Abstract
Background
Ezetimibe-statin combination therapy has been found to reduce low density lipoprotein cholesterol levels and the risk of major adverse cardiovascular events (MACEs) in large trials. We sought to examine the differential effect of ezetimibe on MACEs when added to statins according to the presence of diabetes.
Methods
Randomized clinical trials with a sample size of at least 50 participants and at least 24 weeks of follow-up that compared ezetimibe-statin combination therapy with a statin- or placebo-controlled arm and reported at least one MACE, stratified by diabetes status, were included in the meta-analysis and meta-regression.
Results
A total of seven trials with 28,191 enrolled patients (mean age, 63.6 years; 75.1% men; 7,298 with diabetes [25.9%]; mean follow-up, 5 years) were analysed. MACEs stratified by diabetes were obtained from the published data (two trials) or through direct contact (five trials). No significant heterogeneity was observed among studies (I2=14.7%, P=0.293). Ezetimibe was associated with a greater reduction of MACE risk in subjects with diabetes than in those without diabetes (pooled relative risk, 0.84 vs. 0.93; Pheterogeneity=0.012). In the meta-regression analysis, the presence of diabetes was associated with a greater reduction of MACE risk when ezetimibe was added to statins (β=0.87, P=0.038).
Conclusion
Ezetimibe-statin combination therapy was associated with greater cardiovascular benefits in patients with diabetes than in those without diabetes. Our findings suggest that ezetimibe-statin combination therapy might be a useful strategy in patients with diabetes at a residual risk of MACEs.
Keywords: Ezetimibe, Myocardial infarction, Stroke, Hydroxymethylglutaryl-CoA reductase inhibitors, Diabetes mellitus
INTRODUCTION
Statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) have shown efficacy in lowering cholesterol levels and reducing the risk of cardiovascular events in the setting of primary and secondary prevention [1,2]. Given the major contribution of cardiovascular events to morbidity and mortality in patients with diabetes, high-intensity statins are recommended for patients with diabetes [3,4]. However, individuals with diabetes have substantial residual cardiovascular risk, even when receiving statin therapy, leading to an unmet need for additional lipid-modifying strategies [5].
Ezetimibe, a Niemann-Pick C1-like1 (NPC1L1) inhibitor, blocks intestinal cholesterol absorption, leading to the reduction of circulating cholesterol levels via a distinct mechanism from that of statins [6,7]. A large randomized controlled trial (RCT), the Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT), demonstrated the efficacy of ezetimibe-statin combination therapy on the reduction of cholesterol levels and major cardiovascular adverse events (MACEs) in patients who had recently experienced a myocardial infarction [8]. Notably, in a subgroup analysis, the beneficial effect of ezetimibe added to statins on MACEs was more prominent in patients with diabetes than in patients without diabetes [8]. Results from another large, placebo-controlled trial investigating the efficacy of ezetimibe-statin combination therapy in reducing cardiovascular events in chronic kidney disease patients also found a similar preferential effect of ezetimibe-statin combination therapy in patients with diabetes [9]. Given the high residual cardiovascular risk in patients with diabetes who are receiving treatment, these findings suggest that ezetimibe might provide additional benefits for preventing cardiovascular events, particularly in patients with diabetes. However, this potential differential effect of ezetimibe according to presence of diabetes has not been assessed as a primary outcome in pooled results from RCTs.
In this meta-analysis, we compared the effect of ezetimibe-statin combination therapy on MACEs to that of statins alone or placebo in patients with and without diabetes, based on the pooled results of RCTs.
METHODS
Data sources, search strategy, and selection criteria
We conducted a meta-analysis based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10]. Relevant studies were identified by searching the following data sources: MEDLINE via PubMed, Embase, and the Central Controlled Trials Register of the Cochrane Collaboration (from 1994 to December 2016). The following text words and medical subject headings were used without language restriction: “ezetimibe,” “ezetimibe-simvastatin drug combination,” “simvastatin,” “pravastatin,” “lovastatin,” “atorvastatin,” “rosuvastatin,” “fluvastatin,” “pitavastatin,” and “hydroxymethylglutaryl-CoA reductase inhibitors.” The reference lists of identified studies were also scanned to find potentially relevant studies. Two independent authors (Y.H.L and N.H.) performed the literature search, data extraction, and quality assessment with a standardized method, and a third reviewer (E.S.K.) adjudicated any discrepancies. Quality assessment was done using the Cochrane Collaboration's tool for assessing the risk of bias in randomized trials (Supplemental Fig. S1) [11]. This study was approved by Institutional Review Board of Severance Hospital, Yonsei University (no. 4-2015-0637).
Data extraction and quality assessment
Standard information was extracted from published reports and unpublished data obtained from investigators into a spreadsheet. We requested and received data using a formal question sheet for trials with unpublished information. We collected data on the number of randomized patients and the occurrence of MACEs in each ezetimibe and comparator group in the overall participants, as well as in subgroups divided by the presence of diabetes. Mean age, body mass index, follow-up duration, and the difference in the decrease of serum low density lipoprotein cholesterol (LDL-C) concentration between the ezetimibe and control groups during the study were tabulated for each study.
