Abstract
Introduction
The free and cued selective reminding test is used to identify memory deficits in mild cognitive impairment and demented patients. It allows assessing three processes: encoding, storage, and recollection of verbal episodic memory.
Methods
We investigated the neural correlates of these three memory processes in a large cohort study. The Memento cohort enrolled 2323 outpatients presenting either with subjective cognitive decline or mild cognitive impairment who underwent cognitive, structural MRI and, for a subset, fluorodeoxyglucose–positron emission tomography evaluations.
Results
Encoding was associated with a network including parietal and temporal cortices; storage was mainly associated with entorhinal and parahippocampal regions, bilaterally; retrieval was associated with a widespread network encompassing frontal regions.
Discussion
The neural correlates of episodic memory processes can be assessed in large and standardized cohorts of patients at risk for Alzheimer's disease. Their relation to pathophysiological markers of Alzheimer's disease remains to be studied.
Keywords: Memory, Alzheimer's disease, MRI, Fluorodeoxyglucose-PET, Multicenter cohort
Highlights
-
•
This is the largest cohort ever to be used in the study of the morpho-metabolic correlates of episodic memory in human, ensuring the validity of the obtained results.
-
•
We found that encoding of information is linked to a posterior network previously evidenced to support working memory.
-
•
The storage process was mainly supported in our study by medial temporal regions.
-
•
Spontaneous retrieval of stimuli implicated broad neural networks including the frontal regions.
-
•
These associations were particularly strong in APOE ε4 carriers suggesting that the free and selective reminding test is useful to detect Alzheimer's disease at an early stage.
1. Introduction
Episodic memory refers to memory for personal experience with respect to time and context [1]. The three principal processes involved in episodic memory are encoding, storage, and retrieval of information. It can be impaired in various diseases, for example, depression [2], Parkinson disease [3], frontotemporal dementia [4]. Alzheimer's disease (AD) is the most frequent disorder characterized by memory impairment [5]. Indeed, impairments in episodic memory performance are considered as the first clinical sign of typical AD and have been associated with atrophy of the entorhinal cortex and hippocampus [6], [7], [8].
A few longitudinal studies of cognition in healthy older adults have shown that a subtle decline in episodic memory often occurs before the emergence of the functional and overt cognitive changes required for a clinical diagnosis of AD dementia [9], [10], [11], [12], [13], [14]. These findings led to the amnestic mild cognitive impairment (MCI) [15] concept, a predementia condition in elderly individuals, which is characterized by subjective and objective memory impairments with relatively preserved general cognition and functional abilities. Before this MCI stage of AD, subjective cognitive decline (SCD) can be a symptom of preclinical AD [16], [17].
The Free and Cued Selective Reminding Test (FCSRT) has been proposed as a verbal associative episodic memory test [18]. It aims at exploring the three memory processes in a single neuropsychological test and is used in clinical practice of some memory clinics for AD diagnosis [11], [19]. Its subscores allow the assessment of serial cognitive processes involved in episodic memory: immediate recall (IR) for encoding, index of sensitivity to cueing (ISC) for storage, and total free recall (FR) for retrieval of memorized stimuli. Previous studies have shown that FCSRT is useful for prognosing MCI patients who will decline to dementia stage of AD [11], [20], [21], [22] and for diagnosing typical amnestic AD patients among various neurodegenerative conditions [19]. When storage is impaired (i.e., low FR score and low total recall or ISC scores), an amnestic syndrome [23] termed “of the hippocampal (or medial temporal) type” has been defined. However, only a few imaging studies, mainly in a small number of demented patients, have shown a correlation between hippocampal volumes and FCSRT performances [24], [25]. In addition, little is known on the link between FCSRT performances and other brain regions known to be implicated in episodic memory such as the working memory network [26] or prefrontal areas [27]. There is a large number of studies that tackled the question of the neural correlates of episodic memory (for a recent review see [28]). However, the experimental paradigms frequently differ from one study to the next, which induced some discrepancies, concerning for instance the laterality of the medial temporal lobe involvement found to be mainly left sided in some studies [29], [30], [31] right sided in other [32], [33] and sometimes bilateral [34], [35].
In this study, we investigated structural and metabolic correlates of the three episodic memory processes assessed by the FCSRT in a large French cohort of participants with standardized cognitive assessment as well as structural and metabolic imaging. Within this framework, we hypothesize that encoding and storage phases would be related to hippocampal and parietal regions, and recollection phase would be related to a widespread brain network, including more anterior brain regions. Our large sample size and standardization allow us to draw unequivocal conclusions from our results, shedding some light on previously described discrepancies [36].
2. Material and methods
2.1. Participants
Memento study consecutively enrolled 2323 nondemented outpatients in 28 French expert memory clinics, from 2011 to 2014. The study procedures and participants' baseline characteristics are described elsewhere [37]. At inclusion, participants presented either with cognitive impairment, when performing worse than one standard deviation to the mean of a group (with similar age, age/educational norms) in one or more cognitive domains, this deviation being identified for the first time through cognitive tests performed recently (less than 6 months preceding screening phase), or with isolated cognitive complaints, if participants had subjective cognitive complaint (assessed through visual analogic scale), without any objective cognitive deficit as defined previously, while being 60 years and older, and they all had a Clinical Dementia Rating scale [38] score ≤0.5. Main exclusion criteria were contraindication or refusal to perform magnetic resonance imaging (MRI), neurological disease such as treated epilepsy, treated Parkinson's disease, Huntington disease, or brain tumor, history of head trauma with neurological sequelae, stroke occurring in the past three months, history of schizophrenia, or illiteracy. All examinations (including neuropsychological battery administration, clinical examinations, brain MRI, and fluorodeoxyglucose [FDG] positron emission tomography [PET]) performed through Memento followed standardized procedures.
The analytic sample consists in participants who underwent a brain MRI and a neuropsychological evaluation, including the FCSRT at their inclusion in the cohort (N = 2157). A subsample that additionally performed the optional FDG-PET was considered in a subsequent analysis (N = 1310).
All participants signed an informed consent to participate in the study that was approved by the ethics committee “Comité de Protection des Personnes Sud-Ouest et Outre Mer III.” The study was conducted following standards of the Good Clinical Practice and the Helsinki Declaration. Although not a clinical trial, the protocol was registered in ClinicalTrials.gov (Identifier: NCT01926249, https://clinicaltrials.gov/ct2/show/NCT01926249).
