Abstract
Background
The diagnosis of malnutrition remains controversial. Further, it is unknown if physician diagnosis of malnutrition impacts outcomes. We sought to compare outcomes of patients with physician diagnosed malnutrition to patients recognized as malnourished by registered dietitians but not physicians and to describe the impact of each of six criteria on the diagnosis of malnutrition.
Methods
We conducted a retrospective cohort study of adult patients identified as meeting criteria for malnutrition. Pediatric, psychiatric, maternity and rehabilitation patients were excluded. Patient demographics, clinical data, malnutrition type and criteria, nutritional interventions, and outcomes were abstracted from the electronic medical record
Results
Registered dietitians identified malnutrition for 291 admissions during our study period. This represents 4.1% of hospital discharges. Physicians only diagnosed malnutrition on 93 (32%) of these. Physicians diagnosed malnutrition in 43% of patients with a body mass index (BMI) less than 18.5 but only 26% of patients with BMI higher than 18.5. Patients with a physician diagnosis had a longer length of stay (mean 14.9 days versus 7.1 days) and were more likely to receive total parenteral nutrition (20.4% versus 4.6%). Sixty two percent of patients had malnutrition due to chronic illness. Of six criteria used to identify malnourished patients, weight loss and reduced energy intake were the most common.
Conclusions
Malnutrition is under-recognized by physicians. However, further research is needed to determine if physician recognition and treatment of malnutrition can improve outcomes. The most important criteria for identifying malnourished patients in our cohort were weight loss and reduced energy intake.
Introduction
Malnutrition is widely believed to be an under-recognized issue among patients in the hospital setting with prevalence rates of malnutrition among hospitalized patients estimated to be between 21% and 54%.1 Many organizations, including the Joint Commission, have mandated screening of all hospitalized patients for malnutrition. Those with a malnutrition diagnosis have a five-fold increased risk of death, increased length of stay, and increased costs compared to patients lacking a diagnosis code for malnutrition making the identification of malnutrition of utmost importance.1,2
An increased risk for poor health outcomes due to malnutrition has been documented in both ward and intensive care unit (ICU) patients.3–5 Moreover, treating malnutrition with oral nutritional supplements, enteral nutrition, or parenteral nutrition is beneficial to most patients.6,7 However, among patients in the ICU, feeding to goal versus trophic feeding has failed to show benefit.8 Additionally, within our hospital system, Registered Dietitians (RDs) provide front line support for malnutrition identification as well as determining appropriate nutritional intervention such as adding nutritional supplements to diets of hospitalized patients and making recommendations for enteral and parenteral nutrition. It is unclear whether physician recognized malnutrition has a meaningful impact on patient care.
Challenges in examining associations between malnutrition and patient health outcomes are exacerbated by difficulty in the identification of malnutrition since the definition of malnutrition, in the hospital setting, remains controversial.9 A recent investigation using administrative claims data found the prevalence of a malnutrition diagnosis to be lower than expected at 3.2% whereas other investigations have found rates as high as 54%.1 While there is no gold standard for the diagnosis of malnutrition, the Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition (ASPEN) published a consensus statement in 2012 on characteristics needed to define malnutrition10. The ASPEN consensus statement recommends that malnutrition be identified after evaluation of the following five criteria: energy intake, weight loss, loss of body fat or muscle mass, presence of edema, and grip strength. At least two of these criteria are necessary for a diagnosis of malnutrition. However, routine collection of all these measurements is difficult to obtain during clinical practice. In 2015, the European Society of Clinical Nutrition and Metabolism published its own consensus statement on diagnosis of malnutrition.11 This statement recommends that the diagnosis of malnutrition be based on a very low body mass index (BMI) defined as less than <18.5 kg/m2 by itself, or a combination of weight loss and either low free fat mass index or low BMI (<20 kg/m2 or <22 kg/m2, depending on age). The World Health Organization (WHO) also focuses on low BMI for the identification of malnutrition.12 Despite guidelines that use low BMI to define malnutrition, malnutrition has been documented in patients with high BMI as well.13,14
Grip strength has been one of the most difficult measurements to obtain in a hospitalized population. It requires specialized equipment (dyanometer) which is not always available to RDs and a cooperative patient. Many patients with delirium or sedated in intensive care units cannot participate in this measurement due to mental status. Further, additional data on normal reference ranges are needed.12 An investigation conducted within two tertiary care teaching hospitals found that among 263 patients referred for nutritional evaluation during the month of May, 2012, food intake history was available for 76% of patients, weight history for 67%, physical exam for loss of fat and muscle mass was done in 94%, exam for edema was done in 84%, but none of the patients had hand grip strength measured.15 Since hand grip strength is a difficult measurement it might have limited value in its contribution to the definition of malnutrition among hospitalized populations.
