD25−CD127high (Teff) then analyzed for FOXP3 expression. B, Representative FACS plots of IL-4 and IFN-γ expression in Violetlow (peanut-specific) CD4+ Teff cells. C–F, percentages (Fig 2, C and D) and absolute numbers (Fig 2, E and F) of IL-4 and IFN-γ expression in peanut-responsive (Violetlow) CD4+ Teff cells in all 10 patients responding to OIT treatment measured at weeks 0, 12, 24, 52 and 104. G, Representative FACS plots of IL-4 and IFN-γ expression in Violetlow CD3+CD4+FOXP3+ Treg cells. H–K, percentages (Fig 2, H and I) and absolute numbers (Fig 2, J and K) of IL-4 and IFN-γ expression in peanut-responsive (Violetlow) CD4+FOXP3+ Treg cells in the same subjects as panels C–F at weeks 0, 12, 24, 52, and 104. Results represent means ±SEM. *p<0.05, **p<0.01, ***<0.001, ****<0.0001 by one way ANOVA with post test analysis with comparisons made to week 0 values.
Food allergy is associated with the acquisition of food-allergen specific Treg cells of a Th2 cell-like phenotype that contributes to disease pathogenesis [16, 17, 23]. In agreement with our findings in the murine model and in human subjects with milk allergy [17], the peanut-allergic patients in our study had a high frequency of peanut-specific proliferating Treg cells (CD3+CD4+FOXP3+Violetlow) that expressed IL-4 at baseline (Fig 2, G). Similar to the peanut-specific CD4+ Teff cells, IL-4 expression in the peanut-specific Treg cells also substantially decreased over time on OIT (Fig 2, H and J). By week 104 of the study, IL-4 production in Treg cells was reduced by over 60% as compared to week 0. Similar to their CD4+ Teff cell counterparts, peanut specific Treg cells exhibited no change in their IFNγ expression on OIT (Fig 2, I and K).
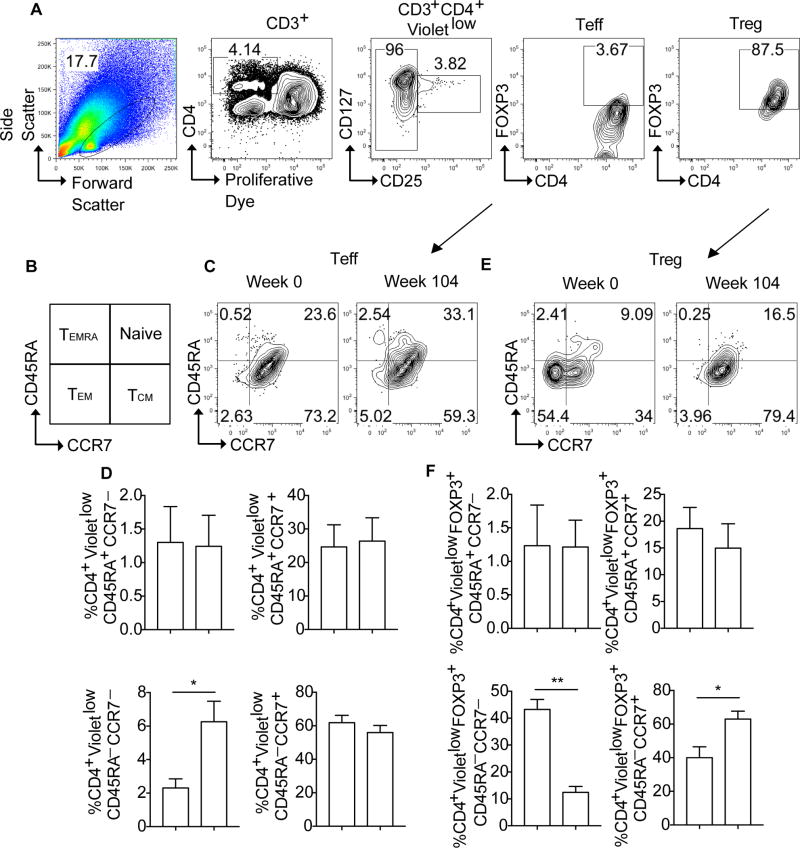
OIT therapy down-regulates the peanut-specific Treg cell effector memory response in favor of a central memory like-phenotype
We next examined the impact of OIT on the peanut-specific Teff and Treg cell memory responses. Naïve T cells differentiate upon successive encounters with antigen into functionally distinct effector and memory subpopulations. These include T effector memory (TEM) cells and a related population of short-lived terminally differentiated T effector cells (or TEMRA) that circulate through blood and peripheral tissues, and central memory T cells (TCM) found in circulation and lymphoid tissues [24–28]. These subpopulations can be segregated based on their expression of the marker CD45RA and the lymphoid tissue homing receptor CCR7 as follows: lymphoid-homing naïve (CD45RA+CCR7+) and TCM (CD45RA−CCR7+) cells, and tissue-homing TEM (CD45RA−CCR7−) and TEMRA (CD45RA+CCR7−) cells [24–28]. We cultured PBMC with peanut protein and analyzed the peanut-specific VioletlowCD4+ Teff and Treg cells for their expression of CD45RA and CCR7. The gating strategy is shown in Fig 3, A, and a scheme illustrating the