Abstract
Background
The ongoing Multicenter Prospective Cohort Study of Active Surveillance on Papillary Thyroid Microcarcinoma (MAeSTro) aims to observe the natural course of papillary thyroid microcarcinoma (PTMC), develop a protocol for active surveillance (AS), and compare the long-term prognosis, quality of life, and medical costs between the AS and immediate surgery groups.
Methods
This multicenter prospective cohort study of PTMC started in June 2016. The inclusion criteria were suspicious of malignancy or malignancy based on fine needle aspiration or core needle biopsy, age of ≥18 years, and a maximum diameter of ≤1 cm. If there was no major organ involvement, no lymph node/distant metastasis, and no variants with poor prognosis, the patients were explained of the pros and cons of immediate surgery and AS before selecting AS or immediate surgery. Follow-up visits (physical examination, ultrasonography, thyroid function, and questionnaires) are scheduled every 6 months during the first 2 years, and then every 1 year thereafter. Progression was defined as a maximum diameter increase of ≥3, ≥2 mm in two dimensions, suspected organ involvement, or lymph node/distant metastasis.
Results
Among 439 enrolled patients, 290 patients (66.1%) chose AS and 149 patients (33.9%) chose immediate surgery. The median follow-up was 6.7 months (range, 0.2 to 11.9). The immediate surgery group had a larger maximum tumor diameter, compared to the AS group (7.1±1.9 mm vs. 6.6±2.0 mm, respectively; P=0.014).
Conclusion
The results will be useful for developing an appropriate PTMC treatment policy based on its natural course and risk factors for progression.
Keywords: Thyroid neoplasms, Active surveillance, Prospective cohort, Papillary thyroid microcarcinoma
INTRODUCTION
The incidence of thyroid cancer has been growing continuously [1,2], with a marked increase in the proportion of papillary thyroid microcarcinoma (PTMC). In the United States, this proportion increased from 25% in 1988–1989 to 39% in 2008–2009, while in Korea it increased from 9% in 1990 to 54% in 2005 [3]. Another Korean report described a similar increment from 14% in 1995 to 56% in 2005 [4]. However, the 5-year relative survival rate has increased from 94.2% in 1993–1995 to almost 100% in 2008–2012 [2,5]. These results have generated debate regarding the over-diagnosis and over-treatment of thyroid cancer [6], although an early diagnosis remains beneficial for some patients [2].
Active surveillance (AS) has begun to be introduced as one of the options in the management of PTMC with a good expected prognosis [7]. This approach was initially used for prostate cancer [8], and targets patients with a low-risk of cancer progression to undergo aggressive observation that minimizes unnecessary surgeries or deteriorations in quality of life (QOL). However, it is always possible that unexpected progression can miss the right timing for appropriate surgical treatment. Therefore, appropriate screening criteria are critical to minimizing risk, and careful follow-up is needed for patients who choose AS instead of immediate surgery.
The 2015 guidelines of the American Thyroid Association [9] suggested that AS could be considered for patients with low-risk PTMC who have no clinical or radiological evidence of invasion or metastasis. A recent report defined progression as new local metastasis to the cervical lymph nodes, distant metastasis, or a size increase of ≥3 mm [7]. However, previous studies have used different definitions of low-risk PTMC and progression during AS, and the definitions have not been established or validated [10,11,12,13]. Furthermore, those studies were limited by their single-center design [10,11,12,13].
The Multicenter Prospective Cohort Study of Active Surveillance on Papillary Thyroid Microcarcinoma (MAeSTro) was designed to observe the natural course of PTMC during AS, and to compare the outcomes of patients who have immediate surgery and who have delayed surgery because of the disease progression during AS. Factors that influenced the decision-making were also evaluated. Healthcare providers provided sufficient information to the participants, who were subsequently allowed to select their management strategy. Patients were carefully followed up using ultrasonography by expertized radiologists. The study is planned to be going on for 10 years, and interim analyses are planned in every 5 years from the start. The findings will be useful in developing an approach to PTMC management that can facilitate early detection of progression to an aggressive status.
In this article, we introduce the protocol of MAeSTro study, and describe the characteristics of the participants in both AS and surgery group. Since there could be many patients and clinicians who want to undergo AS without having officially recommended protocols for AS, the study could be meaningful in suggesting the inclusion and follow-up strategy of AS. Also, the article could be helpful to the researchers by suggesting characteristics of patients in AS and immediate surgery group.
