Abstract
Introduction
We evaluated the selective M1 muscarinic positive allosteric modulator, MK-7622, as adjunctive cognitive enhancing therapy in individuals with Alzheimer's disease.
Methods
A randomized, double-blind, proof-of-concept trial was performed. Participants with mild-to-moderate Alzheimer's disease, being treated with an acetylcholinesterase inhibitor, were randomized 1:1 to 45 mg of MK-7622 or placebo for 24 weeks. Endpoints included the mean change from baseline in Alzheimer's Disease Assessment Scale–Cognitive Subscale (ADAS-Cog11) at 12 weeks and Alzheimer's Disease Cooperative Study–Activities of Daily Living Inventory at 24 weeks.
Results
Two hundred forty participants were randomized. The trial was stopped for futility after meeting prospectively defined stopping criteria. MK-7622 did not improve cognition at 12 weeks (group difference in ADAS-Cog11: 0.18 [95% confidence interval: −1.0 to 1.3]) or function at 24 weeks (group difference in Alzheimer's Disease Cooperative Study–Activities of Daily Living Inventory: 0.06 [95% confidence interval: −2.4 to 2.5]). More participants taking MK-7622 discontinued study medication because of adverse events than those taking placebo (16% vs 6%) and who experienced cholinergically related adverse events (21% vs 8%).
Discussion
MK-7622 (45 mg) does not improve cognition or function when used as adjunctive therapy in mild-to-moderate Alzheimer's disease.
Keywords: MK-7622, Alzheimer's disease, Cholinergic, Muscarinic, Allosteric modulator, Clinical trial
Highlights
-
•
MK-7622 is a positive allosteric modulator of the M1 receptor.
-
•
MK-7622 did not improve cognition or function in Alzheimer's disease patients.
-
•
MK-7622 increased cholinergically related side effects in Alzheimer's disease patients.
-
•
M1 positive allosteric modulation is not useful for treating Alzheimer's disease.
1. Background
Novel symptomatic therapies are needed for the treatment of Alzheimer's disease (AD). Acetylcholinesterase inhibitors and memantine are the current standard of care but exhibit only modest efficacy and dose-limiting side effects. Alternative approaches to improving cholinergic function in patients with AD have focused on either agonism or modulation of the muscarinic or nicotinic cholinergic receptors [1], [2]. Of the five subtypes of muscarinic receptor, the M1 muscarinic receptor is abundantly expressed in the hippocampus, cortex, and other brain regions associated with cognitive function, whereas the other muscarinic receptors are more highly expressed in peripheral tissues [1]. The M1 muscarinic receptor likely mediates the procognitive effects of cholinergic agents, whereas other acetylcholine receptors, particularly M2 and M3, may account for side effects [1], [3].
Multiple muscarinic agonists have been developed, and several have produced cognitive or behavioral benefits in AD [4], [5], [6]. However, these compounds were not selective for M1 and produced intolerable peripheral cholinergic effects such as nausea and salivation. An alternative approach to direct agonism of muscarinic receptors is allosteric modulation [7], [8]. MK-7622 is a novel selective M1-positive allosteric modulator that sensitizes the receptor to acetylcholine in the nanomolar range while having no effect on M2, M3, or M4 receptors up to 100 μM [9]. In preclinical studies, MK-7622 restores cognitive function to scopolamine-challenged or cholinergic-depleted animals, induces gamma wave electroencephalogram activity in the hippocampus and cortex, promotes cerebral blood flow, and increases active wakefulness at the expense of delta sleep (i.e., it is an alerting agent) [9] (unpublished data). Importantly, the doses required to produce these effects did not cause overt peripheral cholinergic stimulation.
In humans, MK-7622 has a Tmax of 2–4 hours and a half-life of ∼25 hours, which permits daily dosing. In a phase-1 healthy-volunteer study, MK-7622 at doses of 10, 40, and 70 mg showed a dose-related tendency to increase sigma (12–15 Hz) band awake electroencephalogram activity versus placebo, indicative of an alerting effect, with statistically significant increases at the 40 and 70 mg doses at 2, 4, and 12 hours after dose administration [9]. Furthermore, in another phase-1 study, MK-7622 at doses of 1, 10, and 70 mg reversed scopolamine impairment as measured by a detection task (an assessment of psychomotor function and information processing) from 1 to 4 hours [9]. These observations suggest blood-brain penetration of MK-7622 with pharmacodynamic effects at the administered doses.