Regardless of challenges in obtaining measurement and the varying definitions of malnutrition in patient populations, the Joint Commission requires that all hospitalized patients be screened for risk of malnutrition.16 Similar to many hospitals, nursing staff at Christiana Care Health System screen patients for nutrition risk on admission. Patients who screen positive, as well as those where a clinician requests evaluation, are subsequently evaluated for malnutrition risk by an RD. Due to the challenges in obtaining grip strength, in 2013 Christiana Care Health System created a modified version of the criteria recommended in the ASPEN consensus recommendations. In this modification, body mass index (BMI) was substituted for grip strength.
The current investigation examined the prevalence of malnutrition and described characteristics of malnourished patients as identified by RDs using our modified ASPEN criteria. Demographic characteristics, clinical characteristics, treatments received, and discharge disposition were explored. Within the population, a subset of all those patients with dietitian-identified malnutrition was also diagnosed as malnourished by the physician. Comparisons between those only identified by the RD to those recognized by both the RD and physician were conducted to understand the characteristics of patients diagnosed by physicians as malnourished. Finally, we examined the impact of each of the six diagnostic criteria on the prevalence of malnutrition.
Methods
Study Setting and Population
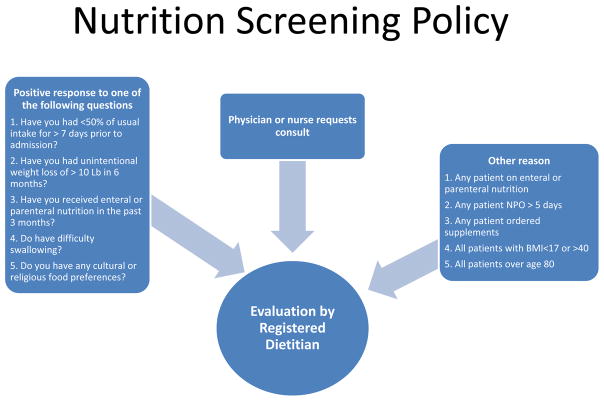
Christiana Care Health System (CCHS) is a 1,100 bed, two-hospital health care system in northern Delaware. The majority of medical inpatients are cared for by hospitalist physicians. All patients admitted to CCHS are screened for risk of malnutrition using criteria shown in figure 1. An adapted ASPEN criterion that replaces grip strength with body mass index less than 18.5 kg/m2 is used by RDs to identify patients meeting criteria for malnutrition. Adult (greater than 18 years of age) patients who were positive for two of the six criteria on evaluation by an RD were included in this study. In contrast to RDs, there was no standard protocol for diagnosis of malnutrition by physicians at our institution. Therefore, diagnosis of malnutrition was left to the individual judgment of each physician. Pediatric, psychiatric, maternity and rehabilitation patients were excluded from the current investigation. RDs write notes in the electronic medical record (EMR) making recommendation for nutritional interventions. RDs at Christiana Care are able to order diet changes and supplement changes independent of physicians but orders for tube feeding and parenteral nutrition must be entered by a physician, physician assistant, or nurse practitioner.
Figure 1.
Nutrition Screening Policy: This figure shows the possible pathways to an evaluation by a dietitian at Christiana Care Healthcare System.
