Abstract
Background
Most patients with differentiated thyroid cancer (DTC) have a favorable prognosis. However, patients with DTC and initial distant metastasis have not been commonly found, and their clinical characteristics have seldom been reported. In this study, we analyzed the clinical features and prognosis of patients with DTC and initial distant metastasis in Korea.
Methods
We retrospectively reviewed the clinical data of 242 patients with DTC and initial distant metastasis treated from 1994 to 2013, collected from five tertiary hospitals in Korea.
Results
The patients' median age was 51 years, and 65% were women. They were followed for a median of 7 years. Lung was the most common site of distant metastasis: only lung 149 patients (62%), only bone 49 (20%), other single site one (pleura), and combined sites 43 (40 were lung and bone, two were bone and other site, and one was lung and other site). At the time of diagnosis, 50 patients (21%) had non-radioactive iodine (RAI) avidity. Five-year disease-specific survival (DSS) was 85% and 10-year DSS was 68%, which were better than those in previous studies. After multivariate analysis, old age, male sex, metastatic site, and histologic type (follicular type) were significant factors for poor prognosis. However, negative RAI avidity status was not a significant prognostic factor after adjusting for other variables.
Conclusion
The prognosis of Korean patients with DTC and initial distant metastasis was better than in previous studies. Old age, male sex, metastasis site, and histologic type were significant prognostic factors.
Keywords: Differentiated thyroid cancer, Neoplasm metastasis, Radioactive iodine, Prognosis
INTRODUCTION
Papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC) are often referred to together as differentiated thyroid cancer (DTC). Recently, the incidence of DTC has been increasing worldwide [1,2,3]. Despite the increase in incidence, most patients with DTC have a favorable prognosis [4,5]. However, 1% to 9% of patients with DTC present with distant metastasis at the time of diagnosis, and the mortality rate is significantly higher when initial distant metastasis is present [6,7,8,9].
Previous studies of DTC with distant metastasis show inconsistent results. Long-term survival rates range from 13% to 100% [9,10,11,12]. This difference is thought to be due to heterogeneity among patients. Because of the rarity of the disease, most of the previous studies did not distinguish distant metastasis at initial diagnosis from distant metastasis found during follow-up. However, these two groups had different disease entities, disease characteristics, and prognoses [10]. Furthermore, primary tumor histology and site of distant metastasis are associated with different disease characteristics and prognosis [8,9,13,14]. Therefore, enrollment of a homogenous group is necessary to evaluate the disease characteristics more clearly.
The epidemiology of thyroid cancer has recently been changing [15,16]. Earlier diagnoses of thyroid cancer have increased since the introduction of high-resolution ultrasound sonography. New therapeutic modalities, such as tyrosine kinase inhibitors (TKIs), have been developed for the treatment of radioactive iodine (RAI)-refractory DTC [17,18]. Therefore, the current prognosis of DTC with distant metastasis might be different from the past. In this study, we analyzed the clinical characteristics and long-term prognoses of Korean patients with DTC and initial distant metastasis.
METHODS
We reviewed the clinical data of 242 patients with DTC and initial distant metastasis at the time of diagnosis that had been treated between 1994 and 2013 at Samsung Medical Center, Asan Medical Center, Chonnam National University Hwasun Hospital, Samsung Changwon Hospital, and Gyeongsang National University Hospital. Duplicated patients were identified by chart review and were treated as one person at the time of analysis. The Institutional Review Board at each participating hospital approved this study (IRB number: SMC 2017-02-058, IRB 2017-0601, CNUHH-2017-069, SCMC2017-11-001, 2017-10-009). Informed consent was waived due to retrospective design.
Initial distant metastasis was defined as distant metastasis detected before or within 6 months after initial treatment. Distant metastasis was found by pathological confirmation or imaging studies such as whole-body scan (WBS), computed tomography (CT), magnetic resonance imaging, bone scan, or positron emission tomography (PET) scans [19]. Distant metastasis was classified according to the metastatic site: (1) only lung, (2) only bone, (3) combined, and (4) other site metastasis. Lung metastasis included micronodular (smaller than 1 cm), macronodular (larger than 1 cm), and miliary lung metastasis. Bone metastasis included solitary or multiple bone metastases. The combined group included cases where two or more different metastatic sites were found. Iodine avidity was determined by visual uptake in the known site of metastatic disease by WBS after RAI therapy [8].
