Abstract
Vitis vinifera cv. Koshu is an indigenous cultivar in Japan and has several characteristics that distinguish it from European V. vinifera. In Japan, Koshu is the most popular cultivar for wine making. We report herein a cell culture established from Koshu for use as a system for the production of resveratrol and its derivatives. Grape cell culture YU-1 was developed from the apex tissues of Koshu. YU-1 growth was favorably compared with BY-2 growth, a standard cell line in plant cell biology. Stilbene production and stilbene synthesis gene expression in YU-1 were upregulated by UV-C irradiation. YU-1 irradiated with UV-C decreased hemolymph sugar levels in model animals. Taken together, this study suggests that YU-1 may be used as a source of valuable medicinal components in plant cell bioreactor systems.
Keywords: Antidiabetic, Hypoglycemic effect, Koshu, Resveratrol, Vitis vinifera
Introduction
Vitis vinifera cv. Koshu is an indigenous cultivar that is thought to have been cultivated in Japan since the 12th century (Fujita et al. 2009). Koshu belongs to the European domesticated grape species V. vinifera and is 70% V. vinifera and that the remaining 30% is derived from the Chinese wild species V. davidii or a closely related species (Goto-Yamamoto et al. 2015). Koshu grape juice has higher total phenolic content, such as hydroxycinnamic acid and monomeric flavonol contents, than juice from other white V. vinifera cultivars (Kobayashi et al. 2011). Morphologically, Koshu differs from major European grape cultivars, such as Cabernet Sauvignon and Chardonnay (Shimazaki et al. 2011). The cluster and berry size of Koshu are larger than those of European cultivars and berry skin color is pink. Koshu vines grow vigorously and because of this, the traditional shelf style (overhead trellis) is used. Thus, Koshu is one of unique bioresources in V. vinifera population.
Grape cell cultures are an alternative system for the production of plant secondary metabolites under various controlled environments. Grape cell cultures have been studied as a means of producing plant secondary metabolites, including tannins, anthocyanins, and stilbenes, for use in medicine or the food industry (Decendit and Mérillon 1996; Decendit et al. 1996). Biotic and abiotic stress enhanced resveratrol production in grape cultured cells (Keskin and Kunter 2008; Santamaria et al. 2011; Belchí-Navarro et al. 2012). Here, we aimed to establish cell cultures from Koshu for production of resveratrol and its derivatives easily and rapidly. Moreover, we demonstrated that Koshu cell culture possesses an antidiabetic effect.
Materials and methods
Cell cultures
Apex tissues (1–2 cm long) were collected from V. vinifera cv. Koshu grown in the experimental vineyard of The Institute of Enology and Viticulture, University of Yamanashi, Japan, in 2007. The tissues were surface-sterilized with 70% ethanol for 5 min and then with a 10% solution of commercial bleach for 10 min. After washing the tissues with sterile water three times, shoot tips containing the meristematic dome and a few leaf primordia were excised from the apex tissues under an air-flow cabinet and placed on the surface of 1/2 Murashige and Skoog medium (Nacalai Tesque, Kyoto, Japan) containing 1 mg/mL 6-benzylaminopurine (Wako, Tokyo, Japan), 3% sucrose (Wako), and 0.6% agar (Wako). Callus induced from the shoot tips was selected and transferred to GB medium containing Gamborg’s B5 medium salt mixture (Wako), 20 mg/L thiamine hydrochloride (Wako), 2 mg/L nicotinic acid (Wako), 2 mg/L pyridoxine hydrochloride (Wako), 200 mg/L myo-inositol (Wako), 58 mM sucrose, 0.54 µM 1-naphthaleneacetic acid (Wako), 0.93 µM kinetin (Wako), and 0.8% agar. Callus of V. vinifera cv. Cabernet Sauvignon was also obtained by the same method for comparison with Koshu callus. Calli were grown at 27 °C in the dark and were maintained by changing the medium every 1 or 2 months.
