Abstract
Background
Blood levels of many hormones show rhythmic fluctuations with variable duration of cycles. Clusterin/apolipoprotein J is a glycoprotein which is highly expressed in the plasma and has modulatory roles in immune and inflammatory reactions, neurobiology, lipid metabolism, and leptin signaling. In this study, we examined the diurnal fluctuations of plasma clusterin concentrations in lean and obese young men.
Methods
For the study, 14 subjects (five lean and five obese men; two lean and two obese women) were admitted to the research ward and blood samples were drawn every 30 minutes during light-on period (6:00 AM to 10:00 PM) and every hour during light-off period.
Results
Notably, plasma clusterin concentrations displayed a unique ultradian rhythm with five cycles a day in both men and women. During the light-on period, circulating clusterin levels showed fluctuating curves with 4 hours regular intervals with sharp peaks and troughs. In contrast, single oscillation curve during light-off exhibited a smoothened/lower peak and longer (8-hour) duration. In obese men, these cycles were phase-advanced by approximately 1 hour, and had reduced amplitude of fluctuating curves and blunted diurnal pattern. Cyclic fluctuations of plasma clusterin were preserved under fasting and unexpected meal condition, suggesting that rhythmic oscillations in plasma clusterin levels are not generated by meal-related cues.
Conclusion
These findings firstly demonstrate a novel pattern of plasma clusterin fluctuations with extremely regular cycles.
Keywords: Clusterin, Obesity, Ultradian rhythm, Circadian rhythm
INTRODUCTION
The levels of many biological substances in bodily fluids constantly fluctuate with certain patterns or rhythms. These rhythmic changes can be categorized according to the temporal length of the cycle [1]. Ultradian rhythms refer to fluctuations with a cycle shorter than a day such as insulin [2], ghrelin [3], and leptin [4]. Diurnal rhythms are fluctuations with a 24-hour-cycle such as cortisol [5] while infradian rhythms are the ones with cycles longer than 24 hours (i.e., female sex hormones). These rhythms are generated by feedback regulation of clock genes and hormones or environmental cues. These fluctuations may be helpful for maintaining the overall homeostasis of the subjects.
Diurnal rhythms of certain hormones or biological molecules provide fundamental information that allow for understanding of unknown physiological functions of hormones or biological substances. For example, the levels of plasma insulin decrease during fasting and increase after meal intake, and these changes are appropriate for its glucose-lowering effects [2]. The appetite-stimulating hormone ghrelin shows a dramatic premeal surge, suggesting its role as a meal initiator [3]. Conversely, the diurnal rhythm of leptin shows a nocturnal rise that may have an appetite-suppressing effect during the night [4].
Clusterin was firstly identified in 1983 from the ram rete testis fluid as a peptide with cluster-forming activity [6,7]. Clusterin is also called apolipoprotein J [8], sulfated glycoprotein-2 [9], serum protein 40 kD, 40 kD (SP-40/40) [10], or complement lysis inhibitor [11]. Clusterin is a heterodimeric glycoprotein composed of disulfide bond-linked α and β chains, with each chain weighing about 40 kDa [12]. Clusterin protein is primarily expressed in epithelial cells of most organs [13]. Specifically, clusterin is highly expressed the testis, epididymis, liver, stomach, brain, heart, blood vessels, kidney, and lung [13]. In addition, clusterin is abundant in bodily fluids such as semen, urine, breast milk, cerebrospinal fluid, and plasma [13]. Clusterin has been implicated in multiple biological processes including reproduction [6,9], apoptosis [14,15], complement pathway [10,16], lipid transport [8,17], and leptin signaling [18]. We have previously reported that fasting plasma clusterin levels showed a significant positive correlation with the markers of adiposity and systemic inflammation [19]. Clusterin in the plasma is known as leptin-binding partner [18] and a component of high density lipoprotein (HDL) [8,17]. In the present study, we aimed to investigate the diurnal profiles of plasma clusterin levels under controlled light-darkness and meal intake in both lean and obese subjects. We also examined the effect of prolonged fasting followed by unexpected meal intake on rhythmic fluctuations of plasma clusterin.
METHODS
This study was approved by the Institutional Review Boards (2008-0314) at the Asan Medical Center (Seoul, Korea) and carried out in accordance with the guidelines proposed in the Declaration of Helsinki.