2.2. Neuropsychological evaluation
A full neuropsychological test battery was administered to participants at baseline [37] including the FCSRT [39] to study the verbal episodic memory. In this associative memory test, the subject has to learn 16 words by groups of four with each corresponding cue provided verbally by the tester (e.g., “fish” is the cue for the word “herring”). In a first step, the subject is asked to recall words just after reading them, four by four (namely IR, scored from 0 to 16). Then, three recall (firstly free and then cued) trials separated from each other by a distractive task (mental calculation during 20 seconds) are successively performed. The FR score ranges from 0 to 16 × 3 = 48. The ISC is computed as 100 * (sum of the three cued recall/[48-FR]). The list of the 16 words and the detailed procedure of execution are available elsewhere [40].
The neuropsychological test battery also included the Rey figure copy [41] that assesses visuospatial and visuoconstructive abilities and was used as a control of the specificity of the morpho-metabolic correlates of the FCSRT subscores.
Using performances at the full neuropsychological tests battery, Petersen criteria [42] were applied to categorize participants' cognitive status as non-MCI (SCD), pure amnestic MCI, multidomain amnestic MCI, pure nonamnestic MCI, multidomain nonamnestic MCI.
2.3. MRI evaluation
Brain magnetic resonance images were acquired after a standardization of the imaging processes (notably the sequences used) by a dedicated neuroimaging specialist team (CATI for “Centre pour l'Acquisition et le Traitement des Images”, http://cati-neuroimaging.com/). MRI machines of 1.5 and 3 Tesla were used for this study (the complete list of machines is provided in Supplementary Appendix A). All MRI scans were centralized, quality checked, and postprocessed by the CATI to obtain standardized measurements for each participant. The MRI protocol included 3D-T1 1 mm isometric sequences that were used to assess the whole-brain, gray matter, and white matter volumes with Statistical Parametric Mapping [43], hippocampal volumes with the SACHA software [44], [45] and cortical thickness with FreeSurfer in Desikan-Killiany atlas [46], [47].
2.4. FDG-PET evaluation
As for MRI, the CATI allowed for intercenter reproducibility through harmonization of FDG-PET protocols and postprocessing [48]. Structural MRI images were coregistered to PET images using Statistical Parametric Mapping 8 with visual inspection to detect any coregistration errors. MRI 3D T1-weighted images were segmented and spatially normalized into the Montreal Neurological Institute space using the VBM8 package (http://dbm.neuro.uni-jena.de/vbm/) implemented in Statistical Parametric Mapping 8. MRI matrix transformation was then used to spatially normalize PET images into Montreal Neurological Institute space. Parametric PET images were created for each individual, by dividing each voxel with the mean activity extracted from the reference region, the pons. Finally, gray matter masks extracted from each individual MRI volume were applied to the parametric PET images before Regions Of Interest (ROIs) analysis. Metabolic FDG-PET indexes were calculated in ROIs from the Automated Anatomical Labeling 2 (AAL2) atlas [49] to the exception of the cerebellum.
2.5. APOE genotyping
Apolipoprotein E (APOE) ε2, ε3, or ε4 alleles were determined for all participants by KBiosciences (Hoddesdon, UK; www.kbioscience.co.uk) as described elsewhere [37].
2.6. Statistical methods
Sample characteristics are reported as median (q1; q3) or frequency, as appropriate. Between-group comparisons were performed through the χ2 test for discrete variables or analysis of variance tests for continuous variables. Multivariable analyses were undertaken on three outcomes (IR, FR, and ISC scores at inclusion). As more than half of the population scored 16 (maximum score) in the IR subscore, it was dichotomized as equal to 16 versus <16, and logistic regressions were computed for analyses. To account for skewed distributions of FR and ISC subscores, their anatomical and metabolic correlates were modeled through median regression. For each outcome, models were built using brain structure as the “exposure” of interest and gender, age, education, number of ε4 alleles of APOE genotype, and type of MRI/PET as adjustment covariates. Due to multiple comparisons in 34 cortical thicknesses (FreeSurfer) MRI ROIs and in the 47 (AAL2) FDG-PET ROIs, a false discovery rate (FDR) was maintained at 0.05 or less by recomputing P-values [50]. A P-value ≤.05 was considered statistically significant. The effect sizes (estimate) of the significant association were then presented graphically on an inflated brain mesh.
Finally, we analyzed jointly the association between the imaging measurements and the three FCSRT subscores, assuming that all three are markers of the episodic memory. As the episodic memory in itself is unmeasured, we used a latent class analysis approach [51]. This method allows linking multiple outcomes of different nature (i.e., binary, ordinal, discrete and continuous) generated by the same underlying latent process and flexible enough to deal with nonlinear associations. We thus can estimate whether imaging measurements are associated with the latent process and, using contrasts, test whether the contribution of the subscores can be considered statistically equivalent or different [52].
Analyses were performed using SAS software version 9.3 (SAS Institute, Cary, NC) and R (LCMM package v1.7.8) for latent class analysis.
Finally, to determine whether APOE ε4 genotype could modify the relation between cortical thickness and FCSRT performances, we introduce APOE ε4 genotype*cortical thickness interaction term and was tested in the models. Uncorrected and FDR-corrected P-values were computed. For uncorrected P-values < .05, results of stratified analyses (Non–APOE ε4 and APOE ε4 carriers) were presented.
3. Results
Of the 2323 participants, 2157 (age median and [interquartile range]: 71.6, [65.6–77.1] years) were administered the FCSRT and had a brain MRI. Among them, 1310 underwent FDG-PET scan (Fig. 1). Table 1 shows the analytical sample baseline characteristics according to FDG data availability. Participants who had a FDG-PET were more likely women (67% vs. 59%, P = .0002), had less frequently a clinical dementia rating score equal to 0.5 (63% vs. 58%, P = .018) and had more frequently an SCD or a nonamnestic MCI profile (P = .045). The scatter plots of raw FCSRT subscores are provided in Supplementary Appendix B both globally (whole cohort) and by APOE and cognitive (SCD or MCI) status.
Fig. 1.
Participants flow chart. Abbreviations: FCSRT, free and cued selective reminding test; FDG-PET, fluorodeoxyglucose-positron emission tomography.
Table 1.
