Abstract
The Alzheimer's Association's Research Roundtable met in November 2016 to explore how best to measure changes in cognition and function in the preclinical stage of Alzheimer's disease. This review will cover the tools and instruments currently available to identify populations for prevention trials, and measure subtle disease progression in the earliest stages of Alzheimer's disease, and will include discussions of suitable cognitive, behavioral, functional, composite, and biological endpoints for prevention trials. Current prevention trials are reviewed including TOMMOROW, Alzheimer's Prevention Initiative Autosomal Dominant Alzheimer's Disease Trial, the Alzheimer's Prevention Initiative Generation Study, and the Anti-Amyloid Treatment in Asymptomatic Alzheimer's to compare current approaches and tools that are being developed.
1. Introduction
As knowledge of Alzheimer's disease (AD) progression improves, the field has recognized that it may be possible, and perhaps necessary, to develop drugs that target early, prodromal stages of the disease and move to secondary prevention as a treatment strategy. To achieve regulatory approval for such therapeutics, appropriate clinically relevant endpoints are needed that enable detection of disease progression and response to therapy in a population that is, by definition, asymptomatic. On November 29 and 30, 2016, the Alzheimer's Association Research Roundtable convened academic, industry, and government scientists to explore cognitive, functional, and biological endpoints that will enable clinical trials to be conducted in the earliest, presymptomatic stages of AD pathophysiology and discuss the challenges that need to be overcome. A number of secondary prevention trials are already underway, providing the forum with preliminary information to guide future development.
2. Cognition across the age and disease continuum
2.1. Normal cognitive aging
Defining normal cognitive function in older adults is surprisingly complicated due to variability in how and in whom it is measured. For example, in a 2002 cross-sectional study of 345 people between the ages of 20 and 92 years, Park et al. showed that while test performance in many cognitive domains—including reasoning, working memory, and processing speed—declined over the lifespan, scores on tests of vocabulary and world knowledge increased [1]. In addition, neuroimaging has revealed that, just as with physical health, brain health declines along a continuum with age, with progressive reductions in brain volume and white matter integrity [2]. In fact, age-related declines in cognitive function are correlated with reductions in the volume and/or thickness of brain structures and white matter integrity, and poor cognition may serve as a proxy for the integrity of brain structure. It also is assumed to serve as a correlate for the ability to function in everyday life.
Besides measuring structural changes in the brain, it is also possible to measure brain activity using functional magnetic resonance imaging. Typically, cognitively normal adults will show increased activity, particularly in the frontal cortex, with age. Park et al. (2009) proposed the Scaffolding Theory of Aging and Cognition to account for this increased brain activity, which posits that age-related neural degradation within brain structures can be counterbalanced by “compensatory scaffolding” (i.e., the engagement of additional neural circuits, neurogenesis, and other active processes, resulting in some protection from decline in cognitive function [2]). The Dallas Lifespan Brain Aging Study was developed to test this model, and other large normative data sets, such as the Virginia Cognitive Aging Project, Health and Retirement Study, and Harvard Aging Brain Study (HABS), have provided further data to describe how cognition changes over the lifespan. In addition to documenting overall age-related declines in cognitive performance, the HABS and Dallas Lifespan Brain Aging studies have also measured the deposition of amyloid, a hallmark protein of AD, in seemingly healthy, cognitively normal adults and have shown a link between early deposition and poorer scores on tests of memory and other cognitive processes [3], [4]. Amyloid deposition is associated also with the apolipoprotein E (APOE) ε4 genotype [5] and with greater decline in cognitive function over time [6].
The methods for studying changes in cognition with age are problematic. Cross-sectional designs are used in most studies of the aging brain and may be confounded by cohort differences and sampling issues. For example, in 1963, a 20-year-old performed better on tests of psychomotor speed, executive function and language than a 20-year-old born in 1922 [7]. Hence, an age difference in cognition between a 20-year-old and an 80-year-old could be either due to decline with age or due to the fact that there was already quite a difference between the two at the age of 20 years. In addition, cohort effects on dementia incidence were recently reported from the Einstein Aging Study [8] suggesting that the adverse effects of brain aging may be diminished in younger compared with older cohorts. A related issue is that obtaining a cognitively normal representative sample of older study participants is difficult. First, older individuals carry many comorbidities that exclude large subsets of individuals from studies; second, the remarkable ability to image neuropathology such as amyloid and tau at early stages of deposition complicates what we mean by “normal cognitive aging” [9]. Another complication in lifespan studies is that middle-aged participants are difficult to recruit and often are represented by cohorts who differ in employment, education, and socioeconomic status relative to both the younger and older adult samples in a cross-sectional study. The alternative is a longitudinal cohort study (LCS) design. Often considered the gold standard for tracking cognitive performance over time, longitudinal testing may be confounded by practice effects. However, a recent analysis indicates that when practice effects are eliminated, age trends in longitudinal studies closely resemble those seen in cross-sectional studies [10].
It is important to recognize that cognition is not a unitary construct, consisting, instead, of a number of discrete domains, such as, for example, attention, memory, and language, to name a few. The accurate measurement of cognitive function requires careful sampling and an assessment of factors that could affect cognition that are unrelated to a therapeutic intervention (e.g., education, past testing, and other, myriad variables, unique to the individual). Young and old adults may rely on different cognitive operations or different sequences of operations to achieve optimal performance on an identical task. For example, young adults may rely on speed and working memory—abilities where they excel—to perform a complex task, whereas older adults may rely on their cognitive strengths—experience and vocabulary—to solve the same task [11], [12].
Finally, the focus on everyday cognition (ECog) and instrumental activities of daily living (IADL) as measurements of cognitive ability has important advantages and disadvantages for measuring change. The advantage, of course, is that it more closely reflects how an individual is performing in the real world. An effect on these measurements after an intervention is therefore ecologically valid, and highly relevant to patients and caregivers. However there are also significant disadvantages—that is, people differ greatly in the types of everyday tasks they perform. For example, some are relatively simple, such as remembering to collect eggs in a farm daily at a specific time, while others are more complex, such as overseeing a bank or dispatching a fleet of trucks to transport materials across a country. Thus, a single scale of daily living activities may not be sensitive enough to measure the outcome of an intervention in very early disease stages in different individuals [13], [14]. An alternative to using tests of ECog might be high-frequency assessments via cell phone or tablet computer in the context of a person's everyday environment [15]. The drawback here, however, is that many older adults have little experience with these devices and so simpler technology (a regular telephone) may be considered for those without more modern technology.
In summary, the measurement of cognition for purposes of a clinical trial is a complex issue and should be tailored to the subject population under study and the particular goals of the project. It may be advisable to include some measures that make a study comparable to others, by using instruments such as the National Institute of Health (NIH) toolbox, which was designed to serve as a common currency among longitudinal and intervention studies [16], [17].
2.2. Lessons learned from LCSs
Two population-based LCSs—the Religious Orders Study and the Rush Memory and Aging Project—have collected more than 20 years of clinical data from more than 3000 older persons who were cognitively normal for age at enrollment. As of late 2016, approximately 650 of the participants have dementia and approximately 850 have mild cognitive impairment (MCI). Neuropathological data have now been obtained from more than 1200 postmortem brain autopsies. These data show that most cognitive decline in older adults is due to well-known pathologies, and that decline progresses most rapidly in people who have a combination of AD, cerebrovascular, and Lewy body disease pathology [18], [19]. These studies have also shown that different cognitive domains decline at different rates depending on the type of pathology. For example, markers of AD neuropathology were associated with a decline in episodic memory, semantic memory, and working memory [16].
