Abstract
Thyroid diseases, including autoimmune thyroid diseases and thyroid cancer, are known to have high heritability. Family and twin studies have indicated that genetics plays a major role in the development of thyroid diseases. Thyroid function, represented by thyroid stimulating hormone (TSH) and free thyroxine (T4), is also known to be partly genetically determined. Before the era of genome-wide association studies (GWAS), the ability to identify genes responsible for susceptibility to thyroid disease was limited. Over the past decade, GWAS have been used to identify genes involved in many complex diseases, including various phenotypes of the thyroid gland. In GWAS of autoimmune thyroid diseases, many susceptibility loci associated with autoimmunity (human leukocyte antigen [HLA], protein tyrosine phosphatase, non-receptor type 22 [PTPN22], cytotoxic T-lymphocyte associated protein 4 [CTLA4], and interleukin 2 receptor subunit alpha [IL2RA]) or thyroid-specific genes (thyroid stimulating hormone receptor [TSHR] and forkhead box E1 [FOXE1]) have been identified. Regarding thyroid function, many susceptibility loci for levels of TSH and free T4 have been identified through genome-wide analyses. In GWAS of differentiated thyroid cancer, associations at FOXE1, MAP3K12 binding inhibitory protein 1 (MBIP)-NK2 homeobox 1 (NKX2-1), disrupted in renal carcinoma 3 (DIRC3), neuregulin 1 (NRG1), and pecanex-like 2 (PCNXL2) have been commonly identified in people of European and Korean ancestry, and many other susceptibility loci have been found in specific populations. Through GWAS of various thyroid-related phenotypes, many susceptibility loci have been found, providing insights into the pathogenesis of thyroid diseases and disease co-clustering within families and individuals.
Keywords: Genome-wide association study, Graves disease, Hashimoto disease, Thyroid neoplasms, Thyroid function
INTRODUCTION
Most thyroid diseases, including autoimmune thyroiditis and thyroid cancer, have been recognized to have high heritability [1,2]. In twin studies, a high concordance rate for Graves' disease (GD) in monozygotic twins was reported, in the range of 50% to 70%, compared with 3% to 25% in dizygotic twins [1,3]. A study of autoimmune hypothyroidism likewise showed a 55% concordance in monozygotic twins [4]. Familial clustering of autoimmune thyroid disease has been consistently reported [5,6,7]. Hemminki et al. [7] showed that the familial standardized incidence ratios for GD were 4.49 for individuals with an affected parent, 5.04 for those whose singleton sibling was affected, 310 when two or more siblings were affected, and 16.45 in twins. For Hashimoto's thyroiditis (HT), the sibling risk ratio was 28 based on data from the National Health and Nutrition Examination Survey III [8], and a similar risk was confirmed in data from Germany [5]. These pieces of evidence suggest the existence of a genetic predisposition to autoimmune thyroid diseases.
Thyroid function, including levels of thyroid hormone and thyroid stimulating hormone (TSH), is regulated within a narrow range in individuals, although the inter-individual variability is large [9]. This suggests that every individual has his or her own set point of thyroid function [10]. About 40% to 60% of variation in thyroid function has been estimated to be determined by genetic factors [10,11,12]. Thyroid cancers also show a high degree of heritability, with genetic factors accounting for more than 50% of the causes of thyroid cancer [2]. Except for medullary thyroid cancer, which is well known to be caused by germline or somatic mutations, the prevalence of familial differentiated thyroid cancer (DTC) accounted for 2.5% to 11.3% cases of DTC [13,14,15,16,17]. Only 5% of cases of nonmedullary familial DTC were reported to be of the syndromic form, which is accompanied by well-known germline mutations, including Cowden syndrome, familial adenomatous polyposis, Gardner syndrome, Carney complex type 1, Werner syndrome, and DICER1 syndrome [18]. Thus, the majority of cases of familial DTC were found not to be caused by germline mutations, despite its pattern of genetic inheritance.
Thus, genetics plays a prominent role in most thyroid-related phenotypes. Research into the genes responsible for thyroid disease has identified several candidates [19]. However, candidate gene studies have been controversial and have shown very few reproducible findings. Panicker [19] published a thorough review of genetic studies of thyroid function and autoimmune thyroid diseases conducted through 2010. In the last decade, genome-wide association studies (GWAS) have been extensively used to identify genes involved in complex diseases [20]. GWAS have facilitated the screening of a large proportion of the genome and discovered a variety of susceptibility genes. GWAS have been widely applied in autoimmune thyroid diseases, thyroid function, and thyroid cancer, and have identified susceptibility genes for thyroid-related phenotypes. Herein, we comprehensively review the wide range of discoveries from GWAS conducted in Western and Asian populations regarding autoimmune diseases, thyroid function, and thyroid cancer.
GWAS FOR AUTOIMMUNE THYROID DISEASES
Several candidate gene studies identified putative susceptibility variants for GD, but only the human leukocyte antigen (HLA) locus and the cytotoxic T-lymphocyte associated protein 4 (CTLA4), thyroid stimulating hormone receptor (TSHR), and protein tyrosine phosphatase, non-receptor type 22 (PTPN22) loci were confirmed in subsequent replication studies [21,22,23,24,25]. The first genome-wide analysis using 14,436 nonsynonymous single-nucleotide polymorphisms (SNPs) for GD was performed by the Wellcome Trust Case Control Consortium, and showed that three loci (HLA, TSHR, and Fc receptor like 3 [FCRL3]) were associated with GD [26]. A subsequent GWAS with >500,000 SNPs confirmed previously reported loci and identified a novel region of susceptibility loci at 6q27 (the ribonuclease T2 [RNASET2]-FGFR1 oncogene partner [FGFR1OP]-CCR6) and an intergenic region at 4p14 (GDCG4p14) [27]. Several GWAS of autoimmune thyroid diseases (GD, HT, and positivity of anti-thyroid peroxidase [TPO] antibody or anti-thyroglobulin [Tg] antibody) and hypothyroidism have further identified susceptibility loci (Table 1) [26,27,28,29,30,31,32,33,34,35,36]. Since GWAS of HT have been performed for a variety of phenotypes including self-reported hypothyroidism, biochemical hypothyroidism with positive antibodies, antibody positivity, and level of antibodies, caution is needed when interpreting the results. Several types of hypothyroidism might not have an autoimmune etiology, and autoimmunity does not necessarily lead to hypothyroidism. Thus, careful consideration regarding the phenotype is required when interpreting the biological mechanisms of the associated genes identified through GWAS of autoimmune thyroid diseases.