Subjects
Ten young (18 to 30 years old) males were recruited in this study through a website advertisement. Five were obese and five were lean according to the Asia-Pacific obesity criteria proposed by the Western Pacific Regional Office of the World Health Organization [20]. The range of body mass index (BMI) in the lean group was 17 to 23 kg/m2 and that of obese group was 27 to 40 kg/m2. To know sexual difference in the diurnal pattern of plasma clusterin levels, four women (two lean and two obese) were also recruited. All subjects had stable body weight (changes less than 3 kg) for at least 3 months before the study. They had no past or present medical problems of diabetes, hypertension, other chronic illnesses, and gastrointestinal surgery. They were neither smokers nor heavy drinkers. Subjects with irregular sleep and diet patterns or night-time and shift workers were excluded.
On the screening visit, all the subjects signed written informed consent and filled out a self-administered questionnaire that included demographic characteristics, general health status, and social history. Anthropometric parameters such as blood pressure, height, weight, and waist circumference were determined by the standard protocols. Subjects also underwent a routine physical examination, measurement of vital signs, electrocardiography, and routine laboratory tests including a complete blood count, electrolytes, fasting glucose, lipid profiles, and a liver function test to assess if they were suitable for this study.
Study design
Subjects were recommended to intake their meals and sleep on the same schedule of our study for 1 week before the study. After the 1 week-diet and sleep rhythm adjustment period, subjects were admitted to the Asan Clinical Research Center at 5:00 PM one day prior to study day. After dinner, an indwelling intravenous catheter was placed in the antecubital vein. Following an overnight fasting, blood was drawn into ethylenediaminetetraacetic acid-coated tubes containing aprotinin (250 kallikrein inhibitor units; Sigma-Aldrich, St. Louis, MO, USA) at 30-minute intervals from 7:00 AM to 9:00 PM and then hourly until 7:00 AM in the next morning. Breakfast was provided at 8:00 AM, lunch at 12:30 PM, and dinner at 6:00 PM during the study day. The meals were Korean standard meals, which consisted of 60% carbohydrates, 25% fats, and 15% proteins. Korean standard meals were composed of streamed rice, one soup, and three small side dishes (one dish of meat or fish and two dishes of vegetables including Kimchi). Lights were turned off at 10:00 PM and turned on at 6:00 AM. During the light-on period, subjects were awake and remained in bed. After 2 to 3 weeks from the first admission, two lean and two obese male subjects were readmitted in order to evaluate the impacts of fasting and unexpected meal on plasma clusterin fluctuations. Before the second admission, the subjects were informed that they would undergo fasting until 6:00 PM on the study day; they were permitted to only have water during the fasting period. However, a meal was provided at 3:00 PM without any notice to avoid the effect of meal expectation. Blood samples were collected every 30 minutes until 6:00 PM. All subjects included in this study were in a good compliance with the protocol before and during the admission.
Assays
Plasma was immediately separated by centrifugation at 4℃ and stored at −70℃ until use. Fasting plasma glucose, total cholesterol, triglyceride, and HDL-cholesterol levels were measured by an auto-analyzer (Hitachi E170, Hitachi Ltd., Tokyo, Japan). Plasma clusterin was assayed with an ELISA (enzyme-linked immunosorbent assay) kit (AdipoGen, San Diego, CA, USA) according to the manufacturer's instructions. The lower and upper limits of detection were 0.001 and 5 µg/mL, respectively, and the intra- and inter-assay coefficients of variation were 7.7% and 8.5%, respectively.
