Abstract
Background
BRAFV600E mutation status and prevalence of non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) has not yet been reported in Korea. The aim of this study was to investigate the significance of the BRAFV600E mutation in the follicular variant of papillary thyroid carcinoma (FVPTC) and to determine the prevalence of NIFTP in BRAFV600E mutation-prevalent Korean patients.
Methods
This study retrospectively analyzed 1,417 consecutive patients who underwent total thyroidectomy with routine prophylactic central lymph node dissection for papillary thyroid carcinoma (PTC). BRAFV600E mutation analysis was performed routinely using multiplex polymerase chain reaction by applying dual priming oligonucleotide. Clinicopathological characteristics and ultrasonographic findings were compared between BRAFV600E mutation-positive and -negative groups for FVPTC. Pathologists reviewed the pathology slides according to consensus diagnostic criteria for the encapsulated FVPTC and NIFTP.
Results
The prevalence of the BRAFV600E mutation in all subtypes of PTC was 61.0% (861/1,411). FVPTC presented a BRAFV600E mutation rate of 27.3%. The FVPTC patients with BRAFV600E mutation were older than those with no BRAFV600E mutation (P = 0.021). The prevalence of NIFTP was 0.18% among all PTC patients (2/1,411) and the proportion of NIFTP among FVPTC was 9.1% (2/22).
Conclusion
The BRAFV600E mutation is prevalent in Korean patients with FVPTC in a region with high frequency of the BRAFV600E mutation and very low prevalence of NIFTP compared with that reported in western studies.
Keywords: Thyroid Carcinoma, B-type Raf (BRAF), Follicular Variant, Papillary Carcinoma, NIFTP
Graphical Abstract
INTRODUCTION
The follicular variant of papillary thyroid carcinoma (FVPTC) is the second most common subtype of papillary thyroid carcinoma (PTC) after the classical type, constituting approximately 20% (11.8%–41%) of all PTCs and the incidence of FVPTC has increased.1,2,3,4 FVPTC is a unique clinical entity and it has hybrid characteristics of classical PTC and follicular thyroid carcinoma (FTC) or adenoma.5,6 FVPTC can be classified into infiltrative and encapsulated FVPTC.6 The infiltrative FVPTC often have BRAF mutations, whereas the encapsulated FVPTC most commonly have RAS mutations.7 The encapsulated FVPTC can be reclassified into invasive and non-invasive encapsulated FVPTC according to presence of capsular or vascular invasion.6 Recently, Nikiforov et al.8 reported that non-invasive encapsulated FVPTC behave in an indolent fashion, and therefore should not be considered malignant. Thus, non-invasive encapsulated FVPTC was renamed as “Non-invasive follicular thyroid neoplasm with papillary-like nuclear features” (NIFTP).
To our knowledge, there has been no report about BRAFV600E status and prevalence or incidence of NIFTP among all PTC in Korea. Nikiforov et al.8 suggested that estimated worldwide incidence of NIFTP was 18.6% among all PTC and the most common clonal molecular alterations were RAS mutations. Although one case of BRAFK601E mutation was detected, there was no BRAFV600E mutation.
However, in Korea, most thyroid cancer is classical PTC, and the BRAFV600E mutation is highly prevalent.9,10,11,12 Consequently, the prevalence of FVPTC is relatively low (2.7%–14.2%) compared with other countries.13,14,15 Recently, Hahn et al.16 firstly reported that the proportion of NIFTP among FVPTC in three tertiary medical centers of Korea for 7 years was 16.3% (34/208). However, they did not report the total number of patients with PTC.16 Therefore, they could not show the estimated incidence of NIFTP in Korea.
The aim of this study was to investigate the BRAFV600E mutation status of FVPTC and to determine prevalence of NIFTP after reclassifying non-invasive encapsulated FVPTC as NIFTP by careful histopathological review based on diagnostic criteria of NIFTP in Korean patients.
METHODS
Patients and clinicopathological data
From January 2011 to December 2012, 1,417 subjects (154 men, 1,263 women) who underwent total thyroidectomy due to PTC at Pusan National University Hospital were enrolled in the current study. In cases of multifocal PTC, only the largest tumor was included. Either synchronous PTC and FTC or PTC and medullary thyroid cancer were excluded in this study. Prophylactic central compartment neck node dissection was routinely performed in all patients, and lateral neck dissection was done in patients with pathologically confirmed lateral lymph node metastasis (LNM) or clinically suspected LNM on preoperative imaging or intraoperative examination. Electronic records that include tumor size, extrathyroidal extension (ETE), LNM, BRAFV600E mutation and the pathological subtype of PTC were reviewed to collect the clinicopathological features. Two pathologists specializing in thyroid pathology reviewed and interpreted the pathology slides according to consensus diagnostic criteria for the encapsulated FVPTC and NIFTP.8 Cancer staging was conducted according to the criteria outlined by the American Joint Committee on Cancer 2010, 7th edition.
