Abstract
In the field of Alzheimer's disease research, the use of biomarkers such as amyloid positron emission tomography (PET) has become widespread over a relatively brief period of time. There is an increasing tendency in research studies and trials to switch from no disclosure under any condition toward a qualified disclosure of individual research results, such as amyloid PET scan results. This perspective article aims to evaluate the possible need for a modification of the available recommendations on amyloid PET scan disclosure, based on recent empirical evidence obtained within the field of amyloid PET. This article also applies the International Guideline for Good Clinical Practice to the field of amyloid PET disclosure. Hence, we propose several recommendations to facilitate amyloid PET disclosure while minimizing possible risks of amyloid disclosure in a research context.
Keywords: Amyloid PET scan, Disclosure, Alzheimer's disease, Recommendations, Amyloid imaging, Disclosing information, Ethics
1. Background
There is currently no obligation for the researcher to disclose individual research results (IRRs) to the research participant. No one favors full disclosure under all circumstances or no disclosure under any condition [1]. In the field of Alzheimer's disease (AD) research, the use of biomarkers such as amyloid positron emission tomography (PET) has become widespread over a relatively brief period of time. There is an increasing tendency to switch from no disclosure under any condition toward a qualified disclosure of IRRs. This switch has been guided partly by the Appropriate Use Criteria of Amyloid Imaging and the Health Authorities approval of amyloid PET imaging in patients with a clinically defined memory impairment [2], [3], [4], [5], [6], [7]. Grill et al showed how disclosure of amyloid status is not a barrier to the recruitment of participants in clinical trials [8]. A qualified disclosure policy implies that disclosure may take place if the result is in line with particular criteria. These criteria take into account the proof of clinical utility of the result and the actionability of the result (possibility to provide a treatment, symptomatic relief, etc.) [1], [9]. Disclosure of results will also vary depending on three other factors: The first factor concerns the issue of active versus passive disclosure. Passive disclosure only takes places after explicit request of the research participant, whereas an active disclosure refers to a process whereby researchers actively offer results to the participant [1]. The second factor concerns the result itself: there is a difference between disclosing an aggregate group result, an incidental finding, or an individual research result [1]. The content of the data also makes a difference, for example, a standard blood value results or disclosure of a genetic risk factor. The third factor concerns the study population: There is a fundamental difference between disclosing information to cognitively healthy participants, participants who have a cognitive deficit, or participants who are already in a more severe stage of AD.
To our knowledge, four studies have developed recommendations about the disclosure of amyloid PET results. The recommendations from Porteri et al, Lingler et al, and Grill et al focus on mild cognitive impairment (MCI) patients, whereas the recommendations by Harkins et al targeted the disclosure of results to cognitively normal adults [10], [11], [12], [13]. Three of four recommendations [10], [11], [13] focused on the disclosure of amyloid PET results, whereas one recommendation [12] pertains to biomarker-based information more generally. Relatively few empirical studies have explored the viewpoints of patients, carers, and stakeholders regarding amyloid PET disclosure [14], [15], [16], [17].
This article aims to evaluate the possible need for a modification of the previously mentioned available recommendations [10], [12], based on recent empirical evidence and the perspective of patients themselves [14], [15], [18]. This perspective article applies the International Guideline for Good Clinical Practice to the field of amyloid PET disclosure in a research context taking into account recent empirical evidence obtained within the field of amyloid PET. The review also takes into account relevant elements that have arisen from recent empirical studies of genetic AD risk disclosure [19]. We propose several recommendations to facilitate amyloid PET disclosure while minimizing possible risks of amyloid disclosure in the research context. Although this article focuses on the use of amyloid PET scans in research, the criteria set forward in this article may also be of interest for clinicians when using amyloid PET scans as part of the clinical diagnostic evaluation of their patients with cognitive problems.
