Abstract
Treatment of NIH 3T3 cells with trichostatin A (TSA), an inhibitor of histone deacetylase (HDAC), resulted in a dose-dependent increase in transcription from a rDNA reporter and from endogenous rRNA genes. Chromatin immunoprecipitation using anti-acetyl-histone H4 antibodies demonstrated a direct effect of TSA on the acetylation state of the ribosomal chromatin. TSA did not reverse inhibition of transcription from the rDNA reporter by retinoblastoma (Rb) protein, suggesting that the main mechanism by which Rb blocks rDNA transcription may not involve recruitment of deacetylases to rDNA chromatin. Overexpression of histone transacetylases p300, CBP and PCAF stimulated transcription in transfected NIH 3T3 cells. Recombinant p300, but not PCAF, stimulated rDNA transcription in vitro in the absence of nucleosomes, suggesting that the stimulation of rDNA transcription by TSA might have a chromatin-independent component. We found that the rDNA transcription factor UBF was acetylated in vivo. Finally, we also demonstrated the nucleolar localization of CBP. Our results suggest that the organization of ribosomal chromatin of higher eukaryotes is not static and that acetylation may be involved in affecting these dynamic changes directly through histone acetylation and/or through acetylation of UBF or one of the other components of rDNA transcription.
INTRODUCTION
The regulation of ribosomal DNA (rDNA) transcription is a major task for the cell. Not only is the scale of rDNA transcription significant (the transcription products constitute ∼80% of the total mass of cellular RNA), but the process of ribosome biogenesis is complex. The regulation of transcription of the 45S precursor by RNA polymerase I must be coordinated with transcription of the 5S RNA genes by RNA polymerase III and transcription/translation of the genes for the more than 80 ribosomal proteins that are transcribed by RNA polymerase II. All of these together are regulated in response to the various conditions that modulate cell growth, some of which require significant changes in the protein synthetic capacity of the cell (reviewed in 1–7). In general, transcriptional regulation results from multiple combinations of interactions between a large but limited number of cis-acting transcription elements and trans-acting regulatory proteins. However, in the case of transcription by RNA polymerase I there are only a limited number of trans-acting regulatory factors. Hence it is likely that regulation of rDNA transcription will involve multiple combinations of modifications of the limited number of transcription factors.
In eukaryotes, the packing of DNA into chromatin (8–11) adds an additional level of complexity to the process of transcription regulation. The nucleosome is the basic subunit of chromatin. In the nucleosome, the DNA is wound around an octamer of histones. Therefore its structure is considerably different from that of ‘free’ DNA (for reviews see 10,11). As a consequence, every DNA-dependent process that takes place, including transcription, is associated with some form of chromatin remodeling. This remodeling (for reviews see 10–14) includes two mechanisms: (i) histone modifications that give transcription factors access to DNA without disrupting nucleosomes by loosening the chromatin structure or by adding ‘tags’ to specific chromatin domains; and (ii) chromatin disruption by molecular machines driven by ATP hydrolysis, e.g. remodeling complexes such as yeast SWI/SNF and DNA and RNA polymerases (14). The state of histone acetylation has been correlated with chromatin remodeling and the activation of transcription (reviewed in 13,14).
Histone acetylation has long been recognized as a mechanism for altering the structure of the nucleosome (15–17). In the past several years direct links between transcription, histone acetylation and the mechanisms by which the state of histone acetylation is regulated have been established (18–20). A significant body of knowledge has accumulated concerning the mechanism(s) through which histone acetylation affects transcription by RNA polymerase II. However, little is known about the role of histone acetylation on the structure or function of rDNA.
Sogo and colleagues (21–25; reviewed in 26) suggested that the organization of ribosomal chromatin in a mammalian cell consisted of a constant number of active, non-nucleosomal repeats and non-active repeats organized as nucleosomes. In this model the control of transcription reflects variations in the frequency of transcription initiation from a set number of open genes. Mutskov et al. (27) extended these observations. They demonstrated that some of the rDNA repeats in rat tumor cells were associated with acetylated histones and these DNA–protein complexes were not organized as ‘nucleosomes’. It is interesting that this observation is consistent with those originally reported by Prior et al. (28), who reported that the transcriptionally active ribosomal genes in Tetrahymena were not organized as typical nucleosomes. Although the experiments of Mutskov et al. (27) are consistent with the model in which the machinery for acetylating and deacetylating the ribosomal chromatin is in place in the nucleolus, they did not demonstrate a correlation between acetylation and rDNA transcription. Moreover, as they did not compare the structure of the ribosomal chromatin in sodium butyrate-treated (29) and untreated cells, they could not discuss the possibility that the structure or organization of ribosomal chromatin might be dynamic. When Chen and Pikaard (30) studied the epigenetic silencing of RNA polymerase I transcription in Brassica hybrids, they demonstrated that histone deacetylase inhibitors derepressed silent ribosomal RNA (rRNA) genes. However, those authors did not examine the acetylation status of the histones associated with the rDNA (30).
rDNA is transcribed by RNA polymerase I (RPI). SL-1 and UBF, the RPI-specific transcription factors, have been shown to be targets for the signal transduction cascades that affect rDNA transcription (31–33). UBF is a highly conserved protein which purifies as two polypeptides, UBF1 (97 kDa) and UBF2 (94 kDa). These proteins are generated by alternative splicing of the transcripts of one gene (34–38). UBF is not absolutely required for specific initiation on the rDNA promoter in vitro, although its addition to UBF-depleted extracts increases the efficiency of transcription in a dose-dependent manner (39–41). In addition, overexpression of UBF1 in either immortal cells or primary cultures of cardiomyocytes is sufficient to directly increase transcription from both a reporter for rDNA transcription and the endogenous genes (42,43). Activation of rDNA transcription by UBF requires the formation of UBF dimers (1,36–38,44,45), binding of UBF to the upstream promoter element (UPE) (1,41,45,46) and an interaction between UBF and the basal rDNA transcription factor SL-1 (1,47–51). UBF contains domains with homology to the DNA-binding domain of HMGI and has been shown to form an alternative chromatin structure (52). Moreover, UBF can compete with H1 for binding to nucleosome cores (53) and may act to suppress the formation of transcriptionally inactive chromatin that requires linker histone binding. Recently, we have proposed that UBF may function as a regulator of chromatin structural transitions (43). UBF can be found complexed to retinoblastoma (Rb) in differentiating or confluent cells (54,55). In vitro studies have demonstrated that, when complexed to Rb, UBF cannot interact with SL-1 and activate rDNA transcription (54–56). This is analogous to the effect of Rb on E2F (57). However, the binding of Rb to E2F can do more than block the ability of E2F to activate transcription. When E2F is complexed with Rb the resulting complex can also act to recruit histone deacetylase (58,59). This results in the repression of gene transcription, due to an alteration of the acetylation state of the local histones. Arguing by analogy, this suggests the hypothesis that in vivo Rb might act to inhibit rDNA transcription by both inhibiting the UBF–SL1 interaction and recruiting histone deacetylase to the ribosomal chromatin.
If the action of Rb includes the recruitment of histone deacetylase activity to the ribosomal chromatin, then a corollary of this hypothesis is that the organization of ribosomal chromatin is not static, as acetylation would regulate its structure. Thus, we carried out a series of experiments designed to determine whether manipulating the acetylation status of histones would affect rDNA transcription. To determine whether acetylation had a direct effect on rDNA transcription, we examined the possibility that treatment with trichostatin A (TSA) would affect the rate of rDNA transcription and/or influence the structure of the ribosomal chromatin, i.e. the association of rDNA with acetylated histones. We observed that treatment with TSA stimulated rDNA transcription and the accumulation of acetylated histone H4 in rDNA chromatin. This suggests that the rate of rDNA transcription reflected the balance between deacetylase and acetylase activity. Interestingly, TSA did not reverse the inhibitory effect of Rb on rDNA transcription. This suggests that the inhibitory effect of Rb on rDNA transcription may not be through the recruitment of a TSA-sensitive histone deacetylase(s). CBP and two other histone acetyltransferases, CBP-related coactivator (p300) and p300/CBP-associated factor (PCAF), stimulated rDNA transcription when overexpressed in NIH 3T3 cells, and we could demonstrate localization of CBP in the nucleolus. p300, but not PCAF, stimulated rDNA transcription in vitro. As this assay was carried out in the absence of histones, we examined the possibility that one of the rDNA transcription factors was a target of acetylation. We found that antibodies to acetyl-lysine could immunoprecipitate UBF. Our data suggest that acetylation may affect rDNA transcription directly by histone acetylation and indirectly through the post-translational modification of UBF.
