Abstract
Introduction
We assessed the feasibility and cognitive effects of a ketogenic diet (KD) in participants with Alzheimer's disease.
Methods
The Ketogenic Diet Retention and Feasibility Trial featured a 3-month, medium-chain triglyceride–supplemented KD followed by a 1-month washout in clinical dementia rating (CDR) 0.5, 1, and 2 participants. We obtained urine acetoacetate, serum β-hydroxybutyrate, food record, and safety data. We administered the Alzheimer's Disease Assessment Scale-cognitive subscale and Mini–Mental State Examination before the KD, and following the intervention and washout.
Results
We enrolled seven CDR 0.5, four CDR 1, and four CDR 2 participants. One CDR 0.5 and all CDR 2 participants withdrew citing caregiver burden. The 10 completers achieved ketosis. Most adverse events were medium-chain triglyceride–related. Among the completers, the mean of the Alzheimer's Disease Assessment Scale-cognitive subscale score improved by 4.1 points during the diet (P = .02) and reverted to baseline after the washout.
Discussion
This pilot trial justifies KD studies in mild Alzheimer's disease.
Keywords: Alzheimer's disease, Cognition, Ketogenic diet, Low-carbohydrate diet, Medium-chain triglyceride
Highlights
-
•
The medium-chain triglyceride–supplemented KD was feasible in very mild (clinical dementia rating [CDR] 0.5) and mild (CDR 1) Alzheimer's disease participants, as 10 of 11 participants adhered to the dietary protocol.
-
•
The medium chain triglyceride-supplemented KD was not feasible in moderate (CDR 2) Alzheimer's disease participants as all four of these participants withdrew from the study.
-
•
Dietary compliant participants had a 4.1-point mean improvement on Alzheimer's Disease Assessment Scale-cognitive subscale scores from baseline to the end of the diet. Alzheimer's Disease Assessment Scale-cognitive subscale improvements diminished after a 1-month diet washout period.
1. Introduction
Aberrant energy metabolism occurs in Alzheimer's disease (AD) animal models and patients [1]. It manifests as perturbed mitochondrial function and structure as well as during fluorodeoxyglucose positron emission tomography evaluations. Fluorodeoxyglucose positron emission tomography reveals focal reductions in brain glucose utilization with advancing age [2], [3], which extend in magnitude and distribution during AD [4], [5]. Neuron loss, synaptic degradation, or bona fide bioenergetic changes could contribute to this, although changes in asymptomatic subjects with AD risk factors suggest that bona fide changes exist [6], [7], [8]. Brain homogenates of AD subjects also show less glucose consumption than control homogenates [9], even though homogenization disrupts synapses and neutralizes their impact; persistence of reduced glucose flux in homogenates suggests that diminished synaptic connectivity does not exclusively account for reduced AD glucose utilization.
In AD, targeting bioenergetic defects and decreased glucose utilization for therapeutic purposes is reasonable [10]. One potential approach includes the ketogenic diet (KD). KDs increase fat and reduce carbohydrate consumption [11]. This reduces insulin, which stimulates liver oxidation of fatty acids to ketone bodies (β-hydroxybutyrate [BHB], acetoacetate, and acetone) that enter the blood. BHB and acetoacetate access the brain and fuel respiration [12]. Acetone also circulates, it may access the brain, and ventilation facilitates its excretion. Given a choice between ketone bodies and glucose, neurons preferentially consume ketone bodies [13], [14], [15]. Although brain glucose utilization declines in AD, ketone body utilization does not [16].
Here, we considered the previously proposed question [17] of whether a KD can benefit cognition in AD. Although studies report that ketosis-like diets improve memory in cognitively intact or mild cognitive impairment participants [18], [19], [20] and that a simple medium chain triglyceride (MCT)-induced ketosis may improve AD cognition [21], [22], whether a true KD affects cognition in actual AD patients or is even feasible remains unclear. Adding to the relevance of this question, KDs differ from MCT-limited approaches in terms of ketone body pharmacokinetics, dietary fat intake, insulin signaling effects, and inflammation effects. We therefore tested the feasibility of maintaining AD participants on a KD and acquired preliminary insight into how a KD affects cognition in AD participants.
2. Methods
2.1. Overall study design and recruitment
The Ketogenic Diet Retention and Feasibility Trial (KDRAFT) was a single-arm, pilot clinical study with a target enrollment of 15 participants with AD. The protocol required participants to maintain an MCT-supplemented KD for 3 months and immediately on completing this intervention to discontinue that diet and resume a normal diet for 1 month (thereby defining a 1-month washout period). Because from a calorie intake perspective our diet increased dietary fat consumption and added MCT to offset lowered carbohydrate consumption, to distinguish our diet from a low-carbohydrate, calorie-restricted KD we herein refer to our diet intervention as a very high-fat KD (VHF-KD).
To recruit for this trial, we first identified potential participants from a registry database maintained by the University of Kansas Alzheimer's Disease Center. Then, we informed potential participants about the study opportunity through one of two approaches. One approach used letters or phone calls to initiate discussion of this opportunity. Alternatively, we invited potential participants to a KD cooking demonstration at the University of Kansas (KU) Clinical Research Center Demonstration Kitchen and used this event to initiate discussion of the research opportunity.
2.2. Participants
The participants met McKhann et al. criteria for AD [23]. Individuals were eligible to participate in the study if they had a clinical dementia rating (CDR) of very mild AD (CDR 0.5), mild AD (CDR 1), or moderate AD (CDR 2). No clinically significant electrolyte, renal, or liver function abnormalities, a body mass index (BMI) ≥ 21 kg/m2, English mastery, and an active study partner were also required. Exclusion criteria included serious medical risks including type 1 diabetes, ongoing or recent cancer, a cardiac event in the past year, or other conditions deemed serious risks by physicians on the study team. The KU Medical Center's Institutional Review Board approved the protocol. Informed consent was obtained from all study participants as per institutional guidelines.
