Abstract
Objective
To assess whether type of milk supplementation provided to breastfeeding late preterm infants (LPIs) was associated with length of stay (LOS) in the hospital or breastfeeding status at discharge.
Design
Retrospective chart review.
Setting
Tertiary care teaching hospital in the southern United States.
Participants
Late preterm infants 350/7-36 6/7 weeks gestational age (N=183) admitted to the mother-baby unit between November 1, 2014 and October 31, 2016.
Methods
The exposure of interest was type of milk supplementation, e.g., expressed human milk (EHM), pasteurized donor human milk (PDHM), and formula. Outcomes measured were LOS and breastfeeding status at discharge. Generalized Poisson regression models were used to compare LOS by type of milk supplementation. Modified Poisson regression models were used to estimate risk ratios and 95% confidence intervals for associations with breastfeeding status at discharge.
Results
The LOS for breastfed infants supplemented with EHM and/or PDHM did not differ significantly from exclusively breastfed infants who received no supplement. Exclusively formula fed infants had longer LOS of 3.2 days compared to 2.6 days for exclusively breastfed infants (p=0.001). Breastfed infants who received any formula supplementation were 16% less likely to continue breastfeeding until day of discharge compared to breastfed infants who received human milk supplementation (RR 0.84, 95% CI 0.77-0.92).
Conclusion
The high prevalence of supplementation among breastfeeding LPIs underscores the potential effect of type of milk supplementation on LOS and breastfeeding outcomes. Our findings suggest that human milk supplementation discourages transition to formula feeding before hospital discharge without increasing LOS.
Keywords: late preterm infant, donor milk, human milk, pasteurized donor human milk, pasteurization, breastfeeding, length of stay, supplementation
Late preterm infants (LPIs) born between 34 and 366/7 weeks gestational age are at increased risk of morbidity and mortality (Academy of Breastfeeding Medicine (ABM), 2016). Approximately 9.6% of U.S. births are preterm (<37 weeks), and approximately 72% of those births are defined as late preterm. This figure represents almost 275,000 of the nearly 4 million annual births in the U. S. (Martin, Hamilton, Osterman, Driscoll & Matthews, 2017). Much of the increased morbidity and mortality in LPIs is related to feeding difficulties. Effective oral feeding behavior is dependent upon the physical maturity of the infant’s brain. Since one third of a neonate’s brain develops between 35 and 41 week gestation (Hallowell & Spatz, 2012), many LPIs are born with immature neurological systems, which results in inconsistent sleep/wake cycles and observable feeding cues and fatigue while feeding at the breast (Briere, Lucas, McGrath, Lussier & Browne, 2015; Cartwell, Atz, Newman, Mueller & Demirci, 2017). Late preterm infants may also be similar in birth weight to healthy, term infants, which leads families and health care providers to assume they can be managed the same way as term newborns. In reality, LPIs are at increased risk of respiratory distress, poor thermoregulation, decreased stamina and alertness, weaker sucking and ineffective milk transfer at breast, all of which can increase risk of hypoglycemia, hyperbilirubinemia, weight loss, delay of discharge, hospital readmission after discharge, and breastfeeding failure (Adamkin, 2006; Boyle et al., 2015).
While breastfeeding, or human milk feeding, is recommended for all infants (excluding contraindications) for the first one to two years of life, it is even more important for optimal growth and brain development in preterm infants (American Academy of Pediatrics [AAP], 2012; Briere et al., 2015). Exclusively breastfed infants have increased white matter development in several brain regions that affects cognitive and behavioral outcomes (Deoni et al., 2013). One theory is that human milk is responsible for the difference in brain development, particularly the long chain polyunsaturated fatty acids that infants accrue rapidly in their brains during the last trimester of pregnancy (Hallowell & Spatz, 2012). Because preterm infants are born before brain growth is complete, they are even more reliant on the human milk fatty acids in their diets. In addition, preterm infants who are not fed human milk diets are at significantly increased risk of necrotizing enterocolitis, sepsis, gastrointestinal and respiratory infections, sudden infant death syndrome and greater number of hospital readmissions in the first year of life (AAP, 2012).
Optimal care to promote breastfeeding during the birth hospitalization includes skin-to-skin contact, early initiation of breastfeeding, rooming-in or non-separation of mother and newborn, limiting formula supplementation and provision of skilled lactation support (Munn, Newman, Mueller, Phillips & Taylor, 2016; World Health Organization, 1991). These practices are even more important for mothers of LPIs who may have delayed lactogenesis or experience challenges with initiation of breastfeeding (Meier, Furman & Degenhardt, 2007). Because LPIs are at increased risk of poor breastfeeding, they are even more likely to receive formula supplementation and to have shorter duration of breastfeeding (Nyqvist et al., 2013). While optimal breastfeeding practices are helpful, LPIs also require closer monitoring of feedings to ensure adequate intake, and mothers need skilled support to protect their milk supplies for long-term breastfeeding (Sables-Baus et al., 2013).
Mothers of LPIs often need to initiate milk expression in the hospital for supplemental feedings until their infants mature enough (38-40 weeks gestational age) to effectively breastfeed consistently (Briere et al., 2015). Some hospitals have policies to define medical indications for supplementation of breastfed infants, particularly for newborns on a mother-baby unit, starting first with mother’s own expressed milk and then use of formula (Mattsson, Hamilton, Osterman, Driscoll, & Matthews, 2015). Many medical organizations and protocols recommend supplementation with expressed mother’s own milk as available and then pasteurized donor human milk (PDHM) to improve outcomes and avoid formula supplementation (AAP, 2012; ABM, 2016; Association of Women’s Health, Obstetric and Neonatal Nurses, 2014). Use of PDHM is standard of care in most U.S. NICUs for very low birth weight infants, yet PDHM is not routinely recognized as an option for medically indicated supplementation of term or late preterm infants (Briere et al., 2015).
