Abstract
Japanese black vinegar (JBV) is a traditional vinegar manufactured with steamed unpolished rice. After screening, beneficial effects of JBV on IgE-mediated allergic responses were found. In this study, acetic acid-free JBV was used to evaluate its antiallergic effects. JBV suppressed degranulation of rat basophilic leukemia RBL-2H3 cells in a dose-dependent manner without cytotoxicity. The inhibitory effect of JBV on the degranulation seemed to be caused by the bioactive ingredients other than proteins, because the activity was not affected by heat treatment or protease digestion. JBV inhibited the elevation in the intracellular Ca2+ concentration induced by antigen. Immunoblot analysis revealed that JBV suppresses degranulation of RBL-2H3 cells by downregulated phosphorylation of PI3K, Akt, and PLCγ1. In addition, oral administration of JBV significantly suppressed passive cutaneous anaphylaxis reaction in mice and an allergic symptom in Cry j1-induced pollinosis model mice. Thus, JBV has a potential as a health-promoting food with the antiallergy effect.
Electronic supplementary material
The online version of this article (10.1007/s10616-018-0208-6) contains supplementary material, which is available to authorized users.
Keywords: Japanese black vinegar, Degranulation, Antiallergic effect, RBL-2H3 cells, Pollinosis
Introduction
Allergy is an excessive immune defense reaction. Allergic symptoms are divided into four types based on the difference in their mechanisms to generate immune responses (Gell and Coombs 1963). Allergic rhinitis is a typical type I allergic disease that causes three characteristic symptoms, namely sneeze, runny nose, and stuffy nose, due to causative antigens reaching the nasal mucosa. Allergic rhinitis is caused by most of inhalable antigens, for example, ticks and molds, which cause perennial allergic rhinitis, and tree and grass pollens, which cause seasonal allergic rhinitis. A survey conducted in 2008 revealed that the prevalence of allergic rhinitis was 38.9% in Japan, among which the prevalence of Japanese cedar pollinosis was 26.5% (Baba and Nakae 2008). The survey also revealed that the prevalence of Japanese cedar pollinosis increased by 10% over a decade, suggesting a rapid increase in patients with Japanese cedar pollinosis (Yamada et al. 2014). Cedar pollinosis is elicited by cedar pollens that reach the nasal mucosa. Antigen-presenting cells, such as macrophages and dendritic cells, phagocytize and present them on MHC class II molecules on their cell surface. Naive CD4+ T cells that receive antigen presentation and cytokines differentiate into Th2 cells and produce cytokines such as interleukin (IL)-4 and IL-13. These cytokines stimulate B cells to promote class switching and to produce specific IgE antibodies. On the other hand, Th1 cells produce interferon (IFN)-γ that plays the opposite role to IL-4 and promotes Th1 cell differentiation. Th1/Th2 balance is well maintained in healthy subjects; however, Th2 predominant condition results in occurrence of allergic diseases (Galli et al. 2008).
Type I allergic reaction is initiated by degranulation of mast cells and basophils provoked by cross-linkage of an allergen to specific IgE antibodies bound to the high-affinity IgE receptor (FcεRI). Aggregation of antigen-IgE-bound FcεRI leads to mast cell degranulation and secretion of inflammatory cytokines through the activated intracellular signaling processes (Amin 2012). The initial signaling event is activation of the Src family non-receptor tyrosine kinases Lyn and Fyn. Activated Lyn induces phosphorylation of another kinase Syk, which leads to Ca2+ mobilization. As a result, chemical mediators such as histamine are released from intracellular granules, and inflammatory cytokines are secreted, which induces contraction of smooth muscle, vasodilation, and increased vascular permeability (Kraft and Kinet 2007). Mast cells thus play a crucial role in the type I allergic reactions, and prevention of mast cell degranulation is of great significance for the relief of allergic symptoms. Because a long-term administration of antihistamine and steroids sometimes causes side effects, an alternative way is required to relieve allergic symptoms without drugs. Recently, antiallergic foods have attracted much attention as alternates for drugs. So far, several food components have been reported to suppress mast cell degranulation (Miyata et al. 2008; Lee et al. 2013; Onishi et al. 2014). As a result of screening a number of foodstuffs, we found Japanese black vinegar called kurozu in Japanese as an antiallergic food material.
Japanese black vinegar is a traditional vinegar manufactured with three components: steamed unpolished rice, a fermentation starter called koji, and water. A fermentation process is saccharification, alcoholic fermentation, and acetic fermentation. Japanese black vinegar is rich in various kinds of amino acids. In addition, various microorganisms such as Aspergillus, yeasts, lactic acid bacteria, and acetic acid bacteria are known to be involved in the process of fermentation and maturation. Thus, Japanese black vinegar contains acetic acid and other organic acids, peptides, amino acids, polysaccharides, and so on. Japanese black vinegar has been reported to have several functions such as antioxidant (Shimoji et al. 2002) and liver function-improving actions (Fujii et al. 1999). Although various functionalities have been reported, the antiallergic effect of Japanese black vinegar has not yet been reported. In the present study, the antiallergic effect of Japanese black vinegar was examined using the rat basophilic leukemia cell line RBL-2H3 cells that have been commonly used for screening of substances inhibiting mast cell degranulation in vitro (Passante and Frankish 2009) and using murine models of passive cutaneous anaphylaxis (PCA) and pollinosis in vivo. Our data suggest that Japanese black vinegar would contribute to attenuation of allergic symptoms. The findings demonstrated in the present study would be of significance in providing a new dimension to the functionality of Japanese black vinegar.
Materials and methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM), RPMI-1640 medium, fetal bovine serum (FBS), penicillin, streptomycin, bovine serum albumin (BSA), mouse anti-dinitrophenol (DNP) monoclonal IgE, DNP-human serum albumin (HSA) conjugate, Triton X-100, Evans blue, and mineral oil were purchased from Sigma-Aldrich (St. Louis, MO, USA). Goat anti-actin antibody and horseradish peroxidase (HRP)-labeled anti-goat IgG antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). HRP-labeled anti-rabbit IgG antibody and rabbit antibodies against phosphoinositide 3-kinase (PI3K) p85, phosphorylated PI3K p85/p55, Syk, phosphorylated Syk, Lyn, phosphorylated Lyn, phospholipase C (PLC) γ1, phosphorylated PLCγ1, PLCγ2, and phosphorylated PLCγ2 were purchased from Cell Signaling Technology (Danvers, MA, USA). Cry j1, a purified allergen of Japanese cedar pollens, was purchased from Hayashibara (Okayama, Japan). All other chemicals were purchased from Wako Pure Chemical Industries (Osaka, Japan) or Nacalai Tesque (Kyoto, Japan) unless otherwise noted.
