Abstract
Introduction
No licensed medications are available to treat vascular dementia (VaD).
Methods
Patients were randomly assigned to experimental groups (SaiLuoTong [SLT] 360 or 240 mg for groups A and B for 52 weeks, respectively) or placebo group (SLT 360 mg and 240 mg for group C only from weeks 27 to 52, respectively).
Results
Three hundred twenty-five patients were included in final analysis. At week 26, the difference in VaD Assessment Scale–cognitive subscale scores was 2.67 (95% confidence interval, 1.54 to 3.81) for groups A versus C, and 2.48 (1.34 to 3.62) for groups B versus C (both P < .0001). However, at week 52, no difference was observed among the groups on the VaD Assessment Scale–cognitive subscale (P = .062) because of the emerging efficacy of SLT in placebo beginning at week 27.
Discussion
This study suggests that SLT is effective for treatment of VaD, and this compound Chinese medicine may represent a better choice to treat VaD.
Keywords: Vascular dementia, Clinical trial, Compound Chinese medicine, SaiLuoTong/SLT
1. Background
Vascular dementia (VaD) is a cognitive dysfunction syndrome caused by ischemic stroke, hemorrhagic stroke, and cerebral vascular disease [1]. In China, the prevalence of VaD is 1.50% [2], and it is estimated that there are approximately three million patients with this disease [2], [3]. Although acetylcholinesterase inhibitors, such as donepezil, galantamine, and rivastigmine, showed positive therapeutic effects on VaD in clinical trials, there are still no licensed medications that meet the criteria of the US Food and Drug Administration or the European Medicine Agency for this disease [1]. This requires that the drugs should show global or functional benefits, in addition to cognitive benefits, for approval [4], [5]. In recent years, an increasing number of clinical trials have been conducted to test the effects of compound Chinese medicines for treating VaD, and many have shown positive effects by improving cognitive or behavioral symptoms [6].
The SaiLuoTong (SLT) capsule is a modern compound Chinese medicine that is manufactured by Shineway Pharmaceutical Group Co., Ltd (Shijiazhuang, China). It consists of active ingredients quantified in milligrams (for details, see eTable 1 in Supplementary 2) and derived from Ginkgo biloba, ginsenosides, and saffron in a 5:5:1 proportion per capsule, based on preclinical studies. Ginkgo biloba has antiinflammatory properties [7] and stimulates hippocampal neurogenesis [8]. Ginsenoside Rg1 inhibits oxidative stress-induced neuronal apoptosis [9], protects against neurodegeneration in cultured hippocampal neurons [10], and improves memory function in Alzheimer's disease (AD) and estrogen-deficient rat models [11], [12]. Saffron has the capacity to scavenge oxygen free radicals [13], improve learning and memory in animal models of chronic stress [14], and alleviate neuronal injury in vitro and in vivo [15]. It also moderately inhibits acetylcholinesterase, which is the main effect of donepezil in AD [16], and a clinical trial showed that saffron has similar cognitive-enhancing effects to donepezil in patients with AD [16]. All of these functions of Ginkgo biloba, ginsenosides, and saffron in SLT are related to potential mechanisms that could help treat VaD.
Therefore, we hypothesized that SLT may have therapeutic efficacy in patients with mild-to-moderate VaD and designed the present clinical trial to test this.
2. Methods
2.1. Study design and participants
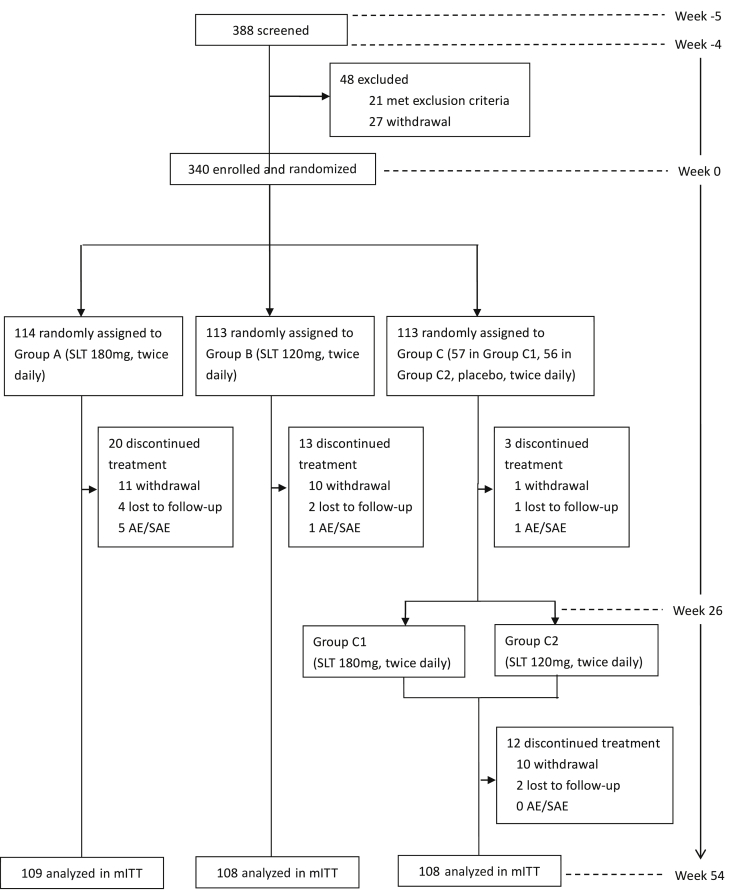
This 59-week, phase II, randomized, controlled, double-blind, parallel-arm study was performed at 16 academic centers throughout China. A protocol amendment was made on April 27, 2013, which increased the follow-up period from ±1 week to ±2 weeks for each visit to reduce the dropout rate. Fig. 1 displays an overall schematic of the design.
Fig. 1.
Trial profile. Abbreviations: AE, adverse event; SAE, serious adverse event; mITT, modified intent to treat.