Table 1. Susceptibility Loci for Autoimmune Thyroid Disease Detected by Genome-Wide Association Studies.
| Phenotypes | Locus | Gene | Protein function | Population | Reference |
|---|---|---|---|---|---|
| GD, HT | 1p13 | PTPN22 | Involvement in T-cell signaling | UK, USA | [28,30] |
| GD | 10p15.1 | IL2RA | Encoding CD25 | UK | [28] |
| GD, HT | 2q33.2 | CTLA4 | Inhibition of T-cell signaling | UK, Chinese Han, USA | [27,28,30,31] |
| GD | 1q23.1 | FCRL3 | Regulation of B-cell signaling | UK, Chinese Han | [26,27,28,31] |
| GD, HT | 6p21 | HLA class I region | Endogenous antigen presentation for recognition by CD8+ T-cells | UK, Chinese Han, USA | [26,27,30,31] |
| GD, HT | 6p21 | HLA class II region | Exogenous antigen presentation for recognition by CD4+ T-helper cells | UK, Chinese Han, USA | [26,27,30,31] |
| GD | 14q31.1 | TSHR | Autoantigenic target in GD | UK, Chinese Han | [26,27,28,31] |
| GD, HT | 6q27 | RNASET2-FGFR1OP | A fusion partner for FGFR1 in the t(6;8) (q27;p11) translocations | UK, Chinese Han | [27,28,31] |
| GD | 4p14 | CHRNA9-RHOH | Negative regulator of hematopoietic cell growth and survival | Chinese Han | [27,31] |
| GD | 1p36.32 | MMEL1 | Role in pain perception, arterial pressure regulation, phosphate metabolism, and homeostasis | UK | [28] |
| GD | 12q12 | PRICKLE1 | Negative regulator of the Wnt/β-catenin signaling pathway | UK | [28] |
| GD | 16p11.2 | ITGAM | Role in leukocyte adhesion to platelets and fibrinogen | UK | [28] |
| GD | Xq21.1 | GPR174-ITM2A | Thymocyte selection and T-cell activation | Chinese Han | [31,32] |
| GD | 22q12.3–13.1 | C1QTNF6-RAC2 | Role in elicitation of immune responses and the induction of peripheral immune tolerance | Chinese Han | [31] |
| GD | 1q23.2 | SLAMF6 | Coreceptor in the process of NK cell activation | Chinese Han | [31] |
| GD | 9q34.2 | ABO | Determination of ABO blood group | Chinese Han | [31] |
| GD | 14q32. | C14orf64 | Long intergenic non-protein coding RNA 1550 (LINC01550) | Chinese Han | [31] |
| GD | 8q24.22 | TG | Encoding thyroglobulin | Chinese Han | [31] |
| HT | 9q22.33 | FOXE1 | Encoding TTF-2, role in thyroid morphogenesis | USA | [29,30] |
| HT | 12q24.12 | SH2B3 | Negative regulator of cytokine signaling | USA | [30] |
| HT | 1p13.3 | VAV3 | Role in actin cytoskeletal rearrangements and transcriptional alterations | US, Japan | [30,36] |
| HT | 1p36.13 | CAPZB | Role in regulating actin filament dynamics | USA | [30] |
| HT | 5q13.3 | PDE8B | Role in hydrolysis of the second messenger cAMP | USA | [30] |
| GD, HT | 2p25.1 | TRIB2 | Role in apoptosis of hematopoietic cells | UK | [28] |
| GD, HT | 3q27.3 | LPP | Involvement in cell-cell adhesion and cell motility | UK | [28] |
| GD, HT, TPOAb | 6q15 | BACH2 | Role in coordinating transcription activation and repression by MAFK | UK, Europeana | [28,34] |
| GD, HT | 11q21 | FAM76B | Role in NEDD8-specific protease activity | UK | [28] |
| TPOAb | 2p25.3 | TPO | Encoding thyroid peroxidase | European, Korea | [33,34] |
| TPOAb | 12q24.12 | ATXN2 | Role in Akt signaling and checkpoint regulation. | European | [34] |
| TPOAb | 1p13.2 | MAGI3 | Role in Sertoli-Sertoli cell junction dynamics and Ras signaling pathway | European | [34] |
| TPOAb | 3q21.1 | KALRN | Role in p75 NTR-mediated signaling and EPH-ephrin signaling | European | [34] |
| TPOAb | 9q31.1 | GRIN3A | Role in circadian entrainment | Croatia | [35] |
| TgAb | 6q27 | DLL1 | Role in mediating cell fate decisions during hematopoiesis | Croatia | [35] |
GD, Graves' disease; HT, Hashimoto's thyroiditis or hypothyroidism; PTPN22, protein tyrosine phosphatase, non-receptor type 22; IL2RA, interleukin 2 receptor subunit alpha; CTLA4, cytotoxic T-lymphocyte associated protein 4; FCRL3, Fc receptor like 3; HLA, human leukocyte antigen; TSHR, thyroid stimulating hormone receptor; RNASET2, ribonuclease T2; FGFR1OP, FGFR1 oncogene partner; FGFR1, fibroblast growth factor receptor 1; CHRNA9, cholinergic receptor nicotinic alpha 9 subunit; RHOH, ras homolog family member H; MMEL1, membrane metalloendopeptidase like 1; PRICKLE1, prickle planar cell polarity protein 1; ITGAM, integrin subunit alpha M; GPR174, G protein-coupled receptor 174; ITM2A, integral membrane protein 2A; C1QTNF6, C1q and TNF related 6; RAC2, Rac family small GTPase 2; SLAMF6, SLAM family member 6; NK, natural killer; TG, anti-thyroglobulin; FOXE1, forkhead box E1; TTF-2, thyroid transcription factor-2; SH2B3, SH2B adaptor protein 3; VAV3, vav guanine nucleotide exchange factor 3; CAPZB, capping actin protein of muscle Z-line subunit beta; PDE8B, phosphodiesterase 8B; cAMP, cyclic adenosine monophosphate; TRIB2, tribbles pseudokinase 2; LPP, LIM domain containing preferred translocation partner in lipoma; TPOAb, anti-thyroid peroxidase antibody; BACH2, BTB domain and CNC homolog 2; MAFK, MAF bZIP transcription factor K; FAM76B, family with sequence similarity 76 member B; NEDD8, neural precursor cell expressed, developmentally down-regulated 8; TPO, anti-thyroid peroxidase; ATXN2, ataxin 2; MAGI3, membrane associated guanylate kinase, WW and PDZ domain containing 3; KALRN, kalirin RhoGEF kinase; NTR, neurotrophin receptor; GRIN3A, glutamate ionotropic receptor NMDA type subunit 3A; TgAb, anti-thyroglobulin antibody; DLL1, delta like canonical Notch ligand 1.
aEuropean refers to European ancestry from various countries.