2.3. The VHF-KD intervention and dietary intake evaluations
Participants received nutrition counseling from the study dietitian at the baseline study visit. Targeted macronutrient composition for the dietary intervention included approximately 70% of energy as fat (including the MCT), 20% of energy as protein, and restriction of carbohydrate to less than 10% of energy; we sought a ketogenic ratio of 1:1 (ratio of lipid consumption in grams to nonlipid consumption in grams) or better. Energy intake requirement and targeted daily MCT dosage was estimated using the Mifflin-St. Jeor equation [24]. A monthly supply of MCT oil (Now Foods, USA), which contained a combination of C8:0 and C10:0 fatty acids, was provided at each study visit. In general, the MCT dosage provided approximately 10% of total energy from fat during the first week and increased by increments of 10% during each successive week until reaching 40%, while allowing for titration adjustments based on participant tolerance. Participants could choose to consume the MCT neat or mix it with food or beverages. We provided daily multivitamin, vitamin D, calcium, and phosphorus supplements to help prevent micronutrient deficiencies. On completion of the VHF-KD, participants were instructed to return to a normal diet to complete a 1-month washout period.
2.4. Objectives and outcomes
Our primary objective was to address the feasibility of implementing a VHF-KD intervention in AD participants. Secondary objectives included evaluating the effects of a VHF-KD on cognition.
2.4.1. Dietary assessment
At the baseline visit, the study dietitian provided instructions to the participant's study partner for completing 3-day food records (3DFRs). Each 3DFR recorded intake for two weekdays and one weekend day. We entered 3DFR data into the Nutrition Data System for Research (version 2016) to analyze nutrient intake. We collected 3DFRs immediately after the baseline visit (just before starting the diet); at months 1, 2, and 3; and during washout visits to characterize dietary intake before, during, and after the dietary intervention.
2.4.2. Biomarker, safety, and anthropometric assessments
Participants self-monitored urine ketones daily, in the early evening, using urine acetoacetate test strips (Ketostix, Bayer, Germany). Daily urine ketone status was recorded as either negative, trace (5–14.9 mg/dL), small (15–39.9 mg/dL), moderate (40–79.9 mg/dL), or large (80+ mg/dL) in a provided diary. Days in which participants did not measure the ketone levels were conservatively tallied as a “negative” ketone response.
All serum biomarker and safety lab tests were collected after a 12-hour fast. Full lipid and metabolic panels were collected at the baseline and month 3 (end of diet intervention) visits, and these assays were performed by the KU Hospital clinical laboratory. Serum electrolytes, renal function tests, liver function tests, and glucose levels were measured by the KU Hospital clinical laboratory at the baseline and month 3 visits. Serum BHB and insulin levels were measured at all visit time points (baseline, month 1, month 2, month 3, and washout) by the KU Hospital clinical laboratory. Homeostatic model assessment 2-insulin resistance (HOMA2-IR) values for each participant were calculated using a HOMA2 Calculator (v. 2.2.3; University of Oxford, United Kingdom).
At each visit, we queried the participants and their care partners for adverse symptoms. Also at each visit, we further encouraged the participants and their care partners to contact the study dietitian for any symptoms or medical complaints they thought might relate to their participation in the study. As part of an additional safety measure, participants received baseline and month 1, 2, and 3 electrocardiogram assessments.
Height, weight, and body composition were measured for all subjects. BMI (kg/m2) was calculated using weight and height measurements. Dual energy x-ray absorptiometry was used to attain and quantify body fat percentage, lean body mass, fat mass, and bone mass at baseline and month 3.
2.4.3. Cognitive testing
A trained psychometrician administered the Mini–Mental State Examination (MMSE) and the Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-cog) at baseline, at the end of the intervention (month 3), and after the 1-month washout. The MMSE is a brief, 30-point questionnaire designed to identify cognitive impairment. The ADAS-cog consists of 11 tasks that measure changes in memory, language, praxis, and attention. Total incorrect answers and cued reminders are reported as the cumulative score, thus higher scores on the ADAS-cog reflect greater cognitive impairment.
2.5. Statistical analyses
Continuous variables were described using their means and standard deviations. Descriptive statistics were utilized to quantify and characterize feasibility of the intervention. Normality of the dependent variables was assessed through Q-Q visualization of residual models. Paired t-tests were used to analyze differences in dietary macronutrient intake before and during the diet intervention. We constructed linear mixed models and tested mean differences with post-hoc pairwise comparisons for cognitive data and serum biomarkers, with the exception of serum BHB where residual assessment indicated the use of Friedman tests with Conover post-hoc pairwise comparisons. Statistical analyses were performed using R (v. 3.3.2; R Foundation, Vienna, Austria). Statistical tests were two-tailed, and significance was set at P < .05. For the cognition analysis, our null hypothesis assumed that a VHF-KD would not affect the cognitive performance of participants with AD.
3. Results
Fifteen participants enrolled in the study over a 31-month period (December 2013–July 2016). The mean age and education levels were 73.1 ± 9.0 and 15.0 ± 2.7 years, respectively. Seven were male. To enroll these 15 participants, we contacted 298 individuals. During the first 19 months, 72 potential participants were contacted by the study coordinator via letter or phone call, and nine enrolled in the study. From months 20 through 31, we invited 185 participants, by letter, to one of two different ketogenic cooking demonstrations at the KU Clinical Research Center Demonstration Kitchen. Thirty-eight total potential participants attended one of two cooking demonstrations; attendance was limited by space limitations and not by invitee interest. These two cooking demonstrations resulted in the enrollment of six new participants. Supplementary Figure 1 depicts recruitment and participant flow.
