Abstract
Four inhaler adherence clusters have been identified using the INCA audio device in COPD patients: (1) regular use/good technique, (2) regular use/frequent technique errors, (3) irregular use/good technique, and (4) irregular use/frequent technique errors. Their relationship with healthcare utilization and mortality was established, but the cost-effectiveness of adherence-enhancing interventions is unknown. In this exploratory study, we aimed to estimate the potential cost-effectiveness of reaching optimal adherence in the three suboptimal adherence clusters, i.e., a theoretical shift of clusters 2, 3, and 4 to cluster 1. Cost-effectiveness was estimated over a 5-year time horizon using the Irish healthcare payer perspective. We used a previously developed COPD health-economic model that was updated with INCA trial data and Irish national economic and epidemiological data. For each cluster, interventions would result in additional quality-adjusted life years gained at reasonable investment. Cost-effectiveness was most favorable in cluster 3, with possible cost savings of €845/annum/person.
Introduction
In chronic obstructive pulmonary disease (COPD), real-world adherence to maintenance therapy can be as low as 20%.1 In contrast, trials report adherence rates of over 80% in most participants.2 Previous studies showed that patients with high adherence have significantly better clinical and economic outcomes.3 Consequently, interventions focusing on adherence enhancement have shown to be effective and cost-effective.4,5 Yet, in these studies, only average adherence was assessed and no distinction was made between the different aspects of non-adherence. Optimal implementation of inhaled therapy involves both regular use as well as good inhaler technique.6 In our previous work, we identified four distinct clusters of inhaler adherence that were associated with differential clinical outcomes.7 Economic evaluations of adherence-enhancing interventions in each of those clusters have not been performed, but could help prioritizing specific target populations for tailored interventions. The aim of this follow-up study is to estimate the potential cost-effectiveness of reaching optimal adherence in each suboptimal adherence cluster.
Results
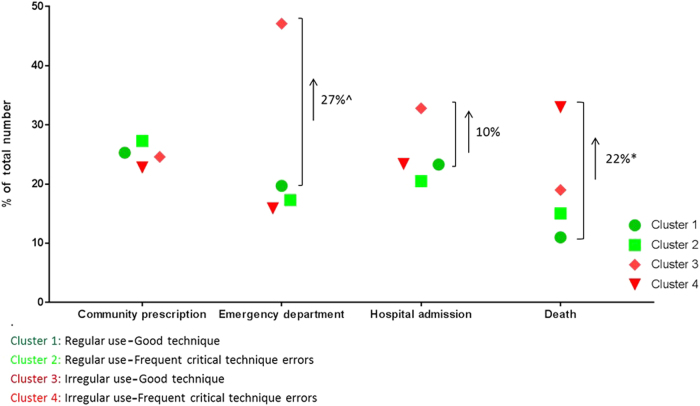
The study’s baseline population characteristics (n = 226) have been described elsewhere.1,7 In short, cluster 1 had the lowest mortality during follow-up. Cluster 2 had the highest rate of antibiotic and/or oral steroids community prescriptions. The highest overall healthcare use was attributable to patients from cluster 3. Cluster 4 had the highest mortality (Fig. 1).
Fig. 1.
Proportional contribution of each adherence cluster to all-cause clinical outcomes over the 12-month follow-up period (adjusted for the number of participants per cluster). Reported differences are the absolute differences in the proportion of events attributable to cluster 3 vs. cluster 1 for emergency department and hospital admission and cluster 4 vs. cluster 1 for death. ^ denotes p = 0.05, *p < 0.05
Cost-effectiveness
Cost-effectiveness analysis results are shown in Table 1. Adherence-enhancing interventions seem generally cost-effective, given all ICERs are below the Irish cost-effectiveness threshold of €45,000/QALY.8 In other words, this means that all adherence-enhancing interventions would cost less than €45,000 to gain one life-year in perfect health. For each cluster, the theoretical intervention would result in additional life years and QALYs gained. Notably, for cluster 3, an intervention would be cost-saving (i.e., less costs and more QALYs).
Table 1.