2. From information to follow-up
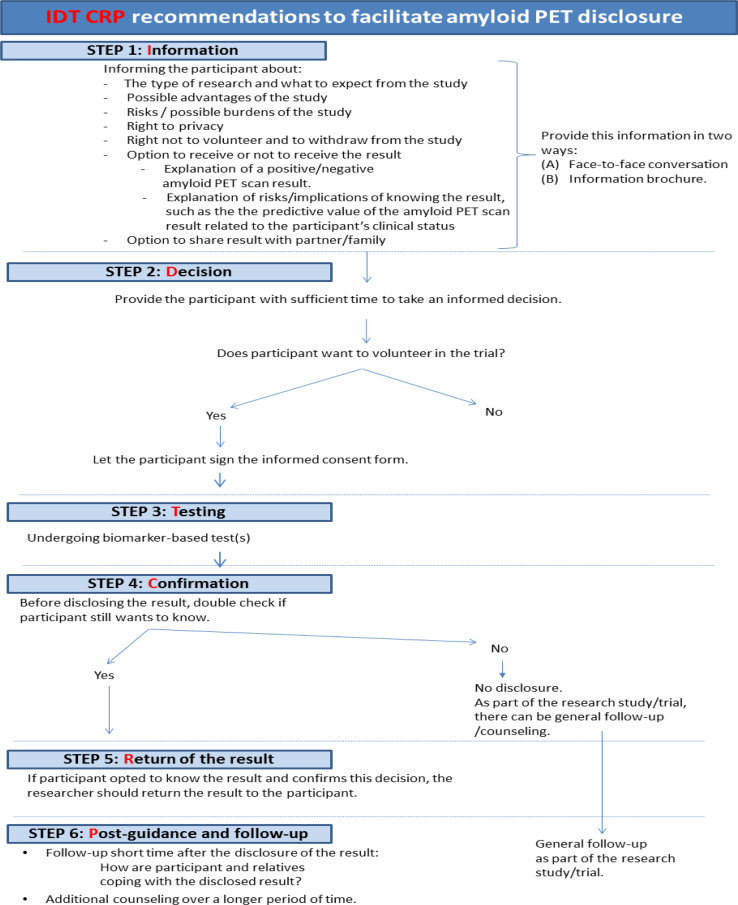
Disclosure of results is associated with multiple ethical challenges. Based on the article of Porteri et al [12] that describes multiple important aspects of the informed consent process, we suggest a six-step recommendation to facilitate disclosure and to minimize possible risks of amyloid PET disclosure. This recommendation results in six concrete steps: Information (I), Decision (D), Testing (T), Confirmation (C), Return of result (R), and Postguidance (P). These are abbreviated as the IDT CRP recommendations (Fig. 1).
Fig. 1.
IDT CRP recommendations (This figure is based on the amyloid PET recommendations from Porteri et al [12] and Harkins et al [10]. However, this figure has been designed by the authors of this publication with the purpose of providing an overview schedule of the recommendations). Abbreviation: PET, positron emission tomography.
2.1. Information
Before testing, it is of key importance to provide accurate, clear, and easily understandable information to the participant [10], [12], [19]. Participants with diverse educational backgrounds might have difficulties to understand the complexity of the research design and the type of individual research result they may opt for [1], [20], [21]. For instance, interviews before amyloid disclosure showed how some participants misused the terminology of a positive and negative amyloid PET scan result, whereby the word “positive” was used by participants to describe “good news” and vice versa [14]. The REVEAL study and a recent study with MCI patients after IRR disclosure highlighted that most participants understood the “take-home-message”, yet many participants could not recall the specific wording of the result as explained by the study physician [5], [15]. Hence, the provided information should not be restricted to a written information brochure. It should also include the opportunity to have a face-to-face conversation with a researcher or study clinician to address any questions and concerns about the study design and the option of being informed of their amyloid PET scan result. The added value of an oral conversation and question moment was mentioned by participants and their carers in the study conducted by Lawrence et al and received positive feedback from amnestic MCI patients in a clinical trial before the amyloid PET disclosure [14], [22].
2.1.1. Information provided to the participant
Before trial participation, the following topics need to be explained to the participant [12]: voluntary decision to participate, the right to withdraw throughout the study without having to provide a reason, and to change their mind about being informed of the result [12]. There are two important nuances. First, the participant does not need to provide a reason for altering his/her mind. Although the reason for withdrawal as provided by the participant sometimes provides additional insight and feedback for the research team about the ongoing trial, it can also benefit the research team when designing, setting up, and recruiting for a new trial. Second, participants may change their mind about disclosure up to the moment of disclosure. Once the result has been disclosed, this is an irreversible process the participant needs to be aware off.
