Abstract
Introduction
Patients with dementia (PWDs) are often subjected to enforced dependency and experience functional decline and emotional distress during hospital stay. Person-centered care (PCC) with specialized psychosocial interventions, minimally obtrusive medical care, and physical restraints-free practice holds potential to improve patient outcomes. We evaluate the effectiveness of an acute hospital dementia unit (Care for Acute Mentally Infirm Elders [CAMIE]) that adopts a PCC protocol.
Methods
Prospective naturalistic cohort study whereby PWDs in the CAMIE unit (n = 170) were compared with a control group in usual care wards (n = 60) over 6 months. Assessments included patient demographics, dementia type and stage, comorbidities (Charlson's Comorbidity Index), acute illness severity, Well-Being, Ill-Being, functional status (Modified Barthel Index), agitation levels (Pittsburgh Agitation Scale), and quality of life (EuroQoL), assessed on admission and discharge. Multivariate analysis of covariance examined the effect of CAMIE versus usual care on pre-post outcomes.
Results
CAMIE patients showed statistically significant greater gains in Modified Barthel Index function and Well-Being, decreased Ill-Being and agitation, and greater improvement in EuroQoL index score (effect size: Δ = 0.18) after adjusting for baseline differences that translated to a quality-adjusted life years gain of 0.045, assuming stability over 3 months. Estimating added cost of CAMIE stay over usual care at SGD 1500 (USD 1040) for average length of stay of 15 days per patient, the incremental cost-effectiveness ratio fell within the threshold for cost-effectiveness at USD 23,111.
Discussion
PCC for PWDs in acute hospitals not only improves clinical outcomes for patients but is also cost-effective. The results support the adoption of PCC on a wider scale for better care of PWDs.
Keywords: Dementia, Geriatric, Person-centered care, Acute hospital, Cost-effectiveness
1. Introduction
With global population aging, the number of patients with dementia (PWDs) is projected to double every 20 years, that is, 65.7 million in 2030 and 115.4 million in 2050 [1]. Consequently, high numbers of PWDs are expected in acute care settings [2]. There is a need to plan ahead to secure best care for PWDs in acute hospitals.
Hospitalization can cause significant distress to PWDs [3], where enforced dependency and unfamiliar environments negatively impact the well-being of patients, leading to functional decline and deficits [4]. In busy, task-focused acute settings, challenging behaviors often emerge and potentially complicate treatment [5]. Therefore, quality care to meet the needs of PWDs is imperative. Adoption of the person-centered care (PCC) approach that has become synonymous with high-quality care for PWDs should be considered.
Research evidence supports the adoption of PCC in residential care facilities that prioritizes PWDs' well-being first, beyond physical and custodial care [6]. PCC promotes the strengths of PWDs and honors their values and choices [7]. Neglecting PWDs' personal and psychosocial needs can result in need-driven dementia-compromised behaviors, as well as social isolation that potentiates accelerated decline [8]. PCC emphasizes shaping the care environment to value personhood and address the unmet needs of PWDs [9]. PCC in long-term care facilities has decreased use of antipsychotic medications [10] and produced positive patient outcomes, including better quality of life and reduced agitation and challenging behaviors [11], [12].
Most research into PCC has been in long-term care facilities [10], [11], [12], with little investigation of its effectiveness in acute care settings [13], [14]. Providing PCC in acute hospitals can be challenging because of priorities placed on diagnostic procedures, close monitoring, and instituting treatment within short lengths of stay [13]. Therefore, examining the effectiveness of PCC for PWDs in the acute hospital is pertinent and forms the aim of this study.
In July 2012, Khoo Teck Puat Hospital became the first acute hospital in Singapore to set up a specialized unit for PWDs that adopts PCC. The unit, known as Care for Acute Mentally Infirm Elders (CAMIE), is set within a home-like environment, which prioritizes the needs of patients beyond care tasks. The unit has 10 beds, a kitchenette, lounge and dining area, and a sizable outdoor space. Care is operationalized under two protocols: (1) enhanced medical care protocol, which includes moderating intrusive interventions (e.g., catheters, feeding tubes), a physical restraints-free policy, appropriate and modest use of psychotropic medications, careful attention to hydration, bowel and bladder care, and encouraging mobilization and (2) enhanced psychosocial care protocol, which includes prioritizing patient needs over tasks, encouraging family members and volunteers to provide companionship, and engaging in daily structured activities (e.g., music therapy, recreational/group activities). These interventions are modeled after the established Hospital Elder Life Program [15], [16] and the philosophy and practice of PCC [9], [17], [18]. Meanwhile, patients in the conventional geriatric ward (control group) receive standard medical care.
Upon admission, a patient's background information is obtained from the patient and his/her family via a “Know Me Better” form, which is placed at the patient's bedside to facilitate individualized care. CAMIE includes flexibility in custodial activities such as shower and feeding times, engaging patients in activities of interest (e.g., music listening), and encouraging social integration (e.g., communal dining). The more physically able patients are encouraged to mobilize and spend more time out of bed engaging in group-based activities such as games and puzzles, music therapy, horticulture therapy, and exercises in the lounge and outdoor areas of the ward. The kitchenette is used for cooking demonstrations by the dieticians, and patients are encouraged to participate in group-cooking sessions. Patients with more advanced dementia who are bed- or chair-fast can also participate in the activities with more individualized attention. Examples of more customized activities include one to one music therapy, sensory stimulation with aroma oil massage, and leisurely conversations.