Statistical analyses
All data are presented as mean±SEM. Parameters of plasma clusterin rhythms were compared between lean and obese groups using the Mann-Whitney U test. The Kruskal-Wallis test was used to confirm temporal variations in plasma clusterin levels during the day-night cycle. The P values less than 0.05 were considered to be statistically significant. SPSS version 18.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
RESULTS
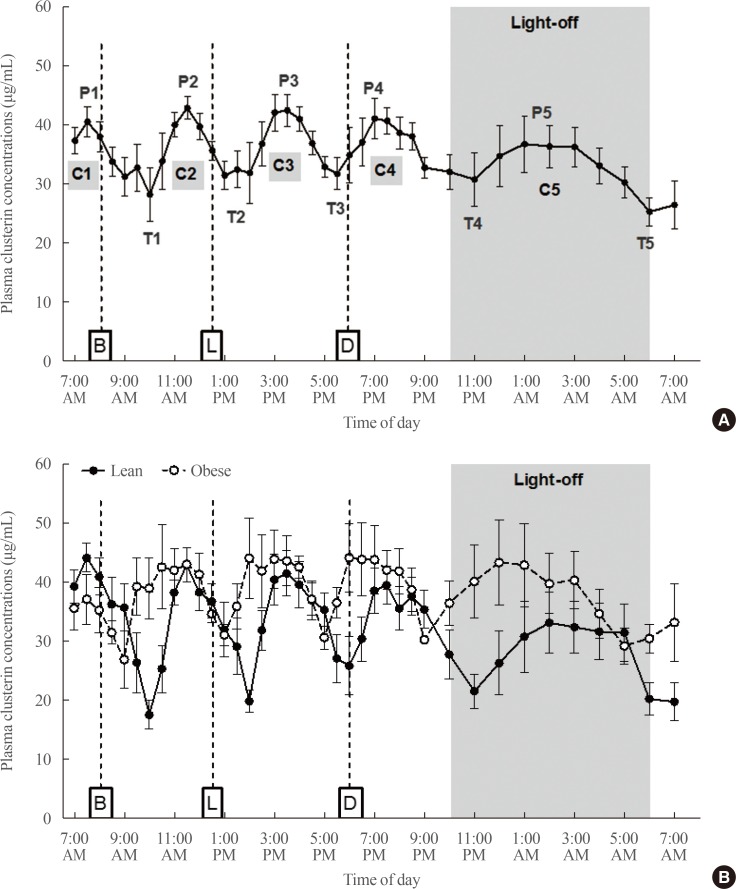
Demographic characteristics and baseline biochemical findings of subjects are shown in Table 1 and in our previous paper [21]. Notably, plasma clusterin levels exhibited five cyclic fluctuations per day: four daytime cycles and one nighttime cycle (Fig. 1A). The Kruskal-Wallis test confirmed a significant temporal variation in plasma clusterin concentrations during the day (P<0.001). Except for the fifth cycle at night, the duration of each cycle was approximately 4 hours. During light-on period, peaks of the curves were sharp and constant. In contrast, their trough levels tended to increase, and thus the amplitude of curves tended to decrease as the time approached evening. During the light-off period, the temporal length of clusterin cycle almost doubled (about 8 hours) and the values of peak, trough, and amplitude were all lower compared to those of curves during light-on period (Fig. 1A).
Table 1. Baseline Demographic and Biochemical Characteristics of Lean and Obese Subjects.
| Characteristic | Lean (n=5) | Obese (n=5) |
|---|---|---|
| Age, yr | 23.0±1.0 | 22.6±2.3 |
| BMI, kg/m2 | 21.9±0.7 | 29.8±1.4a |
| Waist circumference, cm | 80.4±2.1 | 98.4±2.8a |
| Triglyceride, mg/dL | 88.6±16.5 | 78.0±5.7 |
| Total cholesterol, mg/dL | 162.0±9.4 | 186.6±5.3 |
| LDL-C, mg/dL | 100.8±7.3 | 125.8±6.2a |
| HDL-C, mg/dL | 47.4±2.1 | 46.2±1.5 |
| Fasting glucose, mg/dL | 94.0±2.2 | 101.8±4.5 |
Values are expressed as mean±SEM.
BMI, body mass index; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol.
aP<0.05 vs. lean group.
Fig. 1. Ultradian rhythms in plasma clusterin concentrations of (A) total subjects (n=10) and (B) lean and obese subjects (n=5 per group). P, peak; T, trough; C, cycle; B, breakfast; L, lunch; D, dinner.
When we compared the rhythmic oscillations in plasma clusterin levels between lean and obese subjects, the duration of each cycle was similar in both lean and obese subjects (Fig. 1B). Interestingly, clusterin cycles were phase-advanced by one hour in obese subjects (Fig. 1B). The average trough values of curves tended to be higher in obese group than in lean group especially in the afternoon and evening, whereas the peak values did not significantly differ between lean and obese groups (Table 2). As a result, the amplitude of oscillating curves tended to be lower in obese individuals. We also observed loss of diurnal patterns: higher curve amplitude in the morning and lower amplitude in the evening. The relative amplitude was greater in lean group than in obesity group (P=0.028), although the absolute amplitude did not show significant difference. In addition, the peaks flattened, and we could not point out single peak level.