BRAFV600E mutation evaluation
BRAFV600E analysis was performed routinely in paraffin-embedded thyroidectomy specimen sections from the removed thyroid cancer tissue. Representative sections from tumors were dissected on the glass using a clean blade and placed in a 1.5 mL tube. Genomic DNA was isolated from five to ten μm thick tissue sections using the QIAamp DNA Mini kit (Qiagen, Chatsworth, CA, USA) according to the manufacturer's instructions. For BRAFV600E mutation detection, we used Seeplex BRAF ACE detection system by applying dual priming oligonucleotide (DPO) technology (Seegene, Seoul, Korea). DPO-based multiplex polymerase chain reaction (PCR) analysis can reportedly detect the presence of BRAFV600E in as few as 2% of cells in a fine needle aspiration (FNA) specimen of thyroid nodules.17 In the DPO-based multiplex PCR analysis, five successive deoxyinosine linkers were used for 3'-end sensitization of the primer to enhance the specificity for single-base substitution. The shorter 3'-portion is linked to the longer 5'-portion by five successive deoxyinosine linkers. The binding energy of the shorter 3'-portion alone is sufficiently low to distinguish a single-base difference, which enhances the specificity of allele-specific PCR.18
Ultrasonography image analysis
All patients were investigated by ultrasound (US) (LOGIQ E9; General Electric, Waukesha, WI, USA) within a month before surgery in Pusan National University Hospital, and two endocrinologists (B.H.K, J.H.K) with at least 5 years of experience with thyroid US and FNA reviewed the results blinded to the clinical information. Internal content, echogenicity, shape, calcification, and margin of each nodule were evaluated according to the consensus statement and recommendations by the American Thyroid Association.19 Halo sign or hypoechoic rim surrounding nodule was also reviewed because it was histologically comprised of the nodule capsule or pseudo-capsule.20 The nodules were categorized as follows: low or indeterminate suspicion versus high suspicion.
Statistical analysis
Statistical analyses were performed using commercially available software (MedCalc 12.3; MedCalc Software, Mariakerke, Belgium). Continuous data are expressed as mean ± standard deviation for normally distributed values. Categorical data were presented as frequency and percentage. Independent t-test, χ2 test, and Fisher's exact test were used to analyze the demographic features. For FVPTC, patients were classified into two groups, BRAFV600E mutation-positive or -negative. In addition, FVPTC were separated into two major classes: infiltrative and encapsulated. Various clinicopathological characteristics were evaluated using the χ2 test, Fisher's exact test, or Mann-Whitney test between the two groups as appropriate. Statistical significance was defined as P < 0.05.
Ethics statement
This study was approved by the Institutional Review Board of Pusan National University Hospital (approval number: 1110-004-001). Informed consent was waived by the board.
RESULTS
Clinicopathological characteristics according to histological subtype of PTC
Clinicopathological characteristics of patients and tumor are shown in Table 1. Rare variant PTCs (1 insular thyroid cancer, 4 mixed type) were excluded in this study due to their small numbers. Therefore, this analysis included 1,411 patients in total. The classic type, FVPTC, diffuse sclerosing variant (DSV), and tall cell variant (TCV) represented 1,374 (97.3%), 22 (1.6%), 10 (0.7%), and 5 (0.35%) patients, respectively. The mean age was 50.6 ± 11.2 years (range, 14–81 years). The mean tumor size was 0.89 ± 0.64 cm (range, 0.3–6.0 cm). The percentage of patients with papillary thyroid microcarcinoma (PTMC) was 72.9% (1,001/1,374) in classical PTC. DSV PTC was more highly associated with young age (mean 43.7 years) compared with classical PTC and the other variant of PTC. In addition, DSV PTC and TCV PTC were associated with male patients compared with classical PTC (all P value < 0.05). The aggressive variants of PTC (DSV and TCV) showed a larger tumor size, and higher occurrence of ETE, LNM, and advanced stage than did classical and FVPTC (all P value < 0.05) (Table 1).