In Table 1, we represent benefits and risks as reported by previously conducted studies regarding disclosure of results [14], [15], [16], [22], [23], [24], [25], [26], [27]. Table 1 can provide researchers with an overview of possible benefits and risks, which can be explained to the participant. The benefits and risks should not be limited to the elements mentioned in Table 1, as research is still ongoing to explore more participants' views and concrete experiences on this topic.
Table 1.
Reported advantages and disadvantages of knowing amyloid PET result
| Advantages/benefits | Disadvantages/risks |
|---|---|
|
|
Benefits and risks can be interpreted differently by participants compared to the views of researchers [15]. For example, emotional and psychological consequences may be perceived as a compelling reason against disclosure of results. However, studies have reported that not all participants respond negatively to the disclosed result, even when it indicates pathology instead of a normal state. For example, knowing the result can also lead to the feeling of relief and less anxiety [15], [16], [28], [29]. A recent study concluded that the disclosure of amyloid PET scan results to cognitively normal adults had no effects on depressive symptoms. However, anxiety symptoms peaked at a low level on the day of amyloid PET scan disclosure, but this was not sustained at 6 weeks or 6 months after disclosure [30]. Furthermore, test-related distress was slightly higher in participants with elevated amyloid compared with participants with normal amyloid levels [30]. This can indicate that after a certain amount of time to reflect and cope with the news, most participants may respond better to the news than is indicated in the literature as arguments against disclosure.
In particular, the following two disadvantages, consequences, or risks need to be addressed and explained in-depth to the participant:
2.1.1.1. Uncertainty and limitations of the predictive value
The correct meaning of a positive and negative amyloid PET scan needs to be explained to the research participant before any disclosure and this information needs to be tailored to the clinical status of the subject, both content-wise and regarding the way in which the information is explained [10], [12]. For example, in case of MCI, if the amyloid PET scan is positive, this indicates an elevation of the detected amyloid plaque levels in the brain, which implies that the underlying pathology of MCI is due to AD, yet this does not imply that the participant will progress toward AD dementia in the near future. Owing to the difficulty of individual timelines, it is hard to predict which participants will convert to AD dementia over which time period, who will remain stable over the following years or who will develop a different neurodegenerative disease [25], [26]. In addition, age is an important factor as it is associated with a higher prevalence of a positive amyloid PET scan [31]. To avoid false reassurance in case of a negative amyloid PET scan result, the participant needs to be aware that a non-AD cause can be the underlying pathology causing their memory complaints [26]. A negative amyloid PET scan result implies that at the moment of the conducted scan, there was no elevation of amyloid plaques detected. For MCI patients with a negative amyloid PET scan, in a few years, there may be a conversion from a negative to a positive amyloid PET scan, in particular when values are at an intermediate level. Despite the limitation of the predictive value, the information which individuals will most likely progress to clinical AD is of importance and has great relevance for the participant [13], [26]. For example, MCI patients stated that receiving a high or low risk of having underlying AD based on the amyloid PET scan was already perceived as valuable information [14].
2.1.1.2. Lack of an effective disease-modifying treatment
Most individuals are aware that there is currently no treatment for AD. The lack of a cure or prevention for AD may withhold some participants from opting for their amyloid PET scan disclosure. Therefore, the study physician or researcher should clarify the current state of the art in AD therapy and which actions (lifestyle interventions, medication, etc.) the participant may undertake based on the received result [26]. To avoid therapeutic misconception, whereby participants mistakenly assume they will receive treatment as part of the clinical trial, information before trial participation is again of high essence. It may be that participants volunteer in the trial out of a sense of personal urgency to gain access to otherwise unavailable interventions [25].
2.1.2. Who to inform about the result
2.1.2.1. Presence of a relative
Before any disclosure, it is beneficial to ask the participant who needs to be informed of the test result. For some participants, the presence of a trusted relative or close friend can provide the necessary support in case of a positive amyloid PET scan result. It can also allow the participant's family to understand their situation and to assist in any type of financial, legal, health care, or other arrangements [12]. Others may not want to burden their relatives or may, depending on the familial situation or due to privacy reasons, prefer not to involve others in this disclosure process. This is in line with the recommendations by Grill et al, who stated that an informant should be present as the informant can provide social/emotional support [11]. However, Grill et al also mentioned that the preference of MCI patients who refuse bringing an informant should be respected [11].