Cells and cell culture
RBL-2H3 cells were obtained from American Type Culture Collection (Rockville, MD, USA) and cultured in DMEM supplemented with 100 U/mL of penicillin, 100 µg/mL of streptomycin, and 10% FBS at 37 °C under humidified 5% CO2 in air.
Animals
Female BALB/c mice were purchased from Japan SLC (Shizuoka, Japan) and kept in an animal room under 12 h light/dark cycle at a temperature of 24 ± 2 °C. Animals received standard chow and water ad libitum. All animal experiments described in this study were carried out in accordance with the protocols approved by the Laboratory Animal Care Committee of Ehime University (approved protocol numbers: 08U3-1 and 08U7-1). Mice were maintained in accordance with the Guidelines for the Care and Use of Laboratory Animals of Ehime University.
Sample preparation
Japanese black vinegar manufactured by Sakamoto Kurozu Inc. (Kagoshima, Japan) was freeze-dried to remove acetic acid and ten-fold concentrated. It was then centrifuged at 1900×g for 10 min, and the supernatant was used for the experiments in this study as an acetic acid-free Japanese black vinegar sample (JBV). One hundred mL of JBV was yielded from 1000 mL of intact black vinegar. According to the analysis by HPLC, the amount of acetic acid in JBV was 0%, whereas that of intact black vinegar was 4.19% (supplementary data 1). JBV was used by diluting in 10 mM sodium phosphate buffer (NaPB, pH 7.4) to reduce the effect of pH. Dry weight was measured by weighing a portion of freeze-dried JBV. To evaluate the molecular size of bioactive ingredients, JBV was dialyzed using a dialysis membrane with molecular weight cut-off (MWCO) of 3500 (Wako Pure Chemical Industries) against 10 mM NaPB for 24 h at 4 °C. To evaluate the heat stability, JBV was heated at 100 °C for 15 min and used for the degranulation assay described below. To evaluate the protease stability, JBV was treated with 10–500 µg/mL of proteinase K (Wako Pure Chemical Industries) at 37 °C for 15 min or overnight. The samples were then heated at 100 °C for 10 min to inactivate proteases and used for the degranulation assay described below.
Antigen-induced degranulation assay
The assay was performed as previously described with some modifications (Watanabe et al. 2005; Ishida et al. 2013). RBL-2H3 cells suspended in DMEM containing 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 10% FBS were seeded into a 96-well culture plate (Corning, Corning, NY, USA) at 4.0 × 104 cells/well and cultured for 12 h at 37 °C under humidified 5% CO2. The cells were then treated with anti-DNP IgE at 50 ng/mL for 2 h at 37 °C. After washing the cells with modified Tyrode’s (MT) buffer (20 mM HEPES, 135 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, and 0.05% BSA, pH 7.4) twice, anti-DNP IgE-sensitized cells were treated with 120 μL of MT buffer containing various concentrations of JBV or 10 mM NaPB (vehicle) for 10 min at 37 °C. 10 μL of DNP-HSA diluted in MT buffer at 0.625 μg/mL was subsequently added to each well and incubated for 30 min at 37 °C. After incubation, the supernatant was collected from each well, and the cells were sonicated in 130 μL of MT buffer containing 0.1% Triton X-100 for 5 s on ice. Both supernatant and cell lysate were transferred to a new 96-well microplate at 50 μL/well and incubated for 5 min at 37 °C. 100 μL of 3.3 mM 4-nitrophenyl 2-acetamido-2-deoxy-β-d-glucopyranoside (Wako Pure Chemical Industries) dissolved in 0.1 M citrate buffer (pH 4.5) were then added to each well and incubated for 25 min at 37 °C. The enzyme reaction was terminated by adding 100 μL of 2 M glycine buffer (pH 10.4), and the absorbance was measured at 405 nm using a SH-8000Lab microplate reader (Corona Electric, Ibaraki, Japan). β-Hexosaminidase release rate (%) was calculated as follows:
| 1 |
where “A” is the absorbance of each well.
Cell viability
Cytotoxicity of JBV to RBL-2H3 cells was examined using a WST-8 assay kit (Kishida Chemical; Osaka, Japan) according to the manufacturer’s instructions. Anti-DNP IgE-sensitized cells were seeded into a 96-well culture plate, treated with various concentrations of JBV, and stimulated with DNP-HSA as described above. After the cells were washed with phosphate-buffered saline (PBS, pH 7.4) once, 100 μL of 10% FBS-DMEM containing 10% WST-8 solution was added to each well of the culture plate and incubated for 30 min at 37 °C. The absorbance was then measured at 450 nm using a Model 550 microplate reader (Bio-Rad Laboratories, Hercules, CA, USA).
Immunoblot analysis
RBL-2H3 cells were seeded into a 24-well culture plate (BD Falcon) at 2.5 × 105 cells/well and cultured for 12 h at 37 °C under humidified 5% CO2. The cells were then treated with anti-DNP IgE at 50 ng/mL for 2 h at 37 °C. After washing the cells with MT buffer twice, anti-DNP IgE-sensitized cells were treated with 490 μL of MT buffer containing JBV (5.4 mg/mL) or 10 mM NaPB (vehicle) for 10 min at 37 °C. The cells were then stimulated with DNP-HSA at 50 ng/mL and further incubated for 10 min. After removing the added reagents, cells were lysed and immunoblotting was performed with various antibodies as previously described (Nishi et al. 2014).
Measurement of intracellular Ca2+ concentration ([Ca2+]i)
[Ca2+]i was measured using a Calcium Kit Fluo 3 (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer’s instructions. RBL-2H3 cells were seeded into a white 96-well culture plate (Nunc, Roskilde, Denmark) and treated with the anti-DNP IgE as described above. The IgE-sensitized RBL-2H3 cells were then washed with PBS twice and incubated with 100 μL of Fluo-3 AM for 1 h at 37 °C. After washing the cells with PBS, the cells were treated with 120 μL of MT buffer containing JBV (5.4 mg/mL) or 10 mM NaPB (vehicle) for 10 min at 37 °C. The cells were then stimulated by adding 10 μL of MT buffer containing DNP-HSA at 0.625 μg/mL and the fluorescent intensity was immediately monitored with an excitation wavelength of 490 nm and an emission wavelength of 530 nm using a SH-8000Lab microplate reader.
