Abstract
A chemically defined platform basal medium and feed media were developed using a single Chinese hamster ovary (CHO) cell line that produces a monoclonal antibody (mAb). Cell line A, which showed a peak viable cell density of 5.9 × 106 cells/mL and a final mAb titer of 0.5 g/L in batch culture, was selected for the platform media development. Stoichiometrically balanced feed media were developed using glucose as an indicator of cell metabolism to determine the feed rates of all other nutrients. A fed-batch culture of cell line A using the platform fed-batch medium yielded a 6.4 g/L mAb titer, which was 12-fold higher than that of the batch culture. To examine the applicability of the platform basal medium and feed media, three other cell lines (A16, B, and C) that produce mAbs were cultured using the platform fed-batch medium, and they yielded mAb titers of 8.4, 3.3, and 6.2 g/L, respectively. The peak viable cell densities of the three cell lines ranged from 1.3 × 107 to 1.8 × 107 cells/mL. These results show that the nutritionally balanced fed-batch medium and feeds worked well for other cell lines. During the medium development, we found that choline limitation caused a lower cell viability, a lower mAb titer, a higher mAb aggregate content, and a higher mannose-5 content. The optimal choline chloride to glucose ratio for the CHO cell fed-batch culture was determined. Our platform basal medium and feed media will shorten the medium-development time for mAb-producing cell lines.
Keywords: Chinese hamster ovary (CHO) cells, Chemically defined medium, Choline, Fed-batch culture
Introduction
Monoclonal antibodies (mAbs) and Fc fusion proteins have become major drug modalities for the treatment of a wide range of diseases, especially in the areas of autoimmune/inflammatory disorders and oncology (Aggarwal 2014). Because mAb therapies usually require large doses over a long period of time, a large amount of mAb must be produced to meet the demands of clinical development and the commercial market. To meet the strong demand, significant efforts have been made by the biopharmaceutical industry to develop a high-yield cell culture process. Chinese hamster ovary (CHO) cell lines have been used most widely to produce mAbs, and there have been significant improvements in the CHO cell fed-batch culture process over the last few decades (Zhu 2012). Fed-batch cultures are used widely to produce therapeutic mAbs. The advantages of a fed-batch culture over other culture processes, such as batch, perfusion, and continuous cultures, are its higher product concentration, ease of operation, and faster development. Typical product concentrations of fed-batch processes were in the range of 0.1–1 g/L in the 1990s, which was then they improved to 1–10 g/L in the 2000s (Huang et al. 2010; Li et al. 2010; Wurm 2004). However, the advantages of the current high-yield fed-batch cultures over batch cultures have not been reported. Thus, the effect of the feed on the product concentration is not commonly recognized in the current high-yield fed-batch cultures, although previous studies have reported that fed-batch cultures have yielded product titers that are 7.6-fold greater in average than those of batch cultures (Sauer et al. 2000).
Faster development of a high-yield cell culture process has also been an area of focus among biopharmaceutical companies because of the increasing number of therapeutic mAb candidates and to shorten the period needed to reach Phase 1 clinical trials. The platform approach is a practical solution to enable faster development of cell culture processes. Platform fed-batch cell culture process generally consists of company-specific host cell line, expression vector, and cell culture medium and feed media. Platform cell culture medium and feed media are used based on the assumption that production cell lines derived from a common host cell line with a common expression vector will have similar nutritional requirements. However, no studies have examined the development of platform fed-batch culture medium and feed media to date.
Fetal bovine serum and animal-derived hydrolysates were used widely in culture media until the 1990s to produce therapeutic recombinant proteins, including mAbs. However, to eliminate the risk of contamination with adventitious agents, such as transmittable spongiform encephalopathy, serum-free media and animal-derived hydrolysate-free media were developed extensively and became increasingly common by the 2000s. Through these efforts, there was a substantial increase in the understanding of the nutritional requirements of CHO cells. Eventually, the development of chemically defined media and feed formulations, in combination with the adaptation of CHO cells to chemically defined media, led to mAb titers as high as 10 g/L (Huang et al. 2010; Lu et al. 2013).
