Abstract
Lysozyme from hen egg has been reported to possess an anti-inflammatory effect. However, little is known about its detailed mechanism. The mechanism of anti-inflammatory effect of lysozyme was examined in this study. When mouse macrophage-like cell line RAW264.7 cells and mouse peritoneal macrophages were activated with lipopolysaccharide (LPS) and then treated with lysozyme, the production of tumor necrosis factor-α and interleukin-6 was significantly suppressed. The effect was induced by suppressing the gene expression levels of both cytokines. Phagocytosis activity of peritoneal macrophages was not altered by the treatment with lysozyme, suggesting that lysozyme shows the anti-inflammatory effect without inhibiting the phagocytotic response of macrophages. In addition, lysozyme inhibited phosphorylation of c-jun N-terminal kinase (JNK) and was taken up by macrophages within 1 h after treatment of the cells with lysozyme. Overall results suggest that lysozyme is taken up intracellularly and suppresses LPS-induced inflammatory responses by inhibiting JNK phosphorylation.
Electronic supplementary material
The online version of this article (10.1007/s10616-017-0184-2) contains supplementary material, which is available to authorized users.
Keywords: Anti-inflammatory effect, Lysozyme, Peritoneal macrophages, Tumor necrosis factor-α, Interleukin-6
Introduction
In recent years, chronic inflammation closely related to the pathological base of chronic diseases such as cancer and lifestyle-related diseases has garnered attention (Coussens et al. 2013; Manabe 2011). Inflammation is a defensive reaction, which occurs when individuals are infected with pathogens such as viruses and bacteria. Leucocytes, such as macrophages and neutrophils, are related to inflammation. Macrophages are multifunctional leucocytes related to innate immunity and remove foreign substances such as bacteria and dead cells. In addition, lipopolysaccharide (LPS), a cell membrane-constituting component of gram-negative bacteria, is recognized by macrophages and promotes the release of various mediators that trigger inflammatory reactions (Kawai et al. 2001; Yamamoto et al. 2003; Sato et al. 2005). The recognition of LPS by macrophages is caused by its binding to Toll-like receptors (TLR) on the cell surface, which activates the cells and promotes the production of inflammatory mediators such as prostaglandins and inflammatory cytokines. Gene expression of inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), is also induced through the binding of the ligand to TLR and acts on the defense against infection. On the other hand, the overexpression of these genes causes rheumatoid arthritis, insulin resistance, and arteriosclerosis (Keffer et al. 1991; Wellen and Hotamisligil 2003; Xu et al. 2003).
Lysozyme is an anti-bacterial protein that breaks down bacterial cell walls. Hen egg white lysozyme is a basic protein (pI = 11) composed of a single polypeptide chain with 129 amino acid residues. Lysozyme is widely distributed in various biological fluids and tissues, such as tears, saliva, and respiratory secretions, and is also secreted by polymorphonuclear leukocytes (Jollès and Jollès 1984). Hen egg white lysozyme has been reported to promote antibody production by lymphocytes (Murakami et al. 1997; Sugahara et al. 2000). In addition, heat-treated lysozyme also enhances the anti-bacterial and immunostimulatory activity (Sugahara et al. 2002; Carrillo et al. 2014). Heat treatment in the processing step is considered to enhance immunostimulatory activity. Thus, we examined the health function of lysozyme derived from hen egg white in the immune system for application to functional foods.
Lysozyme is well known to exhibit the anti-inflammatory effect in addition to anti-bacterial and immunostimulatory activities (Ogundele 1998; Lee et al. 2015; Carrillo et al. 2016). Lysozyme has also been reported to attenuate inflammation in a porcine model of dextran sodium sulfate-induced colitis (Lee et al. 2009) and to suppress polyphosphate-mediated vascular inflammatory responses (Chung et al. 2016). However, the detailed mechanism of the anti-inflammatory effect of lysozyme is still unknown. Thus, we examined the mechanism of the anti-inflammatory effect of lysozyme derived from hen egg white on macrophages involved in inflammatory responses.
Materials and methods
Reagents
Lysozyme from chicken egg white (≥ 90%), Dulbecco’s modified Eagle’s medium (DMEM), RPMI 1640 medium, penicillin, streptomycin, fetal bovine serum (FBS), and LPS from Escherichia coli 026/B6 were products from Sigma-Aldrich (St. Louis, MO, USA). Goat anti-actin antibody and anti-goat IgG antibody labeled with horseradish peroxidase (HRP) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). HRP-labeled anti-rabbit IgG antibody and rabbit antibodies against extracellular signal-regulated protein kinases (ERK) 1/2, phosphorylated ERK1/2, c-Jun N-terminal kinase (JNK), phosphorylated JNK, p38 mitogen-activated protein kinase (MAPK), and phosphorylated p38 MAPK were purchased from Cell Signaling Technology (Danvers, MA, USA). All other chemicals were purchased from Wako Pure Chemical Industries (Osaka, Japan) or Nacalai Tesque (Kyoto, Japan) unless otherwise noted.