IgE-mediated passive cutaneous anaphylaxis (PCA) in mice
The assay was performed according to the method of Knoops et al. (2005) with some modifications (Yasunaga et al. 2016). After acclimating to their housing environment for 1 week, 7-week-old female BALB/c mice were intradermally injected with 10 µL of PBS containing 100 ng of anti-DNP IgE and with 10 µL of PBS alone into their left and right ears, respectively. After 24 h, 200 μL of PBS containing 200 μg of DNP-HSA and 0.5% Evans blue were injected into their tail vein. One hour before DNP-HSA injection, the anti-DNP IgE-injected mice were orally administered 20 µL of 10 mM NaPB to the control group, 20 µL of JBV at 25 mg/kg body weight to low-dose JBV-administered group, and 20 µL of JBV at 250 mg/kg body weight to high-dose JBV-administered group. The mice were then euthanized 30 min after DNP-HSA injection, and their ears were excised. The extravagated dye was then extracted from each ear with 500 μL of formamide for 16 h at 70 °C, and the absorbance was measured at 620 nm using an Ultrospec 3000 spectrophotometer (Amersham Pharmacia Biotech, Uppsala, Sweden).
A mouse model of Japanese cedar pollinosis
A mouse model of Japanese cedar pollinosis was developed according to Nomiya et al. (2008) by treating mice with Cry j1 as scheduled in Fig. 1. Following adaptation period for 1 week, 6-week-old female BALB/c mice were randomly divided into 4 groups as follows: intact group (8 mice), control group (8 mice), low-dose JBV-administered group (8 mice), high-dose JBV-administered group (8 mice). Control group mice and JBV-administered group mice were intranasally treated with 1.0 μg of Cry j1 dissolved in 10 μL of PBS using a glass microsyringe (Hamilton; Reno, NV, USA) on days 0, 7, 14, and 21 and challenged with 0.2 μg of Cry j1 in 10 μL of PBS for 6 consecutive days from day 28 to day 33, while the intact group mice were treated with 10 μL of PBS alone from day 0 to day 33. The low-dose and the high-dose JBV-administered group mice were orally administered 20 µL of JBV at 25 and 250 mg/kg body weight, respectively, for 7 consecutive days from day 28 to day 34. The intact and control group mice were orally administered 20 µL of 10 mM NaPB. On day 34, all mice, including intact group, were treated with 0.2 μg of Cry j1. Nasal rubbing frequency was counted for 30 min immediately after the final challenge. On day 35, all mice were euthanized and blood and spleen were collected. Splenocytes were prepared by gently passing the spleen through a cell strainer (BD Falcon), hemolyzing with a lysis buffer (155 mM NH4Cl, 15 mM NaHCO3, 1 mM ethylenediaminetetraacetic acid, pH 7.3), and washing with PBS. The cells were then suspended in RPMI-1640 medium supplemented with 100 U/mL of penicillin, 100 µg/mL of streptomycin, 10% FBS, and 10 µg/mL of concanavalin A (Seikagaku, Tokyo, Japan) and seeded into a 48-well culture plate in triplicate at 1.0 × 106 cells/well. After cultivation for 24 h at 37 °C, the concentrations of IL-4 and IFN-γ in the culture medium were determined by enzyme-linked immunosorbent assay (ELISA). Serum IgE and IgG1 levels were measured by in-house-developed ELISA as described previously (Kondo et al. 2015). The IL-4 concentration was determined using an ELISA kit (eBioscience, San Diego, CA, USA) according to the manufacturer’s instructions. The concentration of IFN-γ was determined by in-house-developed ELISA as described below. Although the cell supernatant was used without dilution, it took a long time to obtain sufficient color development. Even when coloring occurred, we could not quantify the amount of immunoglobulins or cytokines because the absorbance of samples was below the detection limit of the calibration curve. Since the amount of immunoglobulins and cytokines could not be quantified using the standard solution, all data were expressed in absorbance.
Fig. 1.
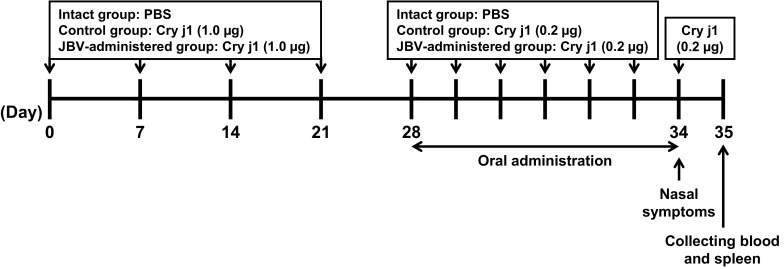
Experimental design for investigation of antiallergy effect of JBV in a mouse model of Japanese cedar pollinosis
Mouse IFN-γ ELISA
Rat anti-mouse IFN-γ antibody (Thermo Scientific, Waltham, MA, USA) diluted in PBS at 1.0 µg/mL was added to each well of a 96-well microtiter plate (Nunc, Roskilde, Denmark) at 100 μL/well and incubated at 4 °C overnight. After washing with 0.05% Tween 20-PBS (PBS-T) three times, each well was blocked with 1% BSA in PBS for 2 h at 37 °C. After washing with PBS-T three times, each well was treated with 50 µL of standards or cell culture medium for 2 h at 37 °C. After washing with PBS-T three times, each well was treated with 100 µL of biotinylated rat anti-mouse IFN-γ antibody (Thermo Scientific) diluted in 1% BSA-PBS at 0.5 µg/mL for 1 h at 37 °C. After washing with PBS-T three times, 100 µL of streptavidin-HRP conjugate (Molecular Probes, Carlsbad, CA, USA) diluted in 1% BSA-PBS at 1.25 µg/mL was added to each well and incubated for 1 h at 37 °C. After washing with PBS-T three times, 0.6 mg/mL of 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid diammonium salt) (Wako Pure Chemical Industries) dissolved in a 0.03% H2O2-0.05 M citrate buffer (pH 4.0) was added at 100 µL/well, and the absorbance was measured at 415 nm after adding 100 µL/well of 1.5% oxalic acid for termination of the coloring reaction. Since the IFN-γ concentration could not be quantified using the standard solution, the data were expressed in absorbance.
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 7.02 (GraphPad Software, La Jolla, CA, USA). Statistical significance was determined via one-way analysis of variance (ANOVA) with Dunnett’s multiple comparison test as indicated. Values with *P < 0.05, **P < 0.01, or ***P < 0.001 were considered statistically significant.
Results
Effect of JBV on degranulation of RBL-2H3 cells
An effect of JBV on degranulation of RBL-2H3 cells was examined. JBV was added at various concentrations to the culture medium of anti-DNP IgE-sensitized RBL-2H3 cells, and degranulation was induced with DNP-HSA as antigen. As shown in Fig. 2a, degranulation was suppressed in a dose-dependent manner by treating cells with JBV. Significant differences against control in the β-hexosaminidase release rate were observed at equal to or higher than 2.7 mg/mL of JBV, suggesting that JBV has a degranulation-suppressive activity on RBL-2H3 cells stimulated by antigen. JBV was found to exhibit no cytotoxicity to RBL-2H3 cells as shown in Fig. 2b. From these results, JBV was used at around 5.4 mg/mL for further experiments.