METHODS
Study design
This is a multicenter prospective cohort study. Data were collected for patients who were diagnosed with PTMC after June 2016, and included cancer progression, QOL questionnaires, imaging results, and specimens of blood and thyroid tissues (NCT02938702).
The participants were recruited from Seoul National University Hospital, Seoul Metropolitan Government Seoul National University Boramae Medical Center (Seoul), Seoul National University Bundang Hospital (Seongnam), and the National Cancer Center (Goyang, Korea). Seoul National University Hospital and Seoul National University Bundang Hospital are tertiary referral hospitals. National Cancer Center and Seoul Metropolitan Government Seoul National University Boramae Medical Center are secondary referral hospitals. Participants were recruited during outpatient visits starting in June 2016, and most participants were referred from a primary care physician because of a diagnosis of PTMC or nodules with highly suspicious features for cancer.
The study protocol was approved by the ethics committee of Seoul National University Hospital (IRB number 1603-044-747), Seoul National University Bundang Hospital (IRB number B-1605-348-402), Seoul Metropolitan Government Seoul National University Boramae Medical Center (IRB number 26-2017-18), and National Cancer Center (IRB number NCC2016-0183). All patients provided informed consent and were informed that they could withdraw or change between the AS and surgery group at any time. According to the International Conference on Harmonization Good Clinical Practice Guidelines, all recorded data were anonymized.
Participants
All PTMC were considered eligible, regardless of the presence of invasion or metastasis. Participants who were eligible for AS received sufficient information regarding AS and immediate surgery. After careful consideration about the pros and cons of each modality, participants were allowed to select their preferred management. Patients who were not eligible for AS were recommended to undergo immediate surgery. However, careful follow-up as same as AS group was recommended if the patient does not want to undergo immediate surgery or had other major health concerns that precluded surgery. Optimal types and extent of surgery was decided for each patient by their surgeons.
Participants are included if all following criteria are met:
- Agreement to participate in the study and signed the consent form
- A diagnosis of suspicious of malignancy or malignancy (Bethesda category V or VI) based on fine needle aspiration (FNA), or suggestive of malignancy or malignancy by core needle biopsy
- Age of ≥18 years
- A maximum nodule diameter of ≤1 cm
The overall exclusion criteria were:
- Unable or unwilling to attend regular follow-ups
- A diagnosis of benign, atypia of undetermined significance, suspicious for follicular neoplasm, or follicular neoplasm (Bethesda category II, III, or IV) based on FNA or, or benign, indeterminate by core needle biopsy
Patients were not excluded if they had:
- ≥2 unilateral or bilateral PTMCs
- Cytologically or histologically confirmed benign >1 cm nodules with intermediate or highly suspicious ultrasonographic findings (Korean Thyroid Imaging Reporting and Data System [K-TIRADS] Class 4–5)
- Cytologically unconfirmed >1 cm nodules with ultrasonographic findings suggestive of benign lesions (K-TIRADS Class 2–3)
- Hypothyroidism or hyperthyroidism
- Pregnancy before or during the study
- A family history of non-medullary thyroid cancer
Participants were allowed to choose AS if they fulfilled the following criteria:
- No evidence of organ involvement (e.g., esophagus, nerves, trachea, major vessels, or muscle) in imaging studies including high-resolution of ultrasonography (PTMC adjacent or abutting to organs were included if it is not suspected of organ involvement)
- No clinically suspicious or pathological diagnosis of lymph node/distant metastasis
- No poorly differentiated cancer or variant with a poor prognosis, such as the tall cell, diffuse sclerosing, columnar cell, or solid variants
Participants were recommended to undergo immediate surgery if they fulfilled the following criteria:
- Suspected organ involvement (e.g., trachea, esophagus, nerves, vessels, or muscles)
- Clinical suspicious or pathological diagnosis of lymph node/distant metastasis
- Poorly differentiated histology or a variant with a poor prognosis such as tall-cell, diffuse sclerosing, columnar cell, or solid variant
- Presence of Graves' disease with an indication for radioactive iodine therapy or surgery
Enrollment and follow-up
In patients who are allowed to choose between AS and immediate surgery, they had shared decision-making after providing information material for patients before choosing their plans for PTMC. To minimize interphysician variability, information material contains balanced information based on published results about risks and benefits of AS and immediate surgery. It made the provided information consistent for each patient to help their decisions. Interview time was at least 15 minutes per patient, and 1 to 2 weeks of consideration period was given to discuss with their families.