Peritoneal macrophages
Peritoneal macrophages were prepared as previously described (Putra et al. 2012) with some modifications. In brief, 8-week-old female BALB/c mice (Japan SLC, Shizuoka, Japan) were injected with 3.0% thioglycollate medium (2 mL/body) into the peritoneum. Four days after injection, mice were sacrificed and injected with 3 mL of RPMI 1640 medium into the peritoneum to harvest thioglycollate-elicited peritoneal macrophages. Collected cells were centrifuged at 160×g for 5 min at 4 °C, and the cell pellet was washed with RPMI 1640 medium and centrifuged again. The cell pellet was then resuspended in RPMI 1640 medium supplemented with 100 U/mL of penicillin, 100 µg/mL of streptomycin, and 10% FBS and cultured in a culture dish (Corning, Corning, NY, USA). After incubation at 37 °C for 1 h, the cells were washed with phosphate-buffered saline (PBS, pH 7.4) three times to remove unattached cells such as neutrophils. In the subsequent experiments, peritoneal macrophages were detached by pipetting in cold PBS. All animals were maintained and examined according to the protocol approved by the Animal Care and Use Committee of Ehime University.
RAW264.7 cells
Mouse macrophage-like cell line RAW264.7 cells were obtained from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan). RAW264.7 cells were cultured in DMEM supplemented with 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 10% FBS at 37 °C under humidified 5% CO2. RAW264.7 cells were detached using PBS containing 0.25% trypsin and 0.02% ethylenediamine-N,N,N′,N′-tetraacetic acid (Dojindo Laboratories, Kumamoto, Japan) for the subsequent experiments.
Cytokine production assay
Lysozyme was dissolved in 10 mM sodium phosphate buffer (pH 7.4) and sterilized by filtration. Peritoneal macrophages suspended in 10% FBS-RPMI 1640 medium or RAW264.7 cells suspended in 10% FBS-DMEM were seeded into a 96-well culture plate (Corning) at 6.0 × 104 cells/well and cultured at 37 °C overnight under humidified 5% CO2. After washing with PBS, peritoneal macrophages were pretreated with 100 ng/mL of LPS in 0.2 mL of 10% FBS-RPMI 1640 medium at 37 °C, whereas RAW264.7 cells were pretreated with 100 ng/mL of LPS in 0.2 mL of 10% FBS-DMEM. After incubation for 1 h, the cells were washed with PBS to remove LPS. Peritoneal macrophages were then treated with various concentrations of lysozyme in 0.2 mL of 10% FBS-RPMI 1640 medium at 37 °C, whereas RAW264.7 cells were treated with various concentrations of lysozyme in 0.2 mL of 10% FBS-DMEM. After incubation for 11 h, the concentrations of IL-6 and TNF-α in culture media were measured by enzyme-linked immunosorbent assay (ELISA) using mouse IL-6 ELISA MAX Standard (BioLegend, San Diego, CA, USA) and mouse TNFα ELISA Ready-SET-GO! (eBioscience, San Diego, CA, USA), respectively, according to the manufacturer’s instructions.
Cell viability
Cytotoxicity of lysozyme to peritoneal macrophages and RAW264.7 cells was examined using a WST-8 assay kit (Nacalai Tesque) according to the manufacturer’s instructions. Peritoneal macrophages suspended in 10% FBS-RPMI 1640 medium or RAW264.7 cells suspended in 10% FBS-DMEM were seeded into a 96-well culture plate at 6.0 × 104 cells/well and cultured at 37 °C overnight under humidified 5% CO2. After washing with PBS, peritoneal macrophages were pretreated with 100 ng/mL of LPS in 0.2 mL of 10% FBS-RPMI 1640 medium at 37 °C, whereas RAW264.7 cells were pretreated with 100 ng/mL of LPS in 0.2 mL of 10% FBS-DMEM. After incubation for 1 h, peritoneal macrophages were washed with PBS and treated with various concentrations of lysozyme in 0.2 mL of 10% FBS-RPMI 1640 medium at 37 °C, whereas RAW264.7 cells were treated with various concentrations of lysozyme in 0.2 mL of 10% FBS-DMEM. After incubation for 11 h, the culture media were removed and peritoneal macrophages were cultured in 100 μL of 10% FBS-RPMI 1640 medium containing 10% WST-8 solution for 40 min at 37 °C under dark condition, whereas RAW264.7 cells were cultured in 100 μL of 10% FBS-DMEM containing 10% WST-8 solution for 15 min. The absorbance was then measured at 450 nm using a microplate reader.
