Abstract
Recent advances in the bioengineering field have introduced new opportunities enabling cell encapsulation in three-dimensional (3D) structures using either various natural or synthetic materials. However, such hydrogel scaffolds have not been fully biocompatible for cell cultivation due to the lack of physical stability or bioactivity. Here, we utilized a uniquely fabricated semi-synthetic 3D polyethylene glycol-fibrinogen (PEG-Fb) hydrogel scaffold, which exhibits both high stability and high bioactivity, to encapsulate HEK293 cells for the production of human recombinant acetylcholine esterase (AChE). To examine the beneficial bioactive effect of the PEG-Fb scaffold over 2D surfaces, an experimental system was established to compare the viability, proliferation and AChE secretion of encapsulated cells versus non-encapsulated surface-adherent cells in serum starvation. Our results show that the transfer of surface-adherent HEK293 cells from fully enriched medium with 10% FCS to 0.2% FCS resulted in an eightfold reduction in cell number and a fourfold reduction in AChE production. In contrast, the encapsulated cells were highly viable and about twofold more efficient in AChE production. In addition, they had round morphology with a twofold larger cell diameter, supporting the observation of increased AChE production. These results suggest a role of the PEG-Fb scaffold in providing a supportive microenvironment in reduced serum conditions that enhances encapsulated cell functions, opening new directions to study the implementation of this platform in large-scale pharmaceutical protein production.
Keywords: Hydrogel, Scaffold, Poly(ethylene glycol), Fibrinogen, 3D cultivation, Continuous cell line (CCL)
Introduction
Mammalian cells have become a vital tool for studying animal cell function and are commonly used in biotechnological processes for the production of vaccines and therapeutic proteins. Over the last 20 years, these processes have mainly been performed using a few continuous cell lines (CCL) that have been cultivated in serum-containing media (Wurm 2004; Barrett et al. 2009; Dumont et al. 2016). Traditionally, the cultivation of mammalian cell lines has been confined to two-dimensional (2D) tissue culture polystyrene surfaces in serum-containing media and was limited by the flask’s surface area. A step forward in up-scaling the technology was achieved by the introduction of microcarrier-based systems. This methodology enabled the growth of anchorage-dependent cells in suspension culture (Kumar et al. 1999; Hu et al. 2000), allowing the transition from cell cultivation in flasks to stirred bioreactors controlled by basic process parameters. However, despite the technical value added to bioprocess developments, microcarrier-based systems have not led to a conceptual shift in the culture platform but, rather, have led to a revised form of cell cultivation in 2D.
Recently, new research directions in biomaterials have been opened for the growth of anchored cells in suspension with the development of three-dimensional (3D) scaffolds based on hydrogels. These hydrogel scaffolds consisted of a variety of different precursor materials belonging to two main categories. The first category is natural derived hydrogels such as collagen (Butcher and Nerem 2004) and alginate (Barralet et al. 2005), which support cell function but have weak mechanical properties and are not physically stable. The second category is fully synthetic hydrogels made of non-biological molecules such as poly(ethylene glycol) (PEG) (Bryant and Anseth 2002). These synthetic hydrogels are mechanically stable, reproducible and are easily modified and processed but lack the bioactivity that natural endogenous ECM molecules exhibit. Thus, neither hydrogel category has performed adequately in fulfilling the requirements for long-term and stable cell cultivation in 3D culture.
Previously, a novel semi-synthetic hydrogel scaffold that combines an endogenous ECM biomolecule—fibrinogen—and a synthetic PEG polymer was designed for 3D cell culture (Almany and Seliktar 2005). The fibrinogen molecules were conjugated to acrylated PEG by a Michael-type addition reaction to form PEG-fibrinogen (PEG-Fb) precursors. Spherical PEG-Fb hydrogels were formed by photocrosslinking the water-born precursors using long-wavelength ultraviolet light. The PEG-Fb hydrogels are biocompatible for encapsulating cells due to the bioactivity of the fibrinogen, yet structurally stable due to the PEG. Different biomedical and tissue engineering implementations using the PEG-Fb platform have been described, such as regulation of neuronal morphogenesis (Berkovitch and Seliktar 2017), regeneration of heart tissue (Plotkin et al. 2014; Kerscher et al. 2016) and modeling of 3D tumor microsphere cultivation (Pradhan et al. 2016).