Five participants did not successfully implement the VHF-KD, withdrew from the study within the first month or shortly after the first month (dropout rate of 33%), and provided only baseline data. Four of the dropouts had moderate AD (CDR 2), and the other had very mild AD (CDR 0.5) (Table 1). The partners of the participants that withdrew from the study uniformly cited caregiver burden as the reason for discontinuing. The remaining 10 participants were compliant with the VHF-KD intervention, although one participant discontinued cholinesterase inhibitor therapy and was therefore technically protocol noncompliant. This was the only participant in whom we documented cognitive decline.
Table 1.
Participants and trial disposition
| Subject ID | CDR | Sex | Data for analysis | Time/reason for dropout | Symptoms/complaints |
|---|---|---|---|---|---|
| Very Mild AD | |||||
| 01∗ | 0.5 | M | X | Diarrhea | |
| 02 | 0.5 | M | Week 2/Caregiver Burden | ||
| 05† | 0.5 | M | X | ||
| 06 | 0.5 | F | X | Nausea, Diarrhea | |
| 07 | 0.5 | F | X | ||
| 11 | 0.5 | F | X | ||
| 13 | 0.5 | M | X | Diarrhea | |
| Mild AD | |||||
| 08 | 1 | F | X | ||
| 10 | 1 | M | X | Diarrhea | |
| 14 | 1 | F | X | Diarrhea | |
| 15 | 1 | F | X | ||
| Moderate AD | |||||
| 03 | 2 | M | Week 4/Caregiver Burden | ||
| 04 | 2 | F | Week 4/Caregiver Burden | ||
| 09 | 2 | M | Week 5/Caregiver Burden | ||
| 12 | 2 | F | Week 4/Caregiver Burden | ||
Abbreviations: CDR, clinical dementia rating; AD, Alzheimer's disease; M, male; F, female.
Subject 01 completed the intervention, but did not contribute washout cognitive data.
Subject 05 was intervention-compliant, but not protocol compliant as cholinesterase inhibitor therapy was discontinued.
Most participants preferred to mix their MCT oil with either food or beverages and used this strategy to enhance MCT tolerability. Participants who completed the intervention generally consumed 1.5–3 tablespoons of MCT oil per day, which constituted 60–80% of the target MCT volume. No one reached 100% of the MCT target volume.
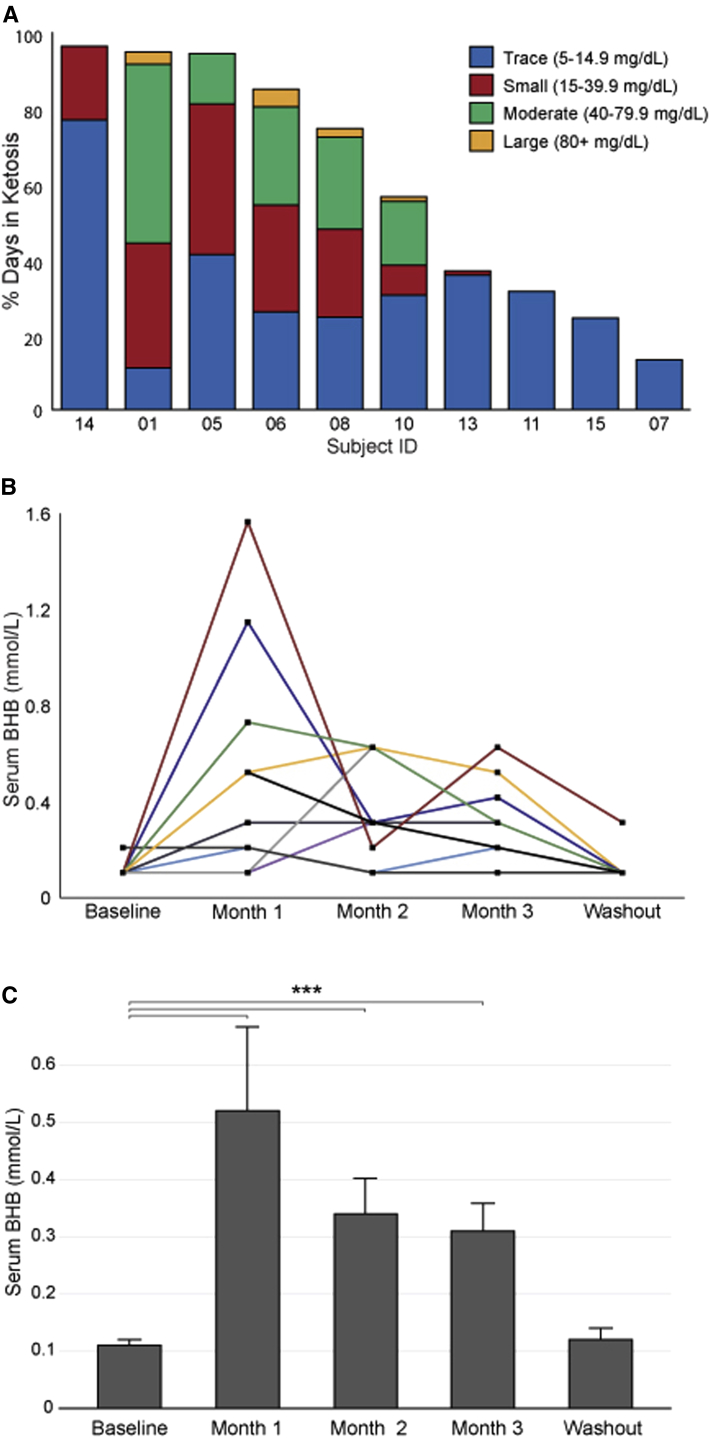
Self-reported urine ketone assessments from the 10 completers revealed each achieved ketosis, although the depth and consistency of ketosis varied. On average, completers detected the presence of at least some degree of urine acetoacetate on 54.5 ± 29.0 days (60.6%) of the 3-month dietary intervention. Forty-nine percent of the positive daily readings were reported as trace, 26% as small, 23% as moderate, and 2% as large. Fig. 1A summarizes each subject's urine ketone data.