MATERIALS AND METHODS
Tissue culture and cell lines
NIH 3T3 cells (ATCC no. CCL163) were obtained from the ATCC. Monolayer cultures of NIH 3T3 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C in a 5% CO2 atmosphere. N1S1 cells were cultured as described previously (31).
Transient transfection assays
NIH 3T3 cells were plated at 9 × 103 cells/cm2. Twenty-four hours later, the exponentially growing cells were transfected using Opti-MEM and Lipofectamine (Life Technologies, Gaithersburg, MD). All transfections were carried out at a constant concentration of DNA. When necessary, varying amounts of the appropriate vector DNA were added to adjust the total DNA in the transfection to 4 µg. Five hours after transfection, the culture medium was replaced with fresh medium containing the indicated concentration of TSA. pSMECAT, a construct containing the mouse rDNA promoter upstream of an internal ribosome entry site and the bacterial chloramphenicol acetyltransferase (CAT) gene, was used to measure rDNA transcription in the transfection experiments. Previous experiments have demonstrated that transcription of the chimeric CAT gene is accurately initiated by RPI and that it reflects activity of the endogenous rRNA genes in several systems (43,60, and references within). pSMECAT-7, a construct with a G/A mutation at –7 that prevents transcription by RPI, is not transcribed in NIH 3T3 cells (60), even when the cells were treated with TSA (data not shown). Twenty-four hours after transfection, cells were harvested and lysates prepared for CAT assay (61).
Isolation of nuclei and DNA concentration determination
NIH 3T3 cells were plated at low density (9 × 103 cells/cm2). Twenty-four hours later, cells were treated with different concentrations of TSA and allowed to grow for an additional 19 h. Cells were than released from tissue culture plates using 0.25% trypsin–EDTA. DMEM containing 10% FBS was added to stop the reaction. The cells were collected by centrifugation (400 g for 10 min), washed once with ice-cold phosphate-buffered saline (PBS) and counted. Samples of 2 × 107 cells were swollen on ice for 10 min in 2 ml of nuclear isolation buffer (10 mM Tris–HCl, pH 7.4, 10 mM NaCl, 10 mM MgCl2, 0.5% NP-40) and lysed by mixing. Nuclei were collected by centrifugation at 1000 g for 5 min and resuspended in 200 µl of nuclear storage buffer (50 mM Tris–HCl, pH 8.3, 40% glycerol, 5 mM MgCl2, 0.1 mM EDTA). Aliquots of 20 µl were removed for determination of DNA using a fluorometric Hoechst dye-binding assay (62).
Nuclear run-on transcription
The synthesis and isolation of de novo synthesized RNA was carried out as described (55) with modifications. Equal numbers of control and TSA-treated NIH 3T3 cell nuclei (30 µg DNA) were incubated for 30 min at 37°C in a 100 µl total volume containing 5 mM Tris–HCl, pH 8, 2.5 mM MgCl2, 150 mM KCl, 0.4 mg heparin, 2.5 mM DDT, 1 mM ATP, CTP and GTP, 20 µCi [α-32P]UTP and 0.5 µl of 40 µg/µl RNasin. The reaction was stopped and RNA isolated using TRIzol Reagent (Life Sciences) according to the manufacturer’s protocol. Unincorporated nucleotides were removed from the sample by centrifugation through a G25 STE Select-G (RF) column (5 Prime–3 Prime) and RNA synthesis was determined by liquid scintillation spectrophotometry. Run-on transcripts were hybridized to the mouse rDNA promoter (–168 to +292 with respect to the transcription initiation site) and the cDNA for mouse GAPDH immobilized on Zeta Probe membranes (Bio-Rad) prepared using a Bio-Dot microfiltration apparatus (Bio-Rad). Hybridizations were carried out at 50°C for 16 h in 50% formamide, 1% SDS, 5× SSC (0.75 M NaCl, 0.075 M trisodium citrate), 5× Denhardt’s solution (1× Denhardt’s solution = 0.02% bovine serum albumin, 0.02% polyvinylpyrrolidone and 0.02% Ficoll). Membranes were then washed sequentially at room temperature for 15 min with each of the following solutions: 2× SSC + 0.1% SDS, 1× SSC + 0.1% SDS and 0.1× SSC + 0.1% SDS. Radioactive hybrids were detected and quantitated using a Molecular Dynamics PhosphorImager.
Chromatin immunoprecipitation (ChIP) assays
ChIP assays (63–65) were carried out using either anti-acetyl-histone H4 or anti-acetyl-histone H3 antibodies as described by the supplier (Upstate Biotechnology). NIH 3T3 cells were plated at low density (5 × 105 cells/100 mm dish) and treated with varying amounts of TSA. After 19 h, cells were crosslinked using 1% formaldehyde (Aldrich Chemical Co.). The cells collected from two 100 mm plates were subject to ChIP as recommended by the manufacturer. Following ChIP, the samples were incubated for 4 h at 65°C to reverse crosslinks and DNA isolated as described (27).
PCR analysis
Equal amounts of DNA purified from immunoprecipitated chromatin were amplified using AmpliTaq Gold Taq polymerase (Perkin Elmer) and two pairs of nested primers for the mouse rDNA promoter. The first pair of primers (primer 1, 5′-AAGCTATGGGCGCGGTTTTCT-3′; primer 2, 5′-GCCCACAGCAAGCACACG-3′) amplify the rDNA promoter region from –231 to + 187. The second pair of primers (primer 3, TTGTCAGGGTCGACCAGTTG; primer 4, AAGCACACGCAAGCAGCAAG) amplify the rDNA promoter region between –177 and +179. Results were quantitated using a Lumi-Imager (Boehringer).
Transfer and hybridization for ChIP analysis
A single-stranded ‘sense’ (to avoid hybridization to RNA) DNA probe was synthesized by asymmetrical PCR (66). The region between –231 and +179 was amplified with primers 1 and 2 (described above) and then amplified with primer 1 in the presence of 75 µCi [α-32P]dCTP, 20 µM dATP, dGTP and dTTP and 0.625 U Taq polymerase (Perkin Elmer) for 30 cycles of amplification. Each cycle consisted of 2 min at 94°C, 2 min at 58°C and 5 min at 72°C. Probe was purified from unincorporated nucleotides by gel filtration (STE Select-D G-25 column; 5 Prime–3 Prime).
Varying amounts of DNA isolated from chromatin were immobilized on Zeta Probe blotting membrane (Bio-Rad) using a Bio-Dot microfiltration apparatus (Bio-Rad). Membranes were either used immediately or baked overnight at 80°C and stored in plastic bags at room temperature for later use. Hybridization was performed at 65°C for 18–24 h in 1 mm EDTA, 7% SDS and 0.5 M Na2HPO4, pH 7.2. Membranes were washed twice for 30 min at 65°C in 1 mM EDTA, 40 mM Na2HPO4 and 5% SDS, followed by two washes for 30 min at 65°C in 1 mM EDTA, 40 mM Na2HPO4 and 1% SDS. Radioactive hybrids were detected and quantitated using a Molecular Dynamics PhosphorImager.
In vitro transcription
In vitro transcription was carried out as described previously (40). Some reactions contained, in addition, purified recombinant p300 or PCAF histone transacetylases. The radioactive products of the in vitro transcription reactions were separated by urea gel electrophoresis. The gel was dried and the 630 nt transcripts were detected and quantitated with a Molecular Dynamics PhosphorImager.
Immunoprecipitation and western blot analysis
Nuclear protein extracts were prepared from N1S1 rat hepatoma cells essentially as described (67). Following the final dialysis, aliquots of the extracts were stored at –80°C until used in immunoprecipitation. Immunoprecipitation with a rabbit polyclonal anti-UBF antiserum or a rabbit polyclonal anti-acetyl-lysine antiserum (Upstate Biotechnology) was performed without detergent as follows. An aliquot of 500 µg nuclear protein extract from rat hepatoma cells, diluted to 1 ml in PBS with protease inhibitors (100 µg/ml aprotinin, 10 µM leupeptin and 1 µg/ml pepstatin A), was precleared by tumbling for 30 min at 4°C with 50 µl of a 50% slurry of protein A–agarose beads (washed with PBS). The beads were removed by centrifugation (5 s pulse in a microcentrifuge) and precleared extracts were incubated overnight at 4°C with 5 µl of appropriate antibody. An aliquot of 50 µl of 50% protein A–agarose beads was then added and samples were tumbled for a further 2 h at 4°C. Beads were then collected by centrifugation as above and washed three times with 500 µl of PBS containing protease inhibitors (as above).