Briere et al. (2015) described actual hospital policies that may exclude LPIs from receipt of PDHM. Several researchers who studied LPIs and breastfeeding outcomes reported use of formula for supplementation as the only option after expressed mother’s milk (Gianni et al 2016; Goyal, Attanasio, & Kozhimannil, 2014; Mattsson et al., 2015). Many clinical protocols or guidelines for breastfeeding LPIs recommend use of PDHM when supplementation is medically indicated, but no published research is referenced to support this recommendation (AAP, 2012; ABM, 2014, 2016; Meier, Patel, Wright & Engstrom, 2013). In their overview of policy statements and practice guidelines related to breastfeeding LPIs, Briere et al. (2015) concluded that more research was needed to establish the use and benefits of donor milk for supplementation when mother’s own milk was not available. Lastly, the U.S. Surgeon General called for development of evidence-based clinical guidelines for the use of banked donor milk, particularly in infants with low or very low birth weight or prematurity (U.S. Department of Health and Human Services, 2011). To address these research gaps, we examined type of milk provided to LPIs admitted to a mother-baby unit, including supplementation with PDHM, expressed human milk, and formula. The primary aim of our study was to evaluate whether length of stay (LOS) in the hospital differed by type of supplemented milk provided. We also examined associations with breastfeeding status at hospital discharge as a secondary outcome of interest.
Methods
Setting and Sample
We conducted a retrospective chart review of late preterm infants at 350/7-366/7 weeks gestational age who were admitted to the mother-baby unit of a tertiary care teaching hospital in Oklahoma during a two-year period, November 1, 2014 through October 31, 2016. This review did not include LPIs born in the 34th week of gestation because of the facility’s policy to directly admit all preterm infants less than 35 weeks gestation age to the NICU. The hospital is formally working toward Baby-Friendly Hospital designation, so standard care practices to support breastfeeding couplets included rooming-in, immediate skin-to-skin contact, and assistance with initiation of breastfeeding within the first hour after birth. The hospital also had policies that defined supplementation of breastfeeding infants when medically necessary that included use of PDHM when own mother’s milk was not available in the NICU and the mother-baby unit. The study period was selected to include the two most recent calendar years of births following the establishment of the state’s nonprofit milk bank in late 2013.
To maintain data integrity, the chart review was limited to births that occurred before the hospital changed to a new documentation system in November 2016. A total of 624 late preterm births were identified from an electronic search of maternal hospital admissions during the study period. Infants directly admitted to the NICU were excluded (n=226). The documentation system for each infant admitted to the mother-baby unit was then manually reviewed to confirm eligibility. Three reviewers were trained to search the mother’s and infant’s electronic records for requested data using an online, secure data collection tool to ensure reliability. Upon review of the newborn clinical assessment for gestational age, 155 newborn infants were excluded for gestational age at birth outside the 350/7-366/7week criterion. Additional exclusions included transfer to the NICU (n=25), infant death due to anencephaly (n=1), multiple gestation (n=26), maternal complications or illness leading to separation (n=5), and infants entering into state custody (n=3). The final sample size for analysis included 183 LPIs.
For power calculations, we assumed a baseline mean length of hospital stay of 2.5 days for LPIs admitted to the mother-baby unit (Harron et al., 2017; Aly et al., 2015; Mattsson et al., 2015) with 70% initiating breastfeeding (Radtke, 2011) and 20% of those feeding exclusively at the breast without supplementation (Zanardo et. al., 2011). We estimated that a sample size of 180 would have 80% power to detect an incidence rate ratio of 1.55 for the comparison of time to hospital discharge for LPIs breastfeeding with supplementation compared to those breastfeeding without supplementation, assuming an alpha of 0.05 and correlation of 0.2 between breastfeeding supplementation and other covariates. This study was reviewed and approved by the study site’s Institutional Review Board.
Measures
Infant feeding status (breastfeeding, formula feeding) was recorded from the infant’s medical record as determined by maternal intent on admission. Among breastfeeding LPIs, supplemental feedings were recorded as expressed human milk, PDHM, or formula, and reason for supplementation was recorded as maternal request, physician order for medical indication, or other. For analysis, milk type was coded and examined as six mutually exclusive categories: breastfeeding only (n=27, 14.8%), breastfeeding with PDHM supplementation only (n=8, 4.4%), breastfeeding with expressed human milk supplementation with or without PDHM (n=12, 6.6%), breastfeeding with supplementation of formula and human milk (expressed human milk or PDHM; n=14, 7.7%), breastfeeding with formula supplementation only (n=79, 43.2%), and formula feeding only (n=43, 23.5%). Because of limited sample size for some categories, milk type categories were collapsed into four categories: breastfeeding only, breastfeeding supplemented with human milk only (expressed human milk or PDHM), breastfeeding with any formula supplementation, and formula feeding only.
The primary outcome of interest, LOS, was calculated by subtracting date of birth from date of discharge and measured in days. Thus, the date of birth was defined as day 0. Feeding status at the time of hospital discharge was classified in the medical record as three categories designating exclusive breastfeeding, supplemented breastfeeding, or formula feeding. Among infants receiving supplementation, this secondary outcome of interest was defined as a binary indicator of any breastfeeding at the time of hospital discharge. Maternal demographic and medical characteristics collected from the medical record included maternal age, race/ethnicity, parity, insurance status, mode of birth with or without labor induction and maternal complications such as hypertension and gestational diabetes. Infant characteristics included gestational age at birth, birth weight, infant sex, and medical complications noted as hypoglycemia, hyperbilirubinemia, and difficulty breastfeeding.