Eligible patients had to be aged ≥40 years, male or female, Han Chinese, have ≥5 years of education, have a diagnosis of probable VaD of mild to moderate severity, and have evidence of ischemic lesions on brain magnetic resonance imaging. Exclusion criteria were non-VaD primary dementia or non-ischemic VaD, disturbances of consciousness, severe aphasia, physical disabilities, or any other factor that could preclude the completion of neuropsychological testing. The full details of the inclusion and exclusion criteria are provided in eAppendix 1 in Supplementary 2.
The study protocol (Supplementary 1) was approved by independent ethics committees at all study sites. Written informed consent was obtained from each patient, or from the patient's legal guardian or representative, before enrollment. This study was registered at ClinicalTrials.gov (NCT01978730).
2.2. Randomization and masking
Randomization was performed using an interactive web response system and stratified according to severity of VaD (two levels: mild and moderate) and center (16 centers in total). Interactive web response system generated the randomization sequence with 33 blocks × 12 (4:4:2:2). The patient randomization file consisted of the trial randomization number and treatment group code. A drug kit number list was generated and subsequently assigned to the patients by interactive web response system. The personnel involved in the execution and data analysis were blinded to the drug kit randomization list. Study participants, their caregivers, and all assessors remained blinded to the treatment assignments throughout the study, and safety assessors were not permitted to be involved in the primary efficacy assessments. The SLT and placebo were identical in appearance, smell, and taste, to maintain blinding.
2.3. Study intervention
The trial began with a 1-week screening period and a 4-week placebo run-in period, and participants were randomly assigned to four groups: group A, SLT 360 mg, and group B, 240 mg SLT, for 52 weeks; group C (C1 and C2), placebo for the first 26 weeks and switched to SLT 360 mg and 240 mg, respectively, for the next 26 weeks (Fig. 1). Treatment compliance was monitored by counting the capsules. The number of capsules taken was recorded in a diary and reviewed at each clinic visit.
2.4. Primary and second outcomes
The coprimary outcomes included the Vascular Dementia Assessment Scale–cognitive subscale (VaDAS-cog) [17] and Alzheimer's Disease Cooperative Study–Clinical Global Impression of Change (ADCS-CGIC) scores [18]. The VaDAS-cog is composed of 14 items related to memory and orientation, language, the ability to practice, attention focus, and executive function (score ranges from 0 [no impairment] to 90 [serious impairment]). The ADCS-CGIC is a version of the clinician's interview-based impression of change plus caregiver input [19], [20] and covers four domains (general, mental cognitive state, activities of daily living [ADLs], and behavior), with scores ranging from 1 (significant improvement) to 7 (severe deterioration). An experienced clinician performed the ADCS-CGIC and was blinded to all of the other psychometric assessments. The secondary outcomes included the Mini–Metal State Examination (MMSE), ADCS-ADLs, and Clinical Dementia Rating (CDR) scale scores, performance on the clock drawing task (CLOX) and the Chinese version of the executive interview (C-EXIT25), and the Neuropsychiatric Inventory (NPI). These scales evaluate global cognition, living ability, dementia severity, executive function, mental status, and behavior. The patients were assessed at baseline and at weeks 13, 26, 39, and 52 with respect to the VaDAS-cog and ADCS-CGIC, and at baseline and weeks 26 and 52 for the MMSE, CDR, ADCS-ADLs [21], CLOX [22], C-EXIT25 [23], and NPI [24]. Brain magnetic resonance imaging scans were performed at baseline and at week 52.
2.5. Evaluation of safety
We monitored patients throughout the study for adverse events (AEs), serious adverse events (SAEs), and concomitant medication use and performed clinical and laboratory examinations including measurements of vital signs, physical and neurological examinations, and 12-lead electrocardiography at all clinical visits (screening, baseline, and weeks 13, 26, 39, 52, and 54).
2.6. Statistical analysis
The power of this study was calculated based on the change in primary endpoint from baseline on the VaDAS-cog. Because the clinical use of SLT in patients with VaD is still in the exploratory stages and no previous trial results are available, the result of a clinical trial of memantine in patients with VaD was used as a reference to calculate sample size, in which the drug-placebo difference in change from baseline on the ADAS-cog was 2.83 (SD 5.72) [25]. The two-sided t-test with a significance level of 5% was used. As a result, 86 patients were needed per group to achieve 90% power. Given an expected dropout rate of 20%, the total number of patients to be randomized was 324.
The primary and secondary outcomes were analyzed using data from the modified intent-to-treat (mITT) population. In this study, the mITT population consisted of all randomly assigned patients who took at least one dose of the study medication and had both a baseline and (at least one) postbaseline efficacy assessment. Missing values for the primary endpoint measures were replaced using the last observation carried forward method. No missing data were imputed for the secondary endpoint measures.
The statistical analysis plan was finalized, and the database was locked in September 2016. The comparison of VaDAS-cog scores within groups, at baseline and at weeks 13, 26, 39, and 52, was done using the paired t-test. For the first phase (weeks 0–26), the changes from baseline in VaDAS-cog and ADCS-CGIC scores, at weeks 13 and 26, were analyzed using analysis of covariance, with groups as a fixed effect, center as a random effect, and the baseline score and degree of disease as covariates. For the second phase (weeks 27–52), changes in coprimary outcomes from baseline and at weeks 39 and 52 were analyzed using the same model. The ADCS-CGIC scores were analyzed as categorical data using the Cochran-Mantel-Haenszel method.
For secondary outcomes, such as the MMSE, CDR, C-EXIT25, CLOX, ADCS-ADLs, and NPI, the paired t-test or the Mann-Whitney U test was used to compare scores before and after treatment within groups. One-way analysis of variance or the Kruskal-Wallis test was conducted to compare the changes at weeks 26 and 52 from baseline between the groups. The safety set consisted of all subjects who took at least one dose of the study medication and had at least one postbaseline safety evaluation. The incidence of AEs at weeks 26 and 52 was compared between the groups using the χ2 test or Fisher's exact test.
All analyses were conducted using SAS software (ver. 9.4; SAS Institute, Cary, NC, USA). All hypothesis tests were two-tailed, and P values ≤ 0.05 were considered significant. All data were overseen by a Data and Safety Monitoring Board.