Fig. 2.
Effect of JBV on degranulation and cell viability of RBL-2H3 cells. a Anti-DNP IgE-sensitized RBL-2H3 cells were treated with JBV at indicated concentrations or with 10 mM NaPB (control). Degranulation was induced by treating the cells with DNP-HSA. Released β-hexosaminidase was used as a marker of degranulation. b Viability of anti-DNP IgE-sensitized RBL-2H3 cells was measured using a WST-8 assay kit after treating the cells with JBV at indicated concentrations or with 10 mM NaPB (control), followed by stimulation with DNP-HSA. Data are presented as mean ± SD (n = 3). **P < 0.01; ***P < 0.001 against control by Dunnett’s multiple comparison test
Characteristics of bioactive ingredients in JBV
JBV was dialyzed using a dialysis membrane with MWCO of 3500 to estimate the molecular size of bioactive ingredients and used for the antigen-induced degranulation assay. JBV was dialyzed for 24 h against 100 times the volume of 10 mM NaPB. During 24 h of dialysis, NaPB was exchanged at least twice. The result of gel filtration suggested that low-molecular-weight substances were removed (supplementary data 2). Since there was no decrease or disappearance of the activity by dialysis (Fig. 3a), it was suggested that the molecular weight of the bioactive ingredient is higher than 3500. To investigate the molecular characteristics of bioactive ingredients, JBV was heated at 100 °C for 15 min and used for the antigen-induced degranulation assay. The result showed that the degranulation-suppressive activity of JBV was not affected by heating (Fig. 3b), indicating that the bioactive ingredients in JBV are stable to heat. In addition, JBV was treated with proteinase K and used for the degranulation assay. As shown in Fig. 3c, proteinase K treatment did not affect the degranulation-suppressive activity of JBV at all at any proteinase K concentrations, indicating that the bioactive ingredients in JBV are proteinase K-resistant.
Fig. 3.
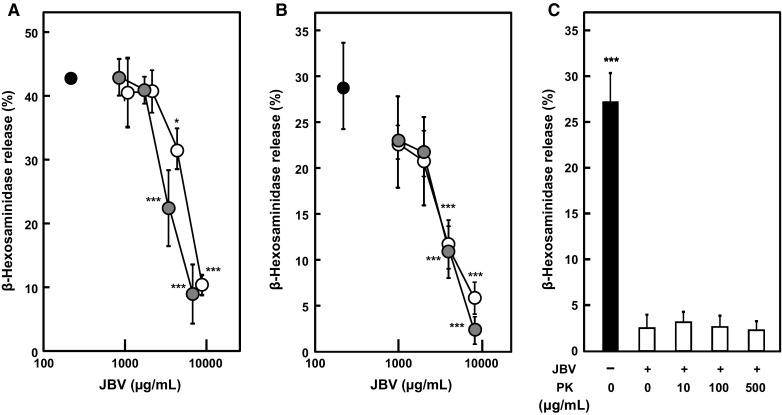
Effect of dialysis, heat treatment, and proteinase-K digestion of JBV on degranulation of RBL-2H3 cells. a JBV was dialyzed using a dialysis membrane of molecular weight cut off of 3500. Then, anti-DNP IgE-sensitized RBL-2H3 cells were treated with dialyzed (gray circle) or non-dialyzed (open circle) JBV at indicated concentrations or with NaPB (closed circle) as control. Degranulation was induced by treating the cells with DNP-HSA. Released β-hexosaminidase was used as a marker of degranulation. Data are presented as mean ± SD (n = 3). *P < 0.05; ***P < 0.001 against control by Dunnett’s multiple comparison test. b JBV was heated at 100 °C for 15 min. Then, anti-DNP IgE-sensitized RBL-2H3 cells were treated with heated (gray circle) or non-heated (open circle) JBV at indicated concentrations or with NaPB (closed circle) as control. Degranulation was induced by treating the cells with DNP-HSA. Released β-hexosaminidase was used as a marker of degranulation. Data are presented as mean ± SD (n = 3). ***P < 0.001 against control by Dunnett’s multiple comparison test. c JBV (2.0 mg/mL) was treated with 10, 100, or 500 μg/mL of proteinase K for 16 h at 37 °C. Then, anti-DNP IgE-sensitized RBL-2H3 cells were treated with proteinase K-digested or non-digested JBV or with NaPB. Degranulation was induced by treating the cells with DNP-HSA. Released β-hexosaminidase was used as a marker of degranulation. Data are presented as mean ± SD (n = 3). ***P < 0.001 against cells treated with non-digested JBV by Dunnett’s multiple comparison test
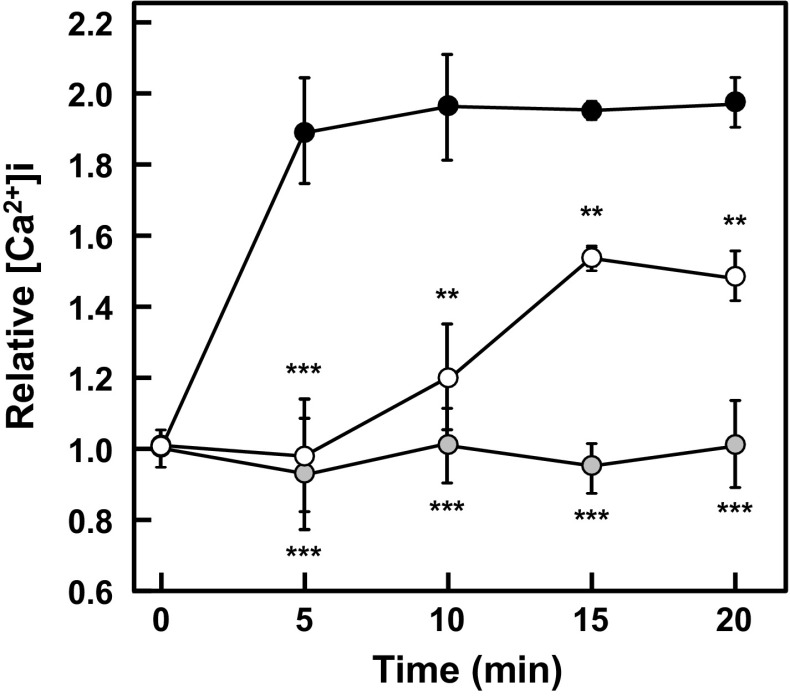
Effect of JBV on [Ca2+]i
Ca2+ is one of major second messengers in the intracellular signaling, and the elevation in [Ca2+]i is a critical process in mast cell degranulation. Thus, the effect of JBV on [Ca2+]i in RBL-2H3 cells was examined. As shown in Fig. 4, the antigen-induced elevation in [Ca2+]i was suppressed by treating RBL-2H3 cells with JBV. This result suggested that JBV would be involved in an intracellular event leading to the elevation in [Ca2+]i to suppress degranulation of RBL-2H3 cells.