2.1.2.2. Deciding on informing others after disclosure of the result
In advance, it is better to address if the participant and trusted relative want to inform others of the disclosed result and, if so, who they will inform. If they decide to inform others, what message will they provide to them? It is important to avoid a situation whereby the participant is confronted with outsiders who have been informed without explicit consent of the participant, with misinterpretation of the received result, and with a possible situation of stigmatization.
2.2. Decision
Participants need to decide themselves on volunteering for the trial, undergoing biomarker-based tests, and whether or not to be informed about the study results. As described in the International Guideline for Good Clinical Practice, this decision should be made voluntarily and without external pressure (research team, relatives, etc.) [19]. It was reported that most MCI patients felt supported by their family members in their decision to volunteer in the trial and to opt for their amyloid PET scan result, yet that there is a thin line between family support and family pressure [18]. Researchers should check whether the participant has made the decision himself freely and what the motivation is to enroll and to opt in for disclosure.
In reality, including family members may create challenges [11]. For example, they might disagree with each other, or the patient might feel pushed by the relative to know the result. To avoid a situation whereby the patient feels pushed or pressured by a relative's decision, the researcher can possibly resolve this problem by using one of the following techniques:
-
(a)
Addressing the question first to the patient and by listening to the patient's questions and decision. In a second step, the researcher addresses the same questions to the relative or informant who is present.
-
(b)
The first option is not always feasible. For example, the relative may take over the conversation, or the patient may not feel comfortable to freely express his/her opinion in the presence of a relative or due to certain familial tensions. When one or more of these difficulties are noticed, the researcher may ask to discuss the aforementioned topics first with the patient alone. After this private face-to-face conversation, the relative may then be asked to share his/her opinion and thoughts.
In case of doubt or clear discordance between the views of the patient and the relative, the researcher should address with both of them together what the impact of the discordance may be. For example, the impact on their relationship when the partner or relative wants to know the result, while the participant clearly feels that he/she is not ready to be informed of this result.
The following studies have provided insight into possible motivational reasons to enroll and to opt for disclosure [14], [16], [18], [27]. We have clustered these motivational reasons in Table 2 as this can provide researchers with an insight into the views of participants.
Table 2.
Reported motivational reasons for trial volunteering and for opting to know their result
| Motivational reasons to volunteer in trial | Motivational reasons to opt for IRR disclosure |
|---|---|
|
|
The notion of “knowledge is power” and the need for information were addressed. In the REVEAL study, participants mentioned the need for information to make informed health care decisions [29]. Information could also fulfill psychological rather than practical needs [27].
2.3. Testing
2.3.1. Conducting tests
After consent of the participant to undergo testing, it is important to describe each step of the test procedure.
Studies have also reported on the issue of trials being time-consuming due to the many tests being conducted [16], [18]. To reduce the disadvantage of trial participation such as time-consuming and to avoid dropout throughout the trial, it is recommended to inform participants before consent about the estimated time/amount of visits and to cluster several tests into one hospital visit. However, clustering as many tests as possible into one hospital visit needs to remain feasible for the researchers and the research setting should not burden the participant nor affect the quality of the obtained research data [18].
For the participants who opted to know their result, additional information about the estimated timeline for test analysis can provide them with insight when to expect the disclosure of their result.
2.3.2. Analysis and validation of the amyloid PET scan
For the visual read of the amyloid PET scan, it is of vital importance that the read is performed in a reliable manner.
In case the amyloid PET result is equivocal, waiting with disclosing the amyloid PET scan result until more information is available from other biomarker tests, such as cerebrospinal fluid results, is recommended. The participant should then be informed about this delay in a proper manner.
2.4. Confirmation
It is important to assess whether the participant still wants to be informed about the result during a confirmation moment prior the actual disclosure [15], [30]. This can be an opportunity to repeat the purpose of the amyloid PET scan, the classification, and interpretation of an amyloid-positive and amyloid-negative PET scan result. This confirmation moment has two concrete benefits: (1) to answer remaining questions and concerns and (2) to minimize the possibility that the participant afterward misinterprets this information.
2.5. Return or disclosure of the result
2.5.1. Disclosure by a trained and skilled researcher/clinician
The Amyloid Imaging Task Force provides criteria for prescribing and disclosing amyloid PET result. They stated that the disclosure should be done by a dementia specialist who devotes at least 25% of their patient contact time to the evaluation and care of adults with cognitive impairment or dementia [6], [7].