For the early detection of a possible progression in participants who choose AS instead of surgery, their PTMCs are closely monitored according to the following protocol. Participants who undergo immediate surgery are also followed after surgery according to the protocol.
In the AS group, follow-up visits are scheduled every 6 months during the first 2 years after the diagnosis, and then every 1 year thereafter. The follow-ups involve a physical examination (palpation of the thyroid and neck), high-resolution thyroid ultrasonography, and thyroid function test. Questionnaires regarding QOL are done at baseline and 6 or 12 months after the diagnosis. Additional imaging or biopsy was performed as necessary. Patients undergo surgery if progression is detected.
Disease progression is defined as:
- A size increase of ≥3 mm in at least one dimension, or ≥2 mm in at least two dimensions
- Suspected organ involvement during the follow-up, such as trachea, esophagus, nerves, vessels, or muscles in imaging study including high-resolution ultrasonography
- Pathological diagnosis of lymph node/distant metastasis
Patients in the AS groups are also recommended to undergo surgery if:
- They elect to undergo surgery in the absence of progression
- Combined Graves' disease is suitable for radioactive iodine ablation therapy or surgery
- Clinical symptoms (hoarseness or dysphagia) during a vocal cord inspection suggest recurrent laryngeal nerve involvement.
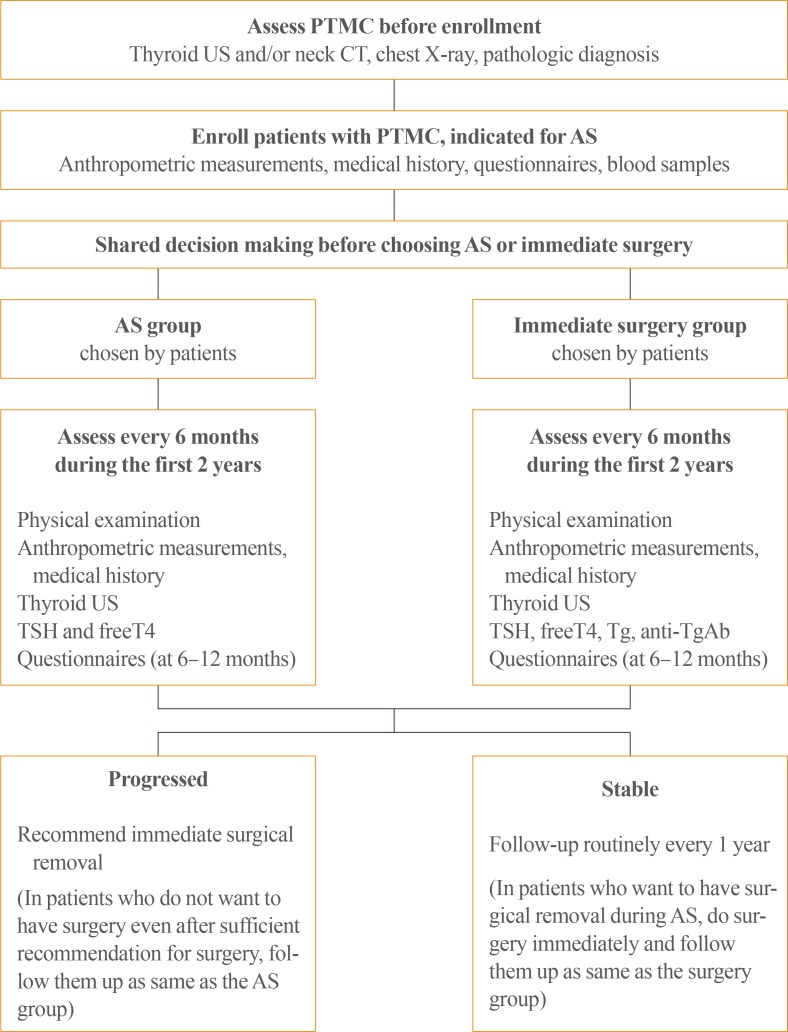
Patients who undergo immediate surgery, or who undergo surgery after being initially included in the AS group, are scheduled for follow-up visits every 6 months during the first 2 years after surgery, and then every 1 year thereafter. The follow-ups involve the same examinations as for the AS group, in addition to laboratory testing of serum thyroglobulin and anti-thyroglobulin antibodies (Fig. 1). However, careful follow-up as same as AS group was recommended if patients with disease progression refuse to undergo surgery. Patients in the surgery group can undergo radioactive iodine therapy if indicated according to the 2016 Korean Thyroid Association guidelines [14,15]. Participants will be followed-up for at least 10 years or until death.