Table 1. Clinicopathological characteristics according to histologic subtypes of PTC.
| Characteristics | Classical (n = 1,374) | Follicular variant (n = 22) | Diffuse sclerosing variant (n = 10) | Tall cell variant (n = 5) |
|---|---|---|---|---|
| Age, yr | 50.0 ± 11.2 | 53.8 ± 9.6 | 43.7 ± 15.1a,b | 53.0 ± 21.7c |
| Sex, female | 1,234 (89.8) | 16 (72.7) | 7 (70.0)a | 3 (60.0)a |
| Tumor size, cm | 0.9 ± 0.6 | 1.4 ± 1.3a | 2.1 ± 1.2a,b | 2.0 ± 1.1a,b |
| ETE | 520 (37.8) | 7 (31.8) | 8 (80.0)a,b | 3 (60.0)a,b |
| LNM | 518 (37.7) | 4 (18.2)a | 9 (90.0)a,b | 3 (60.0)a,b |
| Advanced stage | 393 (28.6) | 9 (40.9) | 9 (90.0)a,b | 4 (80.0)a,b |
| BRAFV600E mutation | 850 (61.9) | 6 (27.3)a | 2 (20.0)a | 3 (60.0)b,c |
Statistical significance was tested by the χ2 test. Data are expressed as mean ± standard deviation and frequency (%) for categorical variables.
PTC = papillary thyroid carcinoma, ETE = extrathyroidal extension, LNM = lymph node metastasis, Advanced stage = American Joint Committee on Cancer (AJCC) stage III + IV.
aP < 0.05 vs. conventional PTC; bP < 0.05 vs. follicular variant PTC; cP < 0.05 vs. diffuse sclerosing variant PTC.
BRAFV600E mutation status according to histologic subtype of PTC
The prevalence of the BRAFV600E mutation in all subtypes of PTC is 61.0% (861/1,411). The BRAFV600E mutation was most commonly detected in conventional PTC (61.9%) and TCV PTC (60%). However, FVPTC and DSV PTC presented a BRAFV600E mutation rate of 27.3% and 20%, respectively (Table 1).
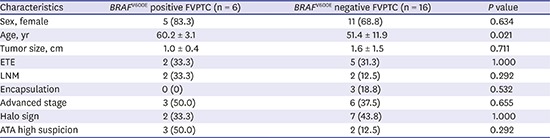
Clinicopathological and ultrasonographic features according to BRAFV600E mutation status in FVPTC
The clinicopathological and ultrasonographic characteristics of BRAFV600E mutation-positive and -negative FVPTC are summarized in Table 2. FVPTC patients with BRAFV600E mutation were older than those with no BRAFV600E mutation (P = 0.021). The FVPTC patients with BRAFV600E mutation had more LNM, advanced stage, and ultrasonographic high suspicion category than those with no BRAFV600E mutation, but those associations were not statistically significant. FVPTC patients with no BRAFV600E mutation showed more chance, although statistically not significant, of encapsulation and halo sign than those with BRAFV600E mutation.
Table 2. Clinicopathological and sonographic characteristics of FVPTC according to BRAFV600E mutation.
| Characteristics | BRAFV600E positive FVPTC (n = 6) | BRAFV600E negative FVPTC (n = 16) | P value |
|---|---|---|---|
| Sex, female | 5 (83.3) | 11 (68.8) | 0.634 |
| Age, yr | 60.2 ± 3.1 | 51.4 ± 11.9 | 0.021 |
| Tumor size, cm | 1.0 ± 0.4 | 1.6 ± 1.5 | 0.711 |
| ETE | 2 (33.3) | 5 (31.3) | 1.000 |
| LNM | 2 (33.3) | 2 (12.5) | 0.292 |
| Encapsulation | 0 (0) | 3 (18.8) | 0.532 |
| Advanced stage | 3 (50.0) | 6 (37.5) | 0.655 |
| Halo sign | 2 (33.3) | 7 (43.8) | 1.000 |
| ATA high suspicion | 3 (50.0) | 2 (12.5) | 0.292 |
Data are expressed as mean ± standard deviation and frequency (%) for categorical variables.
FVPTC = follicular variant papillary thyroid carcinoma, ETE = extrathyroidal extension, LNM = lymph node metastasis, Advanced stage = American Joint Committee on Cancer (AJCC) stage III + IV, ATA = American Thyroid Association.