Several other LCS such as the Alzheimer's Disease Neuroimaging Initiative (ADNI), Mayo Clinic Study of Aging, and the Australian Imaging, Biomarkers and Lifestyle Flagship Study of Ageing (AIBL) have also collected data on participants in the preclinical stages of AD, defined as the presence of one or more positive biomarkers of AD pathophysiology and no symptoms of cognitive impairment. The AIBL study is a prospective LCS of cognitive aging collecting and integrating data from neuroimaging (amyloid positron emission tomography [PET] imaging and magnetic resonance imaging measuring hippocampal volume), biomarkers, lifestyle, as well as clinical and neuropsychological measures. Eligible volunteers, over the age of 60 years and fluent in English, were classified into three groups: (1) individuals meeting the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association, now known as the Alzheimer's Association (NINCDA-ADRDA) criteria for AD [20], (2) individuals meeting criteria for MCI [21], [22], and (3) elderly healthy controls (HC). The 6-year follow-up data confirmed other studies that indicated a 20- to 30-year progression of brain amyloid β (Aβ) accumulation before dementia is diagnosed, after which accumulation begins to plateau as dementia progresses [23]. Among amyloid-positive participants, 28.6% progressed over 6 years compared to 11.9% among amyloid-negative participants. When age is taken into account, the decline accelerates; and carriage of the APOE ε4 allele was also associated with an accelerated rate of decline [24]. Cognitively normal subjects in ADNI have also been tested with several cognitive measures, showing that elevated brain amyloid is associated with decline in cognitive test scores, which, albeit, remain within normal limits for age, in contrast to minimal decline in scores in participants without brain amyloid [25].
2.3. Early changes in the AD continuum
Population-based studies of aging point to the difficulty of distinguishing normal from pathological aging. A decline in cognitive test scores within begins more than a decade before dementia becomes apparent in persons destined to become demented and proceeds in a continuous manner over time [26], [27]. Most standardized tests provide age-corrected norms, and although decline may occur in an individual, test scores may continue to remain within average limits for age, masking the decline. Furthermore, a change in test scores that does not render the individual abnormal for age may correlate poorly with everyday functional abilities. Thus, detecting cognitive change that is of clinical significance in the early preclinical stages of AD is challenging. The role of cognitive reserve further complicates the matter. One strategy to assess these issues is to determine “personally relevant” indices of cognitive decline. One strategy to highlight personally relevant decline is to correct test scores for the individual's peak prior level of cognitive ability as estimated by reading vocabulary scores [28].
Revised diagnostic criteria define preclinical disease by the presence of cerebral amyloid deposits, assessed using amyloid PET imaging or cerebrospinal fluid (CSF) biomarkers [29], [30], [31], yet studies correlating these findings to even sensitive measures of cognition show small changes and substantial variance [32], [33], [34]. A recent analysis from the Mayo Clinic Study of Aging showed that elevated amyloid is associated with poorer cognition across multiple domains, with the greatest change in global cognition, attention, and memory, but not language [35].
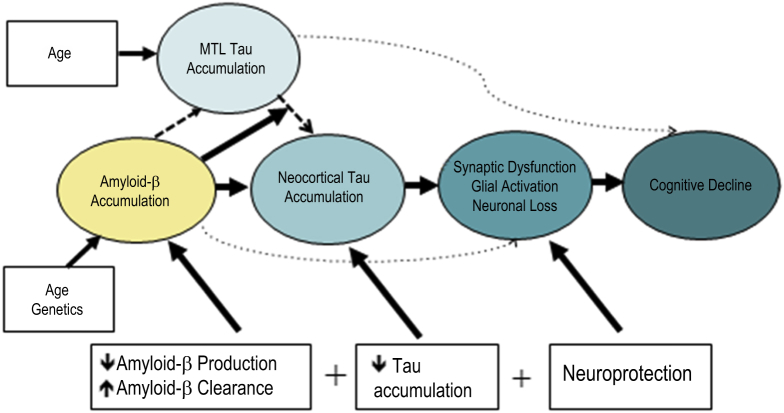
The evidence is substantial that β-amyloidosis, with its 20+ year preclinical time course and its lack of neuroanatomical-cognitive domain relationships, is not the proximate driver of cognitive decline. Instead, evidence of neurodegeneration—assessed by hippocampal atrophy, levels of CSF tau, or tau PET imaging, fludeoxyglucose PET hypometabolism, or magnetic resonance-derived measures of cortical volume or thickness—is also required [36], [37], [38], [39] before cognitive impact is obvious. Fig. 1 illustrates a hypothetical interaction of amyloid and tau, proposed by Sperling et al. in 2014 [6]. Still unknown is whether these represent separate assaults or if one drives the other. But to the extent that these various measures are only modestly correlated (e.g., from [40]: [41], [42], [43], [44], [45], [46]), it is likely that these biomarkers represent at least partially independent aspects of the AD pathophysiologic process.
Fig. 1.
Hypothetical interaction of amyloid and tau in preclinical AD. Abbreviations: AD, Alzheimer's disease; MTL, medial temporal lobe.
From reference [6].
APOE ε4 seems to accelerate amyloid-related decline, suggesting possible synergistic effects of amyloid and APOE ε4 [47]. In addition, deterioration of brain function as a biological consequence of aging may lead to cognitive decline. Subjective cognitive concerns also appear to be associated with preclinical AD, particularly in APOE ε4 carriers, although as the disease progresses, affected individuals become less aware of their declining cognition, a condition known as anosognosia [48], [49].
3. Cognitive composites and batteries as endpoints in prevention trials
Several AD prevention trials are currently underway, using different endpoints as primary outcome measures. These endpoints include time-to-event endpoints, such as time to the first diagnosis of MCI due to AD and continuous measures of cognition. To assess cognition, these studies have all selected cognitive measures most sensitive in the defined study population, combining them into a “battery” or “composite” measure (Table 1). Composites may be theoretically or statistically derived or both.
Table 1.
Comparison of cognitive composites used in prevention trials
| Domain | Test | TOMM | API ADAD | APCC | PACC | PACC-A4 |
|---|---|---|---|---|---|---|
| Global functioning and mental status | MMSE | |||||
| Attention | WAIS-III Digit Span, forward | |||||
| Trails A | ||||||
| Perceptual speed | Symbol digit modalities |  |
||||
| Executive function | Trails B | |||||
| WAIS-III Digit Span, backward | ||||||
| WAIS-R Digit Symbol Substitution Test | ||||||
| Ravens Progressive Matrices (subset) | ||||||
| Orientation | MMSE orientation to place | |||||
| MMSE orientation to time | ||||||
| Language | Multilingual Naming Test | |||||
| Semantic fluency (animals) | ||||||
| Lexical/phonemic fluency (FAS) | ||||||
| Boston Naming Test |  |
|||||
| Visuospatial | Clock drawing | |||||
| Copy of BVMT-R figures | ||||||
| Judgment of line orientation | ||||||
| CERAD–constructional praxis | ||||||
| Ravens Progressive Matrices (subset) | ||||||
| Episodic memory | CVLT | |||||
| Brief Visuospatial Memory Test | ||||||
| CERAD Word List–delayed recall | ||||||
| Logical Memory–delayed recall | ||||||
| FCSRT | ||||||
| Paragraph Recall |
Abbreviations: APCC, API Preclinical Composite; TOMM, TOMMORROW trial; API ADAD, The Alzheimer's Prevention Initiative–Autosomal Dominant Alzheimer's Disease; PACC, Preclinical Alzheimer's Cognitive Composite; A4, Anti-Amyloid treatment in Asymptomatic Alzheimer's; MMSE, Mini-Mental State Examination; WAIS-III, Wechsler Adult Intelligence Scale–Third Edition; WAIS-R, Wechsler Adult Intelligence Scale–Revised; BVMT-R, Brief Visuospatial Memory Test–Revised; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; CVLT, California Verbal Learning Test–Second Edition; FCSRT, Free and Cued Selective Reminding Test; RBANS, Repeatable Battery for Neuropsychological Status.
NOTE. Green color = used in this composite; 1 = replaced by Multilingual Naming Test (MiNT); 2 = replaced by RBANS coding; 3 = replaced by RBANS line orientation; 4 = replaced by RBANS list recall; 5 = replaced by RBANS story recall; 6 = adapted WAIS-R Digit Symbol Substitution Test.