Real-time RT-PCR
Peritoneal macrophages suspended in 10% FBS-RPMI 1640 medium were seeded into a 24-well cell culture plate at 5.0 × 105 cells/well and cultured at 37 °C overnight under humidified 5% CO2. After washing with PBS, the cells were pretreated with 100 ng/mL of LPS in 1.0 mL of 10% FBS-RPMI 1640 medium at 37 °C. After incubation for 1 h, the cells were washed with PBS and treated with 500 μg/mL of lysozyme in 1.0 mL of 10% FBS-RPMI 1640 medium at 37 °C. After incubation for 5 h or 11 h, total RNA was isolated from the cells using Sepasol-RNA I Super G (Nacalai Tesque) according to the manufacturer’s instructions and used as a template for cDNA synthesis with MMLV-reverse transcriptase (Promega, Madison, WI, USA) and an oligo-(dT)20 primer (Toyobo, Osaka, Japan). Real-time PCR was performed using Thunderbird SYBR qPCR Mix (Toyobo), 10 pmol of a forward primer, 10 pmol of a reverse primer, and 0.1 μg of a cDNA sample as previously described (Nishi et al. 2011) with some modifications. Thermal cycling conditions were 20 s at 95 °C, and 40 cycles of 3 s at 95 °C and 30 s at 60 °C. PCR products were measured on a StepOnePlus Real-time PCR System (Applied Biosystems, Foster City, CA, USA), and relative gene expression was calculated based on the comparative CT method using StepOne Software v2.1 (Applied Biosystems). Expression of the β-actin gene was used as an endogenous control. Specific oligonucleotide sequences for each gene are as follows. Mouse β-actin: sense, 5′-CATCCGTAAAGACCTCTATGCCAAC-3′ and antisense, 5′-ATGGAGCCACCGATCCACA-3′; mouse IL-6: sense, 5′-AAGCCAGAGTCCTTCAGAGAGAT-3′ and antisense, 5′-TTGGATGGTCTTGGTCCTTAGC-3′; mouse TNF-α: sense, 5′-CTACTCCCAGGTTCTCTTCAA-3′ and antisense, 5′-GCAGAGAGGAGGTTGACTTTC-3′.
Phagocytosis activity
Phagocytosis activity was measured as previously described (Putra et al. 2012) with some modifications. Peritoneal macrophages suspended in 10% FBS-RPMI 1640 medium were seeded into a 48-well cell culture plate at 2.5 × 105 cells/well and cultured at 37 °C under humidified 5% CO2. After incubation for 3 h, the cells were pretreated with 100 ng/mL of LPS in 0.5 mL of 10% FBS-RPMI 1640 medium. After incubation at 37 °C for 1 h, the cells were washed with PBS and treated with 500 μg/mL of lysozyme in 0.5 mL of 10% FBS-RPMI 1640 medium for 5 h or 11 h. After washing with PBS, 0.5 mL of 10% FBS-RPMI 1640 medium containing 500 µg of Texas Red-conjugated zymosan A (Saccharomyces cerevisiae) BioParticles (Molecular Probes, Eugene, OR, USA) was added to each well and incubated for 1 h under dark condition. After removing the culture medium, the cells were collected in PBS and centrifuged at 160×g for 5 min at 4 °C. The cell pellet was suspended in 1 mL of 2% FBS-PBS, and phagocytosis activity was measured on a flow cytometer (FACSCalibur; BD Biosciences, San Jose, CA, USA).
Confocal microscopy
Lysozyme was directly labeled with fluorescein isothiocyanate (FITC) using a FluoroTag FITC conjugation kit (Sigma-Aldrich). Lysozyme (5.5 mg) was dissolved in 11 mL of 0.1 M carbonate-bicarbonate buffer (pH 9.0) at room temperature, while the FITC isomer I in the kit was dissolved in 0.1 M carbonate buffer at 1 mg/mL. FITC isomer I solution (300 μL) was then dropped into the lysozyme solution and incubated for 2 h at room temperature with gentle shaking. The mixed solution was applied to a PD-10 desalting column (GE Healthcare, Piscataway, NJ, USA), and the eluate was dialyzed against 10 mM sodium phosphate buffer overnight. Peritoneal macrophages and RAW264.7 cells seeded into a culture dish containing a cover glass at 1.0 × 105 cells/dish for 12 h were pretreated with 100 ng/mL of LPS in 2 mL of 10% FBS-RPMI 1640 medium and in 2 mL of 10% FBS-DMEM, respectively, for 1 h. Peritoneal macrophages and RAW264.7 cells were then treated with 500 μg/mL of FITC-labeled lysozyme diluted in 10% FBS-RPMI 1640 medium and in 10% FBS-DMEM, respectively, in a CO2 incubator at 37 °C. After incubation for 1 h, the cells were washed with PBS three times and fixed with 4% paraformaldehyde in PBS for 15 min at room temperature. After fixing, the cells were washed with PBS three times, and the cover glass was mounted on a slide glass. Images were taken using a confocal laser scanning microscope FV10i-DOC (Olympus, Tokyo, Japan).