Statistical analysis
Relative risks (RRs) and 95% confidence intervals (CIs) were calculated from the event numbers, with the total number of patients as the denominator for individual studies. Heterogeneity across studies was estimated using the I2 statistic [12]. I2 values ranging from 0% to 40% were regarded as indicating no important heterogeneity; moderate, substantial, and considerable heterogeneity were defined as I2 values ranging from 30% to 60%, 50% to 90%, and 75% to 100%, respectively [13]. Weighted pooled treatment effects were obtained with a random-effects model to provide a more conservative assessment of the average effect size. The heterogeneity of the pooled effect between subgroups was calculated using the Cochran Q statistic, with the following formula: Q=Σ[(1/variance of individual study)×(effect of individual study−effect of pooled study)]2, where variance of individual study=[(upper limit−lower limit)/(2×z)]2 [14]. A funnel plot with symmetry testing by the Egger linear regression method was used to test for potential publication bias [15]. Sensitivity analyses were performed by repeating analyses while removing one study at a time using the ‘metaninf’ command (STATA). Analyses confined to statin-controlled trials were also performed, with the exclusion of placebo-controlled trials. Random-effects meta-regression models with inverse variance weighting were built to assess whether the presence of diabetes explained the variance in the estimated RR for MACEs observed between trials. Two-sided P values <0.05 were considered to indicate statistical significance. All statistical analyses were performed with STATA version 14.0 (Stata Corp., College Station, TX, USA).
RESULTS
Characteristics of trials
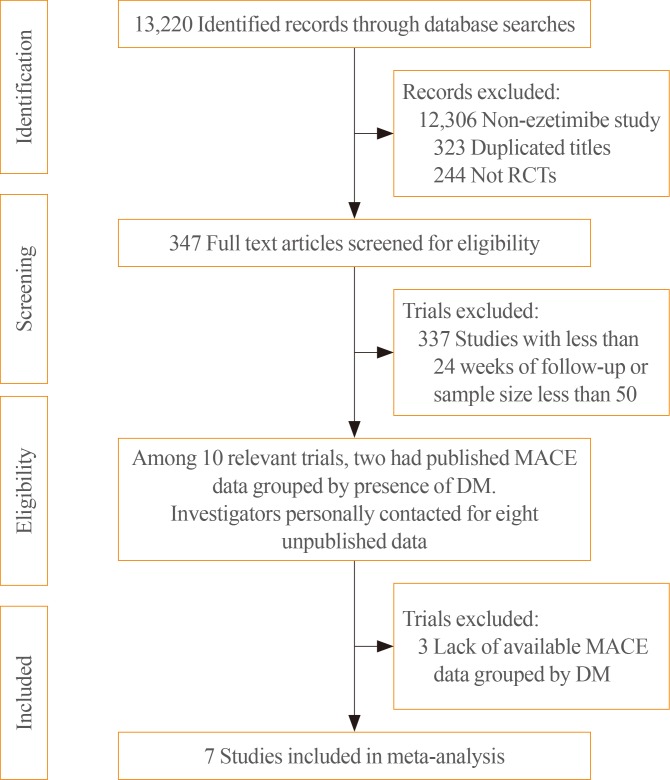
Among the 13,220 identified records, 347 randomized, placebo or statin-controlled endpoint trials of ezetimibe were screened (Fig. 1). Studies were included if they were completed RCTs comparing the effects of adding ezetimibe to any statin or placebo on the incidence of MACEs and if they reported the clinical outcomes in participants stratified by the presence of diabetes. We also contacted investigators from eight potentially relevant trials about unpublished data for incident MACEs in participants stratified by diabetes, and received and included data from five of those trials. Finally, a total of seven studies, two with published data [8,9] and five with previously unpublished data that had not been analysed until our request [16,17,18,19,20], were included in the meta-analysis. The included studies enrolled 28,191 patients (7,298 with diabetes [25.9%]) with stable angina, recent acute coronary syndrome, chronic kidney disease, peripheral arterial occlusive disease, or hypercholesterolemia (Table 1). The mean age of study subjects was 63.6 years and 75.1% were men. The mean follow-up duration of the studies was approximately 5 years, according to the weighted average. The prevalence of diabetes varied from 22.6% to 49.7%. Only one study, the Study of Heart and Renal Protection (SHARP) trial, was a placebo-controlled study (vs. an ezetimibe-simvastatin combination), whereas other trials included statin users as the control group. A greater LDL-C reduction (%) was shown in the ezetimibe and statin combination group than in the control group (statins or placebo), regardless of differences in the intensity and doses in the statin-controlled trials.
Fig. 1. Flow diagram of the literature search to identify randomized controlled trials (RCTs) comparing the differential effect of ezetimibe combination therapy on the reduction of major adverse cardiovascular events (MACEs) according to the presence of diabetes. DM, diabetes mellitus.