Correlation between encapsulation status and clinicopathological characteristics in FVPTC
The clinicopathological characteristics of encapsulated FVPTC and infiltrative FVPTC are summarized in Table 3. Encapsulated FVPTC was marginally associated with large tumor size (P = 0.065) and positive halo sign (P = 0.055) compared with infiltrative FVPTC due to small sample size. Infiltrative FVPTC had a higher frequency of ETE, LNM, BRAFV600E mutation, high suspicious ultrasonographic features, and advanced stage compared with encapsulated FVPTC, however, there was no statistical significance (all P value > 0.05).
Table 3. Clinicopathological characteristics of FVPTC according to presence of encapsulation.
| Characteristics | Encapsulated FVPTC (n = 3) | Infiltrative FVPTC (n = 19) | P value | |
|---|---|---|---|---|
| Sex | 1.000 | |||
| Male | 1 (33.3) | 5 (26.3) | ||
| Female | 2 (66.6) | 14 (73.7) | ||
| Age, yr | 44.7 ± 16.6 | 55.2 ± 9.7 | 0.388 | |
| Old age (> 45 yr) | 2 (66.6) | 16 (84.2) | 0.470 | |
| Tumor size, cm | 2.7 ± 2.9 | 1.2 ± 0.9 | 0.065 | |
| ETE | 0 (0) | 7 (36.8) | 0.523 | |
| LNM | 0 (0) | 4 (21.1) | 1.000 | |
| Multifocality | 0 (0) | 7 (36.8) | 0.709 | |
| Advanced stage | 1 (33.1) | 8 (42.1) | 1.000 | |
| BRAFV600E mutation | 0 (0) | 6 (31.6) | 0.532 | |
| Halo sign | 3 (100) | 6 (31.6) | 0.055 | |
| ATA high suspicion | 0 (0) | 5 (26.3) | 1.000 | |
Data are expressed as mean ± standard deviation and frequency (%) for categorical variables.
FVPTC = follicular variant papillary thyroid carcinoma, ETE = extrathyroidal extension, LNM = lymph node metastasis, Advanced stage = American Joint Committee on Cancer (AJCC) stage III + IV, ATA = American Thyroid Association.
Prevalence and clinical outcome of NIFTP
The prevalence of NIFTP in this study was 0.18% among all PTC patients (2/1,411) and proportion of NIFTP among FVPTC was 9.1% (2/22). The proportion of NIFTP among encapsulated FVPTC was 66.6% (2/3). There was no BRAFV600E mutation in NIFTP. Clinical outcomes and details of follow-up for 2 patients with NIFTP are summarized in Table 4. These two patients were treated with total thyroidectomy with prophylactic central lymph node dissection only, and neither of them received radioactive iodine therapy. Two patients had no adverse events and no evidence of disease during approximately 4 years follow-up period.
Table 4. Clinical outcome and details of follow-up for 2 patients in NIFTP.
| Parameters | Patient 1 | Patient 2 |
|---|---|---|
| Age, yr | 50 | 58 |
| Sex | Female | Female |
| Tumor size, cm | 0.7 | 1.5 |
| FNA cytology result | AUS | PTC |
| ATA US feature | Low suspicion | Low suspicion |
| Halo sign | Yes | Yes |
| Type of surgery | Total thyroidectomy | Total thyroidectomy |
| LNM | None | None |
| TNM stage | I | I |
| RAI therapy | None | None |
| FU duration, yr | 4.5 | 4.1 |
| No evidence of disease | Yes | Yes |
| BRAFV600E mutation | Negative | Negative |
NIFTP = non-invasive follicular thyroid neoplasm with papillary-like nuclear features, FNA = fine needle aspiration, ATA = American Thyroid Association, US = ultrasonography, AUS = atypia of undetermined significance, PTC = papillary thyroid carcinoma, LNM = lymph node metastasis, TNM = tumor, node, metastasis, RAI = radioactive iodine, FU = follow up.
DISCUSSION
We have evaluated the status of the BRAFV600E mutation of FVPTC to determine the prevalence of NIFTP in a BRAFV600E mutation-prevalent area. The classical PTC and FVPTC represented 97.4% and 1.6% of all PTC patients, respectively. The prevalence of the BRAFV600E mutation was 61.9% in classical PTC and 27.3% in FVPTC. BRAFV600E mutation-positive FVPTC was more highly associated with old age than BRAFV600E mutation-negative FVPTC. Encapsulated FVPTC was marginally associated with large tumor size and positive halo sign compared with infiltrative FVPTC. In the current study, the prevalence of NIFTP was 0.18% among all PTC patients. The proportion of NIFTP among FVPTC and encapsulated FVPTC was 9.1% and 66.6%, respectively. There was no BRAFV600E mutation in NIFTP.