A heterogeneity analysis between GD and HT showed that GD and HT share several susceptibility loci (HLA, PTPN22, and CTLA4), while an association with TSHR was exclusively seen in GD patients. The majority of genes associated with autoimmune thyroid disease are thought to play a major role in autoimmune processes, including disrupted T-cell regulation and peripheral immune tolerance [37]. Variants in thyroid-specific loci, including TSHR and forkhead box E1 (FOXE1), could affect the immune recognition of autoantigens and antibody generation [37].
GWAS OF THYROID FUNCTION
Thyroid function, including levels of free thyroxine (T4) and TSH, is highly heritable even in euthyroid subjects. A large meta-analysis of GWAS of serum levels of TSH and free T4, in 26,420 and 17,520 euthyroid European individuals, respectively, was performed, identifying many susceptibility loci for levels of TSH (phosphodiesterase 8B [PDE8B], phosphodiesterase 10A [PDE10A], capping actin protein of muscle Z-line subunit beta [CAPZB], MAP, vascular endothelial growth factor A [VEGFA], nuclear receptor subfamily 3 group C member 2 [NR3C2], insulin like growth factor binding protein 5 [IGFBP5], SRY-box 9 [SOX9], nuclear factor I A [NFIA], fibroblast growth factor 7 [FGF7], PR/SET domain 11 [PRDM11], microRNA 1179 [MIR1179], insulin receptor [INSR], ABO, inositol-tetrakisphosphate 1-kinase [ITPK1], neuregulin 1 [NRG1], MAP3K12 binding inhibitory protein 1 [MBIP], SAM and SH3 domain containing 1 [SASH1], and GLIS family zinc finger 3 [GLIS3]) and levels of free T4 (iodothyronine deiodinase 1 [DIO1], LIM homeobox 3 [LHX3], FOXE1, aminoadipate aminotransferase [AADAT], lysophosphatidylcholine acyltransferase 2 [LPCAT2]/calpain small subunit 2 [CAPNS2], neuropilin and tolloid like 1 [NETO1]/F-box protein 15 [FBXO15]) [38]. A GWAS of TSH levels was also conducted in 1,346 Chinese Han individuals [39]. Zhan et al. [39] confirmed previously reported TSH susceptibility loci near FOXE1 and CAPZB and identified novel variants in XK related 4 (XKR4). Whole-genome sequence-based analysis was performed to examine the genetic architecture for levels of free T4 and TSH, and further identified novel variants on synapsin II (SYN2), PDE8B, and beta-1,4-galactosyltransferase 6 (B4GALT6) [40]. They also found a rare functional variant (minor allele frequency=0.4%) in the transthyretin (TTR) gene, which is located near B4GALT6. This study showed that common variants explained over 20% of the variance in TSH and free T4 and that a substantial amount of heritability of thyroid function could be explained by rare variants with larger effects. Results of GWAS for thyroid function are summarized in Table 2.
Table 2. Susceptibility Loci for Levels of Thyroid Stimulating Hormone or Free Thyroxine Detected by Genome-Wide Association Studies.
| Phenotypes | Locus | Gene | Protein function | Population | Reference |
|---|---|---|---|---|---|
| TSH | 5q13.3 | PDE8B | Role in hydrolysis of the second messenger cAMP | European, USA, Germany, UK | [38,40,48,49] |
| 6q27 | PDE10A | Role in regulation of the intracellular concentration of cyclic nucleotides | European, UK | [38,40] | |
| 1p36.13 | CAPZB | Regulating actin filament dynamics | European, Chinese Han, Germany, UK | [38,39,40,49] | |
| 16q23.2 | MAF | Role in increased T-cell susceptibility to apoptosis | European, UK, Germany | [38,40,49] | |
| 6p21.1 | VEGFA | Proliferation and migration of vascular endothelial cells | European, UK | [38,40] | |
| 4q31.23 | NR3C2 | Role in aldosterone actions | European, Germany, UK | [38,40,49] | |
| 2q35 | IGFBP5 | Encoding insulin like growth factor binding protein 5 | European | [38] | |
| 17q24.3 | SOX9 | Role in chondrocyte differentiation | European | [38] | |
| 1p31.3 | NFIA | Encoding nuclear factor IA | European | [38] | |
| 15q21.2 | FGF7 | Mitogenic and cell survival activities | European | [38] | |
| 11p11.2 | PRDM11 | Role in transcription regulation | European | [38] | |
| 15q26.1 | MIR1179 | MicroRNA 1179 | European | [38] | |
| 19p13.2 | INSR | Encoding insulin receptor | European | [38] | |
| 9q34.2 | ABO | Determination of ABO blood group | European, UK | [38,40] | |
| 14q32.12 | ITPK1 | Regulation of the synthesis of inositol tetraphosphate | European | [38] | |
| 8p12 | NRG1 | Role in the growth and development of multiple organ systems | European | [38] | |
| 14q13.3 | MBIP-NKX2-1 | Encoding TTF-1, binding to TG promoter | European, UK | [38,40] | |
| 6q24.3 | SASH1 | Role in the TLR4 signaling pathway | European | [38] | |
| 9p24.2 | GLIS3 | Role in transcription in thyroid gland | European | [38] | |
| 8q12.1 | XKR4 | Role in apoptosis | Chinese Han | [39] | |
| 9q22.33 | FOXE1 | Encoding TTF-2, role in thyroid morphogenesis | Chinese Han, USA, UK | [39,40,48] | |
| 2q35 | IGFBP2 | Encoding insulin like growth factor binding protein 2 | UK | [40] | |
| 3p25.2 | SYN2 | Binding to small synaptic vesicles | UK | [40] | |
| Free T4 | 1p32.3 | DIO1 | Encoding iodothyronine deiodinase 1 | European, UK | [38,40] |
| 9q34.3 | LHX3 | Role in pituitary development | European, UK | [38,40] | |
| 9q22.33 | FOXE1 | Encoding TTF-2, role in thyroid morphogenesis | European | [38] | |
| 4q33 | AADAT | Role in L-lysine catabolism | European, UK | [38,40] | |
| 16q12.2 | LPCAT2-CAPNS2 | Role in membrane biogenesis | European | [38] | |
| 18q22.3 | NETO1-FBXO15 | Role in spatial learning and memory in the hippocampus | European | [38] | |
| 18q12.1 | B4GALT6 | Role in biosynthesis of glycosphingolipids | UK | [40] |
TSH, thyroid stimulating hormone; PDE8B, phosphodiesterase 8B; cAMP, cyclic adenosine monophosphate; PDE10A, phosphodiesterase 10A; CAPZB, capping actin protein of muscle Z-line subunit beta; VEGFA, vascular endothelial growth factor A; NR3C2, nuclear receptor subfamily 3 group C member 2; IGFBP5, insulin like growth factor binding protein 5; SOX9, SRY-box 9; NFIA, nuclear factor I A; FGF7, fibroblast growth factor 7; PRDM11, PR/SET domain 11; MIR1179, microRNA 1179; INSR, insulin receptor; ITPK1, inositol-tetrakisphosphate 1-kinase; NRG1, neuregulin 1; MBIP, MAP3K12 binding inhibitory protein 1; NKX2-1, NK2 homeobox 1; TTF, thyroid transcription factor; TG, thyroglobulin; SASH1, SAM and SH3 domain containing 1; TLR4, Toll-like receptor 4; GLIS3, GLIS family zinc finger 3; XKR4, XK related 4; FOXE1, forkhead box E1; IGFBP2, insulin like growth factor binding protein 2; SYN2, synapsin II; T4, thyroxine; DIO1, iodothyronine deiodinase 1; LHX3, LIM homeobox 3; AADAT, aminoadipate aminotransferase; LPCAT2, lysophosphatidylcholine acyltransferase 2; CAPNS2, calpain small subunit 2; NETO1, neuropilin and tolloid like 1; FBXO15, F-box protein 15; B4GALT6, beta-1,4-galactosyltransferase 6.