Population baseline characteristics—The Memento cohort
| Characteristics | All participants (N = 2157) | FDG-PET participants (n = 1310) | FDG-PET performed yes versus no (P-value) |
|---|---|---|---|
| Median age in years, (Q1; Q3) | 71.6 (65.6; 77.1) | 72 (65.8; 77.0) | .55 |
| Female gender, n (%) | 1335 (61.9) | 770 (58.8) | .0002 |
| Educational level > 12 years, n (%) | 1172 (54.5) | 732 (56.0) | .070 |
| Number of APOE ε4 allele, n (%) | .99 | ||
| 0 | 1445 (70.4) | 890 (70.4) | |
| 1 | 537 (26.2) | 332 (26.2) | |
| 2 | 70 (3.4) | 43 (3.4) | |
| CDR score, n (%) | .018 | ||
| 0 | 869 (40.3) | 554 (42.3) | |
| 0.5 | 1288 (59.7) | 756 (57.7) | |
| Cognitive status, n (%) | .045 | ||
| SCD | 343 (15.9) | 219 (16.7) | |
| Pure aMCI | 196 (9.1) | 112 (8.5) | |
| Multi-domain aMCI | 924 (42.8) | 533 (40.7) | |
| Pure naMCI | 366 (17.0) | 237 (18.1) | |
| Multi-domain naMCI | 328 (15.2) | 209 (16.0) | |
| Median MMSE score, (Q1; Q3) | 28 (27; 29) | 28 (27; 29) | |
| FCSRT scores, median (Q1; Q3) | |||
| IR (/16) | 16 (15; 16) | 16 (15; 16) | .97 |
| FR (/48) | 27 (21; 32) | 27 (21; 32) | .078 |
| ISC (/100) | 89 (77; 96) | 89 (77; 96) | .25 |
Abbreviations: CDR, clinical dementia rating; MMSE, Mini Mental State Evaluation; FCSRT, Free and Cued Selective Reminding Test; IR, immediate recall; FR, free recall; ISC, index of sensitivity to cueing; SCD, subjective cognitive decline; aMCI, amnestic mild cognitive impairment; naMCI, nonamnestic mild cognitive impairment; FDG-PET, fluorodeoxyglucose-positron emission tomography.
NOTE. Between-group comparisons were performed through χ2 test for discrete variables or analysis of variance tests for continuous variables.
3.1. MRI measures and FCSRT subscores
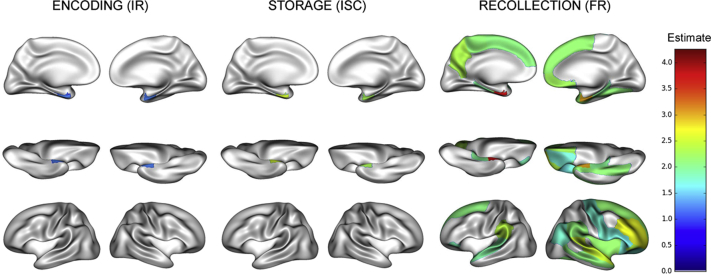
Fig. 2 summarizes results of MRI analyses and FCSRT scores correlations. As expected, greater hippocampal volume was associated with increased odds of having high scores at IR: 1.30 (1.15; 1.46) (odds ratio [<16 vs. = 16] [95% confidence interval {CI}]), FR and ISC: 5.53 (4.72; 6.35) and 3.72 (2.71; 4.73), respectively (differences in median [95% CI]), all FDR corrected P values < .0001 associations. Distinct patterns of associations were observed for the three subscores: FCSRT-IR and FCSRT-ISC were mainly associated with entorhinal and parahippocampal regions, bilaterally; FCSRT-FR was associated with a widespread network encompassing frontal regions. No differences were found in these associations between hemispheres. Rey figure copy score was not associated with any mediotemporal regional cortical thickness (data not shown).
Fig. 2.
Regional pattern of association between FCSRT subscores and cortical thickness (N = 2157). Abbreviations: FCSRT, free and cued selective reminding test; IR, immediate recall; ISC, index of sensitivity to cueing; FR, free recall.
In the latent class analyses (results provided in Supplementary Appendix C), IR was the subscore most strongly associated with cortical thicknesses in the superior temporal, precentral, lingual, precuneus, and latero-occipital regions. By contrast, the ISC was not associated with these structural variations, except for cortical thickness in the superior temporal cortex (coefficient = 0.10 [95% CI 0.02; 0.17]) as compared to IR (coefficient = 0.21 [95% CI 0.10; 0.31]) and FR (coefficient = 0.19 [95% CI 0.13; 0.26]).
APOE ε4 carriers had significantly different associations between FCSRT subscores and regional cortical thicknesses as described in Supplementary Appendices D and E. Most strikingly, the difference in median of the FR associated to the entorhinal cortex thickness was twice as important in APOE ε4 carriers than in noncarriers. However, none of these differences was significant after FDR correction.
3.2. FDG-PET correlates of FCSRT subscores
As there was no difference between the left and right hemisphere associations to FCSRT subscores, symmetrical regions were joined as metaregions of interest to study the associations to each FCSRT subscores in 47 regions (i.e., 94/2 from the AAL2 atlas, excluding the cerebellum).
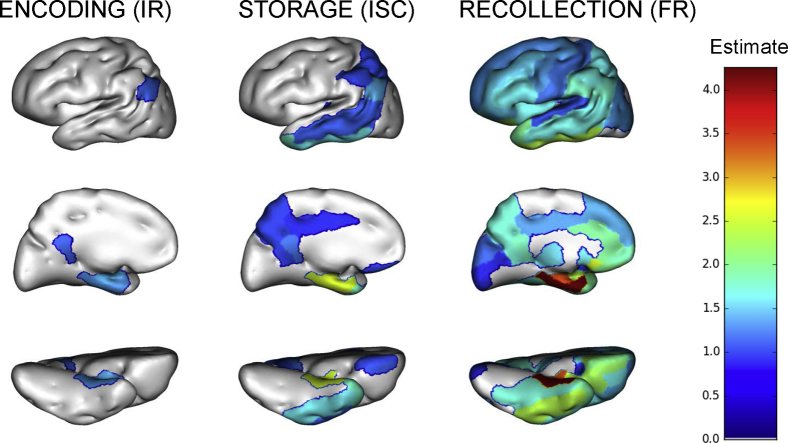
The regions where the brain metabolism was significantly linked to IR and ISC scores were limited to the posterior cingulate gyri, parietotemporal junction, and medial temporal lobes albeit in a more widespread fashion for ISC than for IR (Fig. 3). Conversely, FR score was significantly related to metabolic measures in a diffuse network comprising prefrontal (medial, dorsolateral, and orbitofrontal), as well as parietal (lateral and medial) and temporal (lateral and medial) regions.
Fig. 3.
Regional pattern of association between FCSRT subscores and mean regional FDG uptake values (n = 1310). Abbreviations: FCSRT, free and cued selective reminding test; FDG, fluorodeoxyglucose; ISC, index of sensitivity to cueing; FR, free recall; IR, immediate recall.