Fig. 4.
Effect of JBV on [Ca2+]i. After anti-DNP IgE-sensitized RBL-2H3 cells were incubated with Fluo-3 AM, the cells were treated with JBV (5.4 mg/mL) or with 10 mM NaPB as control. Fluorescence intensity was measured immediately after inducing degranulation by treating with DNP-HSA. Closed circle, NaPB-treated cells stimulated with antigen; open circle, JBV-treated cells stimulated with DNP-HSA; gray circle, NaPB-treated cells not stimulated with antigen. Data are presented as mean ± SD (n = 3). **P < 0.01; ***P < 0.001 against NaPB-treated cells stimulated with antigen by Dunnett’s multiple comparison test
Effect of JBV on signaling pathways involved in antigen-induced degranulation
The effect of JBV on signaling pathways involved in the antigen-induced degranulation was examined by immunoblot analysis. As shown in Fig. 5, phosphorylation of PI3K, Akt, and PLCγ1 significantly decreased by treating RBL-2H3 cells with JBV, whereas there was no effect on the phosphorylation of Lyn, Syk, or PLCγ2 by JBV treatment. These results indicated that JBV suppresses the antigen-induced degranulation of RBL-2H3 cells through inhibiting the signaling pathways involved in degranulation.
Fig. 5.
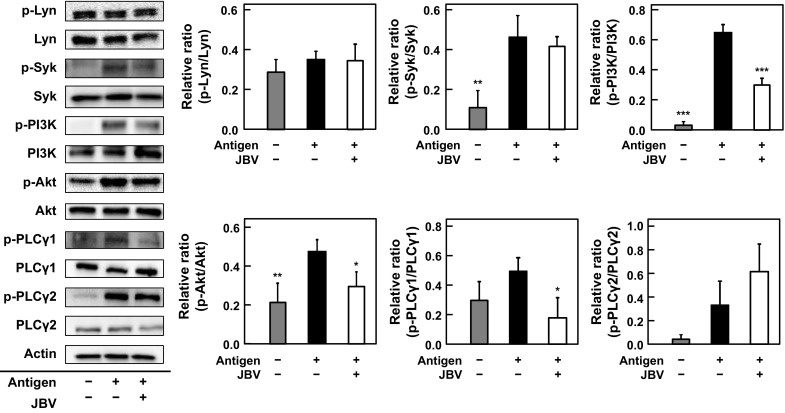
Effect of JBV on signaling pathways involved in degranulation of RBL-2H3 cells stimulated by antigen. After anti-DNP IgE-sensitized RBL-2H3 cells were treated with JBV (5.4 mg/mL) or with 10 mM NaPB as control, degranulation was induced by treating with DNP-HSA. After 10 min, each cell lysate was prepared and immunoblotting was performed. A representative blot from three independent experiments is shown. The p-Lyn, p-Syk, p-PI3K, p-Akt, p-PLCγ1, and p-PLCγ2 indicate phosphorylated Lyn, phosphorylated Syk, phosphorylated PI3K, phosphorylated PLCγ1, and phosphorylated PLCγ2, respectively. The result of densitometric analysis is expressed as the ratio of the amount of phosphorylated protein to that of whole protein. Data are presented as mean ± SD (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001 against NaPB-treated cells stimulated with antigen by Dunnett’s multiple comparison test
Effect of JBV in PCA model mice
The antiallergic activity of JBV was evaluated in vivo using PCA model mice in a blinded manner. Anti-DNP IgE-injected mice were orally administered low-dose JBV (25 mg/kg body weight), high-dose JBV (250 mg/kg body weight), or 10 mM NaPB (vehicle). PCA reaction was induced by intravenous injection of DNP-HSA. As shown in Fig. 6, the PCA reaction was significantly suppressed by oral administration of high-dose JBV (P < 0.05 against control group), indicating that JBV could exhibit the inhibitory effect on mast cell degranulation in vivo.
Fig. 6.
Effect of JBV on IgE-mediated PCA model mice. Six-week-old female BALB/c mice were intravenously injected with DNP-HSA with 0.5% Evans blue 24 h after intradermal injection of anti-DNP IgE and PBS alone into left and right ears of mice, respectively. One hour before DNP-HSA injection, the anti-DNP IgE-injected mice were orally administered 20 µL of 10 mM NaPB to the control group, 20 µL of JBV at 25 mg/kg body weight to low-dose JBV-administered group, and 20 µL of JBV at 250 mg/kg body weight to high-dose JBV-administered group. Evans blue was extracted from each ear and the absorbance of the dye was measured at 620 nm. Absorbance of the dye extracted from left ear was subtracted with that from right ear. Data are presented as mean ± SD (n = 6). *P < 0.05 against control by Dunnett’s multiple comparison test
Effect of JBV in a mouse model of Japanese cedar pollinosis
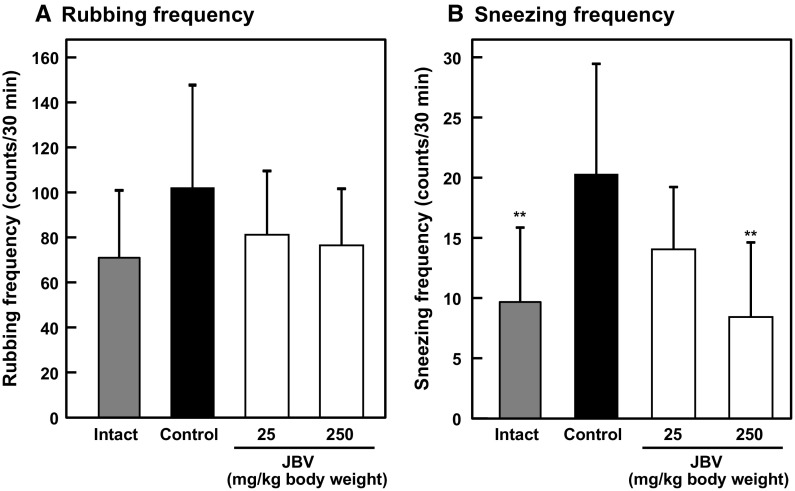
Finally, we investigated the effect of JBV on the IgE-mediated allergic reaction in a mouse model of Cry j1-induced pollinosis. Cry j1 is the major allergen of Japanese cedar (Cryptomeria japonica) pollens. Mice were sensitized and challenged with Cry j1 as shown in Fig. 1. The low-dose and the high-dose JBV-administered group mice were orally administered 20 µL of JBV at 25 and 250 mg/kg body weight, respectively, for 7 consecutive days from day 28 to day 34. The intact and control group mice were orally administered 20 µL of 10 mM NaPB. Oral administration of JBV did not affect the body weight of mice (data not shown). On day 34, all mice were treated with Cry j1, and sneezing and rubbing frequencies were counted in a blinded manner, which are clinical signs of hypersensitivity in pollinosis model mice (Tsunematsu et al. 2007). Although there was no significant difference among any groups, the rubbing frequency tended to decrease in JBV-administered group mice (Fig. 7a). In addition, the sneezing frequency significantly decreased in the high-dose JBV-administered group mice compared with that in the control group mice (P < 0.01) (Fig. 7b). These results suggested that JBV has a potential to attenuate the symptoms of Japanese cedar pollinosis in vivo. Biochemical analysis was performed by comparing the absorbance obtained from ELISA measuring the amount of immunoglobulins and cytokines in serum or culture medium of splenocytes from mice of each group. As shown in Fig. 8, there was a significant decrease in the absorbance of IgE, IgG1, and IL-4 measured in the serum of JBV-administered group mice compared with that of control group mice. On the contrary, the absorbance of IFN-γ was not affected by JBV administration. These results indicated that oral administration of JBV significantly decreased serum IgE, IgG1, and IL-4 levels, whereas the serum IFN-γ level was not affected. In addition, there was a significant decrease in the absorbance of IL-4 measured in the culture medium of splenocytes from high-dose JBV-administered group mice, whereas that of IFN-γ was not affected (Fig. 9). These results indicated that oral administration of high-dose JBV significantly decreased the IL-4 production by splenocytes, whereas the IFN-γ production was not affected. The effect of JBV on cytokine production in JBV-administered group was consistent between serum and splenocytes.