Fig. 1. Conduct of the study. PTMC, papillary thyroid microcarcinoma; US, ultrasonography; CT, computed tomography; AS, active surveillance; TSH, thyroid-stimulating hormone; T4, thyroxine; Tg Ab, thyroglobulin antibody.
Outcomes
This study aimed to evaluate various outcomes, as well as the baseline characteristics of the patients at their PTMC diagnosis and the frequency of AS contraindication. First, we examined the natural course of PTMC, including the tumor's size change and the development of local or distant metastasis. Second, we compared the long-term outcomes (recurrence, metastasis, and mortality) for the immediate surgery and delayed surgery groups. Third, we evaluated the risk factors for PTMC progression during AS or after surgery, based on the ultrasonographic findings, epidemiological factors, and pathological features. Fourth, we compared the QOL results, complications, and medical costs between the AS and immediate surgery groups. Fifth, we examined the factors that influenced the patients' decisions regarding their PRMC management.
Data collection
Clinical data
Each participant had a comprehensive physical examination and shared decision-making regarding their general health, medical history, and QOL responses. The interviews and questionnaires were administered by trained clinical research coordinators. The clinical data included current and past medical history, family history, medication, drinking habits, and smoking habits. Anthropometric data were collected while the participants were wearing light clothing without shoes or accessories. Height was measured to the nearest 0.1 cm in an upright position, and weight was measured to the nearest 0.1 kg using an electronic scale. Body mass index was calculated as weight in kilograms divided by the square of height in meters. Blood pressure and pulse rate were measured at the heart level in the seated position using a standardized cuff, and the measurements were performed after 5 minutes of rest and repeated after another 5 minutes of rest. The Korean version of a thyroid-specific QOL questionnaire has been developed specifically for patients with thyroid cancer [16]. Medical histories and anthropometric measures were evaluated at the enrollment and every follow-up visit. QOL questionnaire responses were obtained at the enrollment and after 6 to 12 months.
Laboratory data
Blood samples were obtained and centrifuged at 30 minutes after sampling for 10 minutes at 3,000 rpm. All samples were analyzed within 24 hours. Serum thyroid-stimulating hormone (TSH) and free thyroxine (T4) levels were measured at the enrollment and every follow-up visit using commercial immunoradiometric kits (Seoul National University and Seoul Metropolitan Government Seoul National University Boramae Medical Center: TSH, DiaSorin SPA, Saluggia, Italy; free T4, Shin Jin Medics, Seoul, Korea; Seoul National University Bundang Hospital and National Cancer Center: TSH, Cisbio International, Gif-sur-Yvette, France; free T4, DiaSorin SPA, Saluggia, Italy). Extra blood samples from the enrollment were stored for future analysis at −80℃ if participants provided informed consent for the storage and analysis.
Imaging data
The initial ultrasonography was performed by radiologists who specialized in thyroid imaging, and patients were followed-up by the same radiologist to minimize interpersonal variation. The images were collected using high-resolution ultrasonography units (IU22, Philips Medical Systems, Bothel, WA, USA; AixPlorer, Supersonic Imagine, Aix en Provence, France) and linear transducers (5 to 12 MHz for IU22; 4 to 15 MHz for AixPlorer). Another thyroid physician confirmed the results that were provided by the radiologist. At least two images were obtained for each nodule: a transverse image to obtain the maximum transverse and perpendicular dimensions, as well as a longitudinal image to obtain the maximum longitudinal dimension. Color doppler images were also obtained for each nodule. Other radiological or pathological tests were performed based on the recommendation of the primary physician or a clinical suspicion of progression.
Sample size
Based on the findings of Japanese studies [17], AS and surgery groups' 10-year rates of clinical progression were set to 9.1% and 3.0%, respectively. The expected enrollment ratio for AS and surgery groups was estimated to be 2:1, based on enrollment status and our prior experience. The α error was set to 0.05 and power was set to 80% based on a two-sided effect. Based on these assumptions, required minimal sample size was 334 and 167 for AS and surgery groups, using Fisher's exact test. Based on a drop-out rate of 0.2, we aimed to recruit 625 patients with PTMC, although recruitment remains open for consenting participants until sufficient data are obtained to develop an appropriate treatment policy. Recruitment will be terminated if an unexpected progression rate were detected at the interim analysis. If the treatment policy changed during the study, based on interim results or evidence from other institutions, enrollment would be stopped at 625 participants.