CD45RA/CCR7marker combinations of the different memory subsets is shown in Fig 3, B. The distribution of memory subsets among Violetlow CD4+ Teff cells, dominated by CD45RA−CCR7+ TCM cells, was mostly unaffected by OIT, except for increased frequency of the otherwise minor population of CD45RA−CCR7− TEM cells. (Fig 3, C and D). In contrast, there were major shifts in the memory subpopulations of Violetlow CD4+ Treg cells following OIT, including a steep decline in the frequencies of CD45RA−CCR7− TEM-like Treg cells and a reciprocal increase in those of CD45RA−CCR7+ TCM-like Treg cells (Fig 3, E and F). These data suggest that OIT down-regulates the TEM cell-like phenotype of peanut-specific Treg cells, congruent with its suppression of their Th2 cell-like phenotype.
Figure 3. OIT therapy results in the contraction of the peanut-specific Treg cell TEM response.
A, Representative FACS plots showing the gating strategy for identifying CD3+CD4+Violetlow Teff and Treg cells as shown by FOXP3 expression. B, Schematic representation of the strategy for the identification of T cell memory subsets by flow cytometry based on CD45RA and CCR7 expression. D–F, Flow cytometric analysis of CD45RA and CCR7 expression on peanut-responsive CD3+CD4+Violetlow Teff (Fig 3, C and D) and Treg cells (Fig 3, E and F) at weeks 0 and 104. Results represent means ±SEM. *p<0.05, **p<0.01 by Student’s two tailed paired t test analysis.
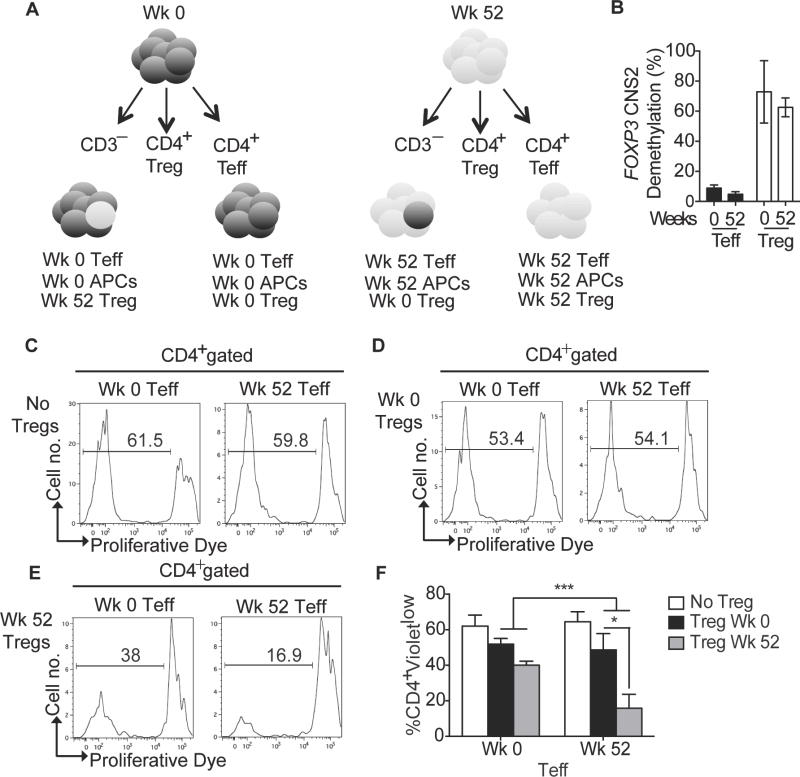
OIT restores peanut-specific Treg cell suppression
To determine whether OIT promoted tolerance by augmenting Treg cell function, we performed paired peanut-specific Treg cell suppression assays at baseline and at week 52 following OIT (Fig 4, A). CD4+CD25+CD127low Treg cells and CD4+CD25−CD127high Teff cells were purified by FACS from PBMC collected at weeks 0 and 52 from four patients who successfully completed therapy (Fig E6, A in the online repository). Due to limitations with cell number availability for sorting Treg cells, we were unable to perform suppression assays for the remaining patients. Purified Treg cells were co-cultured with matched Teff and antigen-presenting cells (APCs) also isolated from the same week 0 and 52 PBMC samples (Fig. 4, A). To confirm the lineage identity of the collected Treg cells, we analyzed the demethylation of CpG motifs in the FOXP3 conserved noncoding sequence 2 (CNS2). Whereas the CpG motifs of CNS2 in Tregs were highly demethylated, as reported previously [29, 30], those of Teff cells were highly methylated (Fig. 4, B). In these experiments, Teff cells were labeled with proliferation dye, and their proliferation was assessed when co-cultured with unlabeled Treg cells. We gated out unlabeled Treg cells and assessed the intracellular expression of FOXP3 in Teff cells to rule out promiscuous FOXP3 expression as a confounding factor in the analysis (Fig E6 in the Online Repository).