Fig. 7.
Effect of JBV on a mouse model of pollinosis. The low-dose and the high-dose JBV-administered group mice were orally administered 20 µL of JBV at 25 and 250 mg/kg body weight, respectively, for 7 consecutive days from day 28 to day 34. The intact and control group mice were orally administered 20 µL of 10 mM NaPB. On day 34, all mice were treated with 0.2 μg of Cry j1. Data are presented as mean ± SD (n = 8). **P < 0.01 against control by Dunnett’s multiple comparison test. a Nasal rubbing frequency was counted for 30 min after the final challenge on day 34. b Sneezing frequency was counted for 30 min after the final challenge on day 34
Fig. 8.
Effect of JBV on serum immunoglobulin and cytokine levels in Japanese cedar pollionosis model mice. Data are presented as mean ± SD (n = 8). *P < 0.05; ***P < 0.001 against control by Dunnett’s multiple comparison test. The absorbance of a IgE, b IgG1, c IFN-γ, and d IL-4 in serum from mice of each group was at 415 and 450 nm, respectively
Fig. 9.
Effect of JBV on cytokine productions by splenocytes isolated from Japanese cedar pollionosis model mice. Data are presented as mean ± standard deviation (n = 8). ***P < 0.001 against control by Dunnett’s multiple comparison test. The absorbance of a IFN-γ and b IL-4 in supernatant of splenocytes from mice of each group was measured at 415 and 450 nm, respectively
Discussion
In this study, JBV was revealed to suppress the antigen-induced degranulation of RBL-2H3 cells (Fig. 2). The bioactive ingredients in JBV were suggested to be heat-stable and protease-resistant, and their molecular weight was estimated to be higher than 3500 (Fig. 3). Based on these data, a certain kind of polysaccharides in JBV was suggested as bioactive ingredients to suppress degranulation of RBL-2H3 cells. Some polysaccharides such as fucoidan and mannobiose have been reported to suppress mast cell degranulation (Yang et al. 2013; Tanino et al. 2016). JBV contains ingredients derived from various fungi during the fermentation process in addition to rice-derived components as raw materials. In addition, the rice-derived components may be modified by the fermentation. Although we are currently analyzing, the bioactive ingredients have not been identified yet.
The mechanism underlying the inhibition of degranulation by JBV was investigated. The signaling pathways involved in degranulation are initiated by phosphorylation of Src family non-receptor tyrosine kinases Lyn and Fyn after aggregation of FcεRI through cross-linkage of antigen to IgE. The activated Lyn phosphorylates another tyrosine kinase Syk, and the activated Syk phosphorylates LAT, which recruits growth-factor-receptor-bound protein 2-related adaptor protein (GADS), SH2-domain-containing leukocyte protein of 76 kDa (SLP76), and PLCγ1/2 and forms a LAT-complex (Kraft and Kinet 2007; Metcalfe et al. 2009). The PLCγ1/2 activation occurs through the LAT-complex formed after Syk activation and through PI3K-mediated membrane recruitment of Btk, subsequently inducing Ca2+ mobilization (Kraft and Kinet 2007). As shown in Fig. 4, JBV suppressed the elevation in [Ca2+]i. From Fig. 5, JBV suppressed the phosphorylation of PI3K, Akt, and PLCγ1, whereas JBV did not affect the phosphorylation of Lyn or Syk. The phosphorylation level of PLCγ2 in JBV-treated cells was higher than that in control cells with no significant difference between JBV-treated cells and control cells (Fig. 5). These results suggested that JBV did not affect the phosphorylation of PLCγ2. JBV suppressed the phosphorylation of PLCγ1, because it partially suppressed the activation of LAT-complex formed after Syk activation. Since Akt exists downstream of PI3K (Kraft and Kinet 2007; Metcalfe et al. 2009), it was suggested that the decreased phosphorylation level of Akt is due to the downregulated PI3K activation by JBV. These results suggested that JBV suppressed the phosphorylation of PI3K, thereby suppressing activation of downstream signals such as Akt and PLCγ1. PI3K is activated after binding to Gab2, which is a cytosolic adapter molecule and phosphorylated by Fyn, Syk, or both (Metcalfe et al. 2009; Siraganian et al. 2010). In addition, JBV did not affect the phosphorylation of Lyn or Syk, suggesting that JBV does not affect antigen–antibody interaction, which is the initial event of degranulation. These findings suggested that JBV suppresses degranulation through downregulating the signaling pathway involved the phosphorylation of PI3K and the elevation in [Ca2+]i.