3.1. TOMMORROW
TOMMORROW is a study designed to test the efficacy of low-dose pioglitazone in delaying the onset of MCI or AD and to qualify a biomarker risk algorithm for identifying cognitively normal older adults at risk for developing MCI due to AD within 5 years, based on TOMM40 rs10524523 genotype, APOE genotype, and age. This study uses a time-to-event design in combination with a linguistically and culturally valid neuropsychological battery of tests, which captures the transition from normal to early-stage symptomatic disease. The battery enables operationalization of the National Institute on Aging/Alzheimer's Association core clinical criteria for MCI due to AD [30] by requiring, fulfillment of the clinical criteria across two consecutive examinations at least 6 months apart, consisting of decline in the Clinical Dementia Rating scale score from 0.0 to 0.5 plus either (1) a decline from baseline on at least one of the two memory tests in the battery with performance falling at least −1.5 standard deviation below the normative mean after age adjustment or (2) a decline from baseline on a memory test and one other test in a domain other than memory with performance falling at least −1.3 standard deviation below the mean. Other potential causes must be ruled out as the proximal cause of cognitive impairment and each assessment is adjudicated by a panel of clinical dementia experts.
The cognitive test battery includes at least two tests in each of five domains: Episodic Memory, Executive Function, Language, Visuospatial Processing, and Attention. The English version has been translated, culturally adapted, validated, and normed for ages 65 to 88 years in German, Italian, and Russian and shown to be psychometrically sound and sensitive in early AD [50]. The battery is designed to mirror a memory evaluation that would be conducted in clinical practice with cognitively healthy adults at risk for developing MCI. Including two tests for each domain guards against judgments of impairment based on single spuriously low scores. In addition, by having dual measures for each domain, an overall global cognitive composite score can be calculated, which incorporates all domains, even in the setting of missing test scores. This is important for purposes of measuring continuous changes in cognition in healthy adults, including signs of cognitive decline or symptomatic improvements.
3.2. Alzheimer's prevention initiative
The Alzheimer's Prevention Initiative (API) had two clinical trials underway at the time of the Roundtable meeting; one, a treatment and biomarker development trial in people with autosomal dominant AD (ADAD) mutation and the other in APOE ε4 homozygotes (the API Generation Study). Both trials are enrolling cognitively normal individuals close to their estimated age of clinical onset. The API ADAD study uses a cognitive composite as the primary endpoint, while the Generation Study uses dual primary endpoints, the API Preclinical Composite, and time to diagnosis of MCI or dementia due to AD. The compounds being tested also differ. The ADAD trial is testing Roche's crenezumab, a monoclonal antibody that targets Aβ; while the Generation trial will test two compounds: CNP520 (developed by Novartis and Amgen), an inhibitor of BACE1, the β-secretase enzyme that cleaves the amyloid precursor protein at an early step in the formation of amyloid plaques [51]; and CAD106, an active immunotherapy that targets Aβ. Participants are randomized to either treatment or its placebo.
The API ADAD Composite was empirically derived using a data set from the world's largest kindred of PSEN1 E280A mutation carriers and noncarriers [52]. Separately, the API Preclinical Composite was empirically derived from three cohorts of unimpaired older adults (Rush Alzheimer's Disease Center's Religious Orders Study, Memory and Aging Project, and Minority Aging Research Study) who progressed or did not progress to AD, thereby controlling for cognitive decline associated with normal aging [53]. The API Preclinical Composite will be used for the API Generation Study and other prevention trials. In each case, the goal was to find the most sensitive combination of test items to detect and track cognitive decline associated with preclinical AD and evaluate the efficacy of preclinical treatment.
3.3. PACC-HABS and A4
The Alzheimer's Disease Cooperative Study Preclinical Alzheimer's Cognitive Composite (ADCS-PACC) is a retrospectively and theoretically derived composite measure designed to track subtle cognitive decline in clinically normal older adults and in a slightly modified form (PACC-A4) to be used as a primary outcome measure for trials conducted in the preclinical stage of AD, including the Anti-Amyloid Treatment in Asymptomatic Alzheimer's (A4) study [25], [54]. The composite, comprising tests of episodic memory, executive function, and global cognition, was derived retrospectively using three data sets: the ADCS–Prevention Instrument (ADCS-PI) study, ADNI, and AIBL. The ADCS-PI studied cognitively normal individuals over the age of 75 years for four years with the goal of developing measures for trials earlier in the AD course. This study did not include measures of amyloid level (CSF or PET) but used the presence of at least 1 APOE ε4 allele as a predictor of decline. More importantly, the ADCS-PI included the Free and Cued Selective Reminding Test, Wechsler Adult Intelligence Scale–Revised Digit Symbol Substitution test, New York Paragraph recall instead of the Wechsler Memory Scale–Revised Logical Memory and the Modified Mini-Mental State Examination instead of the Mini-Mental State Examination (MMSE) that were used to derive this version of the ADCS-PACC. In comparison, ADNI included the ADAS-Cog Word List instead of the Free and Cued Selective Reminding Test, Wechsler Memory Scale–Revised Logical Memory Story A, Wechsler Adult Intelligence Scale–Revised Digit Symbol Substitution test, and MMSE to derive that ADCS-PACC. For AIBL, the ADCS-PACC was derived from the California Verbal Learning Test–Second Edition list A, Wechsler Adult Intelligence Scale–Third Edition Coding, Wechsler Memory Scale–Third Edition Logical Memory Story A, and MMSE. The PACC used in A4 (PACC-A4) differed from all of these three, in that, it comprises an alternative version of the Free and Cued Selective Reminding Test (not the one used in ADCS-PI), Wechsler Adult Intelligence Scale–Third Edition Coding (a shortened version 90 versus the validated 120-second version), nonvalidated Logical Memory Stories, and the MMSE. Data presented at the Research Roundtable demonstrated how the PACC performs over time in a preclinical population, that is, older adults enrolled in the HABS who have evidence of amyloid deposition.
A revised version of PACC is under development and validation. The revised version of PACC has been developed retrospectively from data from preclinical AD in the ADNI, AIBL, Mayo Clinic Study of Aging, and Washington University cohorts at approximately 3-, 4-, and 5-year follow-up points [55], [56]. Lasso Regression models have been applied uniformly to the slopes of performance over time for individual neuropsychological tests in clinically normal adults from each of the different cohorts. Using this approach, and restricting the modeling to tests most readily applied in global trials (i.e., minimizing language/cultural translation and expert test administration and scoring requirements), the resultant revised version of PACC assesses the same cognitive domains as the ADCS-PACC and achieves equivalent or modestly better separation of Aβ+ and Aβ− groups over time periods used in clinical trials of preclinical AD.
3.4. European Prevention of Alzheimer's Disease
The European Prevention of Alzheimer's Disease (EPAD) program recently published a consensus statement recommending cognitive measures comprising the EPAD Neuropsychological Evaluation for planned large-scale drug trials in preclinical AD [57]. This work was predicated on a published literature review, examining existing LCS of nondemented cohorts that went on to develop incident dementia, as well as data from neuroimaging studies to determine the temporal order and brain biomarker correlates of specific cognitive functions [58]. The results of the literature review indicated that there are particular neuropsychological tests and cognitive domains for which subtle cognitive changes are detectable before a clinical diagnosis of MCI, and that with which significant differences between cognitively normal and preclinical populations can be detected in within 5 years of diagnosis. The tests therefore recommended by the EPAD Clinical and Cognitive Outcomes Scientific Advisory Group are shown in Table 2. The EPAD LCS includes the EPAD Neuropsychological Evaluation, and the measures contained within are denoted as primary, secondary, and exploratory. For EPAD, the endpoint designated as primary is the total scale index score, an overall composite of the Repeatable Battery for Neuropsychological Status, which the Scientific Advisory Group deemed to be well validated and having a favorable likelihood of regulatory acceptance. Secondary measures are those with empirical support for their use, but requiring either additional validation by a second independent study (i.e., EPAD LCS), validated alternative forms, or additional normative data. Exploratory measures are those with initial empirical evidence supporting their use, but requiring two well-designed studies (i.e., EPAD LCS and EPAD) Proof of Concept with concordant findings to further validate these measures. The Repeatable Battery for Neuropsychological Status will serve as the criterion measure for the psychometric validation of these secondary and exploratory measures.