Various factors that influence distant metastasis were reviewed for analysis. Age at initial diagnosis, gender, histologic finding of thyroid cancer after surgery, data on additional therapy for distant metastasis, and RAI avidity of the metastatic site were included for analysis. Histologic findings of thyroid cancer included primary tumor histology (PTC, FTC, Hürthle cell carcinoma [HTC]), tumor size, lymph node metastasis, extrathyroidal extension, involvement of resection margin, and lymphovascular invasion. Additional therapy included RAI therapy, TKI, external radiotherapy, and systemic chemotherapy. Disease-specific death and survival were used for prognostic analysis.
Statistical analysis
Continuous data were expressed as mean±standard deviation. Data on categorical characteristics were expressed as percent values or absolute numbers. For comparisons of clinical and pathological characteristics between the initial distant metastasis groups, a chi-sqaure test was used for categorical data and t test was used for continuous data. A multivariate Cox proportional hazard model applying the backward elimination method was used to identify factors associated with disease-specific death. P<0.05 was considered significant. Statistical analysis was performed using SPSS software version 23 (IBM Co., Armonk, NY, USA).
RESULTS
Baseline characteristics
We studied 242 Korean patients with DTC and initial distant metastasis. The incidence of initial distant metastasis was 0.7% during the study period. The baseline characteristics of enrolled patients are shown in Table 1. Their median age was 51.0±17.1 years with a range from 13 to 79 years, and 65% were women. They were followed for a median of 7 years (interquartile range, 4.1 to 10.1). PTC was diagnosed in 175 patients (72%), FTC in 62 (26%), and HTC in five (2%). Among PTC subtypes, classic PTC was 147, follicular variant PTC was six, and other type of PTC was six. The median primary tumor size representing initial distant metastasis was 2.8 cm (interquartile range, 0.1 to 11.5). Gross extrathyroidal extension was found in 87 patients (36%), and positive resection margin was detected in 69 patients (29%). Nearly all patients had undergone total thyroidectomy (97%) and RAI therapy (99%), respectively.
Table 1. Baseline Characteristics of Differentiated Thyroid Cancer Patients with Initial Distant Metastasis.
| Characteristic | Total enrolled patients |
|---|---|
| Age, yr | 51.0±17.1 |
| Female sex | 157 (64.9) |
| Type of thyroid surgery | |
| Total thyroidectomy | 234 (96.7) |
| Lobectomy | 8 (3.3) |
| RAI treatment | |
| Yes | 240 (99.2) |
| No | 2 (0.8) |
| Initial neck dissection | |
| No | 37 (15.3) |
| Yes | 205 (84.7) |
| Tumor histology | |
| PTC | 175 (72.3) |
| FTC | 62 (25.6) |
| HTC | 5 (2.1) |
| Tumor size, cm | 2.8±2.0 |
| ETE | |
| No | 61 (25.1) |
| Microscopic ETE | 94 (38.9) |
| Gross ETE | 87 (36.0) |
| Resection margin | |
| Positive | 69 (28.5) |
| Negative | 173 (71.5) |
| No. of resected LN | 17.5±25.8 |
| No. of initial LN metastasis | 6.0±11.2 |
| Lymphatic invasion | |
| Yes | 70 (28.9) |
| No | 172 (71.1) |
| Vascular invasion | |
| Yes | 45 (18.6) |
| No | 197 (81.4) |
| T stage | |
| T1 | 64 (26.4) |
| T2 | 79 (32.6) |
| T3a | 50 (20.7) |
| T3b | 33 (13.6) |
| T4a | 11 (4.5) |
| T4b | 4 (1.7) |
| N stage | |
| N0 | 77 (31.8) |
| N1a | 38 (15.7) |
| N1b | 126 (52.1) |
Values are expressed as mean±SD or number (%).
RAI, radioactive iodine; PTC, papillary thyroid carcinoma; FTC, follicular thyroid carcinoma; HTC, Hürthle cell carcinoma; ETE, extrathyroidal extension; LN, lymph node.
