Abstract
Perilla frutescens is an Asian dietary herb consumed as an essential seasoning in Japanese cuisine as well as used for a Chinese medicine. Here, we report that a newly found methoxyflavanone derivative from P. frutescens (Perilla-derived methoxyflavanone, PDMF; 8-hydroxy-5,7-dimethoxyflavanone) shows carcinostatic activity on human lung adenocarcinoma, A549. We found that treatment with PDMF significantly inhibited cell proliferation and decreased viability through induction of G2/M cell cycle arrest and apoptosis. The PDMF stimulation induces phosphorylation of tumor suppressor p53 on Ser15, and increases its protein amount in conjunction with up-regulation of downstream cyclin-dependent kinase inhibitor p21Cip1/Waf1 and proapoptotic caspases, caspase-9 and caspase-3. We also found that small interfering RNA knockdown of p53 completely abolished the PDMF-induced G2/M cell cycle arrest, and substantially abrogated its proapoptotic potency. These results suggest that PDMF represents a useful tumor-preventive phytochemical that triggers p53-driven G2/M cell cycle arrest and apoptosis.
Keywords: Apoptosis, Flavanone, G2/M cell cycle arrest, Lung cancer, p53, Perilla frutescens
Introduction
Lung cancer is the most common cause of cancer mortality in male and the second cause in female, which is responsible for 1.38 million annual deaths worldwide (Ridge et al. 2013). Non-small-cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancer cases. For NSCLC patients with early stages, surgery followed by chemotherapy is the optimal treatment (Sandler et al. 2000). Although platinum-based doublet chemotherapy is the first-line standard chemotherapy for advanced NSCLC, its response rate is only 30%, resulting in an average of 9 months of overall survival (Azzoli et al. 2009; Socinski 2014). It is therefore needed to develop safer and more effective therapeutic strategies to treat NSCLC.
Perilla frutescens is an Asian traditional edible plant as well as used for a Chinese medicinal herb. Leaf extract of P. frutescens (PLE) has been reported to possess antioxidant and anti-inflammatory activities (Meng et al. 2009; Ueda and Yamazaki 1997, 2001), and ameliorate hepatotoxicity (Kim et al. 2007), allergy (Makino et al. 2001, 2003), and obesity (Kim and Kim 2009). PLE has also been reported to inhibit growth of human cancer cells (Kwak and Ju 2015; Kwak et al. 2009; Lin et al. 2007), and suppress skin tumor promotion in mice (Ueda et al. 2003). Those anti-cancer activities of PLE are reported to be fulfilled by its constituents including luteolin (Ueda et al. 2002, 2003), rosmarinic acid (Makino et al. 2003; Osakabe et al. 2002; Takano et al. 2004), and triterpene acids (Banno et al. 2004).
Our group has recently reported that a new methoxyflavanone derivative from P. frutescens (8-hydroxy-5,7-dimethoxyflavanone, named Perilla-derived methoxyflavanone; PDMF) directly suppresses the serine/threonine kinase Akt to inhibit type I hypersensitivity reactions (Kamei et al. 2017). Akt, also known as protein kinase B (PKB), is a critical regulator of cell proliferation, and its over-activation leads to cancer development (Manning and Cantley 2007). The targeted action of PDMF to Akt (Kamei et al. 2017) prompted us to investigate whether PDMF is also effective for suppression of cancer cell proliferation and survival. In this study, we report a carcinostatic effect of PDMF on human lung adenocarcinoma A549.
Materials and methods
Cell culture and reagents
The A549 human lung adenocarcinoma cell line and WI-38 normal fibroblast (RCB0702) were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) and RIKEN BRC (Tsukuba, Japan) through the National Bio-Resource Project of the MEXT, Japan, respectively, and were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies, Tokyo, Japan) containing 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, USA), 100 U/ml penicillin, and 100 μg/ml streptomycin (Life Technologies) in a humidified atmosphere with 5% CO2 at 37 °C. Maintained A549 and WI-38 cells between the 3rd and 10th passages were used for this study. Perilla-derived methoxyflavanone (8-hydroxy-5,7-dimethoxyflavanone; PDMF) was chemically synthesized by Tokyo Chemical Industry (Tokyo, Japan), which had a purity of 99.6% confirmed by high performance liquid chromatography analysis. Doxorubicin was purchased from Sigma-Aldrich. All chemicals used in this study were of analytical or cell-culture grade.
Cell proliferation and viability assays
Cell proliferation was quantified as BrdU incorporation using a colorimetric Cell Proliferation ELISA, BrdU Kit (Roche Diagnostics, Indianapolis, IN, USA) according to the manufacturer’s instructions. A549 cells were seeded at 5 × 103 cells/well and cultured overnight in a 96-well plate. The cells were treated with 3, 7.5, 15, 30, 45, 60 or 75 μg/ml PDMF, or either 580 ng/ml (1 μM) doxorubicin as a positive control or dimethyl sulfoxide (DMSO, vehicle) as a negative control for 24 h. BrdU was added to a final concentration of 10 μM and cultured for the last 2 h. BrdU incorporation was quantified by OD450 according to the manufacturer’s instructions.
Trypan blue exclusion assay was used to determine cell viability after treatment with PDMF or doxorubicin. A549 and WI-38 cells (5 × 103 cells/well) were cultured in 6-well plates and treated with 30, 45, 60 or 75 μg/ml PDMF, or either 580 ng/ml doxorubicin or DMSO (vehicle) for 24 h. The cultured cells were harvested and re-suspended in 100 μl DMEM, and the cell suspension was thoroughly mixed with an equal volume of 0.4% trypan blue staining solution (Gibco, Grand Island, NY, USA). Numbers of viable and dead cells were counted using a hemocytometer.
Cell cycle and apoptosis analyses
Cell cycle progression or induction of apoptosis of A549 cells was analyzed by a FACS Calibur flow cytometer (BD Biosciences, San Jose, CA, USA) using the BD Pharmingen™ FITC-BrdU Flow Kit (BD Biosciences) or the annexin V/propidium iodide (PI) staining kit (BioLegend, San Diego, CA, USA), respectively, according to the manufacturer’s instructions. A549 cells were treated with 30, 45, 60 or 75 μg/ml PDMF, or either 580 ng/ml doxorubicin or DMSO (vehicle) for 24 h. Quantitative analysis of the FACS data was performed with FlowJo software (FlowJo, Ashland, OR, USA).