Table 2.
EPAD-ENE: Measures and cognitive domains
| Cognitive domains | Measures |
|---|---|
| Primary | |
| Verbal episodic memory | List learning and story memory (RBANS) |
| Visual episodic memory | Figure recall (RBANS) |
| Visuospatial/constructional | Figure copy and line orientation (RBANS) |
| Language | Picture naming (RBANS) |
| Attention/executive functioning | Semantic fluency (RBANS) |
| Digit span (RBANS) | |
| Coding (RBANS) | |
| Secondary | |
| Working memory | Dot counting (NIH examiner/toolbox) |
| Choice reaction time and set-shifting | Flanker (NIH examiner/toolbox) |
| Paired associate learning | Name/face pairs (NIH examiner/toolbox) |
| Exploratory | |
| Allocentric space | Four mountains task |
| Egocentric space | Supermarket trolley virtual reality |
Abbreviations: EPAD-ENE, EPAD Neuropsychological Evaluation; RBANS, Repeatable Battery for Neuropsychological Status; NIH, National Institute of Health.
Overall, based on the literature, the selected EPAD Neuropsychological Evaluation reflects the Scientific Advisory Group's conclusion that many trials and studies-to-date have tended to focus on episodic memory functions of the hippocampus, while neglecting its other roles in cognition, such as spatial navigation and spatial memory; and that novel tests may be needed to target other brain regions where both tau and Aβ pathologies co-occur in early-stage disease, such as the entorhinal cortex, precuneus, and retrosplenial cortex. Finally, it should be noted that for the EPAD LCS and Proof of Concept studies, the Repeatable Battery for Neuropsychological Status Total Scale Index score serves at the primary endpoint for regulatory purposes.
4. Functional measures
Although functional decline is thought to occur only after cognitive decline, studies suggest that subtle functional decline occurs even in cognitively normal individuals who later progress to MCI or AD [13], [59], [60], [61]. Indeed, the presence of mild or subtle functional changes in persons who meet criteria for MCI [30] has raised some conceptual concerns about how to define the boundaries of MCI. Moreover, everyday function is important to patients and families because functional impairment results in loss of autonomy and independence and is related to quality of life. Among the activities that have been explored in functional assessments, in early AD are complex everyday activities, such as financial capacity, driving, computer usage, and other everyday activities such as shopping. Various methods have been developed to carefully quantify functional changes among older adults. Such methods include performance-based measures, in which older adults are directly observed and rated on their ability to perform various functional tasks. Questionnaire-based measures of everyday function have also been developed, in which a wide range of everyday activities is assessed, and these questionnaires can be completed by the patient (self-report) and/or the study partner (informant report). Recent advances in both types of tools have included a focus on measuring very early changes that occur in MCI and “Pre-MCI.”
The University of California San Diego Performance-Based Skills Assessment (UPSA) was developed as a performance-based measure to assess performance across four domains: communication, comprehension and planning, transportation and mobility, and finance. In a study of cognitively normal elders as well as patients with amnestic MCI and mild-to-moderate AD, UPSA scores demonstrated stepwise impairment across the disease continuum. The effect size in distinguishing HC from MCI was greater with the UPSA compared the Alzheimer's Disease Cooperative Study—Activities of Daily Living Inventory [62]. A short form with only the communication and comprehension domains has been shown to correlate well with the long form and to have acceptable sensitivity and specificity for distinguishing between HC, individuals with MCI, and individuals with AD dementia [63]. Impairments in episodic memory and semantic memory have been shown to predict performance on the UPSA, further supporting the idea that functional and cognitive domains are systematically related [64], [65]. Overall, what remains to be determined is how the UPSA will perform in populations tested earlier in the disease course than those previously studied. There are also a number of other performance-based functional assessments such as the Harvard Automated Phone Task or the Financial Capacity Instrument, which have also shown promise in detecting early, clinically meaningful changes in preclinical AD, although such tasks need to control for prior experience [66], [67].
Alternatives to performance-based measures include both informant-reported and patient-reported ratings of everyday function. The ECog scale is one such assessment tool, which independently queries the patient and an informant about everyday functional abilities across six domains: memory, language, visual perception, planning, organization, and divided attention [68]. Informant-based ratings on the ECog have been shown to be associated with objective measures of disease status (e.g., neuropsychological function, structural imaging measures of brain atrophy, and PET/CSF biomarkers), although self-reports might also have sensitivity in early disease stages [69], [70]. Informant-rated ECog scores are also strongly associated with the risk of progression from MCI to dementia [71].
The relative sensitivity and predictive utility of informant versus patient ratings of functioning remains unclear. Farias et al. have shown that in a mixed diagnostic sample, informant report is consistently more strongly associated with disease biomarkers than self-report [69]. During the meeting, Farias presented data showing that when comparing informant and self-ratings on the ECog to predict risk of progression from MCI to dementia, self-report is a significant but a weaker predictor than the informant report (Table 3). In contrast, in a new study recently published by the same group [72], self-rated ECog was an equally if not better predictor than informant report in terms of progression from normal cognition to MCI. Such findings suggest that changes in self-reported everyday function may be useful early in the disease process but that, as cognitive impairment advances, the informant's report may offer a more robust assessment. Among older adults who live alone, informant report may not be possible. Limitations of self-report are anosognosia (i.e., frank denial and/or unawareness of one's own deficits) and factors such as anxiety or depression that may increase the tendency to report cognitive symptoms [73].
Table 3.
Patient- versus informant-reported everyday function predicting MCI to dementia conversion using E-Cog
| Independent variable | Incident dementia–patient |
Incident dementia–informant |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Everyday planning | 1.7 (1.1–2.6)∗ | 3.0 (2.2–4.1)∗ |
| Everyday memory | 1.7 (1.3–2.4)∗ | 3.0 (2.3–3.8)∗ |
| Everyday visuospatial | 1.9 (1.2–3.0)∗ | 2.7 (1.9–3.6)∗ |
| Everyday language | 1.3 (0.8–1.9) | 2.5 (1.8–3.5)∗ |
| Everyday organization | 1.5 (1.05–2.2)∗ | 2.6 (2.1–3.3) |
| Everyday divided attention | 1.2 (0.8–1.6) | 2.3 (1.8–2.9)∗ |
| ECog total score | 1.8 (1.2–2.8)∗ | 4.2 (3.0–5.8)∗ |
Abbreviations: CI, Confidence interval; E-Cog, everyday cognition; MCI, mild cognitive impairment; HR, hazard ratio.
P value < .05.
Deficits in IADL are typically assessed using rating scales administered to patients or informants. In a systematic review of IADL scales, Sikkes et al concluded that all 12 scales reviewed fell short in important basic psychometric properties [14] such as reliability, validity, and responsiveness to change. Testing these properties is a basic assumption for adequate measurements of both cognition and function. A second systematic review in 2016 concluded that there was limited evidence for content and construct validity [74]. In addition, the review noted that patients were confused by complex questions, which highlights the importance of involving the target population in the development of such scales.
The Amsterdam IADL Questionnaire was designed to correct some of the deficiencies of other IADL questionnaires [75]. The research team started by asking patients and caregivers what activities they thought were relevant and then involved clinicians and researchers to generate IADL-appropriate questions that could be completed on a computer by the study partner. This approach led to a range of everyday activities, including cooking, finances, and everyday technology use [75]. The Amsterdam IADL Questionnaire has been shown to be valid and reliable. In addition, it was shown to correlate longitudinally with cognitive decline, which underlines the ability to detect changes over time [76]. A short version was recently developed [77] and shown to be reliable and valid across the disease spectrum. That is, patients had increasing difficulties with IADL along the disease spectrum. Notably, the short version was able to detect IADL difficulties even in preclinical AD when compared to HC, supporting the inclusion of more complex IADL tasks to improve sensitivity to cognitive decline [77]. Cross-cultural adaptations of the Amsterdam IADL Questionnaire have been developed for several countries, and others are currently being developed.