3. Results
3.1. Study participants
We screened 388 patients from March 28, 2013 to February 25, 2014; the last patient withdrew from the trial on April 21, 2015. Of the 340 patients randomly assigned to treatment, 114 cases (94 finished this study, 82.5%) were in the high-dose group (group A, 180 mg, twice daily), 113 (100, 88.5%) were in the low-dose group (group B, 120 mg, twice daily), and 113 (98, 86.7%) were in the control group C (57 in C1 and 56 in C2). Causes of dropout were withdrawal, loss to follow-up, and AEs. In total, 325 patients (109 cases in group A, 108 cases in group B, 55 cases in group C1, and 53 cases in group C2) received at least one dose of the study drug with a safety assessment and comprised the safety set or received at least one postbaseline efficacy assessment and comprised the mITT population (Fig. 1). The baseline demographic and clinical characteristics of the mITT population are shown in Table 1.
Table 1.
Characteristics of the treatment group at baseline
| Characteristic | Group A (n = 109) | Group B (n = 108) | Group C (n = 108) | P |
|---|---|---|---|---|
| Age, mean (SD), y | 64.9 (9.1) | 66.0 (9.2) | 66.0 (9.3) | .5871 |
| Education distribution, n (%) | .4380 | |||
| 41–50 | 5 (4.6) | 5 (4.6) | 4 (3.7) | |
| 51–60 | 34 (31.2) | 33 (30.6) | 32 (29.6) | |
| 61–70 | 45 (41.23) | 30 (27.8) | 35 (32.4) | |
| 71–80 | 20 (18.6) | 35 (32.4) | 30 (27.8) | |
| 81–90 | 5 (4.6) | 5 (4.6) | 7 (6.5) | |
| Female, n (%) | 42 (38.5) | 39 (36.1) | 29 (26.9) | .1591 |
| Education, mean (SD), y | 9.9 (3.4) | 9.6 (3.5) | 9.8 (3.5) | .7823 |
| Education distribution, n (%) | .8478 | |||
| Primary school | 29 (26.9) | 26 (23.9) | 28 (25.9) | |
| Middle school | 32 (29.6) | 32 (29.3) | 33 (30.6) | |
| High school | 32 (29.6) | 34 (31.2) | 29 (26.9) | |
| College | 15 (13.9) | 17 (15.6) | 18 (16.7) | |
| Medical history, n (%) | ||||
| Hypertension | 93 (85.3) | 94 (87.0) | 87 (80.6) | .3981 |
| Hyperlipidemia | 16 (14.7) | 10 (9.3) | 14 (13.0) | .4628 |
| Diabetes mellitus | 52 (47.7) | 39 (36.1) | 52 (48.2) | .1294 |
| Atrial fibrillation | 2 (1.8) | 0 (0.0) | 3 (2.8) | .0977 |
| Coronary heart disease | 15 (13.8) | 13 (12.0) | 16 (14.8) | .8340 |
| Lung disease | 8 (7.3) | 5 (4.6) | 8 (7.4) | .6381 |
| Gastrointestinal disease | 25 (22.9) | 35 (32.4) | 28 (25.9) | .2762 |
| Stroke | 109 (100.0) | 108 (100.0) | 108 (100.0) | .4924 |
| Large-artery atherosclerosis | 37 (33.9) | 30 (27.8) | 32 (29.6) | |
| Cardioembolism | 2 (1.8) | 0 (0.0) | 0 (0.0) | |
| Small-artery occlusion lacunar | 63 (57.8) | 73 (67.6) | 68 (63.0) | |
| Acute stroke of other determined etiology | 2 (1.8) | 3 (2.8) | 4 (3.7) | |
| Stroke of other undetermined etiology | 5 (4.6) | 2 (1.9) | 4 (3.7) | |
| Personal history, n (%) | ||||
| Alcohol intake | 37 (33.9) | 39 (36.1) | 47 (43.5) | .3134 |
| Smoking | 45 (41.3) | 45 (41.7) | 49 (45.4) | .7984 |
| Concomitant drugs in at least 10 patients, n (%) | 98 (89.9) | 94 (87.0) | 94 (87.0) | .7535 |
| Calcium channel blocker agents | 43 (39.5) | 48 (44.4) | 46 (42.6) | .7634 |
| Lipid regulator agents | 27 (24.8) | 23 (21.3) | 24 (22.2) | .8415 |
| Renin angiotensin system agents | 32 (29.4) | 18 (16.7) | 19 (17.6) | .0465 |
| Analgesics | 47 (43.1) | 38 (35.2) | 40 (37.0) | .4628 |
| Antidiabetic agents | 35 (32.1) | 23 (21.3) | 30 (27.8) | .1859 |
| Other Chinese medicine | 14 (12.8) | 14 (13.0) | 14 (13.0) | 1.0000 |
| Psychometric scores, mean (SD) | ||||
| VaDAS-cog | 31.5 (10.1) | 30.8 (9.5) | 31.8 (9.9) | .7611 |
| MMSE | 19.9 (3.4) | 19.7 (3.7) | 19.8 (3.6) | .8377 |
| CDR | 1.4 (0.5) | 1.4 (0.5) | 1.4 (0.5) | .7984 |
| CDR-SB | 6.6 (2.4) | 6.5 (2.4) | 6.5 (2.4) | .9839 |
| ADCS-ADLs | 50.0 (11.6) | 51.5 (11.1) | 50.8 (9.4) | .5889 |
| CLOX | 10.1 (3.4) | 10.2 (3.0) | 10.2 (2.9) | .9755 |
| C-EXIT25 | 18.3 (7.7) | 17.9 (7.6) | 17.7 (6.8) | .8341 |
| NPI for patients | 5.9 (5.4) | 5.5 (4.6) | 5.7 (4.9) | .8174 |
| NPI for caregivers | 3.1 (3.4) | 2.8 (3.1) | 2.8 (3.4) | .7966 |
| HAMD | 6.7 (3.7) | 6.2 (3.2) | 6.0 (3.7) | .2630 |
| mHIS | 9.5 (1.2) | 9.6 (1.4) | 9.7 (1.3) | .7675 |
Abbreviations: SD, standard deviation; VaDAS-cog, Vascular Dementia Assessment Scale–cognitive subscale; MMSE, Mini–Mental State Examination; CLOX, clock drawing task; C-EXIT25, Chinese version of the executive interview; NPI, Neuropsychiatric Inventory; CDR, Clinical Dementia Rating; CDR-SB, the sum of boxes of the CDR; ADCS-ADLs, Alzheimer's disease cooperative study activities of daily living; HAMD, Hamilton Depression Scale; mHIS, Modified Hachinski Ischemic Scale.