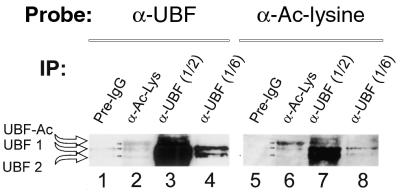
For western analysis the immunoprecipitates were resuspended in 60 µl of 6× SDS sample buffer, heated at 95°C for 10 min, fractionated by 10% SDS–PAGE and electroblotted onto Immobilon-P membranes (Millipore). The membranes were rinsed in PBS (for anti-UBF antibody) or Tris-buffered saline (TBS) (for anti-acetyl-lysine antibody) containing 0.05% Tween-20 (Sigma) and blocked for 1 h at room temperature in buffer A (PBS or TBS, 0.05% Tween-20 and 5% non-fat dry milk powder). Membranes were then incubated with the appropriate antibody in buffer A for either 1 h (rabbit polyclonal anti-UBF antibody and anti-histone H3; Upstate Biotechnology) at room temperature or for 16 h at 4°C (anti-acetyl-lysine antibody). All membranes were then washed three times in PBS or TBS containing 0.05% Tween-20 at room temperature and protein–antibody complexes were detected using ECL western blotting detection reagents (Amersham). The polyclonal rabbit antiserum to UBF was raised against recombinant protein (68). Although the samples were routinely precleared with protein A–agarose beads we have occasionally observed a very minor degree of non-specific binding of UBF to beads loaded with pre-immune sera. This non-specific binding is seen in Figure 7, lane 1. However, it represents a significantly lower amount of UBF than that precipitated with either the antibodies to UBF or antibodies to acetyl-lysine.
Figure 7.
Authentic UBF is acetylated. Proteins were immunopurified from rat hepatoma nuclear extracts using antibodies to UBF or acetyl-lysine bound to protein A–agarose beads (IP) as described in Materials and Methods. The immunoprecipitated proteins were washed, boiled in SDS buffer, fractionated by SDS–PAGE and transferred to Immobilon P membranes. One-half or one-sixth of the respective immunoprecipitates (as indicated) was loaded on the gel. Blots were probed using an antibody to either UBF or acetyl-lysine. The arrows indicate the different forms of UBF (UBF1, UBF2 and a slow migrating form of UBF that appeared to be hyperacetylated, UBF-Ac).
Immunocytochemistry
Mouse embryo fibroblast (MEF) cells grown on glass coverslips were first fixed in 4% paraformaldehyde for 1 h at room temperature and then permeabilized in PBS containing 0.1% Triton X-100, 0.1% sodium citrate and 0.1% BSA at 4°C for 30 min. The cells were then incubated with polyclonal rabbit antibodies directed against CBP (A22; Santa Cruz) and either mouse monoclonal anti-UBF1 or anti-nucleolin antibodies (Santa Cruz). Incubation with the primary antibodies was performed at 37°C for 1 h, followed by three washes in PBS containing 0.1% Triton X-100. The secondary anti-rabbit or anti-mouse antibodies were purchased from Molecular Probes (Alexa 488, green, and Alexa 568, red). Incubation with secondary antibodies was performed at 37°C for 20–30 min. Cells were washed three times, then incubated with DAPI at room temperature for 5 min. After washing, the coverslips were air dried and mounted for microscope analysis.
RESULTS
The effect of TSA on transcription from an rDNA reporter in transient transfection assays
The steady-state level of histone acetylation represents the net of acetylation and deacetylation. Therefore, to determine if acetylation has an effect on rDNA transcription we chose in our first experiments to inhibit histone deacetylase activity with TSA. NIH 3T3 cells were transfected with a rDNA reporter, pSMECAT, and treated with varying doses of TSA for 19 h. As shown in Figure 1, treatment with TSA results in a dose-dependent stimulation of expression from the rDNA reporter. This is consistent with the hypothesis that acetylation is involved in the regulation of rDNA transcription.
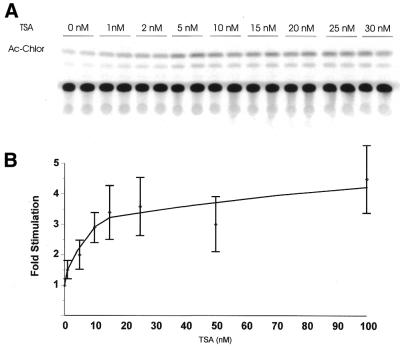
Figure 1.
TSA, an inhibitor of histone deacetylation, stimulates rDNA transcription in transient transfection assays. NIH 3T3 cells were transfected with 1 µg rDNA reporter (pSMECAT) for 5 h and then treated with the indicated concentrations of TSA. After 19 h, cell lysates were prepared and assayed for CAT activity as described in Materials and Methods. Results were visualized and quantified using a Molecular Dynamics PhosphorImager. (A) Image of a CAT assay from a typical experiment. (B) Graph depicting the effect of TSA on the expression of pSMECAT (means ± SD, n = 12).
Other reports have used TSA and transient transfections to successfully demonstrate a role for deacetylases in regulating specific gene expression (69–71). However, we examined the possibility that our observations might reflect a global effect of TSA on transcription. To control for this possibility, NIH 3T3 cells were transfected with the promoter for atrial natriuretic factor (ANP) coupled to a luciferase reporter and treated with TSA. Treatment with TSA did not significantly affect transcription from the ANP promoter (data not shown). This is consistent with other reports that TSA does not have a global effect on transcription.
In the course of these experiments we observed variations in the dose–response curve. In most experiments the maximum stimulatory dose was between 10 and 25 nM TSA. However, in some experiments the maximum stimulatory dose was 100 nM. We found that some of this variability was due to the lot of TSA used, but the greatest cause of variability appeared to correlate with the age of the cells. Cells passed in culture less than 20 times responded to a lower dose of TSA than cells cultured longer. This observation could reflect an increased amount of deacetylase activity in aging cultured NIH 3T3 cells or a decreased permeability to TSA. The former possibility might correlate with a set of observations from Guarente’s laboratory (72). They observed an increase in yeast NAD-dependent histone deacetylase (a TSA-resistant acetylase), the protein product of the Sir2 gene, with longevity (72). Be that as it may, when either low passage or high passage number NIH 3T3 cells were treated with TSA, expression from the rDNA reporter was stimulated. In all other experiments we used cells cultured for no more than 15 passages.
The effect of TSA treatment on rDNA transcription from an endogenous gene
The transient transfection experiments suggested that histone deacetylase activity repressed transcription from the rDNA reporter. Nuclear run-on assays were carried out to determine whether the inhibition of histone deacetylase activity would activate transcription from endogenous rDNA. As shown in Figure 2, treatment with TSA resulted in a dose-dependent increase in the number of counts that hybridized to the 5′-external transcribed spacer of the rDNA repeat, i.e. TSA stimulated transcription from the endogenous rRNA genes. A 4-fold stimulation of rDNA transcription was observed at 5 nM TSA. This was in good agreement with the maximum fold stimulation of rDNA transcription from the rDNA reporter. In parallel we determined that TSA did not affect transcription from the endogenous GAPDH gene (data not shown). As TSA stimulated transcription from both the rDNA reporter and the endogenous rDNA genes, our results were consistent with the hypothesis that acetylation may play a role in rDNA transcription.
Figure 2.

TSA stimulates transcription from the rRNA genes. Nuclei were isolated from NIH 3T3 cells treated for 24 h with the indicated concentrations of TSA and rDNA transcription was measured by nuclear run-on assay as described in Materials and Methods. In vitro labeled RNA was isolated using TRIzol reagent and purified by ethanol precipitation and/or Sephadex G-25 columns. The transcripts were hybridized to a fragment of the mouse rDNA promoter (–168 to +292) (10 µg DNA) immobilized on Zeta-Probe membrane. The results were visualized with a Molecular Dynamics PhosphorImager. (Upper) The results of a typical experiment. (Lower) The results from repeated experiments were quantitated and plotted relative to the control. Each point represents the mean ± SD (n = 7).