Table 1. Data of Subjects in Seven Randomized Controlled Trials of Ezetimibe Combination Therapy with Statin or Placebo Comparator Arms that Reported Incident MACEs Grouped by the Presence of Diabetes.
| Study | DM/Total, no. (%) | Target population | Mean age, yr | Men, % | Mean BMI, kg/m2 | LDL-C reduction in treatment group, % | LDL-C reduction in control group, % | Treatmenta | Control | Median follow-up, wk | MACE definition | MACEs in treatment group, no. (%) | MACEs in control group, no. (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| West | 29/67 (43.2) | PAOD | 63.5 | 55.9 | 29.0 | −42.8 | −26.3 | S40+E10 | S40 or previous statin | 96 | CV death, non-fatal MI, ischemic stroke, and TIA | 16/51 (31) | 6/16 (38) |
| SHARP | 2,094/9,270 (22.6) | CKD | 62.0 | 62.6 | 27.1 | −35.6 | −2.4 | S20+E10 | Placebo | 240 | CV death, non-fatal MI, ischemic stroke, coronary revascularization | 526/4,650 (11) | 619/4,620 (13) |
| Kouvelos | 79/262 (30.2) | Elective vascular surgery | 71.0 | 89.7 | NA | −48.8 | −39.0 | R10+E10 | R10 | 48 | CV death, non-fatal MI, ischemic stroke, hospitalization for USA | 9/126 (7) | 17/136 (13) |
| IMPROVE-IT | 4,933/18,144 (27.2) | ACS | 63.6 | 75.7 | 28.3 | −42.9 | −26.1 | S40+E10 | S40 | 288 | CV death, non-fatal MI, ischemic stroke, hospitalization for USA, coronary revascularization | 2,572/9,067 (28) | 2,742/9,077 (30) |
| Suzuki | 78/157 (49.7) | Hypercholesterolemia | 64.0 | 64.0 | 25.5 | −15.0 | −14.3 | Any statin+E10 | Any statin | 144 | CV death, non-fatal MI, ischemic stroke, hospitalization for USA, coronary revascularization | 1/86 (1) | 4/71 (6) |
| PRECISE-IVUS | 60/202 (29.7) | ACS, stable angina | 66.5 | 79.0 | 25.5 | −19.3 | −4.3 | A10+E10 | A10 | 48 | CV death, non-fatal MI, ischemic stroke, hospitalization for USA, coronary revascularization | 18/100 (18) | 25/102 (25) |
| HEAVEN | 25/89 (25.0) | Stable angina | 64.3 | 71.9 | NA | −35.5 | −3.7 | A80+E10 | Any statin | 48 | CV death, non-fatal MI, ischemic stroke, hospitalization for USA, coronary revascularization | 13/42 (31) | 13/47 (28) |
MACE, major adverse cardiovascular event; DM, diabetes mellitus; BMI, body mass index; LDL-C, low density lipoprotein cholesterol; PAOD, peripheral artery occlusive disease; CV, cardiovascular; MI, myocardial infarction; TIA, transient ischemic attack; SHARP, the Study of Heart and Renal Protection; CKD, chronic kidney disease; NA, not available; USA, unstable angina; IMPROVE-IT, the Improved Reduction of Outcomes: Vytorin Efficacy International Trial; ACS, acute coronary syndrome; PRECISE-IVUS, Plaque Regression With Cholesterol Absorption Inhibitor or Synthesis Inhibitor Evaluated by Intravascular Ultrasound Study; HEAVEN, Virtual histology evaluation of atherosclerosis regression during atorvastatin and ezetimibe administration study.
aA10 atorvastatin (10 mg), S20 simvastatin (20 mg), S40 simvastatin (40 mg), R10 rosuvastatin (10 mg), E10 ezetimibe (10 mg).