Figure 4. Peanut desensitization restores peanut-specific Treg cell suppression.
A, Schematic diagram of the suppression assay set-up. CD3+CD4+CD25− Teff cells, and CD3+CD4+CD25+CD127low Treg cells were purified by FACS and cultured with peanut protein and CD3− antigen-presenting cells in four different combinations: 1- Week 0 Teff with Week 0 Treg cells. 2- Week 0 Teff with Week 52 Treg cells. 3- Week 52 Teff with Week 0 Treg cells. 4- Week 52 Teff with Week 52 Treg cells. As controls, Week 0 and Week 52 Teff cells were cultured absent Treg cells. Teff cells were labeled with CTV proliferative dye at the start of the cultures B, FOXP3 CNS2 methylation analysis in FACS purified Teff and Treg isolated from Weeks 0 and Week 52 samples C–E, Representative histograms showing CTV dye dilution in CD3+CD4+ T cells of the indicate cultured cell combinations. F, Percentages of proliferating CD3+CD4+Violetlow Teff cells for all 4 patients. Results represent means ±SEM of studies on weeks 0 and week 52 samples, as indicated, derived from 4 subjects. *p<0.05, ***<0.001 by two way ANOVA with post-test analysis.
In the absence of Treg cells, Teff cells from weeks 0 and 52 proliferated vigorously in response to peanut, indicating that OIT supplemented with omalizumab did not extinguish the peanut-specific Teff cell response (Fig 4, C). When Treg and Teff cells were co-cultured, the combination of week 0 Teff cells and week 0 Treg cells had the highest proliferative response, while week 52 Teff cells combined with week 52 Treg cells had the lowest proliferative response (Fig 4, C–F). When week 0 Treg cells were added to week 52 effector T cells/APCs, the peanut-specific proliferative response of week 52 Teff was restored to as high as week 0 Teff cells (Fig 4, D and F). When week 52 Treg cells were combined with week 0 Teff, suppression in the proliferation was also noted when compared to week 0 Treg cells co-cultured with week 0 Teff. These results indicate that prior to OIT therapy the Treg cells exhibited poor peanut-specific suppressor function. Following OIT therapy, peanut-specific Treg cells are reprogrammed to gain in allergen-specific suppressor function, an effect that may contribute to oral tolerance induction by OIT.
We further analyzed the impact of OIT on peanut-specific Treg cell suppression in the two patients (007 and 008) that did receive OIT but did not respond to treatment. When analyzed in vitro these patients maintained a low Treg cell percentage throughout the study after the initial transient drop (Fig E2, A). Treg cells isolated from PBMC samples of those subjects at week 52 did not lose their IL-4 expression and were unable to suppress peanut-induced Teff cell proliferation, consistent with failure to promote adequate desensitization in those two subjects (Fig E2, B and C).