Western blot analysis
A549 cells were harvested, washed twice with chilled PBS, and lysed with ice-cold lysis buffer containing 0.1% sodium lauryl sulfate (SDS), 150 mM NaCl, 1 mM ethylene diamine tetraacetic acid (EDTA), 1% Triton X-100, 2 µg/ml aprotinin, 5 µg/ml Pefabloc SC (4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride), a protease inhibitor cocktail (Roche Diagnostics, Tokyo, Japan), 1% phosphatase inhibitor cocktail (Nacalai Tesque, Kyoto, Japan) and 50 mM Tris–HCl (pH 7.4) after 24 h incubation with PDMF, doxorubicin or DMSO (vehicle). The cell lysates were kept on ice for 30 min after gentle vortex, and then were centrifuged at 14,000 g for 15 min at 4 °C. The supernatants were either loaded onto SDS-PAGE gel immediately after protein extraction or stored at −80 °C until use. The protein concentration was determined by Bradford assay (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s instructions. For Western blotting, 30 μg protein weight of cell lysate was loaded onto a 15% SDS-PAGE gel under reduced condition. Proteins separated on the gel were transferred onto a PVDF membrane. The PVDF membrane was incubated in blocking buffer containing 3% nonfat milk powder, 1% bovine serum albumin (Sigma-Aldrich) and 0.5% Tween-20 in PBS for 1 h. Subsequently, the PVDF membrane was incubated with anti-p53 polyclonal antibody (pAb), anti-phospho-p53 (pp53) pAb, anti-p21 monoclonal antibody (mAb, clone no. EA10), anti-Akt pAb, anti-pAkt pAb, anti-caspase-3 pAb (Cell Signaling Technology, Beverly, MA, USA), anti-caspase-8 mAb (clone no. 5D3), anti-caspase-9 mAb (clone no. 5B4) (Medical and Biological Laboratories, Nagoya, Japan) or anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mAb (clone no. H-12, Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight, followed by horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (Cell Signaling Technology) or HRP-conjugated anti-mouse IgG (Becton-Dickinson, Durham, NC, USA) for 1 h with agitation at room temperature. Finally, the specific binding to each primary antibody was detected on X-ray film (Konica Minolta Medical Imaging, Wayne, NJ, USA) with ECL prime immunodetection reagent (GE Healthcare, Little Chalfont, UK).
Knockdown of p53 by small interfering RNA (siRNA) transfection
Cultured A549 cells (2 × 105 cells/well) in a 6-well plate were transfected with 75 pmol p53 siRNA (#106141, Ambion, Austin, TX, USA) or control siRNA (#AM4611, Ambion) with Lipofectamine 2000 (Invitrogen). The selectivity of the siRNA was validated and confirmed by its supplier. After 48 h incubation, 1.5 ml fresh DMEM medium was added and then cultured with 75 μg/ml PDMF, 580 ng/ml doxorubicin, or DMSO (vehicle) for an additional 24 h.
Statistical analysis
Data are represented as mean ± SD. Non-repeated ANOVA with post hoc Dunnett’s test was performed to determine the statistical significance compared to a corresponding negative control. Statistical significance was defined as P < 0.05. The IC50 of PDMF for A549 and WI-38 cell viability was calculated using Graph Pad Prism 5 (Version 5.01, GraphPad Software, San Diego, CA, USA). The data shown in the figures are representative data for three independent experimental results.
Results
Stimulation with PDMF impairs cell proliferation and decreases viability in A549 human lung adenocarcinoma
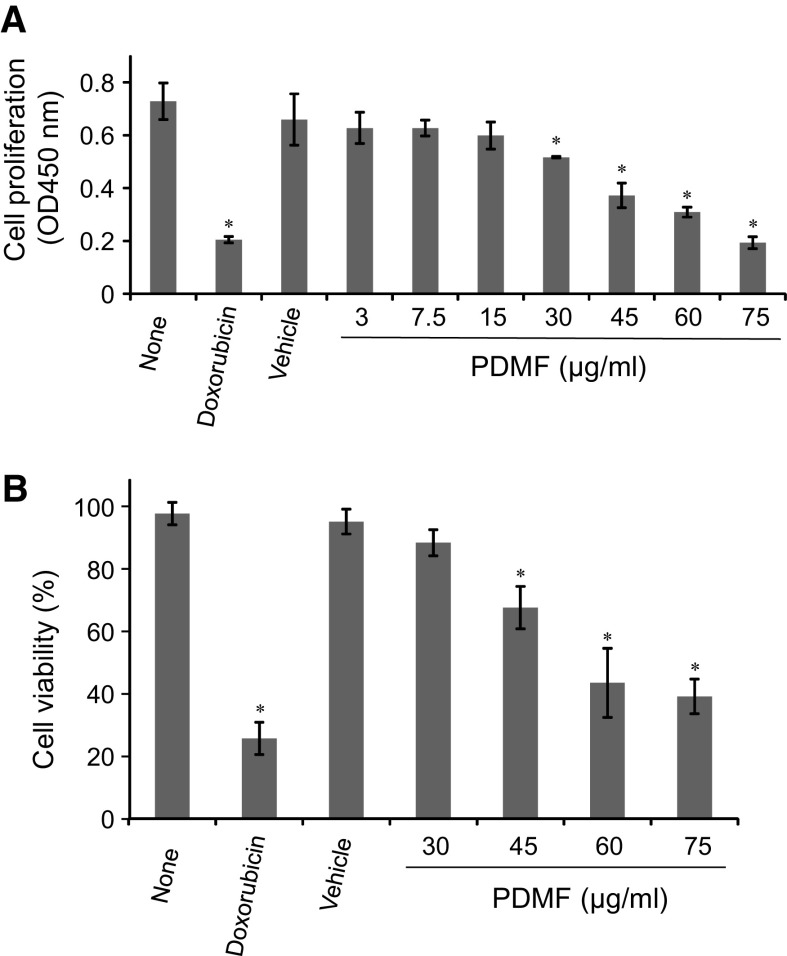
We first analyzed whether stimulation with PDMF affect cell proliferation and viability in A549 cells. BrdU incorporation assay revealed that treatment with PDMF (30–75 μg/ml) significantly inhibited cell proliferation in a dose-dependent manner (Fig. 1a). We also found that stimulation with 45–75 μg/ml of PDMF showed cytotoxicity on A549 cells (Fig. 1b).
Fig. 1.
PDMF suppresses cell proliferation of A549 human lung adenocarcinoma. a Effect of PDMF on the proliferation of A549 cells was assessed by BrdU incorporation assay. A549 cells were incubated with medium (none), 580 ng/ml doxorubicin, or 0 (vehicle), 3, 7.5, 15, 30, 45, 60 or 75 μg/ml of PDMF for 24 h. Representative data of six independent experiments are shown as mean ± SD of three replicates. b The cytotoxic effect of PDMF on A549 cells was determined by trypan blue dye exclusion assay. A549 cells were incubated for 24 h with medium (none), 580 ng/ml doxorubicin, or 0 (vehicle), 30, 45, 60 or 75 μg/ml PDMF. Data are represented as mean ± SD from six independent experiments. * P < 0.05 indicates significant differences compared with vehicle by ANOVA with post hoc Dunnett’s test
PDMF induces G2/M cell cycle arrest in A549 cells
We next investigated the effect of PDMF on cell cycle progression of A549 cells. Flow cytometric analysis of anti-BrdU-FITC- and 7-AAD-stained A549 cells indicated that treatment with PDMF (45–75 μg/ml) triggered cell cycle arrest at G2/M phase in a dose-dependent manner, while cell numbers at the G1 and S phases showed a slightly but statistically significant decrease as compared with those in the vehicle as a negative control (Fig. 2a, b). The potency of PDMF-driven G2/M phase cell cycle arrest was comparable to that seen in doxorubicin (580 ng/ml)-treated A549 cells as a positive control. These results suggest that the PDMF-driven anti-proliferative activity is attributable to the induction of G2/M cell cycle arrest in A549 cells.