Outcome analysis
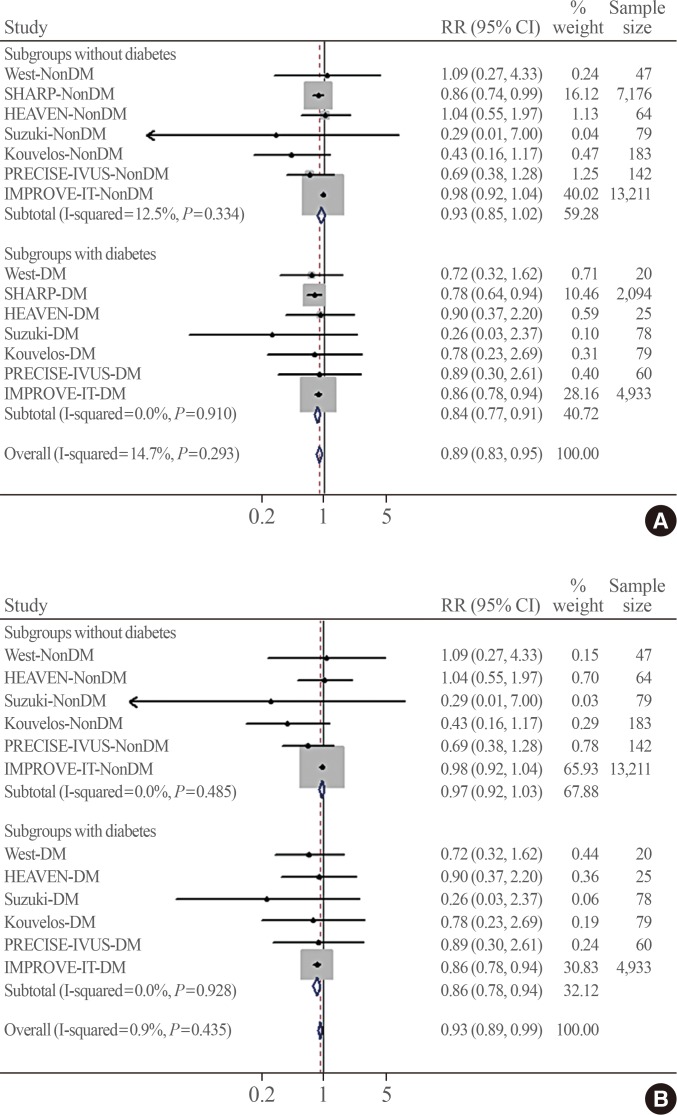
Fig. 2 shows the pooled association of ezetimibe combination therapy with MACE risk according to the presence of diabetes. No significant heterogeneity was observed across the trials (I2=14.7%, P=0.293). In the included patients, a total of 6,581 MACEs occurred during follow-up. The definitions of MACEs were generally consistent among studies. Fig. 2A shows that the association of ezetimibe combination therapy with a lower MACE risk was greater in the pooled RR from subgroups with diabetes (RR, 0.84; 95% CI, 0.77 to 0.91) than in the pooled RR from subgroups without diabetes (RR, 0.93; 95% CI, 0.85 to 1.02; Pheterogeneity=0.012). A similar result was observed when the placebo-controlled study (SHARP) was excluded from the pooled analysis (RR, 0.86; 95% CI, 0.78 to 0.94 vs. RR, 0.97; 95% CI, 0.92 to 1.03; Pheterogeneity=0.022) (Fig. 2B), indicating a statistically significant difference between the two pooled RRs (in the diabetes and no diabetes groups).
Fig. 2. Pooled effects of ezetimibe-statin combination therapy on major adverse cardiovascular events grouped by the presence of diabetes within studies. The test for heterogeneity between subgroups was significant (A) in all studies (P=0.012) and (B) after excluding the placebo-controlled trial (SHARP) (P=0.022). RR, risk ratio; CI, confidence interval; DM, diabetes mellitus; SHARP, the Study of Heart and Renal Protection; HEAVEN, virtual histology evaluation of atherosclerosis regression during atorvastatin and ezetimibe administration study; PRECISE-IVUS, Plaque Regression With Cholesterol Absorption Inhibitor or Synthesis Inhibitor Evaluated by Intravascular Ultrasound Study; IMPROVE-IT, the Improved Reduction of Outcomes: Vytorin Efficacy International Trial.
When all included trials were analysed by meta-regression, there was a trend toward a greater MACE risk reduction by ezetimibe combination therapy when added to statins in patients with diabetes compared to those without diabetes (β=0.89; 95% CI, 0.75 to 1.06; P=0.203). When the placebo-controlled study was excluded from the analysis, ezetimibe-statin combination therapy was associated with a greater reduction of MACE risk in subjects with diabetes than in those without diabetes compared with statin monotherapy (β=0.87; 95% CI, 0.78 to 0.99; P=0.038).
Data on cancer incidence were available in three studies (Kouvelos, SHARP, and IMPROVE-IT) (Supplemental Fig. S2). The pooled RR for cancer incidence was 1.01 (95% CI, 0.94 to 1.09; P=0.794), indicating no difference between the ezetimibe and control groups.
Sensitivity analysis
In the sensitivity analysis (Table 2), the meta-analyses were repeated after removing one study at a time. Omitting individual trials did not significantly affect the pooled risk, and the risk reduction by ezetimibe combination therapy in the diabetes group remained robust even after removal of the largest trial (subanalysis excluding the IMPROVE-IT trial: [RR, 0.77; 95% CI, 0.65 to 0.92 vs. RR, 0.84; 95% CI, 0.77 to 0.91; P=0.372 in the diabetes subgroup]; [RR, 0.85; 95% CI, 0.74 to 0.97 vs. RR, 0.93; 95% CI, 0.85 to 1.02; P=0.278 in the non-diabetes subgroups]). However, the difference between the pooled RRs in the diabetes and non-diabetes groups did not reach statistical significance when the IMPROVE-IT trial was excluded (RR, 0.78; 95% CI, 0.65 to 0.93 in the diabetes subgroup vs. RR, 0.85; 95% CI, 0.74 to 0.97 in the non-diabetes group; Pheterogeneity=0.460), although a nominally consistent pattern was observed with the pooled results of the studies overall.