Cell suspension cultures were established by growing callus in a 100 mL flask with 30 mL of GB medium without agar. The cell suspension cultures were maintained at 27 °C on an orbital shaker (110 rpm) in the dark and subcultured every week with an inoculum dilution of 1/8 (v/v inoculum/fresh medium). Hereafter, grape culture cells of Koshu and Cabernet Sauvignon were referred to as YU-1 and CS, respectively.
Tobacco BY-2 cell line was obtained from RIKEN BioResource Center (Ibaragi, Japan). BY-2 was maintained on Linsmaier and Skoog (LS) medium (Funakoshi, Tokyo, Japan) containing 0.4% gellan gum (Wako) (Linsmaier and Skoog 1965).
Measurement of cell growth
Cell growth of each cell culture on GB medium for YU-1 and CS or LS medium for BY-2 was determined by weighing the callus block every week. Growth of cell suspension culture was estimated by determining the cell count using a counting chamber (Hirschmann, Eberstadt, Germany).
Ultraviolet-C irradiation
To analyze stilbene production by YU-1, 4- to 5-week-old calli cultured on GB medium were used. The cells were irradiated with UV-C in a clean bench for 5, 10, or 15 min at an irradiation distance of 45 cm, and then incubated for 24 or 48 h at 27 °C in the dark. To analyze stilbene synthase gene (STS) expression, 4-day-old cell suspension cultures were used. Four mL of a cell suspension culture was transferred to one well of a six-well plate, and UV-C irradiation was carried out immediately for 15 min at an irradiation distance of 45 cm. After irradiation, the cell suspension cultures were statically incubated for 3, 6, 12, 24, and 48 h at 27 °C in the dark.
Quantification of stilbenes
The cells were pulverized in liquid nitrogen by using a mortar and pestle. The powder was added to four times its volume of ethanol and the resulting mixture was shaken at 60 °C for 30 min in the dark. The mixture was filtered through a 0.45 μm pore size syringe filter (Anotop 10, Whatman, Kent, UK). Stilbenes in the filtrate were analyzed by LC–MS (Shimadzu UFLC-XR LC system, Shimadzu, Kyoto, Japan and Shimadzu LCMS 2020, Shimadzu). A gradient solvent system was used with 0.2% formic acid in ultrapure water as solvent A and acetonitrile as solvent B. Samples were eluted by the following gradient time program: linear gradient elution from 15% B to 40% B within 30 min; linear gradient elution from 40% B to 80% B within 5 min; and return to 15% B and elution of 15% B for 10 min prior to injection of the next sample. Flow rate was 0.2 mL/min. The column was Atlantis T3 (150 × 2.1 mm, Waters, Milford, MA, USA) maintained at 40 °C. Stilbenes concentrations were expressed as μg/g FW (μg stilben per 1 g callus fresh weight).
Real-time RT-PCR
The cells were ground with an SK mill (SK-200, Tokken, Chiba, Japan). Total RNA isolation was performed using RNAiso (Takara, Shiga, Japan) according to the manufacturer’s instructions. First-strand cDNA was synthesized from 1 μg of total RNA using a PrimeScript RT reagent Kit with gDNA Eraser (Takara) according to the manufacturer’s instructions. Real-time RT-PCR was performed using an SYBR Premix Ex Taq II (Takara) with a Thermal Cycler Dice Real Time System (Takara). PCR conditions were as follows: incubation at 95 °C for 30 s, followed by 40 cycles at 95 °C for 5 s and at 60 °C for 1 min. The primers used for amplification were β-actin primers (5′-CAAGAGCTGGAAACTGCAAAGA-3′ and 5′-AATGAGAGATGGCTGGAAGAGG-3′, corresponding to bases 409–430 and 537–516 of V. vinifera β-actin, GenBank accession no. AF369524, respectively) and STS primers (5′-AGGAAGCAGCATTGAAGGCTC-3′ and 5′-TGCACCAGGCATTTCTACACC-3′, corresponding to bases 320–340 and 420–400 of V. vinifera stilbene synthase, GenBank accession no. X76892, respectively). β-Actin was used for normalization. Using the standard curve method and Thermal Cycler Dice Real Time System Single Software ver. 3.00 (Takara), the expression levels of each gene were determined as the number of amplification cycles needed to reach a fixed threshold. Data were expressed as relative values to β-actin.