Fig. 2.
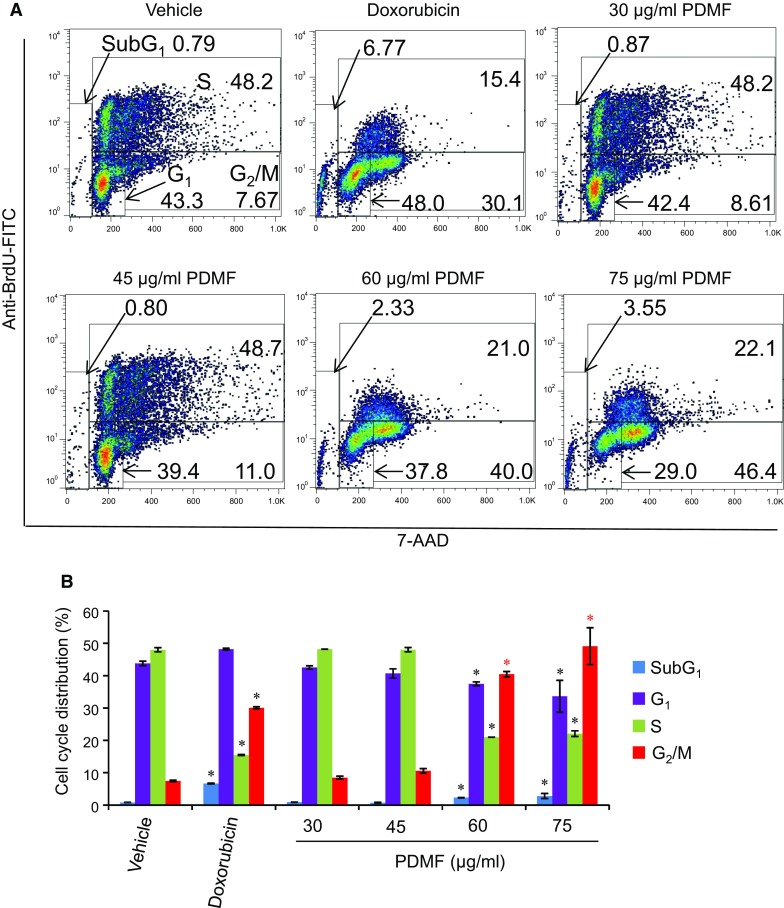
Stimulation with PDMF induces G2/M cell cycle arrest on A549 cells. a Representative cell cycle distribution data are shown by flow cytometric analysis upon incubation with 580 ng/ml doxorubicin, or 0 (vehicle), 30, 45, 60 or 75 μg/ml of PDMF for 24 h. b Quantification of cell cycle distribution upon 24 h incubation with serial concentrations of PDMF. The values are mean ± SD of three different experiments. * P < 0.05 indicate significant differences compared with vehicle
Proapoptotic potency of PDMF
To examine whether PDMF-induced cell death in A549 cells was associated with apoptosis, PDMF-treated A549 cells were analyzed by flow cytometry using a combination of annexin V and propidium iodide (PI) staining. We found that treatment with PDMF (45–75 μg/ml) resulted in a significant accumulation of early (Annexin V+PI−) and late (Annexin V+PI+) apoptotic cells (Fig. 3a), and that total annexin V-positive apoptotic cells significantly increased in a dose-dependent manner (Fig. 3b). These data clearly show proapoptotic potency of PDMF on A549 cells.
Fig. 3.
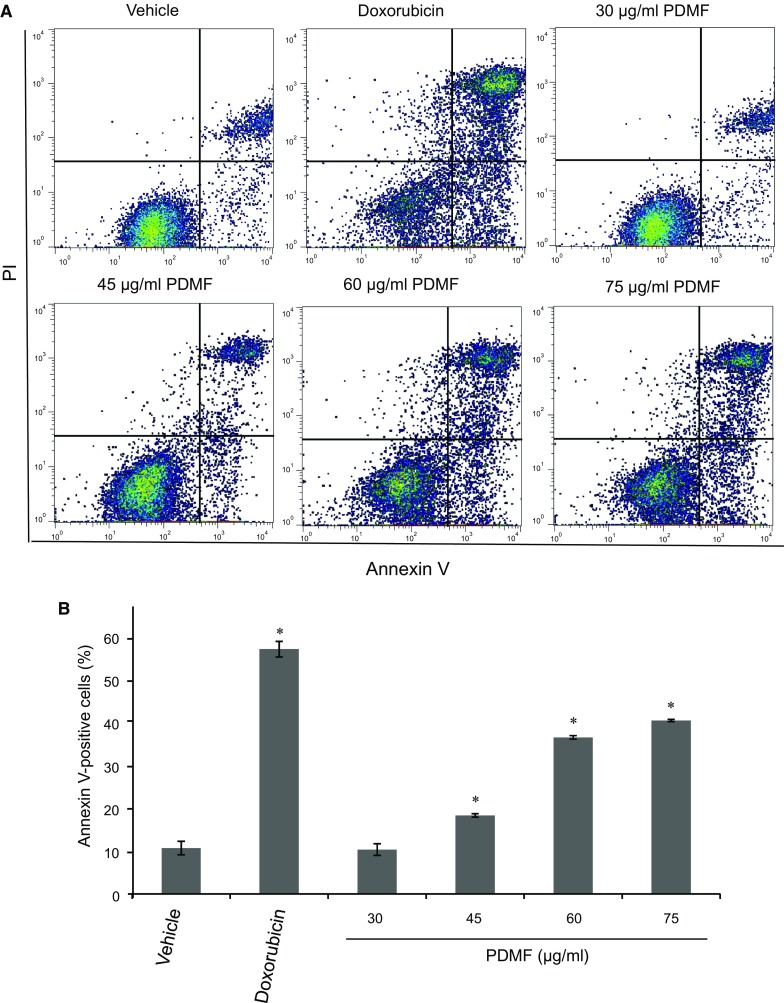
Proapoptotic potency of PDMF on A549 cells. a Representative cytograms of apoptotic A549 cells stimulated with 0 (vehicle), 30, 45, 60 or 75 μg/ml PDMF, or 580 ng/ml doxorubicin as a positive control for 24 h. The lower-right (annexin V+PI− cells) and the upper-right (annexin V+PI+ cells) quadrants show early and late apoptotic cells, and the lower-left (annexin V−PI− cells) and the upper-left (annexin V−PI− cells) quadrants represent viable and necrotic cells, respectively. b Quantification of annexin V-positive apoptotic cells upon stimulation with PDMF. The values are the mean ± SD of three different experiments. * P < 0.05 indicates significant differences compared with vehicle
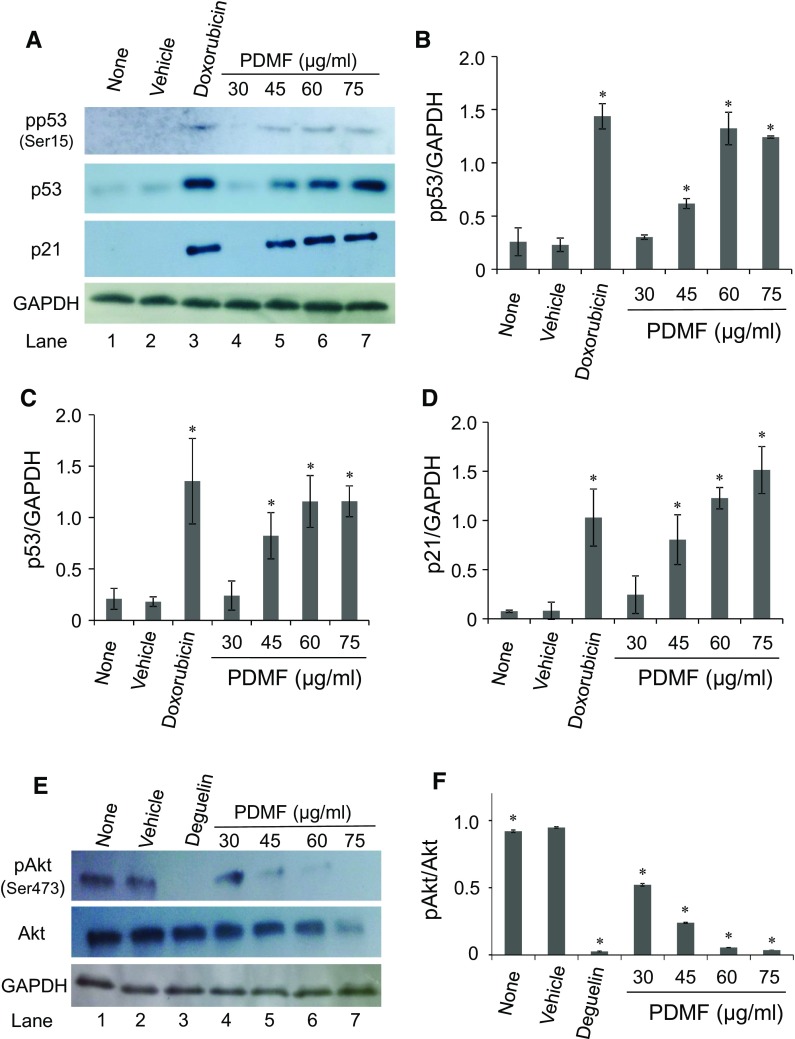
PDMF stimulation induces phosphorylation of p53 and increases its protein amount
To elucidate the molecular mechanism by which PDMF fulfilled carcinostatic activity on A549 cells, we first examined the effect of PDMF on the expression and activation of tumor suppressor p53, since A549 cells carried wild-type p53 alleles (Lehman et al. 1991). We found that stimulation with PDMF (45–75 μg/ml) induced serine phosphorylation of p53 (pp53) on Ser15 (Fig. 4a, b), a critical post-translational modification of p53 by its upstream kinases ataxia telangiectasia mutated (ATM) and ataxia telangiectasia and Rad3 related kinase (ATR) upon DNA damage checkpoint responses (Siliciano et al. 1997; Tibbetts et al. 1999). We also found that PDMF significantly increased the protein amount of p53 in a dose-dependent manner (Fig. 4a, c). These results suggest that stimulation with PDMF up-regulates p53 through its activation and protein induction. Consistent with the up-modulation of p53, we found that PDMF stimulation induced protein levels of p21Cip1/Waf1 (Fig. 4a, d), a p53-responsive cyclin-dependent kinase (CDK) inhibitor (El-Deiry et al. 1993).