Immunoblot analysis
Peritoneal macrophages suspended in 10% FBS-RPMI 1640 medium were seeded into a 35-mm culture dish (Corning) at 5.0 × 105 cells/dish and cultured at 37 °C overnight under humidified 5% CO2. After washing with PBS, the cells were pretreated with 100 ng/mL of LPS in 2 mL of 10% FBS-RPMI 1640 medium at 37 °C. After incubation for 15 min, the cells were washed with PBS and treated with 500 µg/mL of lysozyme in 2 mL of 10% FBS-RPMI 1640 medium at 37 °C. After incubation for 30 min, cytosolic proteins were prepared using a CelLytic NuCLEAR Extraction Kit (Sigma-Aldrich) according to the manufacturer’s instructions. Denatured proteins were then separated using SDS-PAGE and transferred onto a PVDF membrane (Hybond-P; GE Healthcare, Buckinghamshire, UK). Immunoblotting with various antibodies was performed as previously described (Kanda et al. 2012).
Statistical analysis
Data obtained were expressed as mean ± standard deviation. Student’s t test or one-way ANOVA followed by Dunnett’s test or Tukey–Kramer test was used to assess the statistical significance of the difference. Values with *p < 0.05 or **p < 0.01 were considered statistically significant.
Results and discussion
Effect of lysozyme on inflammatory cytokine production
In this study, we used RAW264.7 cells and peritoneal macrophages. The purity of peritoneal macrophages was around 80% (Supplementary data), which is a similar percentage to other papers using the same method to collect peritoneal macrophages (Ding et al. 1988; Vodovotz et al. 1993; Schindler et al. 2001). It has been reported that lysozyme binds to LPS to form a complex and inhibits inflammatory reaction (Takada et al. 1994). In this study, peritoneal macrophages and RAW264.7 cells were thus cultured in a culture medium containing LPS to induce inflammatory responses, and LPS was washed away. The anti-inflammatory effect of lysozyme on activated macrophages was then examined. First, the effect of lysozyme on cytokine production by peritoneal macrophages and RAW264.7 cells was examined. After pretreating with 100 ng/mL of LPS for 1 h, peritoneal macrophages and RAW264.7 cells were treated with lysozyme at various concentrations for 11 h, and the cytokine concentration in the medium was measured using ELISA. As shown in Fig. 1, lysozyme significantly inhibited the production of IL-6 and TNF-α in dose-dependent manners. When RAW264.7 cells and peritoneal macrophages were treated with 500 µg/mL of lysozyme, the production of IL-6 and TNF-α was suppressed by around 50% compared with control (Fig. 1). The cytotoxicity of lysozyme was evaluated using the WST-8 assay. The result showed that lysozyme has no cytotoxicity to peritoneal macrophages or RAW264.7 cells even at 1000 µg/mL (Fig. 1). From these results, further experiments were performed at 500 µg/mL of lysozyme.
Fig. 1.
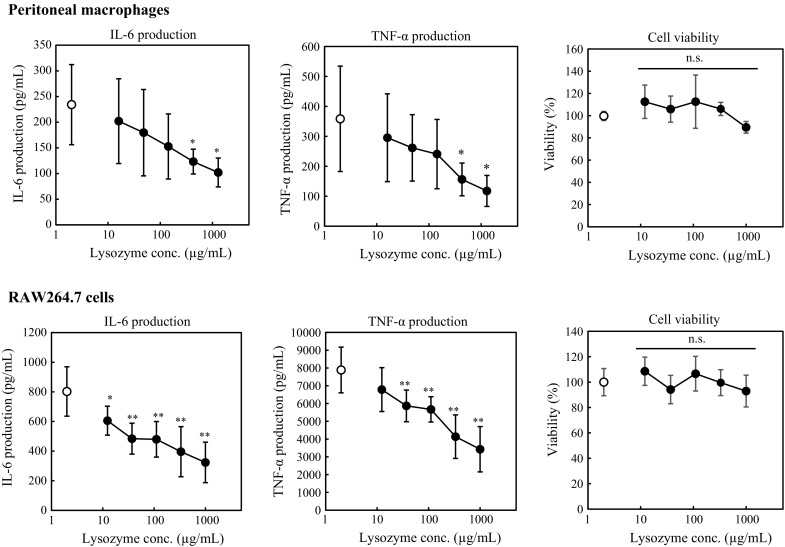