3.2. Coprimary endpoints
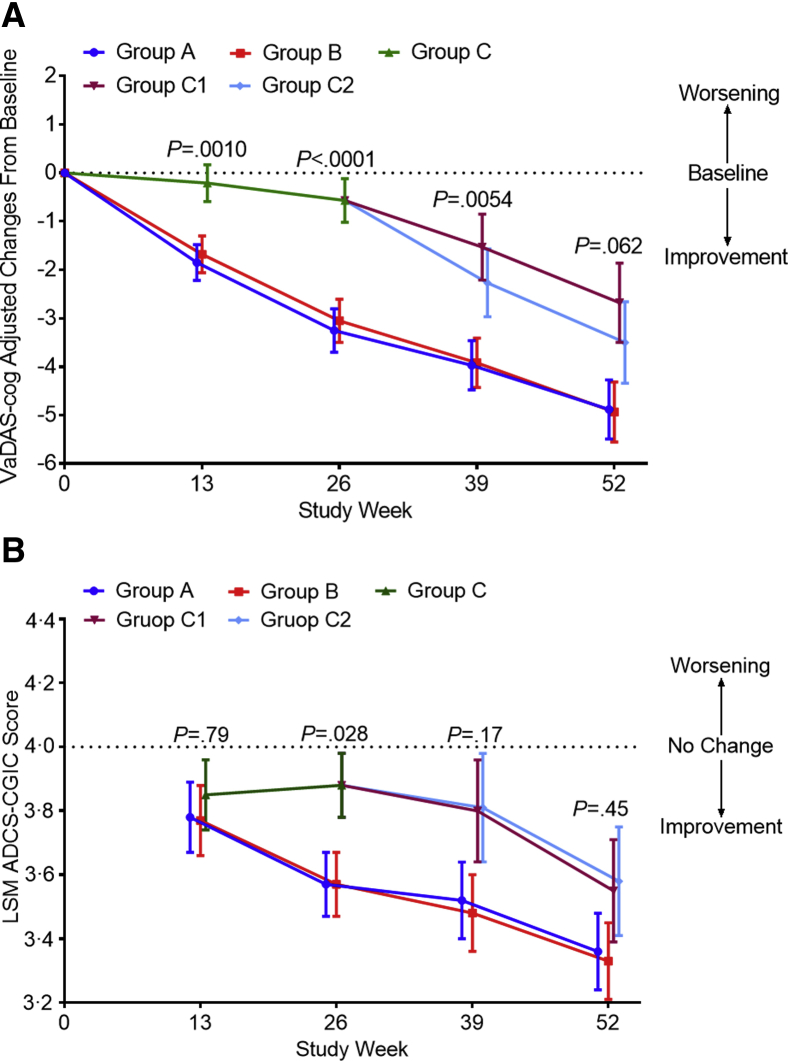
The changes in the least squares mean scores between week 26 and baseline on the VaDAS-cog were −3.25 (standard error [SE] 0.45) for group A, −3.05 (0.45) for group B (both P < .0001), and −0.57 (0.45) for group C (P = .15), with a significant difference among groups (P < .0001) (Fig. 2A). The differences were 2.67 (95% confidence interval, 1.54–3.81) between groups A and C, and 2.48 (1.34–3.62) between groups B and C (both P < .0001). However, the difference between groups A and B was not significant [0.20 (−0.94 to 1.34), P = .73], indicating that they had similar effectiveness. On week 52, the VaDAS-cog scores changed from baseline, by −4.88 (SE 0.61) in group A, −4.93 (0.62) in group B, −2.68 (0.82) in group C1 and −3.50 (0.84) in group C2 (P < .0001 for groups A, B, and C2; P = .00070 for group C1), with no significant difference among the four groups (P = .062) (Fig. 2A). For the ADCS-CGIC, at week 26, the change in the least squares mean score was 3.57 (SE 0.10) for group A, 3.57 (0.10) for group B, and 3.88 (0.10) for group C, with a significant difference among groups (P = .028): groups A and B were more effective than group C [C1 and C2 were combined for the first 26 weeks, i.e., 0.31 (0.05–0.57), P = .028, between groups A and C and 0.31 (0.05 to 0.57), P = .019, between groups B and C]. At week 52, the change in the least squares mean score from baseline was 3.36 (SE 0.12) for group A, 3.33 (0.12) for group B, 3.55 (0.16) for group C1, and 3.58 (0.17) for group C2, with no significant difference among the four groups (P = .45) (Fig. 2B), suggesting that the effect of SLT in the control group was close to that in the active groups.
Fig. 2.
Changes in the VaDAS-cog and ADCS-CGIC scores from baseline to weeks 26 and 52 among the different groups. (A) The change in the VaDAS-cog score from baseline among groups was significantly different (P < .0001) at week 26. No significant difference was seen at week 52 (P < .062), confirming similar efficacy between the active and control groups after using SLT in the second 26 weeks of the study. (B) The change in the ADCS-CGIC score from baseline among groups was significantly different (P = .028) at week 26. Efficacy appears in groups C1 and C2 following use of SLT at week 52. Error bars are 95% confidence intervals. P represents the significance of the difference among groups. Abbreviations: VaDAS-cog, Vascular Dementia Assessment Scale–cognitive subscale; ADCS-CGIC, Alzheimer's Disease Cooperative Study–Clinical Global Impression of Change; LSM, least squares mean; SLT, SaiLuoTong.