Statistical Analysis
Infant and maternal characteristics were compared by milk type using Chi-square tests and Fisher’s exact tests. Associations between milk type and LOS were evaluated using generalized Poisson regression models with robust standard errors (Harris, Yang, & Hardin, 2012). Generalized Poisson models are appropriate for count data with under dispersion (i.e., variance smaller than the mean), a violation of the Poisson distribution which can lead to overestimated standard errors (Harris et al. 2012). The negative dispersion parameter (delta<0) calculated by the generalized Poisson regression model confirmed the presence of under dispersion in these data. Comparisons with zero-truncated Poisson regression models and zero-truncated negative binomial models using Akaike’s (AIC) and Bayesian information criteria (BIC) verified that model fit was improved by the generalized Poisson regression model. Results of LOS analyses were expressed as incidence rate ratios (IRR) and 95% confidence intervals as well as estimated marginal mean days with 95% confidence intervals. Among the subset of breastfed infants who received supplementation (n=113), modified Poisson regression models were used to estimate risk ratios (RR) and 95% confidence intervals for associations with the binary outcome of any breastfeeding at discharge.
Covariates that changed the magnitude of the associations with milk type by more than 10% when included cumulatively in the multivariable model that used a manual forward selection approach were controlled as confounders in the final adjusted models. Covariates evaluated as potential confounders included maternal age (<24, 25-34, 35+ years), race (initially evaluated as White, Black and other and collapsed to White vs non-White to maximize model precision when results were consistent), medical insurance (Medicaid vs other payor), parity (≥1 vs 1), cesarean (yes/no), labor induction (yes/no), maternal hypertension (yes/no), gestational diabetes (yes/no), obesity (body mass index [kg/m2] at admission for birth ≥30 vs <30), maternal smoking (yes/no), gestational age (35 vs 36 weeks), small for gestational age (yes/no according to sex-specific birth weight for gestational age < 10th percentile of national standards) (Duryea, Hawkins, McIntire, Casey, & Leveno, 2014), hyperbilirubinemia (yes/no), hypoglycemia (yes/no), difficulty breastfeeding (yes/no), infant sex (male vs female) and physician ordered supplementation (yes/no).
Covariates that met the criteria for confounding and were controlled in the analyses for LOS included maternal age, race, medical insurance, parity, cesarean section, maternal hypertension, gestational age, hyperbilirubinemia and physician orders for supplementation. No covariates met the 10% criterion for confounding in the analyses of breastfeeding status at discharge; thus, only unadjusted results are reported for this outcome. Statistical analyses were conducted using STATA version 14.2 (College Station, TX) and SAS version 9.4 (Cary, NC) software. Statistical significance was defined as p<0.05.
Results
The maternal and infant characteristics of the study populations are displayed in Table 1. The LPIs were born to mothers who were predominantly less than age 35 (85.8%, n=157), had previous live births (63.9%, n=117), were obese (60.5%, n=107), and whose medical coverage was provided by Medicaid (68.3%, n=125). Most infants were born in the 36th week of gestation (75.4%, n=138). Hyperbilirubinemia (35.5%, n=65) and hypoglycemia (28.4%, n=52) were frequent neonatal complications.
Table 1.
Distribution of maternal and infant characteristics by milk type for 183 late preterm infants admitted to the mother-baby unit
| Milk Type | Breastfeeding Only (n=27) n (%) |
Breastfeeding Supplemented With Human Milk (n=20) n (%) |
Breastfeeding Supplemented with Any Formula (n=93) n (%) |
Formula Only (n=43) n (%) |
Total (n=183) n (% of total) |
pa |
|---|---|---|---|---|---|---|
| Maternal Age | 0.67 | |||||
| < 24 years | 10 (37.0) | 10 (50.0) | 31 (33.3) | 16 (37.2) | 67 (36.6) | |
| 25 to 34 years | 15 (55.6) | 7 (35.0) | 46 (49.5) | 22 (51.2) | 90 (49.2) | |
| ≥ 35 years | 2 (7.4) | 3 (15.0) | 16 (17.0) | 5 (11.6) | 26 (14.2) | |
| Maternal Race | 0.04 | |||||
| White | 16 (59.3) | 11 (55.0) | 40 (43.0) | 12 (27.9) | 79 (43.0) | |
| Non-white | 11 (40.7) | 9 (45.0) | 53 (57.0) | 31 (72.1) | 104 (56.8) | |
| Medical Insurance | 0.13 | |||||
| Other Payor | 12 (44.4) | 7 (35.0) | 31 (33.3) | 8 (18.6) | 58 (31.7) | |
| Medicaid | 15 (55.6) | 13 (65.0) | 62 (66.7) | 35 (81.4) | 125 (68.3) | |
| Parity | 0.002 | |||||
| > 1 | 22 (81.5) | 7 (35.0) | 55 (59.1) | 33 (76.7) | 117 (63.9) | |
| 1 | 5 (18.5) | 13 (65.0) | 38 (40.9) | 10 (23.3) | 66 (36.1) | |