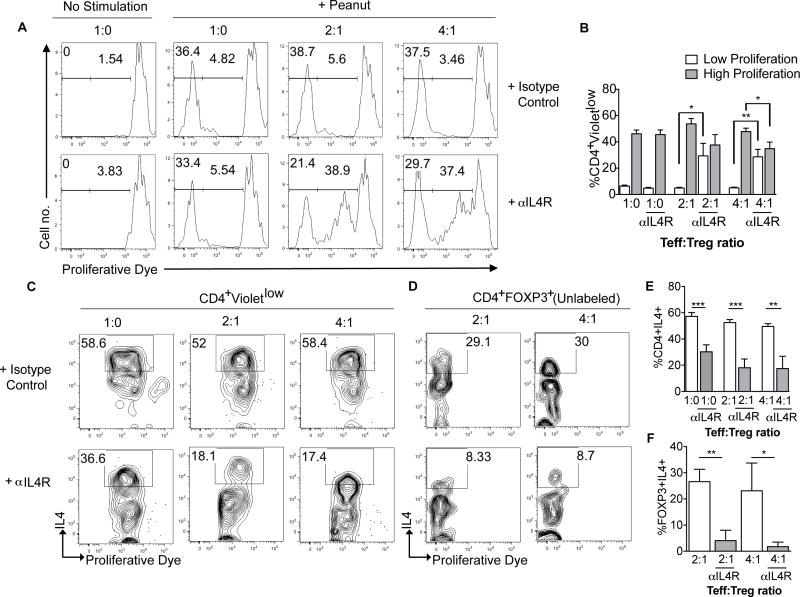
Blockade of IL4R signaling promotes peanut-specific Treg cell suppressor function
Our previous studies on a genetically prone mouse model of food allergy have identified excessive IL-4/IL-4R signaling in Treg cells as mediating their pathogenic Th2 cell-like reprogramming, leading to failure of oral tolerance [17]. To determine whether suppression of IL4/IL-4R signaling rescues the failure of peanut-specific Treg cell suppression of Teff cells (Fig 4, F), we carried out suppression assays on froze-thawed cells in the absence or presence of a neutralizing anti-IL-4Rα chain mAb. To that end, Treg and Teff cells were isolated by FACS from PBMC of treatment-naïve peanut allergic subjects who were not involved in the omalizumab/OIT trial. Peanut-specific Treg cell suppressor activity was found absent in peanut allergic subjects, as evidenced by the complete failure of Treg cells to affect the highly proliferative response of Teff cells to peanut. In the absence of Treg cells, addition of anti-human IL-4Rα mAb to had no effect on the proliferation of Teff cells to peanut. In contrast, treatment of Treg cell-Teff cell co-cultures with anti-IL-4Rα mAb, but not with an isotype control mAb, resulted in enhanced suppression by the Treg cells of peanut-induced Teff cell proliferation (Fig 5, A and B). This effect was evidenced by decreased frequencies of highly proliferating (violetlow) Teff cells in co-cultures treated with anti-IL-4Rα mAb, coupled with a pronounced increase in the frequencies of low-proliferating (violetintermediate) Teff cells. These results indicated that treatment with a neutralizing anti-IL-4Rα mAb augmented the suppressive function of peanut reactive Treg cells by specifically inhibiting IL-4R signaling in the Treg cells.
Figure 5. IL4R blockade rescues peanut-specific Treg suppressor function.
A. In vitro modulation of peanut-specific Treg suppression assays by anti-IL-4Rα mAb treatment. Representative flow cytometric analysis of CTV-loaded Teff cells derived from treatment-naïve peanut-allergic subjects and co-cultured for 5 days with APCs in the absence or presence of peanut protein. Cell cultures were treated with an isotype control or an anti-IL-4Rα mAb, and Treg cells were added at a Teff:Treg cell ratios of 1:0, 2:1 and 4:1, as indicated. Teff cells were then analyzed for CTV dye dilution. B, Percentages of CD3+CD4+Violetlow cells in the respective cultures. C and D, Representative histograms showing Teff and Treg cell production of IL4 respectively. E and F, Percentages of Teff and Treg cell IL4 production. Results shown in panels B, E and F represent means ±SEM of data derived from cultures of 5 peanut allergic subjects. *p<0.05, **<0.01, ***<0.001 by paired one way ANOVA with post-test analysis.
To determine whether treatment with anti-IL-4Rα mAb impacted the Th2 cell skewing of proliferating Teff cells and the Th2 cell-like phenotype of peanut-reactive Treg cells, we examined the expression of IL-4 by the respective cell type in proliferating cultures. Anti-IL-4Rα mAb treatment markedly decreased the expression of IL-4 in proliferating Teff cells in the absence or presence of Treg cells. It profoundly inhibited the expression of IL-4 in Treg cells, consistent with reversal of Th2 cell-like program of peanut reactive Treg cells (Fig 5, C–F). These results established that treatment with a neutralizing anti-IL-4Rα mAb acutely reversed the Th2 cell-like skewing of peanut-reactive Treg cells and augmented their peanut specific suppressive function.