CAMIE is run by a multidisciplinary team of doctors, nurses, and allied health professionals including a social worker, dietician, pharmacist, as well as physio, occupational, and speech and music therapists. There are also one to two volunteers daily who help to feed the patients and engage them in activities and conversations. Family caregivers can participate in patient care as well, and these present opportunities for caregiver training serve to equip caregivers with enhanced skills and confidence to care for the patients after discharge. The team, in particular the social workers and nurses, dedicates time to explore the caregiver's coping and makes attempts to encourage and empower caregivers to care better. There are twice-a-week meetings for team members to update patients' care plans and progress, share experiences, discuss the challenges faced, as well as meet with family caregivers to address their concerns and plan for postdischarge care.
CAMIE has a higher nurse staffing compared with a conventional ward with a staff-to-patient ratio of 4-to-10 (vs. 3-to-10) in the day and 3-to-10 (vs. 2-to-10) at night. The increased staffing allows manpower to be allocated to caring beyond the medical and custodial needs of the patients and is especially important at night when patients with gait instability often attempt to get out of bed unsupervised. All CAMIE staff attend a 2-day in-house training workshop on PCC to learn theoretical and practical applications of PCC in relation to caring for PWDs. Training topics include understanding dementia and its management, challenging behaviors, resolution and validation therapy, and engaging PWDs in purposeful activities. A functional analysis approach to challenging behavior, which considers biological, biographical, psycho-emotional, and situational factors in the understanding the unmet needs of the patient, is emphasized. The training also encompasses an experiential learning activity that helps attendees understand the confusion experienced by PWDs by putting them in a simulated disconcerting environment and making them perform tasks such as wearing diapers and taking medications (a syrup mixture).
We examined the effectiveness of CAMIE care compared with conventional geriatric care as control. Specifically, we hypothesized that PWDs in the CAMIE unit would display greater improvements in general well-being and functional ability, require lower doses of psychotropic medications with greater reduction in agitated behaviors, and have a shorter length of stay (LoS). We also aimed to show that these enhanced outcomes are cost-effective through an economic analysis.
2. Method
2.1. Participants
A total of 230 patients (170 in CAMIE unit and 60 in conventional geriatric ward as controls) admitted to Khoo Teck Puat Hospital, Singapore, were recruited over 6 months. All patients received standard treatment for their respective medical conditions. Patients were admitted to the CAMIE unit if they suffered from confusion due to dementia, with/without delirium based on the confusion assessment method criteria, and concomitant acute medical problems. The exclusion criteria for CAMIE were medically unstable patients, patients requiring high dependency care, or isolation due to infection control or reverse barrier care. The control group consisted of patients who satisfied admission criteria for CAMIE unit but were denied because of the lack of bed availability.
Table 1 displays the demographic and clinical characteristics of the sample. The mean age for the CAMIE and control patients were 82.45 and 84.37 years, respectively, and 54.1% of the CAMIE sample and 56.7% of the control group were females. Both samples consisted of a large proportion of Chinese, followed by Malay, Indian, and other ethnicities, which is the representative of the multiethnic composition of Singapore. Ethics approval for the study was granted by the Domain Specific Research Board of the National Healthcare Group.
Table 1.
Demographic and clinical features of patients in both groups at baseline
| Patient characteristics | CAMIE unit (n = 170) n (%) |
Control group (n = 60) n (%) |
t or χ2 | P value |
|---|---|---|---|---|
| Gender (females) | 92 (54.10) | 34 (56.70) | χ2(1, N = 230) = 0.12 | .733 |
| Age in years, mean ± SD | 82.45 ± 7.96 | 84.37 ± 5.96 | t(228) = −1.70 | .090 |
| Ethnicity | χ2(3, N = 230) = 0.54 | .909 | ||
| Chinese | 118 (69.40) | 40 (66.70) | ||
| Malay | 30 (17.60) | 10 (16.70) | ||
| Indian | 16 (9.40) | 7 (11.70) | ||
| Others | 6 (3.50) | 3 (5.00) | ||
| Dementia type | χ2(3, N = 230) = 2.46 | .483 | ||
| AD | 40 (23.50) | 14 (23.30) | ||
| VaD | 72 (42.40) | 29 (48.30) | ||
| Mixed | 42 (24.70) | 15 (25.00) | ||
| Others | 16 (9.40) | 2 (3.30) | ||
| Dementia severity (DSM-IIIR) | χ2(2, N = 230) = 1.70 | .429 | ||
| Mild | 14 (8.20) | 2 (3.30) | ||
| Moderate | 102 (60.00) | 37 (61.70) | ||
| Severe | 54 (31.80) | 21 (35.00) | ||
| Delirium present | 134 (78.80) | 44 (73.30) | χ2(1, N = 230) = 0.76 | .382 |
| Illness severity (mSII) | χ2(2, N = 230) = 3.27 | .195 | ||
| Level 1 | 26 (15.30) | 8 (13.30) | ||
| Level 2 | 119 (70.00) | 37 (61.70) | ||
| Level 3 | 25 (14.70) | 15 (25.00) | ||
| Comorbidity status (CCI), mean ± SD | 6.12 ± 1.64 | 6.12 ± 1.54 | t(228) = .03 | .977 |
| MMSE, mean ± SD | 6.18 ± 6.56 | 3.00 ± 4.60∗ | t(228) = 3.46 | .001 |
| Admitting diagnosis | χ2(12, N = 230) = 9.25 | .681 | ||
| Falls | 39 (22.90) | 10 (16.70) | ||
| Pneumonia | 25 (14.70) | 15 (25.00) | ||
| Worsening BPSD | 27 (15.90) | 7 (11.70) | ||
| UTI | 16 (9.40) | 6 (10.00) | ||
| Metabolic disorders | 14 (8.20) | 4 (6.70) | ||
| Functional decline | 12 (7.10) | 3 (5.0) | ||
| Stroke | 4 (2.40) | 3 (5.0) | ||
| AMI/Angina | 5 (2.90) | 1 (1.70) | ||
| COPD exacerbation | 4 (2.40) | 1 (1.70) | ||
| Gastroenteritis | 2 (1.20) | 2 (3.30) | ||
| Constipation | 5 (2.90) | 3 (5.00) | ||
| Cellulitis | 4 (2.40) | 1 (1.70) | ||
| Others | 14 (8.20) | 3 (5.0) |
Abbreviations: AD, Alzheimer's disease; AMI, acute myocardial infarction; BPSD, behavioral and psychological symptoms of dementia; CAMIE, Care for Acute Mentally Infirm Elders; CCI, Charlson's Comorbidity Index; COPD, chronic obstructive pulmonary disease; DSM-IIIR, Diagnostic and Statistical Manual of Mental Disorders, 3rd edition revised; mSII, modified severity of illness index; MMSE, Mini–Mental Status Examination; SD, standard deviation; UTI, urinary tract infection; VaD, vascular dementia.