Chemically defined media for mammalian cells have been of great interest among investigators since the 1960s. Ham (1965) first reported clonal growth of CHO cells in a chemically defined synthetic medium, F-12. Since then, the components of F-12 have been used in chemically defined media such as DMEM/F-12, which is the most widely used medium for serum-free cultures (Jayme et al. 1997). Choline chloride is a component of F12 medium. Choline was first identified in ox bile in 1862 (Strecker 1862). The nutritional importance of choline was first reported in 1932, when it was shown that a dietary choline deficiency caused a fatty liver in rodents (Best and Huntsman 1932). The major metabolic use for choline is as a precursor in the biosynthesis of phosphatidylcholine and sphingomyelin, two phospholipids that are components of biological membranes (Zeisel 2006). Choline is also a major source for methyl groups via its metabolite, betaine, which participates in S-adenosylmethionine synthesis pathways. It was reported previously that choline deprivation limited cell growth and induced cell death in a hybridoma cell culture (Ishaque and Al-Rubeai 2002). Fortifying medium with choline chloride in CHO cell batch cultures enhanced cell growth and mAb production (Kim et al. 2005). Despite the aforementioned importance of choline in mammalian cells, little attention has been paid to the optimal choline ratio in cell culture media, and no studies have reported the effect of choline limitation on the quality of recombinant proteins and mAbs.
In this study, we developed a chemically defined basal medium and feed media using a single CHO cell line. During the medium development, we found that a choline limitation during the fed-batch culture caused a lower cell viability, a lower mAb titer, a higher mAb aggregate content, and a higher mannose-5 content. Thus, we optimized the choline to glucose ratio in the feeds. The optimized medium and feeds yielded a 6.4 g/L mAb titer, which is 12-fold higher than that of the batch culture using a commercially available, chemically defined CD CHO medium. To determine whether the developed basal medium and feed media are widely applicable, they were tested on three different cell lines.
Materials and methods
Cell lines and culture medium
Four recombinant CHO cell lines were used in this study. Cell lines A, B, and C, expressing mAbs A, B, and C, respectively, were established using CHOK1SV cells as the host cell and the GS Gene Expression System provided by Lonza Biologics (Slough, Berkshire, UK). mAb A is a humanized IgG, while mAbs B and C are human IgG. Cell line A16 was obtained from cell line A via subcloning. The cell lines were passaged in CD CHO medium (Life Technologies, Carlsbad, CA, USA) containing methionine sulfoximine for selective pressure.
Chemically defined basal medium was developed for the fed-batch process by modifying the CD CHO medium with Takeda’s proprietary supplements. Protein-free, chemically defined feed media developed in-house were used for the fed-batch culture. These feed media contain many components, such as glucose, amino acids, nucleosides, vitamins, minerals and choline.
Culture conditions
A batch suspension culture was conducted in a 250-mL Erlenmeyer flask (Corning Corp., Corning, NY, USA) on an incubator shaker SCS-20RG-2 (Sanki Seiki, Osaka, Japan), with shaking at 140 rpm, at 37 °C, 8% CO2, and 90% humidity to avoid evaporation.
Fed-batch production cultures were conducted using either 3-L or 15-L glass bioreactors equipped with an ABLE Pack controller (ABLE Corp., Tokyo, Japan). Agitation was maintained at 127 rpm in the 3-L bioreactor and 110 rpm in the 15-L bioreactor. The temperature was maintained at 37 °C using a heating blanket. The pH was controlled by CO2 sparging and by adding either 7.5% sodium bicarbonate or 1.0 M sodium carbonate. Dissolved oxygen was controlled at 20% air saturation using an air overlay and oxygen sparging. Starting from day 2 or later, feed medium containing glucose was added continuously into the bioreactors to maintain the glucose concentration at 2 g/L. The feed rates of all feed media were determined in proportion to the feed rate of glucose.
Routine bioreactors offline measurements
Offline dissolved oxygen, dissolved CO2, and pH were measured daily with a RAPIDLab 348 blood gas analyzer (Siemens Healthcare Diagnostics, Frimley, UK). Cell viability was measured by trypan blue exclusion using a Vi-CELL XR cell counter (Beckman Coulter, Brea, CA, USA). Cell density was determined with a Z2 Coulter counter (Beckman Coulter). Viable cell density was calculated from the cell density and the cell viability. A portion of the broth from each daily sampling was centrifuged to obtain the supernatant for metabolite and product titer measurements. Glucose and lactate concentrations were determined with a BF-5 bioanalyzer (Oji Keisoku Kiki, Hyogo, Japan). Osmolality was measured with an Osmostat OM-6040 (Arklay, Kyoto, Japan).