Fig. 1.
Urine and serum BHB values. (A) The figure indicates the percentage of days in which each participant's self-reported urine acetoacetate analysis revealed ketosis, as well as the depth of ketosis achieved. (B) Serum BHB values for each subject. Subject 15, who reported only trace urine acetoacetate for only 24.2% of the intervention, is shown by a yellow line. (C) Mean serum BHB values ± SD. ∗∗∗P < .001. Abbreviations: BHB, β-hydroxybutyrate; SD, standard deviation; ADAS-cog, Alzheimer's Disease Assessment Scale-cognitive subscale; CDR, clinical dementia rating.
Serum BHB levels were significantly elevated at months 1, 2, and 3 compared with that of baseline (0.11 mmol/L at baseline vs. 0.52, 0.34, and 0.31 mmol/L; P < .001) (Fig. 1B and 1C) and returned to the normal range at the end of the washout period (0.12 mmol/L). Serum BHB results generally seemed to reflect urine ketone responses with one exception. One participant's (subject 15) urine acetoacetate reached only trace levels and for just 24.2% of the days on the diet, but throughout the intervention, serum BHB measurements showed a relatively robust induction (see Fig. 1A and 1B).
Table 2 shows values for anthropometric measures, serum biomarkers, safety labs, metabolic measures, and dietary intake. Preintervention and intraintervention energy intake values were comparable. During the intervention, total fat intake increased (fat: 90.6 g vs. 166.7 g; P < .001), and total carbohydrate intake was significantly reduced (209.8 g vs. 46.0 g; P < .001). The mean macronutrient proportion during the VHF-KD intervention was 73.4% fat, 9% carbohydrate, and 17.6% protein as energy.
Table 2.
Anthropometric, blood-derived, and dietary intake data (n = 10)
| Parameter | Baseline | Month 3 | P-value |
|---|---|---|---|
| Anthropometric measures | |||
| Weight, kg | 76.5 (17.4) | 74.7 (16.2) | .81 |
| BMI, kg/m2 | 27.7 (7.5) | 26.9 (6.8) | .81 |
| Body fat, % | 38.6 (11.5) | 38.2 (11.3) | .94 |
| Lean body mass, kg | 43.5 (7.6) | 42.8 (8.6) | .85 |
| Bone mass, kg | 2.7 (0.7) | 2.7 (0.7) | .98 |
| Lipid status | |||
| Total cholesterol, mg/dL | 173.0 (27.5) | 194.4 (36.3) | .16 |
| LDL, mg/dL | 102.1 (18.7) | 117.4 (25.4) | .14 |
| HDL, mg/dL | 55.4 (9.2) | 58.2 (8.6) | .49 |
| Serum biomarkers | |||
| AST, U/L | 21.1 (5.2) | 24.5 (7.0) | .24 |
| ALT, U/L | 15.7 (6.5) | 18.9 (8.1) | .34 |
| Alkaline phosphatase, U/L | 62.6 (21.3) | 55.8 (19.5) | .48 |
| Calcium, mg/dL | 9.4 (0.4) | 9.5 (0.3) | .71 |
| Chloride, mEq/L | 104.1 (1.3) | 105.4 (2.2) | .12 |
| Potassium, mEq/L | 4.0 (0.3) | 4.1 (0.2) | .47 |
| Sodium, mEq/L | 137.2 (1.8) | 137.9 (1.9) | .41 |
| BUN, mg/dL | 20.9 (4.3) | 20.1 (6.1) | .74 |
| Creatinine, mg/dL | 1.0 (0.2) | 1.1 (0.3) | .58 |
| Metabolic status | |||
| Insulin, μU/mL | 5.1 (2.2) | 5.0 (2.2) | .94 |
| Glucose, mg/dL | 92.6 (5.6) | 95.8 (10.0) | .40 |
| HOMA2-IR | 0.7 (0.3) | 0.7 (0.3) | .97 |
| Beta, % | 67.8 (22.1) | 62.3 (19.0) | .56 |
| Sensitivity, % | 169.6 (51.0) | 182.3 (86.4) | .70 |
| Dietary intake | |||
| Energy (kcal) | 1950.7 (450.6) | 2002.8 (660.7) | .80 |
| Fat, g | 90.6 (23.5) | 166.7 (60.0) | <.001∗ |
| Carbohydrate, g | 209.8 (80.3) | 46.0 (26.9) | <.001∗ |
| Protein, g | 81.3 (21.1) | 90.0 (28.2) | .34 |
Abbreviations: BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; HOMA2-IR, homeostatic model assessment 2-insulin resistance; SD, standard deviation.
NOTE. Values are Mean (SD).
Significant difference between time points (P ≤ .05).
No serious adverse events occurred. Participant complaints were minor in magnitude and transient, and mostly limited to MCT-associated diarrhea (in 50%). Weight, cholesterol, blood glucose, insulin, and homeostatic model assessment 2-insulin resistance parameters remained stable. The VHF-KD did not alter electrolyte, renal, or liver function values. BMI, body fat percentage, and bone mass did not change. There were no electrocardiogram changes.