| Cesarean | 0.76 | |||||
| Yes | 8 (29.6) | 4 (20.0) | 25 (26.9) | 14 (32.6) | 51 (27.9) | |
| No | 19 (70.4) | 16 (80.0) | 68 (73.1) | 29 (67.4) | 132 (72.1) | |
| Labor Induction | 0.21 | |||||
| Yes | 2 (7.4) | 6 (30.0) | 20 (21.5) | 11 (25.6) | 39 (21.3) | |
| No | 25 (92.6) | 14 (70.0) | 73 (78.5) | 32 (74.4) | 144 (78.7) | |
| Hypertension | 0.09 | |||||
| Yes | 6 (22.2) | 9 (45.0) | 30 (32.3) | 21 (48.8) | 66 (36.1) | |
| No | 21 (77.8) | 11 (55.0) | 63 (67.7) | 22 (51.2) | 117 (63.9) | |
| Gestational Diabetes | 0.72b | |||||
| Yes | 2 (7.4) | 2 (10.0) | 15 (16.1) | 7 (16.3) | 26 (14.2) | |
| No | 25 (92.6) | 18 (90.0) | 78 (83.9) | 36 (83.7) | 157 (85.8) | |
| Obesityc | 0.61 | |||||
| Body mass index ≥30 | 18 (66.7) | 9 (47.4) | 56 (61.5) | 24 (60.0) | 107 (60.5) | |
| Body mass index <30 | 9 (33.3) | 10 (52.6) | 35 (38.5) | 16 (40.0) | 70 (39.6) | |
| Smoking | 0.62b | |||||
| Yes | 1 (3.7) | 2 (10.0) | 6 (6.5) | 5 (11.6) | 14 (7.7) | |
| No | 26 (96.3) | 18 (90.0) | 87 (93.6) | 38 (88.4) | 169 (92.4) | |
| Gestational Age | 0.49 | |||||
| 35 weeks | 4 (14.8) | 4 (20.0) | 24 (25.8) | 13 (30.2) | 45 (24.6) | |
| 36 weeks | 23 (85.2) | 16 (80.0) | 69 (74.2) | 30 (69.8) | 138 (75.4) | |
| Small for Gestational Age | 0.88b | |||||
| Yes | 1 (3.7) | 2 (10.0) | 8 (8.6) | 3 (7.0) | 14 (7.7) | |
| No | 26 (96.3) | 18 (90.0) | 85 (91.4) | 40 (93.0) | 169 (92.4) | |
| Hyperbilirubinemia | 0.49 | |||||
| Yes | 8 (29.6) | 10 (50.0) | 33 (35.5) | 14 (32.6) | 65 (35.5) | |
| No | 19 (70.4) | 10 (50.0) | 60 (64.5) | 29 (67.4) | 118 (64.5) | |
| Hypoglycemia | 0.001 | |||||
| Yes | 2 (7.4) | 10 (50.0) | 34 (36.6) | 6 (14.0) | 52 (28.4) | |
| No | 25 (92.6) | 10 (50.0) | 59 (63.4) | 37 (86.1) | 131 (71.6) | |
| Difficulty Breastfeeding | 0.12b | |||||
| Yes | 0 (0.0) | 2 (10.0) | 5 (5.4) | 0 (0.0) | 7 (3.8) | |
| No | 27 (100.0) | 18 (90.0) | 88 (94.6) | 43 (100.0) | 176 (96.2) | |
| Reason for Supplementation | <0.0001 | |||||
| Physician Order | 0 (0.0) | 14 (70.0) | 21 (22.6) | 0 (0.0) | 35 (19.1) | |
| Maternal Request | 0 (0.0) | 2 (10.0) | 45 (48.4) | 0 (0.0) | 47 (25.7) | |
| Unknown Reason | 0 (0.0) | 4 (20.0) | 27 (29.0) | 0 (0.0) | 31 (16.9) | |
| Not Supplemented | 27 (100.0) | 0 (0.0) | 0 (0.0) | 43 (100.0) | 70 (38.3) | |
| Breastfeeding Status at Hospital Discharge | <0.0001 | |||||
| Exclusive Breastfeeding | 27 (100.0) | 20 (100.0) | 0 (0.0) | 0(0.0) | 47 (25.7) | |
| Supplemented Breastfeeding | 0 (0.0) | 0 (0.0) | 78 (83.9) | 3 (7.0) | 81 (44.3) | |
| Formula Feeding | 0 (0.0) | 0 (0.0) | 15 (16.1) | 40 (93.0) | 55 (30.1) |
Chi-square test, except where otherwise noted;
Fisher’s exact test;
Six patients were missing information on body mass index.
At birth, most mothers initiated breastfeeding (76.5%, n=140). The majority of breastfed LPIs (113 of 140, 80.7%) received supplementation with human milk or formula during their hospital stays. Among all LPIs, the distribution of milk type was 14.8% (n=27) exclusive breastfeeding, 10.9% (n=20) breastfeeding with human milk supplementation, 50.8% (n=93) breastfeeding with formula supplementation, and 23.5% (n=43) exclusive formula feeding (Table 2). Of the 113 breastfed LPIs who received supplementation, 69.9% (n=79) had formula, 12.4% (n=14) had formula and human milk (expressed human milk or PDHM), 10.6% (n=12) had expressed human milk (with or without PDHM), and 7.1% (n=8) had PDHM only. The mean LOS was 2.91 days (standard deviation=0.92, range=1-6 days, median=3 days; Figure 1). At hospital discharge, 25.7% (n=47) of LPIs were exclusively breastfeeding, 44.3% (n=81) were supplemented and breastfeeding, and 30.1% (n=55) were exclusively formula feeding (Table 1).
Table 2.
Associations between milk type and length of stay among 183 late preterm infants
| Milk Type | Number of Infants by Milk Type n (%) | Mean LOSa Days (95% CI) | Unadjusted IRR (95% CI)b | p | Adjusted IRR (95% CI)b,c | p |
|---|---|---|---|---|---|---|
| Breastfeeding Only | 27 (14.8%) | 2.58 (2.33-2.83) | REFERENCE | REFERENCE | ||
| Breastfeeding Supplemented with EHM or PDHM | 20 (10.9%) | 2.95 (2.56-3.35) | 1.46 (1.23-1.73) | <0.001 | 1.14 (0.96-1.37) | 0.14 |
| Breastfeeding with Any Formula Supplementationd | 93 (50.8%) | 2.86 (2.71-3.00) | 1.23 (1.12-1.36) | <0.001 | 1.11 (1.00-1.23) | 0.06 |
| Formula Only | 43 (23.5%) | 3.19 (2.92-3.45) | 1.34 (1.18-1.52) | <0.001 | 1.23 (1.09-1.40) | 0.001 |
Note. EHM = expressed human milk; PDHM = pasteurized donor human milk.
Predicted mean length of stay (LOS) calculated using Generalized Poisson regression models adjusted for maternal age, race, medical insurance, parity, cesarean section, gestational age, maternal hypertension, physician orders for supplementation and hyperbilirubinemia.