Data analysis
Continuous data were reported as mean±standard deviation or median (interquartile range), as appropriate. Categorical data were reported as numbers (%). Associations were tested using multivariate regression analyses, which were adjusted for potential confounders. Cox proportional hazard models and Kaplan-Meier curves were used to evaluate the time-to-event outcomes (recurrence, metastasis, and mortality). Stratification and sensitivity analyses were also planned to further evaluate the demographic and clinical characteristics among patients who experienced recurrence, metastasis, or mortality in the AS and surgery groups. Variables that were measured multiple times during the follow-up were analyzed using repeated measures regression analyses. All tests used a significance level of 0.05, and all analyses were performed using IBM SPSS software version 22.0 (IBM Co., Armonk, NY, USA).
RESULTS
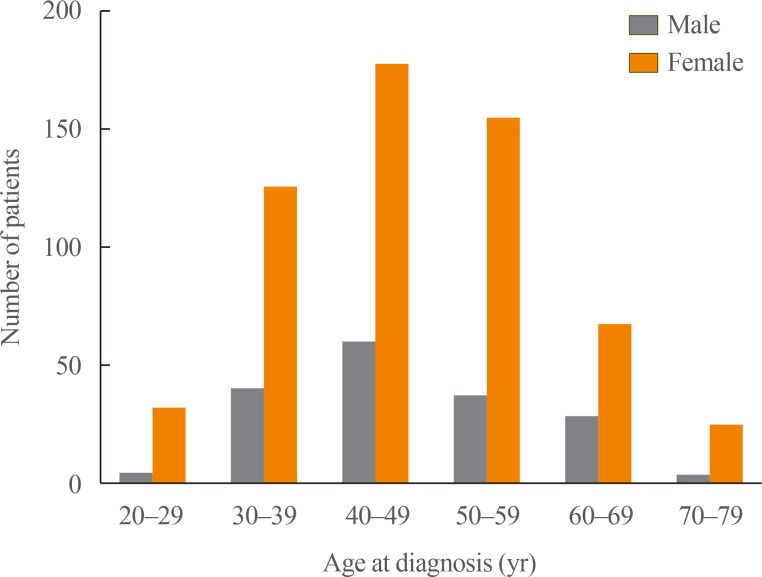
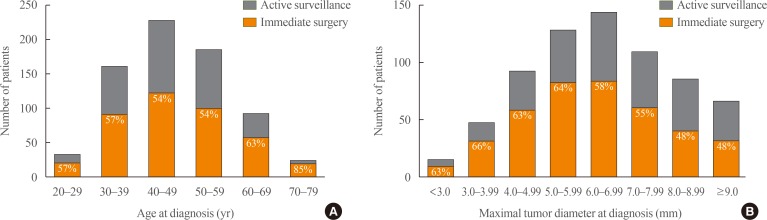
A total of 732 participants with PTMC were enrolled at the participating centers since June 2016, including 419 patients (57.2%) in AS group and 313 patients (42.8%) in immediate surgery group (Table 1). The mean follow-up duration was 6 months (range, 0 to 17). Older patients tended to choose AS group (mean age of patients 48.4±12.2 years in AS group, 46.4±10.6 years in surgery group, P=0.023). The surgery group had a significantly larger maximum tumor diameter at the diagnosis (6.2±1.9 mm in AS group, 6.6±2.0 mm in surgery group, P=0.008). Especially, patients with tumor size ≥7 mm chose surgery more than those with tumor size <7 mm (149 [35.3%] in size ≥7 mm, 144 [46.0%] in size <7 mm, P=0.002). There were no significant differences in sex and thyroid function. Sex and age distribution in the study was similar to the general population of patients with thyroid cancer in Korea (Fig. 2) [18]. Fig. 3 shows the characteristics of AS and immediate surgery groups according to their age and maximum tumor size.