DISCUSSION
In this study, we investigated the immune mechanisms of OIT in a cohort of 13 high-risk peanut allergic children who underwent a rapid high dose oral desensitization protocol facilitated with omalizumab[19]. The cell suppressive function of allergen-specific Treg cells, measured at 1 year after the initiation of therapy, was markedly augmented in patients who were successfully desensitized to peanuts but not in those who failed desensitization. In parallel, our data revealed that peanut-specific Teff cells exhibited decreased IL-4 production following OIT, consistent with reversal of their Th2 skewing and in agreement with results of other OIT studies [7, 21]. Peanut-specific Teff cells did not show evidence of anergy, as revealed by their similar proliferation to peanut in the absence or presence of IL-2. Thus, the persistence of a non-responsive state in peanut allergic subjects may reflect in part augmented immunological suppression mediated by Treg cells.
We have previously shown that food allergy is associated with the acquisition of allergen-specific Treg cells of a Th2 cell-like phenotype with IL-4 production that contributes to disease pathogenesis [17]. In this study, we also found that peanut-reactive Treg cells of peanut allergic subjects exhibited a Th2 cell-like phenotype with increased expression of IL-4 and markers associated with TEM cells, indicative of their Th2-cell like skewing and acquisition of a tissue-homing effector phenotype. In food allergic mice, the acquisition by allergen-specific Treg cells of a Th2 cell-like phenotype is pathogenic, as evidenced by the suppression of the food allergic response upon deletion of Il4/Il13 in Treg cells16. Consistent with these findings, OIT was associated with a reversal of the Th2 cell-like program in peanut–specific Treg cells as evidenced by decreased IL-4 production.
Treg cell suppression assays revealed that prior to start of therapy, peanut-specific FOXP3+ Treg cells were unable to suppress Teff cells. In line with the reversion of the Th2 cell-like phenotype of peanut-specific Treg cells following successful therapy, the suppressor function of these cells was markedly increased at one year following the initiation of therapy, consistent with a durable change in their regulatory capacity. A direct effect by OIT on Teff cell responses to Treg cells may also contribute to enhanced suppression by week 52 Treg cells, but this would require further investigations with larger cohort numbers to discern such an effect. Previous studies on peanut-allergic subjects treated with OIT alone absent omalizumab have revealed that cessation of OIT was associated with the re-emergence of food allergy [6, 7]. Further longer term follow-up studies will be required to determine whether the salutary effects of omalizumab-facilitated OIT on Treg cell function and tolerance to peanut intake persist following the cessation of OIT therapy.
The role of IL-4R signaling in the aberrant Th2 cell-like program and loss of suppressor function of peanut-specific Treg cells was confirmed in separate studies employing Treg and Teff cells of treatment-naïve peanut allergic subjects. While treatment with a neutralizing anti-IL-4Rα mAb did not affect the proliferation of peanut-specific Teff cells in the absence of Treg cells, it restored the capacity of Treg cells of peanut allergic subjects to suppress peanut-specific Teff cell proliferation. Restoration of peanut-specific Treg cell function was associated with suppression of the Th2 cell-like program with decreased IL-4 production. These results are consistent with a critical role for increased IL-4R signaling in the Th2 cell- like Treg cells, effected by IL-4 produced by both the Treg cells and possibly the Teff cells, in the maintenance of their aberrant Th2 cell-like program and the abolition of their peanut-specific suppressor function. Overall, the functional restoration of peanut-specific Treg cell function effected by OIT supplemented by omalizumab in vivo or anti-IL-4Rα treatment in vitro may reflect transcriptional and epigenetic changes at several genetic loci in Treg cells relevant to regulatory and/or Th2 responses [7]. Although no clinical assessment of long-term tolerance has been carried out in our patients, the functional Treg cell changes are consistent with the promotion by omalizumab/OIT treatment of a state of long-term allergen desensitization and possibly tolerance.