The primary efficacy objective of the current trial was to establish proof of concept for MK-7622 as adjunctive therapy to acetylcholinesterase inhibitors in improving cognition in individuals with mild-to-moderate AD after 12 weeks of treatment. The trial also assessed safety and tolerability for up to 24 weeks of treatment. A 45-mg dose of MK-7622 was selected for evaluation based on indirect evidence of target modulation (described previously) and tolerability at this dose in phase-1 studies. Mechanistically, it is hypothesized that acetylcholinesterase inhibitors and MK-7622 have synergistic effects. As a positive allosteric modulator, MK-7622 selectively potentiates the action of acetylcholine at the M1 receptor, but in the absence of acetylcholine it has only modest activity at the M1 receptor. Acetylcholinesterase inhibitors increase synaptic levels of acetylcholine by inhibiting the breakdown of acetylcholine with the enzyme acetylcholinesterase, thereby making more acetylcholine available at the receptor site.
2. Methods
Full details of the study methods and statistical analysis are provided in the study protocol that is available as supplementary material (Supplementary Material 1).
2.1. Participants
Eligible participants were aged 55–85 years and met criteria for a diagnosis of probable AD based on the National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association criteria [10] as well as the criteria for AD dementia in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision [11]. Individuals had an MRI scan consistent with the diagnosis of AD within the last 12 months and a score of 12–24 on the Mini Mental State Examination (MMSE) at screening [12]. Participants were on a stable dose of either donepezil (10 mg daily), rivastigmine (9.5 or 13.3 mg/24 hours for the patch or 6–12 mg total daily dose for the capsule), or galantamine (16–24 mg total daily dose) for at least 2 months before the trial. They also had a reliable and competent trial partner who could accompany them to clinic visits. Major exclusion criteria included: evidence of vascular dementia as suggested by a modified Hachinski Ischemia score of >4 [13], clinically significant stroke, or MRI signs of significant cerebrovascular disease; clinically relevant neurological disorder other than AD; clinically relevant or unstable psychiatric disorder, including schizophrenia or other psychotic disorder, bipolar disorder, major depression (unless in remission), substance abuse disorders, or delirium; or significant laboratory screening test abnormality.
2.2. Study design and treatment
This randomized, placebo-controlled, parallel-group, multicenter, double-blind trial (Merck Protocol MK-7622−012; ClinicalTrials.gov NCT01852110) was conducted at 59 trial centers (55 in the United States of America and four in Canada) from October 2013 to April 2016. The study was conducted in accordance with the principles of Good Clinical Practice and was approved by the relevant institutional review boards. Each participant or their legally acceptable representative provided written informed consent.
The study consisted of a screening phase, a 2-week single-blind placebo run-in period, and a 24-week double-blind treatment period. After the 2-week placebo run-in period, participants were allocated in a double-blind fashion to MK-7622 or placebo in a 1:1 ratio. Participants randomized to MK-7622 began with a 15-mg dose for 1 week before being titrated up to 30 mg for 1 week and then up to 45 mg as the final dose. Randomization was implemented via an interactive voice response system using a computer-generated randomized allocation schedule prepared by a statistician. Randomization was stratified based on MMSE score (12–18 vs. 19–24) and type of acetylcholinesterase inhibitor (donepezil vs. other). In addition, there was a 30% randomization cap on the number of participants with MMSE scores of 21–24. All study personnel, including the investigators, site staff, participants, and sponsor staff remained blinded to treatment allocation throughout the study. Unblinding took place after data collection was complete.
2.3. Assessments
Cognition was assessed by the Alzheimer's Disease Assessment Scale–Cognitive Subscale (ADAS-Cog12) [14], [15] administered at baseline and weeks 4, 8, 12, 18, and 24. While the ADAS-Cog12 was collected, the ADAS-Cog11 was the primary cognitive endpoint. Additional cognitive assessments included the MMSE and a prespecified cognitive composite measure, the Composite Cognition Score-3 Domain (CCS-3D), administered at baseline and weeks 12 and 24. The CCS-3D was constructed using the delayed recall item from the ADAS-Cog as well as the Digit Span Test, Trail Making Test, Digit Symbol Coding, Controlled Oral Word Association Test, and the Verbal Fluency Test. Other assessments included daily function assessed by the Alzheimer's Disease Cooperative Study–Activities of Daily Living Inventory [16] at baseline and weeks 12 and 24, global change as assessed by the Alzheimer's Disease Cooperative Study–Clinical Global Impression of Change [17] at weeks 12 and 24, and neuropsychiatric symptoms as assessed by the Neuropsychiatric Inventory [18] at baseline and weeks 12 and 24. Blood samples for pharmacokinetic analysis were taken at baseline and weeks 4, 12, and 24. Safety was assessed by adverse event reports throughout the study and by routine laboratory analyses, ECGs, and physical examinations at regular clinic visits.