The effect of TSA treatment on acetylation state of histone H4 in rDNA chromatin
The above data demonstrate a role for acetylation in the regulation of rDNA transcription, but did not identify the possible targets of this acetylation. As mentioned, TSA has been used to study the role of histone acetylation in transcription. Histone acetylation, resulting in an alteration in chromatin structure, has been linked to the stimulation of transcription both for endogenous genes and transfected DNA (73–75). However, the above experiments do not discriminate between a direct effect of TSA on rDNA transcription or an indirect effect. Therefore, it was of interest to determine if there was an accumulation of acetylated histones on the rDNA, as that would represent a direct effect of TSA treatment.
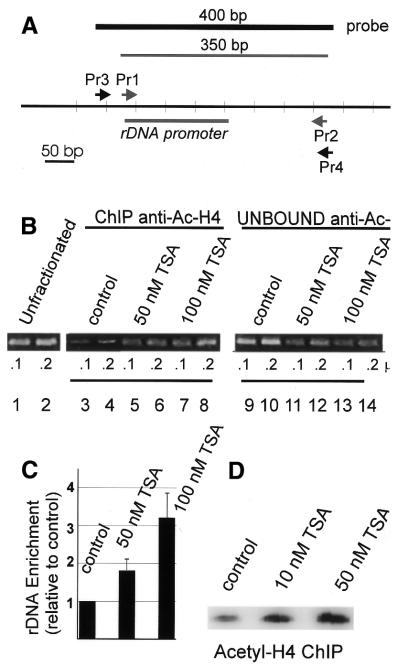
Chromatin immunoprecipitation. ChIP experiments were carried out using chromatin from control and TSA-treated cells. NIH 3T3 cells were treated with various doses of TSA, crosslinked with formaldehyde and the chromatin subjected to immunoprecipitation with either anti-acetyl H4 or anti-acetyl H3 antibodies, as described in Materials and Methods. Following the immunoprecipitation reactions, the crosslinks were reversed, the DNA was purified and equal aliquots of the purified DNA from the control and TSA-treated cells were analyzed either by PCR (Fig. 3B and C) using primers that flanked the transcription initiation site or by hybridization (Fig. 3D) with a probe for the rDNA promoter region. The primers and probes used in the analyses are depicted in Figure 3A.
Figure 3.
TSA treatment results in accumulation of acetylated histone H4 in rDNA chromatin. Analysis of DNA immunoprecipitated with anti-acetyl-histone H4 antibody (Upstate Biotechnology) from chromatin isolated from TSA-treated or control NIH 3T3 cells. Cells were crosslinked in situ, collected and lysed. Resulting protein–DNA complexes were sheared by sonication and immunoprecipitated with antibody to acetylated histone H4 as described in Materials and Methods. After recovery of DNA was determined by spectrophotometry, recovery of rDNA was determined by PCR or dot blotting. (A) Scheme of PCR primers used in these experiments and the rDNA promoter. The resulting size of each product is indicated. (B) Nested PCR analysis of ChIP DNA from control and TSA-treated cells. The first round of PCR was performed with primer pair 3 and 4 and the second round with primer pair 1 and 2. The 350 bp final product is shown. (C) Three independent PCR/ChIP experiments similar to those described in (B) were visualized and quantitated using a Molecular Dynamics scanning densitometer. The results are expressed relative to the level of PCR product in the control sample (means ± SD). (D) Hybridization analysis of ChIP DNA immunoprecipitated from control and TSA-treated cells. After the DNA was immunoprecipitated and purified, it was immobilized on Zeta Probe membranes. The membranes were probed with a 400 bp fragment (PCR primers 3 and 4) synthesized in the presence of [α-32P]dCTP.
PCR analysis of the ChIP experiments was carried out with nested primers (primer pairs 3 and 4 and 1 and 2; Fig. 3A). We analyzed the input DNA, the DNA obtained by ChIP and the DNA that did not immunoprecipitate. Two aliquots of each DNA were subject to analysis to ensure that the amount of PCR product obtained was proportional to the input DNA. As shown in Figure 3B, lanes 3–8, treatment with increasing amounts of TSA resulted in precipitation of increasing amounts of rDNA in the samples precipitated with anti-acetyl-histone H4. Conversely, treatment with TSA resulted in an apparent depletion of the rDNA from the DNA remaining in solution after anti-acetyl-histone H4 antibody immunoprecipitation (lanes 9–14). Several independent PCR/ChIP experiments were quantitated, and the means ± SD are presented in Figure 3C. We did not detect any significant effect of TSA on the amount of acetyl-histone H3 in rDNA chromatin (data not shown). To complement the PCR analyses, we analyzed the ChIP DNA by hybridization. In these experiments, equal amounts of input and anti-acetyl H4 antibody immunoprecipitated DNA from control cells and cells treated with TSA were immobilized on membranes and hybridized to a radioactive probe for the mouse rDNA promoter (Fig. 3D). Quantitation of the blots demonstrated an enrichment of rDNA in the ChIP DNA after treatment with TSA.
Both the PCR and hybridization analyses demonstrated that TSA resulted in an increased association of the rDNA with acetylated H4 histone. These data suggest that the activation of rDNA transcription observed when cells are treated with TSA is associated with an alteration in acetylation state of the ribosomal chromatin. This suggests that the activation of rDNA transcription reflects a direct effect of TSA on the ribosomal chromatin.
The effect of TSA on inhibition of rDNA transcription by Rb
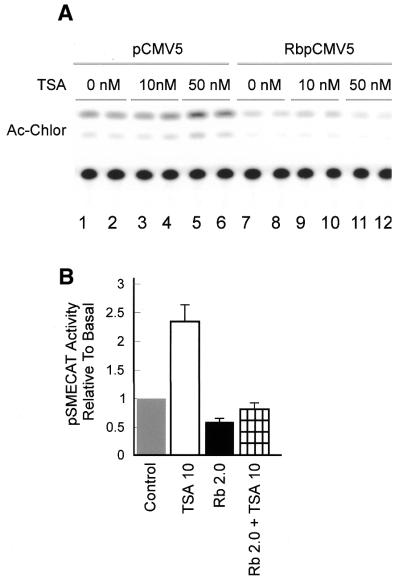
The observation that inhibiting deacetylation affected DNA transcription led us to examine the hypothesis that Rb might act to inhibit rDNA transcription not only by inhibiting UBF, but also by recruiting histone deacetylase to the ribosomal chromatin. To investigate this hypothesis we determined whether TSA would abrogate the inhibitory effect of Rb on rDNA transcription. The overexpression of Rb inhibits rDNA transcription (Fig. 4A, compare the CAT assays in lanes 1 and 2 with those in lanes 7 and 8) as previously reported (54–56). However, the Rb-dependent inhibition of rDNA transcription was not reversed when the cells were exposed to TSA (compare the CAT assays in lanes 9–12 with those presented in lanes 3–6). These results, which are summarized in Figure 4B, suggest the primary mechanism by which Rb inhibits rDNA transcription does not involve deacetylation of the ribosomal chromatin. If one examines these experiments from another perspective, one sees that overexpression of Rb is sufficient to inhibit the TSA-dependent stimulation of rDNA transcription. This would be expected if the primary mechanism of action of Rb is to block the interaction of UBF with the transcription pre-initiation complex, as has been proposed (54,55).
Figure 4.
TSA does not reverse the inhibition of rDNA transcription by Rb in transient transfection assays. NIH 3T3 cells were transfected with 2 µg rDNA reporter (pSMECAT) and 2 µg DNA of an Rb expression plasmid or empty vector (pCMV5) for 5 h and then treated with the indicated concentrations of TSA. After 19 h, cell lysates were prepared and assayed for CAT activity as described in Materials and Methods. Results were visualized and quantitated using a Molecular Dynamics PhosphorImager. (A) CAT assays from a typical experiment. (B) Three independent experiments similar to those described in (A) were quantitated, adjusted for protein in the assay and expressed as a fraction of CAT activity (± SEM) observed in the control sample.
The effects of histone acetyltransferases CBP, p300 and PCAF on rDNA transcription in vivo
Our data are consistent with the presence of acetylated histones in the rDNA chromatin and with a role for histone acetylation in the regulation of rDNA transcription. In the above experiments we examined the effect of inhibiting histone deacetylation on rDNA transcription. The converse of these experiments would be to shift the steady-state level of acetylation by over-xpressing histone acetyltransferase. Consequently, we co-transfected NIH 3T3 cells with expression vectors encoding either CBP (subcloned from CBPpRc/RSV, a generous gift from H. Goodman), p300, PCAF or PCAF with deleted histone acetyltransferase activity (PCAF delHAT) and determined their effects on rDNA transcription. As shown in Figure 5, overexpression of p300 significantly stimulated rDNA transcription. Overexpression of either PCAF or CBP had less prominent effects. These results agree with the observation that overexpression of CBP stimulates rDNA transcription (76) and with the model that increasing the relative ratio of histone acetylase to histone deacetylase activity would stimulate rDNA transcription.