NOTE. Metabolic disorders include hyponatremia, hypokalemia, hypercalcemia, and hypoglycemia. Other disorders include deep vein thrombosis, hypertensive urgency, acute retention of urine, congestive cardiac failure, intestinal obstruction, anemia for investigation, and flare of arthritis.
P < .01.
2.2. Measures
2.2.1. Demographic and clinical variables
Patients' demographics (age, gender, and ethnicity) and clinical information (admitting diagnosis, dementia type and severity, delirium status, use of psychotropic medications, and LoS) were retrieved from medical records.
2.2.2. Diagnostic and Statistical Manual of Mental Disorders, 3rd edition revised
Diagnostic and Statistical Manual of Mental Disorders, 3rd edition revised [19], was used to assess dementia severity. Mild dementia refers to PWDs who retain the capacity for independent living with adequate personal hygiene. Moderate dementia is applied when independent living is unsafe and some degree of supervision is necessary. In severe dementia, activities of daily living are so impaired that continuous supervision is required.
2.2.3. Mini–Mental Status Examination
Mini–Mental Status Examination (MMSE) [20] is a widely used instrument for detecting cognitive impairment, and cutoff scores for the Singapore population have been established [21].
2.2.4. Modified Severity of Illness Index
A modified version of Severity of Illness Index (SII) [22], comprising a four-level scale, assesses severity of acute illness upon admission. Higher levels indicate greater severity and are associated with higher cost of hospitalization and longer LoS [23].
2.2.5. Charlson's Comorbidity Index
Higher Charlson's Comorbidity Index (CCI) scores indicate greater comorbidity and higher mortality risk [24]. CCI contains 19 categories of comorbid conditions, each assigned a severity weighting according to its mortality risk. We used the age-adjusted CCI that entailed an age adjustment of additional points (up to +6) for increasing age.
2.2.6. Bradford Well-Being and Ill-Being Profiling
This scale was adapted from Dementia Care Mapping [25], developed for assessing PCC practice [26]. Well-Being (WB) and Ill-Being (IB) profiling provides an index of the relative state of well-being and ill-being experienced by patients through independent observations on a set of indicators. A higher WB score indicates a better state of well-being, whereas a higher IB score indicates greater ill-being.
2.2.7. Pittsburgh Agitation Scale
The Pittsburgh Agitation Scale (PAS) [27] comprises four behavior groups: (1) aberrant vocalization; (2) motor agitation; (3) aggressiveness; and (4) resistance to care. An intensity score, on a scale of 0 (not present) to 4 (highly present), is assigned to each behavior group. The ratings across the four behavior groups are summed to give the total score, with higher score indicating greater agitation.
2.2.8. Modified Barthel Index
The 10-item Modified Barthel Index (MBI) [28] rating scale assesses functional independence in personal care and mobility. Overall scores range from 0 to 100, with higher scores indicating greater independence and scores ≤60 indicating moderate to severe functional impairment.
2.2.9. EuroQoL
The EuroQoL (EQ-5D) [29] quality of life instrument descriptive system consists of five domains of health states (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression). Each domain is rated on three response options: (1) no problems; (2) some problems; and (3) extreme problems. Responses across the five domains are coded into a five-digit descriptor representing a health state, ranging from 11,111 (best health state) to 33,333 (worst health state). This classification system categorizes 243 unique health states [30] that is converted into a quality of life index score, also known as health-state utility values, on a scale of 0 (deceased) to 1.0 (full health) [31]. EQ-5D has been psychometrically validated in Singapore [32], [33] and extensively used in clinical and epidemiological studies [34], [35].
2.2.10. Confusion Assessment Method
Confusion Assessment Method [36] was used to ascertain the presence of delirium in the patients. It is based on four features: (1) acute onset and fluctuating course; (2) inattention; (3) disorganized thinking; and (4) altered level of consciousness. Delirium diagnosis requires the presence of 1, 2, and either 3 or 4.
2.3. Design and procedure
A mixed 2 × 2 design was used. The between-groups independent variable was ward type (CAMIE unit and conventional geriatric ward), and the within-groups factor was time (admission and discharge). The dependent variables were the patient outcomes (WB, IB, agitation, functional ability, and quality of life) and related measures such as chlorpromazine equivalent dosage and LoS.