Raters for clinical and cognitive assessments were required to meet minimum education and experience standards. Following credentials review, prequalified raters underwent additional training and certification before rating in the trial. Furthermore, rater performance on selected assessments was centrally evaluated and monitored to ensure and maintain adequate reliability throughout the trial.
2.4. Trial governance
This trial was developed in collaboration with a scientific advisory committee comprising sponsor (Merck & Co, Inc, Kenilworth, NJ, USA) and external scientific experts who provided input with respect to trial design, interpretation of trial results, and subsequent peer-reviewed scientific publications. The trial was managed by the study sponsor.
2.5. Statistical analysis
The primary efficacy outcome was change from baseline in ADAS-Cog11 at week 12 in the MK-7622 group compared with that in the placebo group. The secondary outcomes were change from baseline in Alzheimer's Disease Cooperative Study–Activities of Daily Living Inventory at week 24 and change from baseline in the CCS-3D at week 12. The CCS-3D was intended to cover aspects of cognition not well assessed by the ADAS-Cog and was calculated as the mean of three domain z-scores (episodic memory, executive function, and attention processing). Each of these three domain z-scores was calculated as the mean of domain-specific tests, as follows: episodic memory = immediate word recall, delayed word recall, word recognition, and orientation (all from ADAS-Cog); executive function = digits backward (from Digit Span), Trail Making Test part B, Controlled Oral Word Association Test, and Verbal Fluency Test; attention/processing speed = Trail Making Test part A, digits forward (from Digit Span Test), and Digit Symbol Coding. Other efficacy endpoints were exploratory.
The full analysis set population consisted of all randomized participants who received at least one dose of trial medication and either had a baseline measurement or at least one postdose, postrandomization observation for the analysis endpoint. For the analysis of change from baseline in ADAS-Cog11 at week 12, a constrained longitudinal data analysis method was used [19]. This model assumes a common mean across treatment groups at baseline and a different mean for each treatment at each of the postbaseline time points. In this model, the response vector consists of baseline and the values observed at each postbaseline time point. Time is treated as a categorical variable so that no restriction is imposed on the trajectory of any means over time. The analysis model also included categorical factors of treatment, apolipoprotein E ε-4 (APOE4) genotype (positive/carrier, negative/noncarrier), AD medication stratum (donepezil, other acetylcholinesterase inhibitors), gender, the use of memantine (use, no use), and the time-by-treatment interaction, as well as the baseline MMSE score and age as continuous covariates. The treatment difference in terms of mean change from baseline to a given time point was estimated and tested from this model. An unstructured covariance matrix was used to model the correlation among repeated measurements. The same constrained longitudinal data analysis model used for the primary endpoint was used to analyze all continuous secondary and exploratory endpoints. For the Alzheimer's Disease Cooperative Study–Clinical Global Impression of Change, a mixed-effects repeated-measures model was used and included all the covariates used in the primary efficacy analysis model. Additional sensitivity analyses were performed using an intent-to-treat population for the efficacy endpoints.
The all-subjects-as-treated population was employed for safety analyses. The all-subjects-as-treated population consisted of all randomized participants who received at least one dose of trial medication. The number and percentage of participants with adverse events were calculated. As both MK-7622 and acetylcholinesterase inhibitors target the cholinergic system, a “cholinergically related adverse event” term was prespecified for analysis and was composed of the following individual adverse events: nausea, vomiting, diarrhea, salivation, salivary gland pain, abdominal pain, sweating, bradycardia, and atrioventricular conduction block. A participant was considered to have a cholinergically related adverse event if they had at least one of the listed adverse events. The difference between MK-7622 and placebo and 95% confidence intervals were calculated using the Miettinen and Nurminen method [20]. For the endpoint of cholinergically related adverse event only, a P value was provided for the between-group comparison using the Miettinen and Nurminen method.
All statistical analyses were performed using SAS, version 9.3 (SAS Institute, Cary, NC, USA).
The trial planned to randomize 250 participants into MK-7622 45 mg and placebo groups (1:1 ratio). The sample size of 125 participants per group provided 80% power to declare that MK-7622 45 mg was superior to placebo on the primary endpoint, if the underlying treatment difference in mean changes from baseline in ADAS-Cog11 score was two points. The power and sample size were based on an expected dropout rate of approximately 8% by week 12.