Cost-effectiveness of enhancing adherence based on a 5-year time horizon
| Cluster | Total costs | Intervention costsa | Medication costs | Healthcare costs | Life years | QALYs |
|---|---|---|---|---|---|---|
| Cluster 2: Regular use, frequent technique errors | ||||||
| Intervention | €11,386 | €184 | €2901 | €8300 | 3.75 | 3.00 |
| Cluster 2 | €10,150 | €0 | €2723 | €7428 | 3.52 | 2.81 |
| Difference | €1235 | €184 | €179 | €872 | 0.23 | 0.19 |
| ICER | €6520/QALY gained | |||||
| Cluster 3: Irregular use, good technique | ||||||
| Intervention | €11,812 | €184 | €2936 | €8692 | 3.80 | 3.03 |
| Cluster 3 | €12,657 | €0 | €2395 | €10,262 | 3.40 | 2.70 |
| Difference | −€845 | €184 | €541 | −€1570 | 0.39 | 0.33 |
| ICER | Cost-saving (i.e., less costs, more QALYs) | |||||
| Cluster 4: Irregular use, frequent technique errors | ||||||
| Intervention | €13,075 | €184 | €3000 | €9891 | 3.88 | 3.10 |
| Cluster 4 | €10,180 | €0 | €1918 | €8261 | 2.98 | 2.36 |
| Difference | €2896 | €184 | €1082 | €1630 | 0.90 | 0.74 |
| ICER | €3935/QALY gained | |||||
ICER incremental cost-effectiveness ratio, QALY quality-adjusted life year
aNote that the mean per-patient intervention costs are slightly lower given some patients die within the first year and so do not cost the full €200
Discussion
We performed exploratory cost-effectiveness modeling to assess the potential economic benefits of improved adherence in three clusters of patients with suboptimal adherence. In all clusters, interventions seem cost-effective. Moreover, in patients with irregular use but good inhaler technique, a possible cost-saving of €845/annum/person could be yielded despite the higher cost of medication arising from better adherence.
Remote monitoring of adherence on a longitudinal basis provides an accurate evaluation of regularity of use as well as inhaler technique. The use of a technology, such as the one described in this report, identified four patterns of adherence and may give guidance as to how we might approach this challenge in a personalized manner. Hence, in the future, by providing personalized interventions to the highest risk groups we may both improve adherence and have a positive impact on both clinical and economic outcomes in those most in need, such as those in cluster 3. The need for personalized adherence-enhancing interventions has recently been highlighted.9 They could include education and training on inhaler technique for those with frequent technique errors, patient reminders for those with irregular use due to forgetfulness, and shared decision-making and motivational interviewing for those with irregular use due to a conscious, intentional decision.9
This study is a first attempt to estimate the cost-effectiveness of interventions in different adherence clusters, but was limited by the use of post hoc and short-term effectiveness data for the different clusters. Regarding generalizability, absolute costs may differ per country or setting, but we expect relative cluster results to be comparable. Our exploratory cost-effectiveness model estimates should be confirmed when long-term clinical intervention trial data become available, including extensive sensitivity and scenario analyses as well as analyses in a non-admitted primary care COPD population.
Conclusion
Personalized adherence interventions targeting patient-specific regularity of inhaler use and inhaler technique could result in clinical and economic benefits for COPD patients.
Methods
Study design
This was an exploratory cost-effectiveness analysis. Ethical approval for the clinical study was obtained from the Beaumont Hospital Ethics Committee.
Patient population
Patient selection and data collection has been described previously.1 Briefly, hospitalized patients with COPD, prescribed salmeterol/fluticasone propionate (Seretide®, GlaxoSmithKline, Ireland), were included and written informed consent was obtained from all participants.
Inhaler adherence
Adherence was assessed using the INCATM device that can track both timing and quality of inhaler use.1 The INCA acoustic recordings allowed for identification of the following four adherence clusters:7 (1) regular use/good technique, (2) regular use/frequent technique errors, (3) irregular use/good technique, and (4) irregular use/frequent technique errors. Cluster-specific healthcare utilization and mortality was studied previously7 and is summarized in Fig. 1.
Cost-effectiveness analysis
The cost-effectiveness of enhanced adherence, i.e., the theoretical shift of patients from poor adherence (i.e., cluster 2, 3, or 4) to good adherence (i.e., cluster 1), was estimated. Therefore, a previously developed, described, and validated health-economic model5 was used and populated with Irish and INCA-specific cost (for medication, intervention, and healthcare utilization) and effect (mortality and exacerbations) data (Appendix 1). The model has received a high-quality score in the latest COPD model review,10 and estimates the incremental cost-effectiveness ratio (ICER) in terms of costs (2013, €) per quality-adjusted life-year (QALY) gained from the Irish healthcare payer’s perspective assuming fixed per-patient intervention costs of €200 (authors’ estimation). Three ICERs were calculated using cluster 1 as intervention and clusters 2, 3, and 4, respectively, as usual care scenarios. In line with recommendations,11 a policy-relevant time horizon of 5 years was used, taking into account a 5% discount rate for both costs and effects as per Irish Health Information and Quality Authority guidelines (www.hiqa.ie).