Figure 5.
Overexpression of histone acetyltransferases stimulates rDNA transcription in transient transfection assays. NIH 3T3 cells were transfected with 1 µg rDNA reporter (pSMECAT) and different amounts (0.5, 1.0 or 2.0 µg DNA) of p300, PCAF, PCAF delHAT or CBP expression plasmids (all in pCDNA3.1). After 24 h, cell lysates were prepared and assayed for CAT activity. The results were visualized, normalized and quantitated using a Molecular Dynamics PhosphorImager. (Upper) CAT assays from a typical experiment. (Lower) A graph summarizing the CAT assays from three experiments (means ± SD). The results were normalized to the results obtained with pCAF delHAT.
The effect of purified histone transacetylases on rDNA transcription in vitro
Histone acetyltransferases are known to be able to acetylate proteins other than histones, including some transcription factors, e.g. p53 (77,78). Thus, it was possible that at least part of the effect of TSA or overexpressing histone transacetylases might be due to modification of one of the rDNA transcription factors. To examine this question, we added purified recombinant acetyltransferases p300 and PCAF to an in vitro transcription system using a nuclear extract. These assays were carried out in the absence of the core histones (western blot analysis did not detect histone H3 in our nuclear extract; data not shown). Thus, any change in transcription would be due to the modification of one or more of the components of the RPI transcriptional machinery. As shown in Figure 6, the addition of p300 to in vitro transcription reactions resulted in a small (2-fold), but reproducible, stimulation of rDNA transcription. In contrast, we could not detect any effect of adding PCAF. With the caveat that we have added purified individual components of what are, in most instances, macromolecular complexes (79), these results suggest that one of the components of the rDNA transcription apparatus may be a target for acetyltransferase activity. This would suggest that at least one rDNA transcription factor, as well as the ribosomal chromatin, may be the target(s) for histone acetyltransferase activity.
Figure 6.
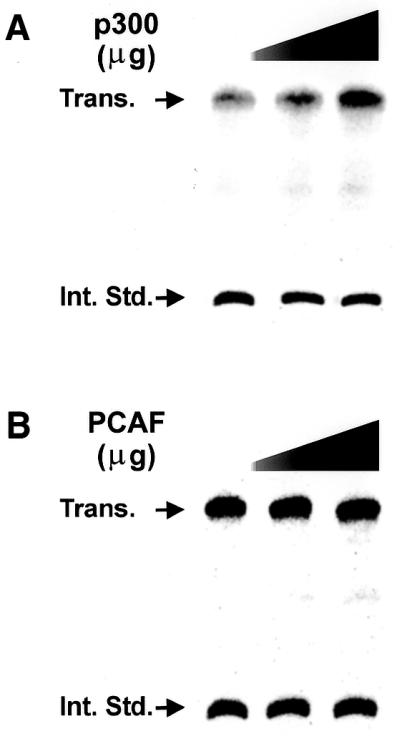
Histone acetyltransferase p300, but not PCAF, stimulates rDNA transcription in vitro. Truncated template assays were carried out using extracts from N1S1 cells, the rat rDNA promoter (–286 +624) and increasing amounts of p300 and PCAF as described in Materials and Methods. After the transcription reaction, an internal standard was added to correct for the recovery of transcripts and the transcripts were purified by phenol extraction and ethanol precipitation. The transcripts were resolved by electrophoresis on urea–acrylamide gels, the gels were dried and and transcription quantitated with a Molecular Dynamics PhosphorImager. The results in each reaction were normalized for recovery of the internal standard and stimulation was determined by comparison of the ratios of the authentic transcript to the internal standard. (A) The in vitro transcription assay shows stimulation of rDNA transcription by p300. The assays included 1.5 and 2.0 µl of affinity-purified p300 (concentration ∼1 mg/ml). (B) Transcription in the presence of 1 and 2 µl (concentration ∼1mg/ml) of affinity-purified PCAF.
Acetylation of UBF
Our in vitro results suggest that acetylation may affect the activity of one or more of the rDNA transcriptional factors. Previous research from our own and other laboratories (31–33) have demonstrated UBF to be a target of various signal transduction cascades. Furthermore, UBF has been shown to interact with Rb and CBP (76) and thus, by analogy with E2F, it may also be a target for regulation by acetylation/deacetylation. Therefore, we investigated whether UBF might be acetylated in vivo. In these experiments (Fig. 7) nuclear extracts of rat hepatoma cells were incubated with either anti-UBF or anti-acetyl-lysine antibodies and the immunopreciptates were analyzed by western blot analysis using antibodies to either UBF (lanes 1–4) or acetyl-lysine (lanes 5–8). The antibodies to acety-lysine recognized both UBF1 and UBF2 in the immunoprecipitates obtained with anti-UBF antibodies (lanes 7 and 8). Further, the anti-acetyl-lysine antibodies immunoprecipitated several proteins recognized by anti-UBF antibodies (lane 2). The data demonstrate that both UBF1 and UBF2 are acetylated (lanes 2 and 8). Interestingly, the anti-acetyl-lysine antibodies immunoprecipitated a band that migrates more slowly than the major forms of UBF (lane 2, UBF-Ac) and also recognized two proteins in the samples immunoprecipitated by anti-UBF that migrate more slowly than the major forms of UBF. If these bands are modified forms of UBF, we have no explanation for this apparently anomalous change in migration. Although acetylation does not usually affect the mobility of proteins, the observed change could be caused by high levels of acetylation and/or the combination of acetylation and other modifications (particularly phosphorylation). In this regard, it should be noted that UBF migrates anomalously on SDS–PAGE. The predicted mass for UBF1 is 87 kDa, but the protein migrates at 97 kDa. Our data suggest that only a small part of the UBF population is acetylated and that the population of UBF present in the cell is a mixture of UBF molecules acetylated to different levels. Although we do not know the biological significance of UBF acetylation, this could represent an additional mechanism for regulating UBF activity.
Localization of CBP in the nucleolus
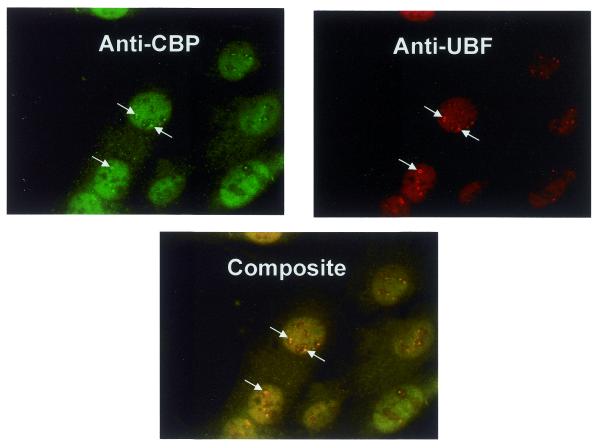
The above results suggest that histone acetyltransferases play a role in RPI transcription, but they do not necessarily mean that the histone acetyltransferases that we used in our experiments, namely CBP, p300 and PCAF, are involved in this process in vivo. If we were to find a histone acetyltransferase activity in the nucleolus, that would provide evidence that it might be involved in rDNA transcription. Therefore, we stained MEF cells with antibodies directed against CBP, p300 and PCAF and either anti-UBF1 or anti-nucleolin antibodies. As shown in Figure 8, we were able to demonstrate that CBP co-localizes with UBF in the nucleolus. Similar immunolocalization experiments have been carried out using anti-p300 antibodies. Although those experiments did not definitively demonstrate the localization of p300 to the nucleolus (possibly due to the quality of the antibodies), we have been able to demonstrate that when p300 is overexpressed it can be found in the nucleolus (data not shown).
Figure 8.
CBP is present in the nucleolus. MRF cells were stained with either anti-CBP (green) or anti-UBF antibody (red) as described in Materials and Methods. In the merged image yellow denotes co-localization of CBP and UBF in the nucleolus.