Interim analyses were conducted for safety (after 60 participants had reached 8 weeks) and for futility (after 188 participants had reached 12 weeks) and were reviewed by a data-monitoring committee comprised of Merck researchers who were not otherwise involved in the study. Criteria were set to conclude futility if the conditional power of observing a significant difference on the 12-week ADAS-Cog11 was less than 20%.
3. Results
3.1. Patient disposition
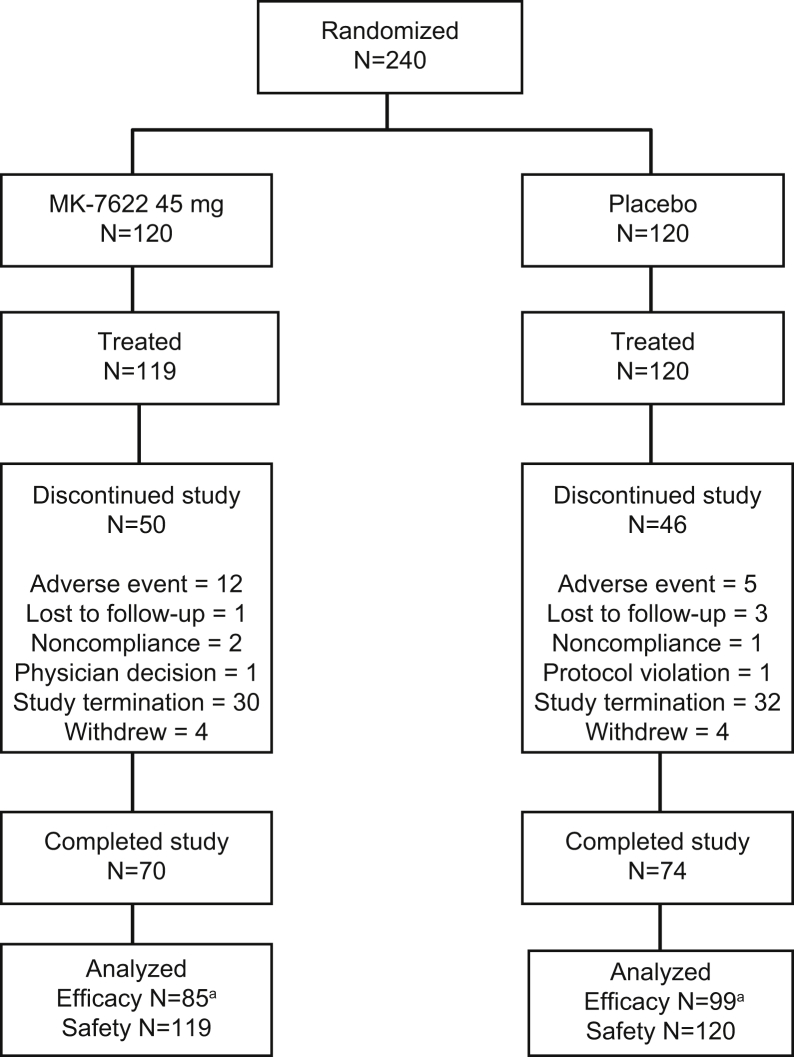
A total of 240 participants were randomized, and 239 received at least one dose of study treatment (Fig. 1). The trial was stopped for futility after meeting the prospectively defined stopping threshold. At the time of study termination, 144 participants had completed the study. The majority of discontinuations were due to the early study termination.
Fig. 1.
Study flowchart. aN = number analyzed for the primary endpoint of ADAS-Cog11 at week 12. Abbreviation: ADAS-Cog, Alzheimer's Disease Assessment Scale–Cognitive Subscale.
Characteristics of treated participants are shown in Table 1. Overall, the participant characteristics were consistent with the targeted mild-to-moderate AD population, and demographics were well balanced between treatment groups.
Table 1.