Table 2. Sensitivity Analyses for Assessing the Effects of Individual Studies on the Pooled Risk Ratio for Major Adverse Cardiovascular Events.
| Pooled RR | 95% CI | |
|---|---|---|
| Omitted study (DM)a | ||
| West | 0.84 | 0.77–0.91 |
| SHARP | 0.86 | 0.78–0.94 |
| HEAVEN | 0.84 | 0.77–0.91 |
| Suzuki | 0.84 | 0.77–0.91 |
| Kouvelos | 0.85 | 0.78–0.93 |
| PRECISE-IVUS | 0.84 | 0.77–0.91 |
| IMPROVE-IT | 0.77 | 0.65–0.92 |
| Combined | 0.84 | 0.77–0.91 |
| Omitted studies (non-DM)b | ||
| West | 0.91 | 0.80–1.02 |
| SHARP | 0.97 | 0.91–1.03 |
| HEAVEN | 0.91 | 0.81–1.02 |
| Suzuki | 0.92 | 0.83–1.02 |
| Kouvelos | 0.96 | 0.91–1.01 |
| PRECISE-IVUS | 0.94 | 0.86–1.02 |
| IMPROVE-IT | 0.85 | 0.74–0.97 |
| Combined | 0.93 | 0.85–1.02 |
RR, risk ratio; CI, confidence interval; DM, diabetes mellitus; SHARP, the Study of Heart and Renal Protection; HEAVEN, Virtual histology evaluation of atherosclerosis regression during atorvastatin and ezetimibe administration study; PRECISE-IVUS, Plaque Regression With Cholesterol Absorption Inhibitor or Synthesis Inhibitor Evaluated by Intravascular Ultrasound Study; IMPROVE-IT, The Improved Reduction of Outcomes: Vytorin Efficacy International Trial.
aDM, subgroup with diabetes in each study; bNon-DM, subgroup without diabetes in each study.
Publication bias
A funnel plot and Egger test of the studies did not reveal any evidence of underlying publication bias for reporting MACEs (Supplemental Fig. S3).
DISCUSSION
In this meta-analysis of seven RCTs, we found a differential association of ezetimibe-statin combination therapy on MACE risk according to the presence of diabetes. Compared with statins alone, ezetimibe combination therapy reduced the risk of MACEs. The benefit of ezetimibe combination therapy was more prominent in patients with diabetes than in patients without diabetes.
Recent reviews and meta-analyses of RCTs, including the IMPROVE-IT study, showed that ezetimibe was likely associated with a reduction of the risk of myocardial infarction and stroke, without affecting the risk of overall or cardiovascular mortality or newly-developed cancer [21,22]. However, published reviews reported marginal cardiovascular benefits of ezetimibe when ezetimibe was added to statins for reducing nonfatal myocardial infarction and stroke (17 fewer myocardial infarctions and six fewer strokes per 1,000 persons treated over 6 years) [21,22,23,24]. This uncertainty is reflected in the absence of a consensus regarding ezetimibe in international guidelines. The 2013 treatment guidelines of the American Heart Association/American College of Cardiology (AHA/ACC) focused on statin monotherapy and did not suggest considering second-line drugs, including ezetimibe, as a treatment option based on a lack of strong evidence [4]. However, European and Korean guidelines for the management of dyslipidaemia permit the use of ezetimibe as a second-line therapy in association with statins when the therapeutic goal is not met despite maximal tolerated statin doses or in subjects intolerant to statins [25,26]. In line with newer evidence, the 2016 AHA/ACC updates on cholesterol treatment commented that second-line cholesterol-lowering drugs can be used to meet LDL-C treatment targets, at least in limited circumstances [27]. Given the current evidence of the ability of ezetimibe to prevent cardiovascular events, it is important to identify the specific populations that might benefit the most from ezetimibe. However, no reviews or meta-analyses have primarily focused on the differential effect of ezetimibe according to the presence of diabetes. In this study, the pooled results of RCTs with a statin control arm showed that patients with diabetes experienced a greater benefit from ezetimibe-statin combination therapy than patients without diabetes, indicating that the presence of diabetes might be a potential indication for adding ezetimibe to the therapeutic regimen of patients with high residual risk.