FR and ISC were significantly associated with precuneus, posterior cingulate cortex, associative parietal cortex, and temporal cortex (both left and right sides), whereas for IR, correlations were significant only in the posterior cingulate and temporal cortices, bilaterally.
The latent class analysis (Supplementary Appendix F) did not indicate singular patterns of regional metabolic association to the three FCSRT subscores. However, we found a global effect on episodic memory as a whole of the metabolic measures in 34/47 of the studied AAL regions (with the exception of the putamen, pallidum, and primary motor and sensitive areas).
Compared to noncarriers, APOE ε4 carriers had higher associations between FCSRT subscores and metabolism in multiple regions encompassing a large occipito-parieto-temporal network for IR and FR and the same network with additional frontal and limbic regions for ISC. In contrast to the same analysis for cortical thicknesses, most of these differences remained significant after the FDR correction and are described in Supplementary Appendices G and H.
4. Discussion
We explored the structural (MRI) and metabolic (FDG-PET) correlates of the three main processes of episodic memory, using a cued memory test, the FCSRT, in a large cohort of elderly participants with cognitive profile ranging from SCD to MCI. We found that the three different processes involved in episodic memory are associated with different brain networks.
4.1. Validity of the FCSRT to study episodic memory
Our findings are in line with the “Attention to memory” model proposed by Cabeza and collaborators [26]. This model stipulates that the parietal cortex is involved in voluntary (top-down) retrieval of information, which is the case in the FCSRT. First, IR (which reflects the registration process) was mostly associated with metabolism in posterior brain areas. These posterior regions are associated with attentional and working memory performances [26]. Second, FR is associated with cortical thickness and metabolism in most of the brain regions and most notably in anterior brain regions. Actually, FR measures the ability to actively recollect information and is linked to executive functions. Finally, the ISC, a subscore representative of storage, is mostly associated with the cortical thickness in temporal regions [28]. This memory process is largely independent of attention and executive functions, which are impaired, for example, in pure brain vascular disease, such as in Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy [53]. In Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy, storage impairment is both rare and occurs at a later course of the disease [54].
Interestingly, the absence of differential associations according to hemispheric side for both hippocampal volumes and medial temporal lobe cortical thicknesses on FCSRT subscores is in line with previous studies [28]. In young healthy subjects, episodic memory rather involves the left hemisphere [55], but in the elderly, it involves both hemispheres. This might reflect a compensatory mechanism necessary to memorize stimuli in aging.
Our results concerning the FCSRT association with brain structures and metabolism are specific. Indeed, we did not find any similar association between the Rey's figure copy score, chosen as a nonmemory/nonlanguage cognitive process control, and the imaging markers in temporomedial regions (data not shown).
Current structural and metabolic correlates of memory exhibited some similarities. However, some regions were rather associated to a memory process on MRI (such as temporopolar regions for encoding) or FDG-PET (such as posterior cingulate cortex for storage). This is probably due both to differences in imaging acquisition and processing and to physiopathological discrepancies in MRI versus FDG-PET. The hippocampal paradox in AD (i.e., compensated metabolism that remains normal in atrophied hippocampus) is an example of such discrepancies [56]. The processing of the images relied on the use of validated pipelines and atlases that differed between the two imaging modalities, namely FreeSurfer [46] for the MRI cortical thickness ROIs and AAL2 [49] for FDG-PET ROIs. However, the macroscopic differences evidenced in our study cannot be attributed solely to the use of these different atlases. As FDG-PET was optional and performed only in a subsample, one could argue that this is the cause of the evidenced discrepancies. However, the Appendix analysis on the subsample having both MRI and FDG-PET showed the same results as in the whole group excluding a selection bias (Supplementary Appendices I and J).
A limitation in the delineation of the neural correlates of episodic memory in our study is that the FCSRT is an associative memory test with semantic cueing. Hence, some of the associated structural or metabolic regions are likely to support semantic rather than episodic processes [57]. This is for instance the case for the temporopolar association to FR. This is however the case with all verbal memory tests, especially those allowing encoding and retrieval facilitation through cueing.
4.2. Linking neural substrates of episodic memory to early AD diagnosis and pathology
A challenge for establishing an early AD diagnosis is to identify the pattern of memory disorder in relation to pathological injury. It has been shown that the FCSRT can quantify and qualify the memory deficits and can therefore distinguish “pure memory impairment” (failure of information storage and new memory formation) from retrieval disorders due to attention/executive changes in normal aging or frontal pathologies [11], [21], [22], [58], [59], [60]. Such a test can identify the amnesic syndrome due to medial temporal damage that we call “the hippocampal type,” and characteristically observed in AD. This syndrome is defined by poor FR and decreased total recall caused by an insufficient effect of cueing. The low performance on total recall, despite retrieval facilitation given by semantic cues, indicates poor storage capacity. Our study indicates that a decline in the retrieval process might be an earlier cognitive marker of AD than the more specific storage deficit.
When looking at the association between storage and retrieval and the regional cortical thickness, AD physiopathology distribution comes to mind.
In typical AD patients, the disease progression is stereotyped: amyloid β lesions are initially neocortical and will diffuse centripetally, and tau neurofibrillary tangles are first evidenced in the medial temporal regions before spreading in a centrifuge way [61].
The medial temporal regions underlying storage is reminiscent of the early Braak stages that can be demonstrated neuropathologically [62] or more recently by way of tau-tracer PET imaging [63]. This medial temporal involvement associated with the storage explains the specificity of the ISC (and of the sum of the total recall) for AD even at an early (prodromal) stage and among multiple neurodegenerative conditions [19]. Conversely, the retrieval process neural substrates encompass both the medial temporal regions (affected early on during AD by the tauopathy as mentioned previously) and the regions in which amyloid deposition begins in AD (medial and dorsal prefrontal, precuneus, anterior and posterior cingulate, parietotemporal junction) [64], [65]. This interpretation is in line with recent evidence suggesting that the FR score declines on average 2 years before the total recall score in cognitively healthy elderly individuals but having a positive amyloid PET scan [66].
Two conditions are considered at high risk for AD: the status of MCI and the status of APOE ε4 carrier. In our study, the metabolic correlates of the storage process are the same regions as those found to be hypometabolic both in MCI who rapidly progress to AD [67] and in asymptomatic APOE ε4 carriers [68]. This strongly suggests that FCSRT can be considered as a valuable surrogate marker of neurodegeneration in subjects at risk for AD. The fact that episodic and semantic memory processes are tested in the FCSRT explains why this test is so sensitive to early AD as both episodic and semantic impairment can be observed in this affection [69].