Characteristics of initial distant metastasis
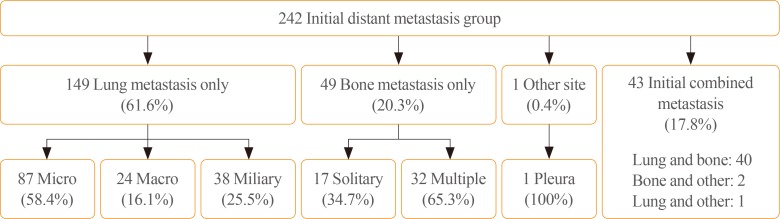
Of the 242 enrolled patients, 149 (62%) were classified as only lung metastasis, 49 patients (20%) were classified as only bone metastasis, and 43 patients (18%) were classified as combined metastasis. One patient presented with only pleural metastasis. Among other site metastasis except lung and bone, three patients showed pleural metastasis and one patient showed brain metastasis. Pleural metastasis was found in CT and brain metastasis was found in PET CT.
Among the only lung metastasis group, 87 patients (58%) were classified with micronodular lung metastasis, 24 (16%) with macronodular lung metastasis, and 38 (26%) with miliary metastasis. Among the only bone metastasis group, 17 patients (35%) were classified with single bone metastasis and 32 (65%) with multiple bone metastases (Fig. 1). The combined metastasis group included 40 patients with lung and bone, two with bone and other site, and one with lung and other site metastasis. Only lung metastasis was more frequent in PTC than in FTC (79% vs. 13%, P<0.001), and only bone metastasis was more frequent in FTC than in PTC (50% vs. 8%, P<0.001).
Fig. 1. Classification of initial distant metastasis in this study. A total of 242 patients were diagnosed with initial distant metastasis. Patients were classified according to site of distant metastasis. Diagnoses were as follows: 149 with lung only, 49 with bone only, and 43 with combined metastasis. One patient was diagnosed with other site only metastasis. Lung metastasis was subdivided into micronodular, macronodular, and miliary metastasis. Bone metastasis was subdivided into solitary and multiple metastases.
Among 242 patients, 50 (21%) had non-RAI avidity. According to the metastatic site, 25 of the patients (17%) with only lung metastasis, six of the patients (12%) with only bone metastasis, 18 of the patients (42%) with combined metastasis, and one of the patients with pleural metastasis had non-RAI avidity, respectively. The combined metastasis group (42%) had a higher rate of non-RAI avidity than the other lung and bone only groups.
Treatment response and survival characteristics