Thyroid function may be affected by the presence of antibodies to TPO or Tg, even in the normal range. In GWAS of thyroid function, data on the presence of antibodies were limited. Therefore, it is difficult to conclude that the genes found in GWAS of thyroid function determine an individual set point of the hypothalamus-pituitary-thyroid axis. Several genetic loci identified in GWAS of thyroid function were also found in GWAS of autoimmune thyroid diseases (FOXE1, CAPZB, and PDE8B). A detailed examination of the presence of antibodies should be considered when performing GWAS of thyroid function in the future. In addition, only very limited GWAS of thyroid function have been performed in Asians, so more research is needed.
GWAS OF THYROID CANCER
The first GWAS of thyroid cancer was reported in 2009 and showed that common variants located on 9q22.33 (FOXE1) and 14q13.3 (NK2 homeobox 1 [NKX2-1]) were associated with DTC [41]. Associations at FOXE1, MBIP/NKX2-1, disrupted in renal carcinoma 3 (DIRC3), and NRG1 have been identified and repeatedly confirmed in individuals of European ancestry [41,42,43,44]. Several markers associated with DTC, including inner mitochondrial membrane peptidase subunit 2 (IMMP2L), retinoic acid receptor responder 1 (RARRES1), small nuclear RNA activating complex polypeptide 4 (SNAPC4), basic leucine zipper ATF-like transcription factor (BATF), DEAH-box helicase 35 (DHX35), UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase-like 4 (GALNTL4), 5-hydroxytryptamine receptor 1B (HTR1B), forkhead box A2 (FOXA2), and WDR11 antisense RNA 1 (WDR11-AS1), were identified but not replicated in other studies [43,44,45,46]. A recent meta-analysis of GWAS including a total of 3,001 DTC patients and 287,550 controls from five study groups of European populations found five novel loci (pecanex-like 2 [PCNXL2], telomerase RNA component [TERC], neuronal regeneration related protein [NREP]-erythrocyte membrane protein band 4.1 like 4A [EPB41L4A], oligosaccharide-binding folds containing 1 [OBFC1], and SMAD family member 3 [SMAD3]) [47]. Table 3 provides the susceptibility loci identified in GWAS of thyroid cancer [38,39,40,48,49]. The most robust signals were detected on 9q22.33 (FOXE1) in Caucasians [41,50]. The FOXE1 locus was also reported to be a susceptibility gene for radiation-related thyroid cancer [50]. A functional study showed that common variants on FOXE1 regulated FOXE1 transcription through the recruitment of the upstream stimulatory factor 1 (USF1)/USF2 transcription factors [51]. Several reports demonstrated that variants of FOXE1 were related to aspects of the clinical aggressiveness of papillary thyroid cancer (PTC), such as tumor stage, size, lymphocytic infiltration, and extrathyroidal extension [52,53].
Table 3. Susceptibility Loci for Thyroid Cancer Detected by Genome-Wide Association Studies.