IRR = Incidence Rate Ratio calculated using modified Poisson regression, CI = 95 % Confidence Interval
Modified Poisson regression model controlling for maternal age, race, medical insurance, parity, cesarean section, gestational age, maternal hypertension, physician orders for supplementation and hyperbilirubinemia.
Breastfeeding with any formula supplementation includes formula supplementation only and formula supplementation plus EHM or PDHM.
Figure 1.
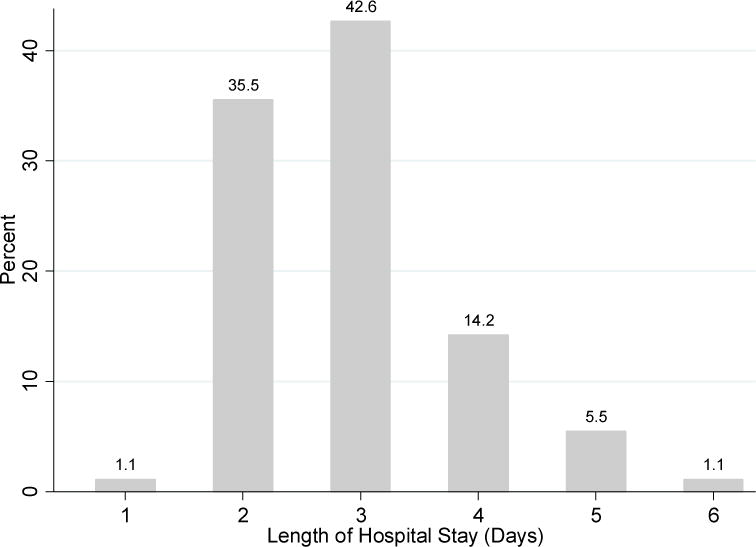
Distribution of length of hospital stay in days for 183 late preterm infants.
Our data indicate that the most frequent reason given for supplementation was maternal request, which occurred for 25.7% (n=47) of all LPIs. Supplementation by physician order was recorded in 19.1% (n=35), and 16.9% (n=31) were supplemented for undetermined reasons. Of the 93 breastfeeding LPIs supplemented with formula, a greater proportion were supplemented because of the mother’s request (48%, n=45) followed by unknown reasons (29%, n=27) and physician order (23%, n=21). Supplementation with human milk (expressed human milk or PDHM, n=20)) was due to physician order (70%, n=14), followed by unknown reasons (20%, n=4), and maternal request (10%, n=2).
In unadjusted analyses, LOS was significantly longer for exclusively formula fed infants (IRR 1.34, 95% CI 1.18-1.52) and breastfeeding infants who received human milk (IRR 1.45, 95% CI 1.23-1.73) or formula (IRR 1.23, 95% CI 1.12-1.36) when compared to exclusively breastfed infants (see Table 2). After controlling for maternal age, race, medical insurance, parity, cesarean, gestational age, maternal hypertension, physician orders for supplementation and hyperbilirubinemia, the LOS for breastfed LPIs supplemented with expressed human milk or PDHM was no longer significantly different than exclusively breastfed LPIs, and the rate ratio was attenuated (IRR 1.14, 95% CI 0.96-1.37). When the human milk supplementation group was further separated into those who supplemented with PDHM only (n=8, 4.4% overall) and those who supplemented with the mother’s own expressed human milk (with or without the addition of PDHM, n=12, 6.6% overall), the adjusted association with PDHM supplementation was further reduced (IRR 1.08, 95% CI 0.88-1.33, p=0.45). Furthermore, the magnitude of association with expressed human milk supplementation remained similar to results for the combined group and not statistically significant (IRR 1.18, 95% CI 0.95-1.47, p=0.14).
The results for these refined milk supplementation categories, however, should be interpreted with caution because of the small numbers within the separate groups. The association between LOS and use of any formula supplementation whether or not accompanied by human milk supplementation was modest and marginally significant (IRR 1.11, 95% CI 1.00-1.23) (Table 2). The results were similar when the formula supplementation group was examined separately for those with formula and human milk supplementation (IRR 1.12, 95% CI 0.95-1.33, p=0.17) and those with formula supplementation only (IRR 1.10, 95% CI 0.99-1.23, p=0.08). However, LOS was estimated to be 23% greater for LPIs who exclusively formula fed compared to those who were exclusively breastfed (IRR 1.23, 95% CI 1.09-1.40). The mean LOS estimated by these adjusted models was 3.19 days (95% CI 2.92-3.45) for exclusively formula fed LPIs and 2.58 days (95% CI 2.34-2.83) for exclusively breastfed LPIs (Table 2). The confidence intervals for mean LOS for these two groups do not overlap, demonstrating the statistical significance of the observed difference.
Analyses were restricted to the 113 breastfed LPIs who received some form of supplementation to compare the proportion of these infants with any breastfeeding at hospital discharge. Any breastfeeding at discharge was defined as exclusive breastfeeding or some breastfeeding. All breastfed LPIs who received human milk supplementation without formula supplementation remained classified as exclusively breastfed at hospital discharge (AAP 2012) whereas 16% (n=15) of breastfed LPIs who received any formula supplementation with or without the addition of human milk supplementation transitioned to solely formula feeding on day of hospital discharge (Table 1). Thus, breastfed LPIs receiving any formula supplementation were less likely, specifically 0.84 times as likely, to be breastfeeding on day of discharge compared to breastfeeding LPIs receiving human milk supplementation (RR 0.84, 95% CI 0.77-0.92, Table 3).
Table 3.
Association between type of milk supplementation provided to late preterm infants and status of any breastfeeding at hospital discharge among 113 breastfeeding infants receiving supplementation
| Milk Type | Total Supplemented n (%) | Any Breastfeeding at Discharge n (% of row) | Unadjusted RR (95% CI)a | p |
|---|---|---|---|---|
| Breastfeeding Supplemented with EHM or PDHM | 20 (17.7%) | 20 (100%) | Reference | |
| Breastfeeding with Any Formula Supplementation | 93 (82.3%) | 78 (83.9%) | 0.84 (0.77-0.92) | 0.0001 |
Note. EHM = expressed human milk; PDHM = pasteurized donor human milk.