In AD, MRI and FDG-PET are considered valuable prognostic tools [5]. In our analytical sample, APOE ε4 had an impact on the degree of association between FCSRT subscores, metabolism and, to a lesser extent, cortical thickness. The stronger associations observed between structure or metabolism and FCSRT subscores in APOE ε4 carriers in our study is an argument to support the claim that this cognitive test can be considered, in this population of elderly SCD or MCI, as a neuropsychological prognostic marker of AD.
Among the three subscores, FR correlates to cortical thickness and metabolism in the largest cortical network (fronto-parieto-temporal associative cortices). Thus, FR is likely to decrease if any part of its associated neural network is injured. This explains why this subscore is the most sensitive in early AD. By contrast, the ISC, which is associated with cortical thickness and metabolism in the medial temporal areas is probably a more specific but less-sensitive marker. As amyloid PET imaging will be soon available for a sample of several hundred of Memento participants, it will be possible to test the hypothesis of an early cognitive impact of brain amyloidosis on the retrieval process of episodic memory. In summary, our results strengthen Wolk and Dickerson's claim that multiple measures of memory tests, underlined by different brain structures, are required to address the full spectrum of impairment that can affect AD patients [70]. These authors' work on the longitudinal follow-up of cognitively normal elderly Alzheimer's Disease Neuroimaging Initiative participants [71] confirms that cortical thickness is an early sign of AD and not only linked with cross-sectional memory impairment as demonstrated by our study but also with longitudinal cognitive decline.
The relation between FCSRT neural substrates and early-stage AD pathological patterns has direct implication for clinical care and trials. It can explain why some trials in amnestic MCI defined with the FCSRT will show some evidence of efficacy, such as the slowing of hippocampal atrophy and cortical thickness with Donepezil [72], [73], whereas other trials in which MCI is not defined with the FCSRT do not, despite being more powered and longer [74], [75]. Our study supports the use of the FCSRT as an important neuropsychological enrichment factor for AD in SCD and MCI trials. The FCSRT can also be used as a clinically meaningful endpoint as in the recently published INSIGHT-PreAD study [76] in which the total recall subscore dramatic decrease is used as a proxy to address the “preclinical” to “clinical” stages transition. This approach is aimed at increasing the specificity of early clinical AD detection (at the prodromal stage) to enrich clinical trial inclusions. Other studies, such as a large U.S. prevention trial Anti-Amyloid Treatment in Asymptomatic Alzheimer's Disease [77], [78] and the French Multidomain Alzheimer Preventive Trial [79] have used the FCSRT as a clinical endpoint not by itself but among other tests in cognitive composite scores, namely the “Preclinical Alzheimer Cognitive Composite” score and the “MAPT-PACC” score, respectively. Although the use of composite scores allow to reduce type one error in statistical analyses, their clinical value and neural underpinnings are not as clear as individual cognitive tests (although most individual tests, and the FCSRT among them, are not purely related to one cognitive domain. In the case of the FCSRT, as mentioned previously, episodic and semantic memory processes are implicated).
4.3. Validity of the methodology
Strengths of our findings are related to the size of the cohort, its multimodality, and the quality of data collected for the Memento cohort, including a high degree of standardization of acquired data in all domains, from neuropsychological tests to imaging. This was organized before, during, and after (postprocessing) acquisition of data, which allowed optimal intercenter reproducibility as already described for PET imaging [48].
We acknowledge that the approach we used cannot bring the same refined information as functional MRI (fMRI) studies, which can for instance indicate which part of the hippocampus is involved in different memorization processes [36]. Our approach is complementary to fMRI delineation of the structural underpinning of memory processes and is likely to yield more robust, if less precise, results. The small number of participants included in most fMRI studies can be seen as a factor of discrepancies observed across studies (i.e., no hippocampal involvement in the retrieval of personal episodic autobiographical memory events [80] versus left hippocampal involvement [29]). The fMRI methodologies (particularly concerning the statistical analysis of results) also vary from one fMRI study to the next, and the inferences derived must be taken with caution [81]. In our study, the added value of a homogeneous population, standardized acquisition process over a relatively short interval of time, standardized quality checking, and postprocessing of data by a unique team (at the CATI [82]) and ultimately, statistical analysis taking into account both the multiple comparisons and adjustment factors allow us to draw valid conclusions from our findings. Also, the choice to study the associations of cognitive tests with predefined cortical areas derived from published atlas greatly decreases the number of statistical tests performed relatively to voxel-based comparisons while the analyzed regions remain pertinent on an anatomical and functional point of view. In any case, both types of studies are bound to provide complementary results, fMRI providing a finer delineation of subtle episodic memory functioning while our innovative methodology gives a more general and robust understanding of the major regions structurally and functionally underlying the cognitive processes of memory in aging.
This type of study has to be considered in the broader spectrum of standardized MRI postprocessing for routine clinical care. Numerous software programs are becoming available to the radiologists to help clinicians in their assessment of brain (particularly neurodegenerative) diseases [83]. This approach yet remains to be studied, but the Memento cohort seems to have the optimal design to validate it further as the participants will be followed longitudinally, allowing to determine the best marker of combination of markers to identify incipient AD or other brain diseases.
Research in Context.
-
1.
Systematic review: We searched the literature (PubMed) for the following terms: episodic memory AND magnetic resonance imaging OR Positron emission tomography (PET) OR structural correlates OR functional correlates revealing that there was no single study nor any meta-analysis with such a large number of participants used to analyze the structural and functional correlates of episodic memory with such a high degree of clinical and imaging standardization.
-
2.
Interpretation: Our study revealed that the free and selective reminding test and a simple and rapid association memory tests can be used to finely analyze the anatomical and functional underpinnings of episodic memory. The stronger association between regional metabolism on fluorodeoxyglucose-PET and memory performances in APOE ε4 carriers strengthens the diagnostic value of FCSRT for Alzheimer’s disease.
-
3.
Future directions: As amyloid PET imaging will be soon available for a sample of several hundred of Memento participants, it will be possible to test the hypothesis of an early cognitive impact of brain amyloidosis on the retrieval process of episodic memory.
Acknowledgments
The Memento cohort was supported by a grant from the Fondation Plan Alzheimer (Alzheimer Plan 2008-15 2012) and sponsored by the Bordeaux University Hospital. This work was also conducted by the following: CIC 1401-EC, Bordeaux University Hospital, Inserm, and Bordeaux University.