| Locus | Gene | Protein function | Population | References |
|---|---|---|---|---|
| 9q22.33 | FOXE1 | Encoding TTF-2, role in thyroid morphogenesis | Iceland, USA, Spain, Netherlands, Belarus, Italy, Poland, Korea | [41,42,46,47,48,49,50,54] |
| 14q13.3 | MBIP-NKX2-1 | Encoding TTF-1 | Iceland, USA, Spain, Netherlands, Italy, Poland, Korea | [41,42,46,47,54] |
| 2q35 | DIRC3 | Non-coding RNA | Iceland, USA, Spain, Netherlands, Italy, Poland, UK, Korea | [42,43,47,54] |
| 8p12 | NRG1 | Role in the growth and development of multiple organ systems | Iceland, USA, Spain, Netherlands, Korea | [42,54] |
| 7q31.1 | IMMP2L | Catalytic activity of the mitochondrial inner membrane peptidase complex | Italy, Poland, UK, Spain | [43] |
| 3q25.32 | RARRES1 | Encoding a type 1 membrane protein. | Italy, Poland, UK, Spain | [43] |
| 9q34 | SNAPC4 | Role in RNA polymerase II and III transcription from small nuclear RNA promoters. | Italy, Poland, UK, Spain | [43] |
| 14q24.3 | BATF | Negative regulator of AP-1/ATF transcriptional events | Italy, Poland | [44] |
| 20q11.23 | DHX35 | Putative RNA helicases | Italy, Poland | [44] |
| 5q14 | ARSB | Role in the regulation of cell adhesion, cell migration and invasion | Italy, Poland, Spain | [44] |
| 13q12 | SPATA13 | Role in regulation of cell migration and adhesion assembly and disassembly | Italy, Poland, Spain | [44] |
| 11p15.3 | GALNTL4 | Role in initial reaction in O-linked oligosaccharide biosynthesis | Italy, Poland, Spain | [45] |
| 20p11 | FOXA2 | Activators for liver-specific genes such as albumin and transthyretin | Italy, Poland, Spain | [45] |
| 10q26.12 | WDR11-AS1 | Non-coding RNA | Italy, Spain | [46] |
| 6q14.1 | HTR1B | Role in activity of adenylate cyclase and the release of serotonin, dopamine, and acetylcholine | Italy, Spain | [46] |
| 1q42.2 | PCNXL2 | Role in tumorigenesis | Iceland, USA, Spain, Netherlands, Korea | [47,54] |
| 10q24.33 | OBFC1 | Role in initiation of DNA replication | Iceland, USA, Spain, Netherlands | [47] |
| 5q22.1 | NREP-EPB41L4A | Role in interactions between the cytoskeleton and plasma membrane | Iceland, USA, Spain, Netherlands | [47] |
| 15q22.33 | SMAD3 | Signal transducers and transcriptional modulator | Iceland, USA, Spain, Netherlands | [47] |
| 3q26.2 | TERC-LRRC34 | Encoding telomerase RNA component | Iceland, USA, Spain, Netherlands | [47] |
| 5p15.33 | TERT | Encoding telomerase reverse transcriptase | Iceland, USA, Spain, Netherlands | [47] |
| 12q14.3 | MSRB3 | Role in reduction of methionine sulfoxide to methionine | Korea | [54] |
| 1p13.3 | VAV3 | Role in actin cytoskeletal rearrangements and transcriptional alterations | Korea | [54] |
| 4q21.1 | SEPT11 | Role in cytokinesis and vesicle trafficking | Korea | [54] |
| 3p14.2 | FHIT | Role in purine metabolism | Korea | [54] |
| 19p13.2 | INSR | Encoding insulin receptor | Korea | [54] |
| 12q24.13 | SLC24A6 | Role in cellular calcium homeostasis | Korea | [54] |
FOXE1, forkhead box E1; TTF, thyroid transcription factor; MBIP, MAP3K12 binding inhibitory protein 1; NKX2-1, NK2 homeobox 1; DIRC3, disrupted in renal carcinoma 3; NRG1, neuregulin 1; IMMP2L, inner mitochondrial membrane peptidase subunit 2; RARRES1, retinoic acid receptor responder 1; SNAPC4, small nuclear RNA activating complex polypeptide 4; BATF, basic leucine zipper ATF-like transcription factor; AP-1, activator protein 1; ATF, activating transcription factor; DHX35, DEAH-box helicase 35; ARSB, arylsulfatase B; SPATA13, spermatogenesis associated 13; GALNTL4, UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase-like 4; FOXA2, forkhead box A2; WDR11-AS1, WDR11 antisense RNA 1; HTR1B, 5-hydroxytryptamine receptor 1B; PCNXL2, pecanex-like 2; OBFC1, oligosaccharide-binding folds containing 1; NREP, neuronal regeneration related protein; EPB41L4A, erythrocyte membrane protein band 4.1 like 4A; SMAD3, SMAD family member 3; TERC, telomerase RNA component; LRRC34, leucine rich repeat containing 34; TERT, telomerase reverse transcriptase; MSRB3, methionine sulfoxide reductase B3; VAV3, vav guanine nucleotide exchange factor 3; SEPT11, septin 11; FHIT, fragile histidine triad; INSR, insulin receptor; SLC24A6, solute carrier family 24 member A6.
Recently, we reported 15 variants from 11 loci associated with DTC in a Korean GWAS including 1,085 cases of DTC and 8,884 controls [54]. The most robust signals were detected in the NRG1 gene, and expression quantitative trait loci analysis showed that variants on NRG1 were also associated with NRG1 expression in thyroid tissues [54]. He et al. [55] also showed that the expression levels of NRG1 isoforms were significantly correlated with genotypes. NRG1 encodes neuregulin-1, which acts on the erb-b2 receptor tyrosine kinase (ERBB) family of tyrosine kinase receptors. In a study of the intrinsic resistance of PTC to a B-Raf inhibitor, ERBB2/ERBB3 activation was found to be dependent on autocrine production of neuregulin-1 [56]. NRG1 dysregulation is also closely related with the phosphoinositide 3-kinase (PI3K)-AKT and mitogen-activated protein kinase (MAPK) signaling pathway via ERBB [57]. Our gene set enrichment analysis data showed that variants on NRG1 were associated with many pathways related to cellular growth or cancer, and the ERBB-MAPK signaling pathway was the most significantly enriched. This evidence indicates that NRG1 expression in thyroid tissue could contribute to increased DTC risk via ERBB signaling.
Our results confirmed previously reported loci (FOXE1, NKX2-1, DIRC3, and PCNXL2) from GWAS of European populations and found novel susceptibility loci (vav guanine nucleotide exchange factor 3 [VAV3], INSR, MRSB3, fragile histidine triad [FHIT], septin 11 [SEPT11], and solute carrier family 24 member A6 [SLC24A6]) associated with DTC. Specially, a variant of SLC24A6 was associated with a specific risk of follicular thyroid cancer, for which the genetic factors that increase the risk of thyroid cancer may vary depending on the cancer subtype. Signals on VAV3, INSR, MRSB3, FHIT, SEPT11, and SLC24A6 were only identified in Koreans, suggesting between-study heterogeneity in GWAS of DTC.
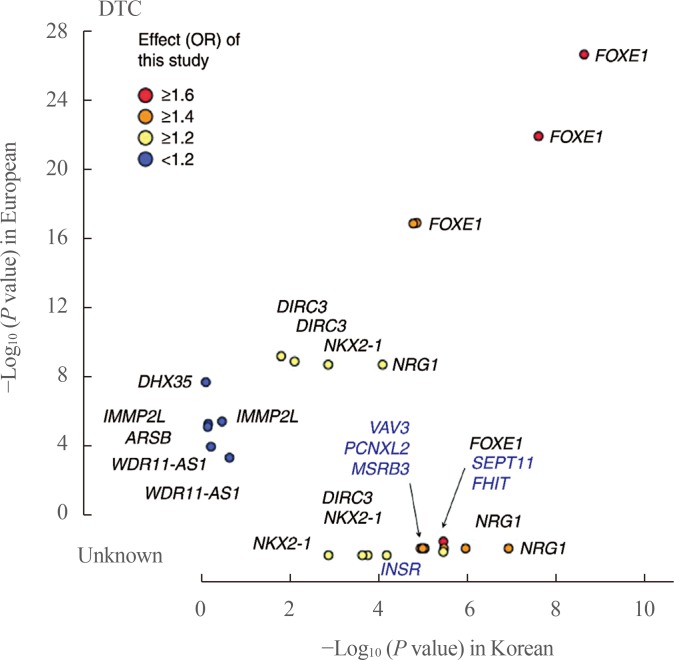
In GWAS in European and Korean populations, some genetic loci (FOXE1, NKX2-1, DIRC3, NRG1, and PCNXL2) were commonly found, while certain susceptibility loci were only found in either the European or Korean population. In addition, the risk allele frequency of commonly found SNPs differs by race, and the DTC risk by genotype varies across ethnicities. For example, the risk allele frequencies of variants on FOXE1 were reported to be 0.14 to 0.34 in Europeans and 0.08 to 0.13 in Asians, suggesting ethnic differences in allele frequencies and a small genetic contribution of variants on FOXE1 to the development of DTC in East Asians [58]. Moreover, common variants on FOXE1 were associated with an increased risk of DTC, with an odds ratio (OR) of 1.80 in the European population, but the OR was 1.35 in East Asians [58]. A comparison of these associations, including effect size (OR) and P values, between Europeans and Koreans is shown in Fig. 1 [54].