The mAb titer was measured using a high-performance liquid chromatography (HPLC) system (Waters, Milford, MA, USA) with a UV detector and a POROS Protein A affinity column (Applied Biosystems, Foster City, CA, USA).
Metabolomics assay
Both cell and spent medium samples were collected for a metabolomics analysis. Cell culture broth containing 1 × 107 viable cells was removed from the bioreactor and centrifuged at 1000×g for 3 min in a refrigerated centrifuge RL-130 (TOMY, Tokyo, Japan). The supernatant was collected as spent medium and stored at − 80 °C. The cell pellet was suspended and washed twice using cold phosphate-buffered saline. After two washes, the cell pellet was frozen in liquid nitrogen and stored at − 80 °C. The cell pellet and spent medium samples were sent on dry ice to Metabolon (Durham, NC, USA). The metabolomics analysis was conducted at Metabolon using gas chromatography—and liquid chromatography–mass spectrometry (Lawton et al. 2008).
Antibody product quality analysis
To analyze the antibody quality, the antibody fraction in the culture supernatant was purified using a Protein A HP Spin-Trap (GE Healthcare, Chalfont St Giles, Buckinghamshire, UK). The percentage of antibody aggregates was determined by size-exclusion chromatography using an HPLC system (Waters) with a UV detector and a TSK Gel G3000 SW XL column (Tosoh, Tokyo, Japan).
Antibody deglycosylation was performed using glycopeptidase F (Takara Bio, Shiga, Japan). The obtained oligosaccharides were derivatized with 2-aminopyridine. After purification, pyridinylamino derivatives of the oligosaccharides were analyzed by reverse-phase HPLC with a fluorescence detector and Shim-pack CLC-ODS18 column (Shimadzu, Kyoto, Japan).
Results
Media development
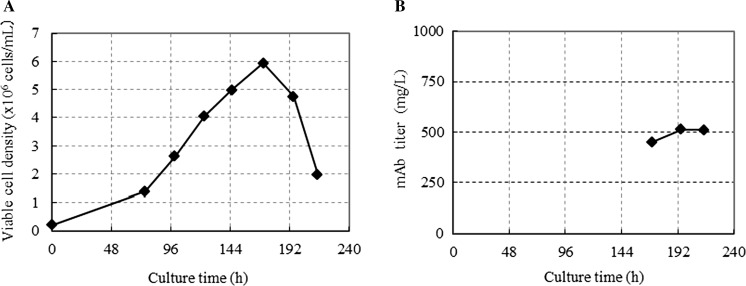
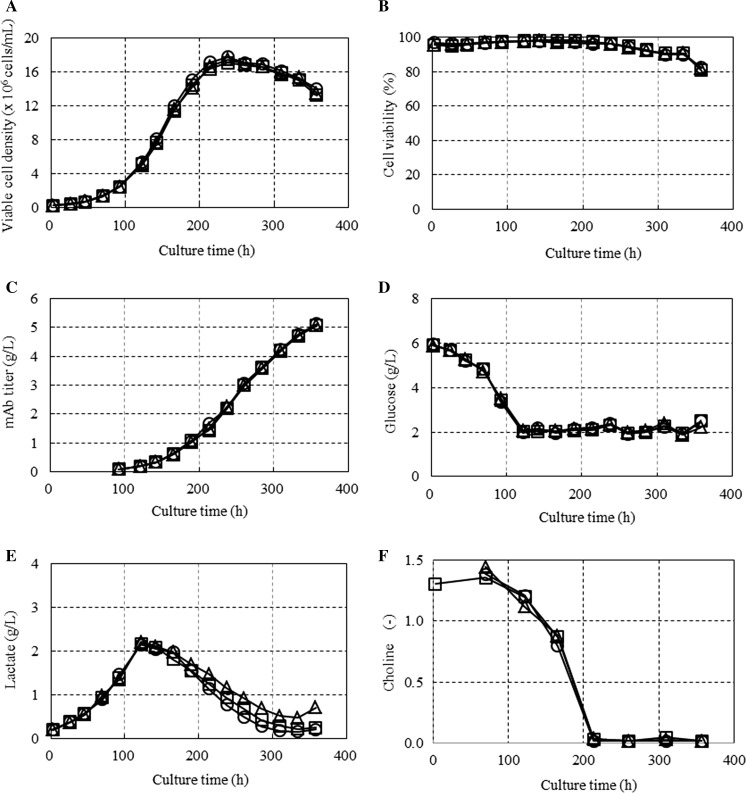
Chemically defined platform basal medium and feed media were developed using a single cell line, cell line A, expressing mAb A. Cell line A was selected for the platform media development because of its high peak-cell density and mAb production yield in a batch culture. The cell line showed a peak viable cell density of 5.9 × 106 cells/mL and a final mAb titer of 510 mg/L in CD CHO medium (Life Technologies) at 37 °C in a shake-flask batch culture (Fig. 1). The first fed-batch culture was conducted using a set of in-house prototype feed media. During the feed media development, the compositions of amino acids and minerals in the platform feed media were balanced stoichiometrically as described by Kuwae et al. (2005), using glucose as an indicator of cell metabolism. Briefly, the feed rate of glucose was determined based on the glucose consumption rate so that the glucose concentration was maintained at 2 g/L via continuous feeding or above 1 g/L via daily bolus feeding during the fed-batch culture. The feed rates of all the feed media were determined in proportion to the feed rate of glucose. After the fed-batch culture, the spent media were analyzed