DISCUSSION
Using both reporter constructs and the endogenous genes we have demonstrated that manipulation of the balance of the state of histone acetylase/deacetylase activities in a cell affects rDNA transcription. The stimulation of rDNA transcription in response to the deacetylase inhibitor TSA is accompanied by accumulation of acetylated histone H4 in the ribosomal chromatin. This accumulation of acetylated histone associated with the rDNA in response to TSA indicates that alteration of the acetylation state of the ribosomal chromatin can directly affect rDNA transcription. This in turn suggests that the structure of ribosomal chromatin is dynamic and not fixed and that there is the potential for changes in the acetylation status of the ribosomal chromatin in response to physiological or biological stimuli.
Our results also provide some interesting correlations with other observations. Low passage number NIH 3T3 cells demonstrated a maximum response at a lower dose of TSA than that required for the maximum response to TSA of high passage number cells. Recent data connect yeast NAD-dependent histone deacetylase, the protein product of the Sir2 gene, with longevity (72). Interestingly, one function of this gene product is silencing of the ribosomal chromatin (for references see 72). One way in which cultured cells, such as NIH 3T3 cells, differ from primary cells is that they are considered to be immortal. Although Sir2p deacetylase is TSA-insensitive, we would argue by analogy that accumulation of some deacetylase activity as the cells ‘age’ in culture might contribute to this phenomenon. Thus, in high passage number cells a higher dose of TSA would be needed to ‘unmask’ the role of acetylation. Another explanation for the correlation of dose–response characteristics and passage number would be that high passage number cells might be less permeable to TSA than are low passage number cells.
We also noted that addition of TSA had no effect on rDNA transcription in confluent cells (data not shown). Interestingly, there is a correlation between the reduction in rDNA transcription associated with confluence-induced cell cycle arrest and an increased association of Rb and the Rb-related protein p130 with UBF in cultured NIH 3T6 cells (55). At least part of the decreased rate of rDNA transcription associated with cell confluence may be attributed to the association of Rb and/or p130 with UBF (54). Rb has been shown to bind to other transcription activators, e.g. E2F, and histone deacetylases simultaneously, resulting in repression of gene expression (57,58). It may be that inhibition of rDNA transcription by Rb results in an elevated level of histone deacetylase associated with the ribosomal chromatin in confluent cells that is too high to respond to TSA. However, our observation that TSA did not reverse the inhibition of transcription by Rb suggests that the primary effect of Rb is to inhibit UBF activity. This does not negate the possibility that Rb may recruit histone deacetylases to ribosomal chromatin. The role of the deacetylases may be to deacetylate UBF (76) and/or SL-1 (80), in coordination with inhibition of UBF-dependent transcription.
The observed stimulation of rDNA transcription by three known histone acetyltransferases, p300, PCAF (this report) and CBP (76), does not necessarily mean that these proteins contribute to the regulation of rDNA transcription in vivo. However, it is consistent with the hypothesis that acetylation plays a role in rDNA transcription. Moreover, our observation that CBP can be demonstrated within the nucleolus strongly suggests that at least CBP is involved in ribosomal transcription in vivo. In addition, the fact that histone acetylases had only a minimal effect on rDNA transcription in vitro in the absence of histones suggests that the main effect of acetylation depends on chromatin. On the other hand, histone acetylases exist in complexes, e.g. PCAF activity itself was recognized as a complex of about 20 proteins (79), including p300. Thus, the lack of an effect in vitro may reflect the lack of one or more of the components of the acetyltransferase complex. Moreover, the observation that CBP can acetylate UBF in vitro (76) and that authentic UBF is acetylated suggest that acetylation may affect at least one of the components of the RPI transcriptional machinery. Recent reports have demonstrated that HMG proteins can be acetylated and that acetylation affects their DNA-bending activity (77,78). UBF contains multiple HMG boxes that may represent the sites of acetylation, and it has been demonstrated that UBF can affect the structure of chromatin. Hence, it is possible that the effect of altering the acetylation state of UBF may affect the nature of the rDNA chromatin.
Jacobson et al. (81) proposed a model linking histone acetyltransferase activities with core promoter recognition by TFIID. They demonstrated a new role for TAF II 250, which has histone acetyltransferase activity (82). TAF II 250 contains a double bromodomain, a structural motif characteristic of proteins involved in chromatin-dependent regulation of transcription. Bromodomain proteins have been identified as integral components of chromatin remodeling complexes and frequently possess histone acetyltransferase activity. This motif in TAF 250 recognizes very specific, transcriptionally relevant acetylation patterns in histone H4. Jacobson et al. (81) suggested that these bromodomains target TFIID to promoters that are organized with acetylated nucleosomes. By analogy, it may be that UBF complexed with a histone acetyltransferase or bromodomain-containing protein might be ‘flagged’ by acetyl-histone H4 on the rDNA promoter. This might then further alter the structure of ribosomal chromatin and stabilize assembly of the pre-initiation complex.
Acknowledgments
ACKNOWLEDGEMENTS
This work was supported by grants GM46991 and 5R01CA83979 from the National Institutes of Health to L.I.R. and M.L.A., respectively, and by funds from the Geisinger Foundation (L.I.R.).
References
- 1.Moss T. and Stefanovsky,V.Y. (1995) Promotion and regulation of ribosomal transcription in eukaryota by RNA polymerase I. Prog. Nucleic Acid Res. Mol. Biol., 50, 25–66. [DOI] [PubMed] [Google Scholar]
- 2.Hannan K., Hannan,R.D. and Rothblum,L.I. (1998) Transcription by RNA polymerase I. Front. Biosci., 3, d376–d398. [DOI] [PubMed] [Google Scholar]
- 3.Paule M.R. (1998) Transcription of Ribosomal RNA Genes by Eucaryotic RNA Polymerase I. Springer-Verlag and R.G. Landes, Georgetown, TX.
- 4.Paule M.R. and White R.J. (2000) Transcription by RNA polymerase I and III. Nucleic Acids Res., 28, 1283–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reeder R.H. (1999) Regulation of RNA polymerase I transcription in yeast and vertebrates. Prog. Nucleic Acid Res. Mol. Biol., 62, 293–327. [DOI] [PubMed] [Google Scholar]
- 6.Grummt I. (1999) Regulation of mammalian ribosomal gene transcription by RNA polymerase I. Prog. Nucleic Acid Res. Mol. Biol., 62, 109–154. [DOI] [PubMed] [Google Scholar]
- 7.Grana X., Garriga,J. and Mayol,X. (1998) Role of the retinoblastoma protein family, pRB, p107 and p130 in the negative control of cell growth. Oncogene, 17, 3365–3383. [DOI] [PubMed] [Google Scholar]
- 8.Han M. and Grunstein,M. (1988) Nucleosome loss activates yeast downstream promoters in vivo. Cell, 55, 1137–1145. [DOI] [PubMed] [Google Scholar]
- 9.Clark-Adams C.D., Norris,D., Osley,M.A., Fassler,J.S. and Winston,F. (1988) Changes in histone gene dosage alter transcription in yeast. Genes Dev., 2, 150–159. [DOI] [PubMed] [Google Scholar]
- 10.Wolffe A.P. and Hayes,J.J. (1999) Chromatin disruption and modification. Nucleic Acids Res., 27, 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolffe A.P. (1998) Chromatin Structure and Function. Academic Press, San Diego, CA.