Effect of lysozyme on cytokine production and cell viability of peritoneal macrophages and RAW264.7 cells. For cytokine production assay, peritoneal macrophages and RAW264.7 cells were pretreated with 100 ng/mL of LPS. After washing, the cells were treated with various concentrations of lysozyme or 10 mM sodium phosphate buffer (control; open circle). After incubation for 11 h, the concentrations of IL-6 and TNF-α in the culture medium were measured. Data are represented as mean ± standard deviations (n = 6). *p < 0.05 or **p < 0.01 against control by Dunnett’s test. For cell viability assay, peritoneal macrophages and RAW264.7 cells were pretreated with 100 ng/mL of LPS. After washing, the cells were treated with various concentrations of lysozyme or 10 mM sodium phosphate buffer (control; open circle). After incubation for 11 h, cell viability was measured using a WST-8 assay kit. Data are represented as mean ± standard deviations (n = 9). n.s. indicates no statistical significance against control by Dunnett’s test
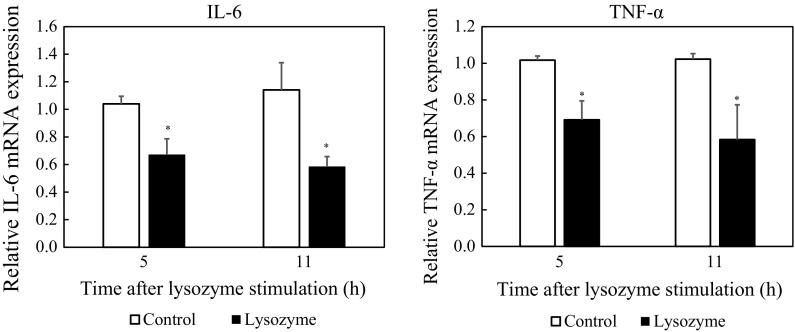
Effect of lysozyme on cytokine gene expression
As described above, lysozyme inhibited cytokine production by peritoneal macrophages and RAW264.7 cells without cytotoxicity. Therefore, the effect of lysozyme on cytokine gene expression was examined. After pretreatment with 100 ng/mL of LPS for 1 h, peritoneal macrophages were treated with lysozyme at 500 µg/mL for 11 h. After that, the transcription level of the cytokine genes was evaluated using real-time RT-PCR. As indicated in Fig. 2, the expression of IL-6 and TNF-α genes was significantly inhibited by lysozyme. In addition, it was examined whether the suppressive effect of lysozyme on gene expression was observed in a shorter period. Expression of IL-6 and TNF-α genes was significantly suppressed by treating the cells with lysozyme for 5 h. These results indicated that lysozyme downregulates the expression of IL-6 and TNF-α genes within 5 h after treatment, resulting in suppressed production of IL-6 and TNF-α.
Fig. 2.
Effect of lysozyme on transcription of cytokine genes in peritoneal macrophages. Peritoneal macrophages were pretreated with 100 ng/mL of LPS for 1 h. After washing, the cells were treated with 500 µg/mL of lysozyme or 10 mM sodium phosphate buffer (control) for 5 or 11 h. The mRNA levels of IL-6 and TNF-α genes were evaluated using real-time RT-PCR. Data are represented as mean ± standard deviations of three independent experiments. *p < 0.05 against control by Student’s t test
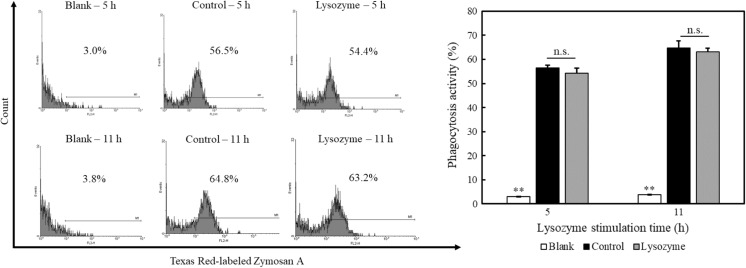
Effect of lysozyme on phagocytotic activity
Macrophages have an important role in innate immunity such as destroying microorganisms, ingesting foreign materials, removing dead cells, and enhancing immune responses. We thus examined the effect of lysozyme on the phagocytosis activity of peritoneal macrophages using Texas Red-labeled zymosan A. Peritoneal macrophages were treated with 500 µg/mL of lysozyme for 5 h or 11 h after pretreating with 100 ng/mL of LPS for 1 h. The cells were then treated with Texas Red-labeled zymosan A for 1 h. As shown in Fig. 3, the zymosan A-mediated phagocytosis activity of the peritoneal macrophages was not affected by lysozyme compared with control. Thus, lysozyme inhibited LPS-induced cytokine production by peritoneal macrophages, but did not modulate phagocytotic activity. These results suggested that lysozyme shows an anti-inflammatory effect without inhibiting the innate immune response by macrophages.
Fig. 3.