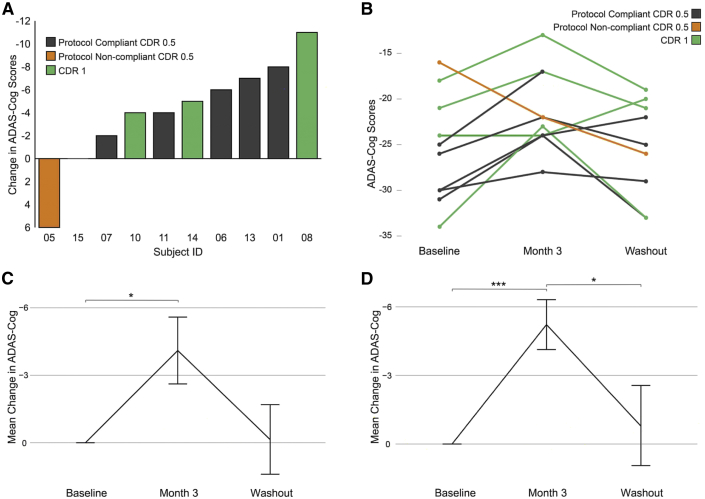
We used a linear mixed model with pairwise analysis to compare cognitive performance at baseline, month 3, and after the 1-month washout period (Fig. 2). Including all diet compliant participants (n = 10), ADAS-cog scores significantly changed from baseline to month 3 with a mean improvement of 4.1 points (25.5 vs. 21.4, P = .02). Excluding the one protocol noncompliant completer (n = 9), ADAS-cog scores changed from baseline to month 3 with a mean improvement of 5.3 points (26.6 vs. 21.3, P = .001). ADAS-cog scores reverted to baseline following the 1-month washout period. After the washout period, the mean ADAS-cog score was 25.3 for all participants (P = .09 for the comparison between the 3-month and the washout values) and also for the protocol compliant subjects (P = .05 for the comparison between the 3 month and washout values). Excluding the protocol noncompliant participant, MMSE scores significantly improved from baseline to month 3 (25.2 vs. 26.3, P = .05) (Table 3).
Fig. 2.
ADAS-cog scores. (A) Waterfall plot showing the difference between the baseline and 3-month VHF-KD ADAS-cog scores for the 10 participants who completed the diet intervention. Subject 15 was CDR 1. (B) The ADAS-cog scores for each participant who completed the diet intervention (baseline score, after 3 months on the VHF-KD, and after a 1 month washout period) are shown. One of the CDR 0.5 participants did not complete washout testing and so an ADAS-cog washout value is not available for this participant. (C) Mean change ± SD ADAS-cog data for all participants who completed the VHF-KD intervention. (D) Mean change ± SD ADAS-cog data for the protocol-compliant participants. ∗P ≤ .05; ∗∗∗P < .005. Abbreviations: ADAS-cog, Alzheimer's Disease Assessment Scale-cognitive subscale; VHF-KD, very high-fat KD; CDR, clinical dementia rating; SD, standard deviation.
Table 3.
KDRAFT cognitive test scores
| Parameter | All participants |
Protocol compliant participants |
||||
|---|---|---|---|---|---|---|
| Baseline (n = 10) | Month 3 (n = 10) | Washout (n = 9) | Baseline (n = 9) | Month 3 (n = 9) | Washout (n = 8) | |
| ADAS-cog | 25.5 (5.9) | 21.4 (4.4)∗ | 25.3 (5.4) | 26.6 (5.1) | 21.3 (4.7)† | 25.3 (5.7) |
| MMSE | 25.5 (1.5) | 26.3 (0.5) | 25.1 (1.8) | 25.2 (1.3) | 26.3 (0.5)‡ | 25.4 (1.7) |
Abbreviations: KDRAFT, Ketogenic Diet Retention and Feasibility Trial; ADAS-cog, Alzheimer's Disease Assessment Scale-cognitive subscale; MMSE, Mini–Mental State Examination; KD, ketogenic diet; SD, standard deviation.
NOTE. Values are Mean (SD).
P = .02, Baseline vs. KD by linear mixed model.
P = .001, Baseline vs. KD by linear mixed model.
P = .05, Baseline vs. KD by linear mixed model.
4. Discussion
This pilot trial investigated the feasibility of a 3-month MCT-supplemented KD intervention in AD participants. The VHF-KD proved feasible in very mild (CDR 0.5) and mild (CDR 1) participants, as 10 of 11 adapted to a restricted carbohydrate and augmented fat nutrient pattern, reached the target macronutrient ratio, demonstrated elevated urine and serum ketone levels, and completed the intervention. We cannot generalize this conclusion to moderate (CDR 2) participants, though, as all four CDR 2 participants failed to achieve ketosis and each withdrew from the study. Secondarily, study completers demonstrated objective improvement on a cognitive assessment (the ADAS-cog), and this objective improvement disappeared after a washout period.
Existing literature documents the challenges of enrolling adults into KD studies [25], [26]. Perceptions of the KD border on undesirable, which make it difficult to recruit participants via traditional contact methods (i.e. letter, phone call, and clinic visit). Placing persons with AD on a KD presents an additional hurdle, as this inevitably contributes to care-provider burden. Based on our study's recruitment experience, we believe that KD studies in adult populations require unique, interactive strategies to generate participation interest. We found that KD cooking demonstrations enhanced interest, altered KD preconceptions, and expedited recruitment.