Upon admission, patients' MMSE, modified Severity of Illness Index, and CCI were administered, along with the emotional, behavioral, and functional assessments (WB, IB, PAS, EQ-5D, and MBI). These measurements were recorded as part of routine assessment conducted by attending nurses, doctors, and therapists in the CAMIE unit and were repeated upon discharge. Administration of the same tests was conducted for patients in the conventional geriatric ward.
2.4. Statistical analyses
The data were analyzed using SPSS, version 21. Preliminary analyses were conducted to compare the demographics and clinical characteristics across patients in the CAMIE and control groups at baseline and to compare the baseline scores for the five dependent variables, using independent samples t-tests. Owing to a significant difference between groups in MMSE scores, these were included as a covariate in the main analysis. Multivariate analysis of covariance (MANCOVA) examined the interaction between ward type and pre-post patient outcomes, covarying for MMSE. Additional between-groups analyses were conducted for other care and discharge-related outcomes. For cost-effectiveness computation, the difference in cost of staying in CAMIE versus conventional ward was computed based on the extra operating cost of CAMIE per patient per day being SGD 100, with costs incurred from the extra staffing required per shift and the cost of more structured activities in the daily routine such as music and recreational activities. The difference between CAMIE versus conventional ward in quality-adjusted life years (QALY) gained during hospitalization was calculated. Subsequently, the incremental cost-effectiveness ratio (ICER; the difference in cost divided by difference in QALY gained) was derived.
3. Results
3.1. Baseline equivalence
As seen in Table 1, there was no significant difference at baseline between the two groups in variables such as age, dementia severity, presence of delirium, comorbidities, admitting diagnoses, and illness severity. There was, however, a difference in MMSE-measured cognition, t(228) = 3.46, P = .001, with patients in the CAMIE unit having significantly higher MMSE scores (M = 6.18, SD = 6.56) compared with those in the conventional geriatric ward (mean [M] = 3.00, standard deviation [SD] = 4.60). However, it should be noted that the mean scores of both groups were within the severe range of cognitive impairment. Significant group differences in pre-intervention scores were also found for WB: t(228) = 3.86, P < .001; PAS: t(228) = −3.89, P < .001; MBI: t(228) = 3.07, P = .002; and EQ-5D: t(228) = 2.36, P = .019, where patients in the CAMIE unit demonstrated significantly higher WB, MBI, and EQ-5D and lower PAS compared with those in the conventional geriatric ward (see Table 2). The use of MANCOVA takes into account the baseline differences between groups.