Effect of lysozyme on phagocytotic activity of peritoneal macrophages. Peritoneal macrophages were pretreated with 100 ng/mL of LPS for 1 h. After washing, the cells were treated with 500 µg/mL of lysozyme or 10 mM sodium phosphate buffer (control) for 5 or 11 h. After washing, the cells were treated with or without Texas Red-labeled zymosan A for 1 h under dark condition, and the phagocytotic activity was measured on a flow cytometer. A representative histogram from three independent experiments is shown. Data are represented as mean ± standard deviations (n = 3). **p < 0.01 against control by Tukey–Kramer test. n.s. indicates no statistical significance against control
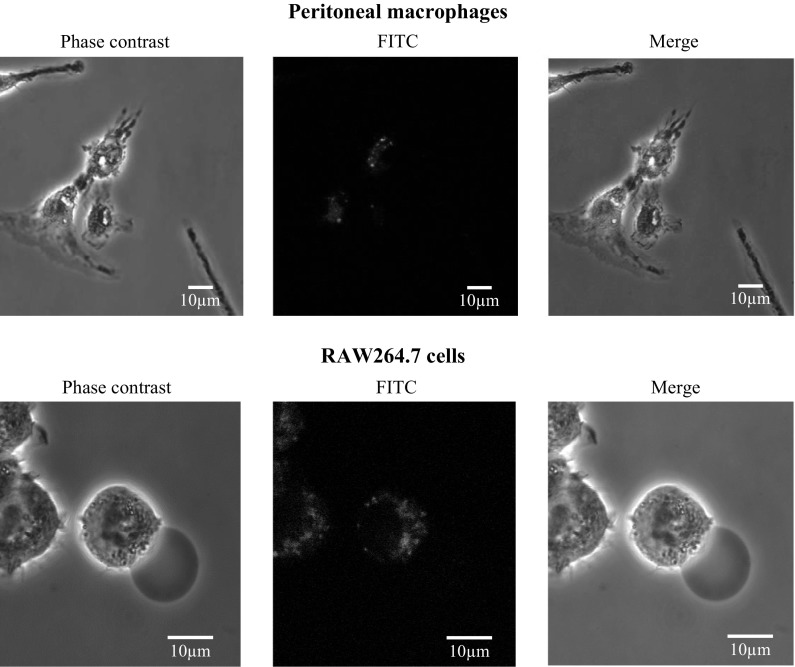
The uptake of lysozyme by macrophages
We have previously reported that lysozyme is taken up by human hybridoma HB4C5 cells to act as an immunoglobulin production stimulating factor (Sugahara et al. 2000). Therefore, we examined whether lysozyme is taken up into macrophages to suppress inflammatory cytokine production. Peritoneal macrophages and RAW264.7 cells were pretreated with 100 ng/mL of LPS for 1 h and treated with FITC-labeled lysozyme for 1 h. As shown in Fig. 4, the uptake of lysozyme by macrophages was observed. This result implied that lysozyme suppresses the production of inflammatory cytokines induced by LPS after being taken up into macrophages.
Fig. 4.
Internalization of lysozyme. Peritoneal macrophages and RAW264.7 cells were pretreated with 100 ng/mL of LPS at 37 °C for 1 h. After washing, the cells were treated with FITC-labeled lysozyme for 1 h. After washing and fixing with 4% paraformaldehyde, images were taken using a confocal laser scanning microscope
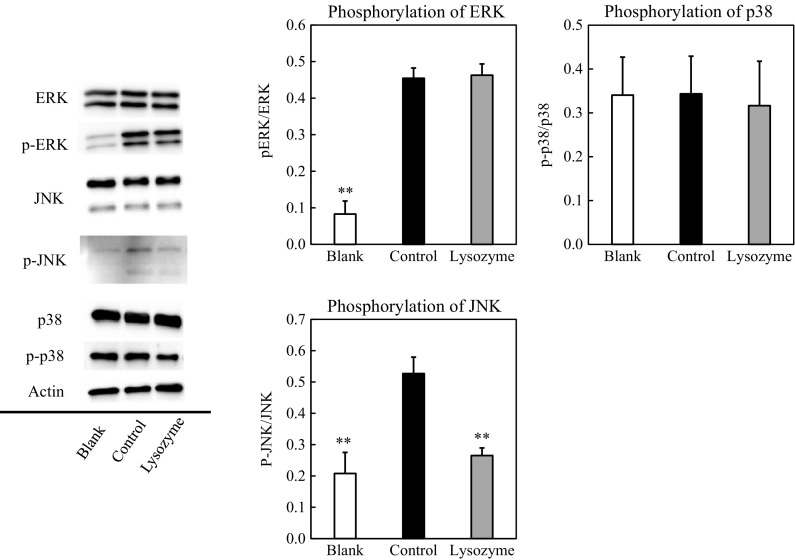
Effect of lysozyme on the signaling pathways in macrophages
Macrophages induce the expression of inflammatory cytokine genes by activating the MAPK cascades. LPS activates MAPK signaling to facilitate inflammatory cytokine production. The effect of lysozyme on MAPK signaling was then examined. Peritoneal macrophages were treated with 500 μg/mL of lysozyme for 30 min after pretreating with 100 ng/mL of LPS for 15 min, and the cytosolic protein levels of the signal molecules were evaluated by immunoblot analysis. As summarized in Fig. 5, the phosphorylation level of JNK was inhibited by lysozyme, whereas that of ERK or p38 was not affected. JNK, one of the MAPK family protein kinases, is involved in cellular responses such as environmental stress, inflammatory cytokines, mitogen stimulation, and apoptosis (Kyriakis and Avruch 1996). Once JNK is phosphorylated and activated, JNK translocates into the nucleus and phosphorylates transcription factors ATF2 and c-Jun. They bind to the cyclic AMP response element response sequence and induce the expression of various inflammatory cytokine genes. As shown in Fig. 5, these results revealed that lysozyme inhibits inflammatory cytokine production through inhibiting the phosphorylation of JNK.