A total of 240 patients (99%) had undergone initial RAI therapy. Two patients were not treated with RAI for personal reasons. TKI was used in 20 patients (8%) and systemic chemotherapy in 10 patients (4%). External radiotherapy was performed on 54 patients (22%) with bone metastasis.
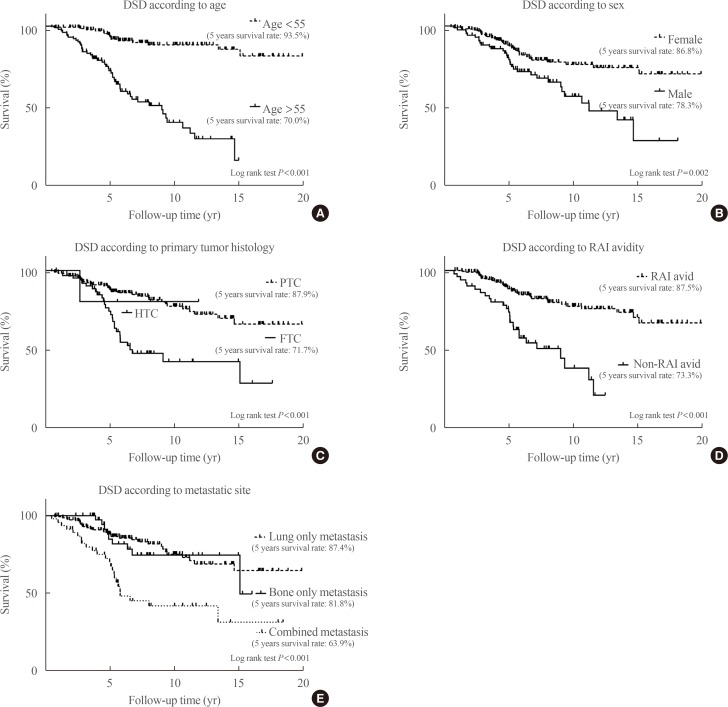
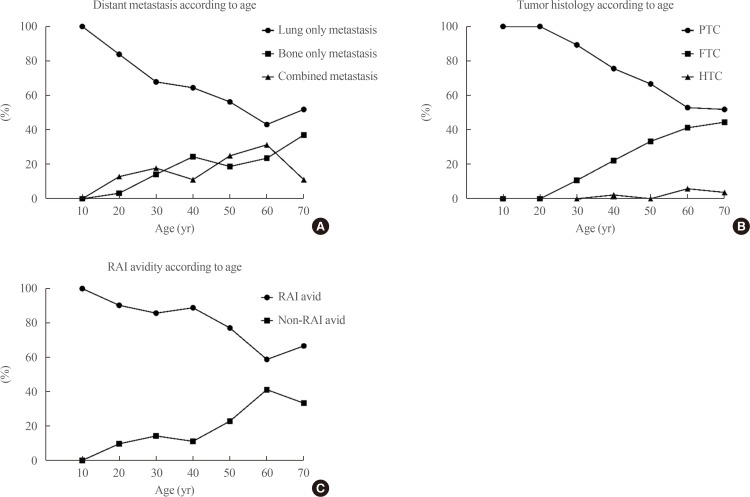
Sixty-three patients (26%) died of thyroid cancer after a median of 5 years (interquartile range, 0.5 to 15.1) after initial surgery. The 5-year survival rate was 84.8%, the 10-year survival rate was 68.1%, and the 15-year survival rate was 59.4%. Kaplan-Meier analysis and a log-rank test were used to evaluate the risk factors for disease-specific death. Age (>55 years), male sex, tumor histology (FTC), site of distant metastasis (combined metastasis), and non-RAI avidity were significant risk factors for disease-specific death (Fig. 2). After adjustment for all other variables using a Cox proportional hazard model, old age, male sex, tumor histology (FTC), and site of distant metastasis were independent risk factors for disease-specific death (Table 2). Combined metastasis had a worse prognosis than single-organ metastasis by univariate log-rank test, but there was no statistical significance according to the multivariate Cox proportional hazard model (hazard ratio, 1.63; 95% confidence interval, 0.78 to 3.41; P=0.195). The following prognostic factors were stratified according to age: site of metastasis, tumor histology, and RAI avidity. After this stratification, bone and combined metastasis, FTC, and non-RAI avidity were increased with age (Fig. 3).
Fig. 2. Survival analysis according to risk factors in patients with differentiated thyroid carcinoma and initial distant metastasis. A Kaplan-Meier analysis and log-rank test were used to evaluate the risk factors for disease-specific death (DSD). (A) Age (>55 years), (B) male sex, (C) tumor histology (follicular thyroid carcinoma [FTC] rather than papillary thyroid carcinoma [PTC]), (D) radioactive iodine (RAI) avidity (non-RAI avidity), and (E) metastatic site were significant risk factors for DSD. HTC, Hürthle cell carcinoma.
Table 2. Prognostic Factors for Disease Specific Death in Patients with Differentiated Thyroid Cancer and Initial Distant Metastasis.