A limitation of our study is that there was no vehicle treatment control for omalizumab, nor a placebo control for OIT. This limitation does not allow us to state with certainty how long the effects of omalizumab on Treg and Teff cell function would last in the absence of OIT. With that in mind, our studies revealed that by the end of the initial phase of the treatment protocol, during which time omalizumab was being employed as a stand-alone therapy, peanut-specific CD4+ Teff and FOXP3+ Treg cell proliferations were sharply reduced. These results are consistent with our previous demonstration of a pivotal role for the IgE-mast cell pathway in driving the Th2 cell response in food allergy [31]. They are also consistent with the demonstration that mono-therapy with omalizumab can increase the threshold for peanut reactivity [32]. Omalizumab prevents free IgE from interacting with its high- and low-affinity receptors (FcεRI and FcεRII, respectively) on mast cells, basophils, macrophages, dendritic cells and B lymphocytes [33]. Our previous published studies in mouse models of food allergy have documented a critical role for mast cell activation via FcεRI in sustaining the Th2 response, in large measure by driving the Th2 cell-like reprogramming of allergen-specific Treg cells, and that treatment with anti-IgE or blockade of FcεRI inhibited the food allergic response [17, 34]. We hypothesize that omalizumab treatment of human subjects may similarly suppress the Th2 cell inflammatory response associated with food allergy. Anti-IgE therapy was only partially effective at reversing the Th2 cell-like phenotype of peanut-specific Treg cells absent additional allergen desensitization. Thus the two approaches appear to be synergistic in inducing desensitization, although more rigorous studies using peanut versus placebo controlled groups would be required to make this distinction.
Both Treg-dependent and -independent mechanisms have been previously been invoked in the induction of tolerance upon oral immunotherapy. In a recent study by Frischmeyer-Guerrerio et al, the addition of omalizumab to OIT in milk allergy did not alter the frequency of casein-specific Treg cells [35]. However, the functional responses of milk-specific Treg and Teff cell responses were not reported in this study [35]. Other proposed mechanisms include anergy and/or deletion of allergen-specific Treg cells[21, 36]. In our study, the in vitro addition of IL-2 failed to rescue the decreased proliferation of peanut-specific CD4+ Teff or Treg cells, suggesting that anergy was not a major mechanism for the observed decline in peanut reactivity. Also, neutralization of IL-10 failed to alter peanut-specific T cell responses. We cannot, however, rule out deletion of peanut-specific T cells as a mechanism by which suppression of peanut reactivity may be occurring in parallel with the enhanced Treg cell-induced suppression[21, 37]. Future studies aiming to determine the frequency of allergen-specific T cells by staining with peanut peptide-presenting recombinant tetrameric complexes of HLA-DR molecules coupled with T cell receptor sequencing approaches would enable determination of the role of T cell clonal deletion in long-term oral tolerance induced by OIT[7].
Of the 13 individuals who underwent OIT with omalizumab, three had to withdraw from the study due to side effects. Analysis of Treg cells of two of those individuals, who dropped out later in their desensitization course (patient 007 and 008, dropped out at weeks 45 and 90, respectively), revealed no improvement in their expression of IL-4 or peanut-induced Teff cell proliferation as compared to the onset of the desensitization protocol (week 0). This data suggests that the loss of peanut-reactive Treg cells expressing a Th2 cell-like phenotype and the subsequent acquisition of increased suppressive capacity are attributes of successful desensitization.
In addition to promoting allergen-specific Treg cell function, peanut-specific OIT in the patients reported herein and in other studies has been demonstrated to generate blocking IgG antibodies that contribute to allergen desensitization by blocking IgE-dependent mast cell responses [31, 38]. Whether the production of blocking antibodies is mechanistically related to improved Treg cell suppression by promoting a switch to blocking antibody responses is currently unknown.
In summary, our studies have elucidated a key relationship between the loss of Th2 cell-like program of peanut specific Treg cells in the course of OIT-induced allergen desensitization and the retrieval by these cells of suppressor function. This was reproduced in vitro by blockade of IL4R signaling directed at peanut-specific Treg cells. These results illuminate immunological tolerogenic mechanisms operative in OIT that may be harnessed to improve future protocols aiming to establish long term tolerance in food allergy.
Supplementary Material
Acknowledgments
This work was supported by NIH NIAID grants 1R56AI117983 and 1R01AI126915 (T.A.C.), the Thrasher Research Foundation, Clinical and Translational Science Center/Harvard Catalyst, the Bunning Food Allergy Fund and the Jasmine and Paul Mashikian Fund. We thank Genentech for providing omalizumab. Genentech was not involved in the design, implementation, or conduct of this study. We thank Dr. Magali Noval Rivas for discussions.