Several biological and clinical findings support the beneficial effect of ezetimibe in diabetes. Patients with a higher risk of cardiovascular events experience greater benefits when ezetimibe is added to statins, as shown in a previous review [22]. Patients with diabetes are more likely to have a higher cardiovascular risk at baseline than patients without diabetes, which might lead to ezetimibe exerting a positive effect in patients with diabetes [8]. Furthermore, pathologic enhancement of NPC1L1 expression, a direct target of ezetimibe, has been reported in patients with diabetes [28,29]. Indeed, ezetimibe was associated with greater decreases in LDL-C and non-high density cholesterol levels in patients with diabetes than in those without diabetes [30,31]. In addition to its favourable effects on the lipid profile of individuals with diabetes, ezetimibe combination therapy was associated with improvements in insulin sensitivity and plasma adiponectin levels compared with statin monotherapy in patients with diabetes [32]. In the Plaque Regression With Cholesterol Absorption Inhibitor or Synthesis Inhibitor Evaluated by Intravascular Ultrasound Study (PRECISE-IVUS) trial, which was included in this analysis, the greater reduction of atherosclerotic plaque progression by ezetimibe could not be entirely explained by its cholesterol-lowering effects [20]. Anti-inflammatory effects, reduction of the plant sterol ratio, inhibition of smooth muscle cell proliferation, and antiplatelet effects have been proposed as potential mechanisms underlying the cardiovascular benefit of ezetimibe [33,34,35,36]. Taken together, these pieces of evidence suggest that ezetimibe combination therapy might have a protective effect on cardiovascular outcomes in patients with diabetes, possibly through its lipid-lowering effects or through a pleiotropic effect; however, the mechanisms of the effects of ezetimibe must be confirmed in further studies.
Concerns regarding increased cancer-related mortality associated with ezetimibe use have been raised. According to a pooled analysis of three large trials (Simvastatin in Aortic Stenosis [SEAS], SHARP, and IMPROVE-IT), ezetimibe use was associated with a nominally increased risk of cancer-related mortality (risk ratio, 1.45; 99% CI, 1.02 to 2.05; uncorrected P=0.007) [37,38]. However, the authors argued that this result might have been due to chance, rather than being a true finding, because a parallel increase in the cancer incidence was not found in the combined analysis. In our study, we also observed no association of ezetimibe use with cancer incidence when SHARP, IMPROVE-IT, and the study by Kouvelos et al. [18] were pooled together, similarly to the meta-analysis performed by Savarese et al. [21]. Although monitoring for mortality due to cancer should be continued in large prospective trials, our findings support the current consensus that ezetimibe is most likely not associated with an increased risk of cancer incidence.
Our study is limited by the small number of eligible RCTs, with a single study representing the majority of enrolled patients. Although we intentionally only analysed RCTs to minimize heterogeneity, it is possible that excluding observational studies with large numbers of subjects and longer follow-up durations might have led to an underestimation of the effect size of ezetimibe. Surrogate outcomes were not analysed in this study. Although we analysed LDL-C levels according to the treatment groups, we could not obtain changes in the LDL-C level for each study stratified by diabetes. A composite endpoint, MACEs, was analysed instead of individual outcomes due to the lack of data stratified by the presence of diabetes, although the scope of this study was to evaluate the heterogeneity of the effects of ezetimibe on cardiovascular events between individuals with diabetes and those without diabetes. The studies that remained after excluding the SHARP and the IMPROVE-IT trials in our study might have been underpowered for detecting a significant additive cardioprotective effect of ezetimibe combination therapy between the diabetes and non-diabetes groups. Meanwhile, a previous meta-analysis of the effects of ezetimibe emphasized that including large studies such as SHARP or IMPROVE-IT in the pooled outcome analyses led to a significantly larger sample of patients, with greater representativeness of real-world patients, when compared to a meta-analysis performed without the results from large trials [21,23]. Furthermore, our study provided a comparison of the pooled risk of cardiovascular outcomes between diabetes and no diabetes groups, based on data that were collected by direct contact. Therefore, we believe that this analysis contributes some novel information on the interaction of the effects of ezetimibe with the presence of diabetes, although further prospective trials are needed to validate this possibility.
In conclusion, the pooled results of RCTs showed that ezetimibe was associated with a greater reduction of MACEs in patients with diabetes than in those without diabetes. This differential effect of ezetimibe was robust across the trials. Given the current evidence regarding ezetimibe as a second-line lipid-lowering agent, ezetimibe-statin combination therapy might provide a feasible treatment option to combat residual cardiovascular risk in patients with diabetes who are intolerant or refractory to statin therapy.
ACKNOWLEDGMENTS
This work was financially supported by a National Research Foundation of Korea grant funded by the Korean government (MEST, Basic Research Promotion Fund; NRF-2010-013-E0008 and NRF-2012000891 to Eun Seok Kang), by the Bio & Medical Technology Development Program of the NRF, Korea, MSIP (2016R1A2B4013029), the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (No. HI14C2476) and the Internal Grant Agency of the Ministry of Health, Czech Republic (IGA NT13224-4/2012). This study was supported by the Korean Endocrine Society of KES Research Award 2017.
The authors are grateful to Hye Sun Lee, PhD and Ji Eun Moon, PhD (Biostatistics Collaboration Unit, Yonsei University College of Medicine, Seoul, Korea) for statistical consultation and to Dong-Su Jang (Medical Illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, Korea) for his help with the figures.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS: All authors had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. Designed the study and wrote the protocol: N.H., Y.H.L., E.S.K. Supervised data collection and synthesis: E.S.K. Wrote the report and final draft of the manuscript: Y.H.L., N.H. Wrote the search strategy and undertook the literature search: K.H. Undertook all data analysis: Y.H.L., N.H. Undertook title screening, data gathering, cleaning and advised on methods, statistical analyses, and interpretation of the findings: K.T., J.A.G., C.M.K., T.K., G.N.K., H.S., C.J.L., S.H.P., B. W.L., B.S.C. Contributed equally to this work: N.H., Y.H.L. Guarantor: E.S.K. All authors contributed to the final manuscript.