Fig. 1. Comparison of associations between Europeans and Koreans. The P values for differentiated thyroid cancer (DTC) between Koreans (x-axis) and Europeans (y-axis) are plotted with the corresponding Korean effect sizes (odd ratio [OR]). The P value shows the −log10 scale, and the P values of novel single-nucleotide polymorphisms from this study are compared as unknown. Adapted from Son et al. [54]. FOXE1, forkhead box E1; DIRC3, disrupted in renal carcinoma 3; NKX2-1, NK2 homeobox 1; NRG1, neuregulin 1; DHX35, DEAH-box helicase 35; IMMP2L, inner mitochondrial membrane peptidase subunit 2; ARSB, arylsulfatase B; WDR11-AS1, WDR11 antisense RNA 1; VAV3, vav guanine nucleotide exchange factor 3; PCNXL2, pecanex-like 2; MSRB3, methionine sulfoxide reductase B3; SEPT11, septin 11; FHIT, fragile histidine triad; INSR, insulin receptor.
CONCLUSIONS
Twin and family studies of autoimmune thyroid diseases and thyroid cancer have indicated high heritability, suggesting that genetic factors play a key role in disease onset. Previous candidate-gene studies have limitations, such as lack of reproducibility and small sample sizes with limited statistical power. In the last decade, GWAS have unraveled the many forms of genetic predisposition to autoimmune thyroid disease, thyroid function, and thyroid cancer. These genetic discoveries provide insight into the pathogenesis of these diseases and provide opportunities to develop new therapies.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Vaidya B, Kendall-Taylor P, Pearce SH. The genetics of autoimmune thyroid disease. J Clin Endocrinol Metab. 2002;87:5385–5397. doi: 10.1210/jc.2002-020492. [DOI] [PubMed] [Google Scholar]
- 2.Czene K, Lichtenstein P, Hemminki K. Environmental and heritable causes of cancer among 9.6 million individuals in the Swedish Family-Cancer Database. Int J Cancer. 2002;99:260–266. doi: 10.1002/ijc.10332. [DOI] [PubMed] [Google Scholar]
- 3.Brix TH, Kyvik KO, Christensen K, Hegedus L. Evidence for a major role of heredity in Graves' disease: a population-based study of two Danish twin cohorts. J Clin Endocrinol Metab. 2001;86:930–934. doi: 10.1210/jcem.86.2.7242. [DOI] [PubMed] [Google Scholar]
- 4.Brix TH, Kyvik KO, Hegedus L. A population-based study of chronic autoimmune hypothyroidism in Danish twins. J Clin Endocrinol Metab. 2000;85:536–539. doi: 10.1210/jcem.85.2.6385. [DOI] [PubMed] [Google Scholar]
- 5.Dittmar M, Libich C, Brenzel T, Kahaly GJ. Increased familial clustering of autoimmune thyroid diseases. Horm Metab Res. 2011;43:200–204. doi: 10.1055/s-0031-1271619. [DOI] [PubMed] [Google Scholar]
- 6.Tamai H, Ohsako N, Takeno K, Fukino O, Takahashi H, Kuma K, et al. Changes in thyroid function in euthyroid subjects with a family history of Graves' disease: a follow-up study of 69 patients. J Clin Endocrinol Metab. 1980;51:1123–1127. doi: 10.1210/jcem-51-5-1123. [DOI] [PubMed] [Google Scholar]
- 7.Hemminki K, Li X, Sundquist J, Sundquist K. The epidemiology of Graves' disease: evidence of a genetic and an environmental contribution. J Autoimmun. 2010;34:J307–J313. doi: 10.1016/j.jaut.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Villanueva R, Greenberg DA, Davies TF, Tomer Y. Sibling recurrence risk in autoimmune thyroid disease. Thyroid. 2003;13:761–764. doi: 10.1089/105072503768499653. [DOI] [PubMed] [Google Scholar]
- 9.Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab. 2002;87:1068–1072. doi: 10.1210/jcem.87.3.8165. [DOI] [PubMed] [Google Scholar]
- 10.Hansen PS, Brix TH, Sorensen TI, Kyvik KO, Hegedus L. Major genetic influence on the regulation of the pituitary-thyroid axis: a study of healthy Danish twins. J Clin Endocrinol Metab. 2004;89:1181–1187. doi: 10.1210/jc.2003-031641. [DOI] [PubMed] [Google Scholar]
- 11.Panicker V, Wilson SG, Spector TD, Brown SJ, Falchi M, Richards JB, et al. Heritability of serum TSH, free T4 and free T3 concentrations: a study of a large UK twin cohort. Clin Endocrinol (Oxf) 2008;68:652–659. doi: 10.1111/j.1365-2265.2007.03079.x. [DOI] [PubMed] [Google Scholar]
- 12.Samollow PB, Perez G, Kammerer CM, Finegold D, Zwartjes PW, Havill LM, et al. Genetic and environmental influences on thyroid hormone variation in Mexican Americans. J Clin Endocrinol Metab. 2004;89:3276–3284. doi: 10.1210/jc.2003-031706. [DOI] [PubMed] [Google Scholar]
- 13.Park YJ, Ahn HY, Choi HS, Kim KW, Park DJ, Cho BY. The long-term outcomes of the second generation of familial nonmedullary thyroid carcinoma are more aggressive than sporadic cases. Thyroid. 2012;22:356–362. doi: 10.1089/thy.2011.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capezzone M, Marchisotta S, Cantara S, Busonero G, Brilli L, Pazaitou-Panayiotou K, et al. Familial non-medullary thyroid carcinoma displays the features of clinical anticipation suggestive of a distinct biological entity. Endocr Relat Cancer. 2008;15:1075–1081. doi: 10.1677/ERC-08-0080. [DOI] [PubMed] [Google Scholar]
- 15.Ito Y, Kakudo K, Hirokawa M, Fukushima M, Yabuta T, Tomoda C, et al. Biological behavior and prognosis of familial papillary thyroid carcinoma. Surgery. 2009;145:100–105. doi: 10.1016/j.surg.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Loh KC. Familial nonmedullary thyroid carcinoma: a meta-review of case series. Thyroid. 1997;7:107–113. doi: 10.1089/thy.1997.7.107. [DOI] [PubMed] [Google Scholar]