Participants with more advanced dementia did not comply with the diet intervention. A priori, one might predict that a positive correlation could exist between dementia severity and compliance because dementia severity should also correlate inversely with the diversity of food choice requests. In other words, moderately or severely demented individuals might generate fewer requests for specific meals or snacks than mildly demented individuals. Conversely, dementia severity also positively correlates with amounts of caregiver burden [27]. Our KD intervention required study partners to assume varying degrees of additional responsibility, including compliance with complex meal preparation instructions, MCT administration, urinary ketone assessment, and food diary completion. Implementing a relatively complex intervention such as a KD may have simply overwhelmed the caregivers of the moderate AD participants. Our data are consistent with this scenario. It is important to note, though, our impression that high levels of study partner burden influenced participant withdrawal is based on informal, anecdotal study partner reporting and not on formal measurements of study partner burden. Future studies of a KD in AD should consider applying quantitative measurements of caregiver burden.
When the study partner was a male, the dropout rate was 25%, and when the study partner was a female, the dropout rate was 43%. These percentages are not significantly different. In general, we could not identify differences between study partners of participants who did and did not comply with the intervention. The relatively small size of this study may account for our inability to identify specific characteristics that predict compliance.
A higher rate of study withdrawal among those with moderate dementia suggests that KD studies of community-based AD participants should focus on individuals with mild and very mild dementia. Studies of moderate or severe AD may still be possible, but should perhaps consider limiting the intervention to participants that live in institutional settings (to minimize diet-associated increases in caregiver burden).
Previously reported KD side effects include constipation, nausea, vomiting, diarrhea, fatigue, and hunger [28]. No participant complained of hunger or fatigue. Some of our participants did report gastrointestinal side effects, particularly diarrhea. Participants and their care-partners, though, tended to associate these complaints with MCT oil consumption and not the KD itself. Utilization of a flexible MCT titration protocol, therefore, likely enhanced compliance and reduced adverse event quality and quantity.
There were no serious adverse events. Laboratory and electrocardiogram tests raised no safety concerns. Weights remained stable and there was no loss of bone mass. We conclude that a VHF-KD is safe in AD, at least over 3 months, and that persons with AD can maintain and tolerate a VHF-KD for at least 3 months.
Because of our small sample size and single-arm design, any interpretation of this study's cognitive performance data requires caution. We may parsimoniously state, though, that a statistically significant improvement in ADAS-cog score coincident with the diet intervention was observed, which is consistent with our research hypothesis of diet-associated changes in this measure. This apparent improvement could reflect a placebo effect, the expectations of an unblinded psychometrician, participant motivation, or a test–retest effect although reversion of the ADAS-cog scores after a washout period argue against the possibility of a test–retest effect.
Only one participant's ADAS-cog score declined while following the diet protocol. This participant's performance continued to deteriorate for at least 1 month after completing the KD intervention, so we do not believe that the KD itself was responsible. Moreover, this participant discontinued cholinesterase inhibitor therapy shortly after starting the KD and was therefore technically protocol noncompliant. We nevertheless included this participant's data in this report for completeness and transparency.
The KDRAFT complements other studies that report ketogenic interventions benefit cognitive performance. A 3-month duration caloric restriction trial found that CR improved memory in healthy adults [18]. Although the investigators did not assess ketone body production, caloric restriction does induce ketosis [29]. Another study reported a single MCT-supplemented “ketogenic meal” acutely boosted cognitive performance in nondemented elderly adults [20]. An exploratory study randomized 23 subjects with mild cognitive impairment, a frequent AD-precursor state, to a 6-week carbohydrate-restricted or high-carbohydrate diet and found memory test performance improved in the carbohydrate-restricted (n = 12) but not in the high-carbohydrate intervention [19]. Although the authors did not formally refer to their carbohydrate-restricted diet as a KD, they did show that cognitive improvement positively correlated with ketone body levels. Two studies report that simply consuming an MCT supplement outside of a KD context, which also boosts serum BHB levels, at least temporarily improves cognition in AD subjects [21], [22]. In a case report, Newport et al. noted administering a BHB-promoting ketone monoester to an AD patient coincided with cognitive improvement [30].
KDs and ketone bodies have protean physiologic effects. KDs can increase ketone body levels and ketone utilization, reduce brain glucose consumption, lower insulin, alter insulin signaling, increase long- and medium-chain fatty acids, affect lipid handling, and reduce inflammation [11], [31], [32], [33], [34], [35], [36], [37]. Ketone bodies change bioenergetic infrastructures in neurons and astrocytes, mediate glia-neuron interactions, affect energy homeostasis, post-translationally modify proteins (directly and indirectly) to influence their function, modify gene expression, and act as signaling molecules [31], [38], [39], [40], [41], [42]. Our study does not address the mechanisms through which a KD might influence cognition. It does not address whether KDs and non-KD ketogenic interventions are functionally or therapeutically interchangeable.
Because of the KDRAFT's exploratory nature, small size, and single-arm structure, we cannot definitively conclude that a KD benefits AD patients. Feasibility and efficacy data from this 3-month pilot trial, though, justify further studies of KDs in mild AD participants. Although positive results from definitive research studies might lead some to consider a KD for the treatment of AD, in the routine clinical setting, its challenges may hinder its implementation. On a fundamental level, definitive positive studies would nevertheless provide insight into and justify evaluating other metabolic manipulations for the treatment of AD.
Research in context.
-
1.
Systematic review: Literature was reviewed using PubMed and revealed, although proposed as a potential therapy, no published studies assessing feasibility and efficacy of a ketogenic diet therapy in humans with Alzheimer's disease (AD).
-
2.