Table 1. Baseline Clinicopathologic Features in Patients with Papillary Thyroid Microcarcinoma According to Active Surveillance and Immediate Surgery Group.
| Variable | AS (n=419) | Surgery (n=313) | P value |
|---|---|---|---|
| Age at diagnosis, yr | 48.4±12.2 | 46.4±10.6 | 0.023 |
| Age >55 years | 237 (56.5) | 190 (60.7) | 0.289 |
| Female sex | 311 (74.4) | 247 (79.4) | 0.133 |
| Maximal tumor diameter at diagnosis, mm | 6.2±1.9 | 6.6±2.0 | 0.008 |
| Maximal tumor diameter ≥7 mm | 149 (35.3) | 144 (46.0) | 0.002 |
| Follow-up duration, mo | 6.0 (5.0–8.0) | 5.0 (4.0–10.0) | <0.001 |
| TSH, µIU/mL | 1.54 (1.01–2.23) | 1.28 (0.77–2.06) | 0.136 |
| Free T4, ng/dL | 1.23 (1.12–1.35) | 1.27 (1.04–1.41) | 0.344 |
| Total T3, ng/dL | 116 (94–130) | 99 (76–116) | 0.707 |
| Thyroglobulin, ng/mL | 6.5 (2.7–10.9) | 3.5 (0.5–9.5) | 0.949 |
Values are expressed as mean±SD, number (%), or median (interquartile range).
AS, active surveillance; TSH, thyroid-stimulating hormone; T4, thyroxine; T3, triiodothyronine.
Fig. 2. Age and gender distribution of participants.
Fig. 3. Distribution of (A) age and (B) tumor diameter at diagnosis according to active surveillance and immediate surgery group.
DISCUSSION
This report describes the protocol for the MAeSTro study, which was designed to develop a protocol for the earliest possible detection of PTMC progression in cases where the patient refused or was reluctant to undergo immediate surgery [19,20]. Thus, the MAeSTro study was designed to provide patients with sufficient information to select AS or immediate surgery, based on previously reported data [7,11,13,21,22]. The patients are also allowed to consider about their decision for 1 to 2 weeks after the initial visit. Furthermore, the study examines the natural course of PTMC, the risk factors for progression, changes in QOL, and cost differences between the AS and immediate surgery group. Thus, in addition to providing clinical and biological data, the results will be helpful for evaluating the effects of AS on lifestyle and economic factors.
Only a few studies have examined AS for thyroid cancer [10,11,12,13]. One recent Japanese report of 1,179 patients who selected AS for PTMC, and detected progression during a median follow-up of 47 months based on size increases (2.3%) or novel lymph node metastasis (0.5%) [13]. Another study of 230 Japanese patients during a median follow-up of 5 years revealed small proportions of progression based on size increases (7%) and novel lymph node metastasis (1%) [12]. Interestingly, large proportions of progression were observed among relatively young or pregnant patients [10,11]. However, none of those studies reported mortality or distant metastasis during AS, and a recent American study revealed a similar result [23]. Although one of the previous studies started in 1993 [10], most of the patients were enrolled after 2010 and the results were from a single country. Thus, the overall long-term prognosis of AS remains unclear [12,13].
There have been some changes in the criteria for selecting AS, which suggests that it can be difficult to establish an adequate protocol or criteria. The Japanese protocol recommended AS for patients with PTMC but without lymph node/distant metastasis, invasion of the recurrent laryngeal nerve or trachea, a clinical suspicion of high-grade malignancy based on FNA biopsy, and poorly differentiated carcinoma [24]. Nevertheless, some researchers have recently suggested that tumors located near the recurrent laryngeal nerve, or attached to the trachea at an obtuse angle, could be suitable for AS [21]. Moreover, Brito et al. [7] suggested adding the following criteria for AS: age of >18 years, likely to attend follow-ups, and a medical team that includes an experienced endocrinologist or surgeon with routine access to neck ultrasonography. However, there remains no established and official recommendation regarding the AS inclusion criteria or protocol. In addition, each country or region may require unique considerations regarding their specific QOL and cost-effectiveness factors [22,25]. A few protocols have been suggested [7,17], and there are ongoing prospective trials involving cases of PTMC with a good expected prognosis (NCT-01392222, NCT02609685, NCT02952612, NCT02363595, NCT01974284, and NCT02938702). These studies are expected to provide meaningful clinical and sociological results that are useful throughout the world, in addition to our findings among Korean patients.