Assessment of antidiabetic effects of YU-1
To evaluate antidiabetic effects, 3-week-old YU-1 was used. YU-1 was irradiated with or without UV-C for 15 min, and incubated for 24 h, after that lyophilized. The lyophilized powders were dissolved at 2.5 mg/mL with saline buffer and extracted at 100 °C for 5 min. The mixtures were centrifuged and the supernatants were collected. We evaluated the antidiabetic effects of YU-1 extract by using the hyperglycemic silkworm model (Matsumoto et al. 2011). Briefly, 50 μL YU-1 extracts or saline were injected into the hemolymph at the second abdominal segment of fifth-instar silkworm larva, that was fed a diet containing glucose for 1 h, with syringes (1 mL) and needles (27G × 3/4). After 6 h, hemolymph was collected and total sugar was determined using the phenol–sulfuric acid method. In the hyperglycemic silkworm model, saline injections were the negative control, insulin injections were the positive control and UV-C irradiated or unirradiated YU-1 extractions were assayed.
Statistical analysis
Data are presented as means ± standard errors of at least three replicates. Statistical analysis was performed with the Student’s t test using Excel 2010 (Microsoft, USA) with the add-in software Statcel 3 (Yanai 2011). A p-value of less than 0.05 was considered statistically significant.
Results and discussion
Optimization of culture condition of YU-1
Calli were developed from the apex tissues of Koshu on the callus initiation medium. One callus that showed the fastest growth and the shiniest appearance among all the calli was transferred to GB medium and designated as YU-1. The optimum temperature for YU-1 growth on GB medium was 27 °C, while light was not involved in YU-1 growth. YU-1 growth on 10 g/L sucrose medium tended to show lower fresh weight, whereas no significant difference was noted in the fresh weight of cells grown on 20 and 30 g/L sucrose media. However, the cells grown on 30 g/L sucrose medium sometimes became brown and ceased growing. A high concentration of sucrose in the culture medium was found to induce polyphenol production (Decendit and Mérillon 1996; Larronde et al. 1998). Thus, the 30 g/L sucrose medium might not be suitable for YU-1 growth. Taken together, we determined that YU-1 grew well on GB medium containing 20 g/L sucrose at 27 °C in the dark.
YU-1 cell suspension culture was prepared by incubating YU-1 in GB liquid medium. YU-1 cell suspension culture exhibited a typical sigmoidal growth curve (Fig. 1). YU-1 cell number increased up to tenfold at day 7 of incubation, and YU-1 cell density was similar to the density of grape cells in the HM1 medium described by Decendit and Mérillon (1996). This result suggested that the cell suspension culture of YU-1 was established.
Fig. 1.
Growth curve of YU-1 cell suspension culture. YU-1 growth in GB liquid medium was expressed as cell number per mL of liquid medium. Data indicate means ± standard errors of three replicates
YU-1 shows a high growth rate
We compared cell growth rates among YU-1, CS, and BY-2 (Fig. 2). CS showed significantly slower growth than YU-1 (Fig. 2a). On the other hand, YU-1 grew approximately 11-fold at week 3 of incubation as compared to that before incubation (week 0). BY-2 is a standard cell line having a high growth rate. This was also evident from the growth curves (Fig. 2b). BY-2 had higher growth rate than YU-1. At the end of week 3, the growth rate of BY-2 fell, while it continued to increase for YU-1. This shows that at the end of incubation time of 4 weeks, the total increase in fresh weight (g) was higher for YU-1 than BY-2. This is one of the advantages of our established YU-1 for the production of plant second metabolites.