Data availability statement
Detailed data and characteristics of the study population are available in ref. 1 and other data that support the findings of this study are available from the corresponding author on reasonable request.
Electronic supplementary material
Appendix 1: Input parameters for cost-effectiveness model
Author contributions
J.F.M.v.B. and R.W.C. designed the study. I.S., M.M., and E.M. were primarily involved in patient recruitment and management of the patients, R.B.R. was primarily involved in the audio analysis required for this manuscript. B.C., G.G., and K.B. were primarily involved in data collection and analysis. J.F.M.v.B. was primarily involved in the health-economic analysis and wrote the first draft of the manuscript. All authors provided input and approved the final manuscript. J.F.M.v.B. is the guarantor of the study.
Funding
This was a researcher-initiated study, funded by the Health Research Board of Ireland, Clinical Scientist Award (RC), and the Irish Research Council (REPRO/2015/90). The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Competing interests
R.W.C. and R.B.R. are named on a patent for the INCA device.
Footnotes
Electronic supplementary material
Supplementary Information accompanies the paper on the npj Primary Care Respiratory Medicine website (10.1038/s41533-018-0092-8).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sulaiman I, et al. Objective assessment of adherence to inhalers by patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2017;195:1333–1343. doi: 10.1164/rccm.201604-0733OC. [DOI] [PubMed] [Google Scholar]
- 2.Vestbo J, et al. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax. 2009;64:939–943. doi: 10.1136/thx.2009.113662. [DOI] [PubMed] [Google Scholar]
- 3.van Boven JF, et al. Clinical and economic impact of non-adherence in COPD: a systematic review. Respir. Med. 2014;108:103–113. doi: 10.1016/j.rmed.2013.08.044. [DOI] [PubMed] [Google Scholar]
- 4.Klijn S, et al. Effectiveness and success factors of educational inhaler technique interventions in asthma & COPD patients: a systematic review. NPJ Prim. Care Respir. Med. 2017;27:24. doi: 10.1038/s41533-017-0022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Boven JF, et al. Improving inhaler adherence in patients with chronic obstructive pulmonary disease: a cost-effectiveness analysis. Respir. Res. 2014;15:66. doi: 10.1186/1465-9921-15-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vrijens B, et al. What we mean when we talk about adherence in respiratory medicine. J. Allergy Clin. Immunol. Pract. 2016;4:802–812. doi: 10.1016/j.jaip.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Cushen, B, et al. The clinical impact of different adherence behaviours in patients with severe COPD. Am. J. Respir. Crit. Care Med. 10.1164/rccm.201712-2469LE (2018). [DOI] [PubMed]
- 8.O’Mahony JF, Coughlan D. The Irish cost-effectiveness threshold: does it support rational rationing or might it lead to unintended harm to Ireland’s health system? Pharmacoeconomics. 2016;34:5–11. doi: 10.1007/s40273-015-0336-1. [DOI] [PubMed] [Google Scholar]
- 9.Van Boven JF, et al. Urging Europe to put non-adherence to inhaled respiratory medication higher on the policy agenda: a report from the First European Congress on Adherence to Therapy. Eur. Respir. J. 2017;49:170076. doi: 10.1183/13993003.00076-2017. [DOI] [PubMed] [Google Scholar]
- 10.Zafari Z, et al. A systematic review of health economics simulation models of chronic obstructive pulmonary disease. Value Health. 2017;20:152–162. doi: 10.1016/j.jval.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 11.van der Schans S, et al. Systematic review and quality appraisal of cost-effectiveness analyses of pharmacologic maintenance treatment for chronic obstructive pulmonary disease: methodological considerations and recommendations. Pharmacoeconomics. 2017;35:43–63. doi: 10.1007/s40273-016-0448-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: Input parameters for cost-effectiveness model
Data Availability Statement
Detailed data and characteristics of the study population are available in ref. 1 and other data that support the findings of this study are available from the corresponding author on reasonable request.