Abbreviations
- DBPCFC
double blind placebo controlled food challenge
- CTV
Cell Trace Violet
- FACS
fluorescence-activated cell sorting
- FcεRI
High affinity receptor for IgE
- FcεRII
Low affinity receptor for IgE
- OIT
oral Immunotherapy
- PBMC
peripheral blood mononuclear cells
- Teff cells
effector T cells
- Treg cells
regulatory T cells
Footnotes
Conflict of Interest
Dale Umetsu currently holds a position at Genentech, Inc. Lynda C. Schneider has received funding for research from Genentech, Inc., and DBV Technologies, is a consultant for Aimmune Therapeutics and is on the Medical Advisory Board Executive Committee for FARE (Food Allergy Research and Education). The other authors have no conflict of interest to declare.
References
- 1.Eigenmann PA, Beyer K, Wesley Burks A, Lack G, Liacouras CA, Hourihane JO, et al. New visions for food allergy: an iPAC summary and future trends. Pediatr Allergy Immunol. 2008;19(Suppl 19):26–39. doi: 10.1111/j.1399-3038.2008.00765.x. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer SH. Food allergy. Mt Sinai J Med. 2011;78(5):683–96. doi: 10.1002/msj.20292. [DOI] [PubMed] [Google Scholar]
- 3.Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133(2):291–307. doi: 10.1016/j.jaci.2013.11.020. quiz 8. [DOI] [PubMed] [Google Scholar]
- 4.Varney VA, Hamid QA, Gaga M, Ying S, Jacobson M, Frew AJ, et al. Influence of grass pollen immunotherapy on cellular infiltration and cytokine mRNA expression during allergen-induced late-phase cutaneous responses. J Clin Invest. 1993;92(2):644–51. doi: 10.1172/JCI116633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinthrajah RS, Hernandez JD, Boyd SD, Galli SJ, Nadeau KC. Molecular and cellular mechanisms of food allergy and food tolerance. J Allergy Clin Immunol. 2016;137(4):984–97. doi: 10.1016/j.jaci.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vickery BP, Scurlock AM, Kulis M, Steele PH, Kamilaris J, Berglund JP, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. 2014;133(2):468–75. doi: 10.1016/j.jaci.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Syed A, Garcia MA, Lyu SC, Bucayu R, Kohli A, Ishida S, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3) J Allergy Clin Immunol. 2014;133(2):500–10. doi: 10.1016/j.jaci.2013.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussey Freeland DM, Fan-Minogue H, Spergel JM, Chatila TA, Nadeau KC. Advances in food allergy oral immunotherapy: toward tolerance. Current opinion in immunology. 2016;42:119–23. doi: 10.1016/j.coi.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berin MC, Shreffler WG. Mechanisms Underlying Induction of Tolerance to Foods. Immunol Allergy Clin North Am. 2016;36(1):87–102. doi: 10.1016/j.iac.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Bryce PJ. Balancing Tolerance or Allergy to Food Proteins. Trends Immunol. 2016;37(10):659–67. doi: 10.1016/j.it.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124(2):292–300. e1–97. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127(3):654–60. doi: 10.1016/j.jaci.2010.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karlsson MR, Rugtveit J, Brandtzaeg P. Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow’s milk allergy. J Exp Med. 2004;199(12):1679–88. doi: 10.1084/jem.20032121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shreffler WG, Wanich N, Moloney M, Nowak-Wegrzyn A, Sampson HA. Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. J Allergy Clin Immunol. 2009;123(1):43–52. e7. doi: 10.1016/j.jaci.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 15.Qamar N, Fishbein AB, Erickson KA, Cai M, Szychlinski C, Bryce PJ, et al. Naturally occurring tolerance acquisition to foods in previously allergic children is characterized by antigen specificity and associated with increased subsets of regulatory T cells. Clin Exp Allergy. 2015;45(11):1663–72. doi: 10.1111/cea.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noval Rivas M, Burton OT, Oettgen HC, Chatila T. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J Allergy Clin Immunol. 2016;138(3):801–11. e9. doi: 10.1016/j.jaci.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noval Rivas M, Burton OT, Wise P, Charbonnier LM, Georgiev P, Oettgen HC, et al. Regulatory T Cell Reprogramming toward a Th2-Cell-like Lineage Impairs Oral Tolerance and Promotes Food Allergy. Immunity. 2015;42(3):512–23. doi: 10.1016/j.immuni.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadeau KC, Schneider LC, Hoyte L, Borras I, Umetsu DT. Rapid oral desensitization in combination with omalizumab therapy in patients with cow’s milk allergy. J Allergy Clin Immunol. 2011;127(6):1622–4. doi: 10.1016/j.jaci.