Fig. 4.
PDMF induces phosphorylation of p53 and increases its protein amount in A549 cells. a Protein levels of phosphorylated p53 (pp53, on Ser15), p53, and p21Cip1/Waf1 upon treatment with PDMF were determined by western blot analysis. GAPDH was immunostained as a loading control. b, c, and d, The relative protein expression levels of pp53 (Ser15), p53 and p21 Cip1/Waf1 were quantified upon normalization with that of GAPDH. e PDMF suppresses Akt phosphorylation. Protein levels of pAkt (on Ser473) and Akt were detected by western blot analysis. f The relative protein expression levels of pAkt (Ser473) normalized with those of total Akt were quantified by the Image J software. The values are the mean ± SD of three different experiments. * P < 0.05 indicates significant differences compared with vehicle
We next sought to see a possible mechanism by which PDMF increased p53 protein levels in A549 cells (Fig. 4a). It has been well-known that Akt enhances MDM2-mediated degradation of p53 (Ogawara et al. 2002), and our group has recently demonstrated that PDMF directly acts on Akt to suppress its phosphorylation (Kamei et al. 2017). These results raise a possibility that PDMF could induce p53 protein levels via its direct inhibition of Akt, which negatively regulates the MDM2-driven p53 degradation pathway in A549 cells. Indeed, we confirmed that stimulation with PDMF significantly suppressed phosphorylation of Akt on serine 473 (Ser473) in a dose-dependent manner (Fig. 4e, f).
These results indicate that PDMF activates p53 and increases its protein amount, both of which could be involved in its carcinostatic effect on A549 cells.
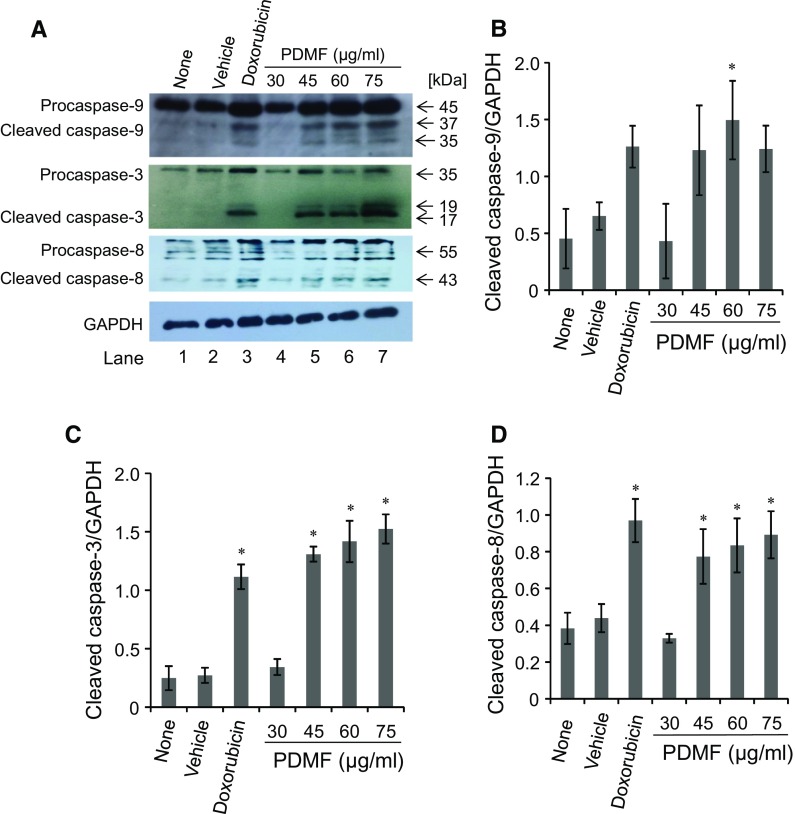
PDMF activates proapoptotic caspases
Proapoptotic action of p53 is fulfilled by downstream caspases: caspase-9 and caspase-3 (Schuler et al. 2000). We next tested whether PDMF activates those caspases to fulfill its proapoptotic potency. Western blot analysis revealed that stimulation with PDMF triggered cleavage of caspase-9 and caspase-3 in comparison to the vehicle as a negative control (Fig. 5a, b, and c). We also found that PDMF induced cleavage of caspase-8 (Fig. 5a, d), another proapoptotic caspase involved in the death receptor-mediated induction of apoptosis upon cross-linking with TNF family member ligands (Elmore 2007). These results suggest that PDMF activates proapoptotic caspase cascades downstream of p53 and cell surface death receptors.