The study presents percentages of recently recruited patients' decisions, and characteristics of the patients according to their decisions. In the study, percentages of patients' choice between AS and immediate surgery group was similar. Considering that most physicians recommended surgery before the study, the percentage of AS group was higher than authors' expectation. It could be because patients who wants AS are more likely to visit the hospitals included in the study, or because not all patients who received surgery participated in the study. In terms of tumor size, patients who have larger tumor tends to have surgery more than AS, especially when tumor is larger than 7 mm. In terms of age, older patients tend to choose AS because emotional and physical burden of surgery with consideration of life expectancy.
The protocol has its originality in several ways. First, the study enrolls both AS and immediate surgery group and has follow-up visits with same interval schedule, which makes it possible to compare groups precisely for disease course of PTMC, especially progression. Second, QOL, which could be the ultimate reason for AS, was also assessed in both groups which has never been assessed in other studies. Third, the recruitment of patients from 4 centers including secondary and tertiary referral hospitals provides a regionally and clinically diverse patient population. It could reduce selection bias and increase representativeness.
In addition to these originalities, the study has strengths. First, prospective cohort design can detect causal associations between PTMC progression and various risk factors. Second, the multidisciplinary MAeSTro team includes endocrinologists, thyroid radiologists, and thyroid surgeons from the departments of endocrine surgery or head and neck surgery. Thus, the team can provide adequate and specialized explanation to facilitate the patients' unbiased selection of the AS or immediate surgery, and the surgeons can perform the optimal treatment in cases that require surgery. Also, each patient is examined by the same radiologist, which minimizes interpersonal variation. Third, face-to-face interview for making shared decision with information material can provide accurate information, because they are performed by experienced healthcare provider, and can also reduce the risk of hospital-based selection bias. Fourth, the emphasis of patient choice provides information regarding the prognosis of PTMC in various states, which can help establish AS inclusion criteria and a definition of progression. However, a limitation is the selection of only voluntary participants, which could introduce selection bias and cause the results not to reflect the findings among the entire Korean population of patients with PTMC.
In conclusion, the MAeSTro study will provide data to develop an AS protocol for patients with PTMC, based on the natural course of PTMC, the risk factors for progression, QOL outcomes, and medical cost.
ACKNOWLEDGMENTS
This work was supported by Seoul National University Hospital (grant #25-2016-0010).
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS: Drafting the work or revising: J.H.M., J.H.K., E.K.L., K.E.L., S.H.K. Acquisition, analysis, or interpretation of data: Y.K.K., W.J.J., C.Y.L., R.E.Y., Y.H., Y.S.S., M.J.K., S.W.C., S.J.K., E.J.J., J.Y.C., C.H.R., Y.J.L., J.H.H., Y.S.J., J.R., Y.H., S.K.P., H.K.S., K.H.Y. Conception or design: D.J.P., Y.J.P. Final approval of the manuscript: Y.J.P.
References
- 1.Wartofsky L, Van Nostrand D. Thyroid cancer: a comprehensive guide to clinical management. 3rd ed. New York: Springer; 2016. Chapter 2, Epidemiology of thyroid cancer; pp. 9–15. [Google Scholar]
- 2.Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015;47:127–141. doi: 10.4143/crt.2015.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho BY, Choi HS, Park YJ, Lim JA, Ahn HY, Lee EK, et al. Changes in the clinicopathological characteristics and outcomes of thyroid cancer in Korea over the past four decades. Thyroid. 2013;23:797–804. doi: 10.1089/thy.2012.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SH, Kim TY, Ryu JS, Gong G, Kim WB, Kim SC, et al. Trends analysis of characteristics of thyroid cancer patients in one medical center. J Korean Endocr Soc. 2008;23:35–43. [Google Scholar]