- 17.Uchino S, Noguchi S, Kawamoto H, Yamashita H, Watanabe S, Yamashita H, et al. Familial nonmedullary thyroid carcinoma characterized by multifocality and a high recurrence rate in a large study population. World J Surg. 2002;26:897–902. doi: 10.1007/s00268-002-6615-y. [DOI] [PubMed] [Google Scholar]
- 18.Peiling Yang S, Ngeow J. Familial non-medullary thyroid cancer: unraveling the genetic maze. Endocr Relat Cancer. 2016;23:R577–R595. doi: 10.1530/ERC-16-0067. [DOI] [PubMed] [Google Scholar]
- 19.Panicker V. Genetics of thyroid function and disease. Clin Biochem Rev. 2011;32:165–175. [PMC free article] [PubMed] [Google Scholar]
- 20.Wellcome Trust. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simmonds MJ, Howson JM, Heward JM, Cordell HJ, Foxall H, Carr-Smith J, et al. Regression mapping of association between the human leukocyte antigen region and Graves disease. Am J Hum Genet. 2005;76:157–163. doi: 10.1086/426947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simmonds MJ, Howson JM, Heward JM, Carr-Smith J, Franklyn JA, Todd JA, et al. A novel and major association of HLA-C in Graves' disease that eclipses the classical HLA-DRB1 effect. Hum Mol Genet. 2007;16:2149–2153. doi: 10.1093/hmg/ddm165. [DOI] [PubMed] [Google Scholar]
- 23.Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 24.Brand OJ, Barrett JC, Simmonds MJ, Newby PR, McCabe CJ, Bruce CK, et al. Association of the thyroid stimulating hormone receptor gene (TSHR) with Graves' disease. Hum Mol Genet. 2009;18:1704–1713. doi: 10.1093/hmg/ddp087. [DOI] [PubMed] [Google Scholar]
- 25.Velaga MR, Wilson V, Jennings CE, Owen CJ, Herington S, Donaldson PT, et al. The codon 620 tryptophan allele of the lymphoid tyrosine phosphatase (LYP) gene is a major determinant of Graves' disease. J Clin Endocrinol Metab. 2004;89:5862–5865. doi: 10.1210/jc.2004-1108. [DOI] [PubMed] [Google Scholar]
- 26.Wellcome Trust Case Control Consortium; Australo-Anglo-American Spondylitis Consortium (TASC) Burton PR, Clayton DG, Cardon LR, Craddock N, et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet. 2007;39:1329–1337. doi: 10.1038/ng.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu X, Pan CM, Zhao SX, Liang J, Gao GQ, Zhang XM, et al. A genome-wide association study identifies two new risk loci for Graves' disease. Nat Genet. 2011;43:897–901. doi: 10.1038/ng.898. [DOI] [PubMed] [Google Scholar]
- 28.Cooper JD, Simmonds MJ, Walker NM, Burren O, Brand OJ, Guo H, et al. Seven newly identified loci for autoimmune thyroid disease. Hum Mol Genet. 2012;21:5202–5208. doi: 10.1093/hmg/dds357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denny JC, Crawford DC, Ritchie MD, Bielinski SJ, Basford MA, Bradford Y, et al. Variants near FOXE1 are associated with hypothyroidism and other thyroid conditions: using electronic medical records for genome- and phenome-wide studies. Am J Hum Genet. 2011;89:529–542. doi: 10.1016/j.ajhg.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eriksson N, Tung JY, Kiefer AK, Hinds DA, Francke U, Mountain JL, et al. Novel associations for hypothyroidism include known autoimmune risk loci. PLoS One. 2012;7:e34442. doi: 10.1371/journal.pone.0034442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao SX, Xue LQ, Liu W, Gu ZH, Pan CM, Yang SY, et al. Robust evidence for five new Graves' disease risk loci from a staged genome-wide association analysis. Hum Mol Genet. 2013;22:3347–3362. doi: 10.1093/hmg/ddt183. [DOI] [PubMed] [Google Scholar]
- 32.Chu X, Shen M, Xie F, Miao XJ, Shou WH, Liu L, et al. An X chromosome-wide association analysis identifies variants in GPR174 as a risk factor for Graves' disease. J Med Genet. 2013;50:479–485. doi: 10.1136/jmedgenet-2013-101595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwak SH, Park YJ, Go MJ, Lee KE, Kim SJ, Choi HS, et al. A genome-wide association study on thyroid function and anti-thyroid peroxidase antibodies in Koreans. Hum Mol Genet. 2014;23:4433–4442. doi: 10.1093/hmg/ddu145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medici M, Porcu E, Pistis G, Teumer A, Brown SJ, Jensen RA, et al. Identification of novel genetic Loci associated with thyroid peroxidase antibodies and clinical thyroid disease. PLoS Genet. 2014;10:e1004123. doi: 10.1371/journal.pgen.1004123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matana A, Popovic M, Boutin T, Torlak V, Brdar D, Gunjaca I, et al. Genome-wide meta-analysis identifies novel gender specific loci associated with thyroid antibodies level in Croatians. Genomics. 2018 Apr 18; doi: 10.1016/j.ygeno.2018.04.012. [Epub] [DOI] [PubMed] [Google Scholar]
- 36.Oryoji D, Ueda S, Yamamoto K, Yoshimura Noh J, Okamura K, Noda M, et al. Identification of a Hashimoto thyroiditis susceptibility locus via a genome-wide comparison with Graves' disease. J Clin Endocrinol Metab. 2015;100:E319–E324. doi: 10.1210/jc.2014-3431. [DOI] [PubMed] [Google Scholar]
- 37.Simmonds MJ. GWAS in autoimmune thyroid disease: redefining our understanding of pathogenesis. Nat Rev Endocrinol. 2013;9:277–287. doi: 10.1038/nrendo.2013.56. [DOI] [PubMed] [Google Scholar]
- 38.Porcu E, Medici M, Pistis G, Volpato CB, Wilson SG, Cappola AR, et al. A meta-analysis of thyroid-related traits reveals novel loci and gender-specific differences in the regulation of thyroid function. PLoS Genet. 2013;9:e1003266. doi: 10.1371/journal.pgen.1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhan M, Chen G, Pan CM, Gu ZH, Zhao SX, Liu W, et al. Genome-wide association study identifies a novel susceptibility gene for serum TSH levels in Chinese populations. Hum Mol Genet. 2014;23:5505–5517. doi: 10.1093/hmg/ddu250. [DOI] [PubMed] [Google Scholar]