FVPTC is the most common variant of PTC and it has different clinicopathological characteristics and molecular alterations compared to classical PTC. Most PTCs such as classic or TCVs with the BRAFV600E mutation showed a papillary growth pattern, whereas the BRAFV600E mutation was uncommon in FVPTC, but detection rate of RAS mutation was high compared to classical PTC.14,21 In addition, the infiltrative FVPTC often have BRAF mutations, whereas the encapsulated FVPTC most commonly have RAS mutations.7
The prevalence of the BRAFV600E mutation which is the most common genetic alteration in PTC, has wide variation (30%–90%) depending on detection method, ethnic and geographic backgrounds, and study populations.10,22,23,24,25 The increase in both the prevalence of the BRAFV600E mutation, in accordance with iodine consumption, and in the number of cases of PTC has been reported in different countries.26,27,28 Particularly, in Korea, where iodine consumption is very high, the prevalence of the BRAFV600E mutation in PTC is much higher than that in western countries.13,14,15 In the present study, the prevalence of the BRAFV600E mutation was 61.9% in classical PTC, 27.3% in FVPTC. Although the reported prevalence of the BRAFV600E mutation in FVPTC has varied (17%–40%) in different detection analyses and study populations, some Korean studies on the BRAFV600E mutation of FVPTC showed higher detection rates (40%) than those of western countries (17%–31%).14,29,30 However, recent studies suggest that almost one third of FVPTC harbor BRAF mutations.13,31 These data are accordant with the result of the present study, in which 27.3% of FVPTC cases were BRAFV600E mutation-positive. The reason for the high prevalence of the BRAFV600E mutation in Korean patients with PTC is still unclear. A possible explanation is that iodine-rich diets or chronic thyroiditis in the Korean population may be associated with the BRAFV600E mutation.26 In view of the positive association with high iodine consumption, a recent Chinese report showed that the prevalence of the BRAFV600E mutation in PTC was significantly higher in iodine-rich areas than in iodine-normal areas in China.31 In contrast, a very recent study showed no differences in genetic alterations of PTC from iodine-rich (Japan) and iodine-deficient (Vietnam) countries.32 Thus, there is still no consensus regarding the association of iodine intake and the high prevalence of the BRAFV600E mutation in patients with PTC in iodine sufficient areas. Therefore, these associations should be further elucidated in future studies.
Recent studies reported that an association exists between the BRAFV600E mutation and poor clinicopathological outcomes of FVPTC.13,29 However, in the current study, only old age was significantly associated with BRAFV600E mutant FVPTC compared with BRAFV600E mutation-negative FVPTC. Some findings in the present study conflict with a report that the BRAFV600E mutation was associated with poor prognostic factors in FVPTC.13,29 However, there is still debate about the correlation between BRAFV600E mutation status and poor clinicopathological features in FVPTC due to limited studies.
The prognosis of FVPTC seems to be more dependent on whether it is completely encapsulated or infiltrative than on BRAFV600E mutation status. The infiltrative FVPTC was more likely to have ETE and LNM and generally behaved like classical PTC. In contrast, the encapsulated, noninvasive FVPTC behaved in an indolent fashion, similar to benign follicular adenomas. The encapsulated FVPTC with capsular or vascular invasion behaved more like a FTC.5,6 In the current study, infiltrative FVPTC showed a higher frequency of LNM. Although the frequency of ETE and advanced stage was higher in infiltrative FVPTC than in encapsulated FVPTC, this did not reach statistical significance due to small sample size. There was no statistical difference between BRAFV600E mutation and infiltrative FVPTC because only one (50%) among two patients had the BRAFV600E mutation in this study.
After Nikiforov et al.8 reported a first new nomenclature and estimated worldwide incidence of NIFTP (18.6% among all PTC), a multicenter study from the 9 institutions from 6 Asian countries including Korea was very recently reported that the mean calculated as an average of NIFTP was 1.5% (range 0%–4.7%).33 A single institution study from Japan also reported that incidence of noninvasive encapsulated FVPTC (EFVPTC) was 0.4% of all PTC cases.34 In the current our study, the prevalence of NIFTP was 0.18% among all PTC patients in accordance with these Asian studies. Such a huge discrepancy of incidence of NIFTP between Western and Asian studies might be considered by several factors such as geographic and ethnic differences in type of thyroid cancers, incidences of FVPTC, differences in histologic interpretation, and variable diagnostic threshold.34 Therefore, low rate of NIFTP in Asian countries should be further elucidated in future large studies.