We further investigated whether the outcomes of in vitro studies are involved in those of in vivo studies. We revealed that oral administration of high-dose JBV significantly inhibits the PCA symptom (Fig. 6) and reduced the sneezing frequency compared with the control group mice (Fig. 7b). Thus, JBV was revealed to exhibit the antiallergy activity in vivo. In PCA reaction, antigen-induced degranulation was induced only in ears of mice. Therefore, the result in this experiment showed that JBV suppresses the degranulation in ears of mice. It was suggested that JBV is carried by the blood stream to ears and acts on mast cells after being absorbed from digestive organ. Type I allergic reactions, including Japanese cedar pollinosis, are induced by a couple of immunoglobulin classes, which are vital biomarkers of allergy (Platts-Mills 2001). In addition, type I allergic state is involved in the production of IL-4 and IFN-γ, because IgE is a biomarker of allergic state and IL-4 is produced by Th2 cells and promotes IgE production by plasma cells. To improve the balance of Th1/Th2 cells, inhibition of Th2 cell function by Th1 cell activation has been reported (Zhu et al. 2006). The administration of polysaccharide contained in black vinegar to mice has been reported to increase the production of IFN-γ and IL-12 by splenocytes (Ueno et al. 2010). It has also been reported that black vinegar contains proteinaceous substances that induce IFN-γ production (Hashimoto et al. 2013). Our data, however, suggested that the suppression of Th2 cell function such as IL-4 production by JBV is not due to the activation of Th1 cells, because there was no change in the serum IFN-γ level (Fig. 8c) or in the IFN-γ production by splenocytes (Fig. 9a). JBV might suppress the function of Th2 cells by directly acting on Th2 cells without the activation of Th1 cells. In addition, it might interfere with the function of Th2 cells through a mechanism of action not involving Th1 cells. Its mechanism of action is currently under investigation. From these data, it was suggested that JBV directly and/or indirectly affects Th2 cells to suppress their function and to maintain the Th1/Th2 balance.
JBV completely inhibited degranulation in vitro, whereas the inhibition rate in vivo was about 50%. The absorption of JBV might be insufficient in 1 h, or the JBV metabolites may be less effective than JBV. Since the bioactive ingredient has not been identified yet, its absorption kinetics and metabolites are unknown. However, from our finding, we consider that there are two mechanisms of action that JBV shows antiallergy effect in vivo. First, the components derived from JBV are carried to each tissue by blood and directly suppress degranulation. Secondly, JBV activates intestinal bacteria and improves Th1/Th2 balance since the activity of Th2 cells was suppressed by the oral administration of JBV, leading to suppression of allergic reactions. To clarify the mechanism of action of JBV, it is necessary to analyze the metabolites in the blood of JBV-administered mice. Another possibility is the involvement of regulatory T cells (Treg cells). Treg cells differentiate from CD4+ naive T cells and produce transforming growth factor β and IL-10 to suppress the function of other CD4+ effector T cells such as Th1, Th2, and Th17 cells (Noval Rivas and Chatila 2016). Lactic acid bacteria have been reported to induce Treg cells (Shah et al. 2012). Similarly, JBV might have an effect to induce Treg cell activation.
Conclusion
JBV suppressed degranulation of RBL-2H3 cells. The bioactive ingredients in JBV were expected to be heat-stable and protease-resistant, and their molecular weight was estimated to be higher than 3500. The effect of JBV was shown to result from inhibition of the Lyn signaling pathway and of subsequent intracellular Ca2+ mobilization. Oral administration of JBV significantly suppressed an allergic reaction in PCA model mice and the sneezing frequency in a mouse model of Japanese cedar pollinosis. Taken together, these findings suggest that JBV has an antiallergy effect that controls mast cell degranulation and would be valuable as a functional food factor.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Animal experiments were accomplished at the Division of Genetic Research of the Advanced Research Support Center (ADRES), Ehime University.
Abbreviations
- BSA
Bovine serum albumin
- [Ca2+]i
Intracellular Ca2+ concentration
- DMEM
Dulbecco’s modified Eagle’s medium
- DNP
Dinitrophenol
- ELISA
Enzyme-linked immunosorbent assay
- FBS
Fetal bovine serum
- FcεRI
High-affinity IgE receptor
- HSA
Human serum albumin
- IFN-γ
Interferon-γ
- IgE
Immunoglobulin E
- IL-4
Interleukin-4
- JBV
Japanese black vinegar
- NaPB
Sodium phosphate buffer
- PBS
Phosphate-buffered saline
- PCA
Passive cutaneous anaphylaxis
- PI3K
Phosphoinositide 3-kinase
Compliance with ethical standards
Conflict of interest
Masanobu Nagano, Kazunori Hashiguchi, Akira Fujii, and Takuya Sugahara are inventors in a patent application filed by Ehime University, which disclose bioactive agents targeting mast cell degranulation described in the present article. The remaining authors declare no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s10616-018-0208-6) contains supplementary material, which is available to authorized users.