Table 2.
Mean (M) and standard deviation (SD) of patient outcomes for both groups
| Outcome measures | CAMIE unit (N = 170) |
Control group (N = 60) |
||
|---|---|---|---|---|
| Pre M (SD) |
Post M (SD) |
Pre M (SD) |
Post M (SD) |
|
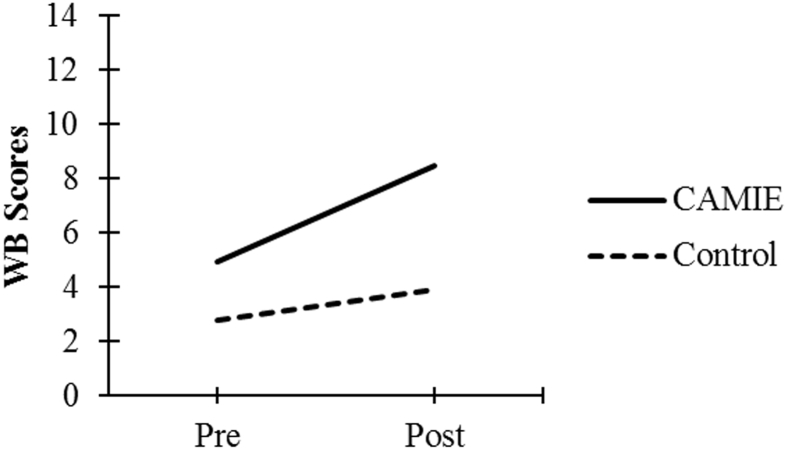
| WB Score | 4.94 (3.95) | 8.46 (3.49) | 2.78 (3.00) | 3.88 (3.51) |
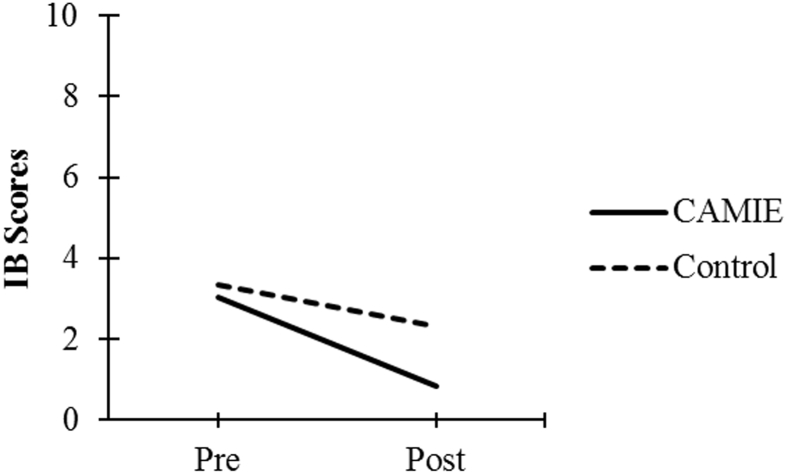
| IB Score | 3.04 (2.11) | 0.84 (1.26) | 3.33 (1.71) | 2.32 (1.72) |
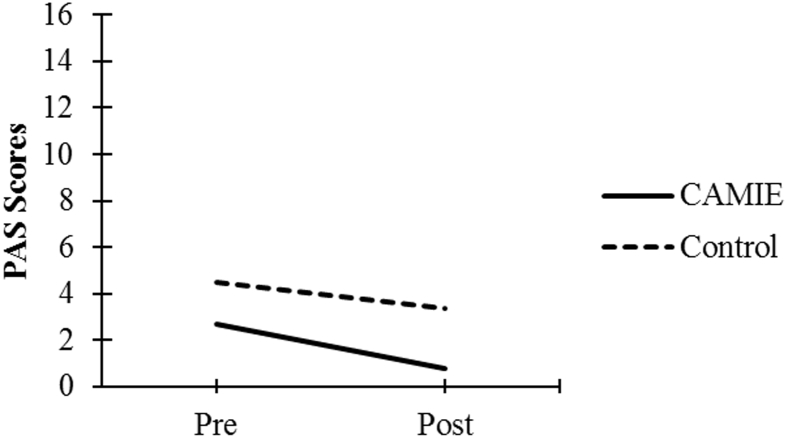
| PAS Score | 2.70 (2.92) | 0.79 (1.39) | 4.48 (3.38) | 3.37 (3.26) |
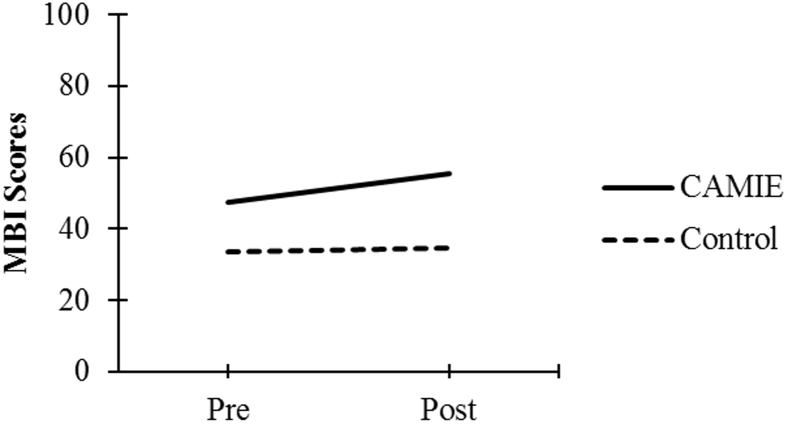
| MBI Score | 47.31 (28.90) | 55.58 (29.37) | 33.68 (31.36) | 34.67 (32.39) |
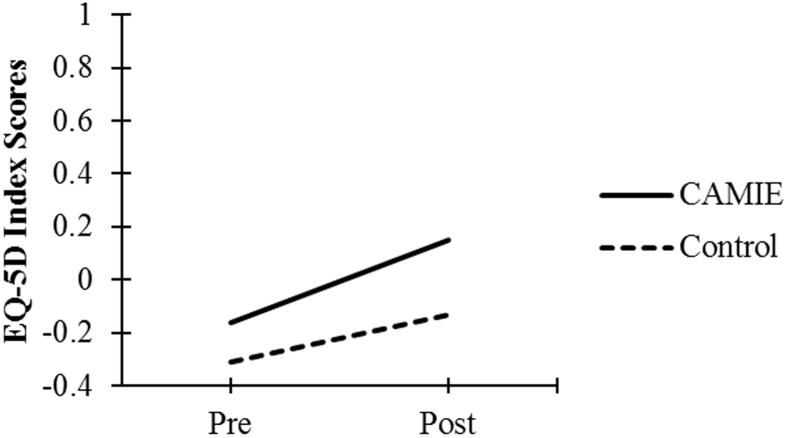
| EQ-5D Index Score | −0.16 (0.43) | 0.15 (0.41) | −0.31 (0.40) | −0.13 (0.46) |
Abbreviations: CAMIE, Care for Acute Mentally Infirm Elders; EQ-5D, EuroQoL; IB, ill-being; MBI, Modified Barthel Index; PAS, Pittsburg Agitation Scale; WB, Well-Being.
3.2. Patient outcomes
A statistically significant interaction of ward type and time was found on the combined outcome variables, Pillai's V = 0.11, F(5, 223) = 5.57, P < .001, ηp2 = .11. Univariate analyses of individual dependent variables revealed statistically significant interactions of group and time for all dependent variables, WB: F(1, 227) = 22.79, P < .001, ηp2 = .09; IB: F(1, 227) = 16.20, P < .001, ηp2 = .07; PAS: F(1, 227) = 4.10, P = .044, ηp2 = .02; MBI: F(1, 227) = 9.89, P = .002, ηp2 = .04; and EQ-5D: F(1, 227) = 5.86, P = .016, ηp2 = .03. Table 2 displays the means and standard deviations of patient outcomes for the two groups.
As seen in Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, patients in the CAMIE unit displayed greater pre-post improvements in WB, MBI, and EQ-5D and greater pre-post reductions in IB and PAS, as compared with control group patients who only had significant pre-post improvements in WB: t(59) = −2.88, P = .005, and EQ-5D: t(59) = −4.44, P < .001, as well as a pre-post reduction in IB: t(59) = 4.47, P < .001. No significant pre-post difference was found in the control group for PAS: t(59) = 2.30, P = .025 or MBI: t(59) = −0.62, P = .540.