The authors acknowledge the fruitful discussion of these results with Pr Laurent Cohen, Pr Lionel Naccache, and Dr David Bendetowicz.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2018.03.010.
Supplementary data
References
- 1.Tulving E. Episodic and semantic memory. In: Tulving E., Donaldson W., editors. Organization of Memory. Academic Press; New York, USA: 1972. [Google Scholar]
- 2.Birrer R.B., Vemuri S.P. Depression in later life: a diagnostic and therapeutic challenge. Am Fam Physician. 2004;69:2375–2382. [PubMed] [Google Scholar]
- 3.Bronnick K., Alves G., Aarsland D., Tysnes O.B., Larsen J.P. Verbal memory in drug-naive, newly diagnosed Parkinson's disease. The retrieval deficit hypothesis revisited. Neuropsychology. 2011;25:114–124. doi: 10.1037/a0020857. [DOI] [PubMed] [Google Scholar]
- 4.Hornberger M., Piguet O., Graham A.J., Nestor P.J., Hodges J.R. How preserved is episodic memory in behavioral variant frontotemporal dementia? Neurology. 2010;74:472–479. doi: 10.1212/WNL.0b013e3181cef85d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubois B., Feldman H.H., Jacova C., Hampel H., Molinuevo J.L., Blennow K. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 6.Hirni D.I., Kivisaari S.L., Monsch A.U., Taylor K.I. Distinct neuroanatomical bases of episodic and semantic memory performance in Alzheimer's disease. Neuropsychologia. 2013;51:930–937. doi: 10.1016/j.neuropsychologia.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Killiany R.J., Hyman B.T., Gomez-Isla T., Moss M.B., Kikinis R., Jolesz F. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002;58:1188–1196. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- 8.Xu Y., Jack C.R., Jr., O'Brien P.C., Kokmen E., Smith G.E., Ivnik R.J. Usefulness of MRI measures of entorhinal cortex versus hippocampus in AD. Neurology. 2000;54:1760–1767. doi: 10.1212/wnl.54.9.1760. [DOI] [PubMed] [Google Scholar]
- 9.Amieva H., Le Goff M., Millet X., Orgogozo J.M., Peres K., Barberger-Gateau P. Prodromal Alzheimer's disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64:492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- 10.Bateman R.J., Xiong C., Benzinger T.L., Fagan A.M., Goate A., Fox N.C. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Stefano F., Epelbaum S., Coley N., Cantet C., Ousset P.J., Hampel H. Prediction of Alzheimer's disease dementia: data from the GuidAge Prevention Trial. J Alzheimers Dis. 2015;48:793–804. doi: 10.3233/JAD-150013. [DOI] [PubMed] [Google Scholar]
- 12.Kawas C.H., Corrada M.M., Brookmeyer R., Morrison A., Resnick S.M., Zonderman A.B. Visual memory predicts Alzheimer's disease more than a decade before diagnosis. Neurology. 2003;60:1089–1093. doi: 10.1212/01.wnl.0000055813.36504.bf. [DOI] [PubMed] [Google Scholar]
- 13.Small B.J., Fratiglioni L., Viitanen M., Winblad B., Backman L. The course of cognitive impairment in preclinical Alzheimer disease: three- and 6-year follow-up of a population-based sample. Arch Neurol. 2000;57:839–844. doi: 10.1001/archneur.57.6.839. [DOI] [PubMed] [Google Scholar]
- 14.Weintraub S., Wicklund A.H., Salmon D.P. The neuropsychological profile of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006171. doi: 10.1101/cshperspect.a006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen R.C., Stevens J.C., Ganguli M., Tangalos E.G., Cummings J.L., DeKosky S.T. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 16.Epelbaum S., Genthon R., Cavedo E., Habert M.O., Lamari F., Gagliardi G. Preclinical Alzheimer's disease: a systematic review of the cohorts underlying the concept. Alzheimers Dement. 2017;13:454–467. doi: 10.1016/j.jalz.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Jessen F., Amariglio R.E., van Boxtel M., Breteler M., Ceccaldi M., Chetelat G. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tulving E., Osler S. Effectiveness of retrieval cues in memory for words. J Exp Psychol. 1968;77:593–601. doi: 10.1037/h0026069. [DOI] [PubMed] [Google Scholar]
- 19.Teichmann M., Epelbaum S., Samri D., Levy Nogueira M., Michon A., Hampel H. Free and Cued Selective Reminding Test - accuracy for the differential diagnosis of Alzheimer's and neurodegenerative diseases: a large-scale biomarker-characterized monocenter cohort study (ClinAD) Alzheimers Dement. 2017;13:913–923. doi: 10.1016/j.jalz.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Derby C.A., Burns L.C., Wang C., Katz M.J., Zimmerman M.E., L'Italien G. Screening for predementia AD: time-dependent operating characteristics of episodic memory tests. Neurology. 2013;80:1307–1314. doi: 10.1212/WNL.0b013e31828ab2c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemos R., Maroco J., Simoes M.R., Santiago B., Tomas J., Santana I. The free and cued selective reminding test for predicting progression to Alzheimer's disease in patients with mild cognitive impairment: a prospective longitudinal study. J Neuropsychol. 2015;11:40–55. doi: 10.1111/jnp.12075. [DOI] [PubMed] [Google Scholar]
- 22.Sarazin M., Berr C., De Rotrou J., Fabrigoule C., Pasquier F., Legrain S. Amnestic syndrome of the medial temporal type identifies prodromal AD: a longitudinal study. Neurology. 2007;69:1859–1867. doi: 10.1212/01.wnl.0000279336.36610.f7. [DOI] [PubMed] [Google Scholar]
- 23.Dubois B., Feldman H.H., Jacova C., Dekosky S.T., Barberger-Gateau P., Cummings J. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 24.Deweer B., Lehericy S., Pillon B., Baulac M., Chiras J., Marsault C. Memory disorders in probable Alzheimer's disease: the role of hippocampal atrophy as shown with MRI. J Neurol Neurosurg Psychiatry. 1995;58:590–597. doi: 10.1136/jnnp.58.5.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarazin M., Chauvire V., Gerardin E., Colliot O., Kinkingnehun S., de Souza L.C. The amnestic syndrome of hippocampal type in Alzheimer's disease: an MRI study. J Alzheimers Dis. 2010;22:285–294. doi: 10.3233/JAD-2010-091150. [DOI] [PubMed] [Google Scholar]
- 26.Cabeza R., Ciaramelli E., Olson I.R., Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nyberg L., Cabeza R., Tulving E. PET studies of encoding and retrieval: the HERA model. Psychon Bull Rev. 1996;3:135–148. doi: 10.3758/BF03212412. [DOI] [PubMed] [Google Scholar]