Study Design
A retrospective cohort study of malnourished patients identified during two separate one-month periods, August of 2014 and September of 2015, by RDs at Christiana Care Healthcare System was conducted. There were 11 patients admitted more than once during our study period. Since their malnutrition type and/or physician diagnosis may have changed from one admission to the next, we chose to analyze the data by admission rather than by patient. However, we repeated the analysis excluding these 11 patients and found no changes in our findings. In an effort to improve physician diagnosis of malnutrition, in March of 2015, a letter was sent to physician groups encouraging them to review the RDs notes and document a diagnosis of malnutrition when appropriate. However, no training or protocols for the diagnosis of malnutrition were provided. This project was reviewed and approved by the Christiana Care Institutional Review Board.
Data Collection
Patient information, including patient demographics, clinical data, malnutrition type and criteria, any nutritional interventions, and patient health outcomes, were extracted from the electronic medical record (EMR) and entered in a REDCap database17. The length of stay was calculated by subtracting date of admission and date of discharge as recorded in the EMR. Discharge dispositions included: home, home with healthcare, skilled nursing facility, non-skilled facility, short-term acute care, another health care facility, hospice, or death. For analysis purposes, we combined these dispositions to create three discharge disposition categories: home (home and home with healthcare), nursing facility (skilled nursing facility, non-skilled facility, short-term acute care, another health care facility), and death or discharge to hospice (hospice or death).
The admitting diagnosis of each patient was classified into one of the following categories: cardiovascular, renal, gastrointestinal, neurological, endocrine, respiratory, infectious disease, peripheral vascular disease, surgical, or trauma. Admission and discharge diagnoses as well as major comorbidities were abstracted. Co-morbidities abstracted were categorized as follows: none, anemia, arthritis, cancer, congestive heart failure, stroke, chronic obstructive pulmonary disease, coronary artery disease, diabetes, venous thromboembolism, gastrointestinal disorders, hyperlipidemia, hypertension, hypotension, infectious diseases, psychiatric diseases, renal disease, seizure disorder, thyroid disorder and other. We also determined whether the patient was initially admitted to the intensive care unit (ICU) or to the floor. Code status was classified into three categories: full code, comfort care, or do not resuscitate but still receiving other medical therapies to treat disease.
The dietitians collected data that identified whether malnutrition was moderate or severe as defined in the ASPEN consensus statement and if malnutrition was the result of acute illness/injury, chronic illness/injury, or social/environmental circumstances. A positive indication of physician diagnosis of malnutrition was made when malnutrition was listed on the patient’s problem and diagnosis list. The type of nutritional intervention recommended was classified into six categories: tube feedings, total parenteral nutrition (TPN), supplements, diet education, continuing current diet, and monitoring the patient. Of these interventions, only tube feeding and parenteral nutrition require physician orders. We tracked both how often dietitians wrote a recommendation for tube feeding or TPN and how often physicians wrote orders for these interventions. The prevalence of malnutrition was calculated by dividing the total number of admissions where a patient was identified as malnourished by the total number of hospital discharges during the study period excluding pediatric, maternity, psychiatric or rehabilitation patients. Finally, we computed average length of stay for the malnourished patients and compared this number to hospital-wide average length of stay during the study period.
Statistical Analysis
Descriptive statistics were used to examine the demographic characteristics, clinical characteristics, discharge locations and admitting diagnosis of patients identified as malnourished during the study period. Means and standard deviations are reported for continuous variables and frequencies and percentages for categorical variables. Comparisons of these variables between the dietitian only diagnosis group and the physician and dietitian group were made using a chi-square statistic for categorical variables and t-test and/or Mann-Whitney U test for continuous variables. Cohen’s kappa was used to evaluate agreement among the six criteria used to define malnutrition. To examine the validity of each of the modified criteria in classifying malnutrition in our cohort, we computed the sensitivity and 95 percent confidence interval (CI) for the scale after removing each of the criteria one at a time. All statistical analyses were performed using Stata version 14.0 (StataCorp, College Station, TX) and a p-value of < 0.05 was considered statistically significant.