Fig. 2.
Comparison of proliferation activity among cell cultures. a Comparison of proliferation activity between YU-1 (left) and CS cells (right). Scale bar shows 2 cm. b Growth curves of YU-1 on GB medium (square) and BY-2 on LS medium (circle), expressed as fresh weight. BY-2 growth curve is the average of three replicates. Data indicate means ± standard errors of three replicates
Stilbene production by YU-1
V. vinifera cell cultures are a good model system for studies of secondary metabolites, such as stilbenes and anthocyanins (Larronde et al. 1998; Zamboni et al. 2009; Gagné et al. 2011). We determined stilbene production by UV-C irradiated YU-1 because stilbene accumulation in grape skins was found to be induced by UV-C irradiation (Takayanagi et al. 2004). UV-C unirradiated YU-1 contained small amounts of piceid, resveratrol, and ε-viniferin (Table 1). In contrast, UV-C irradiation increased production of resveratrol, piceatannol, and ε-viniferin in YU-1. Maximum stilbene production in YU-1 was realized by UV-C irradiation for 15 min and incubation for 24 h. No difference was observed in the stilbene production between incubation for 24 h and 48 h irrespectively of UV-C irradiation time. This suggested that the prolonged incubation didn’t affect stilbene production in UV-C irradiated YU-1. Piceid content in YU-1 was not affected by UV-C irradiation, although piceid content was highest in UV-C irradiated YU-1 for 15 min and incubated for 48 h.
Table 1.
Concentrations of stilbenes in UV-C irradiated YU-1
| UV light (min) | Incubation (h) | Piceid (μg/g FW) | Resveratrol (μg/g FW) | Piceatannol (μg/g FW) | ε-viniferin (μg/g FW) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trans | Cis | Total | Trans | Cis | Total | Trans | Cis | Total | Trans | Cis | Total | ||
| 0 | 24 | 4.602 | 1.113 | 5.715 | 0.198 | 0.044 | 0.242 | ND | ND | ND | 0.189 | 0.031 | 0.220 |
| 0 | 48 | 7.655 | 2.126 | 9.781 | ND | ND | ND | ND | ND | ND | ND | 0.005 | 0.005 |
| 5 | 24 | 4.482 | 1.134 | 5.616 | 4.056 | 0.468 | 4.524 | 0.078 | ND | 0.078 | 0.603 | 0.068 | 0.671 |
| 5 | 48 | 3.979 | 0.994 | 4.973 | 1.488 | 0.149 | 1.637 | 0.034 | 0.053 | 0.087 | 1.109 | 0.101 | 1.210 |
| 10 | 24 | 5.626 | 2.137 | 7.763 | 3.822 | 0.473 | 4.295 | 0.062 | 0.016 | 0.078 | 0.062 | 0.010 | 0.072 |
| 10 | 48 | 4.272 | 0.853 | 5.125 | 2.184 | 0.710 | 2.894 | 0.096 | 0.048 | 0.144 | 0.422 | 0.139 | 0.561 |
| 15 | 24 | 5.556 | 0.920 | 6.473 | 8.694 | 0.900 | 9.594 | 0.162 | 0.036 | 0.198 | 1.398 | 0.138 | 1.536 |
| 15 | 48 | 10.71 | 2.335 | 13.04 | 2.962 | 0.543 | 3.505 | 0.039 | ND | 0.039 | 0.689 | 0.118 | 0.807 |
FW callus fresh weight, ND not detected
STS expression in YU-1 was upregulated by UV-C irradiation (Fig. 3), although no clear expression pattern by UV-C irradiation was observed in STS expression. At 3 h after UV irradiation, STS expression in UV-C irradiated YU-1 was 2.9-fold of that in the unirradiated YU-1. At 6 and 12 h after UV-C irradiation, STS expression in the UV-C irradiated YU-1 returned to the initial level, whereas a significant increase of STS expression was noted at 48 h after UV-C irradiation. STS expression in leaves was induced by UV-C irradiation, and the expression had two peaks (Borie et al. 2004). Taken together, these results suggest that YU-1 yields physiological responses similar to grapevine tissues, and that YU-1 can be used as a system for the production of the stilbene biosynthesis.