Table 2. Comparison of Plasma Clusterin Ultradian Rhythm Parameters between Lean and Obese Subjects.
| Lean (n=5) | Obese (n=5) | P value | |
|---|---|---|---|
| Clusterin, μg/mL | |||
| Average peak value | 41.7±2.9 | 46.6±2.9 | 0.261 |
| Average trough value | 19.1±2.0 | 26.0±2.6 | 0.066 |
| Average absolute amplitudea | 22.6±1.5 | 20.6±0.6 | 0.251 |
| Average relative amplitudeb | 1.3±0.2 | 0.9±0.1 | 0.028 |
Values are expressed as mean±SEM.
aAbsolute amplitude=peak level-next trough level; bRelative amplitude=absolute amplitude/trough level, comparison between lean and obese groups were performed using the Mann-Whitney U test.
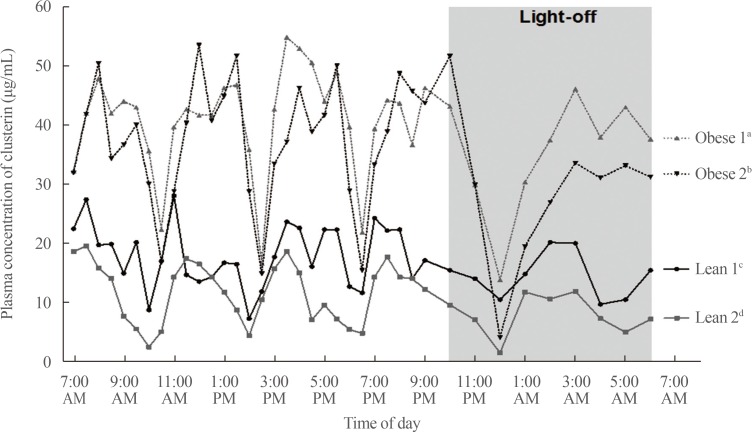
We determined the diurnal patterns of plasma clusterin in two lean and two obese women. Peak and trough values of plasma clusterin in two lean women were lower compared to those in men and obese women (Fig. 2). Similarly to men, four women had striking ultradian oscillations with five cycles. In contrast to obese men, a phase advance in the rhythmic fluctuations of plasma clusterin was not observed in two obese women. Moreover, the amplitude of fluctuations were higher in two obese women than in two lean women (Fig. 2).
Fig. 2. Ultradian rhythms in plasma clusterin concentrations of four woman subjects (two lean and two obese). aObese 1: age 28 years, body mass index (BMI) 32.9; bObese 2: age 28 years, BMI 30.4; cLean 1: age 31 years, BMI 19.1; dLean 2: age 23 years, BMI 18.9.
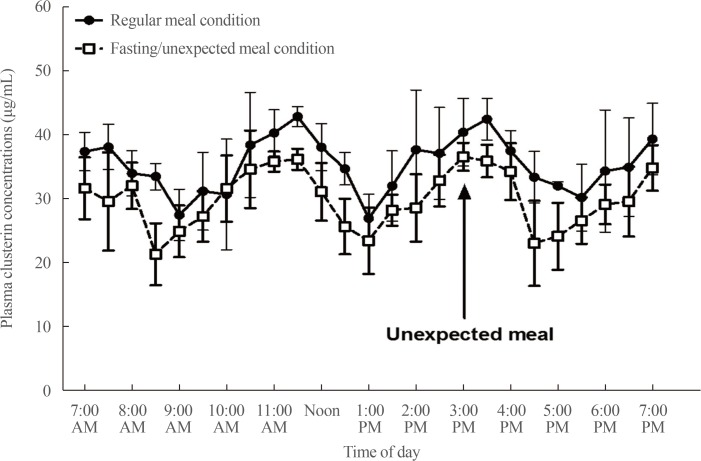
Fating-unexpected meal study revealed that ultradian rhythms of clusterin oscillation remained under prolonged fasting condition and was unaffected by meal intake (Fig. 3). Peak and trough levels of clusterin curves tended to decrease under prolonged fasting condition. Moreover, the temporal phase of fluctuations tended to be advanced by about 30 minutes to one hour under fasting condition.
Fig. 3. Comparison of plasma clusterin diurnal fluctuations between regular three meal condition and fasting-unexpected meal condition (n=4).