to determine whether the concentrations of amino acids and minerals were limiting or excessive for cell growth. In this study, a limiting nutrient was defined as a nutrient that decreased to zero mmol/L in the spent medium during the fed-batch culture. An excessive nutrient was defined as a nutrient that increased to above the initial concentration in the spent medium during the fed-batch culture. When limiting or excess components were found, the compositions of those nutrients in the feed media were balanced stoichiometrically against glucose. Meanwhile, the ratios of other nutrients, such as vitamins and amines, relative to glucose in the feed media were determined through titration in a group of nutrients in a fed-batch culture because of the lack of an in-house capability to quantitatively analyze them in the spent media. Formulations of the feed media were designed to prevent precipitation and discoloration of the feed components so that the feed media could be stored at room temperature for at least 1 month. To this end, some nutrients were eliminated from the feed media and then enriched in the basal medium as needed. Thus, the basal medium for the fed-batch culture was developed in parallel with the feed media to maximize the mAb production titer. To evaluate the performance of the platform basal medium and feed media, a fed-batch culture of cell line A was performed using a 10-L bioreactor in triplicate as described in the Materials and Methods. The cells were seeded at 2.5 × 105 cells/mL, and they reached a peak viable cell density of 1.7 × 107 cells/mL at 237 h of cultivation (Fig. 2a). The feed medium containing glucose was fed continuously after 93 h of cultivation to maintain the glucose concentration at 2 g/L (Fig. 2d). The cell viability was maintained above 80% until 357 h of cultivation (Fig. 2b). The mAb titer reached 5 g/L at 357 h of cultivation (Fig. 2c). Thus, the first platform basal medium and feed medium increased the mAb production titer 10-fold compared with the batch culture in CD CHO medium in which the final MAb titer was 510 mg/L. The lactate profile showed that lactate metabolism changed from the production phase to the consumption phase at 122 h of cultivation (Fig. 2e). To further improve the basal medium and feed medium, a metabolome analysis was performed on the cell lysate and the spent media of the fed-batch culture. Among the metabolites analyzed, choline was found to be one of the potential limiting nutrients in the fed-batch culture. The choline concentration in the spent medium declined to almost zero at 213 h of cultivation (Fig. 2f), while the feed medium containing choline was fed continuously after 93 h of cultivation. The timepoint of choline depletion nearly coincided with the timepoint of the peak viable cell density and the onset of the decrease in cell viability.
Fig. 1.
Batch culture of cell line A in CD CHO medium. Cells were cultured at 37 °C in a shake-flask. a Cell growth; and b mAb titer
Fig. 2.