This study has some limitations. First, the retrospective single-center nature of our study may limit the generalization of our results. Second, although the total sample size of the study was large, only a limited number of FVPTC cases were included in this study. The prevalence of FVPTC (4.9%–41.2%) was varied according to study populations and methods.35 Although FVPTC has been increasingly diagnosed in recent years, a previous large population study in Korea demonstrated very low prevalence of FVPTC (2.6%), this result was concordant with our result (1.6%).14 In addition, FVPTC has more benign ultrasonographic features than classical PTC, making the diagnostic efficacy lower for FVPTC. Because ultrasonographic differences between FVPTC and benign adenoma or FTC are not always clear, occasionally a FVPTC will mistakenly be classified as a benign follicular adenoma.36 Therefore, selection bias may have existed. The lower rate of suspicious findings in FVPTC lesions may have caused less evaluation by FNA biopsy (FNAB), resulting in no necessity of surgery. In addition, benign findings of ultrasonography of FVPTC lesions may have caused evaluation of larger FVPTC lesions by FNAB, resulting in the detection of these lesions at a later stage. In view of this, our study included many PTMC patients (n = 1,034, 73.3%). The percentage of FVPTC after excluding PTMC was 5.8%. Third, considerable inter-observer variability in the diagnosis of FVPTC based on histology was not considered in this study. The diagnosis of FVPTC can be quite difficult and controversial. Fourth, recurrence or survival outcomes could not be evaluated in relation to BRAFV600E mutation status because of the relatively short follow-up period. Lastly, we could not evaluate a more detailed molecular profile by analyzing RAS mutation because it was not available in our hospital. In addition, although BRAFK601E is known as having high association with encapsulated FVPTC, we did not perform BRAFK601E analysis. Despite these limitations, the strength of this study is that BRAFV600E mutation analysis was performed routinely in all consecutive patients with PTC who underwent total thyroidectomy and routine prophylactic central compartment neck node dissection in the BRAFV600E mutation-prevalent area.
In conclusion, this study has found that the BRAFV600E mutation is prevalent in Korean patients with FVPTC in a region with high frequency of the BRAFV600E mutation and very low prevalence of NIFTP compared with western studies. Further prospective research involving a large number of cases is required to conclusively establish the prevalence of NIFTP from Asian countries especially in Korea.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Kim BH, Kim IJ. Investigation: Kim H, Kim JM, Kim YK, Kim EH, Lee MJ, Kim JH. Formal analysis: Kim H, Jeon YK, Kim SS. Writing - original draft: Kim H, Kim BH. Writing - review & editing: Kim BH, Lee BJ, Kim YK.
References
- 1.Lang BH, Lo CY, Chan WF, Lam AK, Wan KY. Classical and follicular variant of papillary thyroid carcinoma: a comparative study on clinicopathologic features and long-term outcome. World J Surg. 2006;30(5):752–758. doi: 10.1007/s00268-005-0356-7. [DOI] [PubMed] [Google Scholar]
- 2.Passler C, Prager G, Scheuba C, Niederle BE, Kaserer K, Zettinig G, et al. Follicular variant of papillary thyroid carcinoma: a long-term follow-up. Arch Surg. 2003;138(12):1362–1366. doi: 10.1001/archsurg.138.12.1362. [DOI] [PubMed] [Google Scholar]
- 3.Zidan J, Karen D, Stein M, Rosenblatt E, Basher W, Kuten A. Pure versus follicular variant of papillary thyroid carcinoma: clinical features, prognostic factors, treatment, and survival. Cancer. 2003;97(5):1181–1185. doi: 10.1002/cncr.11175. [DOI] [PubMed] [Google Scholar]