2.5.2. The terminology of the disclosed result
The terminology used throughout the disclosure process and how these words could impact the participants and his relatives is an ethical challenge in amyloid disclosure. The amyloid PET scans is most commonly classified as positive/negative, yet alternative terms have been used, such as elevated/nonelevated levels, presence or absence of amyloid, buildup or no significant buildup, and normal/abnormal levels of amyloid plaques in the brain [10], [11], [13]. Researchers ought to be aware that some of the used terminology may be misinterpreted by the participant or may have a negative emotional connotation for the participant. For instance, an amyloid PET scan classified as abnormal does not imply that the participant is abnormal yet implies that the level of amyloid detected in the brain is outside the normal range.
Hence, the use of easy-to-understand examples, pictographic and visual information, can provide additional clarification for the participant and may enhance their capabilities to accurately recall and interpret their result. Showing the scan (visual read) to the participant may help for some participants to understand the result, yet researchers ought to be aware that for some patients, scan images with the visual appearance of elevated amyloid levels in the brain might trigger emotional reactions [25]. Checking beforehand with participants if they would like to see the scan and by explaining that this might be confrontational for some participants can be seen as a preventive measure. However, it does not guarantee that the participant will not emotionally respond to these viewed images.
The disclosed result with the correct interpretation of the amyloid PET scan result should also be provided in a written document to the patient and to the patient's general practitioner [11], [15]. This information should explain the imaging technique and in particular what the amyloid PET scan measures, how the amyloid PET scan result needs to be interpreted, and what this result implies on the patient's level regarding the lack of good predictive individual timeline for future cognitive decline and possible treatment options.
After the disclosure, it is beneficial to provide the participant with the possibility of follow-up and counseling and to explain the purpose and benefit of follow-up. This can provide participants with a moment of reflection and a moment to receive an answer to any additional questions that may occur after disclosure.
2.6. Postguidance and follow-up
The available recommendations highlight the importance of follow-up after disclosure [10], [12]. This practical need was also addressed in empirical studies [16]. The opportunity for counseling and follow-up may be valued more by the participant depending on the following three aspects:
-
(a)
The outcome of the result. The disclosure of both amyloid-positive and amyloid-negative PET scans requires follow-up, as both can have implications. In case of a positive scan, the knowledge that one has an elevated level of amyloid in the brain, which Roberts et al described as “viewable image of amyloid growing in the brain,” might trigger different reactions [5]. Not knowing whether and when the individual will cognitively deteriorate might provoke fear in these individuals.
Vice versa, persons who have received a negative amyloid PET scan result may be left with unanswered questions regarding the cause of their memory deficit or may feel overly reassured. The latter attitude may result in postponing arrangements for the future. This issue has been addressed in a qualitative study whereby some MCI patients did not feel a sense of urgency anymore to make practical arrangements for the future in light of their received negative amyloid PET scan result [15]. However, making arrangements for the future is important for anyone of advancing age, also for those who are not at increased genetic risk for AD [27].
Furthermore, the postguidance phase can also provide an opportunity to ask how relatives and carers are coping with the news.
-
(b)
The expectations of participants. If the test results match expectations, even positive results for severe disorders are not necessarily overwhelming. For example, studies reported on the feeling of “relief” after receiving a positive amyloid PET scan result [15], [16]. However, if the test results are unexpected (e.g., screening in healthy adult populations), the psychological consequences may be different [28], [32].
-
(c)
The perception of the disclosed result. Studies have reported that participants may accurately recall their disclosed risk information, yet not all participants match their perceived personal risk with the disclosed objective risk of a disease [33], [34]. This may be the case in participants who have a memory deficit as they may be influenced by their own concrete memory experiences or via emotional experiences through family history of AD [15], [28]. An interview study with amnestic MCI patients revealed that some patients who had received a negative amyloid PET scan result started to doubt the result due to their experienced memory complaints [15]. Another explanation for different perception of the disclosure was provided by the study of Linnenbringer et al whereby apolipoprotein E ε4 (APOE ε4)–positive individuals minimized their risk as a way to cope with unfavorable risk information. Counseling and follow-up may improve accuracy of participants' risk perception [34].