DISCUSSION
In this study, we show a previously-unreported, novel pattern of oscillations in plasma clusterin concentrations. The oscillations consisted of five cycles over 24 hours—four daytime cycles and one nighttime cycle. Interestingly, the temporal lengths of cycles were strikingly regular—4 hours during light-on and 8 hours during light-off. In addition, the amplitudes of cyclic fluctuations exhibited a distinct diurnal pattern: they were highest in the morning and declined until next early morning. These oscillation patterns were conserved in both men and women. At present, there is lack of knowledge for mechanism underlining circadian or ultradian oscillations of circulating clusterin concentration. Because hepatocytes secrete clusterin, fluctuations in circulating clusterin levels might be generated by rhythmic secretion of clusterin from the liver [22]. The amplitudes and temporal lengths of oscillating cycles were unaltered during prolonged fasting. However, clusterin cycles in fasted condition showed phase-advancement by 30 to 60 minutes. Thus, these rhythmic fluctuations may be governed by light-dark cycles or the suprachiasmatic clock neurons rather than nutritional cues.
Our results suggest that obesity can affect plasma clusterin oscillations. Of note, we found that in obese men, the temporal phase of clusterin rhythms was advanced by one hour without alteration in temporal lengths. Moreover, the shape of clusterin curves in obese men was characterized by smoothened peaks, shallow falls, and lower amplitude of cyclic oscillations. Another interesting characteristic in the clusterin curve of obese men subjects was the loss of reduced amplitude of curve during sleep. It will be interesting to study the impact of altered clusterin oscillations on human health including sleep quality.
In our study, obese women did not show obesity-related changes in ultradian and circadian oscillation patterns of plasma clusterin, which were observed in obese men. Notably, two obese women showed higher amplitude of fluctuations compared to those of two lean women subjects. However, our observation was made in only four women and thus the diurnal patterns of blood clusterin in women should be studied in a larger number of women subjects in the future.
Single measurement of plasma clusterin levels in the morning fasting condition revealed that plasma clusterin levels were positively correlated with the adiposity marker BMI [19], although other studies showed no significant correlation between plasma clusterin and BMI [18]. Our current study adds crucial information on diurnal fluctuations in plasma clusterin levels, and we suggest that the timing of blood sample collection should be taken into consideration in future studies on plasma clusterin.
Our findings are limited by the small sample size but strengthened by the unified condition of blood sampling such as light-dark cycles, feeding, and physical activity. In addition, our study was conducted in young Koreans with no metabolic disorders and either normal weight or mild to moderate obesity. Therefore, further studies will be warranted to confirm ultradian and circadian rhythms of plasma clusterin in different ethnic groups and subjects with different metabolic characteristics or the different degree of obesity. Despite these limitations, plasma clusterin concentrations displayed extremely regular, meal-unrelated oscillations. The physiological implications of cyclic oscillations in circulating clusterin should be investigated in the future.
ACKNOWLEDGMENTS
This work was supported by grants from the Korea Science and Engineering Foundation (2015M3A9E7029177, 2017R1A2B3007123), and the Asan Institute for Life Science (17-327). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
AUTHOR CONTRIBUTION: Conception or design: E.J., B.S.Y., M.S.K. Acquisition, analysis, or interpretation of data: J.H.C., E.J., B.S.Y., M.S.K. Drafting the work or revising: J.H.C., M.S.K. Final approval of the manuscript: J.H.C., M.S.K.