- 4.Yu XM, Schneider DF, Leverson G, Chen H, Sippel RS. Follicular variant of papillary thyroid carcinoma is a unique clinical entity: a population-based study of 10,740 cases. Thyroid. 2013;23(10):1263–1268. doi: 10.1089/thy.2012.0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniels GH. Follicular variant of papillary thyroid carcinoma: hybrid or mixture? Thyroid. 2016;26(7):872–874. doi: 10.1089/thy.2016.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Singh B, Tallini G, Carlson DL, Katabi N, Shaha A, et al. Follicular variant of papillary thyroid carcinoma: a clinicopathologic study of a problematic entity. Cancer. 2006;107(6):1255–1264. doi: 10.1002/cncr.22138. [DOI] [PubMed] [Google Scholar]
- 7.Rivera M, Ricarte-Filho J, Knauf J, Shaha A, Tuttle M, Fagin JA, et al. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol. 2010;23(9):1191–1200. doi: 10.1038/modpathol.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016;2(8):1023–1029. doi: 10.1001/jamaoncol.2016.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim TY, Kim WG, Kim WB, Shong YK. Current status and future perspectives in differentiated thyroid cancer. Endocrinol Metab (Seoul) 2014;29(3):217–225. doi: 10.3803/EnM.2014.29.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim TY, Kim WB, Rhee YS, Song JY, Kim JM, Gong G, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2006;65(3):364–368. doi: 10.1111/j.1365-2265.2006.02605.x. [DOI] [PubMed] [Google Scholar]
- 11.Jeong D, Jeong Y, Park JH, Han SW, Kim SY, Kim YJ, et al. BRAF (V600E) mutation analysis in papillary thyroid carcinomas by peptide nucleic acid clamp real-time PCR. Ann Surg Oncol. 2013;20(3):759–766. doi: 10.1245/s10434-012-2494-0. [DOI] [PubMed] [Google Scholar]
- 12.Kwon MJ, Lee SE, Kang SY, Choi YL. Frequency of KRAS, BRAF, and PIK3CA mutations in advanced colorectal cancers: Comparison of peptide nucleic acid-mediated PCR clamping and direct sequencing in formalin-fixed, paraffin-embedded tissue. Pathol Res Pract. 2011;207(12):762–768. doi: 10.1016/j.prp.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Chai YJ, Kim SJ, Kim SC, Koo DH, Min HS, Lee KE, et al. BRAF mutation in follicular variant of papillary thyroid carcinoma is associated with unfavourable clinicopathological characteristics and malignant features on ultrasonography. Clin Endocrinol (Oxf) 2014;81(3):432–439. doi: 10.1111/cen.12433. [DOI] [PubMed] [Google Scholar]
- 14.Lim JY, Hong SW, Lee YS, Kim BW, Park CS, Chang HS, et al. Clinicopathologic implications of the BRAF(V600E) mutation in papillary thyroid cancer: a subgroup analysis of 3130 cases in a single center. Thyroid. 2013;23(11):1423–1430. doi: 10.1089/thy.2013.0036. [DOI] [PubMed] [Google Scholar]
- 15.Min HS, Lee C, Jung KC. Correlation of immunohistochemical markers and BRAF mutation status with histological variants of papillary thyroid carcinoma in the Korean population. J Korean Med Sci. 2013;28(4):534–541. doi: 10.3346/jkms.2013.28.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahn SY, Shin JH, Lim HK, Jung SL, Oh YL, Choi IH, et al. Preoperative differentiation between noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) and non-NIFTP. Clin Endocrinol (Oxf) 2017;86(3):444–450. doi: 10.1111/cen.13263. [DOI] [PubMed] [Google Scholar]
- 17.Kwak JY, Kim EK, Kim JK, Han JH, Hong SW, Park TS, et al. Dual priming oligonucleotide-based multiplex PCR analysis for detection of BRAFV600E mutation in FNAB samples of thyroid nodules in BRAFV600E mutation-prevalent area. Head Neck. 2010;32(4):490–498. doi: 10.1002/hed.21210. [DOI] [PubMed] [Google Scholar]
- 18.Kim SW, Lee JI, Kim JW, Ki CS, Oh YL, Choi YL, et al. BRAFV600E mutation analysis in fine-needle aspiration cytology specimens for evaluation of thyroid nodule: a large series in a BRAFV600E-prevalent population. J Clin Endocrinol Metab. 2010;95(8):3693–3700. doi: 10.1210/jc.2009-2795. [DOI] [PubMed] [Google Scholar]
- 19.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology Consensus statement and recommendations. Korean J Radiol. 2016;17(3):370–395. doi: 10.3348/kjr.2016.17.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith RA, Salajegheh A, Weinstein S, Nassiri M, Lam AK. Correlation between BRAF mutation and the clinicopathological parameters in papillary thyroid carcinoma with particular reference to follicular variant. Hum Pathol. 2011;42(4):500–506. doi: 10.1016/j.humpath.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 22.Cohen Y, Xing M, Mambo E, Guo Z, Wu G, Trink B, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003;95(8):625–627. doi: 10.1093/jnci/95.8.625. [DOI] [PubMed] [Google Scholar]