From a biotechnological perspective, the PEG-Fb microsphere technique could provide advantages in processes of protein production using 3D mammalian cell culture systems due to their exceptional combined attributes. These include a supportive microenvironment and protection against physical and chemical stress, separation of encapsulated cells from the secreted product, operation in fed batch culture and continuous culture, fewer of handling steps and process robustness. Another aspect is the growing demand in the pharmaceutical industry to limit the use of serum due to the potential risk of transmitting agents such as viruses to the final product (Grachev et al. 1998). Today, the common method of adapting cells to grow in serum-free media involves a gradual serum reduction that might result in genotype and phenotype transformations (Barnes et al. 2006; Abdeen et al. 2011) or reduced cell productivity. There is a great need to support cell viability and productivity in a reduced serum environment via other pathways, and the supportive microenvironment of the PEG-Fb hydrogel system can potentially address this need. Herein, we addressed those issues and examined whether cells encapsulated in 3D PEG-Fb hydrogels have bioprocessing and protein production advantages over 2D surface-adherent cells. To that end we used HEK293 cells producing acetylcholine esterase (AChE) as a model and cultivated them under extreme conditions of serum starvation to evaluate the added value of encapsulation. The results showed that the encapsulated cells were more resistant to a direct transfer to serum starvation media with higher viability and AChE productivity compared with 2D surface-adherent cells.
Materials and methods
Cell culture and maintenance
Human embryonic kidney (HEK)-293 cells genetically manipulated to stably produce recombinant human acetylcholine esterase (rHuAChE) (Kronman et al. 1992) were cultured in DMEM (Biological Industries, Beit HaEmek, Israel) supplemented with 10% (v/v) Fetal Calf Serum (FCS) (Biological Industries), 0.2% (v/v) penicillin/streptomycin (Pen/Strep) (Biological Industries), and 2 mM l-glutamine (Biological Industries). The cells were expanded in T/C flasks (Corning, Corning, NY, USA) and were removed with trypsin EDTA (Biological Industries) for further use. Trypan blue reagent (Biological Industries) was used to count cells and to determine cell viability.
Fabrication of PEG-fibrinogen hydrogel scaffolds
PEG-DA molecules were synthesized and covalently conjugated to bovine fibrinogen (Fb) as described previously (Almany and Seliktar 2005; Dikovsky et al. 2006). PEG-Fb precursor molecules were then used for the preparation of hydrogel scaffolds using a dual-photoinitiator emulsion-based method as was described previously (Pradhan et al. 2016) with minor modifications. HEK293 cells were removed from a tissue culture (T/C) flask using trypsin, washed and counted. Five million cells with at least 90% viability were mixed in an aqueous phase containing PEG-Fb precursor molecules (8 mg/ml protein, 108% PEGylation) dissolved in PBS, 1.5% (v/v) triethanolamine (TEOA) (Sigma-Aldrich, St. Louis, MO, USA) as a co-initiator, 1% (v/v) PEG-DA, 1% (v/v) Pluonic F68 (Sigma-Aldrich) as the surfactant and Igracure® 2959 (Cibia Specialty Chemicals, Tarrytown, NY, USA) photoinitiator in a total volume of 100 μl. The aqueous suspension (100 μl) was added to an oil phase solution in a glass test tube containing 1 ml mineral oil (Sigma-Aldrich) and 5 μl Igracure® 651 (I651) (Cibia) photoinitiator solution (300 mg I651 in 1-vinyl-2-pyrrolidinone). The aqueous and oil phases were then vortexed for 2 s to form an emulsion and immediately subjected to photocrosslinking using a UV lamp (365 nm wavelength, 4–5 mW/cm2) for 25 s. Then, the emulsion was washed twice with DMEM medium containing 10% FCS, 1% l-glutamine and 0.2% Pen/strep and then centrifuged at 200×g for 5 min. The upper oil phase was then aspirated, and the remaining aqueous phase was washed again to ensure complete oil removal. After the second wash, the hydrogel pellet was resuspended in DMEM medium and was left for 5–10 min to allow passive sedimentation of the hydrogel scaffolds. The supernatant containing the non-encapsulated cells was removed, and the pellet of hydrogels was taken for further experiments.