Results
A total of 279 patients with 291 admissions were identified as malnourished during the 2 months studied. During the same period, there were 7,184 hospital discharges excluding maternity, pediatric, psychiatric and rehabilitation patients. Thus, malnutrition was identified in 4.1% (291/7,184) of hospital admissions.
The mean age of the malnourished patients was 64.9 ± 17.7 years. Characteristics of these patients are shown in Table 1. Malnutrition attributable to chronic illness was found in a majority (62%) of admissions whereas a surgical procedure was performed in only 12% of admissions during the hospitalization. Of 35 patients who underwent any surgical procedure during their hospitalization, 10 had major abdominal surgery, 6 had cardiac or thoracic surgery, 8 had orthopedic surgery, 4 had urologic surgery, 2 had plastic surgery for skin wounds, 1 had neurologic surgery, 1 had vascular surgery and 3 had other procedures such as lymph node biopsies.
Table 1.
Demographic characteristics of total sample and by physician agreement
| Total Sample N=279 patients | Physician Recognized Malnutrition N=92 | Physician did not recognize malnutrition N=187 | P-value | |
|---|---|---|---|---|
| Demographic and Clinical Characteristics | ||||
| Age, Mean (SD) | 64.9 (17.7) | 65.6 (18.2) | 64.5 (17.5) | 0.63 |
| Male, N (%) | 125 (44.8) | 35 (38.0) | 90 (48.1) | 0.11 |
| Race | ||||
| White | 208 (75) | 69 (75) | 139 (74) | 0.35 |
| Black | 62 (22) | 22 (24) | 40 (21) | |
| Other | 9 (3) | 1 (1) | 8 (4) | |
| Ethnicity | ||||
| Hispanic/Latino, N (%) | 14 (5.0) | 4 (4.3) | 10 (5.3) | 0.56 |
| Total Sample N=291 admissions | Physician Recognized Malnutrition N=93 | Physician did not recognize malnutrition N=198 | P-value | |
| BMI, Mean (SD) | 22.4 (7.2) | 20.9 (5.8) | 23.1 (7.6) | 0.01 |
| Length of stay, Mean (SD) | 9.6 (14.9) | 14.9 (23.7) | 7.1 (6.6) | <0.001 |
| Length of stay, Median (IQR) | 6 (7) | 7 (10) | 5 (5) | 0.019 |
| Surgical procedure during admission, N (%) yes | 35 (12.0) | 12 (12.9) | 23 (11.6) | 0.75 |
| Admission location, ICU, N (%) yes | 27 (9.3) | 4 (4.3) | 23 (11.6) | 0.05 |
| Number of Co-morbidities, Mean (SD) | 4.9 (2.1) | 4.9 (2.3) | 4.8 (2.0) | 0.76 |
| Number of Co-morbidities, Median (IQR) | 5 (3) | 5 (3) | 5 (3) | 0.57 |
| Admitting Diagnosis | ||||
| Surgical disease | 3 (1.0) | 1 (1.1) | 1 (1.0) | |
| Trauma | 16 (5.5) | 5 (5.4) | 11 (5.6) | |
| Respiratory disease | 28 (9.6) | 12 (12.9) | 16 (8.1) | |
| Cardiac disease | 32 (11.0) | 6 (6.5) | 26 (13.1) | |
| Infectious disease | 51 (17.5) | 13 (14.0) | 38 (19.2) | |
| Neurologic | 20 (6.9) | 8 (8.6) | 12 (6.1) | |
| Gastrointestinal | 56 (19.2) | 22 (23.7) | 34 (17.2) | |
| Endocrine | 10 (3.4) | 3 (3.2) | 7 (3.5) | |
| Renal | 26 (8.9) | 5 (5.4) | 21 (10.6) | |
| Peripheral Vascular Disease | 4 (1.4) | 2 (2.2) | 2 (1.0) | |
| Other | 45 (15.5) | 16 (17.2) | 29 (14.7) | 0.45 |
| Malnutrition Type | ||||
| Moderate due to acute injury/illness | 39 (13.4) | 11 (11.8) | 28 (14.1) | 0.59 |
| Severe due to acute injury/illness | 69 (23.7) | 24(25.8) | 45 (22.7) | 0.44 |
| Moderate due to chronic illness | 76 (26.1) | 24 (25.8) | 52 (26.3) | 0.93 |
| Severe due to chronic illness | 103 (35.4) | 33 (35.4) | 70 (35.3) | 0.614 |
| Moderate due to social/environmental circumstance | 3 (1.0) | 0 | 3 (1.5) | n/a |
| Severe due to social/environmental circumstance | 1 (0.3) | 1 (1.1) | 0 | n/a |
| All Moderate types combined | 118 (40.5) | 36 (38.7) | 82 (41.4) | 0.66 |
| All Severe types combined | 173 (59.5) | 57 (62.2) | 116 (58.6) | 0.66 |
BMI, body mass index; SD, standard deviation; N, number; IQR, interquartile range; ICU, intensive care unit