- 12.Grunstein M. (1997) Histone acetylation in chromatin structure and transcription. Nature, 389, 349–352. [DOI] [PubMed] [Google Scholar]
- 13.Wolffe A.P. (1999) Gene control by targeted histone acetylation. Curr. Biol., 8, 422–424. [DOI] [PubMed] [Google Scholar]
- 14.Vignali M., Hassan,A.H., Neely,K.E. and Workman,J.L. (2000) ATP-dependent chromatin-remodeling complexes. Mol. Cell. Biol., 20, 1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vidali G., Boffa,L.C., Bradbury,E.M. and Allfrey,V.G. (1978) Butyrate suppression of histone deacetylation leads to accumulation of multiacetylated forms of histones H3 and H4 and increased DNase I sensitivity of the associated DNA sequences. Proc. Natl Acad. Sci. USA, 75, 2239–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson D., Perry,M.E. and Chalkley,R. (1979) A correlation between nucleosome spacer region susceptibility to DNase I and histone acetylation. Nucleic Acids Res., 6, 561–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bode J., Henco,K. and Wingender,E. (1980) Modulation of the nucleosome structure by histone acetylation. Eur. J. Biochem., 110, 143–152. [DOI] [PubMed] [Google Scholar]
- 18.Wang L., Liu,L. and Berger,S. (1998) Critical residues for histone acetylation by Gcn5p, functioning in Ada and Saga complexes are also required for transcriptional function in vivo. Genes Dev., 12, 640–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuo M.-H., Zhou,J., Jambeck,P., Churchill,M.E.A. and Allis,C.D. (1998) Histone acetyltransferase activity of yeast Gcn5p is required for the activation of target genes in vivo. Genes Dev., 12, 627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tse C., Georgieva,E.I., Ruiz-Garcia,A.B., Sendra,R. and Hansen,J.C. (1998) Gcn5p, a transcription-related histone acetyltransferase, acetylates nucleosomes and folded nucleosomal arrays in the absence of other protein subunits. J. Biol. Chem., 273, 32388–32392, [DOI] [PubMed] [Google Scholar]
- 21.Conconi A., Widmer,R.M., Koller,T.H. and Sogo,J.M. (1989) Two different chromatin structures coexist in ribosomal RNA genes throughout the cell cycle. Cell, 57, 753–761. [DOI] [PubMed] [Google Scholar]
- 22.Luccini R. and Sogo,J.M. (1992) Different chromatin structures along the spacers flanking active and inactive Xenopus rRNA genes. Mol. Cell. Biol., 12, 4288–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dammann R., Lucchini,R., Koller,T. and Sogo,J.M. (1993) Chromatin structures and transcription of rDNA in yeast Saccharomyces cerevisiae. Nucleic Acids Res., 10, 2331–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dammann R., Luchini,R., Koller,T. and Sogo,J.M. (1995) Transcription in the yeast rRNA gene locus: distribution of the active gene copies and chromatin structure of their flanking regulatory sequences. Mol. Cell. Biol., 15, 5294–5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stancheva I., Lucchini,R., Koller,T. and Sogo,J.M. (1997) Chromatin structure and methylation of rat rRNA genes studied by formaldehyde fixation and psoralen cross-linking. Nucleic Acids Res., 25, 1727–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucchini T. and Sogo,J.M. (1998) In Paule,M.P. (ed.), Transcription of Ribosomal RNA Genes by Eucaryotic RNA Polymerase I. Springer-Verlag and R.G. Landes, Georgetown, TX.
- 27.Mutskov V.J., Russanova,V.R., Dimitrov,S.I. and Pashev,I.G. (1996) Histones associated with nonnucleosomal rat ribosomal genes are acetylated while those bound to nucleosome-organized gene copies are not. J. Biol. Chem., 271, 11852–11857. [DOI] [PubMed] [Google Scholar]
- 28.Prior C.P., Cantor,C.,R. Johnson,E.M., Littau,V.C. and Allfrey,V.G. (1983) Reversible changes in nucleosome structure and histone H3 accessibility in transcriptionally active and inactive states of rDNA chromatin. Cell, 34, 1033–1042. [DOI] [PubMed] [Google Scholar]
- 29.Boffa L.C., Vidali,G., Mann,R.S. and Allfrey,V.G. (1978) Suppression of histone deacetylation in vivo and in vitro by sodium butyrate. J. Biol. Chem., 253, 3364–3366. [PubMed] [Google Scholar]
- 30.Chen Z.J. and Pikaard,C.S. (1997) Epigenetic silencing of RNA polymerase I transcription: a role for DNA methylation and histone modification in nucleolar dominance. Genes Dev., 11, 2124–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hannan R.D., Luyken,J. and Rothblum,L.I. (1995) Regulation of rDNA transcription factors during cardiomyocyte hypertrophy induced by adrenergic agents. J. Biol. Chem., 270, 8290–8297. [DOI] [PubMed] [Google Scholar]
- 32.Glibetic M., Taylor,L., Larson,D., Hannan,R., Sells,B. and Rothblum,L. (1995) The RNA polymerase I transcription factor UBF is the product of a primary response gene. J. Biol. Chem., 270, 4209–4212. [DOI] [PubMed] [Google Scholar]
- 33.Luyken J., Hannan,R.D., Cheung,J.Y. and Rothblum,L.I. (1996) Regulation of rDNA transcription during endothelin-1-induced hypertrophy of neonatal cardiomyocytes. Hyperphosphorylation of upstream binding factor, an rDNA transcription factor. Circ. Res., 78, 354–361. [DOI] [PubMed] [Google Scholar]
- 34.Hisatake K., Nishimura,T., Maeda,Y., Hanada,K., Song,C.Z. and Muramatsu,M. (1991) Cloning and structural analysis of cDNA and the gene for mouse transcription factor UBF. Nucleic Acids Res., 19, 4631–4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Mahony D.J. and Rothblum,L.I. (1991) Identification of two forms of the RNA polymerase I transcription factor UBF. Proc. Natl Acad. Sci. USA, 88, 3180–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Putnam C.D. and Pikaard,C.S. (1992) Cooperative binding of the Xenopus RNA polymerase I transcription factor xUBF to repetitive ribosomal gene enhancers. Mol. Cell. Biol., 12, 4970–4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuhn A., Voit,R., Stefanovsky,V., Evers,R., Bianchi,M. and Grummt,I. (1994) Functional differences between the two splice variants of the nucleolar transcription factor UBF: the second HMG box determines specificity of DNA binding and transcriptional activity. EMBO J., 13, 416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paule, M.R.(1994) Transcription of ribosomal RNA by eucaryotic RNA polymerase I. In Conaway,R.C. and Conaway,J.W. (eds), Transcription: Mechanisms and Regulation. Raven Press, New York, NY.
- 39.Jantzen H.M., Admon,A., Bell,S.P. and Tjian,R. (1990) Nucleolar transcription factor hUBF contains a DNA-binding motif with homology to HMG proteins. Nature, 344, 830–836. [DOI] [PubMed] [Google Scholar]
- 40.Smith S.D., Oriahi,E., Lowe,D., Yang-Yen,H.-F., O’Mahony,D., Rose,K., Chen,K. and Rothblum,L.I. (1990) Characterization of factors that direct transcription of rat ribosomal DNA. Mol. Cell. Biol., 10, 3105–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie W.Q. and Rothblum,L.I. (1992) Domains of the rat rDNA promoter must be aligned stereospecifically. Mol. Cell. Biol., 12, 1266–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hannan R.D., Luyken,J. and Rothblum,L.I. (1996) Regulation of ribosomal DNA transcription during contraction-induced hypertrophy of neonatal cardiomyocytes. J. Biol. Chem., 271, 3213–3220. [DOI] [PubMed] [Google Scholar]
- 43.Hannan R., Stefanovsky,V., Arino,T., Rothblum,L. and Moss,T. (1999) Cellular regulation of ribosomal DNA transcription: both rat and Xenopus UBF1 stimulate rDNA transcription in 3T3 fibroblasts. Nucleic Acids Res., 27, 1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McStay B., Frazier,M.W. and Reeder,R.H. (1991) xUBF contains a novel dimerization domain essential for RNA polymerase I transcription. Genes Dev., 5, 1957–1968. [DOI] [PubMed] [Google Scholar]
- 45.Jantzen H.M., Chow,A.M., King,D.S. and Tjian R. (1992) Multiple domains of the RNA polymerase I activator hUBF interact with the TATA-binding protein complex hSL1 to mediate transcription. Genes Dev., 6, 1950–1963. [DOI] [PubMed] [Google Scholar]
- 46.Copenhaver G.P., Putnam,C.D., Denton,M.L. and Pikaard,C.S. (1994) The RNA polymerase I transcription factor UBF is a sequence-tolerant HMG-box protein that can recognize structured nucleic acids. Nucleic Acids Res., 22, 2651–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Learned R.M., Learned,T.K., Haltiner,M.M. and Tjian,R.T. (1986) Human rRNA transcription is modulated by the coordinate binding of two factors to an upstream control element. Cell, 45, 847–857. [DOI] [PubMed] [Google Scholar]