Fig. 3.
STS expression in YU-1 irradiated with UV-C. Total RNA was isolated from the YU-1 at the indicated periods and subjected to real-time RT-PCR analysis. Data indicate means ± standard errors of three replicates
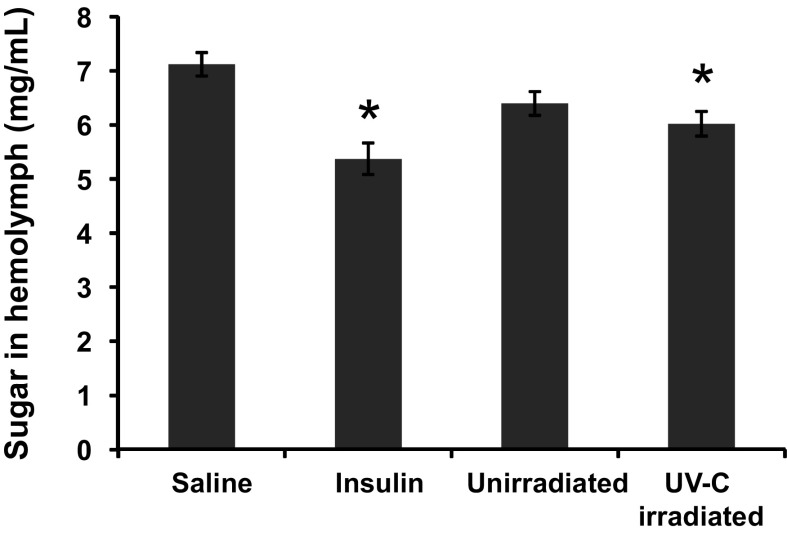
Decrease in hemolymph sugar level of hyperglycemic silkworm by YU-1
Resveratrol and its derivatives display a wide range of biological effects, notably as a cardioprotective, antidiabetic, antitumor or neuroprotective agent, as well as an antifungal or antibacterial compound (Delaunois et al. 2009; Chung et al. 2012). We tested whether UV-C irradiated YU-1, which enhances the production of resveratrol and its derivatives, possesses a hypoglycemic effect by using the hyperglycemic silkworm model. Hemolymph sugar levels in silkworms fed a glucose diet were significantly decreased by human insulin injection (Fig. 4), suggesting that the hypoglycemic effect could be reproduced by this model in this study. Hemolymph sugar level was decreased by injecting the extract of UV-C irradiated YU-1 (p = 0.013), but not by injecting the unirradiated YU-1 extract (p = 0.057). This result demonstrated that UV-C irradiated YU-1 contained hypoglycemic substances. YU-1 did not demonstrate significant cytotoxicity in silkworms (data not shown). Resveratrol content in YU-1 was enhanced by UV-C irradiation (Table 1), and it was reported that resveratrol exhibited anti-hyperglycemic activity in diabetic rats (Szkudelski and Szkudelska 2011). Taken together, we assumed that resveratrol and its derivatives induced in YU-1 by UV-C irradiation might decrease hemolymph sugar level. However, so far, we could not exclude that any bioactive compounds other than the stilbenes decreased hemolymph sugar level. For example, hydroxytyrosol and melatonin show antidiabetic activity (Fernández-Mar et al. 2012). Quercetin and myricetin, which are the major polyphenols in red grapes, are effective in a complimentary role for diabetes management (Pandey and Rizvi 2014). In fact, Koshu, which is a source of YU-1, accumulate more quercetin and its glucosides in berries than red cultivar Merlot (Kobayashi et al. 2011). Further investigations are necessary to elucidate what is the complete composition in YU-1 extract that decrease hemolymph sugar level.