Overall, physicians only recognized malnutrition on 93 admissions (32.0%). Physicians diagnosed malnutrition in 44 of 127 (34.7%) admissions identified by RDs before a letter was sent to physicians asking them to review RD notes and enter a diagnosis of malnutrition when appropriate. After the letter, physicians diagnosed malnutrition in 49 out of 164 (29.9%) admissions where RDs identified malnutrition. This difference was not statistically significant (p = 0.39).
Patient characteristics were similar between physician recognized and unrecognized cases of malnutrition. Physicians were no more likely to diagnose malnourished patients identified by RDs as severely malnourished than those identified as moderately malnourished. However, physicians diagnosed 43.0% of patients with a BMI less than 18.5 compared to only 26.2% of patients with BMI greater than 18.5 (p = 0.003). Patients with a physician diagnosis of malnutrition had a longer hospitalization (mean 14.9 ± 23.7days) compared with malnourished patients without a physician diagnosis (mean 7.1 ± 6.6 days) (Table 1). Both groups of malnourished patients stayed longer than the mean length of stay for hospitalized patients during the study period, which was approximately 5 days.
Of the six criteria used to identify malnutrition, weight loss was the most commonly documented (78.7%). Energy intake was documented in 78.3% of admissions, muscle loss in 39.5%, fat loss in 35.0%, low body mass index in 29.9% and fluid accumulation in 9.6%. One hundred sixty-six patient admissions (57.0%) met only 2 criteria while the remainder (43.0%) met more than 2 criteria for malnutrition. As shown in Table 2, there is poor agreement between the individual criteria. Muscle loss and fat loss had the best agreement with a kappa of 0.64. However, muscle loss and fat loss had poor correlation with weight loss with kappa coefficients of 0.24 and 0.12 respectively.
Table 2.
Agreement between criteria used to identify malnutrition (Cohen’s kappa)
| Criteria | BMI | Energy Intake | Weight loss | Muscle loss | Fat loss | Fluid Accumulation |
|---|---|---|---|---|---|---|
| BMI | 0.2593 | 0.2062 | 0.3245 | 0.3360 | 0.1500 | |
| Energy Intake | 0.3378 | 0.3207 | 0.1753 | 0.0100 | ||
| Weight Loss | 0.2392 | 0.1205 | 0.0755 | |||
| Muscle Loss | 0.6407 | 0.0342 | ||||
| Fat Loss | 0.0034 |
BMI, body mass index
Most patients identified as malnourished received some nutritional intervention (Table 3). The most common interventions were nutritional supplementations. Patients with a physician diagnosis of malnutrition were also more likely to receive total parenteral nutrition (20.4% vs 4.6%, p < 0.01) but rate of tube feeding (23.7% in physician recognized group versus 15.2%) was not significantly different (p= 0.07). The majority of the time (69%) when RDs recommended tube feeding, it was ordered by physicians. Similarly, 64% of the time when RDs recommended TPN, it was ordered by the physician. In addition, 18.2% of the cohort died in the hospital or went to hospice (Table 3). The frequency of hospice and hospital death combined was not different between the two groups. To understand the impact of each criteria on our ability of identify malnutrition in this cohort, we calculated the sensitivity of defining malnutrition eliminating each criterion sequentially (i.e., assuming six criteria as the standard for identifying malnutrition with a sensitivity of 100%, we calculated the sensitivity of using the remaining five). The sensitivity after removal of energy intake was 77%, after removal of weight loss was 74%, after removal of BMI was 87%, after removal of muscle loss was 64%, fat loss was 99%, and after removal of fluid accumulation was 99.6%.