3.3. Secondary endpoints
MMSE scores increased from baseline by 1.85 ± 1.29 in group A, 1.81 ± 1.55 in group B and 0.37 ± 2.53 in group C at week 26 (P < .0001 for groups A and B; P = .15 for group C), with a significant difference among the groups (P < .0001). At week 52, MMSE scores increased significantly and were 2.35 ± 2.63 for group C1, and 2.09 ± 1.52 for group C2 (both P < .0001) after using SLT. The CDR, CDR-sum of boxes, CLOX, C-EXIT25, and ADCS-ADLs produced results similar to those of the MMSE, supporting the positive results of the primary outcomes (Table 2). The change in the NPI score from baseline was significantly different within groups A and B, but not among all groups at week 26 (P = .35) or 52 (P = .84) (Table 2). The details of the results for all outcomes are listed in eAppendix 2 in Supplementary 2.
Table 2.
Scores of the primary and secondary outcomes at weeks 26 and 52 in the mITT population
| Week 26 |
Week 52 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) change from baseline |
Mean (SD) change from baseline |
||||||||
| Psychometric scores | Group A (n = 109) | Group B (n = 108) | Group C (n = 108) | P value (among groups) | Group A (n = 109) | Group B (n = 108) | Group C1 (n = 55) | Group C2 (n = 53) | P value (among groups) |
| Primary outcomes | |||||||||
| VaDAS-cog | −3.26 ± 4.30 | −3.06 ± 4.88 | −0.53 ± 3.75 | <.0001 | −4.96 ± 5.78 | −4.99 ± 6.56 | −2.75 ± 5.65 | −3.45 ± 5.81 | .062 |
| ADCS-CGIC | 3.62 ± 1.01 | 3.62 ± 1.05 | 3.93 ± 0.85 | .028 | 3.38 ± 1.19 | 3.38 ± 1.22 | 3.58 ± 1.07 | 3.62 ± 1.18 | .45 |
| Secondary outcomes | |||||||||
| MMSE | 1.85 ± 1.29 | 1.81 ± 1.55 | 0.37 ± 2.53 | <.0001 | 3.26 ± 2.31 | 3.33 ± 2.43 | 2.35 ± 2.63 | 2.09 ± 1.52 | .0028 |
| CDR | −1.05 ± 0.56 | −1.06 ± 0.55 | −1.13 ± 0.51 | .020 | −0.88 ± 0.50 | −0.89 ± 0.55 | −0.90 ± 0.48 | −0.89 ± 0.57 | .75 |
| CDR-SB | −0.85 ± 1.15 | −0.77 ± 1.17 | −0.28 ± 0.96 | .00040 | −1.46 ± 1.44 | −1.39 ± 1.21 | −0.98 ± 1.33 | −0.94 ± 1.22 | .041 |
| ADCS-ADLs | 4.18 ± 4.79 | 4.01 ± 5.11 | 1.80 ± 5.11 | .00080 | 7.40 ± 5.10 | 7.41 ± 5.31 | 5.78 ± 5.60 | 5.47 ± 5.03 | .061 |
| CLOX | 1.00 ± 1.87 | 1.01 ± 2.10 | 0.02 ± 2.01 | .00030 | 1.70 ± 2.44 | 1.72 ± 2.31 | 0.96 ± 2.02 | 1.09 ± 2.71 | .15 |
| C-EXIT25 | −2.00 ± 2.94 | −1.83 ± 2.94 | −0.33 ± 2.85 | <.0001 | −3.13 ± 3.72 | −3.19 ± 3.41 | −1.96 ± 2.91 | −2.00 ± 3.15 | .052 |
| NPI | −0.82 ± 2.46 | −0.94 ± 2.63 | −0.43 ± 2.87 | .35 | −1.37 ± 3.70 | −1.37 ± 3.15 | −1.29 ± 3.23 | −0.87 ± 3.31 | .84 |
Abbreviations: SD, standard deviation; VaDAS-cog, Vascular Dementia Assessment Scale–Cognitive subscale; ADCS-CGIC, Alzheimer's Disease Cooperative Study–Clinical Global Impression of Change; MMSE, Mini–Mental State Examination; CLOX, clock drawing task; C-EXIT25, Chinese version of the executive interview; CDR, Clinical Dementia Rating; CDR-SB, the sum of boxes of the CDR; ADCS-ADLs, Alzheimer's Disease cooperative study activities of daily living; NPI, Neuropsychiatric Inventory; mITT, modified intent to treat.
3.4. Safety
In total, 287 patients (88.3%) experienced at least one AE at week 26: group A, 91 (83.5%); group B, 101 (93.5%); and group C, 95 (88.0%). No significant difference was seen among the three groups (P = .066) (Table 3). At week 52, 247 patients (76.0%) experienced at least one AE: group A, 81 (74.3%); group B, 80 (74.1%); and group C1, 44 (80.0%), and group C2, 42 (79.3%), with no significant difference among the four groups (P = .78) (Table 3). Among all of the AEs, 43 cases were judged by the investigators to be related to SLT, with symptoms including mild gastrointestinal intolerance (two in group A, one in group B, and two in group C), abnormal alanine aminotransferase (six in group A, one in group B, and two in group C), abnormal aspartate aminotransferase (three in group A), increased thrombin time (eight in group A, three in group B, and four in group C), and dreaminess (one in group A, three in group B, and seven in group C). SAEs occurred in eight subjects, including five cerebral infarctions (three in group A and two in group C), one with acute coronary syndrome in group A, one with acute bronchitis in group B, and one with lung cancer in group A, which were deemed by the investigator to be being unrelated to the study medication. Details are provided in Table 3.
Table 3.