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider LC, Rachid R, LeBovidge J, Blood E, Mittal M, Umetsu DT. A pilot study of omalizumab to facilitate rapid oral desensitization in high-risk peanut-allergic patients. J Allergy Clin Immunol. 2013;132(6):1368–74. doi: 10.1016/j.jaci.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacGinnitie AJ, Rachid R, Gragg H, Little SV, Lakin P, Cianferoni A, et al. Omalizumab facilitates rapid oral desensitization for peanut allergy. J Allergy Clin Immunol. 2017;139(3):873–81. e8. doi: 10.1016/j.jaci.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bedoret D, Singh AK, Shaw V, Hoyte EG, Hamilton R, DeKruyff RH, et al. Changes in antigen-specific T-cell number and function during oral desensitization in cow’s milk allergy enabled with omalizumab. Mucosal Immunol. 2012;5(3):267–76. doi: 10.1038/mi.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charbonnier LM, Wang S, Georgiev P, Sefik E, Chatila TA. Control of peripheral tolerance by regulatory T cell-intrinsic Notch signaling. Nat Immunol. 2015;16(11):1162–73. doi: 10.1038/ni.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noval Rivas M, Chatila TA. Regulatory T cells in allergic diseases. J Allergy Clin Immunol. 2016;138(3):639–52. doi: 10.1016/j.jaci.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 25.Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E. The who’s who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol. 2013;43(11):2797–809. doi: 10.1002/eji.201343751. [DOI] [PubMed] [Google Scholar]
- 26.Thome JJ, Yudanin N, Ohmura Y, Kubota M, Grinshpun B, Sathaliyawala T, et al. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 2014;159(4):814–28. doi: 10.1016/j.cell.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. 2014;14(1):24–35. doi: 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchez Rodriguez R, Pauli ML, Neuhaus IM, Yu SS, Arron ST, Harris HW, et al. Memory regulatory T cells reside in human skin. J Clin Invest. 2014;124(3):1027–36. doi: 10.1172/JCI72932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baron U, Floess S, Wieczorek G, Baumann K, Grutzkau A, Dong J, et al. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3(+) conventional T cells. Eur J Immunol. 2007;37(9):2378–89. doi: 10.1002/eji.200737594. [DOI] [PubMed] [Google Scholar]
- 30.Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5(2):e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burton OT, Logsdon SL, Zhou JS, Medina-Tamayo J, Abdel-Gadir A, Noval Rivas M, et al. Oral immunotherapy induces IgG antibodies that act through FcgammaRIIb to suppress IgE-mediated hypersensitivity. J Allergy Clin Immunol. 2014;134(6):1310–7. e6. doi: 10.1016/j.jaci.2014.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Savage JH, Courneya JP, Sterba PM, Macglashan DW, Saini SS, Wood RA. Kinetics of mast cell, basophil, and oral food challenge responses in omalizumab-treated adults with peanut allergy. J Allergy Clin Immunol. 2012;130(5):1123–9. e2. doi: 10.1016/j.jaci.2012.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Logsdon SL, Oettgen HC. Anti-IgE therapy: clinical utility and mechanistic insights. Curr Top Microbiol Immunol. 2015;388:39–61. doi: 10.1007/978-3-319-13725-4_3. [DOI] [PubMed] [Google Scholar]
- 34.Burton OT, Noval Rivas M, Zhou JS, Logsdon SL, Darling AR, Koleoglou KJ, et al. Immunoglobulin E signal inhibition during allergen ingestion leads to reversal of established food allergy and induction of regulatory T cells. Immunity. 2014;41(1):141–51. doi: 10.1016/j.immuni.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frischmeyer-Guerrerio PA, Masilamani M, Gu W, Brittain E, Wood R, Kim J, et al. Mechanistic correlates of clinical responses to omalizumab in the setting of oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2017;140(4):1043–53. e8. doi: 10.1016/j.jaci.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan JF, Hovde R, Glanville J, Lyu SC, Ji X, Gupta S, et al. Successful immunotherapy induces previously unidentified allergen-specific CD4+ T-cell subsets. Proc Natl Acad Sci U S A. 2016;113(9):E1286–95. doi: 10.1073/pnas.1520180113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berin MC, Mayer L. Can we produce true tolerance in patients with food allergy? J Allergy Clin Immunol. 2013;131(1):14–22. doi: 10.1016/j.jaci.2012.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santos AF, James LK, Bahnson HT, Shamji MH, Couto-Francisco NC, Islam S, et al. IgG4 inhibits peanut-induced basophil and mast cell activation in peanut-tolerant children sensitized to peanut major allergens. J Allergy Clin Immunol. 2015;135(5):1249–56. doi: 10.1016/j.jaci.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.