Fed-batch culture of cell line A. Cells were seeded at 2.5 × 105 cells/mL in a 10-L bioreactor and cultured at 37 °C in triplicate. Feed medium containing glucose was fed continuously. a Cell growth; b cell viability; c the mAb titer; d the glucose concentration in the spent medium; e the lactate concentration in the spent medium; and f the choline concentration in the spent medium
Effect of choline enrichment
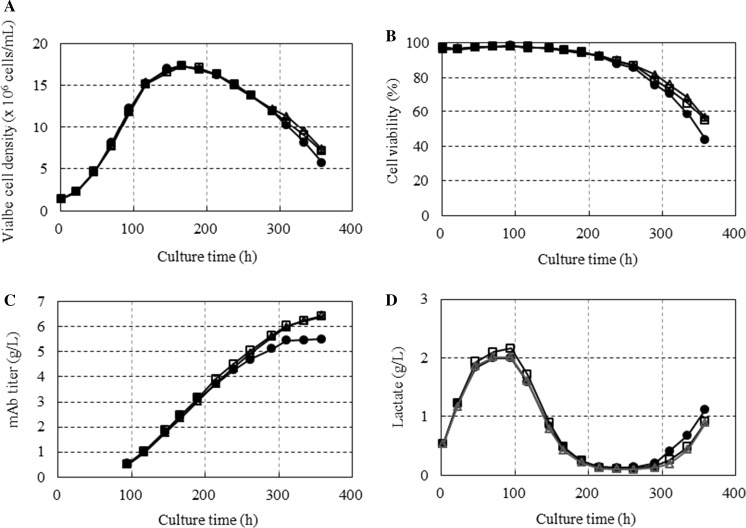
To determine whether choline was a limiting nutrient in the fed-batch culture, twofold and fourfold choline-enriched feed media were prepared and evaluated in fed-batch cultures using cell line A. The twofold and fourfold choline enriched feed medium, respectively, contained twofold and fourfold choline concentration of the original feed medium. The cells were seeded at 1.4 × 106 cells/mL in a 2-L bioreactor, and they reached a peak viable cell density of 1.7 × 107 cells/mL at 166 h of cultivation (Fig. 3a). A sixfold increase in the initial viable cell density resulted in the cell density peaking 3 days earlier compared with the previous cultures shown in Fig. 2a. Although there was no difference in the peak viable cell density among the three different choline conditions, the twofold and fourfold choline-enriched cultures showed a milder decrease in the slope of the cell viability compared with the onefold choline culture (Fig. 3b). The cell viabilities at 358 h of cultivation were 43.7, 55.0, and 56.4%, respectively, for the onefold, twofold, and fourfold choline-enriched cultures. A 16% increase in the mAb titer was observed for the twofold and fourfold choline-enriched cultures compared with the onefold choline culture (Fig. 3c). Both the twofold and fourfold choline-enriched cultures yielded a 6.4 g/L mAb titer at 358 h of cultivation. Thus, the choline-enriched fed-batch culture process increased the mAb production titer 12-fold compared with the batch culture in CD CHO medium, in which the final mAb titer was 510 mg/L. The time courses of the lactate concentration in the culture were very similar among the three choline concentrations. However, the lactate concentrations of the two choline-enriched cultures were slightly lower than that of the onefold choline culture after 300 h of cultivation.
Fig. 3.
Fed-batch cultures of cell line A under three different choline concentrations. Cells were seeded at 1.4 × 106 cells/mL in a 2-L bioreactor and cultured at 37 °C. a Cell growth; b cell viability; c mAb titer; and d lactate concentration in the spent medium. The 1×, 2×, and 4× choline-enriched fed batch cultures are represented by (circle), (square), and (triangle), respectively. The choline concentration of the feed medium used in the 1× choline fed batch culture was the same as that of the original feed medium
Because the choline-enriched cultures improved cell viability and the mAb titer, we evaluated the effect of the choline-enriched culture on the quality of mAb A. Among the quality attributes, we paid attention to both the mAb aggregate content and the glycosylation profile because unusually high levels of mAb aggregates and mannose-5 were observed during the development of multiple cell lines expressing mAb A (data not shown). As shown in Table 1, the aggregate content of mAb A decreased with increasing choline contents in the feed medium. The aggregate content decreased from 13.3 to 9.1% by enriching choline fourfold in the feed medium. For the glycosylation profile, the mannose-5 content of mAb A also decreased from 6.62 to 3.63% following a fourfold enrichment of choline in the feed medium (Table 2). The results described above show that the choline enrichment improved the mAb titer and product quality. These results suggest that choline was a limiting nutrient in the first platform fed-batch process. Other potential limiting nutrients that were found by the metabolome analysis were evaluated in the same manner as for choline, but no other limiting nutrients were found (data not shown). Thus, the feed formulation with the fourfold choline enrichment was finally selected and incorporated into the second platform basal medium and feed media.