| Characteristic | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age at diagnosis, yr | 1.08 (1.05–1.10) | <0.001 | 1.07 (1.05–1.10) | <0.001 |
| Male sex | 2.19 (1.33–3.61) | 0.002 | 2.60 (1.49–4.53) | 0.001 |
| LN dissection | ||||
| No | Reference | 0.126 | - | - |
| CND | 1.96 (1.03–3.73) | 0.042 | - | - |
| LND | 1.27 (0.72–2.23) | 0.408 | - | - |
| Distant metastasis | ||||
| Lung only | Reference | <0.001 | Reference | 0.003 |
| Bone only | 1.14 (0.56–2.34) | 0.852 | 0.43 (0.16–1.13) | 0.088 |
| Combined | 3.36 (1.95–5.81) | <0.001 | 1.63 (0.78–3.41) | 0.195 |
| Other site | 0.00 (0.00–0.00) | 0.974 | 0.00 (0.00–0.00) | 0.977 |
| Tumor histology | ||||
| PTC | Reference | <0.001 | Reference | 0.007 |
| FTC | 3.00 (1.81–4.97) | <0.001 | 3.25 (1.51–7.01) | 0.003 |
| HTC | 1.04 (0.14–7.63) | 0.968 | 0.95 (0.12–7.53) | 0.964 |
| Tumor size | 1.22 (1.11–1.35) | <0.001 | - | - |
| No. of initial LN metastases | 0.97 (0.95–1.00) | 0.047 | - | - |
| Positive lymphatic invasion | 2.36 (1.41–3.95) | 0.001 | - | - |
| Positive blood vessel invasion | 1.45 (0.80–2.64) | 0.221 | - | - |
| Positive resection margin | 0.97 (0.57–1.65) | 0.899 | - | - |
| ETE | ||||
| No | Reference | 0.363 | - | - |
| Microscopic ETE | 0.63 (0.33–1.19) | 0.156 | - | - |
| Gross ETE | 0.81 (0.44–1.48) | 0.490 | - | - |
| Non-RAI avidity | 3.69 (2.19–6.23) | <0.001 | - | - |
Cox proportional hazard regression model was performed.
OR, odds ratio; CI, confidence interval; LN, lymph node; CND, central neck dissection; LND, lateral neck dissection; PTC, papillary thyroid carcinoma; FTC, follicular thyroid carcinoma; HTC, Hürthle cell carcinoma; ETE, extrathyroidal extension; RAI, radioactive iodine.
Fig. 3. Prognostic factors according to change of age. When the prognostic factors site of distant metastasis, tumor histology, and radioactive iodine (RAI) avidity were stratified according to age, (A) the bone only/combined metastasis group, (B) follicular thyroid carcinoma (FTC), and (C) non-RAI avidity were increased with age. However, lung only group, papillary thyroid carcinoma (PTC), and RAI avidity decreased according to age. HTC, Hürthle cell carcinoma.
DISCUSSION
We analyzed 242 patients with DTC and initial distant metastasis. To the best of our knowledge, this study was the largest study investigating DTC and initial distant metastasis, specifically regarding long-term prognosis and recent treatment status. The 10-year survival rate associated with DTC and initial distant metastasis was 68.1%, which was higher than in previous reports. Age, gender, histology, and site of distant metastasis were revealed to be the prognostic factors.
Survival rates in this study were higher than in other studies. Ruegemer et al. [20] reported that the 5- and 10-year survival rates of DTC with distant metastasis were 35% and 25%, respectively. Recently, Nixon et al. [14] reported that the 5-year disease-specific survival rate was 68% in 52 patients with DTC and initial distant metastasis at the Memorial Sloan-Kettering Cancer Center. In our study, the 5- and 10-year disease-specific survival rates were 84.8% and 68.1%, respectively. These discrepancies could be explained by a few differences. First, the study population is different. In a study by Shaha et al. [21], 43% (19/44) of the patients had PTC, 41% (18/44) had FTC, and 16% (7/44) had HTC, respectively. However, in our study, patients with PTC were predominant (PTC 72% [175/242] vs. FTC/HTC 28% [67/242]). In another study in Korea, patients with PTC were also predominant (72%) [22]. The survival rate of patients with DTC with distant metastasis in our study should be better than that in the Western studies, because of good prognosis associated with PTC compared to FTC and HTC [8,9,13,14]. Second, some patients with early diagnosis of DTC, such as those with papillary thyroid microcarcinomas (PTMC) less than 1 cm in diameter, were enrolled in our study [15,16]. Patients with PTMC accounted for 11% (19/174) of total PTC patients. Therefore, early detection of metastatic cancer may affect better prognosis than previous studies. Third, new therapeutic modalities such as TKI were available in our study and they might have increased the survival rate, although a limited number of patients had undergone additional therapy (TKI 8%, chemotherapy 4%) [17,18].