- 23.Fukushima T, Suzuki S, Mashiko M, Ohtake T, Endo Y, Takebayashi Y, et al. BRAF mutations in papillary carcinomas of the thyroid. Oncogene. 2003;22(41):6455–6457. doi: 10.1038/sj.onc.1206739. [DOI] [PubMed] [Google Scholar]
- 24.Xing M, Alzahrani AS, Carson KA, Shong YK, Kim TY, Viola D, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol. 2015;33(1):42–50. doi: 10.1200/JCO.2014.56.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SK, Kim DL, Han HS, Kim WS, Kim SJ, Moon WJ, et al. Pyrosequencing analysis for detection of a BRAFV600E mutation in an FNAB specimen of thyroid nodules. Diagn Mol Pathol. 2008;17(2):118–125. doi: 10.1097/PDM.0b013e31815d059d. [DOI] [PubMed] [Google Scholar]
- 26.Hong AR, Lim JA, Kim TH, Choi HS, Yoo WS, Min HS, et al. The frequency and clinical implications of the BRAF(V600E) mutation in papillary thyroid cancer patients in Korea over the past two decades. Endocrinol Metab (Seoul) 2014;29(4):505–513. doi: 10.3803/EnM.2014.29.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romei C, Fugazzola L, Puxeddu E, Frasca F, Viola D, Muzza M, et al. Modifications in the papillary thyroid cancer gene profile over the last 15 years. J Clin Endocrinol Metab. 2012;97(9):E1758–E1765. doi: 10.1210/jc.2012-1269. [DOI] [PubMed] [Google Scholar]
- 28.Mathur A, Moses W, Rahbari R, Khanafshar E, Duh QY, Clark O, et al. Higher rate of BRAF mutation in papillary thyroid cancer over time: a single-institution study. Cancer. 2011;117(19):4390–4395. doi: 10.1002/cncr.26072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin DY, Kim KJ, Chang S, Kim H, Hwang S, Kim W, et al. Follicular variant of papillary thyroid carcinoma with B-type Raf(V600E) showing higher frequency of suspicious sonographic features and multifocality. Head Neck. 2015;37(11):1590–1595. doi: 10.1002/hed.23793. [DOI] [PubMed] [Google Scholar]
- 30.McFadden DG, Dias-Santagata D, Sadow PM, Lynch KD, Lubitz C, Donovan SE, et al. Identification of oncogenic mutations and gene fusions in the follicular variant of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2014;99(11):E2457–E2462. doi: 10.1210/jc.2014-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guan H, Ji M, Bao R, Yu H, Wang Y, Hou P, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009;94(5):1612–1617. doi: 10.1210/jc.2008-2390. [DOI] [PubMed] [Google Scholar]
- 32.Vuong HG, Kondo T, Oishi N, Nakazawa T, Mochizuki K, Inoue T, et al. Genetic alterations of differentiated thyroid carcinoma in iodine-rich and iodine-deficient countries. Cancer Med. 2016;5(8):1883–1889. doi: 10.1002/cam4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bychkov A, Hirokawa M, Jung CK, Liu Z, Zhu Y, Hong SW, et al. Low rate of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice. Thyroid. 2017;27(7):983–984. doi: 10.1089/thy.2017.0079. [DOI] [PubMed] [Google Scholar]
- 34.Liu Z, Zhou G, Nakamura M, Koike E, Li Y, Ozaki T, et al. Encapsulated follicular thyroid tumor with equivocal nuclear changes, so-called well-differentiated tumor of uncertain malignant potential: a morphological, immunohistochemical, and molecular appraisal. Cancer Sci. 2011;102(1):288–294. doi: 10.1111/j.1349-7006.2010.01769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J, Gong Y, Yan S, Shi Q, Zhu J, Li Z, et al. Comparison of the clinicopathological behavior of the follicular variant of papillary thyroid carcinoma and classical papillary thyroid carcinoma: a systematic review and meta-analysis. Mol Clin Oncol. 2015;3(4):753–764. doi: 10.3892/mco.2015.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeon EJ, Jeong YJ, Park SH, Cho CH, Shon HS, Jung ED. Ultrasonographic characteristics of the follicular variant papillary thyroid cancer according to the tumor size. J Korean Med Sci. 2016;31(3):397–402. doi: 10.3346/jkms.2016.31.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]