Interpretation: We investigated the feasibility of a medium-chain triglyceride–supplemented KD intervention in AD participants and coincident effects on cognition. The dietary protocol was feasible in very mild (clinical dementia rating [CDR] 0.5) and mild (CDR 1) AD patients. All patients with moderate (CDR 2) AD withdrew from the study, citing caregiver burden. Alzheimer's Disease Assessment Scale-cognitive subscale scores significantly improved in dietary compliant participants from baseline to the end of the intervention and returned to baseline level after a 1-month discontinuance of the diet. Our findings suggest that the medium chain triglyceride-supplemented KD is feasible in AD patients up to CDR 1 and may elicit potential cognitive benefit.
-
3.
Future directions: These findings justify further investigation into the ketogenic diet as a therapy in AD.
Acknowledgments
This study was supported by the University of Kansas Alzheimer's Disease Center (P30AG035982), the University of Kansas Heartland Clinical Translational Science Award Center, the University of Kansas Department of Dietetics and Nutrition, and the Stop Alzheimer's Now Foundation.
Footnotes
M.K.T. and D.K.S. report no disclosures. J.D.M. is supported by P30AG035982 and otherwise reports no disclosures. J.M.B. is supported by P30AG035982 and receives or has received research support in the last 2 years for clinical trials from Lilly, Avid Radiopharmaceuticals, Toyama Chemical Company, Merck, and Biogen. R.H.S. is supported by P30AG035982 and within the last 2 years has received an honorarium from Accera and clinical trial support from Ausio Pharmaceuticals, LLC and the Alzheimer's Association.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2017.11.002.
Supplementary data
References
- 1.Swerdlow R.H. Mitochondria and cell bioenergetics: increasingly recognized components and a possible etiologic cause of Alzheimer's disease. Antioxid Redox Signal. 2012;16:1434–1455. doi: 10.1089/ars.2011.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Santi S., de Leon M.J., Convit A., Tarshish C., Rusinek H., Tsui W.H. Age-related changes in brain: II. Positron emission tomography of frontal and temporal lobe glucose metabolism in normal subjects. Psychiatr Q. 1995;66:357–370. doi: 10.1007/BF02238755. [DOI] [PubMed] [Google Scholar]
- 3.Nugent S., Tremblay S., Chen K.W., Ayutyanont N., Roontiva A., Castellano C.A. Brain glucose and acetoacetate metabolism: a comparison of young and older adults. Neurobiol Aging. 2014;35:1386–1395. doi: 10.1016/j.neurobiolaging.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 4.Ferris S.H., de Leon M.J., Wolf A.P., Farkas T., Christman D.R., Reisberg B. Positron emission tomography in the study of aging and senile dementia. Neurobiol Aging. 1980;1:127–131. doi: 10.1016/0197-4580(80)90005-6. [DOI] [PubMed] [Google Scholar]
- 5.Foster N.L., Chase T.N., Fedio P., Patronas N.J., Brooks R.A., Di Chiro G. Alzheimer's disease: focal cortical changes shown by positron emission tomography. Neurology. 1983;33:961–965. doi: 10.1212/wnl.33.8.961. [DOI] [PubMed] [Google Scholar]
- 6.Reiman E.M., Caselli R.J., Yun L.S., Chen K., Bandy D., Minoshima S. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 7.Small G.W., Mazziotta J.C., Collins M.T., Baxter L.R., Phelps M.E., Mandelkern M.A. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA. 1995;273:942–947. [PubMed] [Google Scholar]
- 8.Mosconi L., Brys M., Switalski R., Mistur R., Glodzik L., Pirraglia E. Maternal family history of Alzheimer's disease predisposes to reduced brain glucose metabolism. Proc Natl Acad Sci U S A. 2007;104:19067–19072. doi: 10.1073/pnas.0705036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swerdlow R., Marcus D.L., Landman J., Kooby D., Frey W., 2nd, Freedman M.L. Brain glucose metabolism in Alzheimer's disease. Am J Med Sci. 1994;308:141–144. doi: 10.1097/00000441-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Swerdlow R.H. Bioenergetic medicine. Br J Pharmacol. 2014;171:1854–1869. doi: 10.1111/bph.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kossoff E.H., Zupec-Kania B.A., Rho J.M. Ketogenic diets: an update for child neurologists. J Child Neurol. 2009;24:979–988. doi: 10.1177/0883073809337162. [DOI] [PubMed] [Google Scholar]
- 12.Owen O.E., Morgan A.P., Kemp H.G., Sullivan J.M., Herrera M.G., Cahill G.F., Jr. Brain metabolism during fasting. J Clin Invest. 1967;46:1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edmond J., Robbins R.A., Bergstrom J.D., Cole R.A., de Vellis J. Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J Neurosci Res. 1987;18:551–561. doi: 10.1002/jnr.490180407. [DOI] [PubMed] [Google Scholar]
- 14.Hasselbalch S.G., Knudsen G.M., Jakobsen J., Hageman L.P., Holm S., Paulson O.B. Blood-brain barrier permeability of glucose and ketone bodies during short-term starvation in humans. Am J Physiol. 1995;268:E1161–E1166. doi: 10.1152/ajpendo.1995.268.6.E1161. [DOI] [PubMed] [Google Scholar]
- 15.Cunnane S.C., Courchesne-Loyer A., Vandenberghe C., St-Pierre V., Fortier M., Hennebelle M. Can ketones help rescue brain fuel supply in later life? Implications for cognitive health during aging and the treatment of Alzheimer's disease. Front Mol Neurosci. 2016;9:53. doi: 10.3389/fnmol.2016.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castellano C.A., Nugent S., Paquet N., Tremblay S., Bocti C., Lacombe G. Lower brain 18F-fluorodeoxyglucose uptake but normal 11C-acetoacetate metabolism in mild Alzheimer's disease dementia. J Alzheimers Dis. 2015;43:1343–1353. doi: 10.3233/JAD-141074. [DOI] [PubMed] [Google Scholar]