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Martini L. Encyclopedia of endocrine diseases. Amsterdam: Elsevier Academic Press; 2004. [Google Scholar]
- 2.Lambert AE, Hoet JJ. Diurnal pattern of plasma insulin concentration in the human. Diabetologia. 1966;2:69–72. doi: 10.1007/BF01106976. [DOI] [PubMed] [Google Scholar]
- 3.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 4.Sinha MK, Ohannesian JP, Heiman ML, Kriauciunas A, Stephens TW, Magosin S, et al. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97:1344–1347. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab. 1971;33:14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- 6.Blaschuk O, Burdzy K, Fritz IB. Purification and characterization of a cell-aggregating factor (clusterin), the major glycoprotein in ram rete testis fluid. J Biol Chem. 1983;258:7714–7720. [PubMed] [Google Scholar]
- 7.Fritz IB, Burdzy K, Setchell B, Blaschuk O. Ram rete testis fluid contains a protein (clusterin) which influences cell-cell interactions in vitro. Biol Reprod. 1983;28:1173–1188. doi: 10.1095/biolreprod28.5.1173. [DOI] [PubMed] [Google Scholar]
- 8.de Silva HV, Stuart WD, Park YB, Mao SJ, Gil CM, Wetterau JR, et al. Purification and characterization of apolipoprotein J. J Biol Chem. 1990;265:14292–14297. [PubMed] [Google Scholar]
- 9.Collard MW, Griswold MD. Biosynthesis and molecular cloning of sulfated glycoprotein 2 secreted by rat Sertoli cells. Biochemistry. 1987;26:3297–3303. doi: 10.1021/bi00386a008. [DOI] [PubMed] [Google Scholar]
- 10.Murphy BF, Kirszbaum L, Walker ID, d'Apice AJ. SP-40,40, a newly identified normal human serum protein found in the SC5b-9 complex of complement and in the immune deposits in glomerulonephritis. J Clin Invest. 1988;81:1858–1864. doi: 10.1172/JCI113531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tobe T, Minoshima S, Yamase S, Choi NH, Tomita M, Shimizu N. Assignment of a human serum glycoprotein SP-40,40 gene (CLI) to chromosome 8. Cytogenet Cell Genet. 1991;57:193–195. doi: 10.1159/000133144. [DOI] [PubMed] [Google Scholar]
- 12.Choi-Miura NH, Takahashi Y, Nakano Y, Tobe T, Tomita M. Identification of the disulfide bonds in human plasma protein SP-40,40 (apolipoprotein-J) J Biochem. 1992;112:557–561. doi: 10.1093/oxfordjournals.jbchem.a123938. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg ME, Silkensen J. Clusterin: physiologic and pathophysiologic considerations. Int J Biochem Cell Biol. 1995;27:633–645. doi: 10.1016/1357-2725(95)00027-m. [DOI] [PubMed] [Google Scholar]
- 14.Viard I, Wehrli P, Jornot L, Bullani R, Vechietti JL, Schifferli JA, et al. Clusterin gene expression mediates resistance to apoptotic cell death induced by heat shock and oxidative stress. J Invest Dermatol. 1999;112:290–296. doi: 10.1046/j.1523-1747.1999.00531.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Kim JK, Edwards CA, Xu Z, Taichman R, Wang CY. Clusterin inhibits apoptosis by interacting with activated Bax. Nat Cell Biol. 2005;7:909–915. doi: 10.1038/ncb1291. [DOI] [PubMed] [Google Scholar]
- 16.Kirszbaum L, Bozas SE, Walker ID. SP-40,40, a protein involved in the control of the complement pathway, possesses a unique array of disulphide bridges. FEBS Lett. 1992;297:70–76. doi: 10.1016/0014-5793(92)80330-j. [DOI] [PubMed] [Google Scholar]
- 17.de Silva HV, Stuart WD, Duvic CR, Wetterau JR, Ray MJ, Ferguson DG, et al. A 70-kDa apolipoprotein designated ApoJ is a marker for subclasses of human plasma high density lipoproteins. J Biol Chem. 1990;265:13240–13247. [PubMed] [Google Scholar]
- 18.Arnold T, Brandlhofer S, Vrtikapa K, Stangl H, Hermann M, Zwiauer K, et al. Effect of obesity on plasma clusterin, [corrected] a proposed modulator of leptin action. Pediatr Res. 2011;69:237–242. doi: 10.1203/PDR.0b013e31820930cb. [DOI] [PubMed] [Google Scholar]
- 19.Won JC, Park CY, Oh SW, Lee ES, Youn BS, Kim MS. Plasma clusterin (ApoJ) levels are associated with adiposity and systemic inflammation. PLoS One. 2014;9:e103351. doi: 10.1371/journal.pone.0103351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization Regional Office for the Western Pacific. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia; 2000. [Google Scholar]
- 21.Jeong E, Youn BS, Kim DW, Kim EH, Park JW, Namkoong C, et al. Circadian rhythm of serum vaspin in healthy male volunteers: relation to meals. J Clin Endocrinol Metab. 2010;95:1869–1875. doi: 10.1210/jc.2009-1088. [DOI] [PubMed] [Google Scholar]
- 22.Burkey BF, Stuart WD, Harmony JA. Hepatic apolipoprotein J is secreted as a lipoprotein. J Lipid Res. 1992;33:1517–1526. [PubMed] [Google Scholar]