In sum, both performance-based measures and questionnaire-based assessments of functional activities have an added value to cognitive measurements. The best approach may be to include both types of functional outcomes in clinical trials if at all practical. While older functional instruments were designed for use with individuals who already had dementia, new instruments have been specifically developed to be sensitive to early functional changes that predate a dementia diagnosis. Continued advancement of functional tools might consider the merits of including items that measure the use of technology in daily life to assess early change. In addition, with the diversity of older adults in the United States increasing, functional tools need to be shown to be culturally relevant to a wide range of older adults.
5. Biomarkers as predictors of clinical change in preclinical AD
The National Institute on Aging-Alzheimer's Association Preclinical Workgroup proposed a conceptual framework for preclinical AD in 2011. In the 2011 framework, cognitive functioning in the preclinical state was defined only when both amyloid and neurodegeneration biomarkers were abnormal. However, abnormal cognition was left undefined when only one or none of the biomarker classes was abnormal. While the 2011 Workgroup's efforts represented a novel approach to relating cognition to biomarkers in the preclinical state, work in the past 6 years has required a major revision of the strategy to relate biomarkers to cognition. For example, the need to integrate tau PET imaging into the biomarker framework and the recognition that there was a sizable group, dubbed Suspected Non-Alzheimer Pathology not accounted for [78], has led the Alzheimer's Association and the National Institute on Aging to convene a new workgroup to develop a more flexible and inclusive framework (cite Website version). Despite the inevitable limitations that appeared in ground-breaking efforts of the 2011 preclinical framework, there was one very clear and consistent finding that emerged. Those persons who were considered cognitively normal and who had both abnormally elevated amyloid levels and abnormal levels of a neurodegeneration biomarker have the worst cognitive outcomes compared to all of the other biomarker combination groups [79].
6. Is it possible to detect a clinically meaningful change in preclinical AD?
At least eight preclinical AD prevention trials are currently underway: A4 [54], the two API trials [80], a third API trial sponsored by Novartis, and Amgen in heterozygote E4 carriers (generation 2), the Janssen EARLY trial in asymptomatic individuals with cerebral amyloid burden, the Dominantly Inherited Alzheimer's Network Trial [81], TOMMORROW [82], and EPAD. Each of the groups planning these trials has wrestled with the question of how to detect a clinically meaningful change in individuals who are, by definition, cognitively and clinically normal. On the one hand, population-based studies have detected subtle cognitive changes in verbal fluency, learning, and recall among individuals in the preclinical stage, and prospective LCSs indicate that a decline in global cognition can be detected in people at risk for AD [9], [27]. Focus groups conducted by the Patient-Reported Outcome Consortium also suggest that persons in the preclinical stages of AD notice problems (also referred to as subjective complaints or concerns) in memory, language, attention, comprehension, processing speed, task performance, and interpersonal function [83]. While declining cognitive function is not specific for AD, is heterogeneous, and is modified by age, education, and comorbid diseases, it remains the best surrogate for disease progression.
Composite measures have been proposed as a means of better demonstrating clinical meaningfulness by using a broader range of measures sensitive to a heterogeneous set of symptoms and signs. For example, cognitive and functional measures can be combined [84]. However, composites have the potential to dilute some effects and thereby reduce the ability to capture treatment effects. Different trials are likely to require different measures, including combinations of functional, cognitive, and biomarker assessments that are sensitive at different points along the disease spectrum. Trialists will also need to address the feasibility of administering novel functional measures consistently across multiple centers.
Regulators are also grappling with identifying acceptable endpoints for preclinical trials and harmonizing their guidance across countries. The U.S. Food and Drug Administration issued a draft guidance document in 2013 on developing drugs for early-stage AD [50] and is now working to update that document. The European Medicines Agency has released similar guidelines [85], and the two agencies are working together to align. Health Canada generally follows the Food and Drug Administration guidelines. Both the Food and Drug Administration and European Medicines Agency guidelines emphasize the importance of incorporating the patient voice in developing acceptable measures that are clinically meaningful.
7. Conclusions
The effort to prevent Alzheimer's disease is still in early stages of developing measures sensitive to preclinical disease. While a number of cognitive composites and functional measures have shown some promise, it remains to be seen whether existing tests have adequate sensitivity or if new tests are required. There are also concerns about the feasibility of certain measures in secondary prevention studies that are likely to be large and lengthy. Repeated neuropsychological testing raises concerns about learning effects and participant burden. However, properly designed clinical trials could leverage and maximize practice effects during the prescreening period, which would potentially increase power to detect an interventional effect during the trial and/or decrease the needed sample size. Alternatively, it may be beneficial to reduce the amount of time spent on testing by measuring fewer constructs but measuring them more accurately. At some point, it will also be critical to link change on cognitive and functional composites to clinically meaningful outcomes, such as the duration of delay to the onset of a diagnosis of MCI or dementia or to delay in functional decline.
Heterogeneity of disease presents challenges in the design of clinical trials, yet is a reality and, thus, must be accounted for in trials. Thus, for example, some individuals with AD may have more prominent visuospatial deficits in early stages, while others may have more prominent amnesia or aphasia [86]. This will mean developing measures capable of capturing the heterogeneity of clinical or cognitive change and the effect of a treatment on that change. Moreover, increased efforts are needed to obtain study participants representative of all ages, races, and ethnicities and of different educational and socioeconomic levels.
Roundtable participants also called for increased research to improve understanding of the course of cognition and function from normal to impaired, including increased investment in large-scale, multisite studies of normal aging that involve repeated neuropsychological and functional testing of large groups. Continued investment in computerized tools, such as the NIH toolbox [16], could help achieve this goal through the use of more standardized measures with enhanced sensitivity to subtle cognitive changes. The NIH toolbox also offers measures of noncognitive domains, such as emotion, motor and sensory functions, which may expand the scope of variables that could lead to earlier detection. For example, visual, olfactory, and motor changes have been associated with increased risk of progression from a state of normal aging to MCI and dementia [87], [88], [89], [90], [91], [92], [93], [94], [95]. In addition, there is a need for a lingua franca of what constitutes benefit to enable alignment of researchers, clinicians, patients, regulators, payers, and policy makers to move forward in developing preventive treatments. A case has been made that public and private interests are currently well aligned. It is an ideal time for the field to coalesce on what constitutes a treatment benefit in preclinical AD [96].
Research in Context.
-
1.
Systematic review: This review summarizes the presentations made at the November 2016 Research Roundtable meeting. Each presenter reviewed the literature of recent work in their specific topic areas within the overall area of measuring Alzheimer's disease progression in the preclinical stage.
-
2.
Interpretation: The information covered in this article summarizes, in the view of industry drug developers, the areas of work that need to be addressed in research to achieve success in the development of Alzheimer's disease therapeutics to stop or slow progression at the earliest stages of disease.
-
3.
Future directions: Guidelines are presented to direct future efforts in the measurement of preclinical Alzheimer's disease in individuals who are biomarker positive for the disease.
Acknowledgments
The authors thank Meredith McNeil for logistical planning of the Research Roundtable meeting as well as contributing speakers: Paul Aisen, M.D.; Rachelle Doody, M.D., Ph.D.; Michael Irizarry, M.D.; Jason Karlawish, M.D.; Jessica Langbaum, Ph.D.; Paul Maruff, Ph.D.; Eric McDade, M.D.; Dorene Rentz, PsyD.; Reisa Sperling, M.D.; Robert Wilson, Ph.D.