Table 3.
Intervention types and discharge locations for 291 admissions
| Intervention Type | All, N (%) | Physician Recognized n=93 | Physician Unrecognized n=198 | p-value |
|---|---|---|---|---|
| Tube feedings | 52 (17.9) | 22 (23.7) | 30 (15.2) | 0.07 |
| TPN | 28 (9.6) | 19 (20.4) | 9 (4.6) | <0.001 |
| Supplements | 175 (60.1) | 58 (33.1) | 117 (66.9) | 0.60 |
| Diet Education | 31 (10.7) | 11 (11.8) | 20 (10.1) | 0.66 |
| None/Continue Current Diet/Monitor Patient | 181 (62.2) | 55 (59.1) | 126 (63.6) | 0.46 |
| Discharge Location | 0.93 | |||
| Home (includes AMA, Home and home with health care) | 169 (58) | 54 (58.1) | 115 (58.1) | |
| Healthcare facility (includes non-skilled and skilled nursing facilities, another healthcare facility) | 69 (23.7) | 23 (24.7) | 46 (23.2) | |
| Hospice and Death combined | 53 (18.2) | 16 (17.2) | 37 (18.7) |
TPN, total parenteral nutrition; N, number; AMA, discharge against medical advice
Discussion
We estimated a lower prevalence of malnutrition than many prior studies. However, our estimate is similar to the 3.2% reported in a 2010 study.1 Older studies may use different definitions of malnutrition such as low albumin which is now known to be associated with acute illness and inflammation.
In our cohort, the most frequent etiology of malnutrition was chronic illness. There is often more attention paid to acute malnutrition as might result from acute pancreatitis or surgical treatments such as bowel resection than to chronic malnutrition. Our findings suggest that additional attention to medical patients with chronic malnutrition is needed. Moreover, since the majority of our malnourished patients are discharged home, community resources focusing on care of these patients in the home environment may be needed.
We found that physicians often fail to recognize that their hospitalized patients are malnourished. While we cannot determine the reasons for this from our data, this may reflect inadequacies of medical education regarding nutrition. In fact, a recent cross sectional survey of medical, surgical and obstetrical interns, revealed that medical school education in nutrition is perceived by recent graduates as inadequate.18 Our finding that physicians were more likely to diagnosis underweight patients with malnutrition suggests a need for additional education for physicians on the potential for normal and overweight patients to be malnourished. Further, we found that simply sending a letter asking physicians to review RD notes does not appear to be effective at increasing the recognition of malnutrition.
In addition to providing additional physician education on this topic, promoting collaboration between RDs and physicians is imperative to improve recognition of malnutrition by physicians. It may be that physicians were not reading the notes where RDs were documenting their identification of malnutrition, reasoning, and recommendations. We are planning changes with our electronic medical record so that physicians will be alerted to recommendations from RDs in hopes of improving this collaboration.
The association between physician diagnosis of malnutrition and longer length of stay deserves further study. It is possible that physicians are simply more likely to diagnose malnutrition in sicker patients. However, if physicians delay discharge for treatment in patients who are malnourished then this would mean that physician recognition has treatment implications. Physician recognition of malnutrition was also associated with receipt of TPN. This is not surprising since dietitians are not able to initiate TPN without a physician order. However, the same is true for tube feeding and the association between tube feeding and physician recognition of malnutrition independently of the dietitian was not significant. Our study may lack sufficient power to detect an impact of physician recognition on tube feeding. Both TPN and tube feeding are also often prescribed to patients who are not malnourished to prevent negative nitrogen balance and complications such as poor healing and loss of functional status. Because we do not know the rationale for the interventions that patients received, we cannot ascertain if physician recognition of malnutrition changes treatment. Additionally, we do not know the reasons for nutrition recommendations such as TPN or tube feedings not being followed. Possible reasons could include impending discussion on withdrawal of life support, a belief that ability to tolerate alternative feedings would improve rapidly, or disagreement with the recommendation. This emphasizes the importance of collaboration between RDs and physicians. Further investigation into interventions that physicians could make for malnourished patients is important particularly given the current efforts to improve physician recognition of malnutrition.