Fig. 5.
PDMF activates proapoptotic caspases in A549 cells. a The protein levels of cleaved caspase-9, caspase-3, and caspase-8 were analyzed by western blot analysis. GAPDH served as a loading control. b, c, and d, The relative protein levels of cleaved caspase-9, caspase-3, and caspase-8 were quantified upon normalization with those of GAPDH. The values are the mean ± SD of three different experiments. * P < 0.05 indicate significant differences compared with vehicle
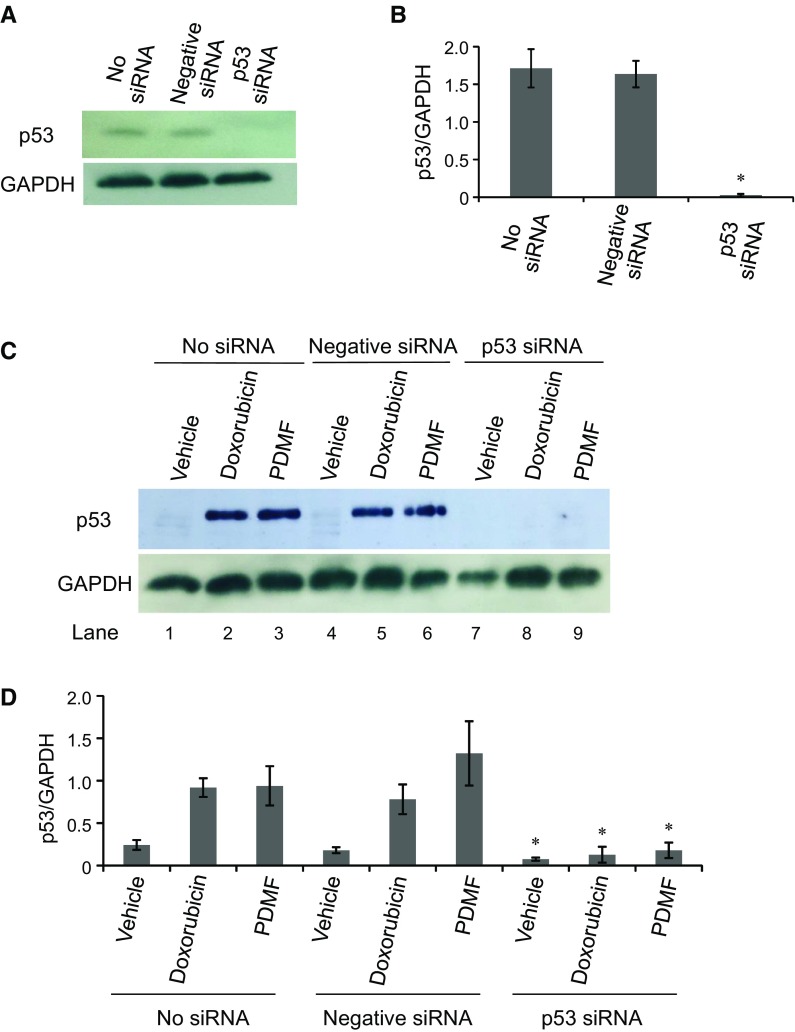
p53 is essential for PDMF-induced G2/M cell cycle arrest and apoptosis in A549 cells
To test whether p53 was needed for PDMF-driven G2/M cell cycle arrest and apoptosis, we knocked down p53 expression in A549 cells by siRNA transfection. We first confirmed that p53 protein expression was successfully suppressed upon transfection with p53 siRNA, but not with negative control siRNA (Fig. 6a, b). We also found that stimulation with PDMF or doxorubicin (a positive control anticancer agent for p53-driven cell cycle arrest and apoptosis) normally induced p53 protein expression in control A549 cells transfected with negative siRNA and sham-treated with no siRNA (Fig. 6c, d), while transfection with p53 siRNA fully abrogated induction of p53 protein upon stimulation with PDMF or doxorubicin (Fig. 6c, d). These results clearly indicate that our p53 knockdown experiment is successfully done with siRNA transfection, and that those experimental settings would offer an appropriate in vitro system to evaluate possible involvement of p53 in the carcinostatic effect of PDMF.
Fig. 6.
Successful siRNA knockdown of p53 in A549 cells. a and b Western blot analysis of p53 upon transfection with p53 siRNA. GAPDH served as a loading control, and relative protein expression levels of p53 were normalized by those of GAPDH. c and d, The protein levels of p53 and GAPDH in p53-silenced and PDMF (75 μg/ml)-stimulated A549 cells were analyzed by western blot analysis. Doxorubicin (580 ng/ml) was set as a positive control for induction of p53. The relative protein levels of p53 were normalized with those of GAPDH. The values are the mean ± SD of three different experiments. * P < 0.05 indicates a significant difference compared with each No siRNA
We next analyzed the impact of p53 knockdown on the G2/M cell cycle arrest of A549 cells upon stimulation with PDMF. As shown in Fig. 7a and b, transfection with p53 siRNA completely abolished the G2/M cell cycle arrest induced by PDMF. We also examined the effect of p53 knockdown on apoptosis induction of A549 cells upon stimulation with PDMF. Transfection with p53 siRNA substantially decreased the annexin V-positive apoptotic cells induced by PDMF (Fig. 7c, d), but the proapoptotic action of PDMF was not fully abrogated upon knockdown of p53. These results suggest that p53 is required for the induction of PDMF-driven G2/M cell cycle arrest in A549 cells, while the proapoptotic potency of PDMF was fulfilled in p53-dependent and -independent manners.
Fig. 7.