References
- Amin K. The role of mast cells in allergic inflammation. Respir Med. 2012;106:9–14. doi: 10.1016/j.rmed.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Baba K, Nakae K. National epidemiological survey of nasal allergy 2008 (compared with 1998) in otolaryngologists and their family members. Progress in Medicene. 2008;28:2001–2012. [Google Scholar]
- Fujii M, Hou D-X, Arimura M, Chiwata T, Suzuki M, Nagano M. Effect of Kurozu (brewed rice vinegar) on maintenance of primary culture of rat hepatocytes. Food Sci Technol Res. 1999;5:97–98. doi: 10.3136/fstr.5.97. [DOI] [Google Scholar]
- Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gell PGH, Coombs RRA (eds) (1963) The classification of allergic reactions underlying disease. In: Clinical aspects of immunology, Blackwell, London, pp 317–337
- Hashimoto M, Obara K, Ozono M, Furuyashiki M, Ikeda T, Suda Y, Fukase K, Fujimoto Y, Shigehisa H. Separation and characterization of the immunostimulatory components in unpolished rice black vinegar (kurozu) J Biosci Bioeng. 2013;116:688–696. doi: 10.1016/j.jbiosc.2013.05.029. [DOI] [PubMed] [Google Scholar]
- Ishida M, Nishi K, Watanabe H, Sugahara T. Inhibitory effect of aqueous spinach extract on degranulation of RBL-2H3 cells. Food Chem. 2013;136:322–327. doi: 10.1016/j.foodchem.2012.08.079. [DOI] [PubMed] [Google Scholar]
- Knoops L, Louahed J, Van Snick J, Renauld JC. IL-9 promotes but is not necessary for systemic anaphylaxis. J Immunol. 2005;175:335–341. doi: 10.4049/jimmunol.175.1.335. [DOI] [PubMed] [Google Scholar]
- Kondo M, Nishi K, Sugahara T. Ishizuchi dark tea suppresses IgE-mediated degranulation of RBL-2H3 cells and nasal rubbing behavior of pollinosis in mice. J Funct Foods. 2015;14:659–669. doi: 10.1016/j.jff.2015.02.045. [DOI] [Google Scholar]
- Kraft S, Kinet J-P. New developments in FcεRI regulation, function and inhibition. Nat Rev Immunol. 2007;7:365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- Lee S-H, Shin HJ, Kim D-Y, Shim D-W, Kim T-J, Ye S-K, Won H-S, Koppula S, Kang T-B, Lee K-H. Streptochlorin suppresses allergic dermatitis and mast cell activation via regulation of Lyn/Fyn and Syk signaling pathways in cellular and mouse models. PLoS ONE. 2013;8:e74194. doi: 10.1371/journal.pone.0074194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe DD, Peavy RD, Gilfillan AM. Mechanisms of mast cell signaling in anaphylaxis. J Allergy Clin Immunol. 2009;124:639–646. doi: 10.1016/j.jaci.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata N, Gon Y, Nunomura S, Endo D, Yamashita K, Matsumoto K, Hashimoto S, Ra C. Inhibitory effects of parthenolide on antigen-induced microtubule formation and degranulation in mast cells. Int Immunopharmacol. 2008;8:874–880. doi: 10.1016/j.intimp.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Nishi K, Teranishi M, Yasunaga S, Iitsuka M, Matsumoto S, Sugahara T. The major whey protein β-lactoglobulin inhibits IgE-mediated degranulation of RBL-2H3 cells and passive cutaneous anaphylaxis in mice. Int Dairy J. 2014;39:89–95. doi: 10.1016/j.idairyj.2014.05.006. [DOI] [Google Scholar]
- Nomiya R, Okano M, Fujiwara T, Maeda M, Kimura Y, Kino K, Yokoyama M, Hirai H, Nagata K, Hara T, Nishizaki K, Nakamura M. CRTH2 plays an essential role in the pathophysiology of Cry j 1-induced pollinosis in mice. J Immunol. 2008;180:5680–5688. doi: 10.4049/jimmunol.180.8.5680. [DOI] [PubMed] [Google Scholar]
- Noval Rivas M, Chatila TA. Regulatory T cells in allergic diseases. J Allergy Clin Immunol. 2016;138:639–652. doi: 10.1016/j.jaci.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi S, Nishi K, Yasunaga S, Muranaka A, Maeyama K, Kadota A, Sugahara T. Nobiletin, a polymethoxy flavonoid, exerts anti-allergic effect by suppressing activation of phosphoinositide 3-kinase. J Funct Foods. 2014;6:606–614. doi: 10.1016/j.jff.2013.12.005. [DOI] [Google Scholar]
- Passante E, Frankish N. The RBL-2H3 cell line: its provenance and suitability as a model for the mast cell. Inflamm Res. 2009;58:737–745. doi: 10.1007/s00011-009-0074-y. [DOI] [PubMed] [Google Scholar]
- Platts-Mills TAE. The role of immunoglobulin E in allergy and asthma. Am J Respir Crit Care Med. 2001;164:S1–S5. doi: 10.1164/ajrccm.164.supplement_1.2103024. [DOI] [PubMed] [Google Scholar]
- Shah MM, Saio M, Yamashita H, Tanaka H, Takami T, Ezaki T, Inagaki N. Lactobacillus acidophilus strain L-92 induces CD4+ CD25+ Foxp3+ regulatory T cells and suppresses allergic contact dermatitis. Biol Pharm Bull. 2012;35:612–616. doi: 10.1248/bpb.35.612. [DOI] [PubMed] [Google Scholar]
- Shimoji Y, Tamura Y, Nakamura Y, Nanda K, Nishidai S, Nishikawa Y, Ishihara N, Uenakai K, Ohigashi H. Isolation and identification of DPPH radical scavenging compounds in Kurosu (Japanese unpolished rice vinegar) J Agric Food Chem. 2002;50:6501–6503. doi: 10.1021/jf020458f. [DOI] [PubMed] [Google Scholar]
- Siraganian RP, Castro RO, Barbu EA, Zhang J. Mast cell signaling: the role of protein tyrosine kinase Syk, its activation and screening methods for new pathway participants. FEBS Lett. 2010;584:4933–4940. doi: 10.1016/j.febslet.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanino Y, Hashimoto T, Ojima T, Mizuno M. F-fucoidan from Saccharina japonica is a novel inducer of galectin-9 and exhibits anti-allergic activity. J Clin Biochem Nutr. 2016;59:25–30. doi: 10.3164/jcbn.15-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunematsu M, Yamaji T, Kozutsumi D, Murakami R, Kimura S, Kino K. Establishment of an allergic rhinitis model in mice for the evaluation of nasal symptoms. Life Sci. 2007;80:1388–1394. doi: 10.1016/j.lfs.2006.12.038. [DOI] [PubMed] [Google Scholar]
- Ueno T, Fujii A, Nagano M, Hou D-X, Fujii M. Antitumor and immunostimulation effects of Kurozu in tumor-bearing mice. Nippon Shokuhin Kagaku Kogaku Kaishi. 2010;57:408–413. doi: 10.3136/nskkk.57.408. [DOI] [Google Scholar]
- Watanabe J, Shinmoto H, Tsushida T. Coumarin and flavone derivatives from estragon and thyme as inhibitors of chemical mediator release from RBL-2H3 cells. Biosci Biotechnol Biochem. 2005;69:1–6. doi: 10.1271/bbb.69.1. [DOI] [PubMed] [Google Scholar]
- Yamada T, Saito H, Fujieda S. Present state of Japanese cedar pollinosis: the national affliction. J Allergy Clin Immunol. 2014;133:632–639. doi: 10.1016/j.jaci.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Yang C, Rupa P, Kanatani H, Nakamura A, Ibuki M, Mine Y. Therapeutic effects of β1, 4 mannobiose in a Balb/c mouse model of intranasally-induced pollen allergy. Allergol Int. 2013;62:65–76. doi: 10.2332/allergolint.12-OA-0473. [DOI] [PubMed] [Google Scholar]
- Yasunaga S, Kadota A, Kikuchi T, Kubo C, Nishi K, Sugahara T. Effect of concurrent administration of nobiletin and β-lactoglobulin on the symptoms of Japanese cedar pollinosis models in mice. J Funct Foods. 2016;22:389–397. doi: 10.1016/j.jff.2016.01.045. [DOI] [Google Scholar]
- Zhu J, Yamane H, Cote-Sierra J, Guo L, Paul WE. GATA-3 promotes Th2 responses through three different mechanisms: induction of Th2 cytokine production, selective growth of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res. 2006;16:3–10. doi: 10.1038/sj.cr.7310002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.