In the previous study, the prognostic factors associated with initial distant metastasis were age, number of involved organs, RAI avidity, extent of metastasis, and symptoms [10,12,14,20,23]. In our study, older age, male sex, FTC, combined metastasis, and non-RAI avidity were associated with poor prognosis in a univariate log-rank test (Fig. 2). The non-RAI avidity and combined metastasis groups had poor prognosis (P<0.001). However, when the multivariate Cox proportional hazard model was used to adjust for the other variables, RAI avidity and combined metastasis were no longer significant (Table 2). As age increased, the ratio of FTC, combined metastasis, and non-RAI avidity were increased, which had a negative impact on prognosis (Fig. 3). This likely reflects that older patients tended to present with more aggressive clinical behaviors of metastatic DTC. When the patients were divided into PTC and FTC (including HTC) to analyze the factors affecting the prognosis, significant factors which affecting prognosis were different (Table 3). The factors affecting disease specific death of PTC were old age, site of metastasis (combined>lung metastasis), positive resection margin, and non-RAI avidity. However, only old age and site of metastasis (lung only>bone only) were significant prognostic factor for FTC. Because the numbers of FTC patients were relatively small, reassignment of a few cases might have changed the results.
Table 3. Prognostic Factors for Disease Specific Death in Patients with Differentiated Thyroid Cancer and Initial Distant Metastasis According to Pathologic Subtype.
| Characteristic | PTC | FTC (including HTC) | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age at diagnosis, yr | 1.09 (1.05–1.13) | <0.001 | 1.06 (1.01–1.11) | 0.025 |
| Male sex | 5.88 (2.10–16.44) | 0.001 | 0.93 (0.30–2.88) | 0.906 |
| LN dissection | ||||
| No | Reference | 0.048 | Reference | 0.552 |
| CND | 0.15 (0.03–0.87) | 0.035 | 1.71 (0.61–4.81) | 0.303 |
| LND | 0.11 (0.02–0.67) | 0.017 | 1.51 (0.36–6.43) | 0.568 |
| Distant metastasis | ||||
| Lung only | Reference | 0.114 | Reference | 0.004 |
| Bone only | 0.66 (0.07–5.80) | 0.704 | 0.18 (0.04–0.79) | 0.023 |
| Combined | 3.45 (1.24–9.57) | 0.017 | 1.30 (0.36–4.72) | 0.692 |
| Other site | 0.00 (0.00–0.00) | 0.978 | 0.00 (0.00–0.00) | - |
| Tumor size | 1.22 (1.11–1.35) | 0.103 | 1.23 (0.94–1.60) | 0.134 |
| No. of initial LN metastases | 0.97 (0.95–1.00) | 0.539 | 0.98 (0.88–1.10) | 0.765 |
| Positive lymphatic invasion | 2.36 (1.41–3.95) | 0.265 | 2.41 (0.76–7.64) | 0.135 |
| Positive blood vessel invasion | 1.45 (0.80–2.64) | 0.807 | 1.10 (0.37–3.25) | 0.866 |
| Positive resection margin | 3.73 (1.42–9.84) | 0.008 | 0.33 (0.07–1.53) | 0.158 |
| ETE | ||||
| No | Reference | 0.761 | Reference | 0.455 |
| Microscopic ETE | 1.22 (0.23–6.44) | 0.815 | 0.53 (0.15–1.80) | 0.303 |
| Gross ETE | 0.86 (0.20–3.62) | 0.837 | 0.41 (0.10–1.73) | 0.226 |
| Non-RAI avidity | 3.04 (1.24–7.35) | 0.014 | 1.12 (0.41–3.05) | 0.827 |
Multivariate Cox proportional hazard regression model was performed.
PTC, papillary thyroid carcinoma; FTC, follicular thyroid carcinoma; HTC, Hürthle cell carcinoma; OR, odds ratio; CI, confidence interval; LN, lymph node; CND, central neck dissection; LND, lateral neck dissection; ETE, extrathyroidal extension; RAI, radioactive iodine.
In our study, there were two noteworthy features. One was that the youngest patient diagnosed with initial distant metastasis was 13 years old. This finding indicates that distant metastasis can occur even in children. The other was that initial distant metastasis was found in a patient whose primary tumor size was 0.1 cm in diameter, although this was an extremely rare finding. Distant metastases have usually been observed in DTC patients with tumors ≥0.8 cm [24]. Therefore, careful evaluation should be necessary even in young patients or patients with very small tumors.