Cell counting and viability determination
To microscopically evaluate the viability of the encapsulated cells, hydrogels were washed with PBS (1200 rpm, 3 min) and then resuspended in 1 ml PBS. Calcein (1 μl) (Sigma-Aldrich), a green stain for live cells, and ethidium bromide (5 µl) (Sigma-Aldrich), a red stain for dead cells, were added. After a 15 min incubation, the hydrogels were washed and visualized in an inverted Nikon Ts2 fluorescence microscope. To determine the number of encapsulated cells, hydrogels were washed three times with PBS, resuspended with 1 mg/ml collagenase (Sigma-Aldrich) in PBS and incubated for 15 min at 37 °C until complete degradation of the hydrogels was observed. The released cells were counted and examined for viability by trypan blue staining.
Determination of secreted AChE activity
Supernatants of surface-adherent or encapsulated HEK293 cells were examined using Ellman’s assay (Ellman et al. 1961), in which secreted AChE induces the hydrolysis of the substrate acetylcholine to acetate and thiocholine. Thiocholine in the presence of the highly reactive dithiobisnitro-benzoate (DTNB) (Sigma-Aldrich) ion reacts to generate 5-thio-2-nitrobenzoate anion with yellow color. The yellow color was quantified by its absorbance at 405 nm at 27 °C using a Thermomax microplate reader (Molecular Devices, Sunnyvale, CA, USA). Assays were performed in the presence of 0.5 mM acetylcholine. AChE activity is defined as the amount of enzyme that degrades 1 µmol substrate in 1 min. Thus, 1 mU/ml is equal to 1 mO.D per min × 3.3 (converting value). Accordingly, AChE activity (U/ml) was calculated using the extinction coefficient (ε) of 13,600 M−1 cm−1 and light path (l) of 0.5 cm in the following equation:
Image acquisition and cell size analysis
Images of surface-adherent and encapsulated cells were acquired using a phase contrast inverted Nikon microscope with camera. Cell diameters were measured using NIS-f software (Nikon, Melville, NY, USA). At least 50 cells were measured in a representative image.
Statistical analysis
The Prisma software was used for statistical analysis. Statistical analysis was performed using the un-paired Welch correction t test. Differences were considered statistically significant at p < 0.05.
Results
Establishing an experimental model
The aim of this study was to examine whether 3D PEG-Fb hydrogel scaffolds can support the viability and productivity of encapsulated cells in reduced serum conditions. To this end, we established an experimental model in which non-encapsulated surface-adherent HEK293 cells genetically manipulated to produce recombinant human AChE were put under stress conditions of reduced serum in medium. The cells were seeded on 24-well plates (3 × 104 cells per well) with decreasing amounts of FCS in the medium: 10% (full medium), 5, 1, 0.2%, or no FCS (0%), then counted at 24-h intervals over 96 h. As shown in Fig. 1a, cells in 10% FCS supplementation (black line) proliferated exponentially, while the number of cells cultured without FCS in the medium (0%) dramatically declined. In the intermediate FCS concentrations (5 and 1%) the cell proliferation rate gradually decreased but remained positive. A decline below the initial number was observed in 0.2% FCS after 48, 72 and 96 h, reflecting a negative proliferation rate (Fig. 1a). Thus, the 48 h time point, in which cell growth has arrested but viability could still be observed, was chosen to compare encapsulated cells with surface-adherent cells. Figure 1b presents microscope pictures showing the appearance of the cells at 48 h.
Fig. 1.
HEK293 cells producing recombinant human AChE were cultivated on 24-well plates (3 × 104 cells/well) in quadruplicate in DMEM medium supplemented with various concentrations of FCS (10–0.2%). The cells were harvested and counted every 24 h. a Cultivated cell concentrations in logarithmic scale over time in different FCS concentrations: 10% (black), 5% (red), 1% (purple), 0.5% (green), 0.2% (light purple), or 0% (no FCS) (orange). b Representative microscopy pictures of three independent experiments with cells cultivated in different FCS concentrations after 48 h
Effects of reduced serum stress on proliferation and AChE production in surface-adherent cells and encapsulated cells
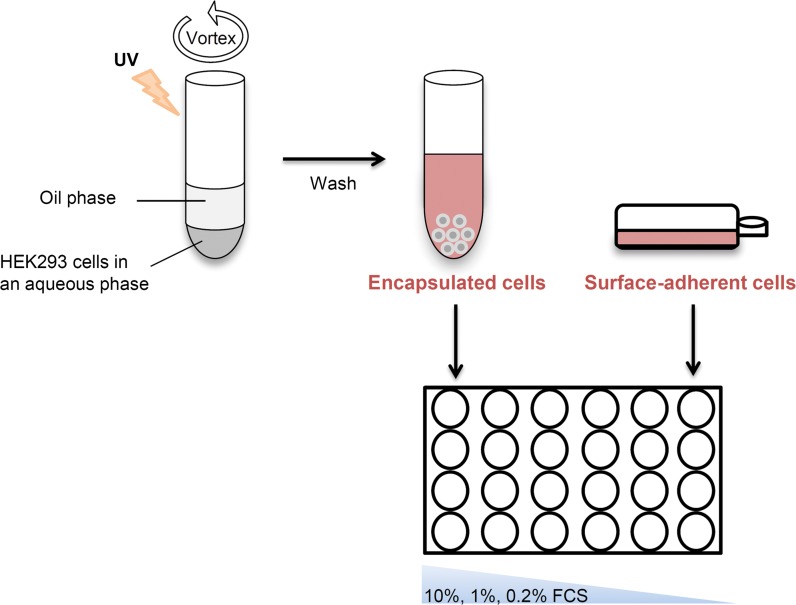
To determine whether cell encapsulation has an advantageous effect over surface adherent cultures, a comparative experiment was set to examine the proliferation and productivity of encapsulated and surface-adherent HEK293 cells under serum stress conditions. Figure 2 illustrates the experimental design; PEG-Fb hydrogels were fabricated using a dual-phase emulsion-based and photocrosslinking technique. An aqueous phase that includes HEK293 cells and PEG-Fb precursor molecules was vortexed with an oil phase to form an emulsion. The emulsion was then shortly UV irradiated to induce photocrosslinking of the PEG-Fb molecules leading to the formation of encapsulated HEK293 cells in hydrogel scaffolds. Non-encapsulated cells that remained outside the hydrogels were washed out. Then, a comparative experiment was conducted in which cells from the same batch, encapsulated or not, were equally seeded on 24-well plates (3 × 104 cells per well). In accordance with the cell growth kinetics observed in 2D culture (Fig. 1), three different conditional media were examined for encapsulated cells in 3D culture: 10, 1 and 0.2% FCS. After 48 h in culture, encapsulated and surface-adherent cells were harvested, and supernatants were collected for further analysis. As was expected, the number of surface-adherent cells was the highest in 10% FCS, and as the serum concentration was reduced to 1 and 0.2%, fewer cells covered the surface. In contrast, in the 3D hydrogel cultures, there was a non-significant decrease in cell numbers as a result of the serum reduction (Fig. 3a). The viability of the cells encapsulated within the hydrogel scaffolds was evaluated with calcein, a green fluorescent dye of cytoplasmic proteins in living cells, and with ethidium bromide, a red fluorescent dye of the DNA in dead cells. Indeed, as shown in Fig. 3b, it can be appreciated that most of the cells inside the hydrogels were viable.
Fig. 2.
An illustration of the experimental setup: cell encapsulation in PEG-Fb hydrogels was done by mixing precursor, an aqueous solution containing PEG-Fb molecules, and HEK293 cells with an oil phase in strong vortex to form an emulsion. Then, the emulsion was photocrosslinked under short-wavelength UV irradiation at 365 nm to form hydrogels. The hydrogels were washed, and an equal number of encapsulated cells and non-encapsulated (surface-adherent) cells were cultivated on a 24-well plate (3 × 104 cells/well) in quadruplicate in DMEM medium supplemented with 10, 1 or 0.2% FCS
Fig. 3.
a Representative microscopy pictures (× 10) of three independent experiments with surface-adherent (2D) and encapsulated (3D) HEK293 cells after 48 h of cultivation in DMEM medium supplemented with 10, 1 or 0.2% FCS. b Viability evaluation of encapsulated cells cultivated inside hydrogel scaffolds in DMEM medium supplemented with 10, 1 or 0.2% FCS. Cells were stained with Calcein, a green fluorescent dye for living cells, and ethidium bromide, a red fluorescent dye for dead cells. Fluorescence microscope images (× 10) of the green or red staining alone and in combination (merged) were obtained
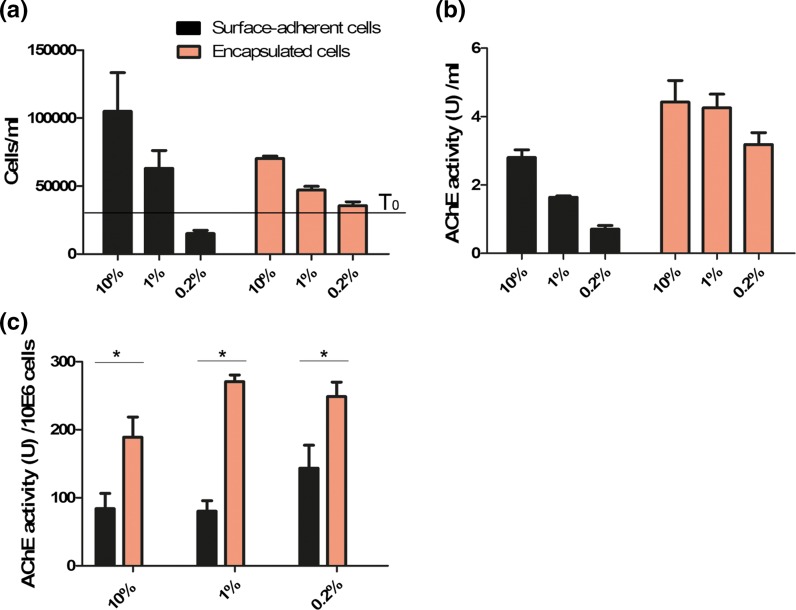
To quantify the number of cells in the 2D and 3D cultures, the surface-adherent cells were harvested using trypsin, and the encapsulated cells were released from the hydrogels using collagenase. Neither enzymatic reaction harmed the viability of the cells (data not shown). The cells were then stained with trypan blue and living cells were counted. As expected, the surface-adherent cells in 10% FCS proliferated normally and after 48 h tripled in number, whereas in 1% FCS, they proliferated less and only doubled in number. In 0.2% FCS, however, the number of cells was below the initial number at time 0 (T0) due to massive cell death and was eightfold less than that in 10% FCS (Fig. 4a). In contrast, the encapsulated cells were significantly less affected by the FCS reduction, showing minor reduction in cell number of only 1.5-fold in 1% FCS and 1.8-fold in 0.2% FCS. Notably, the proliferation rate of the encapsulated cells was relatively less pronounced in 10 and 1% FCS compared with surface-adherent cells, probably due to the limited area for expansion. Importantly, the viability of the encapsulated cells was above 85% in all samples, whereas the viability of the surface-adherent cells decreased to 30% in 0.2% FCS (data not shown). These results clearly demonstrate that PEG-Fb hydrogel scaffolds support the viability and proliferation of encapsulated cells in extremely reduced serum conditions.
Fig. 4.
a Concentrations of surface-adherent cells (black) and encapsulated cells (orange) after 48 h of cultivation in DMEM medium supplemented with 10, 1 or 0.2% FCS. The black line indicates the initial cell concentration at time 0 (T0) (3 × 104 cells/ml). b AChE activity (U)/ml in supernatant of the cultivated cells. c AChE activity (U) per 106 cells calculated by dividing the AChE activity (U/ml) by cell concentration after 48 h. *p < 0.05 tested in t-test. The results are representative of three independent experiments
Cells grown in 3D biocompatible hydrogels have higher AChE production
Next, we examined the effect of reduced serum conditions on the production of a recombinant protein by the cells. To this end, the supernatants from the above samples were collected and examined for AChE activity using Ellman’s assay. As seen in Fig. 4b, the specific AChE activity in the supernatant of surface-adherent cells in 1 and 0.2% FCS significantly declined (1.7-fold to 4-fold), whereas no significant effect was determined in the encapsulated cells. This suggests that the hydrogel scaffolds support the functionality of encapsulated cells under serum stress conditions. To calculate the net specific AChE activity per cell, the values of AChE activity in the supernatant were divided by the cell number. As seen in Fig. 4c, the net AChE activity per cell was significantly higher in encapsulated cells than in surface-adherent cells with 10, 1 or 0.2% FCS in the medium (p < 0.05 by t test), demonstrating that the hydrogel scaffolds not only had a supporting effect on the production of AChE by encapsulated cells in starvation but also enhanced their productivity.
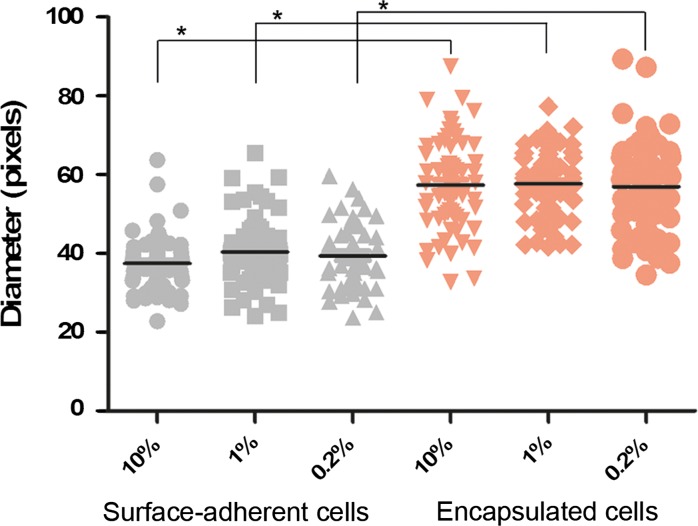
Because a correlation between cell size and specific productivity has been previously reported (Lloyd et al. 2000), we compared the size of the main cell body of encapsulated and surface-adherent cells by measuring the diameters of at least 50 cells in 3 independent pictures from each group. As seen in Fig. 5, the diameter of encapsulated cells was significantly larger than that of surface-adherent cells, 1.5-fold on average, in the 10, 1 and 0.2% FCS groups (p < 0.01 in T test). This suggests that the supportive microenvironment of the hydrogel scaffolds contributes to an increase in cell size that is associated with enhanced AChE production and secretion. Interestingly, visual observation revealed that the morphology of the encapsulated cells is round without extensions, similar to the morphology of single cells in suspension.
Fig. 5.
Cell diameter of surface-adherent cells (gray) and encapsulated cells (orange) after 48 h of cultivation in DMEM medium supplemented with 10, 1 or 0.2% FCS. The diameter of the main body of 60 cells was arbitrarily measured using NIS-f software (Nikon), and the black lines are the average size. The results are representative of three independent experiments. *p < 0.01 tested in t-test
Discussion
The uniquely designed PEG-Fb scaffold used in this study combines endogenous ECM molecules together with synthetic polymers and thus exhibits both bioactivity and enhanced physical stability. In this study, we examined whether this biomaterial technology has added value in the context of a biotechnology application, mainly the production of recombinant proteins. Processes involving cell cultivation strive for robustness; hence, any variation in the composition of the culture medium, such as reduction of serum supplements for regulatory requirements, might result in a decrease in cell productivity and yield and a loss of process robustness. Cell cultivation strategies with the inherent capability of offsetting adversities associated with stressful process modifications offer pronounced advantages over traditional methods. Here, we demonstrated that encapsulating cells in PEG-Fb hydrogels supports their cultivation in starvation conditions of extremely reduced FCS supplementation from 10 to 0.2% without the need for pre-adaptation. Remarkably, encapsulated HEK293-AChE cells were significantly less susceptible to serum reduction, showing high viability and AChE production capability in 1 and 0.2% serum. In contrast, non-encapsulated, surface-adherent cells that were transferred to serum-limited medium responded with a dramatic decrease in viability and productivity. Importantly, the specific AChE activity per cell was twofold higher in encapsulated cells than in surface-adherent cells. These results exhibit important advantages by which encapsulated cells can endure stress conditions posed in biotechnological processes, giving advantages over 2D conditions.
Interestingly, the cells encapsulated in the PEG-Fb hydrogels had round or spherical morphology like single cells grown in suspension, in contrast to the surface-adherent cells that were flat with extensions. This suggests a different mechanism of interfacial interaction between the PEG-Fb scaffold and the encapsulated cells that likely provides the supportive microenvironment for enhanced protein production. Quantitative measurement of cell diameter revealed that the encapsulated cells were twice the size of the surface-adherent cells. It is known that in certain conditions, such as temperature change or external signals, cells skew their cellular resources from proliferation to protein production by enhancing endoplasmic reticulum machinery and increasing cell size (Sunstrom et al. 2000; Lloyd 2013; Kaufmann et al. 1999). Thus, we speculate that the supportive microenvironment of the PEG-Fb scaffold induces the increase in size of encapsulated cells, enhancing the cellular machinery to produce more AChE. Such a protective 3D scaffold microenvironment could also allow for other advantages, including continuous culture for long-term fermentation. Continuous culture would enable sequential harvesting of the secreted AChE out of the hydrogel structure directly from the medium, while protecting the cells from shear forces, by-products, and other harmful conditions. Furthermore, in preliminary experiments, PEG-Fb scaffolds successfully underwent a freeze and thaw cycle with high viability of the encapsulated cells and continued AChE secretion (data not shown). Therefore, pre-made batches of encapsulated cells in PEG-Fb hydrogels can be cryogenically stored and thawed for immediate use without any additional processes, enabling the formation of reproducible working batches for biotechnological purposes.
Further studies to better understand the interaction of cells encapsulated with PEG-Fb scaffold can shed light on the specific effect of the fibrinogen molecule on cellular functions. For example, it is known that fibrin(ogen) molecules can bind growth factors via heparin-binding domains (with varying affinity) and thereby promote cell proliferation in a wound healing model (Martino et al. 2013). It is possible that the fibrinogen molecules in the scaffold bind factors from the medium and release these to proximal cells, so their local concentration is relatively high and therefore compensates for the low-serum environment. The surface-adherent cells, on the other hand, experience low concentrations of these factors from the low-serum medium.
To conclude, this study demonstrates the added value in using PEG-Fb hydrogel scaffolds over traditional 2D surface-adherence cell growth platforms, highlighting the possible implementation of this technology not only in tissue engineering and biomedical applications but also as a promising tool in the biotechnology field.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Noam Cohen and Einat Toister have contributed equally to this work.
References
- Abdeen SH, Abdeen AM, El-Enshasy HE, El Shereef AA. HeLa-S3 cell growth conditions in serum-free medium and adaptability for proliferation in suspension culture. J Biol Sci. 2011;11:124–134. doi: 10.3923/jbs.2011.124.134. [DOI] [Google Scholar]
- Almany L, Seliktar D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials. 2005;26:2467–2477. doi: 10.1016/j.biomaterials.2004.06.047. [DOI] [PubMed] [Google Scholar]
- Barnes LM, Moy N, Dickson AJ. Phenotypic variation during cloning procedures: analysis of the growth behavior of clonal cell lines. Biotechnol Bioeng. 2006;94:530–537. doi: 10.1002/bit.20856. [DOI] [PubMed] [Google Scholar]
- Barralet JE, Wang L, Lawson M, Triffitt JT, Cooper PR, Shelton RM. Comparison of bone marrow cell growth on 2D and 3D alginate hydrogels. J Mater Sci Mater Med. 2005;16:515–519. doi: 10.1007/s10856-005-0526-z. [DOI] [PubMed] [Google Scholar]
- Barrett PN, Mundt W, Kistner O, Howard MK. Vero cell platform in vaccine production: moving towards cell culture-based viral vaccines. Expert Rev Vaccines. 2009;8:607–618. doi: 10.1586/erv.09.19. [DOI] [PubMed] [Google Scholar]
- Berkovitch Y, Seliktar D. Semi-synthetic hydrogel composition and stiffness regulate neuronal morphogenesis. Int J Pharm. 2017;25:545–555. doi: 10.1016/j.ijpharm.2016.11.032. [DOI] [PubMed] [Google Scholar]
- Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59:63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- Butcher JT, Nerem RM. Porcine aortic valve interstitial cells in three-dimensional culture: comparison of phenotype with aortic smooth muscle cells. J Heart Valve Dis. 2004;13:478–485. [PubMed] [Google Scholar]
- Dikovsky D, Bianco-Peled H, Seliktar D. The effect of structural alterations of PEG-fibrinogen hydrogel scaffolds on 3-D cellular morphology and cellular migration. Biomaterials. 2006;27:1496–1506. doi: 10.1016/j.biomaterials.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Dumont J, Euwart D, Mei B, Estes S, Kshirsagar R. Human cell lines for biopharmaceutical manufacturing: history, status, and future perspectives. Crit Rev Biotechnol. 2016;36:1110–1122. doi: 10.3109/07388551.2015.1084266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres VJ, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Grachev V, Magrath D, Griffiths E. WHO requirements for the use of animal cells as in vitro substrates for the production of biologicals (requirements for biological susbstances no. 50) Biologicals. 1998;26:175–193. doi: 10.1006/biol.1998.0153. [DOI] [PubMed] [Google Scholar]
- Hu X, Xiao C, Huang Z, Guo Z, Zhang Z, Li Z. Pilot production of u-PA with porous microcarrier cell culture. Cytotechnology. 2000;1–3:13–19. doi: 10.1023/A:1008127310890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann H, Mazur X, Fussenegger M, Bailey JE. Influence of low temperature on productivity, proteome and protein phosphorylation of CHO cells. Biotechnol Bioeng. 1999;63:573–582. doi: 10.1002/(SICI)1097-0290(19990605)63:5<573::AID-BIT7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Kerscher P, Turnbull IC, Hodge AJ, Kim J, Seliktar D, Easley CJ, Costa KD, Lipke EA. Direct hydrogel encapsulation of pluripotent stem cells enables ontomimetic differentiation and growth of engineered human heart tissues. Biomaterials. 2016;83:383–395. doi: 10.1016/j.biomaterials.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronman C, Velan B, Gozes Y, Leitner M, Flashner Y, Lazar A, Marcus D, Sery T, Papier Y, Grosfeld H, Cohen S, Shafferman A. Production and secretion of high levels of recombinant human acetylcholinesterase in cultured cell lines: microheterogeneity of the catalytic subunit. Gene. 1992;121:295–304. doi: 10.1016/0378-1119(92)90134-B. [DOI] [PubMed] [Google Scholar]
- Kumar A, Goel AS, Payne JK, Evans C, Mikolajczyk SD, Kuus-Reichel K, Saedi MS. Large-scale propagation of recombinant adherent cells that secrete a stable form of human glandular kallikrein, hK2. Protein Expr Purif. 1999;15:62–68. doi: 10.1006/prep.1998.0998. [DOI] [PubMed] [Google Scholar]
- Lloyd AC. The regulation of cell size. Cell. 2013;154:1194–1205. doi: 10.1016/j.cell.2013.08.053. [DOI] [PubMed] [Google Scholar]
- Lloyd DR, Holmes P, Jackson LP, Emery AN, Al-Rubeai M. Relationship between cell size, cell cycle and specific recombinant protein productivity. Cytotechnology. 2000;34:59–70. doi: 10.1023/A:1008103730027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino MM, Briquez PS, Ranga A, Lutolf MP, Hubbell JA. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc Natl Acad Sci USA. 2013;110:4563–4568. doi: 10.1073/pnas.1221602110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin M, Vaibavi SR, Rufaihah AJ, Nithya V, Wang J, Shachaf Y, Kofidis T, Seliktar D. The effect of matrix stiffness of injectable hydrogels on the preservation of cardiac function after a heart attack. Biomaterials. 2014;35:1429–1438. doi: 10.1016/j.biomaterials.2013.10.058. [DOI] [PubMed] [Google Scholar]
- Pradhan S, Clary JM, Seliktar D, Lipke EA. A three-dimensional spheroidal cancer model based on PEG-fibrinogen hydrogel microspheres. Biomaterials. 2016;115:141–154. doi: 10.1016/j.biomaterials.2016.10.052. [DOI] [PubMed] [Google Scholar]
- Sunstrom NA, Gay RD, Wong DC, Kitchen NA, DeBoer L, Gray PP. Insulin-like growth factor-I and transferrin mediate growth and survival of Chinese hamster ovary cells. Biotechnol Prog. 2000;16:698–702. doi: 10.1021/bp000102t. [DOI] [PubMed] [Google Scholar]
- Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004;22:1393–1398. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]