Fig. 1.
Pre-post mean WB scores for both groups. Abbreviations: CAMIE, Care for Acute Mentally Infirm Elders; WB, Well-Being.
Fig. 2.
Pre-post mean IB scores for both groups. Abbreviations: CAMIE, Care for Acute Mentally Infirm Elders; IB, Ill-Being.
Fig. 3.
Pre-post mean PAS scores for both groups. Abbreviations: CAMIE, Care for Acute Mentally Infirm Elders; PAS, Pittsburg Agitation Scale.
Fig. 4.
Pre-post mean MBI scores for both groups. Abbreviations: CAMIE, Care for Acute Mentally Infirm Elders; MBI, Modified Barthel Index.
Fig. 5.
Pre-post mean EQ-5D index scores for both groups. Abbreviations: CAMIE, Care for Acute Mentally Infirm Elders; EQ-5D, EuroQoL.
Although the mean chlorpromazine equivalent dosage (mg) was lower in the CAMIE unit (M = 14.31, SD = 34.44) than conventional ward (M = 15.63, SD = 36.16), it was not statistically significant, t(228) = −0.25, P = .802. In addition, CAMIE patients had an average LoS (M = 14.84 days, SD = 13.77) of 2 days shorter than the conventional ward (M = 16.92 days, SD = 13.19), although it did not reach statistical significance, t(228) = −1.02, P = .310.
3.3. Cost-effectiveness
The computed difference in mean EQ-5D index score between patients of CAMIE and usual care was 0.18. Assuming the difference holds for 3 months (0.25 years), the difference in QALY is 0.18 × 0.25 = 0.045. Given the average LoS of 15 days in CAMIE, the total additional cost of caring for a CAMIE patient per admission is SGD 1500 (USD 1040). The ICER, defined as the cost difference between CAMIE and usual care per unit difference in QALY, is 1040/0.045 = USD 23,111.
4. Discussion
The CAMIE unit implements evidence-based practice in delirium and dementia care within a PCC framework to cater for PWDs. Overall, the results show that patients in the CAMIE unit displayed superior pre-post outcomes compared with patients in the conventional geriatric ward, even after covarying for baseline group difference in MMSE scores. PWDs in the CAMIE unit showed greater pre-post improvements in WB, functional ability, and quality of life, as well as greater pre-post reductions in IB and agitation. These findings are consistent with those of previous studies [10], [12], which showed reduction in need-driven dementia-compromised agitation following implementation of PCC in long-term residential facilities. The improvement in functional ability of patients in the CAMIE unit, but not in the conventional ward, lends additional credence to the effectiveness of PCC in the acute care setting.
The likely factors that contributed to better outcomes in CAMIE patients include the enhanced medical protocol that stressed early mobilization; restraints-free care; careful attention to hydration; and bladder and bowel care. Enhanced psychosocial care that operationalized PCC philosophy was important in procuring improved well-being and behaviors in the patients by emphasizing the importance of knowing the patients well including their life histories, preferences and values, endeavoring to individualize care, upholding patient dignity and autonomy, and responding to situational factors and unmet needs in patients with challenging behaviors.
The mean dosage of antipsychotics and patient's average hospital LoS were not significantly different between the groups. Although the chlorpromazine equivalent dosage was lower in CAMIE than conventional ward, the difference did not achieve statistical significance and adds to the mixed findings of antipsychotic drug use associated with PCC [10], [11]. However, the antipsychotic dosages were modest, and in a specialized acute care unit for PWDs where a sizable proportion of patients present with delirium (>70%), it is conceivable that some patients presented with very challenging behaviors. Avoidance of antipsychotics may have been difficult to achieve, especially in the initial period of hospitalization, when medical interventions such as intravenous hydration and antibiotics would have been necessary. With regard to LoS, patients in CAMIE had a shorter mean LoS of 2 days compared with those in the conventional geriatric ward. In this context, the absolute difference in LoS matters clinically and operationally, despite the absence of statistical significance. The modest reduction in LoS could be attributed not only to better patient function and well-being but was likely the result of increased engagement, enablement, and support for family caregivers. Overall, the data support the hypothesis that CAMIE care improves patient outcomes, including a shorter LoS.
Importantly, better outcomes came at a modest cost of SGD 100 more per patient daily, which was further shown to be cost-effective. Given the threshold for cost-effectiveness stands at ICER ≤3 times the gross domestic product (GDP) per capita of a country, the ICER value of USD 23,111 compares very favorably with the latest GDP per capita estimate of USD 80,192 for Singapore [37]. Even with a more conservative assumption of stability in QALY difference between PWDs in the two wards for only 1 month, rather than 3 months, the computed ICER value of USD 57,777 still falls under 1 GDP per capita of Singapore, thereby suggesting PCC as a cost-effective form of care in the acute setting.
A few limitations should be noted. Given that this was a naturalistic observational study, there was little control over the comparability of patients' baseline variables between the groups. It is important to note that there were significant group differences at baseline on MMSE and on four out of five dependent variables, specifically WB, agitation, functional ability, and quality of life. While a fully randomized controlled trial may have yielded equivalence of groups at baseline, this was not possible in a naturalistic observational study. This engenders the possibility that as CAMIE patients were better off than those in the conventional geriatric ward at baseline, they may have had greater potential for improvement. However, the block control design used in this study is reflective of the real-world acute care setting of the clinical service for PWDs being evaluated. The baseline differences were taken into account with the use of a mixed repeated measures MANCOVA model, which included pre-post factor as a within-subject independent variable. It appears that, despite baseline group differences, the pre-post changes observed in the CAMIE patients were greater than those observed in the conventional geriatric ward patients.