- 40.Taylor PN, Porcu E, Chew S, Campbell PJ, Traglia M, Brown SJ, et al. Whole-genome sequence-based analysis of thyroid function. Nat Commun. 2015;6:5681. doi: 10.1038/ncomms6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gudmundsson J, Sulem P, Gudbjartsson DF, Jonasson JG, Sigurdsson A, Bergthorsson JT, et al. Common variants on 9q22.33 and 14q13.3 predispose to thyroid cancer in European populations. Nat Genet. 2009;41:460–464. doi: 10.1038/ng.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gudmundsson J, Sulem P, Gudbjartsson DF, Jonasson JG, Masson G, He H, et al. Discovery of common variants associated with low TSH levels and thyroid cancer risk. Nat Genet. 2012;44:319–322. doi: 10.1038/ng.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohler A, Chen B, Gemignani F, Elisei R, Romei C, Figlioli G, et al. Genome-wide association study on differentiated thyroid cancer. J Clin Endocrinol Metab. 2013;98:E1674–E1681. doi: 10.1210/jc.2013-1941. [DOI] [PubMed] [Google Scholar]
- 44.Figlioli G, Kohler A, Chen B, Elisei R, Romei C, Cipollini M, et al. Novel genome-wide association study-based candidate loci for differentiated thyroid cancer risk. J Clin Endocrinol Metab. 2014;99:E2084–E2092. doi: 10.1210/jc.2014-1734. [DOI] [PubMed] [Google Scholar]
- 45.Figlioli G, Chen B, Elisei R, Romei C, Campo C, Cipollini M, et al. Novel genetic variants in differentiated thyroid cancer and assessment of the cumulative risk. Sci Rep. 2015;5:8922. doi: 10.1038/srep08922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mancikova V, Cruz R, Inglada-Perez L, Fernandez-Rozadilla C, Landa I, Cameselle-Teijeiro J, et al. Thyroid cancer GWAS identifies 10q26.12 and 6q14.1 as novel susceptibility loci and reveals genetic heterogeneity among populations. Int J Cancer. 2015;137:1870–1878. doi: 10.1002/ijc.29557. [DOI] [PubMed] [Google Scholar]
- 47.Gudmundsson J, Thorleifsson G, Sigurdsson JK, Stefansdottir L, Jonasson JG, Gudjonsson SA, et al. A genome-wide association study yields five novel thyroid cancer risk loci. Nat Commun. 2017;8:14517. doi: 10.1038/ncomms14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malinowski JR, Denny JC, Bielinski SJ, Basford MA, Bradford Y, Peissig PL, et al. Genetic variants associated with serum thyroid stimulating hormone (TSH) levels in European Americans and African Americans from the eMERGE Network. PLoS One. 2014;9:e111301. doi: 10.1371/journal.pone.0111301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rawal R, Teumer A, Volzke H, Wallaschofski H, Ittermann T, Asvold BO, et al. Meta-analysis of two genome-wide association studies identifies four genetic loci associated with thyroid function. Hum Mol Genet. 2012;21:3275–3282. doi: 10.1093/hmg/dds136. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi M, Saenko VA, Rogounovitch TI, Kawaguchi T, Drozd VM, Takigawa-Imamura H, et al. The FOXE1 locus is a major genetic determinant for radiation-related thyroid carcinoma in Chernobyl. Hum Mol Genet. 2010;19:2516–2523. doi: 10.1093/hmg/ddq123. [DOI] [PubMed] [Google Scholar]
- 51.Landa I, Ruiz-Llorente S, Montero-Conde C, Inglada-Perez L, Schiavi F, Leskela S, et al. The variant rs1867277 in FOXE1 gene confers thyroid cancer susceptibility through the recruitment of USF1/USF2 transcription factors. PLoS Genet. 2009;5:e1000637. doi: 10.1371/journal.pgen.1000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jendrzejewski J, Liyanarachchi S, Nagy R, Senter L, Wakely PE, Thomas A, et al. Papillary thyroid carcinoma: association between germline DNA variant markers and clinical parameters. Thyroid. 2016;26:1276–1284. doi: 10.1089/thy.2015.0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Penna-Martinez M, Epp F, Kahles H, Ramos-Lopez E, Hinsch N, Hansmann ML, et al. FOXE1 association with differentiated thyroid cancer and its progression. Thyroid. 2014;24:845–851. doi: 10.1089/thy.2013.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Son HY, Hwangbo Y, Yoo SK, Im SW, Yang SD, Kwak SJ, et al. Genome-wide association and expression quantitative trait loci studies identify multiple susceptibility loci for thyroid cancer. Nat Commun. 2017;8:15966. doi: 10.1038/ncomms15966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He H, Li W, Liyanarachchi S, Wang Y, Yu L, Genutis LK, et al. The role of NRG1 in the predisposition to papillary thyroid carcinoma. J Clin Endocrinol Metab. 2018;103:1369–1379. doi: 10.1210/jc.2017-01798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montero-Conde C, Ruiz-Llorente S, Dominguez JM, Knauf JA, Viale A, Sherman EJ, et al. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov. 2013;3:520–533. doi: 10.1158/2159-8290.CD-12-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fernandez-Cuesta L, Thomas RK. Molecular pathways: targeting NRG1 fusions in lung cancer. Clin Cancer Res. 2015;21:1989–1994. doi: 10.1158/1078-0432.CCR-14-0854. [DOI] [PubMed] [Google Scholar]
- 58.Zhu H, Xi Q, Liu L, Wang J, Gu M. Quantitative assessment of common genetic variants on FOXE1 and differentiated thyroid cancer risk. PLoS One. 2014;9:e87332. doi: 10.1371/journal.pone.0087332. [DOI] [PMC free article] [PubMed] [Google Scholar]