Our study has several limitations. First, it was a retrospective multicenter study, and therefore some data were missing during the long-term follow-up. Also, the centers did not use the same guidelines for evaluation and treatment. Second, the proportion of patients with initial distant metastasis might have been overestimated because patients were selected from the institutional databases of tertiary care centers. Third, molecular markers such as BRAFV600E or telomerase reverse transcriptase (TERT) promoter mutations could not be used for prognostic analysis. Fourth, despite the relatively large number of DTC patients with initial distant metastasis, the numbers in some subgroups were small. Additional nationwide studies will be needed for detailed subgroup analyses. Nevertheless, this study has strength as the first large cohort study in patients with DTC and initial distant metastasis in Korea. This study presented a prevalence of initial distant metastasis in DTC patients, and also showed the distribution of initial metastasis sites, rate of initial RAI refractoriness in initial distant metastasis patients. Also it showed recent trend in treatment for advanced metastatic DTC patients and mortality. These results may be helpful in further clinical research and patient management.
In conclusion, this study showed the clinical characteristics and long-term prognosis of Korean patients with DTC and initial distant metastasis. Age, gender, tumor histology, and metastatic site were the prognostic factors for patients with DTC and initial distant metastasis, and their survival rates were better compared to those in other reports.
ACKNOWLEDGMENTS
This work was supported by the Korean Endocrine Society of EnM Research Award 2017.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS: Conception or design: T.H.K., J.H.C. Acquisition, analysis, or interpretation of data: H.K., H.I.K., S.W.K., J.J., M.J.J., W.G.K., T.Y.K., H.K.K., H.C.K., J.M.H., Y.Y.C. Drafting the work or revising: H.K., T.H.K. Final approval of the manuscript: T.H.K., J.H.C.
References
- 1.Durante C, Montesano T, Torlontano M, Attard M, Monzani F, Tumino S, et al. Papillary thyroid cancer: time course of recurrences during postsurgery surveillance. J Clin Endocrinol Metab. 2013;98:636–642. doi: 10.1210/jc.2012-3401. [DOI] [PubMed] [Google Scholar]
- 2.Olaleye O, Ekrikpo U, Moorthy R, Lyne O, Wiseberg J, Black M, et al. Increasing incidence of differentiated thyroid cancer in South East England: 1987–2006. Eur Arch Otorhinolaryngol. 2011;268:899–906. doi: 10.1007/s00405-010-1416-7. [DOI] [PubMed] [Google Scholar]
- 3.Pires BP, Alves PA, Jr, Bordallo MA, Bulzico DA, Lopes FP, Farias T, et al. Prognostic factors for early and long-term remission in pediatric differentiated thyroid carcinoma: the role of sex, age, clinical presentation, and the newly proposed American Thyroid Association risk stratification system. Thyroid. 2016;26:1480–1487. doi: 10.1089/thy.2016.0302. [DOI] [PubMed] [Google Scholar]
- 4.Cho BY, Choi HS, Park YJ, Lim JA, Ahn HY, Lee EK, et al. Changes in the clinicopathological characteristics and outcomes of thyroid cancer in Korea over the past four decades. Thyroid. 2013;23:797–804. doi: 10.1089/thy.2012.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim H, Kim TH, Choe JH, Kim JH, Kim JS, Oh YL, et al. Patterns of initial recurrence in completely resected papillary thyroid carcinoma. Thyroid. 2017;27:908–914. doi: 10.1089/thy.2016.0648. [DOI] [PubMed] [Google Scholar]
- 6.Massin JP, Savoie JC, Garnier H, Guiraudon G, Leger FA, Bacourt F. Pulmonary metastases in differentiated thyroid carcinoma. Study of 58 cases with implications for the primary tumor treatment. Cancer. 1984;53:982–992. doi: 10.1002/1097-0142(19840215)53:4<982::aid-cncr2820530427>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 7.Pittas AG, Adler M, Fazzari M, Tickoo S, Rosai J, Larson SM, et al. Bone metastases from thyroid carcinoma: clinical characteristics and prognostic variables in one hundred forty-six patients. Thyroid. 2000;10:261–268. doi: 10.1089/thy.2000.10.261. [DOI] [PubMed] [Google Scholar]