RR = Risk Ratio, CI = 95 % Confidence Interval
Discussion
Although researchers have established a link between human milk supplementation and improved outcomes among very preterm and low birth weight infants in the NICU (Abrams, Schanler, Lee, & Rechtman, 2014; Corpeleijn et al., 2016; Quigley & McGuire 2014; Sullivan et al. 2010), the effects of milk supplementation in LPIs have not been adequately assessed. The results of our study reveal similar LOS for breastfed LPIs supplemented with expressed human milk and/or PDHM when compared to exclusively breastfed LPIs who received no supplement. Exclusively formula fed LPIs, however, had a statistically significant longer LOS of 3.2 days compared to 2.6 days for exclusively breastfed LPIs. Given the high percentage of LPIs supplemented solely with formula when expressed human milk and/or PDHM were available options, these findings suggest that an opportunity may exist to reduce LOS in LPIs by increasing exclusive breastfeeding rates at discharge. These findings are generally consistent with those of Mattsson el al. (2015), who evaluated the effects of milk supplementation on LOS among LPIs. In their study of 77 healthy LPIs (345/7-366/7 weeks gestational age) born in a Swedish hospital and cared for in family rooms, they reported a higher mean LOS in LPIs who received formula supplementation on a regular basis than those who were exclusively breastfed. In our study, formula supplementation was also associated with a modestly increased LOS compared to exclusive breastfeeding, but this finding only approached statistical significance with a p value of 0.06. Mattsson et al. (2015), evaluated LOS as the unadjusted difference of means and thus did not take potentially confounding factors into account or apply statistical methods considered applicable for count data. Furthermore, neither donor human milk nor expressed human milk were evaluated because of a hospital policy that reserved the limited supply of PDHM for use only in the NICU. Thus, our study expands upon the findings of Mattsson et al. (2015) by addressing methodologic limitations to control for suspected confounders with the use of a multivariable regression technique for count data. Our findings mitigate concerns that the use of PDHM may discourage mothers from breastfeeding (Bertino et al., 2013). Further research is needed to confirm the absence of an adverse effect of PDHM supplementation on hospital LOS for LPIs and its potential effect on other neonatal outcomes as well as breastfeeding duration after hospital discharge.
The results of this retrospective chart review highlight the challenges in breastfeeding LPIs because more than 80% of breastfeeding LPIs admitted to the mother-baby unit and rooming-in with their mothers received some form of supplementation. Despite the use of PDHM in this facility since 2011 and the supply of PDHM from the state’s own nonprofit accredited milk bank since 2013, less than 1 out of 5 (17.7%) breastfeeding LPIs who were provided supplementation in this study received PDHM as a supplement (with or without other types of milk). Most breastfeeding LPIs who received supplementation were supplemented with formula alone or in combination with human milk. These findings are consistent with those from a 2015 Centers for Disease Control and Prevention survey in which only 26% of the responding U.S. hospitals reported that they limited non-breast milk feeding of breastfed infants (Perrine et al., 2015). This pattern of practice in our study is particularly concerning since the hospital has a policy that clearly established availability of PDHM for medically indicated supplementation on the mother-baby unit, i.e., not restricted to only those infants admitted to the NICU. Although we do not know why mothers asked for their infants to be supplemented or whether the type of supplementation received corresponded with the type requested, the mothers’ requests to supplement could stem from lack of intent to exclusively breastfeed or failure of the hospital staff to provide adequate breastfeeding education that included safety and efficacy of PDHM.
Limitations
Several limitations of this study were identified. Because of the small number of LPIs supplemented with human milk, the precision of our estimates and statistical power for the detection of differences between groups was limited, particularly when we further subdivided the group to analyze PDHM and expressed human milk separately. In addition, the eligible study population was initially identified using gestational age recorded at maternal admission. Subsequent manual review of the selected records confirmed the late preterm birth status of the study population that used the gestational age determination after birth. As a retrospective chart review, the study was limited to data recorded for clinical rather than research purposes, which may have impacted the quality and detail of some measures. In addition, data on infant feeding were extracted from nursing documentation and reason for supplementation was not always clearly stated. This was addressed by analyzing unknown reason as a separate category. As a result, the proportion of supplementation attributed to maternal request and physician orders is likely underestimated. Furthermore, data for the covariate smoking is likely to be incompletely recorded in the medical record. The low prevalence of maternal smoking in our study population suggests that this measure was susceptible to measurement error that may have influenced our ability to adequately assess potential confounding by smoking. Other covariates of potential interest such as any prior preterm live birth or previous breastfeeding experience were not available for evaluation.
The results of this study are generalizable to predominantly low income, multiparous women with high prevalence of obesity in similar tertiary care hospitals. Similar research should be conducted for infants representative of other LPI populations, including those born to primiparous, non-obese women and in households with moderate socioeconomic status. The majority of mothers had a predisposing risk for not breastfeeding as they were obese and low-income (Dieterich, Felice, O’Sullivan, & Rasmussen, 2013; Kair & Colaizy, 2016), yet 76% initiated breastfeeding. This result may reflect the study hospital’s pursuit of Baby-Friendly Hospital designation and the implementation of many practices that provide evidence-based support for breastfeeding couplets.
Implications for Practice and Research
Supplementation with formula during the hospital stay is a known risk factor for early cessation of breastfeeding in term infants (Perrine, Scanlon, Ruowei, Odom, & Grummer-Strawn, 2012), and our study demonstrated similar results with LPIs. Given the known importance of breastfeeding in this vulnerable population (AAP, 2012), our findings reinforced the evidence base that includes supplementation with expressed human milk or PDHM when medical indications exist. Maternal education on the importance of exclusive breastfeeding for LPIs (Kair & Colaizy 2016) and the potential value of supplementation with PDHM in addition to expressed maternal milk may also reduce the high percentage of maternal requests for formula supplementation.