- 48.Bell S.P., Learned,R.M., Jantzen,H.M. and Tjian,R. (1988) Functional cooperativity between transcription factors UBF1 and SL1 mediates human ribosomal RNA synthesis. Science, 241, 1192–1197. [DOI] [PubMed] [Google Scholar]
- 49.McStay B., Hu,C.H., Pikaard,C.S. and Reeder,R.H. (1991) xUBF and Rib 1 are both required for formation of a stable polymerase I promoter complex in X.laevis. EMBO J., 10, 2297–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beckmann H., Chen,J.L., O’Brien,T. and Tjian,R. (1995) Coactivator and promoter-selective properties of RNA polymerase I TAFs. Science, 270, 1506–1509. [DOI] [PubMed] [Google Scholar]
- 51.Hempel W.M., Cavanaugh,A.H., Hannan,R.D., Taylor,L. and Rothblum,L.I. (1996) The species-specific RNA polymerase I transcription factor SL-1 binds to upstream binding factor. Mol. Cell. Biol., 16, 557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grosschedl R., Giese,K. and Pagel,J. (1994) HMG domain proteins: architectural elements in the assembly of nucleoprotein structures. Trends Genet., 10, 94–100. [DOI] [PubMed] [Google Scholar]
- 53.Kermekchiev M., Workman,J.L. and Pikaard,C.S. (1997) Nucleosome binding by the polymerase I transactivator upstream binding factor displaces linker histone H1. Mol. Cell. Biol., 50, 5833–5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cavanaugh A.H., Hempel,W.M., Taylor,L.J., Rogalsky,V., Todorov,G. and Rothblum,L.I. (1995) Activity of RNA polymerase I transcription factor UBF blocked by Rb gene product. Nature, 374, 177–180. [DOI] [PubMed] [Google Scholar]
- 55.Hannan K.M., Kennedy,B.K., Cavanaugh,A.H., Hannan,R.D., Hirschler-Laszkiewicz,I., Jefferson,L.S. and Rothblum,L.I. (2000) RNA polymerase I transcription in confluent cells: Rb downregulates rDNA transcription during confluence-induced cell cycle arrest. Oncogene, 19, 3487–3497. [DOI] [PubMed] [Google Scholar]
- 56.Voit R., Schafer,K. and Grummt,I. (1997) Mechanism of repression of RNA polymerase I transcription by the retinoblastoma protein. Mol. Cell. Biol., 17, 4230–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chellappan S.P., Hiebert,S., Mudryj,M., Horiwitz,J.M. and Nevins,J.R. (1991) The E2F transcription factor is a cellular target for the RB protein. Cell, 65, 1053–1061. [DOI] [PubMed] [Google Scholar]
- 58.Brem A., Miska,E.A., McCance,D.J., Reid,J.L., Bannister,A.J. and Kouzarides,T. (1998) Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature, 391, 597–601. [DOI] [PubMed] [Google Scholar]
- 59.Magnaghi-Jaulin L., Groisman,R., Naguibneva,I., Robin,P., Lorain,S., Le Villain,J.P., Troalen,F., Trouche,D. and Harel-Bellan,A. (1998) Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature, 391, 601–605. [DOI] [PubMed] [Google Scholar]
- 60.Hannan R.D., Stefanovsky,V., Taylor,L., Moss,T. and Rothblum,L.I. (1996) Overexpression of the transcription factor UBF1 is sufficient to increase ribosomal DNA transcription in neonatal cardiomyocytes. Implications for cardiac hypertrophy. Proc. Natl Acad. Sci. USA, 93, 8750–8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ausubel F., Brent,R., Kingston,R., Moore,D., Seidman,J., Smith,J. and Struhl,K. (1993) Current Protocols in Molecular Biology. Wiley, New York, NY.
- 62.Cesarone C.F., Bolognesi,C. and Santi,L. (1979) Improved micro fluorometric DNA determination in biological material using 33258 Hoechst. Anal. Biochem., 100, 188–197. [DOI] [PubMed] [Google Scholar]
- 63.Braunstein M., Rose,A.B., Holmes,S.G., Allis,C.D. and Broach,J.R. (1993) Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev., 7, 592–604. [DOI] [PubMed] [Google Scholar]
- 64.Hebbes T.R., Thorne,A.W. and Crane-Robinson,C. (1988) A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J., 7, 1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alberts A.S., Geneste,O. and Treisman,R. (1998) Activation of SRF-regulated chromosomal templates by Rho-family GTPases requires a signal that induces H4 hyperacetylation. Cell, 92, 475–487. [DOI] [PubMed] [Google Scholar]
- 66.Konat G., Laszkiewicz,I., Bednarczuk,T.A., Kanoch,M. and Wiggins,R.C. (1991) Generation of radioactive and non radioactive ssDNA hybridization probes by polymerase chain reaction. Technique, 3, 311–319. [Google Scholar]
- 67.Haglund R.E. and Rothblum,L.I. (1987) Isolation, fractionation and reconstitution of a nuclear extract capable of transcribing ribosomal DNA. Mol. Cell. Biochem., 73, 11–25. [DOI] [PubMed] [Google Scholar]
- 68.O’Mahony D.J., Xie,W.Q., Smith,S.D., Singer,H.A. and Rothblum,L.I. (1992) Differential phosphorylation and localization of the transcription factor UBF in vivo in response to serum deprivation. In vitro dephosphorylation of UBF reduces its transactivation properties. J. Biol. Chem., 267, 35–38. [PubMed] [Google Scholar]
- 69.Xiao H., Hasegawa,T. and Isobe,K. (1999) Both Sp1 and Sp3 are responsible for p21 waf 1 promoter activity induced by histone deacetylase inhibitor in NIH3T3 cells. J. Cell. Biochem., 73, 291–302. [PubMed] [Google Scholar]
- 70.Van Lint ,C., Emiliani,S. and Verdin,E. (1996) The expression of a small fraction of cellular genes is changed in response to histone hyperacetylation. Gene Expr., 5, 245–253. [PMC free article] [PubMed] [Google Scholar]
- 71.Arts J., Lansink,M., Grimbergen,J., Toet,K.H. and Kooistra,T. (1995) Stimulation of tissue-type plasminogen activator gene expression by sodium butyrate and trichostatin A in human endothelial cells involves histone acetylation. Biochem. J., 310, 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Imai S.-I., Armstrong,C.M., Kaeberiein,M. and Guarente,L. (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature, 403, 795–799. [DOI] [PubMed] [Google Scholar]
- 73.Herrera J.E., Sakaguchi,K., Bergel,M., Trieschmann,L., Nakatani,Y. and Bustin,M. (1999) Specific acetylation of chromosomal protein HMG-17 by PCAF alters its interaction with nucleosomes. Mol. Cell. Biol., 19, 3466–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Munshi N., Merika,M., Yie,J., Senger,K., Chen,G. and Thanos,D. (1998) Acetylation of HMG I(Y) by CBP turns off IFN beta expression by disrupting the enchanceosome. Mol. Cell, 2, 457–467. [DOI] [PubMed] [Google Scholar]
- 75.Luo R.X., Postigo,A.A. and Dean,D.C. (1998) Rb interacts with histone deacetylase to repress transcription. Cell, 92, 463–473. [DOI] [PubMed] [Google Scholar]
- 76.Pelletier G., Stefanovsky,V.Y., Faubladier,M., Hirschler-Laszkiewicz,I., Savard,J., Rothblum,L.I., Cote,J. and Moss,T. (2000) Competitive recruitment of CBP and Rb-HDAC regulates UBF acetylation and ribosomal transcription. Mol. Cell, 6, 1059–1066. [DOI] [PubMed] [Google Scholar]
- 77.Gu W. and Roeder,R.G. (1997) Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell, 90, 595–606. [DOI] [PubMed] [Google Scholar]
- 78.Liu L., Scolnick,D.M., Trievel,R.C., Zhang,H.B., Marmorstein,R., Halazonetis,T.D. and Berger,S.L. (1999) p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol. Cell. Biol., 19, 1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ogryzko V.V., Kotani,T., Zhang,X., Schlitz,R.L., Howard,T., Yang,X.J., Howard,B.H., Qin,J. and Nakatani,Y. (1998) Histone-like TAFs within the PCAF histone acetylase complex. Cell, 94, 35–44. [DOI] [PubMed] [Google Scholar]
- 80.Muth V., Nadaud,S., Grummt,I. and Voit,R.(2001) Acetylation of TAF(I)68, a subunit of TIF-IB/SL1, activates RNA polymerase I transcription. EMBO J., 20, 1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jacobson R.H., Ladurner,A.G., King,D.S. and Tjian R. (2000) Structure and function of a human TAFII250 double bromodomain module. Science, 288, 1422–1425. [DOI] [PubMed] [Google Scholar]
- 82.Mizzen C., Yang,X., Kokubo,T., Brownell,J., Bannister,A., Owen-Hughes,T., Workman,J., Wang,L., Berger,S., Kouzarides,T., Nakatani,Y. and Allis,C. (1996) The TAF(II)250 subunit of TAFIID has histone acetyltransferase activity. Cell, 87, 1261–1270. [DOI] [PubMed] [Google Scholar]