SUPPLEMENTARY MATERIALS
Quality assessment of randomized controlled trials included in the meta-analysis by the Cochrane assessment of risk of bias.
Risk ratios (RRs) for newly-developed cancer. The pooled RR is plotted using the empty diamond. The box size is proportional to the weight of each study. CI, confidence interval; SHARP, the Study of Heart and Renal Protection; IMPROVE-IT, the Improved Reduction of Outcomes: Vytorin Efficacy International Trial.
Funnel plot of enrolled studies stratified by the presence of diabetes. MACE, major adverse cardiovascular event; CI, confidence interval.
References
- 1.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 2.Cholesterol Treatment Trialists' (CTT) Collaboration. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerstein HC, Yusuf S. Dysglycaemia and risk of cardiovascular disease. Lancet. 1996;347:949–950. doi: 10.1016/s0140-6736(96)91420-8. [DOI] [PubMed] [Google Scholar]
- 4.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 5.ACCORD Study Group. Ginsberg HN, Elam MB, Lovato LC, Crouse JR, 3rd, Leiter LA, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park SW. Intestinal and hepatic niemann-pick c1-like 1. Diabetes Metab J. 2013;37:240–248. doi: 10.4093/dmj.2013.37.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sudhop T, Lutjohann D, Kodal A, Igel M, Tribble DL, Shah S, et al. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106:1943–1948. doi: 10.1161/01.cir.0000034044.95911.dc. [DOI] [PubMed] [Google Scholar]
- 8.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 9.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sedgwick P. Meta-analyses: heterogeneity and subgroup analysis. BMJ. 2013;346:f4040. doi: 10.1136/bmj.h1435. [DOI] [PubMed] [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.West AM, Anderson JD, Meyer CH, Epstein FH, Wang H, Hagspiel KD, et al. The effect of ezetimibe on peripheral arterial atherosclerosis depends upon statin use at baseline. Atherosclerosis. 2011;218:156–162. doi: 10.1016/j.atherosclerosis.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovarnik T, Mintz GS, Skalicka H, Kral A, Horak J, Skulec R, et al. Virtual histology evaluation of atherosclerosis regression during atorvastatin and ezetimibe administration: HEAVEN study. Circ J. 2012;76:176–183. doi: 10.1253/circj.cj-11-0730. [DOI] [PubMed] [Google Scholar]
- 18.Kouvelos GN, Arnaoutoglou EM, Matsagkas MI, Kostara C, Gartzonika C, Bairaktari ET, et al. Effects of rosuvastatin with or without ezetimibe on clinical outcomes in patients undergoing elective vascular surgery: results of a pilot study. J Cardiovasc Pharmacol Ther. 2013;18:5–12. doi: 10.1177/1074248412445506. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki H, Watanabe Y, Kumagai H, Shuto H. Comparative efficacy and adverse effects of the addition of ezetimibe to statin versus statin titration in chronic kidney disease patients. Ther Adv Cardiovasc Dis. 2013;7:306–315. doi: 10.1177/1753944713513222. [DOI] [PubMed] [Google Scholar]
- 20.Tsujita K, Sugiyama S, Sumida H, Shimomura H, Yamashita T, Yamanaga K, et al. Impact of dual lipid-lowering strategy with ezetimibe and atorvastatin on coronary plaque regression in patients with percutaneous coronary intervention: the multicenter randomized controlled PRECISE-IVUS trial. J Am Coll Cardiol. 2015;66:495–507. doi: 10.1016/j.jacc.2015.05.065. [DOI] [PubMed] [Google Scholar]
- 21.Savarese G, De Ferrari GM, Rosano GM, Perrone-Filardi P. Safety and efficacy of ezetimibe: a meta-analysis. Int J Cardiol. 2015;201:247–252. doi: 10.1016/j.ijcard.2015.08.103. [DOI] [PubMed] [Google Scholar]
- 22.Fei Y, Guyatt GH, Alexander PE, El Dib R, Siemieniuk RA, Vandvik PO, et al. Addition of Ezetimibe to statins for patients at high cardiovascular risk: systematic review of patient-important outcomes. J Eval Clin Pract. 2018;24:222–231. doi: 10.1111/jep.12663. [DOI] [PubMed] [Google Scholar]