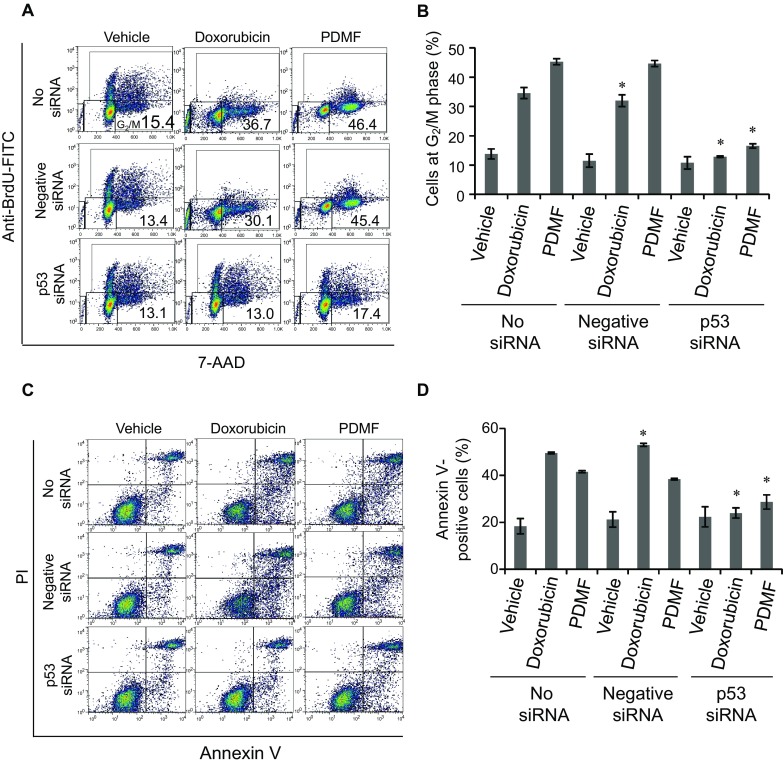
p53 is required for the PDMF-driven G2/M cell cycle arrest and apoptosis induction in A549 cells. a and b Knockdown of p53 completely abolishes the PDMF-mediated G2/M cell cycle arrest. Proportion of cells at G2/M phase upon p53 knockdown and 24 h incubation with PDMF (75 μg/ml) or doxorubicin (580 ng/ml, positive control) was analyzed by flow cytometry and quantified. c and d PDMF-induced apoptosis is substantially abrogated upon siRNA knockdown of p53. Apoptotic cells in p53-silenced and PDMF-stimulated A549 cells were analyzed by flow cytometry, and total annexin V-positive cells were quantified. The values are the mean ± SD of three different experiments. * P < 0.05 indicates a significant difference compared with each No siRNA
Discussion
Chinese herbal medicines exhibit effective anti-tumor activities with minimal toxicity and are a valuable source of novel chemotherapeutic agents (Li et al. 2015). P. frutescens is an active medicinal plant with various pharmacological effects on antitumor activity. Our group has recently discovered a new P. frutescens-derived methoxyflavanone derivative (8-hydroxy-5,7-dimethoxyflavanone), named Perilla-derived methoxyflavanone (PDMF), that directly suppresses Akt to inhibit type I hypersensitivity reactions (Kamei et al. 2017). Since Akt is a critical molecular target of carcinogenesis and tumor development (Manning and Cantley 2007), here we sought to investigate whether PDMF shows antitumor potency on A549 human lung adenocarcinoma and to elucidate its carcinostatic mechanisms.
Some plant-derived flavanones have carcinostatic activity on human cancer cells. Eriodictyol shows less carcinostatic capacity (IC50: >58 µg/ml) to B16 melanoma cells (Habtemariam and Dagne 2010) than that seen in PDMF against A549 cells. Naringenin may also have anti-tumor potential less than or comparable to PDMF (IC50: 27–238 µg/ml in hepatoma and breast cancer cell lines) (Arul and Subramanian 2013; Ayob et al. 2014). These evidences implicate that PDMF represents an additional flavanone derivative whose anti-tumor potency is superior or comparable to other known flavanones.
PDMF preferentially induced G2/M cell cycle arrest and a slight increase in sub-G1 cells. Corresponding to the significant increase in G2/M cells, population of cells at S phase was significantly and dominantly decreased upon treatment with more than 60 µg/ml PDMF compared with vehicle control (Fig. 2b). The cell cycle distribution of PDMF-treated A549 cells was similar to that of doxorubicin-treated cells (Fig. 2). These data imply a similar anti-proliferative action between PDMF and doxorubicin.
We showed that PDMF significantly inhibited tumor cell growth through induction of G2/M cell cycle arrest and apoptosis in A549 cells in a p53-dependent manner (Figs. 2, 3, 7). p53 plays a key role in cell cycle regulation and apoptosis. Activated p53 induces G1 and/or G2/M cell cycle arrest by activating its target genes, or by its direct negative regulation of corresponding cyclins and CDKs (Agarwal et al. 1998; Flatt et al. 2000). Although our data clearly show the PDMF-driven carcinostatic effects on A549 cells are mainly fulfilled by p53, detailed molecular mechanism underlying the p53-driven G2/M cell cycle arrest is largely unknown. Especially, involvement of p21Cip1/Waf1 on the G2/M arrest needs to be clarified in future studies. In addition, further analysis is needed to elucidate upstream molecular events by which PDMF activates p53. Such candidate molecules are ATM and ATR, that up-modulate p53 through phosphorylation on Ser15. Since ATM and ATR work as DNA damage sensors upstream of p53 (Siliciano et al. 1997; Tibbetts et al. 1999), to test whether PDMF induces DNA damage to activate p53 pathway is another important issue to be addressed.
We also have analyzed carcinostatic effect of PDMF on four human leukemia cell lines (CCRF-CEM, Jurkat, KG1a, and K-562 cells), but no effect is seen against these cells whose p53 is inactivated or deficient (data not shown). One possibility for this non-effectiveness is that the efficacy of PDMF is dependent on p53 status in cancer cells, since p53 is indeed essential for the anti-tumor effect of PDMF (Fig. 7). Further analysis on other human cancer cells is needed to confirm this possibility.
Induction of apoptosis is also important for the effective therapeutic strategy for cancer treatment (Riedl and Salvesen 2007; Ma et al. 2013; Srinivasan et al. 2009). Two convergent cascades have been reported to induce apoptosis: extrinsic pathway is executed by death receptor-initiated proapoptotic signals transduced via Fas/Fas ligand system and TNF family cytokines (Ashkenazi and Dixit 1999; Hengartner 2000), and the other intrinsic pathway is activated by genotoxic stresses leading to activation of caspase-9 (Hengartner 2000). Gain-of-function of p53 can activate both pathways, and cause apoptosis via caspase-3 activation (Kim et al. 2009; Amaral et al. 2010). A signaling pathway which links those two proapoptotic signaling events was also known as a cross-talk pathway (Chen et al. 2009; Cohen 1997). The PDMF-induced apoptosis in A549 cells is accompanied by activation of caspases-3, 8 and 9 in a dose-dependent manner (Fig. 5), suggesting that the PDMF-driven apoptotic action is fulfilled by both extrinsic and intrinsic signaling pathways. Our result that partial abrogation of the PDMF-induced apoptosis by knockdown of p53 (Fig. 7c, d) supports the idea that PDMF can also induce apoptosis via p53-independent proapoptotic pathways such as the death receptor-driven mechanisms.