Patients experiencing adverse events at weeks 26 and 52 in the SS population∗
| Event | Week 26 |
Week 52 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group A (n = 109) | Group B (n = 108) | Group C (n = 108) | P value | Group A (n = 109) | Group B (n = 108) | Group C1 (n = 53) | Group C2 (n = 55) | P value | ||
| AEs, number of patients experiencing event (%) | 91 (83.5) | 101 (93.5) | 95 (88.0) | .066 | 81 (74.3) | 80 (74.1) | 44 (80.0) | 42 (79.3) | .78 | |
| AEs occurring in at least 10 patients in either treatment group, n (%) | ||||||||||
| Increased triglyceride level | 22 (20.2) | 23 (21.3) | 29 (26.9) | .47 | Increased triglyceride level | 18 (16.5) | 11 (10.2) | 11 (20.0) | 5 (9.4) | .22 |
| Increased blood glucose | 24 (22.0) | 23 (21.3) | 27 (25.0) | .82 | Decreased high-density lipoprotein | 14 (12.8) | 12 (11.1) | 11 (20.0) | 3 (5.7) | .16 |
| Increased low-density lipoprotein | 18 (16.5) | 24 (22.2) | 29 (26.9) | .18 | Increased blood glucose | 16 (14.7) | 12 (11.1) | 6 (10.9) | 5 (9.4) | .79 |
| Increased total cholesterol level | 20 (18.4) | 20 (18.5) | 23 (21.3) | .85 | Increased total cholesterol level | 9 (8.3) | 12 (11.1) | 7 (12.7) | 7 (13.2) | .69 |
| Decreased high-density lipoprotein | 17 (15.6) | 12 (11.1) | 11 (10.2) | .46 | Urinary leukocyte positive | 10 (9.2) | 10 (9.3) | 6 (10.9) | 8 (15.1) | .66 |
| Urinary leukocyte positive | 13 (11.9) | 10 (9.3) | 6 (5.6) | .27 | Increased low-density lipoprotein | 11 (10.1) | 11 (10.2) | 8 (14.6) | 3 (5.7) | .51 |
| Increased blood uric acid | 4 (3.7) | 11 (10.2) | 7 (6.5) | .15 | ||||||
| Possibly drug-related AEs, n (%) | ||||||||||
| Mild gastrointestinal intolerance | 1 (0.9) | 1 (0.9) | 2 (1.9) | .85 | Mild gastrointestinal intolerance | 1 (0.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.0 |
| Abnormal alanine aminotransferase | 4 (3.7) | 0 (0.0) | 2 (1.9) | .17 | Abnormal alanine aminotransferase | 2 (1.83) | 1 (0.9) | 0 (0.0) | 0 (0.0) | .89 |
| Abnormal aspartate aminotransferase | 2 (1.83) | 0 (0.0) | 0 (0.0) | .33 | Abnormal aspartate aminotransferase | 1 (0.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.0 |
| Increased thrombin time | 4 (3.7) | 1 (0.9) | 1 (0.9) | .38 | Increased thrombin time | 4 (3.7) | 2 (1.9) | 1 (1.8) | 2 (3.8) | .77 |
| Dreaminess | 1 (0.9) | 1 (0.9) | 3 (2.8) | .54 | Dreaminess | 0 (0.0) | 2 (1.9) | 2 (3.6) | 2 (3.8) | .11 |
| Drug-related AEs resulting in treatment discontinuation, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.0 | |
| Any SAEs, n (%) | ||||||||||
| Acute cerebral infarction | 1 (0.9) | 0 (0.0) | 1 (0.9) | Acute cerebral infarction | 2 (1.8) | 0 (0.0) | 1 (1.8) | 0 (0.0) | ||
| Chronic bronchitis | 0 (0.0) | 1 (0.9) | 0 (0.0) | Small cell carcinoma of lung | 1 (0.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Acute coronary syndromes | 1 (0.9) | 0 (0.0) | 0 (0.0) | |||||||
Abbreviations: AEs, adverse events, SAEs, serious adverse events; SS, safety set.
There is no significant difference among the groups.
4. Discussion
Our findings suggest that SLT improved cognition and daily functioning in Chinese patients with mild-to-moderate VaD. The scores on the VaDAS-cog and ADCS-CGIC in the active groups were significantly superior at week 26 compared to those of the control group. At week 52, the benefits seen over the first 26 weeks in groups A and B were reproduced in the second 26 weeks in control groups C (C1 and C2) after using SLT. These results indicate that SLT can improve functioning in multiple domains, such as memory, orientation, language and executive function. The changes from baseline in the active groups were significant at weeks 26 and 52 for scores on the MMSE, CDR, ADCS-ADLs, CLOX, and C-EXIT25, indicating that SLT significantly enhanced global cognitive function, particularly executive function and ADLs. Taken together, most of the primary and secondary outcomes were consistent in supporting the potential efficacy of SLT for VaD, particularly in confirming efficacy in the control subjects, who were switched to an active dose during the second 26 weeks. The results were reproduced in the second cohort within the same trial under the same conditions, which lends added credence to the findings.
The VaD disease mechanisms are complex and mixed. In general, the major pathogenesis of VaD has been attributed to (chronic or acute) global or local hypoperfusion and thromboembolic events, oxidative stress, and the inflammatory response [26]. In recent years, Chinese medicine ingredients have been combined in complex formulations to treat cognitive disorders. Researchers have argued that the reason for the efficacy of modern compound Chinese medicines is that the bioactive components interact synergistically leading to greater pharmacological effects or better clinical outcomes than predicted by the activity of single components [27]. As all of the active ingredients in Ginkgo biloba, ginsenosides, and saffron in the SLT have potential efficacy against the complex disease pathways underlying VaD, their combination was considered to have the potential to maximize the effects. Because SLT has useful effects on hypoperfusion, inflammatory changes, oxidative stress, cholinergic system dysfunction, calcium overload, apoptosis and platelet aggregation, we assume it has multiple potential targets. The multiple effects might represent a particular advantage of SLT with multiple ingredients. Although the present findings suggest that SLT may be beneficial, a further rigorous controlled study is required.
No significant differences were observed in the frequency of most common AEs among the active groups and control, but we could not ascertain an association between SLT usage and these abnormal laboratory results. This may be due to our elderly participants, who had many comorbidities, such as diabetes, high blood pressure, hyperlipidemia, and other common diseases. Among the AEs, the symptoms we considered possibly related to SLT were head discomfort, insomnia, decreased appetite, dizziness and abnormal coagulation. No satisfactory explanation has been given for these symptoms. The SAEs were not different between the SLT and placebo groups, or between the high- and low-dose groups. All SAEs were considered unrelated to the study drug. In general, we conclude that SLT may be safe and tolerable for the treatment of mild-to-moderate VaD.