- 28.Eustache F., Viard A., Desgranges B. The MNESIS model: memory systems and processes, identity and future thinking. Neuropsychologia. 2016;87:96–109. doi: 10.1016/j.neuropsychologia.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Daselaar S.M., Rice H.J., Greenberg D.L., Cabeza R., LaBar K.S., Rubin D.C. The spatiotemporal dynamics of autobiographical memory: neural correlates of recall, emotional intensity, and reliving. Cereb Cortex. 2008;18:217–229. doi: 10.1093/cercor/bhm048. [DOI] [PubMed] [Google Scholar]
- 30.Oddo S., Lux S., Weiss P.H., Schwab A., Welzer H., Markowitsch H.J. Specific role of medial prefrontal cortex in retrieving recent autobiographical memories: an fMRI study of young female subjects. Cortex. 2010;46:29–39. doi: 10.1016/j.cortex.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 31.St Jacques P., Rubin D.C., LaBar K.S., Cabeza R. The short and long of it: neural correlates of temporal-order memory for autobiographical events. J Cogn Neurosci. 2008;20:1327–1341. doi: 10.1162/jocn.2008.20091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okuda J., Fujii T., Ohtake H., Tsukiura T., Tanji K., Suzuki K. Thinking of the future and past: the roles of the frontal pole and the medial temporal lobes. Neuroimage. 2003;19:1369–1380. doi: 10.1016/s1053-8119(03)00179-4. [DOI] [PubMed] [Google Scholar]
- 33.Steinvorth S., Corkin S., Halgren E. Ecphory of autobiographical memories: an fMRI study of recent and remote memory retrieval. Neuroimage. 2006;30:285–298. doi: 10.1016/j.neuroimage.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piolino P., Desgranges B., Hubert V., Bernard F.A., Matuszewski V., Chetelat G. Reliving lifelong episodic autobiographical memories via the hippocampus: a correlative resting PET study in healthy middle-aged subjects. Hippocampus. 2008;18:445–459. doi: 10.1002/hipo.20406. [DOI] [PubMed] [Google Scholar]
- 35.Rabin J.S., Gilboa A., Stuss D.T., Mar R.A., Rosenbaum R.S. Common and unique neural correlates of autobiographical memory and theory of mind. J Cogn Neurosci. 2010;22:1095–1111. doi: 10.1162/jocn.2009.21344. [DOI] [PubMed] [Google Scholar]
- 36.Viard A., Desgranges B., Eustache F., Piolino P. Factors affecting medial temporal lobe engagement for past and future episodic events: an ALE meta-analysis of neuroimaging studies. Brain Cogn. 2012;80:111–125. doi: 10.1016/j.bandc.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Dufouil C., Dubois B., Vellas B., Pasquier F., Blanc F., Hugon J. Cognitive and imaging markers in non-demented subjects attending a memory clinic: study design and baseline findings of the MEMENTO cohort. Alzheimers Res Ther. 2017;9:67. doi: 10.1186/s13195-017-0288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris J.C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 39.Grober E., Buschke H., Crystal H., Bang S., Dresner R. Screening for dementia by memory testing. Neurology. 1988;38:900–903. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- 40.Van der Linden M., Coyette F., Poitrenaud J., Kalafat M., Calicis F., Wyns C. L'épreuve de rappel libre/rappel indicé à 16 items (RL/RI-16) In: Solal, editor. L'évaluation des troubles de la mémoire: présentation de quatre tests de mémoire épisodique avec leur étalonnage. Solal; Marseille, France: 2004. pp. 25–47. [Google Scholar]
- 41.Rey A. L'examen psychologique dans les cas d'encéphalopathie traumatique. Arch de Psychol. 1941;28:286–340. [Google Scholar]
- 42.Petersen R.C. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 43.Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 44.Chupin M., Hammers A., Liu R.S., Colliot O., Burdett J., Bardinet E. Automatic segmentation of the hippocampus and the amygdala driven by hybrid constraints: method and validation. Neuroimage. 2009;46:749–761. doi: 10.1016/j.neuroimage.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chupin M., Mukuna-Bantumbakulu A.R., Hasboun D., Bardinet E., Baillet S., Kinkingnehun S. Anatomically constrained region deformation for the automated segmentation of the hippocampus and the amygdala: method and validation on controls and patients with Alzheimer's disease. Neuroimage. 2007;34:996–1019. doi: 10.1016/j.neuroimage.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 46.Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 47.Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Habert M.O., Marie S., Bertin H., Reynal M., Martini J.B., Diallo M. Optimization of brain PET imaging for a multicentre trial: the French CATI experience. EJNMMI Phys. 2016;3:6. doi: 10.1186/s40658-016-0141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rolls E.T., Joliot M., Tzourio-Mazoyer N. Implementation of a new parcellation of the orbitofrontal cortex in the automated anatomical labeling atlas. Neuroimage. 2015;122:1–5. doi: 10.1016/j.neuroimage.2015.07.075. [DOI] [PubMed] [Google Scholar]
- 50.Hochberg Y., Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 51.Proust-Lima C., Amieva H., Dartigues J.F., Jacqmin-Gadda H. Sensitivity of four psychometric tests to measure cognitive changes in brain aging-population-based studies. Am J Epidemiol. 2007;165:344–350. doi: 10.1093/aje/kwk017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Proust C., Jacqmin-Gadda H., Taylor J.M., Ganiayre J., Commenges D. A nonlinear model with latent process for cognitive evolution using multivariate longitudinal data. Biometrics. 2006;62:1014–1024. doi: 10.1111/j.1541-0420.2006.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buffon F., Porcher R., Hernandez K., Kurtz A., Pointeau S., Vahedi K. Cognitive profile in CADASIL. J Neurol Neurosurg Psychiatry. 2006;77:175–180. doi: 10.1136/jnnp.2005.068726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Epelbaum S., Benisty S., Reyes S., O'Sullivan M., Jouvent E., During M. Verbal memory impairment in subcortical ischemic vascular disease: a descriptive analysis in CADASIL. Neurobiol Aging. 2011;32:2172–2182. doi: 10.1016/j.neurobiolaging.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 55.Cabeza R., Dolcos F., Graham R., Nyberg L. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuroimage. 2002;16:317–330. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- 56.Alsop D.C., Casement M., de Bazelaire C., Fong T., Press D.Z. Hippocampal hyperperfusion in Alzheimer's disease. Neuroimage. 2008;42:1267–1274. doi: 10.1016/j.neuroimage.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davies R.R., Halliday G.M., Xuereb J.H., Kril J.J., Hodges J.R. The neural basis of semantic memory: evidence from semantic dementia. Neurobiol Aging. 2009;30:2043–2052. doi: 10.1016/j.neurobiolaging.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Auriacombe S., Helmer C., Amieva H., Berr C., Dubois B., Dartigues J.F. Validity of the free and cued selective reminding test in predicting dementia: the 3C study. Neurology. 2010;74:1760–1767. doi: 10.1212/WNL.0b013e3181df0959. [DOI] [PubMed] [Google Scholar]