PDMF induces G2/M cell cycle arrest and apoptosis in A549 cells in dose more than 45 μg/ml (IC50: 53.5 μg/ml, Fig. 1a, b). Although the dosages seem to be a little bit high for pharmaceutical use of chemotherapy to NSCLC, and could be decreased by use of chemical derivatives from PDMF. Another possible application of PDMF for cancer chemoprevention is to be used for prevention of NSCLC by oral intake of PDMF per se or PDMF-enriched P. frutescens extract. To further gain insight into the therapeutic and preventive effects by PDMF, additional in vivo experiments are needed to analyze the anti-tumor potency of PDMF and PDMF-derivatives. We are also trying to test the efficacy of low dose PDMF upon co-administration with established anti-cancer drugs. PDMF shows less cytotoxicity to human normal cells (WI-38 lung fibroblast) than to A549 cells (IC50 for WI-38: >75 µg/ml, data not shown), while this observation still warrants its possible side effect on normal cells. Thus, a decrease in PDMF dosage would be preferable for its ideal application to cancer prevention. Our preliminary study indicates that a combination treatment with low dose PDMF and anti-cancer tyrosine kinase inhibitors shows a synergistic tumor suppressive effect in vitro as well as in vivo (Abd El-Hafeez A.A., Fujimura T., and Kawamoto S., unpublished data). Further investigation is needed for the development of cancer preventive strategies using PDMF.
In summary, here we found that PDMF, a methoxyflavanone derivative from P. frutescens, showed a carcinostatic effect on A549 human lung adenocarcinoma through its action to tumor suppressor p53. Strikingly, the PDMF-driven G2/M cell cycle arrest and its proapoptotic potency is dependent on p53, suggesting that PDMF would be useful for prevention of carcinogenesis and/or elimination of pre-cancer lesions carrying wild type p53 alleles. These findings provide a new insight into the cancer chemoprevention by edible plant phytochemicals.
Acknowledgements
This work was financially supported by the Mishima Food Co., Ltd. N. Hirakawa and K. Baba are employees of the Mishima Food Co., Ltd. Abd El-Hafeez A. A. was supported by the Ministry of Education, Culture, Sports, Science, and Technology, MEXT, Japan.
Compliance with ethical standards
Conflict of interest
None of other authors have conflict of interest.
Footnotes
Amer Ali Abd El-Hafeez and Takashi Fujimura have contributed equally to this work.
References
- Agarwal ML, Agarwal A, Taylor WR, Chernova O, Sharma Y, Stark GR. A p53-dependent S-phase checkpoint helps to protect cells from DNA damage in response to starvation for pyrimidine nucleotides. Proc Natl Acad Sci USA. 1998;95:14775–14780. doi: 10.1073/pnas.95.25.14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral JD, Xavier JM, Steer CJ, Rodrigues CM. The role of p53 in apoptosis. Discov Med. 2010;9:145–152. [PubMed] [Google Scholar]
- Arul D, Subramanian P. Naringenin (citrus flavonone) induces growth inhibition, cell cycle arrest and apoptosis in human hepatocellular carcinoma cells. Pathol Oncol Res. 2013;19:763–770. doi: 10.1007/s12253-013-9641-1. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–260. doi: 10.1016/S0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- Ayob Z, Mohd Bohari SP, Abd Samad A, Jamil S. Cytotoxic activities against breast cancer cells of local justicia gendarussa crude extracts. Evid Based Complement Alternat Med. 2014;2014:732980. doi: 10.1155/2014/732980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzoli CG, Baker S, Jr, Temin S, Pao W, Aliff T, Brahmer J, Johnson DH, Laskin JL, Masters G, Milton D, Nordquist L, Pfister DG, Piantadosi S, Schiller JH, Smith R, Smith TJ, Strawn JR, Trent D, Giaccone G. American society of clinical oncology clinical practice guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol. 2009;27:6251–6266. doi: 10.1200/JCO.2009.23.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banno N, Akihisa T, Tokuda H, Yasukawa K, Higashihara H, Ukiya M, Watanabe K, Kimura Y, Hasegawa JI, Nishino H. Triterpene acids from the leaves of Perilla frutescens and their anti-inflammatory and antitumor-promoting effects. Biosci Biotechnol Biochem. 2004;68:85–90. doi: 10.1271/bbb.68.85. [DOI] [PubMed] [Google Scholar]
- Chen JC, Lu KW, Tsai ML, Hsu SC, Kuo CL, Yang JS, Hsia TC, Yu CS, Chou ST, Kao MC, Chung JG. Gypenosides induced G0/G1 arrest via CHk2 and apoptosis through endoplasmic reticulum stress and mitochondria-dependent pathways in human tongue cancer SCC-4 cells. Oral Oncol. 2009;45:273–283. doi: 10.1016/j.oraloncology.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-P. [DOI] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt PM, Tang LJ, Scatena CD, Szak ST, Pietenpol JA. p53 regulation of G2 checkpoint is retinoblastoma protein dependent. Mol Cell Biol. 2000;20:4210–4223. doi: 10.1128/MCB.20.12.4210-4223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habtemariam S, Dagne E. Comparative antioxidant, prooxidant and cytotoxic activity of sigmoidin A and eriodictyol. Planta Med. 2010;76:589–594. doi: 10.1055/s-0029-1240604. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Kamei R, Fujimura T, Matsuda M, Kakihara K, Hirakawa N, Baba K, Ono K, Arakawa K, Kawamoto S. A flavanone derivative from the Asian medicinal herb (Perilla frutescens) potently suppresses IgE-mediated immediate hypersensitivity reactions. Biochem Biophys Res Commun. 2017;483:674–679. doi: 10.1016/j.bbrc.2016.12.083. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Kim HK. Perilla leaf extract ameliorates obesity and dyslipidemia induced by high-fat diet. Phytother Res. 2009;23:1685–1690. doi: 10.1002/ptr.2811. [DOI] [PubMed] [Google Scholar]
- Kim MK, Lee HS, Kim EJ, Won NH, Chi YM, Kim BC, Lee KW. Protective effect of aqueous extract of Perilla frutescens on tert-butyl hydroperoxide-induced oxidative hepatotoxicity in rats. Food Chem Toxicol. 2007;45:1738–1744. doi: 10.1016/j.fct.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kim SH, Lee SC, Song YS. Involvement of both extrinsic and intrinsic apoptotic pathways in apoptosis induced by genistein in human cervical cancer cells. Ann N Y Acad Sci. 2009;1171:196–201. doi: 10.1111/j.1749-6632.2009.04902.x. [DOI] [PubMed] [Google Scholar]