We found that energy intake and weight loss are the most common criteria used to identify malnutrition. Our finding of poor agreement between criteria used to diagnosis malnutrition suggests that multiple criteria are still needed. However, elimination of any one of fat loss or fluid accumulation criteria still results in sensitivity greater than 90%. This suggests that future studies attempting to validate criteria for malnutrition diagnosis could consider using fewer criteria.
This study has several limitations. RDs only assessed patients whom nurses screened positive or when other clinicians requested evaluation. Therefore, in estimating incidence of malnutrition during our study period, we are assuming that the screening is sufficiently sensitive to minimize the number of missed malnourished patients. This is a retrospective chart review so we do not know why clinical decisions were made. For example, while we know if a patient received TPN, we do not know if the diagnosis of malnutrition was the reason or even part of the reason for this intervention. Additionally, we do not know why physicians did not diagnose malnutrition in many patients identified by RDs as malnourished. Because there is no gold standard for the diagnosis of malnutrition, we cannot exclude the possibility that physicians used different criteria than the dietitians’ criteria. Finally, we were only able to assess the performance of our local criteria for the identification of malnutrition and do not know how the results would be different if functional status measurements such as hand grip were included in the criteria.
Using a modified version of ASPEN criteria for malnutrition, we estimated a prevalence of 4.1% among our institutions inpatients. We found that many malnourished patients are not recognized by physicians but that physician recognition is not associated with improved outcomes such as mortality. Future studies should explore if physician recognition and interventions for malnourished patients can improve patients’ outcomes.
Clinical Relevancy Statement.
The best approach to the diagnosis of malnutrition is still debated. We examined the impact of each of the following six criteria on the diagnosis of malnutrition: energy intake, weight loss, muscle loss, fat loss, fluid accumulation and body mass index. We also examined the impact of physician diagnosis of malnutrition, in addition to recognition of malnutrition by registered dietitians, on patient outcomes and treatment. We found that energy intake and weight loss were the criteria most commonly used to diagnosis malnutrition. Further, we found that physicians only recognized 32% of malnourished patients identified by registered dietitians. Efforts to help physicians better recognize malnutrition among hospitalized patients are supported by this finding. Future research to determine if improvements in communication between physicians and dietitians increase physician diagnosis of malnutrition, if a smaller number of criteria could be used to identify malnourished patients, and if physician diagnosis of malnutrition impacts outcomes, is indicated.
Acknowledgments
Work supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number U54-GM104941 (PI: Binder-Macleod) and in part by the NIH NIGMS IDeA Program Grant #P20 GM103446 (PI: Stanhope).