In addition, the assessments of outcomes were short-term and not blinded, as the individuals (doctors, nurses, and therapists) involved in administering the measures (WB, PAS, and MBI) were also involved in providing the care. Patients' post-intervention outcomes were assessed only upon discharge without serial assessments during the hospital stay. It would have been ideal to chart the outcomes periodically as well as conduct postdischarge assessments to derive more robust conclusions on the outcomes. As such, cost-effectiveness computation assumed stability of the outcomes for 3 months after discharge without a formal assessment performed. Follow-up studies would be necessary to determine the long-term sustainability of the benefits gained, and this points toward a useful direction for further research.
5. Conclusion
Overall, this evaluation of clinical and economic outcomes of the CAMIE unit has provided valuable insights into the potential benefits of implementing PCC in an acute care setting. It not only addresses a gap in extant literature but also has very practical implications for informing hospital practice and health care policy. The inclusion of multiple outcomes, use of robust statistical methods, and a cost-effectiveness assessment has enabled a rigorous investigation into the impact of care offered in CAMIE. The findings call for wider adoption of PCC models of enhanced care for PWDs in the acute hospital setting.
Research in Context.
-
1.
Systematic review: A literature search was conducted using specific keywords and search terms related to “person/patient-centered care”, “hospital/acute care for persons/patients with dementia/delirium”, “acute/hospital care for older persons/patients”, and “geriatric hospital/acute care”.
-
2.
Interpretation: Our findings showed that person-centered care for patients with dementia in the acute hospital resulted in better well-being and function compared with usual care. Patients who received person-centered care showed less behavioral problems, and the intervention was also cost-effective.
-
3.
Future directions: Given the limitations of the current naturalistic observational cohort study, future studies with randomized controlled trials and larger samples could better secure homogeneity between groups at baseline and provide adequate statistical power to investigate the effect on outcomes that did not achieve statistical significance in this study such as the use of psychotropic medications and length of stay. Longer term follow-up is necessary to ascertain the sustainability of the outcomes.
Acknowledgments
Study conceptualization and design were performed by F.H.E.T., H.M.K., J.J.C.T., C.M.N., and P.L.K.Y. Acquisition of subjects and data was done by F.H.E.T., H.M.K., J.J.C.T., and C.M.N. Analysis and interpretation of data were by F.H.E.T. and C.L.T. Preparation of manuscript was done by F.H.E.T., C.L.T., C.M.N, C.C.N., and P.L.K.Y. All authors performed the revision and vetting of manuscript. Special thanks to Sandy Shen, patients, and nurses of our geriatric wards and CAMIE unit.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Prince M., Bryce R., Albanese E., Wino A., Ribeiro W., Ferri C.P. The global prevalence of dementia: A systematic review and meta-analysis. Alzheimers Dement. 2013;9:63. doi: 10.1016/j.jalz.2012.11.007. 75.e2. [DOI] [PubMed] [Google Scholar]
- 2.Moyle W., Borbasi S., Wallis M., Olorenshaw R., Gracia N. Acute care management of older people with dementia: A qualitative perspective. J Clin Nurs. 2011;20:420–428. doi: 10.1111/j.1365-2702.2010.03521.x. [DOI] [PubMed] [Google Scholar]
- 3.Dewing J. Care for older people with a dementia in acute hospital settings. Nurs Old People. 2001;13:18–20. doi: 10.7748/nop2001.05.13.3.18.c2177. [DOI] [PubMed] [Google Scholar]
- 4.Covinsky K.E., Pierluissi E., Johnston C.B. Hospitalization-associated disability: “She was probably able to ambulate, but I'm not sure”. JAMA. 2011;306:1782–1793. doi: 10.1001/jama.2011.1556. [DOI] [PubMed] [Google Scholar]
- 5.McGillick J., Murphy-White M. Getting to yes, or how to encourage person-centered dementia care in hospitals. Generations. 2013;37:92–96. [Google Scholar]
- 6.Webster J. Improving care for people with dementia in acute hospital: The role of person-centered assessment. Qual Ageing Old Adults. 2011;12:86–94. [Google Scholar]
- 7.McCance T., McCormack B., Dewing J. An exploration of person-centredness in practice. Online J Issues Nurs. 2011;16:1. [PubMed] [Google Scholar]
- 8.Brooker D.J., Wooley R.J., Lee D. Enriching opportunities for people living with dementia in nursing homes: An evaluation of a multi-level activity-based model of care. Aging Ment Health. 2007;11:361–370. doi: 10.1080/13607860600963679. [DOI] [PubMed] [Google Scholar]
- 9.Kitwood T. McGraw-Hill; Maidenhead, UK: 1997. Dementia Reconsidered: The Person Comes First. [Google Scholar]
- 10.Fossey J., Ballard C., Juszczak E., James I., Alder N., Jacoby R. Effect of enhanced psychosocial care on antipsychotic use in nursing home residents with severe dementia: Cluster randomized trial. BMJ. 2006;332:756–761. doi: 10.1136/bmj.38782.575868.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chenoweth L., King M.T., Jeon Y.-H., Brodaty H., Stein-Parbury J., Norman R. Caring for aged dementia care resident study (CADRES) of person-centered care, dementia-care mapping, and usual care in dementia: A cluster-randomized trial. Lancet Neurol. 2009;8:317–325. doi: 10.1016/S1474-4422(09)70045-6. [DOI] [PubMed] [Google Scholar]