- 59.Grober E., Sanders A.E., Hall C., Lipton R.B. Free and cued selective reminding identifies very mild dementia in primary care. Alzheimer Dis Assoc Disord. 2010;24:284–290. doi: 10.1097/WAD.0b013e3181cfc78b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mura T., Proust-Lima C., Jacqmin-Gadda H., Akbaraly T.N., Touchon J., Dubois B. Measuring cognitive change in subjects with prodromal Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2014;85:363–370. doi: 10.1136/jnnp-2013-305078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jucker M., Walker L.C. Pathogenic protein seeding in alzheimer disease and other neurodegenerative disorders. Ann Neurol. 2011;70:532–540. doi: 10.1002/ana.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 63.Scholl M., Lockhart S.N., Schonhaut D.R., O'Neil J.P., Janabi M., Ossenkoppele R. PET Imaging of Tau Deposition in the Aging Human Brain. Neuron. 2016;89:971–982. doi: 10.1016/j.neuron.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thal D.R., Rub U., Orantes M., Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 65.Villeneuve S., Rabinovici G.D., Cohn-Sheehy B.I., Madison C., Ayakta N., Ghosh P.M. Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. 2015;138:2020–2033. doi: 10.1093/brain/awv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Papp K.V., Rentz D.M., Mormino E.C., Schultz A.P., Amariglio R.E., Quiroz Y. Cued memory decline in biomarker-defined preclinical Alzheimer disease. Neurology. 2017;88:1431–1438. doi: 10.1212/WNL.0000000000003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chetelat G., Desgranges B., de la Sayette V., Viader F., Eustache F., Baron J.C. Mild cognitive impairment: can FDG-PET predict who is to rapidly convert to Alzheimer's disease? Neurology. 2003;60:1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 68.Reiman E.M., Chen K., Alexander G.E., Caselli R.J., Bandy D., Osborne D. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci U S A. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grober E., Lipton R.B., Hall C., Crystal H. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 2000;54:827–832. doi: 10.1212/wnl.54.4.827. [DOI] [PubMed] [Google Scholar]
- 70.Wolk D.A., Dickerson B.C. Alzheimer's Disease Neuroimaging I. Fractionating verbal episodic memory in Alzheimer's disease. Neuroimage. 2011;54:1530–1539. doi: 10.1016/j.neuroimage.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dickerson B.C., Wolk D.A. Alzheimer's Disease Neuroimaging I. MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults. Neurology. 2012;78:84–90. doi: 10.1212/WNL.0b013e31823efc6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cavedo E., Dubois B., Colliot O., Lista S., Croisile B., Tisserand G.L. Reduced regional cortical thickness rate of change in Donepezil-treated subjects with suspected prodromal Alzheimer's disease. J Clin Psychiatry. 2016;77:e1631–e1638. doi: 10.4088/JCP.15m10413. [DOI] [PubMed] [Google Scholar]
- 73.Dubois B., Chupin M., Hampel H., Lista S., Cavedo E., Croisile B. Donepezil decreases annual rate of hippocampal atrophy in suspected prodromal Alzheimer's disease. Alzheimers Dement. 2015;11:1041–1049. doi: 10.1016/j.jalz.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 74.Jack C.R., Jr., Petersen R.C., Grundman M., Jin S., Gamst A., Ward C.P. Longitudinal MRI findings from the vitamin E and donepezil treatment study for MCI. Neurobiol Aging. 2008;29:1285–1295. doi: 10.1016/j.neurobiolaging.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petersen R.C., Thomas R.G., Grundman M., Bennett D., Doody R., Ferris S. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 76.Dubois B., Epelbaum S., Nyasse F., Bakardjian H., Gagliardi G., Uspenskaya O. Cognitive and neuroimaging features and brain beta-amyloidosis in individuals at risk of Alzheimer's disease (INSIGHT-preAD): a longitudinal observational study. Lancet Neurol. 2018;17:335–346. doi: 10.1016/S1474-4422(18)30029-2. [DOI] [PubMed] [Google Scholar]
- 77.Donohue M.C., Sperling R.A., Salmon D.P., Rentz D.M., Raman R., Thomas R.G. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. 2014;71:961–970. doi: 10.1001/jamaneurol.2014.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Papp K.V., Rentz D.M., Orlovsky I., Sperling R.A., Mormino E.C. Optimizing the preclinical Alzheimer's cognitive composite with semantic processing: the PACC5. Alzheimers Dement (N Y) 2017;3:668–677. doi: 10.1016/j.trci.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chhetri J.K., de Souto Barreto P., Cantet C., Cesari M., Coley N., Andrieu S. Trajectory of the MAPT-PACC-preclinical Alzheimer cognitive composite in the Placebo Group of a Randomized Control Trial: results from the MAPT Study: lessons for Further Trials. J Prev Alzheimer's Dis. 2018;5:31–35. doi: 10.14283/jpad.2017.21. [DOI] [PubMed] [Google Scholar]
- 80.D'Argembeau A., Stawarczyk D., Majerus S., Collette F., Van der Linden M., Feyers D. The neural basis of personal goal processing when envisioning future events. J Cogn Neurosci. 2010;22:1701–1713. doi: 10.1162/jocn.2009.21314. [DOI] [PubMed] [Google Scholar]
- 81.Eklund A., Nichols T.E., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Operto G., Chupin M., Batrancourt B., Habert M.O., Colliot O., Benali H. CATI: a Large distributed infrastructure for the neuroimaging of cohorts. Neuroinformatics. 2016;14:253–264. doi: 10.1007/s12021-016-9295-8. [DOI] [PubMed] [Google Scholar]
- 83.Suppa P., Hampel H., Spies L., Fiebach J.B., Dubois B., Buchert R. Fully automated atlas-based hippocampus volumetry for clinical routine: validation in subjects with mild cognitive impairment from the ADNI cohort. J Alzheimers Dis. 2015;46:199–209. doi: 10.3233/JAD-142280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.