Optimal care for LPIs must also include close monitoring of feedings and maternal education to discern when supplementation is truly medically indicated. Nurses can promote breastfeeding through education on LPI behavior, feeding ability, and normal volume of intake. In particular, educating mothers on supplementation options when medically indicated and the importance of human milk supplementation to support LPI brain development and continued breastfeeding could empower more mothers to sustain exclusive breastfeeding. Nurses and physicians have an opportunity to educate themselves and families about the importance of exclusive breastfeeding for this vulnerable infant population that includes the safety and potential efficacy of PDHM.
Conclusion
The high prevalence of supplementation among breastfeeding LPIs underscores the effect of type of milk supplementation on outcomes in this high-risk population. Our findings suggest that human milk supplementation (expressed human milk and/or PDHM) discourages transition to formula feeding before hospital discharge without an associated increase in the LOS. Future studies are needed to examine how organizational and clinical practice patterns positively or negatively impact human milk supplementation patterns among late preterm infants and to evaluate the continuum of neonatal and breastfeeding outcomes associated with pasteurized donor human milk supplementation as compared to expressed human milk and formula supplementation.
Callout 1
Late preterm infants need human milk for optimal growth and brain development but are at increased risk of poor breastfeeding and shorter breastfeeding duration.
Callout 2
Length of stay was similar for late preterm infants exclusively breastfed or human milk supplemented but increased by 23% among exclusively formula fed infants.
Callout 3
Hospital practices that support human milk supplementation in late preterm infants may deter transition to formula feeding before hospital discharge without increasing hospital stay.
Acknowledgments
N/A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors report no conflict of interest or relevant financial relationships.
Note
Supplementation with human milk discourages transition to formula feeding before hospital discharge without increasing length of stay for late preterm infants.
Contributor Information
Rebecca Mannel, Clinical assistant professor in the Department of Obstetrics and Gynecology and Director of the Oklahoma Breastfeeding Resource Center, University of Oklahoma Health Sciences Center, Oklahoma City, OK.
Jennifer D. Peck, Professor in the Department of Biostatistics and Epidemiology, College of Public Health, University of Oklahoma Health Sciences Center, Oklahoma City, OK.
References
- Aly H, Hoffman H, El-Dib M, Said L, Mohamed M. Factor affecting length of stay in late preterm infants: a US national database study. Journal of Maternal-Fetal & Neonatal Medicine. 2015;28(5):598–604. doi: 10.3109/14767058.2014.927428. [DOI] [PubMed] [Google Scholar]
- Abrams SA, Schanler RJ, Lee ML, Rechtman DJ. Greater mortality and morbidity in extremely preterm infants fed a diet containing cow milk protein products. Breastfeeding Medicine. 2014;9(6):281–5. doi: 10.1089/bfm.2014.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Academy of Breastfeeding Medicine. ABM clinical protocol #1: Guidelines for blood glucose monitoring and treatment of hypoglycemia in term and late-preterm neonates, Revised 2014. Breastfeeding Medicine. 2014;9(4):173–179. doi: 10.1089/bfm.2014.9986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Academy of Breastfeeding Medicine. ABM clinical protocol #10: Breastfeeding the late preterm (34–36 6/7 weeks of gestation) and early term infants (37–38 6/7 weeks of gestation), second revision 2016. Breastfeeding Medicine. 2016;11(10):494–500. doi: 10.1089/bfm.2016.29031.egb. [DOI] [PubMed] [Google Scholar]
- Adamkin DH. Feeding problems in the late preterm infant. Clinics in Perinatology. 2006;33(4):831–837. doi: 10.1016/j.clp.2006.09.003. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):600–603. doi: 10.1542/pes.2011-3552. [DOI] [Google Scholar]
- Association of Women’s Health, Obstetric and Neonatal Nurses. Breastfeeding. AWHONN Position statement. Journal of Obstetric, Gynecologic, & Neonatal Nursing. 2014;44(1):145–150. doi: 10.1111/1552-6909.12530. [DOI] [Google Scholar]
- Bertino E, Giuliani F, Baricco M, Nicola PD, Peila C, Vassia C, Coscia A. Benefits of donor milk in the feeding of preterm infants. Early Human Development. 2013;89(Suppl 2):S3–S6. doi: 10.1016/j.earlhumdev.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Boyle EM, Johnson S, Manktelow B, Seaton SE, Draper ES, Smith LK, Field DJ. Neonatal outcomes and delivery of care for infants born late preterm or moderately preterm: A prospective population-based study. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2015;100(6):F479–F485. doi: 10.1136/archdischild-2014-307347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briere CE, Lucas R, McGrath JM, Lussier M, Brownell E. Establishing breastfeeding with the late preterm infant in the NICU. Journal of Obstetric, Gynecologic & Neonatal Nursing. 2015;44(1):102–113. doi: 10.1111/1552-6909.12536. [DOI] [PubMed] [Google Scholar]
- Cartwright J, Atz T, Newman S, Mueller M, Demirci JR. An integrative review of interventions to promote breastfeeding in the late preterm infant. Journal of Obstetric, Gynecologic & Neonatal Nursing. 2017;46(3):347–356. doi: 10.1016/j.jogn.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpeleijn WE, de Waard M, Christmann V, van Goudoever JB, Jansen-van der Weide MC, Kooi EM, van Zoeren-Grobben D. Effect of donor milk on severe infections and mortality in very low-birth-weight infants: The early nutrition study randomized clinical trial. JAMA Pediatrics. 2016;170(7):654–61. doi: 10.1001/jamapediatrics.2016.0183105. [DOI] [PubMed] [Google Scholar]