References
- 1.Park D.C., Lautenschlager G., Hedden T., Davidson N.S., Smith A.D., Smith P.K. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- 2.Park D.C., Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mormino E.C., Kluth J.T., Madison C.M., Rabinovici G.D., Baker S.L., Miller B.L. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132(Pt 5):1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodrigue K.M., Kennedy K.M., Devous M.D., Sr., Rieck J.R., Hebrank A.C., Diaz-Arrastia R. beta-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology. 2012;78:387–395. doi: 10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holtzman D.M., Herz J., Bu G. Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006312. doi: 10.1101/cshperspect.a006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sperling R., Mormino E., Johnson K. The evolution of preclinical Alzheimer's disease: implications for prevention trials. Neuron. 2014;84:608–622. doi: 10.1016/j.neuron.2014.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodge H.H., Zhu J., Lee C.W., Chang C.C., Ganguli M. Cohort effects in age-associated cognitive trajectories. J Gerontol A Biol Sci Med Sci. 2014;69:687–694. doi: 10.1093/gerona/glt181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derby C.A., Katz M.J., Lipton R.B., Hall C.B. Trends in dementia incidence in a birth cohort analysis of the Einstein Aging Study. JAMA Neurol. 2017;74:1345–1351. doi: 10.1001/jamaneurol.2017.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villemagne V.L., Burnham S., Bourgeat P., Brown B., Ellis K.A., Salvado O. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12:357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 10.Salthouse T.A. Why are there different age relations in cross-sectional and longitudinal comparisons of cognitive functioning? Curr Dir Psychol Sci. 2014;23:252–256. doi: 10.1177/0963721414535212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hedden T., Lautenschlager G., Park D.C. Contributions of processing ability and knowledge to verbal memory tasks across the adult life-span. Q J Exp Psychol A. 2005;58:169–190. doi: 10.1080/02724980443000179. [DOI] [PubMed] [Google Scholar]
- 12.Chen X., Hertzog C., Park D.C. Cognitive predictors of everyday problem solving across the lifespan. Gerontology. 2017;63:372–384. doi: 10.1159/000459622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jekel K., Damian M., Wattmo C., Hausner L., Bullock R., Connelly P.J. Mild cognitive impairment and deficits in instrumental activities of daily living: a systematic review. Alzheimers Res Ther. 2015;7:17. doi: 10.1186/s13195-015-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sikkes S.A., de Lange-de Klerk E.S., Pijnenburg Y.A., Scheltens P., Uitdehaag B.M. A systematic review of Instrumental Activities of Daily Living scales in dementia: room for improvement. J Neurol Neurosurg Psychiatry. 2009;80:7–12. doi: 10.1136/jnnp.2008.155838. [DOI] [PubMed] [Google Scholar]
- 15.Sliwinski M.J., Mogle J.A., Hyun J., Munoz E., Smyth J.M., Lipton R.B. Reliability and validity of ambulatory cognitive assessments. Assessment. 2018;25:14–30. doi: 10.1177/1073191116643164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weintraub S., Dikmen S.S., Heaton R.K., Tulsky D.S., Zelazo P.D., Bauer P.J. Cognition assessment using the NIH Toolbox. Neurology. 2013;80(11 Suppl 3):S54–S64. doi: 10.1212/WNL.0b013e3182872ded. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weintraub S., Dikmen S.S., Heaton R.K., Tulsky D.S., Zelazo P.D., Slotkin J. The cognition battery of the NIH toolbox for assessment of neurological and behavioral function: validation in an adult sample. J Int Neuropsychol Soc. 2014;20:567–578. doi: 10.1017/S1355617714000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyle P.A., Wilson R.S., Yu L., Barr A.M., Honer W.G., Schneider J.A. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Ann Neurol. 2013;74:478–489. doi: 10.1002/ana.23964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyle P.A., Yu L., Wilson R.S., Schneider J.A., Bennett D.A. Relation of neuropathology with cognitive decline among older persons without dementia. Front Aging Neurosci. 2013;5:50. doi: 10.3389/fnagi.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task-Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 21.Winblad B., Palmer K., Kivipelto M., Jelic V., Fratiglioni L., Wahlund L.O. Mild cognitive impairment - beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 22.Petersen R.C., Smith G.E., Waring S.C., Ivnik R.J., Tangalos E.G., Kokmen E. Mild cognitive impairment–Clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 23.Villemagne V.L., Burnham S.C., Bourgeat P., Dore V., Brown B.M., Laws S.M. Revisiting, revising, and refining the natural history of Abeta deposition and its effects on neurodegeneration and cognitive decline in sporadic Alzheimer's disease. Alzheimers Dement. 2016;12:P350. [Google Scholar]
- 24.Harrington K.D., Lim Y.Y., Ames D., Hassenstab J., Rainey-Smith S., Robertson J. Using Robust Normative Data to Investigate the Neuropsychology of Cognitive Aging. Arch Clin Neuropsychol. 2017;32:142–154. doi: 10.1093/arclin/acw106. [DOI] [PubMed] [Google Scholar]
- 25.Donohue M.C., Sperling R.A., Salmon D.P., Rentz D.M., Raman R., Thomas R.G. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. 2014;71:961–970. doi: 10.1001/jamaneurol.2014.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amieva H., Le Goff M., Millet X., Orgogozo J.M., Peres K., Barberger-Gateau P. Prodromal Alzheimer's disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64:492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- 27.Bateman R.J., Xiong C., Benzinger T.L., Fagan A.M., Goate A., Fox N.C. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rentz D.M., Weintraub S. Neuropsychological detection of early probably Alzheimer's disease. In: Scinto L.F.M., Daffner K.R., editors. Early diagnosis and treatment of Alzheimer's disease. Humana Press, Inc; Totowa, New Jersey: 2000. [Google Scholar]
- 29.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging and the Alzheimer's Association workgroup. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albert M.S., Dekosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging and the Alzheimer's Association workgroup. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner M., Wolf S., Reischies F.M., Daerr M., Wolfsgruber S., Jessen F. Biomarker validation of a cued recall memory deficit in prodromal Alzheimer disease. Neurology. 2012;78:379–386. doi: 10.1212/WNL.0b013e318245f447. [DOI] [PubMed] [Google Scholar]
- 33.Doraiswamy P.M., Sperling R.A., Johnson K., Reiman E.M., Wong T.Z., Sabbagh M.N. Florbetapir F 18 amyloid PET and 36-month cognitive decline: a prospective multicenter study. Mol Psychiatry. 2014;19:1044–1051. doi: 10.1038/mp.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim Y.Y., Maruff P., Pietrzak R.H., Ames D., Ellis K.A., Harrington K. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer's disease. Brain. 2014;137:221–231. doi: 10.1093/brain/awt286. [DOI] [PubMed] [Google Scholar]
- 35.Petersen R.C., Wiste H.J., Weigand S.D., Rocca W.A., Roberts R.O., Mielke M.M. Association of elevated amyloid levels with cognition and biomarkers in cognitively normal people from the community. JAMA Neurol. 2016;73:85–92. doi: 10.1001/jamaneurol.2015.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burnham S.C., Bourgeat P., Dore V., Savage G., Brown B., Laws S. Clinical and cognitive trajectories in cognitively healthy elderly individuals with suspected non-Alzheimer's disease pathophysiology (SNAP) or Alzheimer's disease pathology: a longitudinal study. Lancet Neurol. 2016;15:1044–1053. doi: 10.1016/S1474-4422(16)30125-9. [DOI] [PubMed] [Google Scholar]
- 37.Mormino E.C., Papp K.V. Cognitive decline in preclinical stage 2 Alzheimer disease and implications for prevention trials. JAMA Neurol. 2016;73:640–642. doi: 10.1001/jamaneurol.2016.0281. [DOI] [PubMed] [Google Scholar]