Characteristics of treated participants
| Characteristic | MK-7622 (45 mg) (N = 119) | Placebo (N = 120) |
|---|---|---|
| Gender | ||
| Male | 58 (48.7) | 52 (43.3) |
| Female | 61 (51.3) | 68 (56.7) |
| Age, years | ||
| Mean (SD) | 72.5 (7.1) | 71.7 (8.3) |
| ≤65 | 20 (16.8) | 30 (25.0) |
| >65 | 99 (83.2) | 90 (75.0) |
| Race | ||
| White | 108 (90.8) | 109 (90.8) |
| Black | 8 (6.7) | 4 (3.3) |
| Asian | 2 (1.7) | 5 (4.2) |
| Other | 1 (0.8) | 2 (1.7) |
| APOE4 genotype | ||
| Negative | 46 (38.7) | 55 (45.8) |
| Positive | 73 (61.3) | 65 (54.2) |
| AD severity by MMSE score | ||
| 12–18 (moderate) | 63 (52.9) | 65 (54.2) |
| 19–24 (mild) | 56 (47.1) | 55 (45.8) |
| Time of initial AD diagnosis | ||
| <6 months ago | 9 (7.6) | 7 (5.8) |
| 6–12 months | 45 (37.8) | 37 (30.8) |
| >24 months | 65 (54.6) | 76 (63.3) |
| Use of memantine at screening | ||
| No | 70 (58.8) | 58 (48.3) |
| Yes | 49 (41.2) | 62 (51.7) |
| Prior AD medication | ||
| Donepezil | 103 (86.6) | 104 (86.7) |
| Other AchEI | 16 (13.4) | 16 (13.3) |
Abbreviations: APOE4, apolipoprotein E ε-4; AD, Alzheimer's disease; MMSE, mini mental state examination; AchEI, acetylcholinesterase inhibitors; SD, standard deviation.
NOTE. Data are represented as number (%) of participants, except for mean (SD) age.
3.2. Efficacy
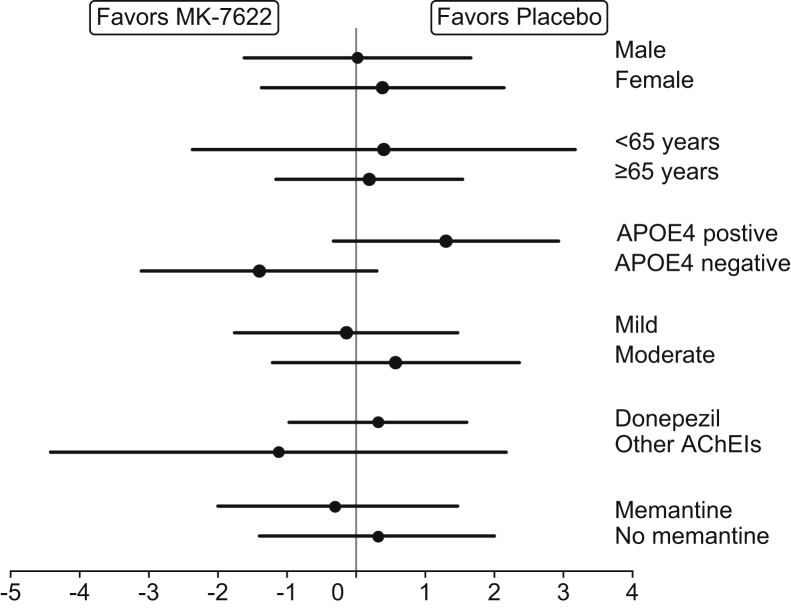
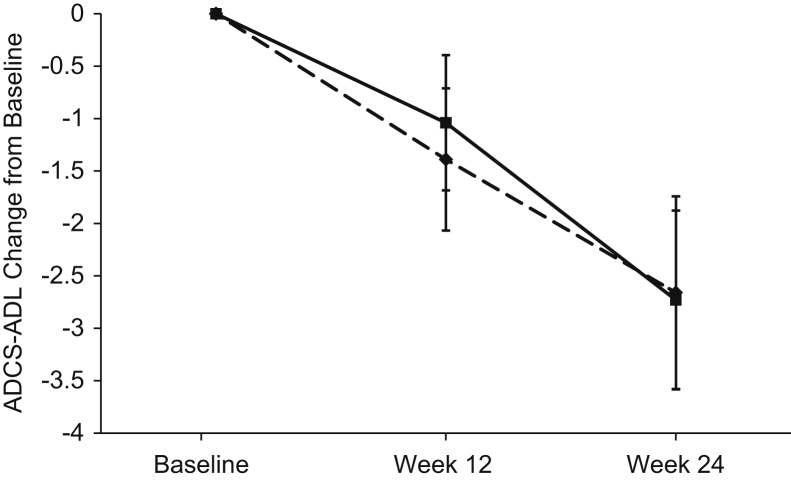
Efficacy findings are summarized in Table 2. Participants treated with MK-7622 45 mg, as compared with those treated with placebo, did not show statistically significant improvement on the primary endpoint of the ADAS-Cog11 at week 12 (change from baseline = 0.18 points; 95% confidence interval: −1.00 to 1.37; P value = .762). No treatment differences on the ADAS-Cog11 were observed at other time points (Fig. 2) or in subgroups (Fig. 3). MK-7622 did not significantly improve function as assessed by the secondary endpoint of Alzheimer's Disease Cooperative Study–Activities of Daily Living Inventory score at week 24 or at week 12 (Fig. 4). No treatment differences were seen on other endpoints (Table 2). Sensitivity analyses performed using an intent-to-treat population for the efficacy endpoints did not show meaningfully different findings from the primary approach (data not shown).