- Deoni SC, Dean DC, III, Piryatinsky I, O’Muircheartaigh J, Waskiewicz N, Lehman K, Dirks H. Breastfeeding and early white matter development: A cross-sectional study. NeuroImage. 2013;82:77–86. doi: 10.1016/j.neuroimage.2013.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich CM, Felice JP, O’Sullivan E, Rasmussen KM. Breastfeeding and health outcomes for the mother-infant dyad. Pediatric Clinics of North America. 2013;60(1):31–48. doi: 10.1016/j.pcl.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duryea EL, Hawkins JS, McIntire DD, Casey BM, Leveno KJ. A revised birth weight reference for the United States. Obstetrics & Gynecology. 2014;124(1):16–22. doi: 10.1097/AOG.0000000000000345. [DOI] [PubMed] [Google Scholar]
- Giannì ML, Bezze E, Sannino P, Stori E, Plevani L, Roggero P, Mosca F. Facilitators and barriers of breastfeeding late preterm infants according to mothers’ experiences. BMC Pediatrics. 2016;16(1):179. doi: 10.1186/s12887-016-0722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal NK, Attanasio LB, Kozhimannil KB. Hospital care and early breastfeeding outcomes among late preterm, early-term, and term infants. Birth. 2014;41(4):330–8. doi: 10.1111/birt.12135. [DOI] [PubMed] [Google Scholar]
- Hallowell SG, Spatz DL. The relationship of brain development and breastfeeding in the late-preterm infant. Journal of Pediatric Nursing. 2012;27(2):154–162. doi: 10.1016/j.pedn.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Harris T, Yang Z, Hardin JW. Modeling underdispersed count data with generalized Poisson regression. The Stata Journal. 2012;12(4):736–747. [Google Scholar]
- Harron K, Gilbert R, Cromwell D, Oddie S, van der Meulen J. Newborn length of stay and risk of readmission. Paediatric and Perinatal Epidemiology. 2017;31(3):221–232. doi: 10.1111/ppe.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kair LR, Colaizy T. Breastfeeding continuation among late preterm infants: Barriers, facilitators, and any association with NICU admission? Hospital Pediatrics. 2016;6(5):261–268. doi: 10.1542/hpeds.2015-0172. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Mathews TJ. Births: final data for 2015. National Vital Statistics Report. 2017;66(1) Retrieved from https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_01.pdf. [PubMed] [Google Scholar]
- Mattsson E, Funkquist EL, Wickström M, Nyqvist KH, Volgsten H. Healthy late preterm infants and supplementary artificial milk feeds: Effects on breastfeeding and associated clinical parameters. Midwifery. 2015;31(4):426–431. doi: 10.1016/j.midw.2014.12.004\. [DOI] [PubMed] [Google Scholar]
- Meier PP, Furman LM, Degenhardt M. Increased lactation risk for late preterm infants and mothers: Evidence and management strategies to protect breastfeeding. Journal of Midwifery & Women’s Health. 2007;52(6):579–587. doi: 10.1016/j.jmwh.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Meier P, Patel AL, Wright K, Engstrom JL. Management of breastfeeding during and after the maternity hospitalization for late preterm infants. Clinics in Perinatology. 2013;40(4):689–705. doi: 10.1016/j.clp.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn AC, Newman SD, Mueller M, Phillips SM, Taylor SN. The impact in the United States of the baby-friendly hospital initiative on early infant health and breastfeeding outcomes. Breastfeeding Medicine. 2016;11(5):222–230. doi: 10.1089/bfm.2015.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyqvist KH, Haggkvist AP, Hansen MN, Kylberg E, Frandsen AL, Maastrup R, Haiek LN. Expansion of the Baby-Friendly Hospital Initiative Ten Steps to Successful Breastfeeding into neonatal intensive care: Expert group recommendations. Journal of Human Lactation. 2013;29:300–309. doi: 10.1177/0890334413489775. [DOI] [PubMed] [Google Scholar]
- Perrine CG, Scanlon KS, Ruowei L, Odom E, Grummer-Strawn LM. Baby-Friendly hospital practices and meeting exclusive breastfeeding intention. Pediatrics. 2012;130(1):54–60. doi: 10.1542/peds.2011-3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine CG, Galuska DA, Dohack JL, Shealy KR, Murphy PE, Grummer-Strawn LM, Scanlon KS. Vital signs: Improvements in maternity care policies and practices that support breastfeeding — United States, 2007–2013. Morbidity and Mortality Weekly Report. 2015;64(39):1112–1127. doi: 10.15585/mmwr.mm6439a5. [DOI] [PubMed] [Google Scholar]
- Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Systematic Reviews. 2014;4:CD002971. doi: 10.1002/14651858.CD002971.pub3106. [DOI] [PubMed] [Google Scholar]
- Radtke JV. The paradox of breastfeeding-associated morbidity among late preterm infants. Journal of Obstetric, Gynecologic, & Neonatal Nursing. 2011;40(1):9–24. doi: 10.1111/j.1552-6909.2010.01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sables-Baus S, DeSanto K, Henderson S, Kunz JL, Morris AC, Shields L, McGrath JM. Infant-directed oral feeding for premature and critically ill hospitalized infants: Guideline for practice. Chicago, IL: National Association of Neonatal Nurses; 2013. [Google Scholar]
- Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawöger R, KiechlKohlendorfer U, Lucas A. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. Journal of Pediatrics. 2010;156(4):562–7. doi: 10.1016/j.jpeds.2009.10.040107. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The Surgeon General’s call to action to support breastfeeding. 2011 Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK52682/pdf/Bookshelf_NBK52682.pdf.
- World Health Organization & United Nations Children’s Fund. Baby-Friendly Hospital Initiative. Geneva, Switzerland: World Health Organization; 1991. [Google Scholar]
- Zanardo V, Gambina I, Begley C, Litta P, Cosmi E, Giustardi A, Trevisanuto D. Psychological distress and early lactation performance in mothers of late preterm infants. Early Human Development. 2011;87(4):321–3. doi: 10.1016/j.earlhumdev.2011.01.0. [DOI] [PubMed] [Google Scholar]