- 5.Choi YM, Kim TY, Jang EK, Kwon H, Jeon MJ, Kim WG, et al. Standardized thyroid cancer mortality in Korea between 1985 and 2010. Endocrinol Metab (Seoul) 2014;29:530–535. doi: 10.3803/EnM.2014.29.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahn HS, Kim HJ, Welch HG. Korea's thyroid-cancer “epidemic”: screening and overdiagnosis. N Engl J Med. 2014;371:1765–1767. doi: 10.1056/NEJMp1409841. [DOI] [PubMed] [Google Scholar]
- 7.Brito JP, Ito Y, Miyauchi A, Tuttle RM. A clinical framework to facilitate risk stratification when considering an active surveillance alternative to immediate biopsy and surgery in papillary microcarcinoma. Thyroid. 2016;26:144–149. doi: 10.1089/thy.2015.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tosoian JJ, Trock BJ, Landis P, Feng Z, Epstein JI, Partin AW, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29:2185–2190. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 9.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014;24:27–34. doi: 10.1089/thy.2013.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shindo H, Amino N, Ito Y, Kihara M, Kobayashi K, Miya A, et al. Papillary thyroid microcarcinoma might progress during pregnancy. Thyroid. 2014;24:840–844. doi: 10.1089/thy.2013.0527. [DOI] [PubMed] [Google Scholar]
- 12.Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, Fujimoto Y. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg. 2010;34:1222–1231. doi: 10.1007/s00268-009-0359-x. [DOI] [PubMed] [Google Scholar]
- 13.Oda H, Miyauchi A, Ito Y, Yoshioka K, Nakayama A, Sasai H, et al. Incidences of unfavorable events in the management of low-risk papillary microcarcinoma of the thyroid by active surveillance versus immediate surgery. Thyroid. 2016;26:150–155. doi: 10.1089/thy.2015.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi KH, Lee EK, Kang HC, Koh Y, Kim SW, Kim IJ, et al. 2016 Revised Korean thyroid association management guidelines for patients with thyroid nodules and thyroid cancer. Int J Thyroidol. 2016;9:59–126. [Google Scholar]
- 15.Yi KH. The revised 2016 Korean Thyroid Association guidelines for thyroid nodules and cancers: differences from the 2015 American Thyroid Association guidelines. Endocrinol Metab (Seoul) 2016;31:373–378. doi: 10.3803/EnM.2016.31.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryu CH, Park B, Ryu J, Ryu YM, Jo SA, Lee YJ, et al. Development and evaluation of a Korean version of a thyroid-specific quality-of-life questionnaire scale in thyroid cancer patients. Cancer Res Treat. 2018;50:405–415. doi: 10.4143/crt.2017.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg. 2010;34:28–35. doi: 10.1007/s00268-009-0303-0. [DOI] [PubMed] [Google Scholar]
- 18.Korean Central Cancer Registry. Annual report of cancer statistics in Korea in 2014 [Internet] Goyang: National Cancer Center; 2017. [updated 2017 Jan 17]. [cited 2018 May 10]. Available from: http://ncc.re.kr/cancerStatsList.ncc?sea. [Google Scholar]
- 19.Sugitani I, Fujimoto Y. Management of low-risk papillary thyroid carcinoma: unique conventional policy in Japan and our efforts to improve the level of evidence. Surg Today. 2010;40:199–215. doi: 10.1007/s00595-009-4034-5. [DOI] [PubMed] [Google Scholar]
- 20.Kim JS, Moon HJ, Han JS, Kim MJ. Importance of regular follow-up examination during active surveillance: a case of anaplastic transformation of papillary thyroid microcarcinoma. Int J Thyroidol. 2016;9:185–189. [Google Scholar]
- 21.Ito Y, Miyauchi A, Oda H. Low-risk papillary microcarcinoma of the thyroid: a review of active surveillance trials. Eur J Surg Oncol. 2018;44:307–315. doi: 10.1016/j.ejso.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Oda H, Miyauchi A, Ito Y, Sasai H, Masuoka H, Yabuta T, et al. Comparison of the costs of active surveillance and immediate surgery in the management of low-risk papillary microcarcinoma of the thyroid. Endocr J. 2017;64:59–64. doi: 10.1507/endocrj.EJ16-0381. [DOI] [PubMed] [Google Scholar]
- 23.Tuttle RM, Minkowitz G, Wong R, Roman B, Patel S, Untch B, et al. Successful implementation of an active surveillance management approach to low risk papillary thyroid cancer in the United States. Thyroid. 2016;26(Suppl 1):A–140. [Google Scholar]
- 24.Ito Y, Miyauchi A. Nonoperative management of low-risk differentiated thyroid carcinoma. Curr Opin Oncol. 2015;27:15–20. doi: 10.1097/CCO.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venkatesh S, Pasternak JD, Beninato T, Drake FT, Kluijfhout WP, Liu C, et al. Cost-effectiveness of active surveillance versus hemithyroidectomy for micropapillary thyroid cancer. Surgery. 2017;161:116–126. doi: 10.1016/j.surg.2016.06.076. [DOI] [PubMed] [Google Scholar]