This study had limitations and strengths. Because preclinical studies showed that SLT had the ability to increase cerebral perfusion, reduce the inflammation cascade and inhibit acetylcholinesterase, it may be necessary to measure these pathophysiological changes in vivo during a clinical trial. If such changes can be matched with the psychometric outcomes, it would help to provide objective support for the multiple potential mechanisms of action of SLT. A strength of our study was the two-stage efficacy evaluation. The exploratory phase during the first 26 weeks was designed to test whether SLT was effective, and the repetition phase during the second 26 weeks was designed to retest whether the effectiveness obtained during the first 26 weeks could be repeated in the second 26 weeks in a cohort originally randomized to receive the placebo. Although the second phase lacked a corresponding control, the similarity of the changes seen during the second phase provided support for the findings seen in the first phase.
In conclusion, our results demonstrate that SLT may be safe and effective for treating mild to moderate VaD. This study suggests that a modern compound Chinese medicine with multiple targets might be a good choice for the development of anti-VaD drugs in the future.
Research in Context.
-
1.
Systematic review: We searched PubMed and clinicaltrials.gov on February 29, 2017, for vascular dementia (VaD) trials published in English journals since January 1, 1990, using the search terms “vascular dementia,” “clinical trial,” “compound Chinese medicine,” and “SaiLuoTong/SLT” in any field. However, we did not find any clinical trials related to SaiLuoTong (SLT).
-
2.
Interpretation: Our findings suggest that SLT had larger effect sizes than seen previously for VaD. As SLT is a compound Chinese medicine that contains several active ingredients, from its components of Ginkgo biloba, ginsenosides, and saffron, we speculated that SLT might have multiple targets for treating VaD. This forms a basis for better explaining the effectiveness of SLT compared to single targets for VaD, as published previously. Our study shows that a compound Chinese medicine can be used to treat VaD.
-
3.
Future directions: Another trial with a longer duration, larger sample size, and more markers of VaD progression are warranted.
Acknowledgments
Sources of support: This study was supported by the Key Project of the National Natural Science Foundation of China (81530036); the National Key Scientific Instrument and Equipment Development Project (31627803); Mission Program of Beijing Municipal Administration of Hospitals (SML20150801); Beijing Scholars Program; Beijing Brain Initiative from Beijing Municipal Science & Technology Commission (Z161100000216137); CHINA-CANADA Joint Initiative on Alzheimer's Disease and Related Disorders (81261120571); and Beijing Municipal Commission of Health and Family Planning(PXM2017_026283_000002).
Role of the funding source: Shineway Pharmaceutical Group Co., Ltd. provided the study medication and funding but played no role in designing the protocol or analyzing or interpreting the data. All data entry and statistical analyses were performed independently by the Data Management Centre of Shanghai Second Military Medical University.
Authors' contributions: J.J., C.W., S.G., S.C., Fangyu Li, and Y.T. contributed to the conception and design of the research work. J.J., C.W., Y.T., F.W., A.Z., C.C., X.Z., and W.Q. participated in the execution and management of the entire protocol. L.S., M.G., H.X., J.L., Fang Li, H.S., S.Y., and L.J. contributed to the data acquisition. J.J., S.C., Fangyu Li, and J.H. performed the data analyses and interpreted the results. All authors are responsible for the drafting and revision of the article. All authors have approved the final version of the article to be published and agreed to take responsibility for all aspects of the research to ensure that the accuracy and integrity of any part of the article are properly investigated and resolved.
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. No other disclosures are reported.
Disclaimer: The content of this article is solely the responsibility of the authors and does not necessarily represent official views.
Additional contributions: The authors thank all investigators of the 16 research centers: Jianping Jia, Xuan Wu Hospital, Capital Medical University; Baojun Wang and Fengfei Ren, Baotou Central Hospital; Yingzhen Xie, Dongzhimen Hospital, Beijing University of Chinese Medicine; Yuangao Liao and Haibing Hu, The First People's Hospital of Chenzhou; Dongdong Yang, Affiliated Hospital of Chengdu University of TCM; Zhijun Zhang and Yongmei Shi, Zhongda Hospital Southeast University; Yefeng Cai and Xinchun Zhang, Guangdong Provincial Hospital of Traditional Chinese Medicine; Desheng Zhou, The First Hospital of Hunan University of Chinese Medicine; Jiang Wu and Fengna Chu, The First Hospital of Jilin University; Changshan Ai and Tieying Zhu, Jilin Integrated Traditional Chinese and Western Medicine Hospital; Yajun Jiang and Guran Yu, Jiangsu Provincial Hospital of TCM; Wei Xie, Nanfang Hospital of South University of Science and Technology; Xiaofei Yu and Yongmei Guo, Shuguang Hospital Affiliated to Shanghai University of TCM; Jimei Li and Yaming Zhao, Beijing Friendship Hospital, Capital Medical University; Jianming Lv and Junfeng Xu, First Teaching Hospital of Tianjin University of TCM and Benyan Luo and Guoping Peng, The First Affiliated Hospital of Zhejiang University. The authors are grateful for the support of Xiuqiang Ma and Yingyi Qin at the Second Military Medical University for the statistical analysis. The authors thank all patients and their families who have given their time for this trial.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2018.02.004.