Fig. 4.
Decrease in hemolymph sugar level of hyperglycemic silkworm by feeding YU-1. Five to eight silkworms were used in one experiment and four replications were made. Data indicate means ± standard errors of four replicates. *p < 0.05 versus saline-injected silkworm
Grape cell cultures are expected as an alternative source of bioactive substances, such as resveratrol, to grape berries and leaves (Nivelle et al. 2017). The optimum conditions and cultivars for bioreactor culture were studied (Ferri et al. 2011). In conclusion, YU-1 is an excellent cell culture for the production of resveratrol and its derivatives. YU-1 has high growth rate and produces large amounts of stilbenes with hypoglycemic effect. This novel grape cell culture established in this study may produce a large amount of resveratrol in a bioreactor, thereby becoming a source of valuable medicinal components.
Acknowledgements
We thank Dr. Hironori Kobayashi and Dr. Ryoji Takata (Kirin Company, Ltd.) for valuable discussion.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- Belchí-Navarro S, Almagro L, Lijavetzky D, Bru R, Pedreño MA. Enhanced extracellular production of trans-resveratrol in Vitis vinifera suspension cultured cells by using cyclodextrins and methyljasmonate. Plant Cell Rep. 2012;31:81–89. doi: 10.1007/s00299-011-1141-8. [DOI] [PubMed] [Google Scholar]
- Borie B, Jeandet P, Parize A, Bessis R, Adrian M. Resveratrol and stilbene synthase mRNA production in grapevine levels treated with biotic and abiotic phytoalexin elicitors. Am J Enol Vitic. 2004;55:60–64. [Google Scholar]
- Chung JH, Manganiello V, Dyck JRB. Resveratrol as a calorie restriction mimetic: therapeutic implications. Trends Cell Biol. 2012;22:546–554. doi: 10.1016/j.tcb.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decendit A, Mérillon JM. Condensed tannin and anthocyanin production in Vitis vinifera cell suspension cultures. Plant Cell Rep. 1996;15:762–765. doi: 10.1007/BF00232224. [DOI] [PubMed] [Google Scholar]
- Decendit A, Ramawat KG, Waffo P, Deffieux G, Badoc A, Mérillon JM. Anthocyanins, catechins, condensed tannins and piceid production in Vitis vinifera cell bioreactor cultures. Biotechnol Lett. 1996;18:659–662. doi: 10.1007/BF00130761. [DOI] [Google Scholar]
- Delaunois B, Cordelier S, Conreux A, Clément C, Jeandet P. Molecular engineering of resveratrol in plants. Plant Biotechnol J. 2009;7:2–12. doi: 10.1111/j.1467-7652.2008.00377.x. [DOI] [PubMed] [Google Scholar]
- Fernández-Mar MI, Mateos R, García-Parrilla MC, Puertas B, Cantos-Villar E. Bioactive compounds in wine: resveratrol, hydroxytyrosol and melatonin: a review. Food Chem. 2012;130:797–813. doi: 10.1016/j.foodchem.2011.08.023. [DOI] [Google Scholar]
- Ferri M, Dipalo SCF, Bagni N, Tassoni A. Chitosan elicits mono-glucosylated stilbene production and release in fed-batch bioreactor cultures of grape cells. Food Chem. 2011;124:1473–1479. doi: 10.1016/j.foodchem.2010.07.114. [DOI] [Google Scholar]
- Fujita K, Shimazaki M, Furiya T, Takayanagi T, Suzuki S. Genetic variation among Koshu (Vitis vinifera L.) accessions generated by retrotransposon insertion into genome. Am J Enol Vitic. 2009;60:490–496. [Google Scholar]
- Gagné S, Cluzet S, Mérillon JM, Gény L. ABA initiates anthocyanin production in grape cell cultures. J Plant Growth Regul. 2011;30:1–10. doi: 10.1007/s00344-010-9165-9. [DOI] [Google Scholar]