- 8.Sampson E, Brierley JD, Le LW, Rotstein L, Tsang RW. Clinical management and outcome of papillary and follicular (differentiated) thyroid cancer presenting with distant metastasis at diagnosis. Cancer. 2007;110:1451–1456. doi: 10.1002/cncr.22956. [DOI] [PubMed] [Google Scholar]
- 9.Schlumberger M, Tubiana M, De Vathaire F, Hill C, Gardet P, Travagli JP, et al. Long-term results of treatment of 283 patients with lung and bone metastases from differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1986;63:960–967. doi: 10.1210/jcem-63-4-960. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Soh EY. Differentiated thyroid carcinoma presenting with distant metastasis at initial diagnosis clinical outcomes and prognostic factors. Ann Surg. 2010;251:114–119. doi: 10.1097/SLA.0b013e3181b7faf6. [DOI] [PubMed] [Google Scholar]
- 11.Shaha AR, Ferlito A, Rinaldo A. Distant metastases from thyroid and parathyroid cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:243–249. doi: 10.1159/000055749. [DOI] [PubMed] [Google Scholar]
- 12.Shoup M, Stojadinovic A, Nissan A, Ghossein RA, Freedman S, Brennan MF, et al. Prognostic indicators of outcomes in patients with distant metastases from differentiated thyroid carcinoma. J Am Coll Surg. 2003;197:191–197. doi: 10.1016/S1072-7515(03)00332-6. [DOI] [PubMed] [Google Scholar]
- 13.Lang BH, Wong KP, Cheung CY, Wan KY, Lo CY. Evaluating the prognostic factors associated with cancer-specific survival of differentiated thyroid carcinoma presenting with distant metastasis. Ann Surg Oncol. 2013;20:1329–1335. doi: 10.1245/s10434-012-2711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nixon IJ, Whitcher MM, Palmer FL, Tuttle RM, Shaha AR, Shah JP, et al. The impact of distant metastases at presentation on prognosis in patients with differentiated carcinoma of the thyroid gland. Thyroid. 2012;22:884–889. doi: 10.1089/thy.2011.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn HS, Kim HJ, Kim KH, Lee YS, Han SJ, Kim Y, et al. Thyroid cancer screening in South Korea increases detection of papillary cancers with no impact on other subtypes or thyroid cancer mortality. Thyroid. 2016;26:1535–1540. doi: 10.1089/thy.2016.0075. [DOI] [PubMed] [Google Scholar]
- 16.Lubitz CC, Sosa JA. The changing landscape of papillary thyroid cancer: epidemiology, management, and the implications for patients. Cancer. 2016;122:3754–3759. doi: 10.1002/cncr.30201. [DOI] [PubMed] [Google Scholar]
- 17.Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319–328. doi: 10.1016/S0140-6736(14)60421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372:621–630. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- 19.Haugen BR. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: what is new and what has changed? Cancer. 2017;123:372–381. doi: 10.1002/cncr.30360. [DOI] [PubMed] [Google Scholar]
- 20.Ruegemer JJ, Hay ID, Bergstralh EJ, Ryan JJ, Offord KP, Gorman CA. Distant metastases in differentiated thyroid carcinoma: a multivariate analysis of prognostic variables. J Clin Endocrinol Metab. 1988;67:501–508. doi: 10.1210/jcem-67-3-501. [DOI] [PubMed] [Google Scholar]
- 21.Shaha AR, Shah JP, Loree TR. Differentiated thyroid cancer presenting initially with distant metastasis. Am J Surg. 1997;174:474–476. doi: 10.1016/s0002-9610(97)00158-x. [DOI] [PubMed] [Google Scholar]
- 22.Jeon MJ, Kim WG, Kim TH, Kim HK, Kim BH, Yi HS, et al. Disease-specific mortality of differentiated thyroid cancer patients in Korea: a multicenter cohort study. Endocrinol Metab (Seoul) 2017;32:434–441. doi: 10.3803/EnM.2017.32.4.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–2899. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 24.Roti E, Rossi R, Trasforini G, Bertelli F, Ambrosio MR, Busutti L, et al. Clinical and histological characteristics of papillary thyroid microcarcinoma: results of a retrospective study in 243 patients. J Clin Endocrinol Metab. 2006;91:2171–2178. doi: 10.1210/jc.2005-2372. [DOI] [PubMed] [Google Scholar]