Fig. 5.
Effect of lysozyme on signaling pathways involved in macrophage activation. Peritoneal macrophages were pretreated with 100 ng/mL of LPS or with 10 mM sodium phosphate buffer (blank) for 15 min. After washing, the cells were treated with 500 µg/mL of lysozyme or 10 mM sodium phosphate buffer (blank, control) for 30 min. The protein levels of ERK, JNK, and p38 were then evaluated using immunoblot analysis. The p-ERK, p-JNK, and p-p38 represent phosphorylated ERK, phosphorylated JNK, and phosphorylated p38, respectively. A representative blot from three independent experiments is shown. The result of densitometric analysis is expressed as the ratio of (phosphorylated JNK protein amount)/(whole JNK protein amount). Data are represented as mean ± standard deviations of three independent experiments. *p < 0.01 against control by Tukey–Kramer test
There are various proteins with the anti-inflammatory activity other than lysozyme. For example, lactoferrin has been reported to have anti-inflammatory activity. The results of this study are thus considered specific effects by lysozyme because we used lysozyme with a purity of over 90%. Ibrahim et al. (2017) reported that several peptides derived from human lysozyme bind to Toll like receptor (TLR) 4 to show an antagonistic anti-inflammatory effect. On the other hand, LPS was washed away with PBS before the cells were treated with lysozyme in this study, suggesting that the anti-inflammatory effect of lysozyme observed in this study would not be due to its antagonism by TLR4. We found that lysozyme is taken up to macrophages (Fig. 4) and inhibits phosphorylation of JNK (Fig. 5). Because JNK expression in macrophages has been reported to promote obesity-induced insulin resistance and inflammation (Han et al. 2013), lysozyme might suppress insulin resistance-related inflammation by acting on macrophages after being absorbed from intestinal lumen to blood in vivo as functional foods.
Macromolecules, such as proteins, are not readily absorbed as an intact form from the intestinal lumen when ingested orally. However, it is known that some intact macromolecules are absorbed from the intestinal lumen to the blood; lysozyme is one of such molecules absorbed in an intact form in the proximal intestinal tract (Yokooji et al. 2013). Thus, it can be considered that lysozyme that has avoided being degraded by proteases in digestive tract could be absorbed as an intact form and shows anti-inflammatory effect in vivo. In fact, it has been reported that oral intake of lysozyme is capable of exerting its pharmacological activity (Ohbayashi et al. 2016), suggesting that part of orally administered lysozyme reaches the intestine as an intact form and exerts its anti-inflammatory activity.
Conclusion
Although lysozyme is well known to exhibit the anti-inflammatory effect, the detailed mechanism of its effect is still unknown. In this study, we found that lysozyme is taken up and inhibits phosphorylation of JNK to suppress pro-inflammatory cytokines. Taken together, our data indicate that lysozyme exerts the anti-inflammatory effect on macrophages by another mechanism in addition to its antagonistic anti-inflammatory effect.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by a JSPS KAKENHI Grant-in-Aid for Scientific Research C (15K07432). Animal experiments were accomplished at the Division of Genetic Research of the Advanced Research Support Center (ADRES), Ehime University.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s10616-017-0184-2) contains supplementary material, which is available to authorized users.