Footnotes
Conflicts of Interest: None declared
References
- 1.Corkins MR, Guenter P, DiMaria-Ghalili RA, et al. Malnutrition diagnoses in hospitalized patients: United States, 2010. JPEN J Parenter Enteral Nutr. 2014;38:186–95. doi: 10.1177/0148607113512154. [DOI] [PubMed] [Google Scholar]
- 2.Mauldin K, O’Leary-Kelley C. New Guidelines for Assessment of Malnutrition in Adults: Obese Critically Ill Patients. Crit Care Nurse. 2015;35:24–30. doi: 10.4037/ccn2015886. [DOI] [PubMed] [Google Scholar]
- 3.Mogensen KM, Robinson MK, Casey JD, et al. Nutritional Status and Mortality in the Critically Ill. Crit Care Med. 2015;43:2605–15. doi: 10.1097/CCM.0000000000001306. [DOI] [PubMed] [Google Scholar]
- 4.Cederholm T, Jagren C, Hellstrom K. Outcome of protein-energy malnutrition in elderly medical patients. Am J Med. 1995;98:67–74. doi: 10.1016/S0002-9343(99)80082-5. [DOI] [PubMed] [Google Scholar]
- 5.Lew CCH, Yandell R, Fraser RJL, Chua AP, Chong MFF, Miller M. Association Between Malnutrition and Clinical Outcomes in the Intensive Care Unit: A Systematic Review [Formula: see text] JPEN J Parenter Enteral Nutr. 2017;41:744–58. doi: 10.1177/0148607115625638. [DOI] [PubMed] [Google Scholar]
- 6.Sriram K, Sulo S, VanDerBosch G, et al. A Comprehensive Nutrition-Focused Quality Improvement Program Reduces 30-Day Readmissions and Length of Stay in Hospitalized Patients. JPEN J Parenter Enteral Nutr. 2017;41:384–91. doi: 10.1177/0148607116681468. [DOI] [PubMed] [Google Scholar]
- 7.Gallagher-Allred CR, Voss AC, Finn SC, McCamish MA. Malnutrition and clinical outcomes: the case for medical nutrition therapy. J Am Diet Assoc. 1996;96:361–6. 9. doi: 10.1016/s0002-8223(96)00099-5. quiz 7–8. [DOI] [PubMed] [Google Scholar]
- 8.Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307:795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hand RK, Murphy WJ, Field LB, et al. Validation of the Academy/A.S.P.E.N. Malnutrition Clinical Characteristics. J Acad Nutr Diet. 2016;116:856–64. doi: 10.1016/j.jand.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 10.White JV, Guenter P, Jensen G, Malone A, Schofield M. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) JPEN J Parenter Enteral Nutr. 2012;36:275–83. doi: 10.1177/0148607112440285. [DOI] [PubMed] [Google Scholar]
- 11.Cederholm T, Bosaeus I, Barazzoni R, et al. Diagnostic criteria for malnutrition - An ESPEN Consensus Statement. Clin Nutr. 2015;34:335–40. doi: 10.1016/j.clnu.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Matarese LE, Charney P. Capturing the Elusive Diagnosis of Malnutrition. Nutr Clin Pract. 2017;32:11–4. doi: 10.1177/0884533616671856. [DOI] [PubMed] [Google Scholar]
- 13.Kaidar-Person O, Person B, Szomstein S, Rosenthal RJ. Nutritional deficiencies in morbidly obese patients: a new form of malnutrition? Part A: vitamins. Obes Surg. 2008;18:870–6. doi: 10.1007/s11695-007-9349-y. [DOI] [PubMed] [Google Scholar]
- 14.Kaidar-Person O, Person B, Szomstein S, Rosenthal RJ. Nutritional deficiencies in morbidly obese patients: a new form of malnutrition? Part B: minerals. Obes Surg. 2008;18:1028–34. doi: 10.1007/s11695-007-9350-5. [DOI] [PubMed] [Google Scholar]
- 15.Nicolo M, Compher CW, Still C, Huseini M, Dayton S, Jensen GL. Feasibility of accessing data in hospitalized patients to support diagnosis of malnutrition by the Academy-A.S.P.E.N. malnutrition consensus recommended clinical characteristics. JPEN J Parenter Enteral Nutr. 2014;38:954–9. doi: 10.1177/0148607113514613. [DOI] [PubMed] [Google Scholar]
- 16.Guenter P, Jensen G, Patel V, et al. Addressing Disease-Related Malnutrition in Hospitalized Patients: A Call for a National Goal. Jt Comm J Qual Patient Saf. 2015;41:469–73. doi: 10.1016/s1553-7250(15)41061-x. [DOI] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frantz DJ, McClave SA, Hurt RT, Miller K, Martindale RG. Cross-Sectional Study of U.S. Interns’ Perceptions of Clinical Nutrition Education. JPEN J Parenter Enteral Nutr. 2016;40:529–35. doi: 10.1177/0148607115571016. [DOI] [PubMed] [Google Scholar]