- Kwak Y, Ju J. Inhibitory activities of Perilla frutescens britton leaf extract against the growth, migration, and adhesion of human cancer cells. Nutr Res Pract. 2015;9:11–16. doi: 10.4162/nrp.2015.9.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak CS, Yeo EJ, Moon SC, Kim YW, Ahn HJ, Park SC. Perilla leaf, Perilla frutescens, induces apoptosis and G1 phase arrest in human leukemia HL-60 cells through the combinations of death receptor-mediated, mitochondrial, and endoplasmic reticulum stress-induced pathways. J Med Food. 2009;12:508–517. doi: 10.1089/jmf.2008.1103. [DOI] [PubMed] [Google Scholar]
- Lehman TA, Bennett WP, Metcalf RA, Welsh JA, Ecker J, Modali RV, Ullrich S, Romano JW, Appella E, Testa JR, Gerwin BA, Harris C. p53 mutations, ras mutations, and p53-heat shock 70 protein complexes in human lung carcinoma cell lines. Cancer Res. 1991;51:4090–4096. [PubMed] [Google Scholar]
- Li M, Zhang F, Wang X, Wu X, Zhang B, Zhang N, Wu W, Wang Z, Weng H, Liu S, Gao G, Mu J, Shu Y, Bao R, Cao Y, Lu J, Gu J, Zhu J, Liu Y. Magnolol inhibits growth of gallbladder cancer cells through the p53 pathway. Cancer Sci. 2015;106:1341–1350. doi: 10.1111/cas.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CS, Kuo CL, Wang JP, Cheng JS, Huang ZW, Chen CF. Growth inhibitory and apoptosis inducing effect of Perilla frutescens extract on human hepatoma HepG2 cells. J Ethnopharmacol. 2007;112:557–567. doi: 10.1016/j.jep.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Ma GF, Chen SY, Sun ZR, Miao Q, Liu YM, Zeng XQ, Luo TC, Ma LL, Lian JJ, Song DL. FoxP3 inhibits proliferation and induces apoptosis of gastric cancer cells by activating the apoptotic signaling pathway. Biochem Biophys Res Commun. 2013;430:804–809. doi: 10.1016/j.bbrc.2012.11.065. [DOI] [PubMed] [Google Scholar]
- Makino T, Furuta Y, Fujii H, Nakagawa T, Wakushima H, Saito K, Kano Y. Effect of oral treatment of Perilla frutescens and its constituents on type-I allergy in mice. Biol Pharm Bull. 2001;24:1206–1209. doi: 10.1248/bpb.24.1206. [DOI] [PubMed] [Google Scholar]
- Makino T, Furuta Y, Wakushima H, Fujii H, Saito K, Kano Y. Anti-allergic effect of Perilla frutescens and its active constituents. Phytother Res. 2003;17:240–243. doi: 10.1002/ptr.1115. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng LH, Lozano YF, Gaydou EM, Li B. Antioxidant zctivities of polyphenols extracted from Perilla frutescens varieties. Molecules. 2009;14:133–140. doi: 10.3390/molecules14010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawara Y, Kishishita S, Obata T, Isazawa Y, Suzuki T, Tanaka K, Masuyama N, Gotoh Y. Akt enhances Mdm2-mediated ubiquitination and degradation of p53. J Biol Chem. 2002;277:21843–21850. doi: 10.1074/jbc.M109745200. [DOI] [PubMed] [Google Scholar]
- Osakabe N, Yasuda A, Natsume M, Sanbongi C, Kato Y, Osawa T, Yoshikawa T. Rosmarinic acid, a major polyphenolic component of Perilla frutescens, reduces lipopolysaccharide (LPS)-induced liver injury in D-Galactosamine (D-GalN)-sensitized mice. Free Radic Biol Med. 2002;33:798–806. doi: 10.1016/S0891-5849(02)00970-X. [DOI] [PubMed] [Google Scholar]
- Ridge CA, McErlean AM, Ginsberg MS. Epidemiology of lung cancer. Semin Intervent Radiol. 2013;30:93–98. doi: 10.1055/s-0033-1342949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl SJ, Salvesen GS. The apoptosome: signaling platform of cell death. Nat Rev Mol Cell Biol. 2007;8:405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- Sandler AB, Nemunaitis J, Denham C, von Pawel J, Cormier Y, Gatzemeier U, Mattson K, Manegold C. Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2000;18:122–130. doi: 10.1200/JCO.2000.18.1.122. [DOI] [PubMed] [Google Scholar]
- Schuler M, Bossy-Wetzel E, Goldstein JC, Fitzgerald P, Green DR. p53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. J Biol Chem. 2000;275:7337–7342. doi: 10.1074/jbc.275.10.7337. [DOI] [PubMed] [Google Scholar]
- Siliciano JD, Canman CE, Taya Y, Sakaguchi K, Appella E, Kastan MB. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 1997;11:3471–3481. doi: 10.1101/gad.11.24.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socinski MA. Update on taxanes in the first-line treatment of advanced non-small-cell lung cancer. Curr Oncol. 2014;21:e691–e703. doi: 10.3747/co.21.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Koduru S, Kumar R, Venguswamy G, Kyprianou N, Damodaran C. Diosgenin targets Akt-mediated prosurvival signaling in human breast cancer cells. Int J Cancer. 2009;125:961–967. doi: 10.1002/ijc.24419. [DOI] [PubMed] [Google Scholar]
- Takano H, Osakabe N, Sanbongi C, Yanagisawa R, Inoue KI, Yasuda A, Natsume M, Baba S, Ichiishi EI, Yoshikawa T. Extract of Perilla frutescens enriched for rosmarinic acid, a polyphenolic phytochemical, inhibits seasonal allergic rhinoconjunctivitis in humans. Exp Biol Med. 2004;229:247–254. doi: 10.1177/153537020422900305. [DOI] [PubMed] [Google Scholar]
- Tibbetts RS, Brumbaugh KM, Williams JM, Sarkaria JN, Cliby WA, Shieh SY, Taya Y, Prives C, Abraham RT. A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev. 1999;13:152–157. doi: 10.1101/gad.13.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Yamazaki M. Inhibition of tumor necrosis factor-alpha production by orally administering a Perilla leaf extract. Biosci Biotechnol Biochem. 1997;61:1292–1295. doi: 10.1271/bbb.61.1292. [DOI] [PubMed] [Google Scholar]
- Ueda H, Yamazaki M. Anti-inflammatory and anti-allergic actions by oral administration of a Perilla leaf extract in mice. Biosci Biotechnol Biochem. 2001;65:1673–1675. doi: 10.1271/bbb.65.1673. [DOI] [PubMed] [Google Scholar]
- Ueda H, Yamazaki C, Yamazaki M. Luteolin as an anti-inflammatory and anti-allergic constituent of Perilla frutescens. Biol Pharm Bull. 2002;25:1197–1202. doi: 10.1248/bpb.25.1197. [DOI] [PubMed] [Google Scholar]
- Ueda H, Yamazaki C, Yamazaki M. Inhibitory effect of Perilla leaf extract and luteolin on mouse skin tumor promotion. Biol Pharm Bull. 2003;26:560–563. doi: 10.1248/bpb.26.560. [DOI] [PubMed] [Google Scholar]