- 38.Soldan A., Pettigrew C., Cai Q., Wang M.C., Moghekar A.R., O'Brien R.J. Hypothetical preclinical Alzheimer disease groups and longitudinal cognitive change. JAMA Neurol. 2016;73:698–705. doi: 10.1001/jamaneurol.2016.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mormino E.C., Papp K.V., Schultz A.P., Hanseeuw B.J., Munro C.E., Jaimes S.Y. Alzheimer's Association International Conference; Toronto, Canada: 2016. AB+ clinically normal participants with elevated tau show greatest decline in the preclinical Alzheimer's disease cognitive composite; p. P333. [Google Scholar]
- 40.Jack C.R., Jr., Wiste H.J., Weigand S.D., Knopman D.S., Mielke M.M., Vemuri P. Different definitions of neurodegeneration produce similar amyloid/neurodegeneration biomarker group findings. Brain. 2015;138(Pt 12):3747–3759. doi: 10.1093/brain/awv283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexopoulos P., Kriett L., Haller B., Klupp E., Gray K., Grimmer T. Limited agreement between biomarkers of neuronal injury at different stages of Alzheimer's disease. Alzheimers Dement. 2014;10:684–689. doi: 10.1016/j.jalz.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Caroli A., Prestia A., Wade S., Chen K., Ayutyanont N., Landau S.M. Alzheimer disease biomarkers as outcome measures for clinical trials in MCI. Alzheimer Dis Assoc Disord. 2015;29:101–109. doi: 10.1097/WAD.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chetelat G. Alzheimer disease: Abeta-independent processes-rethinking preclinical AD. Nat Rev Neurol. 2013;9:123–124. doi: 10.1038/nrneurol.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chetelat G., Desgranges B., Landeau B., Mezenge F., Poline J.B., de la Sayette V. Direct voxel-based comparison between grey matter hypometabolism and atrophy in Alzheimer's disease. Brain. 2008;131(Pt 1):60–71. doi: 10.1093/brain/awm288. [DOI] [PubMed] [Google Scholar]
- 45.Toledo J.B., Weiner M.W., Wolk D.A., Da X., Chen K., Arnold S.E. Neuronal injury biomarkers and prognosis in ADNI subjects with normal cognition. Acta Neuropathol Commun. 2014;2:26. doi: 10.1186/2051-5960-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wirth M., Madison C.M., Rabinovici G.D., Oh H., Landau S.M., Jagust W.J. Alzheimer's disease neurodegenerative biomarkers are associated with decreased cognitive function but not beta-amyloid in cognitively normal older individuals. J Neurosci. 2013;33:5553–5563. doi: 10.1523/JNEUROSCI.4409-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mormino E.C., Betensky R.A., Hedden T., Schultz A.P., Ward A., Huijbers W. Amyloid and APOE ε4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology. 2014;82:1760–1767. doi: 10.1212/WNL.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amariglio R.E., Mormino E.C., Pietras A.C., Marshall G.A., Vannini P., Johnson K.A. Subjective cognitive concerns, amyloid-beta, and neurodegeneration in clinically normal elderly. Neurology. 2015;85:56–62. doi: 10.1212/WNL.0000000000001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samieri C., Proust-Lima C., Glymour M M., Okereke O.I., Amariglio R.E., Sperling R.A. Subjective cognitive concerns, episodic memory, and the APOE ε4 allele. Alzheimers Dement. 2014;10:752–759.e1. doi: 10.1016/j.jalz.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Food and Drug Administration . 2013. Draft Guidance for Industry. Alzheimer's disease: Developing drugs for the treatment of early stage disease.http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM338287.pdf Available at. [Google Scholar]
- 51.Vassar R., Kuhn P.H., Haass C., Kennedy M.E., Rajendran L., Wong P.C. Function, therapeutic potential and cell biology of BACE proteases: current status and future prospects. J Neurochem. 2014;130:4–28. doi: 10.1111/jnc.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ayutyanont N., Langbaum J.B., Hendrix S.B., Chen K., Fleisher A.S., Friesenhahn M. The Alzheimer's prevention initiative composite cognitive test score: sample size estimates for the evaluation of preclinical Alzheimer's disease treatments in presenilin 1 E280A mutation carriers. J Clin Psychiatry. 2014;75:652–660. doi: 10.4088/JCP.13m08927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Langbaum J.B., Hendrix S.B., Ayutyanont N., Chen K., Fleisher A.S., Shah R.C. An empirically derived composite cognitive test score with improved power to track and evaluate treatments for preclinical Alzheimer's disease. Alzheimers Dement. 2014;10:666–674. doi: 10.1016/j.jalz.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sperling R.A., Rentz D.M., Johnson K.A., Karlawish J., Donohue M., Salmon D.P. The A4 study: stopping AD before symptoms begin? Sci Transl Med. 2014;6:228fs13. doi: 10.1126/scitranslmed.3007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hassenstab J. Paper presented at ADPD meeting, April 2017; reference to be added. 2017.
- 56.Hassenstab J, Hagen C, Han B, Lim YY, Maruff P, Mielke MM, et al. Reliability and reproducibility of cognitive composites for Alzheimer's disease secondary prevention trials: The power PACC. Paper presented at ADPD 2017; Vienna, Austria.
- 57.Ritchie K., Ropacki M., Albala B., Harrison J., Kaye J., Kramer J. Recommended cognitive outcomes in preclinical Alzheimer's disease: Consensus statement from the European Prevention of Alzheimer's Dementia project. Alzheimers Dement. 2017;13:186–195. doi: 10.1016/j.jalz.2016.07.154. [DOI] [PubMed] [Google Scholar]
- 58.Mortamais M., Ash J.A., Harrison J., Kaye J., Kramer J., Randolph C. Detecting cognitive changes in preclinical Alzheimer's disease: A review of its feasibility. Alzheimers Dement. 2017;13:468–492. doi: 10.1016/j.jalz.2016.06.2365. [DOI] [PubMed] [Google Scholar]
- 59.Farias S.T., Chou E., Harvey D.J., Mungas D., Reed B., DeCarli C. Longitudinal trajectories of everyday function by diagnostic status. Psychol Aging. 2013;28:1070–1075. doi: 10.1037/a0034069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marshall G.A., Amariglio R.E., Sperling R.A., Rentz D.M. Activities of daily living: where do they fit in the diagnosis of Alzheimer's disease? Neurodegener Dis Manag. 2012;2:483–491. doi: 10.2217/nmt.12.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sikkes S.A., Visser P.J., Knol D.L., de Lange-de Klerk E.S., Tsolaki M., Frisoni G.B. Do instrumental activities of daily living predict dementia at 1- and 2-year follow-up? Findings from the Development of Screening guidelines and diagnostic Criteria for Predementia Alzheimer's disease study. J Am Geriatr Soc. 2011;59:2273–2281. doi: 10.1111/j.1532-5415.2011.03732.x. [DOI] [PubMed] [Google Scholar]
- 62.Goldberg T.E., Koppel J., Keehlisen L., Christen E., Dreses-Werringloer U., Conejero-Goldberg C. Performance-based measures of everyday function in mild cognitive impairment. Am J Psychiatry. 2010;167:845–853. doi: 10.1176/appi.ajp.2010.09050692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gomar J.J., Harvey P.D., Bobes-Bascaran M.T., Davies P., Goldberg T.E. Development and cross-validation of the UPSA short form for the performance-based functional assessment of patients with mild cognitive impairment and Alzheimer disease. Am J Geriatr Psychiatry. 2011;19:915–922. doi: 10.1097/JGP.0b013e3182011846. [DOI] [PubMed] [Google Scholar]
- 64.Kirchberg B.C., Cohen J.R., Adelsky M.B., Buthorn J.J., Gomar J.J., Gordon M. Semantic distance abnormalities in mild cognitive impairment: their nature and relationship to function. Am J Psychiatry. 2012;169:1275–1283. doi: 10.1176/appi.ajp.2012.12030383. [DOI] [PubMed] [Google Scholar]
- 65.Sousa A., Gomar J.J., Ragland J.D., Conejero-Goldberg C., Buthorn J., Keehlisen L. The relational and item-specific encoding task in mild cognitive impairment and Alzheimer disease. Dement Geriatr Cogn Disord. 2016;42:265–277. doi: 10.1159/000448170. [DOI] [PubMed] [Google Scholar]