Supplementary data
References
- 1.O'Brien J.T., Thomas A. Vascular dementia. Lancet. 2015;386:1698–1706. doi: 10.1016/S0140-6736(15)00463-8. [DOI] [PubMed] [Google Scholar]
- 2.Jia J., Wang F., Wei C., Zhou A., Jia X., Li F. The prevalence of dementia in urban and rural areas of China. Alzheimers Dement. 2014;10:1–9. doi: 10.1016/j.jalz.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Wang W., Jiang B., Sun H., Ru X., Sun D., Wang L. Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480 687 Adults. Circulation. 2017;135:759–771. doi: 10.1161/CIRCULATIONAHA.116.025250. [DOI] [PubMed] [Google Scholar]
- 4.Division of Neuropharmacologic Drug Products, US Food and Drug Administration (FDA) 2001. Issues Paper on Vascular Dementia.http://www.fda.gov/ohrms/dockets/ac/01/briefing/3724b2_01_VasDementia.pdf Peripheral and Central Nervous System Advisory Committee meeting. Available at: [Google Scholar]
- 5.Committee for Proprietary Medicinal Products of the European Agency for the Evaluation of Medicinal Products, Human Medicines Evaluation Unit . 1997. Note for Guidance on Medicinal Products in the Treatment of Alzheimer's Disease.http://www.ema.europa.eu/ema/index.jsp?curl=pages/home/Home_Page.jsp&mid= Available at: [Google Scholar]
- 6.Man S.C., Chan K.W., Lu J.H., Durairajan S.S., Liu L.F., Li M. Systematic review on the efficacy and safety of herbal medicines for vascular dementia. Evid Based Complement Alternat Med. 2012;2012:426215. doi: 10.1155/2012/426215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He G.Y., Yuan C.G., Hao L., Xu Y., Zhang Z.X. GBE50 attenuates inflammatory response by inhibiting the p38 MAPK and NF- kappa B pathways in LPS-stimulated microglial cells. Evid Based Complement Alternat Med. 2014;2014:368598. doi: 10.1155/2014/368598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tchantchou F., Xu Y., Wu Y., Christen Y., Luo Y. EGb 761 enhances adult hippocampal neurogenesis and phosphorylation of CREB in transgenic mouse model of Alzheimer's disease. FASEB J. 2007;21:2400–2408. doi: 10.1096/fj.06-7649com. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y., Liu Q., Xu Y., Zhang Y., Lv Y., Tan Y. Ginsenoside Rg1 protects against oxidative stress-induced neuronal apoptosis through myosin IIA-actin related cytoskeletal reorganization. Int J Biol Sci. 2016;12:1341–1356. doi: 10.7150/ijbs.15992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang L., Liu L., Liu J., Dou L., Wang G., Liu X. Ginsenoside Rg1 protects against neurodegeneration by inducing neurite outgrowth in cultured hippocampal neurons. Neural Regen Res. 2016;11:319–325. doi: 10.4103/1673-5374.177741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li F., Wu X., Li J., Niu Q. Ginsenoside Rg1 ameliorates hippocampal long-term potentiation and memory in an Alzheimer's disease model. Mol Med Rep. 2016;13:4904–4910. doi: 10.3892/mmr.2016.5103. [DOI] [PubMed] [Google Scholar]
- 12.Xu Y., Chen T., Li X., Qu Y., An J., Zheng H. Salvia miltiorrhiza bunge increases estrogen level without side effects on reproductive tissues in immature/ovariectomized mice. Aging (Albany NY) 2016;9:156–172. doi: 10.18632/aging.101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mashmoul M., Azlan A., Khaza'ai H., Yusof B.N., Noor S.M. Saffron: A natural potent antioxidant as a promising anti-obesity drug. Antioxidants (Basel) 2013;2:293–308. doi: 10.3390/antiox2040293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghadrdoost B., Vafaei A.A., Rashidy-Pour A., Hajisoltani R., Bandegi A.R., Motamedi F. Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur J Pharmacol. 2011;667:222–229. doi: 10.1016/j.ejphar.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Ochiai T., Shimeno H., Mishima K., Iwasaki K., Fujiwara M., Tanaka H. Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo. Biochim Biophys Acta. 2007;1770:578–584. doi: 10.1016/j.bbagen.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Akhondzadeh S., Sabet M.S., Harirchian M.H., Togha M., Cheraghmakani H., Razeghi S. Saffron in the treatment of patients with mild to moderate Alzheimer's disease: a 16-week, randomized and placebo-controlled trial. J Clin Pharm Ther. 2010;35:581–588. doi: 10.1111/j.1365-2710.2009.01133.x. [DOI] [PubMed] [Google Scholar]
- 17.Mohs R.C., Knopman D., Petersen R.C., Ferris S.H., Ernesto C., Grundman M. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer's disease assessment scale that broaden its scope. Alzheimer Dis Assoc Disord. 1997;11:S13–S21. [PubMed] [Google Scholar]
- 18.Schneider L.S., Olin J.T., Doody R.S., Clark C.M., Morris J.C., Reisberg B. Validity and reliability of the Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;2:S22–S32. doi: 10.1097/00002093-199700112-00004. [DOI] [PubMed] [Google Scholar]
- 19.Schneider L.S., Olin J.T. Clinical global impressions in Alzheimer's clinical trials. Int Psychogeriatr. 1996;8:277–288. doi: 10.1017/s1041610296002645. discussion 88–90. [DOI] [PubMed] [Google Scholar]
- 20.Olin J.T., Schneider L.S., Doody R.S., Clark C.M., Ferris S.H., Morris J.C. Clinical evaluation of global change in Alzheimer's disease: identifying consensus. J Geriatr Psychiatry Neurol. 1996;9:176–180. doi: 10.1177/089198879600900404. [DOI] [PubMed] [Google Scholar]
- 21.Lawton M.P., Brody E.M. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 22.Royall D.R., Cordes J.A., Polk M. CLOX: an executive clock drawing task. J Neurol Neurosurg Psychiatr. 1998;64:588–594. doi: 10.1136/jnnp.64.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan S.M., Chiu F.K., Lam C.W. Correlational study of the Chinese version of the executive interview (C-EXIT25) to other cognitive measures in a psychogeriatric population in Hong Kong Chinese. Int J Geriatr Psychiatry. 2006;21:535–541. doi: 10.1002/gps.1521. [DOI] [PubMed] [Google Scholar]
- 24.Cummings J.L., Mega M., Gray K., Rosenberg-Thompson S., Carusi D.A., Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 25.Orgogozo J.M., Rigaud A.S., Stoffler A., Mobius H.J., Forette F. Efficacy and safety of memantine in patients with mild to moderate vascular dementia: a randomized, placebo-controlled trial (MMM 300) Stroke. 2002;33:1834–1839. doi: 10.1161/01.str.0000020094.08790.49. [DOI] [PubMed] [Google Scholar]
- 26.Venkat P., Chopp M., Chen J. Models and mechanisms of vascular dementia. Exp Neurol. 2015;272:97–108. doi: 10.1016/j.expneurol.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang D., Liu J., Bilinski K., Xu L., Steiner G.Z., Seto S.W. Herbal medicine for the treatment of vascular dementia: An overview of scientific evidence. Evid Based Complement Alternat Med. 2016;2016:7293626. doi: 10.1155/2016/7293626. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.