References
- Carrillo W, García-Ruiz A, Recio I, Moreno-Arribas MV. Antibacterial activity of hen egg white lysozyme modified by heat and enzymatic treatments against oenological lactic acid bacteria and acetic acid bacteria. J Food Prot. 2014;77:1732–1739. doi: 10.4315/0362-028X.JFP-14-009. [DOI] [PubMed] [Google Scholar]
- Carrillo W, Spindola H, Ramos M, Recio I, Carvalho JE. Anti-inflammatory and anti-nociceptive activities of native and modified hen egg white lysozyme. J Med Food. 2016;19:978–982. doi: 10.1089/jmf.2015.0141. [DOI] [PubMed] [Google Scholar]
- Chung J, Ku SK, Lee S, Bae JS. Suppressive effects of lysozyme on polyphosphate-mediated vascular inflammatory responses. Biochem Biophys Res Commun. 2016;474:715–721. doi: 10.1016/j.bbrc.2016.05.016. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- Han SM, Jung YD, Morel C, Lakhani AS, Kim KJ, Flavell AR, Davis JR. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339:218–222. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim HR, Hamasaki K, Miyata T. Novel peptide motifs from lysozyme suppress pro-inflammatory cytokines in macrophages by antagonizing toll-like receptor and LPS-scavenging action. Eur J Pham Sci. 2017;107:240–248. doi: 10.1016/j.ejps.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Jollès P, Jollès J. What’s new in lysozyme research? Always a model system, today as yesterday. Mol Cell Biochem. 1984;63:165–189. doi: 10.1007/BF00285225. [DOI] [PubMed] [Google Scholar]
- Kanda K, Nishi K, Kadota A, Nishimoto S, Liu MC, Sugahara T. Nobiletin suppresses adipocyte differentiation of 3T3-L1 cells by an insulin and IBMX mixture induction. Biochim Biophys Acta. 2012;1820:461–468. doi: 10.1016/j.bbagen.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Kawai T, Takeuchi O, Fujita T, Inoue J, Mühlradt PF, Sato S, Hoshino K, Akira S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol. 2001;167:5887–5894. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, Kollias G. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991;10:4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis MJ, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. BioEssays. 1996;18:567–577. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- Lee M, Kovacs-Nolan J, Yang C, Archbold T, Fan MZ, Mine Y. Hen egg lysozyme attenuates inflammation and modulates local gene expression in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J Agric Food Chem. 2009;57:2233–2240. doi: 10.1021/jf803133b. [DOI] [PubMed] [Google Scholar]
- Lee W, Ku SK, Na DH, Bae JS. Anti-inflammatory effects of lysozyme against HMGB1 in human endothelial cells and in mice. Inflammation. 2015;38:1911–1924. doi: 10.1007/s10753-015-0171-8. [DOI] [PubMed] [Google Scholar]
- Manabe I. Chronic inflammation links cardiovascular, metabolic and renal diseases. Circ J. 2011;75:2739–2748. doi: 10.1253/circj.CJ-11-1184. [DOI] [PubMed] [Google Scholar]
- Murakami F, Sasaki T, Sugahara T. Lysozyme stimulates immunoglobulin production by human-human hybridoma and human peripheral blood lymphocytes. Cytotechnology. 1997;24:177–182. doi: 10.1023/A:1007936629501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi K, Kondo A, Okamoto T, Nakano H, Daifuku M, Nishimoto S, Ochi K, Takaoka T, Sugahara T. Immunostimulatory in vitro and in vivo effects of a water-soluble extract from kale. Biosci Biotechnol Biochem. 2011;75:40–46. doi: 10.1271/bbb.100490. [DOI] [PubMed] [Google Scholar]
- Ogundele MO. A novel anti-inflammatory activity of lysozyme: modulation of serum complement activation. Mediators Inflamm. 1998;7:363–365. doi: 10.1080/09629359890893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohbayashi H, Setoguchi Y, Fukuchi Y, Shibata K, Sakata Y, Arai T. Pharmacological effects of lysozyme on COPD and bronchial asthma with sputum: a randomized, placebo-controlled, small cohort, cross-over study. Pulm Pharmacol Ther. 2016;37:73–80. doi: 10.1016/j.pupt.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Putra ABN, Morishige H, Nishimoto S, Nishi K, Shiraishi R, Doi M, Sugahara T. Effect of collagens from jellyfish and bovine Achilles tendon on the activity of J774.1 and mouse peritoneal macrophage cells. J Funct Foods. 2012;4:504–512. doi: 10.1016/j.jff.2012.02.011. [DOI] [Google Scholar]
- Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- Schindler H, Lutz MB, Röllinghoff M, Bogdan C. The production of IFN-gamma by IL-12/IL-18-activated macrophages requires STAT4 signaling and is inhibited by IL-4. J Immunol. 2001;166:3075–3082. doi: 10.4049/jimmunol.166.5.3075. [DOI] [PubMed] [Google Scholar]
- Sugahara T, Murakami F, Yamada Y, Sasaki T. The mode of actions of lysozyme as an immunoglobulin production stimulating factor. Biochim Biophys Acta. 2000;1475:27–34. doi: 10.1016/S0304-4165(00)00041-6. [DOI] [PubMed] [Google Scholar]
- Sugahara T, Yamada Y, Yano S, Sasaki T. Heat denaturation enhanced immunoglobulin production stimulating activity of lysozyme from hen egg white. Biochim Biophys Acta. 2002;1572:19–24. doi: 10.1016/S0304-4165(02)00272-6. [DOI] [PubMed] [Google Scholar]
- Takada K, Ohno N, Yadomae T. Detoxification of lipopolysaccharide (LPS) by egg white lysozyme. FEMS Immunol Med Microbiol. 1994;9:255–263. doi: 10.1111/j.1574-695X.1994.tb00360.x. [DOI] [PubMed] [Google Scholar]
- Vodovotz Y, Bogdan C, Paik J, Xie QW, Nathan C. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor β. J Exp Med. 1993;178:605–613. doi: 10.1084/jem.178.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI200319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol. 2003;4:1144–1150. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- Yokooji T, Hamura K, Matsuo H. Intestinal absorption of lysozyme, an egg-white allergen, in rats: kinetics and effect of NSAIDs. Biochem Biophys Res Commun. 2013;438:61–65. doi: 10.1016/j.bbrc.2013.07.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.