- 66.Marshall G.A., Dekhtyar M., Bruno J.M., Jethwani K., Amariglio R.E., Johnson K.A. The Harvard Automated Phone Task: new performance-based activities of daily living tests for early Alzheimer's disease. J Prev Alzheimers Dis. 2015;2:242–253. doi: 10.14283/jpad.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Triebel K.L., Martin R., Griffith H.R., Marceaux J., Okonkwo O.C., Harrell L. Declining financial capacity in mild cognitive impairment: A 1-year longitudinal study. Neurology. 2009;73:928–934. doi: 10.1212/WNL.0b013e3181b87971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Farias S.T., Mungas D., Reed B.R., Cahn-Weiner D., Jagust W., Baynes K. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology. 2008;22:531–544. doi: 10.1037/0894-4105.22.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rueda A.D., Lau K.M., Saito N., Harvey D., Risacher S.L., Aisen P.S. Self-rated and informant-rated everyday function in comparison to objective markers of Alzheimer's disease. Alzheimers Dement. 2015;11:1080–1089. doi: 10.1016/j.jalz.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Farias S.T., Park L.Q., Harvey D.J., Simon C., Reed B.R., Carmichael O. Everyday cognition in older adults: associations with neuropsychological performance and structural brain imaging. J Int Neuropsychol Soc. 2013;19:430–441. doi: 10.1017/S1355617712001609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lau K.M., Parikh M., Harvey D.J., Huang C.J., Farias S.T. Early cognitively based functional limitations predict loss of independence in instrumental activities of daily living in older adults. J Int Neuropsychol Soc. 2015;21:688–698. doi: 10.1017/S1355617715000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Farias S.T., Lau K., Harvey D., Denny K.G., Barba C., Mefford A.N. Early functional limitations in cognitively normal older adults predict diagnostic conversion to mild cognitive impairment. J Am Geriatr Soc. 2017;65:1152–1158. doi: 10.1111/jgs.14835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weber M.T., Mapstone M., Staskiewicz J., Maki P.M. Reconciling subjective memory complaints with objective memory performance in the menopausal transition. Menopause. 2012;19:735–741. doi: 10.1097/gme.0b013e318241fd22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sikkes S.A., Hooghiemstra A.M., Pijnenburg Y.A., Scheltens P. AAIC; Toronto, CA: 2016. The past, present, and future of instrumental activities of daily living assessments in Alzheimer's disease; p. P372. Alzheimer's & Dementia; 2016. [Google Scholar]
- 75.Sikkes S.A., de Lange-de Klerk E.S., Pijnenburg Y.A., Gillissen F., Romkes R., Knol D.L. A new informant-based questionnaire for instrumental activities of daily living in dementia. Alzheimers Dement. 2012;8:536–543. doi: 10.1016/j.jalz.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 76.Koster N., Knol D.L., Uitdehaag B.M., Scheltens P., Sikkes S.A. The sensitivity to change over time of the Amsterdam IADL Questionnaire(©) Alzheimers Dement. 2015;11:1231–1240. doi: 10.1016/j.jalz.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 77.Jutten R.J., Peeters C.F.W., Leijdesdorff S.M.J., Visser P.J., Maier A.B., Terwee C.B. Detecting functional decline from normal aging to dementia: Development and validation of a short version of the Amsterdam IADL Questionnaire. Alzheimers Dement (Amst) 2017;8:26–35. doi: 10.1016/j.dadm.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jack C.R., Jr., Knopman D.S., Weigand S.D., Wiste H.J., Vemuri P., Lowe V. An operational approach to National Institute on Aging-Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71:765–775. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jack C.R., Jr., Knopman D.S., Chetelat G., Dickson D., Fagan A.M., Frisoni G.B. Suspected non-Alzheimer disease pathophysiology–concept and controversy. Nat Rev Neurol. 2016;12:117–124. doi: 10.1038/nrneurol.2015.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reiman E.M., Langbaum J.B., Fleisher A.S., Caselli R.J., Chen K., Ayutyanont N. Alzheimer's Prevention Initiative: a plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis. 2011;26(Suppl 3):321–329. doi: 10.3233/JAD-2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mills S.M., Mallmann J., Santacruz A.M., Fuqua A., Carril M., Aisen P.S. Preclinical trials in autosomal dominant AD: implementation of the DIAN-TU trial. Rev Neurol (Paris) 2013;169:737–743. doi: 10.1016/j.neurol.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roses A.D., Saunders A.M., Lutz M.W., Zhang N., Hariri A.R., Asin K.E. New applications of disease genetics and pharmacogenetics to drug development. Curr Opin Pharmacol. 2014;14:81–89. doi: 10.1016/j.coph.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 83.Ropacki M. Placeholder of clinical meaningfulness paper in press. 2017.
- 84.Jutten R.J., Harrison J., de Jong F.J., Aleman A., Ritchie C.W., Scheltens P. A composite measure of cognitive and functional progression in Alzheimer's disease: Design of the Capturing Changes in Cognition study. Alzheimers Dement (N Y) 2017;3:130–138. doi: 10.1016/j.trci.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.European Medicines Agency . (CHMP) CfMPfHU. 2016. Draft guideline on the clinical investigation of medicines for the treatment of Alzheimer's disease and other dementia. EMA: London, United Kingdom. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/02/WC500200830.pdf. [Google Scholar]
- 86.Rogalski E., Sridhar J., Rader B., Martersteck A., Chen K., Cobia D. Aphasic variant of Alzheimer disease: Clinical, anatomic, and genetic features. Neurology. 2016;87:1337–1343. doi: 10.1212/WNL.0000000000003165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Albers M.W., Gilmore G.C., Kaye J., Murphy C., Wingfield A., Bennett D.A. At the interface of sensory and motor dysfunctions and Alzheimer's disease. Alzheimers Dement. 2015;11:70–98. doi: 10.1016/j.jalz.2014.04.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barnes L.L., Mendes de Leon C.F., Wilson R.S., Bienias J.L., Evans D.A. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63:2322–2326. doi: 10.1212/01.wnl.0000147473.04043.b3. [DOI] [PubMed] [Google Scholar]
- 89.Beauchet O., Allali G., Launay C., Herrmann F.R., Annweiler C. Gait variability at fast-pace walking speed: a biomarker of mild cognitive impairment? J Nutr Health Aging. 2013;17:235–239. doi: 10.1007/s12603-012-0394-4. [DOI] [PubMed] [Google Scholar]
- 90.Buracchio T., Dodge H.H., Howieson D., Wasserman D., Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2010;67:980–986. doi: 10.1001/archneurol.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gras L.Z., Kanaan S.F., McDowd J.M., Colgrove Y.M., Burns J., Pohl P.S. Balance and gait of adults with very mild Alzheimer disease. J Geriatr Phys Ther. 2015;38:1–7. doi: 10.1519/JPT.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Montero-Odasso M., Casas A., Hansen K.T., Bilski P., Gutmanis I., Wells J.L. Quantitative gait analysis under dual-task in older people with mild cognitive impairment: a reliability study. J Neuroeng Rehabil. 2009;6:35. doi: 10.1186/1743-0003-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Uhlmann R.F., Larson E.B., Rees T.S., Koepsell T.D., Duckert L.G. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA. 1989;261:1916–1919. [PubMed] [Google Scholar]
- 94.Wilson R.S., Schneider J.A., Arnold S.E., Tang Y., Boyle P.A., Bennett D.A. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007;64:802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]
- 95.Yan J.H., Rountree S., Massman P., Doody R.S., Li H. Alzheimer's disease and mild cognitive impairment deteriorate fine movement control. J Psychiatr Res. 2008;42:1203–1212. doi: 10.1016/j.jpsychires.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 96.Karlawish J., Langa K.M. Unfinished Business in Preventing Alzheimer Disease. JAMA Intern Med. 2016;176:1739–1740. doi: 10.1001/jamainternmed.2016.6310. [DOI] [PMC free article] [PubMed] [Google Scholar]