Table 1.
Effect of enriched choline on the mAb titer and aggregate content
| Relative level of choline in the feed medium | 1× | 2× | 4× |
|---|---|---|---|
| mAb titer (g/L) | 5.5 | 6.4 | 6.4 |
| Aggregates (%) | 13.3 | 10.5 | 9.1 |
Table 2.
Effect of choline on the glycosylation profile of mAb A
| Relative level of choline in the feed medium | 1× | 2× | 4× |
|---|---|---|---|
| Mannose 5 | 6.62 | 3.74 | 3.63 |
| G0a | 16.67 | 17.28 | 17.66 |
| G1a | 3.90 | 3.22 | 2.98 |
| G2a | 0.69 | 0.31 | 0.32 |
| G0Fb | 54.13 | 59.08 | 60.02 |
| G1Fb | 11.30 | 12.01 | 11.35 |
| G2Fb | 1.02 | 1.11 | 0.97 |
aG0, G1 and G2 are non-fucosylated oligosaccharides terminated by 0, 1 and 2 galactoses, respectively
bG0F, G1F and G2F are fucosylated oligosaccharides terminated by 0, 1 and 2 galactoses, respectively
Evaluation of platform media and feeds
As described above, the platform basal medium and feed media were developed using cell line A. To determine whether the second platform basal medium and feed media can work for other cell lines, fed-batch cultures of three different cell lines, A16, B, and C, were performed using a 2-L bioreactor. Cell line A16 is a cloned cell line from cell line A. Cell lines B and C express mAbs B and C, respectively. The seeding viable cell densities of cell lines A16, B, and C were 1.2 × 106, 5 × 105, and 3 × 105 cells/mL, respectively. The peak viable cell density of each cell line was greater than 1.3 × 107 cells/mL (Fig. 4a). Cell lines A16, B, and C yielded mAb titers of 8.4, 3.3, and 6.2 g/L, respectively, at harvest (Fig. 4c). Although cell line A16 showed the lowest peak viable cell density and the lowest viability after 200 h of cultivation among the three cell lines, it showed the highest mAb titer (Fig. 4b). Lactate was produced by the three cell lines until 100 h of cultivation, but it was consumed after 100 h of cultivation and then its concentration decreased to less than 1 g/L (Fig. 4e). The osmolality was maintained between 300 and 450 mOsm/kg throughout the cultivation of each cell line (Fig. 4f). These results show that the platform basal medium and feed medium developed using cell line A worked well for the other CHO cell lines.
Fig. 4.
Fed-batch culture of three different cell lines in the platform basal medium and feeds. Cell lines A16, B, and C were seeded at 1.2 × 106, 5 × 105, and 3 × 105 cells/mL, respectively, and cultured at 37 °C. a Cell growth; b cell viability; c mAb titer; d glucose concentration in the spent medium; e lactate concentration in the spent medium; and f osmolality of the spent medium. Cell lines A16, B, and C are represented by (□), (▲), and (●), respectively
Discussion
To shorten the time required to move an antibody drug candidate from the laboratory bench to a clinical trial, the establishment of a high-yield platform basal medium and feed medium for cell culture is highly desired because the development of cell culture medium takes at least several months for each mAb production cell line. To develop an in-house platform basal medium and feed medium we used the following three strategies. The first strategy was to develop a chemically defined formulation because it enables the rational development of a stoichiometrically balanced feed to avoid both limiting and excess nutrients during a fed-batch culture. In the second strategy, we selected a single CHO cell line that showed a high peak viable cell density in a batch culture in a chemically defined medium to develop a platform basal medium and feed formulation, because we assumed that a cell line that grows well in batch culture has an efficiently balanced nutrient requirement ratio and this balanced nutrient requirement ratio is potentially common to other high mAb-producing CHO cell lines that grow well. Third, we selected glucose as an indicator of cell metabolism to determine the feed rate of all other nutrients because glucose can be measured easily and accurately off-line and on-line. The successful use of glucose as an indicator of cell metabolism to determine the feed rates of other nutrients was reported previously (Kuwae et al. 2005; Lu et al. 2013). The validity of these three strategies was proven by this study, as the mAb titers obtained by the three different cell lines in the platform medium reached 8.4, 3.3, and 6.2 g/L, respectively, without any optimization for each cell line. The peak viable cell densities of the three cell lines ranged from 1.3 × 107 to 1.8 × 107 cells/mL. The metabolic shift from lactate production to lactate consumption was observed during the fed-batch culture of the three cell lines. This type of lactate metabolic shift was typically observed previously in a nutritionally well-balanced fed-batch culture (Kuwae et al. 2005; Luo et al. 2012; Ma et al. 2009, Zhou et al. 1997).