- 17.Swerdlow R., Marcus D.M., Landman J., Harooni M., Freedman M.L. Brain glucose and ketone body metabolism in patients with Alzheimer's disease. Clin Res. 1989;37:461A. [Google Scholar]
- 18.Witte A.V., Fobker M., Gellner R., Knecht S., Floel A. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci U S A. 2009;106:1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krikorian R., Shidler M.D., Dangelo K., Couch S.C., Benoit S.C., Clegg D.J. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol Aging. 2012;33:425.e19–425.e27. doi: 10.1016/j.neurobiolaging.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ota M., Matsuo J., Ishida I., Hattori K., Teraishi T., Tonouchi H. Effect of a ketogenic meal on cognitive function in elderly adults: potential for cognitive enhancement. Psychopharmacology (Berl) 2016;233:3797–3802. doi: 10.1007/s00213-016-4414-7. [DOI] [PubMed] [Google Scholar]
- 21.Reger M.A., Henderson S.T., Hale C., Cholerton B., Baker L.D., Watson G.S. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging. 2004;25:311–314. doi: 10.1016/S0197-4580(03)00087-3. [DOI] [PubMed] [Google Scholar]
- 22.Henderson S.T., Vogel J.L., Barr L.J., Garvin F., Jones J.J., Costantini L.C. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer's disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond) 2009;6:31. doi: 10.1186/1743-7075-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mifflin M.D., St Jeor S.T., Hill L.A., Scott B.J., Daugherty S.A., Koh Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 25.Cervenka M.C., Henry B.J., Felton E.A., Patton K., Kossoff E.H. Establishing an Adult Epilepsy Diet Center: Experience, efficacy and challenges. Epilepsy Behav. 2016;58:61–68. doi: 10.1016/j.yebeh.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 26.Mosek A., Natour H., Neufeld M.Y., Shiff Y., Vaisman N. Ketogenic diet treatment in adults with refractory epilepsy: a prospective pilot study. Seizure. 2009;18:30–33. doi: 10.1016/j.seizure.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Yu H., Wang X., He R., Liang R., Zhou L. Measuring the caregiver burden of caring for community-residing people with Alzheimer's disease. PLoS One. 2015;10:e0132168. doi: 10.1371/journal.pone.0132168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neal E.G., Chaffe H., Schwartz R.H., Lawson M.S., Edwards N., Fitzsimmons G. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia. 2009;50:1109–1117. doi: 10.1111/j.1528-1167.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 29.Lin A.L., Zhang W., Gao X., Watts L. Caloric restriction increases ketone bodies metabolism and preserves blood flow in aging brain. Neurobiol Aging. 2015;36:2296–2303. doi: 10.1016/j.neurobiolaging.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newport M.T., VanItallie T.B., Kashiwaya Y., King M.T., Veech R.L. A new way to produce hyperketonemia: use of ketone ester in a case of Alzheimer's disease. Alzheimers Dement. 2015;11:99–103. doi: 10.1016/j.jalz.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puchalska P., Crawford P.A. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017;25:262–284. doi: 10.1016/j.cmet.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selfridge J.E., Wilkins H.M., Lezi E., Carl S.M., Koppel S., Funk E. Effect of one month duration ketogenic and non-ketogenic high fat diets on mouse brain bioenergetic infrastructure. J Bioenerg Biomembr. 2015;47:1–11. doi: 10.1007/s10863-014-9570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dupuis N., Curatolo N., Benoist J.F., Auvin S. Ketogenic diet exhibits anti-inflammatory properties. Epilepsia. 2015;56:e95–e98. doi: 10.1111/epi.13038. [DOI] [PubMed] [Google Scholar]
- 34.McDaniel S.S., Rensing N.R., Thio L.L., Yamada K.A., Wong M. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52:e7–e11. doi: 10.1111/j.1528-1167.2011.02981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bough K.J., Wetherington J., Hassel B., Pare J.F., Gawryluk J.W., Greene J.G. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–235. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- 36.Hartman A.L., Gasior M., Vining E.P., Rogawski M.A. The neuropharmacology of the ketogenic diet. Pediatr Neurol. 2007;36:281–292. doi: 10.1016/j.pediatrneurol.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noh H.S., Lee H.P., Kim D.W., Kang S.S., Cho G.J., Rho J.M. A cDNA microarray analysis of gene expression profiles in rat hippocampus following a ketogenic diet. Brain Res Mol Brain Res. 2004;129:80–87. doi: 10.1016/j.molbrainres.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 38.Guzman M., Blazquez C. Is there an astrocyte-neuron ketone body shuttle? Trends Endocrinol Metab. 2001;12:169–173. doi: 10.1016/s1043-2760(00)00370-2. [DOI] [PubMed] [Google Scholar]
- 39.Newman J.C., Verdin E. β-hydroxybutyrate: much more than a metabolite. Diabetes Res Clin Pract. 2014;106:173–181. doi: 10.1016/j.diabres.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menzies K.J., Zhang H., Katsyuba E., Auwerx J. Protein acetylation in metabolism - metabolites and cofactors. Nat Rev Endocrinol. 2016;12:43–60. doi: 10.1038/nrendo.2015.181. [DOI] [PubMed] [Google Scholar]
- 41.Xie Z., Zhang D., Chung D., Tang Z., Huang H., Dai L. Metabolic regulation of gene expression by histone lysine beta-hydroxybutyrylation. Mol Cell. 2016;62:194–206. doi: 10.1016/j.molcel.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cullingford T.E. The ketogenic diet; fatty acids, fatty acid-activated receptors and neurological disorders. Prostaglandins Leukot Essent Fatty Acids. 2004;70:253–264. doi: 10.1016/j.plefa.2003.09.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.