- 12.Terada S., Oshima E., Yokota O., Ikeda C., Nagao S., Takeda N. Person-centered care and quality of life of patients with dementia in long-term care facilities. Psychiatry Res. 2013;205:103–108. doi: 10.1016/j.psychres.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 13.Clissett P., Porock D., Harwood R.H., Gladman J.R. The challenges of achieving person-centered care in acute hospitals: A qualitative study of people with dementia and their families. Int J Nurs Stud. 2013;50:1495–1503. doi: 10.1016/j.ijnurstu.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Ross H., Tod A.M., Clarke A. Understanding and achieving person-centered care: The nurse perspective. J Clin Nurs. 2014;24:1223–1233. doi: 10.1111/jocn.12662. [DOI] [PubMed] [Google Scholar]
- 15.Inouye S.K., Bogardus S.T., Charpentier P.A., Leo-Summers L., Acampora D., Holford T.R. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 16.Inouye S.K., Bogardus S.T., Baker D., Leo-Summers L., Cooney L.M., Jr. The Hospital Elder Life Program: A model of care to prevent cognitive and functional decline in older hospitalized patients. J Am Geriatr Soc. 2000;48:1697–1706. doi: 10.1111/j.1532-5415.2000.tb03885.x. [DOI] [PubMed] [Google Scholar]
- 17.Brooker D. Jessica Kingsley Publishers; London, UK: 2007. Person-Centered Dementia Care: Making Services Better. [Google Scholar]
- 18.Edvardsson D., Winblad B., Sandman P.O. Person-centred care of people with severe Alzheimer's disease. Lancet Neurol. 2008;7:362–367. doi: 10.1016/S1474-4422(08)70063-2. [DOI] [PubMed] [Google Scholar]
- 19.American Psychiatric Association . 3rd ed., revised. American Psychiatric Association; Washington, DC: 1987. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 20.Folstein M.F., Folstein S.E., McHugh P.R. Mini mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Feng L., Chong M.S., Lim W.S., Ng T.P. The modified Mini-Mental State Examination test: Normative data for Singapore Chinese older adults and its performance in detecting early cognitive impairment. Singapore Med J. 2012;53:458–462. [PubMed] [Google Scholar]
- 22.Horn S.D., Horn R.A. Reliability and validity of the Severity of Illness Index. Med Care. 1986;24:159–178. doi: 10.1097/00005650-198602000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Wong W.C., Sahadevan S., Ding Y.Y., Tan H.N., Chan S.P. Resource consumption in hospitalized, frail older patients. Ann Acad Med Singapore. 2010;39:830–836. [PubMed] [Google Scholar]
- 24.Charlson M., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Bradford Dementia Group . 7th ed. University of Bradford; Bradford, UK: 1997. Evaluating Dementia Care: The DCM Method. [Google Scholar]
- 26.Brooker D. Dementia care mapping: A review of the research literature. Gerontologist. 2005;45:11–18. doi: 10.1093/geront/45.suppl_1.11. [DOI] [PubMed] [Google Scholar]
- 27.Rosen J., Burgio L., Kollar M., Cain M., Allison M., Fogleman M. A user-friendly instrument for rating agitation in dementia patients. Am J Geriatr Psychiatry. 1994;2:52–59. [PubMed] [Google Scholar]
- 28.Mahoney F.I., Barthel D.W. Functional evaluation: The Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 29.Brooks R. EuroQOL: The current state of play. Health Policy. 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 30.Rabin R., de Charro F. EQ-5D: A measure of health status from the EuroQoL group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 31.Luo N., Wang P., Thumboo J., Lim Y.W., Vrijhoef H.J. Valuation of EQ-5D-3L health states in Singapore: Modeling of time trade-off values for 80 empirically observed health states. Pharmacoeconomics. 2014;32:495–507. doi: 10.1007/s40273-014-0142-1. [DOI] [PubMed] [Google Scholar]
- 32.Luo N., Chew L.H., Fong K.Y., Koh D.R., Ng S.C., Yoon K.H. Validity and reliability of the EQ-5D self-report questionnaire in English-speaking Asian patients with rheumatic diseases in Singapore. Qual Life Res. 2003;12:87–92. doi: 10.1023/a:1022063721237. [DOI] [PubMed] [Google Scholar]
- 33.Luo N., Low S., Lau P.N., Au W.L., Tan C.S.L. Is EQ-5D a valid quality of life instrument in patients with Parkinson's disease? A study in Singapore. Ann Acad Med Singapore. 2009;38:521–528. [PubMed] [Google Scholar]
- 34.Abdin E., Subramaniam M., Vaingankar J.A., Luo N., Chong S.A. Measuring health-related quality of life among adults in Singapore: Population norms for the EQ-5D. Qual Life Res. 2013;22:2983–2991. doi: 10.1007/s11136-013-0405-x. [DOI] [PubMed] [Google Scholar]
- 35.Chong S.A., Abdin E., Luo N., Vaingankar J.A., Supramaniam M. Prevalence and impact of mental and physical comorbidity in the adult Singapore population. Ann Acad Med Singap. 2012;41:105–114. [PubMed] [Google Scholar]
- 36.Inouye S.K., van Dyck C.H., Alessi C.A., Balkin S., Siegal A.P., Horwitz R.I. Clarifying confusion: The confusion assessment method. A new method for detecting delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 37.Trading Economics Singapore GDP per capita PPP. http://www.tradingeconomics.com/singapore/gdp-per-capita-ppp/forecast Available at: