Abstract
Mushrooms possess various bioactivities and are used as nutritional supplements and medicinal products. Twenty-nine bioactive components have been extracted recently from mushrooms grown in Nepal. In this study, we evaluated the ability of these mushroom extracts to augment SIRT1, a mammalian SIR2 homologue localized in cytosol and nuclei. We established a system for screening food ingredients that augment the SIRT1 promoter in HaCaT cells, and identified a SIRT1-augmenting mushroom extract (number 28, Trametes versicolor). UVB irradiation induced cellular senescence in HaCaT cells, as evidenced by increased activity and expression of cellular senescence markers including senescence-associated β-galactosidase, p21, p16, phosphorylated p38, and γH2AX. Results clearly showed that the mushroom extract (No. 28) suppressed the ultraviolet B irradiation-induced cellular senescence in HaCaT cells possibly through augmenting SIRT1 expression.
Electronic supplementary material
The online version of this article (10.1007/s10616-018-0229-1) contains supplementary material, which is available to authorized users.
Keywords: SIRT1, Mushroom, Cellular senescence, Keratinocytes, Skin, Ultraviolet B
Introduction
Accumulating evidence has demonstrated that ultraviolet radiation (UV) is the most common environmental factor that damages the human skin, leading to conditions such as sunburn, solar erythema, inflammation, skin carcinogenesis, and premature senescence (Pillai et al. 2005). In particular, UVB (a wavelength range of 280–320 nm) has been shown to permeate through the epidermis into the dermis and induce reactive oxygen species (ROS) production in the skin and cultured skin cells, thus contributing to gene mutations, abnormal cellular proliferation, and skin aging (Lavker et al. 1995; Hattori et al. 1996; Ahmed et al. 1999).
Sirtuins, a family of NAD+-dependent enzymes, are well-known modulators of lifespan in many species (Kaeberlein et al. 1999). Sirtuin1 (SIRT1), the closest homologue to yeast Sir2, is the most widely studied of the seven sirtuin family members (Frye 2000). Evidence has shown that SIRT1 is involved in cardiovascular disease, neurodegenerative diseases such as Alzheimer’s disease and amyotrophic lateral sclerosis, and inflammatory diseases (Lavu et al. 2008). Furthermore, several lines of evidence have shown that SIRT1 prevents cellular senescence in endothelial cells and fibroblasts via various mechanisms (Huang et al. 2008; Zu et al. 2010; Yamashita et al. 2012). However, experimental evidence regarding the role of SIRT1 in stress-induced fibroblast senescence is sparse, particularly with respect to the protective effect of SIRT1 against UVB-induced premature cell senescence.
Mushrooms, as one of the potential sources of dietary antioxidants, have been valued not only for their nutritional properties (Barros et al. 2008), but also for various medicinal benefits (Lindequist et al. 2005; Ajith and Janardhanan 2007). Around 140,000 species of mushrooms are believed to exist (Wasser 2002); among these, wild mushrooms from Nepal are report to be diverse and play vital roles in many local communities (Adhikari et al. 2006). However, few studies of the pharmacological potential or bioactivity of mushrooms grown in Nepal have been published. Recently, Hai Bang and colleagues described the antioxidant activity of 29 mushrooms collected from the mountainous areas of Nepal (Hai Bang et al. 2014; Tamrakar et al. 2016). To our knowledge, the effects of such mushroom extracts on cellular senescence have not been investigated to date. In this study, we evaluated the protective effects of these twenty-nine mushroom extracts on UVB-induced cellular senescence in human keratinocytes.
Materials and methods
Cell culture and reagents
The HaCaT human keratinocyte cell line (Riken Bioresource Center, Tsukuba, Japan) was cultured in Dulbecco’s modified Eagle’s medium (DMEM; Nissui, Tokyo, Japan) supplemented with 10% fetal bovine serum (Life Technologies, Gaithersburg, MD, USA) at 37 °C in an atmosphere containing 5% CO2.
Screening system for foods that activate the SIRT1 promoter
We amplified the human SIRT1 promoter (− 1593 to − 1) by PCR using human genomic DNA as the template; the amplified fragment was cloned into the promoter-less pEGFP-C3 (Takara, Shiga, Japan) and designated pSIRT1p-EGFP (Harada et al. 2016). HaCaT cells transduced with pSIRT1p-EGFP (HaCaT (SIRT1p-EGFP)) were treated with mushroom extracts, and human SIRT1 promoter activity was then evaluated. Changes in the EGFP fluorescence derived from pSIRT1p-EGFP were monitored using an IN Cell Analyzer 1000 (GE Healthcare, Amersham Place, UK) (Udono et al. 2012).
Ultraviolet B (UVB) irradiation
The medium was removed and cells were exposed to UVB (Integrated Irradiance Level: 10 mJ/cm2; CL-1000 Ultraviolet Crosslinker, UVP, Upland, CA, USA). This exposure was repeated at an interval of 24 h. Soon after UVB irradiation, the medium in which cells were cultured was replaced with fresh medium.
Mushroom samples
Mushrooms were collected from several forests in various parts of Nepal. Scientific names, locations, and accession numbers of the mushrooms are provided in Table S1. Mushroom samples were identified on the basis of morphological features and/or genetic analysis, as described previously (Tamrakar et al. 2016). The samples were dried in an air-ventilated oven at 35 °C for 10 h, followed by at 45 °C for 1 h. The dried samples were ground to a fine powder and extracted with ethanol. The ethanol extracts were rotary-evaporated at 45 °C.
Fluorescence senescence-associated β-galactosidase (SA-β-Gal) assay
Fluorescence SA-β-Gal assay was carried out, as described previously (Udono et al. 2012). Briefly, HaCaT cells were fixed with 2% formaldehyde/0.2% glutaraldehyde, then stained with 33 μM ImaGene Green C12FDG (Life Technologies) and 1 μg/mL Hoechst 33342 solution (Dojin, Kumamoto, Japan). Image acquisition and analysis of imaging data was performed using an IN Cell Analyzer 1000 (GE Healthcare), as described previously. The imaging data were reported as SA-β-Gal intensity (mean fluorescence intensity per cell) and the SA-β-Gal-positive/negative cells by setting the threshold intensity of the SA-β-Gal staining. The threshold of SA-β-Gal intensity was set to the point at which about 75% of total cells were negative in the control cells. The data collected using Developer were analyzed by Spotfire DecisionSite Client 8.2 (PerkinElmer, Waltham, MA, USA) software to visualize the results. This experiment was repeated at least 3 times, and the representative data are shown.
Immunofluorescence
Immunofluorescence was performed as described previously (Udono et al. 2012). Briefly, HaCaT cells were seeded onto a μClear Fluorescence Black plate (Greiner Bio-One, Tokyo, Japan). After fixing the cells with 4% paraformaldehyde, cells were blocked with 5% goat serum and 0.3% Triton-X100. After washing the cells, specific antibodies (anti-p16INK4a (ab81278, Abcam, Cambridge, UK), anti-p21 (2947, Cell Signaling, Danvers, MA, USA), anti-phospho p38 (4511, Cell Signaling), and anti-phospho histone H2A.X (9718, Cell Signaling) were added. After washing the cells, secondary antibody (Alexa Fluor 555 F(ab’) fragments of goat anti-rabbit IgG (Life Technologies) were added. After washing the cells, Hoechst 33342 solution was added, and an image of each well was acquired using an IN Cell Analyzer 1000. Imaging data were analysed using the Multi Target Analysis tool and visualized using Spotfire DecisionSite Client 8.2 software. This experiment was repeated at least 3 times, and the representative data were shown.
Quantitative RT-PCR (qRT-PCR)
RNA was isolated using the High Pure RNA Isolation Kit (Roche, Mannheim, Germany). cDNA was generated from the isolated RNA using the ReverTra Ace (Toyobo, Osaka, Japan). qRT-PCR was performed using the KAPA SYBR Fast qPCR Kit (KAPA Biosystems, Woburn, MA, USA) and the Thermal Cycler Dice Real Time System TP-800 instrument, as described previously (Udono et al. 2012). The samples were analyzed in triplicate, and the SIRT1 level was normalized to the corresponding β-actin level. The PCR primer sequences used were as follows: SIRT1 forward primer 5′-GCCTCACATGCAAGCTCTAGTGAC-3′ and reverse primer 5′-TTCGAGGATCTGTGCCAATCATAA-3′; β-actin forward primer 5′-TGGCACCCAGCACAATGAA-3′ and reverse primer 5′-CTAAGTCATAGTCCGCCTAGA AGCA-3′.
Retrovirus production and transduction
Viral supernatants were produced after transfecting 293 T cells with pGag-pol, pVSV-G, and individual expression vectors (pBABE-puro, pBABE-puro-SIRT1) using the HilyMax reagent (Dojindo), as previously described (Udono et al. 2012). The cells were cultured at 37 °C in DMEM supplemented with 10% FBS for 24 h. Medium was then replaced with fresh DMEM supplemented with 2% FBS and incubated for an additional 24 h. Viral supernatant was collected and supplemented with 10 mg/mL polybrene (Merck Millipore, Billerica, MA, USA). The target cells were infected with this viral supernatant for 24 h at 37 °C. After infection, the cells were selected using 3 μg/mL puromycin (Enzo Life Sciences, Farmingdale, NY, USA) for 3 days.
Statistical analysis
All experiments were performed at least 3 times. The results are presented as mean ± SD. Statistical significance was determined using a two-sided Student’s t test. Multiple comparisons between groups were made by one-way ANOVA with Turkey’s post hoc test. Statistical significance was defined as p < 0.05.
Result and discussion
SIRT1 overexpression protects HaCaT cells from UVB-induced senescence
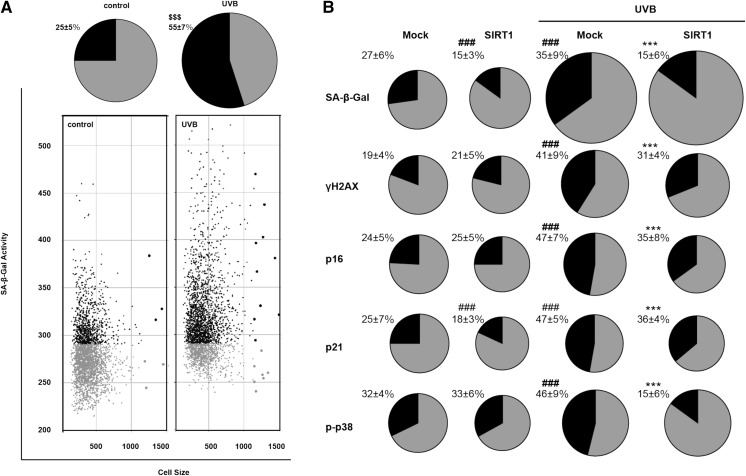
Although UVB irradiation is known to induce apoptosis in HaCaT cells (Li et al. 2017), keratinocytes possess the ability to completely repair DNA damage induced by low-energy UVB (Integrated Irradiance Level: 5 mJ/cm2) exposure and maintain normal cellular function (Kim et al. 2016). In the present study, we repeatedly treated HaCaT cells with low-energy UVB, and evaluated the induction of cellular senescence by measuring SA-β-Gal activity, a biomarker of cellular senescence, using an IN Cell Analyzer 1000 (Udono et al. 2012). Cellular senescence induction was evaluated by analyzing cell size (x-axis) as well as the percentage of SA-β-Gal-positive/negative cells (y-axis) (Fig. 1a). In comparison with that observed in non-treated HaCaT cells, the percentage of SA-β-Gal-positive cells markedly increased among the UVB-irradiated population (Fig. 1a; pie chart). In addition, as shown in Fig. 1a, an increase in the size of UVB-exposed HaCaT cells was observed (Fig. 1a; pie chart). These data indicate that repeated irradiation with low-energy UVB radiation induced cellular senescence in HaCaT cells.
Fig. 1.
Effects of SIRT1 on UVB-induced cellular senescence in HaCaT cells. a Effects of UVB on SA-β-Gal activity in HaCaT cells. Cells (9 × 105 cells) were seeded on to culture dish (φ 35 mm), and cultured for 24 h. Medium was removed and cells were irradiated with UVB (Integrated Irradiance Level: 10 mJ/cm2) and cultured in DMEM supplemented with 10% serum. Next day this UVB irradiation procedure was repeated, and cells were cultured for 1 day, followed by detection of SA-β-Gal activity using an IN Cell Analyzer 1000. Cellular SA-β-Gal (y-axis) and cell size (x-axis) were plotted on a scatter plot. The relative number of cells with high SA-β-Gal activity are shown in the pie chart. Statistical significance was determined using a two-sided Student’s t test. Significant difference is denoted by $$$ p < 0.001. b Effects of SIRT1 on UVB-induced cellular senescence in HaCaT cells. Recombinant HaCaT cells with ectopic SIRT1 expression or mock expression were irradiated with UVB (10 mJ/cm2). Senescence-related biomarkers, including SA-β-Gal activity, γH2AX, p16, p21, and phospho-p38 MAP kinase, were detected using an IN Cell Analyzer 1000. The relative number of cells with high activity or expression of senescence biomarkers is shown in black in the pie chart. The size of the pie chart demonstrates the average cellular size. Multiple comparisons against non-treated mock-transfected HaCaT (#) or against UVB-treated mock-transfected HaCaT (*) were calculated by one-way ANOVA with Turkey’s post hoc test. Significant differences are denoted by ###, *** p < 0.05
SIRT1, popularly known as the longevity gene, has been expressed in cultured skin keratinocytes; the activity of this gene was shown to be downregulated by both UV and H2O2 treatment in a time- and dose-dependent manner (Cao et al. 2009). To investigate the effects of SIRT1 on UVB-induced senescence in HaCaT cells, we first established recombinant HaCaT cells with ectopic expression of SIRT1. Senescence-related biomarkers, such as SA-β-Gal activity, γH2AX, p16, p21, and phospho-p38 (p-p38), were examined in HaCaT cells irradiated with UVB using an IN Cell Analyzer 1000. Figure 1b clearly shows that UVB induced cellular senescence in HaCaT cells, and, further, that SIRT1 attenuated UVB-induced cellular senescence. Taken together, these data suggest that SIRT1 protects HaCaT cells from UVB-induced cellular senescence.
Identification of mushrooms that augment SIRT1 transcription in HaCaT cells
Previously, we established a system for screening foods/food ingredients that augment SIRT1 and SIRT3 expression in Caco-2 cells, and identified Lactobacillus brevis T2102 and pomegraniin A as SIRT1- and SIRT3-activating food components (Zhao et al. 2016; Harada et al. 2016). Here, we established a similar system for screening foods/food ingredients that activate SIRT1 expression using HaCaT cells that express EGFP under the control of the SIRT1 promoter. We tested the SIRT1-augmenting activity of twenty-nine extracts of mushrooms grown in Nepal using this cell line (Table S1). As shown in Fig. 2a, extract numbers 9, 27, and 28 significantly augmented SIRT1 promoter activity in HaCaT cells. In this assay, EGFP fluorescence intensity depends on promoter activity of SIRT1 and cell condition. Thus, we tested whether these mushroom extracts augment endogenous SIRT1 expression in HaCaT cells by qRT-PCR (Fig. 2b). Results showed that extract number 28 significantly augmented endogenous SIRT1 expression in HaCaT cells.
Fig. 2.
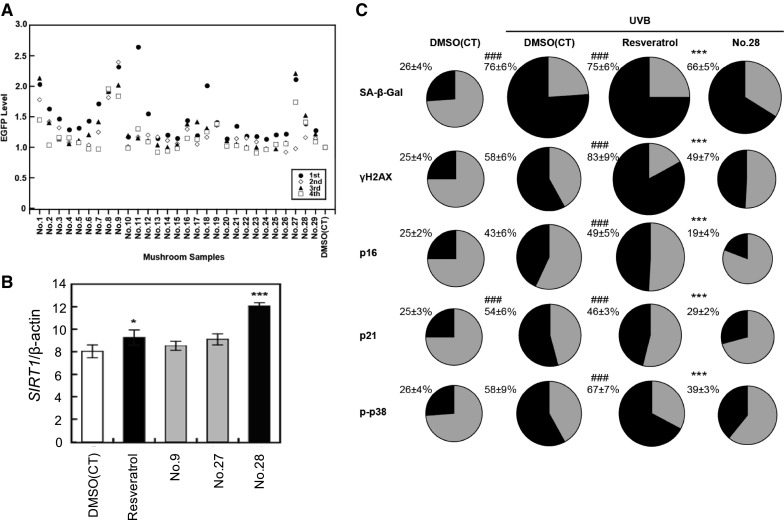
Effects of mushroom extract number 28 on UVB-induced senescence in HaCaT cells. a Identification of mushroom extracts that augment SIRT1 transcription in HaCaT cells. HaCaT cells transduced with vector expressing EGFP under the control of the SIRT1 promoter (2.0 × 104 cells) were seeded on to 96-well μClear Fluorescence Black plate and cultured for 24 h. Mushroom extracts (10 μg/mL) were added and changes in EGFP fluorescence were monitored using an IN Cell Analyzer 1000. b Effects of mushroom extracts on the expression of endogenous SIRT1 in HaCaT cells. HaCaT cells were treated with 10 μg/mL of mushroom extracts. Next day, RNA was prepared and quantitative RT-PCR was conducted. The results are expressed as mean ± SD. Statistical significance was defined as *p < 0.05, ***p < 0.001. c Effects of mushroom extract number 28 on UVB-induced senescence in HaCaT cells. Cells were firstly treated with resveratrol and mushroom extract number 28 (10 μg/mL) every 3 days, irradiated with 10 mJ/cm2 UVB in the presence of extract, and cultured for 3 days. Senescence-related biomarkers, including SA-β-Gal activity, γH2AX, p16, p21, and phospho-p38 MAP kinase, were detected using an IN Cell Analyzer 1000. Resveratrol (5 μM) was used as a positive control. The relative number of cells with high activity or expression of senescence biomarkers is shown in black in the pie chart. Multiple comparisons against non-treated HaCaT (#) or against UVB-treated HaCaT ($) were calculated by one-way ANOVA with Turkey’s post hoc test. Significant differences are denoted by ###, $$$ p < 0.001
Mushroom extract inhibits UVB-induced senescence in HaCaT cells
Next, we tested whether mushroom extract number 28 inhibited UVB-induced senescence in HaCaT cells. UVB induced cellular senescence in HaCaT cells, as evidenced by the activation of SA-β-Gal activity and increase in cellular senescence markers (Fig. 2c). Extract number 28 (Trametes versicolor) markedly attenuated UVB-induced cellular senescence in HaCaT cells; however, resveratrol, which is a known SIRT1-activating polyphenol, had no effects on UVB-induced cellular senescence.
Trametes versicolor, also known as Yunzhi, is known to possess a wide range of beneficial medical properties such as protective effects against oxidative damage, cancer, and bacterial/viral infection as well as immune-potentiating activity; further, this mushroom has been shown to elicit improvement in bone properties in diabetic rats (Lindequist et al. 2005; Chen et al. 2015; Jhan et al. 2016). The best-known commercial polysaccharopeptide preparations derived from T. versicolor are polysaccharopeptide Krestin (PSK) and polysaccharopeptide (PSP). PSPs, which are among the main bioactive constituents of T. versicolor, are recognized as biological response modifiers that are useful adjuncts to conventional therapy. PSPs have been reported to increase the activity and gene expression of antioxidant enzymes and reduce lipid peroxidation in senescence-accelerated mice (Li et al. 2007), as well as exert immunomodulatory effects on blood lymphocytes and the breast cancer cell line MCF-7 (Kowalczewska et al. 2016). Until now, several responsible bioactive compounds in mushrooms including polysaccharides, triterpenoids, proteins, phenolic compounds have been reported (Hai Bang et al. 2014). We need to identify active compounds in the extract in the future study. In the present study, we identified novel activity of extracts derived from T. versicolor, which is expected to be of value in applications related to skin maintenance and repair of skin damage induced by UVB, as well as in the production of novel cosmetics targeting skin aging.
UVB-exposed edible white button mushrooms have been proven to be a safe and effective source of vitamin D2, which supports bone growth and regulates the immune response (Kalaras et al. 2012; Calvo et al. 2013). In the present study, mushrooms were collected from high-altitude regions in Nepal, and therefore considered to possess strong pharmacological potential as a result of UV exposure.
ROS production represents the main source of damage in UVB-irradiated cells. Numerous bioactive components protect HaCaT cells from UVB-induced photo-damage through ROS clearance (Zheng et al. 2016; Oh et al. 2016; Yuan et al. 2017; Zhu et al. 2017; Li et al. 2017). Furthermore, it has been reported that SIRT1 expression is downregulated by UV irradiation through ROS-mediated JNK activation (Cao et al. 2009), and that SIRT1 overexpression prevents UVB-induced fibroblast senescence by suppressing oxidative stress via the deacetylation of FOXO3α and p53 (Chung et al. 2015). However, mushroom extract number 28 (T. versicolor) with strong SIRT1-augmenting ability has been previously reported to possess limited antioxidant activity (Hai Bang et al. 2014), suggesting that this mushroom extract inhibited UVB-induced cellular senescence via a ROS-independent pathway. Further studies aimed at elucidating the underlying mechanisms are therefore warranted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors like to thank Dr. K. Fukami (Kyushu Univ.) and T. H. Bang (Kyushu Univ.) for their assistance.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s10616-018-0229-1) contains supplementary material, which is available to authorized users.
Zhao Chong and Haruka Matsuo have contributed equally to this work.
References
- Adhikari MK, Devkota S, Tiwari RD. Ethnomycolgical knowledge on uses of wild mushrooms in western and central Nepal. Our Nat. 2006;3:13–19. [Google Scholar]
- Ahmed NU, Ueda M, Ichihashi M. Induced expression of p16 and p21 proteins in UVB-irradiated human epidermis and cultured keratinocytes. J Dermatol Sci. 1999;19:175–181. doi: 10.1016/S0923-1811(98)00068-1. [DOI] [PubMed] [Google Scholar]
- Ajith TA, Janardhanan KK. Indian medicinal mushrooms as a source of antioxidant and antitumor agents. J Clin Biochem Nutr. 2007;40:157–162. doi: 10.3164/jcbn.40.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros L, Cruz T, Baptista P, et al. Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food Chem Toxicol. 2008;46:2742–2747. doi: 10.1016/j.fct.2008.04.030. [DOI] [PubMed] [Google Scholar]
- Calvo MS, Babu US, Garthoff LH, et al. Vitamin D2 from light-exposed edible mushrooms is safe, bioavailable and effectively supports bone growth in rats. Osteoporos Int. 2013;24:197–207. doi: 10.1007/s00198-012-1934-9. [DOI] [PubMed] [Google Scholar]
- Cao C, Lu S, Kivlin R, et al. SIRT1 confers protection against UVB- and H2O2-induced cell death via modulation of p53 and JNK in cultured skin keratinocytes. J Cell Mol Med. 2009;13:3632–3643. doi: 10.1111/j.1582-4934.2008.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-H, Kang L, Lo H-C, et al. Polysaccharides of Trametes versicolor improve bone properties in diabetic rats. J Agric Food Chem. 2015;63:9232–9238. doi: 10.1021/acs.jafc.5b02668. [DOI] [PubMed] [Google Scholar]
- Chung KW, Choi YJ, Park MH, et al. Molecular insights into SIRT1 protection against UVB-induced skin fibroblast senescence by suppression of oxidative stress and p53 acetylation. J Gerontol A Biol Sci Med Sci. 2015;70:959–968. doi: 10.1093/gerona/glu137. [DOI] [PubMed] [Google Scholar]
- Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun. 2000;273:793–798. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- Hai Bang T, Suhara H, Doi K, et al. Wild mushrooms in Nepal: some potential candidates as antioxidant and ACE-inhibition sources. Evid Based Complement Altern Med. 2014;2014:195305–195311. doi: 10.1155/2014/195305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada G, Pattarawat P, Ito K, et al. Lactobacillus brevis T2102 suppresses the growth of colorectal cancer cells by activating SIRT1. J Funct Foods. 2016;23:444–452. doi: 10.1016/j.jff.2016.01.016. [DOI] [Google Scholar]
- Hattori Y, Nishigori C, Tanaka T, et al. 8-hydroxy-2′-deoxyguanosine is increased in epidermal cells of hairless mice after chronic ultraviolet B exposure. J Invest Dermatol. 1996;107:733–737. doi: 10.1111/1523-1747.ep12365625. [DOI] [PubMed] [Google Scholar]
- Huang J, Gan Q, Han L, et al. SIRT1 overexpression antagonizes cellular senescence with activated ERK/S6k1 signaling in human diploid fibroblasts. PLoS ONE. 2008;3:e1710. doi: 10.1371/journal.pone.0001710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhan M-H, Yeh C-H, Tsai C-C, et al. Enhancing the antioxidant ability of Trametes versicolor polysaccharopeptides by an enzymatic hydrolysis process. Molecules. 2016;21:1215. doi: 10.3390/molecules21091215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaras MD, Beelman RB, Elias RJ. Effects of postharvest pulsed UV light treatment of white button mushrooms (Agaricus bisporus) on vitamin D2 content and quality attributes. J Agric Food Chem. 2012;60:220–225. doi: 10.1021/jf203825e. [DOI] [PubMed] [Google Scholar]
- Kim M, Park KY, Lee M-K, et al. Adiponectin suppresses UVB-induced premature senescence and hBD2 overexpression in human keratinocytes. PLoS ONE. 2016;11:e0161247. doi: 10.1371/journal.pone.0161247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczewska M, Piotrowski J, Jędrzejewski T, Kozak W. Polysaccharide peptides from Coriolus versicolor exert differential immunomodulatory effects on blood lymphocytes and breast cancer cell line MCF-7 in vitro. Immunol Lett. 2016;174:37–44. doi: 10.1016/j.imlet.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Lavker RM, Gerberick GF, Veres D, et al. Cumulative effects from repeated exposures to suberythemal doses of UVB and UVA in human skin. J Am Acad Dermatol. 1995;32:53–62. doi: 10.1016/0190-9622(95)90184-1. [DOI] [PubMed] [Google Scholar]
- Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins–novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008;7:841–853. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]
- Li L, Ng TB, Song M, et al. A polysaccharide-peptide complex from abalone mushroom (Pleurotus abalonus) fruiting bodies increases activities and gene expression of antioxidant enzymes and reduces lipid peroxidation in senescence-accelerated mice. Appl Microbiol Biotechnol. 2007;75:863–869. doi: 10.1007/s00253-007-0865-4. [DOI] [PubMed] [Google Scholar]
- Li H, Li Z, Peng L, et al. Lycium barbarum polysaccharide protects human keratinocytes against UVB-induced photo-damage. Free Radic Res. 2017;51:200–210. doi: 10.1080/10715762.2017.1294755. [DOI] [PubMed] [Google Scholar]
- Lindequist U, Niedermeyer THJ, Jülich W-D. The pharmacological potential of mushrooms. Evid Based Complement Altern Med. 2005;2:285–299. doi: 10.1093/ecam/neh107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MC, Piao MJ, Fernando PMDJ, et al. Baicalein protects human skin cells against ultraviolet B-induced oxidative stress. Biomol Ther (Seoul) 2016;24:616–622. doi: 10.4062/biomolther.2016.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S, Oresajo C, Hayward J. Ultraviolet radiation and skin aging: roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—a review. Int J Cosmet Sci. 2005;27:17–34. doi: 10.1111/j.1467-2494.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- Tamrakar S, Tran HB, Nishida M, et al. Antioxidative activities of 62 wild mushrooms from Nepal and the phenolic profile of some selected species. J Nat Med. 2016;70:769–779. doi: 10.1007/s11418-016-1013-1. [DOI] [PubMed] [Google Scholar]
- Udono M, Kadooka K, Yamashita S, Katakura Y. Quantitative analysis of cellular senescence phenotypes using an imaging cytometer. Methods. 2012;56:383–388. doi: 10.1016/j.ymeth.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Wasser SP. Review of medicinal mushrooms advances: Good news from old allies. HerbalGram. 2002;56:28–33. [Google Scholar]
- Yamashita S, Ogawa K, Ikei T, et al. SIRT1 prevents replicative senescence of normal human umbilical cord fibroblast through potentiating the transcription of human telomerase reverse transcriptase gene. Biochem Biophys Res Commun. 2012;417:630–634. doi: 10.1016/j.bbrc.2011.12.021. [DOI] [PubMed] [Google Scholar]
- Yuan X-Y, Pang X-W, Zhang G-Q, Guo J-Y. Salidroside’s protection against UVB-mediated oxidative damage and apoptosis is associated with the upregulation of Nrf2 expression. Photomed Laser Surg. 2017;35:49–56. doi: 10.1089/pho.2016.4151. [DOI] [PubMed] [Google Scholar]
- Zhao C, Sakaguchi T, Fujita K, et al. Pomegranate-derived polyphenols reduce reactive oxygen species production via SIRT3-mediated SOD2 activation. Oxid Med Cell Longev. 2016;2016:2927131–2927139. doi: 10.1155/2016/2927131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Hewage SRKM, Piao MJ, et al. Photoprotective effect of carpomitra costata extract against ultraviolet B-induced oxidative damage in human keratinocytes. J Environ Pathol Toxicol Oncol. 2016;35:11–28. doi: 10.1615/JEnvironPatholToxicolOncol.2016014003. [DOI] [PubMed] [Google Scholar]
- Zhu X, Li N, Wang Y, et al. Protective effects of quercetin on UVB irradiation-induced cytotoxicity through ROS clearance in keratinocyte cells. Oncol Rep. 2017;37:209–218. doi: 10.3892/or.2016.5217. [DOI] [PubMed] [Google Scholar]
- Zu Y, Liu L, Lee MYK, et al. SIRT1 promotes proliferation and prevents senescence through targeting LKB1 in primary porcine aortic endothelial cells. Circ Res. 2010;106:1384–1393. doi: 10.1161/CIRCRESAHA.109.215483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.