During the feed medium optimization, we found that the choline limitation during the fed-batch culture caused a lower cell viability, a lower mAb titer, a higher mAb aggregate content, and a higher mannose-5 content. Choline is required for the biosynthesis of phosphatidylcholine, which is a major constituent of cell membranes. Choline limitation may produce defective Golgi membranes, thereby leading to more mAb aggregates and a higher mannose-5 content. Choline is also an essential component of cell culture media, and it has been included as choline chloride in Eagle’s minimal essential medium (MEM) (Eagle 1959), Dulbecco’s modified Eagle medium (DMEM) (Dulbecco and Freeman 1959), and Ham’s F12 nutrient mixture (Ham 1965). However, the ratios of choline chloride to glucose (g-choline chloride/g-d-glucose) in MEM, DMEM, and F-12 were 0.001, 0.00089, and 0.0079, respectively (up to a ninefold difference). For our feed medium, the ratio of choline chloride to glucose was 0.00285 in the onefold choline culture condition (Fig. 5), and this ratio was choline-limiting during the fed-batch culture of cell line A. Both the twofold and fourfold choline culture resulted in a 16% increase in the mAb titer and also resulted in the similar mAb quality improvement compared with the onefold choline culture. Therefore we concluded that choline to glucose ratios of 0.0057 and 0.0114 were optimal in terms of the mAb production titer and the mAb quality for the fed-batch culture of cell line A. To date, no studies have addressed the optimal choline ratio in the medium for CHO cell fed-batch cultures. This is the first report that describes the optimal choline ratio in the medium and its effect on the mAb production titer and mAb quality in a CHO cell fed-batch culture. We found that both a high mAb aggregate content and a high mannose-5 content are inherent characteristics of mAb A; a high mAb aggregate content and a high mannose-5 content were not observed for mAbs B and C that were produced using the platform basal medium and feed media (data not shown). Thus, the selection of cell line A that produces mAb A revealed that choline limitation can impact the mAb aggregate content and the mannose-5 content.
Fig. 5.