Furthermore, research is needed on the caregivers' perception after disclosure. We recommend assessing the perception of the relative to see how they are coping with the IRRs. This reflection moment with relatives can avoid possible misinterpretation and possible harm of undergoing “unnecessary” arrangements after the disclosed news.
3. Conclusion
In the field of genetics, disclosure of IRRs is a much debated and explored topic. In the field of AD therapeutic trials, disclosure of results is a domain that received far less research attention. Researchers may fear implementing disclosure as part of the trial design as disclosure could have a negative impact on the retrieved study data.
Based on the limited recommendations and research studies on amyloid PET disclosure, we can conclude that it is nonetheless feasible to disclose amyloid PET scan results to the research participant. Nevertheless, awareness of potential pitfalls and risks on amyloid PET disclosure is critical, such as the importance of a reliable amyloid PET scan read, the potential of causing emotional harm to the research participant, and the limitations in predictive value of the result. Hence, the presented IDT CRP method can provide necessary insight and guidance for researchers and facilitate amyloid PET disclosure to the research participant in a proper manner.
Owing to the novelty of disclosure in AD research, there are unexplored or underinvestigated areas where future research should focus on: first, the need to further address the impact of amyloid PET disclosure on both the participant and the researcher/research setting; second, the long-term impact (e.g., 5–10 years) of the disclosed result; third, limited information is available on how a participant who received a negative amyloid PET scan responds to a positive amyloid PET scan conversion, nor on how a false positive/negative result was perceived by the participant; fourth, awareness into how the result was disclosed is of utter importance (e.g., which words were used, which steps or disclosure procedures were followed, etc.) as this could impact the design of future trial-disclosures; and fifth, AD therapeutic trials commonly use an implicit disclosure design whereby a positive amyloid PET scan result is an inclusion criterion for trial participation. Research should explore whether this implicit disclosure is the way forward in therapeutic trials and whether participants are well aware of the meaning, implications, and consequences of an implicit disclosure. Because studies have reported on the value of knowing the amyloid PET scan result by the patients, it would be interesting to explore the perspective and views of research participants toward the implicit disclosure policy in AD therapeutic trials.
Research in Context.
-
1.
Systematic review: The ethical recommendations to facilitate amyloid PET disclosure while minimizing possible risks of amyloid disclosure in a research context were based on available recommendations, recent empirical evidence and the perspective of patients.
-
2.
Interpretation: The information covered in this article summarizes the available literature (recommendations, empirical and qualitative research). Ethical recommendations were added, which resulted into a method (abbreviated as the IDT-CRP method) that consists of six steps to facilitate amyloid PET disclosure in a research context.
-
3.
Future directions: A section of unanswered challenges and future research directions of amyloid PET disclosure in the research context is discussed.
Acknowledgments
The authors would like to acknowledge Hugh Desmond for the linguistic editing of this article.
Ethics approval: No ethics approval was needed for this publication.
Funding: This project was funded by the IWT (Agentschap voor Innovatie door Wetenschap en Technologie, Belgium). The project number is 120836: “Biomarker-Based Adaptive Development in AD.” R.B. is a postdoctoral fellow, and R.V. is a senior clinical investigator of the Research Foundation Flanders (FWO).
Authors' contributions: The first draft of the article was based on critical discussions and raised insights via face-to-face meetings between all the authors of the article. The first draft of the article was made by G.V., which was revised by K.D., J.S., R.B., and R.V. All authors read and approved the final article.
Footnotes
R.V. has been the principal investigator of the phase 1 and 2 studies with 18F-flutemetamol, one of the amyloid PET ligands approved for clinical use. R.V. has received consultancy fees from GE Healthcare. R.V./R.B.'s institution has a clinical trial agreement with Merck, Eli Lilly, and Biogen (R.V. as a local PI). The authors declare no other conflicts of interest.