- Goto-Yamamoto N, Sawler J, Myles S. Genetic analysis of east Asian grape cultivars suggests hybridization with wild Vitis. PLoS ONE. 2015;10:e0140841. doi: 10.1371/journal.pone.0140841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskin N, Kunter B. Production of trans-resveratrol in ‘Cabernet Sauvignon’ (Vitis vinifera L.) callus culture in response to ultraviolet-C irradiation. Vitis. 2008;47:193–196. [Google Scholar]
- Kobayashi H, Suzuki Y, Ajimura K, Konno T, Suzuki S, Saito H. Characterization of phenolic compounds biosynthesized in pink-colored skin of Japanese indigenous Vitis vinifera cv. Koshu grape. Plant Biotechnol Rep. 2011;5:79–88. doi: 10.1007/s11816-010-0162-z. [DOI] [Google Scholar]
- Larronde F, Krisa S, Decendit A, Chèze C, Deffieux G, Mérillon JM. Regulation of polyphenol production in Vitis vinifera cell suspension cultures by sugars. Plant Cell Rep. 1998;17:946–950. doi: 10.1007/s002990050515. [DOI] [PubMed] [Google Scholar]
- Linsmaier EM, Skoog F. Organic growth factor requirements of tobacco tissue culture. Physiol Plant. 1965;18:100–127. doi: 10.1111/j.1399-3054.1965.tb06874.x. [DOI] [Google Scholar]
- Matsumoto Y, Sumiya E, Sugita T, Sekimizu K. An invertebrate hyperglycemic model for the identification of anti-diabetic drugs. PLoS ONE. 2011;6:e18292. doi: 10.1371/journal.pone.0018292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nivelle L, Hubert J, Courot E, Jeandet P, Aziz A, Nuzillard JM, Renault JH, Clément C, Martiny L, Delmas D, Tarpin M. Anti-cancer activity of resveratrol and derivatives produced by grapevine cell suspensions in a 14 L stirred bioreactor. Molecules. 2017;22:474. doi: 10.3390/molecules22030474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey KB, Rizvi SI. Role of red grape polyphenols as antidiabetic agents. Integr Med Res. 2014;3:119–125. doi: 10.1016/j.imr.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria AR, Mulinacci N, Valletta A, Innocenti M, Pasqua G. Effects of elicitors on the production of resveratrol and viniferins in cell cultures of Vitis vinifera L. cv Italia. J Agric Food Chem. 2011;59:9094–9101. doi: 10.1021/jf201181n. [DOI] [PubMed] [Google Scholar]
- Shimazaki M, Fujita K, Kobayashi H, Suzuki S. Pink-colored grape berry is the result of short insertion in intron of color regulatory gene. PLoS ONE. 2011;6:e21308. doi: 10.1371/journal.pone.0021308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szkudelski T, Szkudelska K. Anti-diabetic effects of resveratrol. Ann N Y Acad Sci. 2011;1215:34–39. doi: 10.1111/j.1749-6632.2010.05844.x. [DOI] [PubMed] [Google Scholar]
- Takayanagi T, Okuda T, Mine Y, Yokotsuka K. Induction of resveratrol biosynthesis in skins of three grape cultivars by ultraviolet irradiation. J Jpn Soc Hortic Sci. 2004;73:193–199. doi: 10.2503/jjshs.73.193. [DOI] [Google Scholar]
- Yanai H. Statcel, available: the useful add-in software 2 forms on Excel. 3. Tokyo: OMC; 2011. pp. 172–175. [Google Scholar]
- Zamboni A, Gatto P, Cestaro A, Pilati S, Viola R, Mattivi F, Moser C, Velasco R. Grapevine cell early activation of specific responses to DIMEB, a resveratrol elicitor. BMC Genom. 2009;10:363. doi: 10.1186/1471-2164-10-363. [DOI] [PMC free article] [PubMed] [Google Scholar]