The choline chloride to glucose ratio. The choline chloride to glucose ratio of the in-house feed media with 1×, 2×, and 4× choline concentrations were compared to Eagle’s minimal essential medium (MEM), F-12 medium, and Dulbecco’s modified Eagle medium (DMEM)
Acknowledgements
The authors thank Prof. Takeshi Omasa at Tokushima and Osaka University for valuable suggestions and discussions in writing the manuscript. We thank Scott Lloyd, Ph.D., from Edanz Group (www.edanzediting.com/ac), for editing a draft of this manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Aggarwal RS. What’s fueling the biotech engine—2012 to 2013. Nat Biotechnol. 2014;32:32–39. doi: 10.1038/nbt.2794. [DOI] [PubMed] [Google Scholar]
- Best CH, Huntsman MF. The effects of the components of lecithine upon deposition of fat in the liver. J Physiol Lond. 1932;75:405–412. doi: 10.1113/jphysiol.1932.sp002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R, Freeman G. Plaque production by the polyoma virus. Virology. 1959;8:396–397. doi: 10.1016/0042-6822(59)90043-1. [DOI] [PubMed] [Google Scholar]
- Eagle H. Amino acid metabolism in mammalian cell cultures. Science. 1959;130:432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Ham RG. Clonal growth of mammalian cells in a chemically-defined, synthetic medium. Proc Natl Acad Sci USA. 1965;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YM, Hu W, Rustandi E, Chang K, Yusuf-Makagiansar H, Ryll T. Maximizing productivity of CHO cell-based fed-batch culture using chemically defined media conditions and typical manufacturing equipment. Biotechnol Prog. 2010;26:1400–1410. doi: 10.1002/btpr.436. [DOI] [PubMed] [Google Scholar]
- Ishaque A, Al-Rubeai M. Role of vitamins in determining apoptosis and extent of suppression by bcl-2 during hybridoma cell culture. Apoptosis. 2002;7:231–239. doi: 10.1023/A:1015343616059. [DOI] [PubMed] [Google Scholar]
- Jayme D, Watanabe T, Shimada T. Basal medium development for serum-free culture: a historical perspective. Cytotechnology. 1997;23:95–101. doi: 10.1023/A:1007967602484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Lee JC, Chang HN, Oh DJ. Effects of supplementation of various medium components on Chinese hamster ovary cell cultures producing recombinant antibody. Cytotechnology. 2005;47:37–49. doi: 10.1007/s10616-005-3775-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwae S, Ohda T, Tamashima H, Miki H, Kobayashi K. Development of a fed-batch culture process for enhanced production of recombinant human antithrombin by Chinese hamster ovary cells. J Biosci Bioeng. 2005;100:502–510. doi: 10.1263/jbb.100.502. [DOI] [PubMed] [Google Scholar]
- Lawton KA, Berger A, Mitchell M, Milgram KE, Evans AM, Guo L, Hanson RW, Kalhan SC, Ryals JA, Milburn MV. Analysis of the adult human plasma metabolome. Pharmacogenomics. 2008;9:383–397. doi: 10.2217/14622416.9.4.383. [DOI] [PubMed] [Google Scholar]
- Li F, Vijayasankaran N, Shen A, Kiss R, Amanullah A. Cell culture processes for monoclonal antibody production. mAbs. 2010;2:466–477. doi: 10.4161/mabs.2.5.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Toh PC, Burnett I, Li F, Hudson T, Amanullah A, Li J. Automated dynamic fed-batch process and media optimization for high productivity cell culture process development. Biotechnol Bioeng. 2013;55:191–205. doi: 10.1002/bit.24602. [DOI] [PubMed] [Google Scholar]
- Luo J, Vijayasankaran N, Autsen J, Santuray R, Hudson T, Amanullah A, Li F. Comparative metabolic analysis to understand lactate metabolism shift in Chinese hamster ovary cell culture process. Biotechnol Bioeng. 2012;109:146–156. doi: 10.1002/bit.23291. [DOI] [PubMed] [Google Scholar]
- Ma N, Ellet J, Okediadi C, Hermes P, McCormick E, Casnocha S. A single nutrient feed supports both chemically defined NS0 and CHO fed-batch process: improved productivity and lactate metabolism. Biotechnol Prog. 2009;25:1353–1363. doi: 10.1002/btpr.238. [DOI] [PubMed] [Google Scholar]
- Sauer PW, Burky JE, Wesson MC, Sternard HD, Qu L. A high-yielding, generic fed-batch cell culture process for production of recombinant antibodies. Biotechnol Bioeng. 2000;67:585–597. doi: 10.1002/(SICI)1097-0290(20000305)67:5<585::AID-BIT9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Strecker A. Uber eingige neue bestandtheile der schweinegalle. Ann Chem Pharmacie. 1862;183:964–965. [Google Scholar]
- Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004;22:1393–1398. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]
- Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Chen C-C, Buckland B, Aunins J. Fed-batch culture of recombinant NS0 myeloma cells with high monoclonal antibody production. Biotechnol Bioeng. 1997;55:783–792. doi: 10.1002/(SICI)1097-0290(19970905)55:5<783::AID-BIT8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Zhu J. Mammalian cell protein expression for biopharmaceutical production. Biotechnol Adv. 2012;30:1158–1170. doi: 10.1016/j.biotechadv.2011.08.022. [DOI] [PubMed] [Google Scholar]