References
- 1.Bredenoord A.L., Kroes H.Y., Cuppen E., Parker M., van Delden J.J.M. Disclosure of individual genetic data to research participants: the debate reconsidered. Trends Genet. 2011;27:41–47. doi: 10.1016/j.tig.2010.11.004. Elsevier Ltd. [DOI] [PubMed] [Google Scholar]
- 2.FDA Approves AmyvidTM (Florbetapir F 18 Injection) for Use in Patients Being Evaluated for Alzheimer's Disease and Other Causes of Cognitive Decline. Eli Lilly and Company; 2015. https://investor.lilly.com/releasedetail2.cfm?ReleaseID=662647 Available at: Accessed February 5, 2017. [Google Scholar]
- 3.EMA . 2017. Why has Amyvid Been Approved?http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002422/human_med_001611.jsp&mid=WC0b01ac058001d124 Available at: Accessed February 17, 2017. [Google Scholar]
- 4.European Medicines Agency . 2014. Vizamyl Marketing Authorisation Holder: GE HEALTHCARE LIMITED Assessment Report for an Initial Marketing Authorisation application. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002557/WC500172953.pdf. Accessed February 17, 2017. [Google Scholar]
- 5.Roberts J.S., Dunn L.B., Rabinovici G.D. Amyloid imaging, risk disclosure and Alzheimer's disease: ethical and practical issues. Neurodegener Dis Manag. 2013;3:219–229. doi: 10.2217/nmt.13.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson K.A., Minoshima S., Bohnen N.I., Donohoe K.J., Foster N.L., Herscovitch P. Update on appropriate use criteria for amyloid PET imaging: dementia experts, mild cognitive impairment, and education. Amyloid Imaging Task Force of the Alzheimer's Association and Society for Nuclear Medicine and Molecular Imaging. Alzheimers Dement. 2013;9:e106–e109. doi: 10.1016/j.jalz.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Johnson K.A., Minoshima S., Bohnen N.I., Donohoe K.J., Foster N.L., Herscovitch P. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer's Association. Alzheimers Dement. 2013;9:e1–e16. doi: 10.1016/j.jalz.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grill J.D., Zhou Y., Elashoff D., Karlawish J. Disclosure of amyloid status is not a barrier to recruitment in preclinical Alzheimer's disease clinical trials. Neurobiol Aging. 2016;39:147–153. doi: 10.1016/j.neurobiolaging.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bredenoord A.L., Onland-Moret N.C., Van Delden J.J. Feedback of individual genetic results to research participants: in favor of a qualified disclosure policy. Hum Mutat. 2011;32:861–867. doi: 10.1002/humu.21518. [DOI] [PubMed] [Google Scholar]
- 10.Harkins K., Sankar P., Sperling R., Grill J.D., Green R.C., Johnson K.A. Development of a process to disclose amyloid imaging results to cognitively normal older adult research participants. Alzheimers Res Ther. 2015;7:26. doi: 10.1186/s13195-015-0112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grill J.D., Apostolova L.G., Bullain S., Burns J.M., Cox C.G., Dick M. Communicating mild cognitive impairment diagnoses with and without amyloid imaging. Alzheimers Res Ther. 2017;9:1–8. doi: 10.1186/s13195-017-0261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porteri C., Frisoni G.B. Biomarker-based diagnosis of mild cognitive impairment due to Alzheimer's disease: how and what to tell. A kickstart to an ethical discussion. Front Aging Neurosci. 2014;6:41. doi: 10.3389/fnagi.2014.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lingler J.H., Butters M.A., Gentry A.L., Hu Lu, Hunsaker A.E., Klunk W.E. Development of a standardized approach to disclosing amyloid imaging research results in mild cognitive impairment. J Alzheimers Dis. 2016;52:17–24. doi: 10.3233/JAD-150985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanderschaeghe G., Schaeverbeke J., Vandenberghe R., Dierickx K. Amnestic MCI patients' perspectives toward disclosure of amyloid PET results in a research context. Neuroethics. 2017;10:281–297. doi: 10.1007/s12152-017-9313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanderschaeghe G., Schaeverbeke J., Bruffaerts R., Vandenberghe R., Dierickx K. Amnestic MCI patients' experiences after the disclosure of their amyloid PET result in a research context. Alzheimers Res Ther. 2017;9 doi: 10.1186/s13195-017-0321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grill J.D., Cox C.G., Kremen S., Mendez M.F., Teng E., Shapira J. Patient and caregiver reactions to clinical amyloid imaging. Alzheimers Demen. 