- 23.Battaggia A, Donzelli A, Font M, Molteni D, Galvano A. Clinical efficacy and safety of Ezetimibe on major cardiovascular endpoints: systematic review and meta-analysis of randomized controlled trials. PLoS One. 2015;10:e0124587. doi: 10.1371/journal.pone.0124587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomopoulos C, Skalis G, Michalopoulou H, Tsioufis C, Makris T. Effect of low-density lipoprotein cholesterol lowering by ezetimibe/simvastatin on outcome incidence: overview, meta-analyses, and meta-regression analyses of randomized trials. Clin Cardiol. 2015;38:763–769. doi: 10.1002/clc.22441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 26.Committee for the Korean Guidelines for the Management of Dyslipidemia. 2015 Korean guidelines for the management of dyslipidemia: executive summary (English translation) Korean Circ J. 2016;46:275–306. doi: 10.4070/kcj.2016.46.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nayor M, Vasan RS. Recent update to the US cholesterol treatment guidelines: a comparison with international guidelines. Circulation. 2016;133:1795–1806. doi: 10.1161/CIRCULATIONAHA.116.021407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravid Z, Bendayan M, Delvin E, Sane AT, Elchebly M, Lafond J, et al. Modulation of intestinal cholesterol absorption by high glucose levels: impact on cholesterol transporters, regulatory enzymes, and transcription factors. Am J Physiol Gastrointest Liver Physiol. 2008;295:G873–G885. doi: 10.1152/ajpgi.90376.2008. [DOI] [PubMed] [Google Scholar]
- 29.Lally SE, Owens D, Tomkin GH. Sitosterol and cholesterol in chylomicrons of type 2 diabetic and non-diabetic subjects: the relationship with ATP binding cassette proteins G5 and G8 and Niemann-Pick C1-like 1 mRNA. Diabetologia. 2007;50:217–219. doi: 10.1007/s00125-006-0504-0. [DOI] [PubMed] [Google Scholar]
- 30.Vaverkova H, Farnier M, Averna M, Missault L, Viigimaa M, Dong Q, et al. Lipid-altering efficacy of ezetimibe/simvastatin 10/20 mg compared to rosuvastatin 10 mg in high-risk patients with and without type 2 diabetes mellitus inadequately controlled despite prior statin monotherapy. Cardiovasc Ther. 2012;30:61–74. doi: 10.1111/j.1755-5922.2010.00181.x. [DOI] [PubMed] [Google Scholar]
- 31.Leiter LA, Betteridge DJ, Farnier M, Guyton JR, Lin J, Shah A, et al. Lipid-altering efficacy and safety profile of combination therapy with ezetimibe/statin vs. statin monotherapy in patients with and without diabetes: an analysis of pooled data from 27 clinical trials. Diabetes Obes Metab. 2011;13:615–628. doi: 10.1111/j.1463-1326.2011.01383.x. [DOI] [PubMed] [Google Scholar]
- 32.Koh KK, Oh PC, Sakuma I, Kim EY, Lee Y, Hayashi T, et al. Vascular and metabolic effects of ezetimibe combined with simvastatin in patients with hypercholesterolemia. Int J Cardiol. 2015;199:126–131. doi: 10.1016/j.ijcard.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 33.Tie C, Gao K, Zhang N, Zhang S, Shen J, Xie X, et al. Ezetimibe attenuates atherosclerosis associated with lipid reduction and inflammation inhibition. PLoS One. 2015;10:e0142430. doi: 10.1371/journal.pone.0142430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel SB. Ezetimibe: a novel cholesterol-lowering agent that highlights novel physiologic pathways. Curr Cardiol Rep. 2004;6:439–442. doi: 10.1007/s11886-004-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin L, Yang YB, Yang YX, Gong YZ, Li XL, Li GY, et al. Inhibition of smooth muscle cell proliferation by ezetimibe via the cyclin D1-MAPK pathway. J Pharmacol Sci. 2014;125:283–291. doi: 10.1254/jphs.13239fp. [DOI] [PubMed] [Google Scholar]
- 36.Hussein O, Minasian L, Itzkovich Y, Shestatski K, Solomon L, Zidan J. Ezetimibe's effect on platelet aggregation and LDL tendency to peroxidation in hypercholesterolaemia as monotherapy or in addition to simvastatin. Br J Clin Pharmacol. 2008;65:637–645. doi: 10.1111/j.1365-2125.2007.03080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peto R, Emberson J, Landray M, Baigent C, Collins R, Clare R, et al. Analyses of cancer data from three ezetimibe trials. N Engl J Med. 2008;359:1357–1366. doi: 10.1056/NEJMsa0806603. [DOI] [PubMed] [Google Scholar]
- 38.Drazen JM, D'Agostino RB, Ware JH, Morrissey S, Curfman GD. Ezetimibe and cancer: an uncertain association. N Engl J Med. 2008;359:1398–1399. doi: 10.1056/NEJMe0807200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quality assessment of randomized controlled trials included in the meta-analysis by the Cochrane assessment of risk of bias.
Risk ratios (RRs) for newly-developed cancer. The pooled RR is plotted using the empty diamond. The box size is proportional to the weight of each study. CI, confidence interval; SHARP, the Study of Heart and Renal Protection; IMPROVE-IT, the Improved Reduction of Outcomes: Vytorin Efficacy International Trial.
Funnel plot of enrolled studies stratified by the presence of diabetes. MACE, major adverse cardiovascular event; CI, confidence interval.