2017;13:924–932. doi: 10.1016/j.jalz.2017.01.001. Elsevier Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gooblar J., Roe C.M., Selsor N.J., Gabel M.J., Morris J.C. Attitudes of research participants and the general public regarding disclosure of Alzheimer disease research results. JAMA Neurol. 2015;72:1–7. doi: 10.1001/jamaneurol.2015.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanderschaeghe G., Schaeverbeke J., Vandenberghe R., Dierickx K. Amnestic MCI patients' perspectives on volunteer participation in a research context. J Clin Res Bioeth. 2017;8 doi: 10.1007/s12152-017-9313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ICH Expert Working Group . ICH Harmonised Tripartite Guideline; 1996. Guideline for Good Clinical Practice E6 (ICH-GCP) [Google Scholar]
- 20.Heaney C., Tindall G., Lucas J., Haga S.B. Researcher practices on returning genetic research results. Genet Test Mol Biomarkers. 2010;14:821–827. doi: 10.1089/gtmb.2010.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shalowitz D.I., Miller F.G. Disclosing individual results of clinical research: implications of respect for participants. JAMA. 2005;294:737–740. doi: 10.1001/jama.294.6.737. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence V., Pickett J., Ballard C., Murray J. Patient and carer views on participating in clinical trials for prodromal Alzheimer's disease and mild cognitive impairment. Int J Geriatr Psychiatry. 2014;29:22–31. doi: 10.1002/gps.3958. [DOI] [PubMed] [Google Scholar]
- 23.Gooblar J., Roe C.M., Selsor N.J., Gabel M., Morris J. Disclosure of individual research results to cognitively normal research participants. Alzheimers Dement. 2014;10:P812. Elsevier Ltd. [Google Scholar]
- 24.Klein E.P., Kaye J. Dementia specialists and early adoption of amyloid imaging. J Alzheimers Dis. 2013;33:445–450. doi: 10.3233/JAD-2012-121216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grill J.D., David K.J., Burns J.M. Should we disclose amyloid imaging results to cognitively normal individuals? Neurodegener Dis Manag. 2013;3:43–51. doi: 10.2217/nmt.12.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lingler J.H., Klunk W.E. Disclosure of amyloid imaging results to research participants: has the time come? Alzheimers Dement. 2013;9:741–744.e2. doi: 10.1016/j.jalz.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gooding H.C., Linnenbringer E.L., Burack J., Scott Roberts J., Green R.C., Biesecker B.B. Genetic susceptibility testing for Alzheimer disease: motivation to obtain information and control as precursors to coping with increased risk. Patient Educ Couns. 2006;64:259–267. doi: 10.1016/j.pec.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Roberts J.S., Tersegno S.M. Estimating and disclosing the risk of developing Alzheimer's disease: challenges, controversies and future directions. Future Neurol. 2010;5:501–517. doi: 10.2217/fnl.10.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts J.S., Cupples L.A., Relkin N.R., Whitehouse P.J., Green R.C. Genetic risk assessment for adult children of people with Alzheimer's disease: the Risk Evaluation and Education for Alzheimer's Disease (REVEAL) study. J Geriatr Psychiatry Neurol. 2005;18:250–255. doi: 10.1177/0891988705281883. [DOI] [PubMed] [Google Scholar]
- 30.Burns J.M., Johnson D.K., Liebmann E.P., Bothwell R.J., Morris J.K., Vidoni E.D. Safety of disclosing amyloid status in cognitively normal older adults. Alzheimers Dement. 2017;13:1024–1030. doi: 10.1016/j.jalz.2017.01.022. Elsevier Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jansen W.J., Ossenkoppele R., Knol D.L., Tijms B.M., Scheltens P., Verhey F.R. Prevalence of cerebral amyloid pathology in persons without dementia. JAMA. 2015;313:1924. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green R.C., Roberts J.S., Cupples L.A., Relkin N.R., Whitehouse P.J., Brown T. Disclosure of APOE genotype for risk of Alzheimer's disease. N Engl J Med. 2009;361:245–254. doi: 10.1056/NEJMoa0809578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Senay I., Kaphingst K.A. Anchoring-and-adjustment bias in communication of disease risk. Med Decis Making. 2009;29:193–201. doi: 10.1177/0272989X08327395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linnenbringer E., Scott Roberts J., Hiraki S., Adrienne Cupples L., Green R.C. “I know what you told me, but this is what I think:” perceived risk of Alzheimer disease among individuals who accurately recall their genetics-based risk estimate. Genet Med. 2010;12:219–227. doi: 10.1097/GIM.0b013e3181cef9e1. [DOI] [PMC free article] [PubMed] [Google Scholar]