Table 2.
Efficacy results at weeks 12 and 24
| Assessment | Baseline |
Week 12 |
Week 24 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline, mean (SD) | N | Week 12, mean (SD)∗ | CFB, mean (SE)† | Difference versus placebo, estimate (95% CI)† | N | Week 24, mean (SD) | CFB, mean (SE)† | Difference versus placebo, estimate (95% CI)† | |
| ADAS-Cog11 | |||||||||
| MK-7622 | 21.8 (7.1) | 85 | 21.9 (8.2) | 0.39 (0.44) | 0.18 (−1.00 to 1.37) | 64 | 22.7 (9.1) | 1.55 (0.60) | −0.28 (−1.90 to 1.34) |
| Placebo | 23.6 (8.7) | 99 | 23.6 (8.5) | 0.21 (0.42) | 75 | 24.5 (9.2) | 1.83 (0.56) | ||
| ADCS-ADL | |||||||||
| MK-7622 | 60.7 (11. 2) | 86 | 58.7 (11. 9) | −1.39 (0.68) | −0.35 (−2.18 to 1.48) | 64 | 58.1 (14.5) | −2.66 (0.92) | 0.06 (−2.42 to 2.54) |
| Placebo | 59. 4 (11.9) | 95 | 59.0 (12.2) | −1.04 (0.65) | 76 | 58.1 (12.4) | −2.73 (0.85) | ||
| CCS-3D | |||||||||
| MK-7622 | −0.11 (0.74) | 80 | −0.03 (0.80) | 0.13 (0.04) | 0.10 (−0.01 to 0.22) | 56 | −0.07 (0.98) | 0.17 (0.06) | 0.02 (−0.14 to 0.19) |
| Placebo | 0.02 (0.82) | 85 | −0.03 (0.87) | 0.03 (0.04) | 69 | 0.05 (0.82) | 0.14 (0.06) | ||
| MMSE | |||||||||
| MK-7622 | 18.4 (3.4) | 92 | 18.2 (4.4) | −0.47 (0.34) | −0.02 (−0.94 to 0. 90) | 64 | 17.7 (4.9) | −1.28 (0.39) | −0.39 (−1.43 to 0.65) |
| Placebo | 18.3 (3.6) | 100 | 18.0 (4.8) | −0.45 (0.32) | 77 | 17.8 (4.7) | −0.89 (0.36) | ||
| NPI | |||||||||
| MK-7622 | 9.2 (11.0) | 91 | 10.4 (12. 5) | 1.07 (1.02) | 2.10 (−0.57 to 4.78) | 64 | 10.6 (12. 9) | 1.11 (1.23) | 0.26 (−2.98 to 3.50) |
| Placebo | 11.5 (11. 4) | 101 | 10.1 (10. 7) | −1.03 (0.97) | 77 | 12.0 (12. 3) | 0.85 (1.13) | ||
| ADCS-CGIC∗ | |||||||||
| MK-7622 | 91 | 4.43 (0.12) | −0.07 (−0.32 to 0.18) | 83 | 4.70 (0.12) | 0.02 (−0.25 to 0.30) | |||
| Placebo | 99 | 4.50 (0.11) | 92 | 4.67 (0.12) | |||||
Abbreviations: ADAS-Cog11, Alzheimer's Disease Assessment Scale–Cognitive subscale (11-item version); ADCS-ADL, Alzheimer's Disease Cooperative Study–Activities of Daily Living Inventory; CCS-3D, Composite Cognition Score-3 Domain; MMSE, mini mental state examination; NPI, Neuropsychiatric Inventory; ADCS-CGIC, Alzheimer's Disease Cooperative Study–Clinical Global Impression of Change; CFB, change from baseline; SD, standard deviation; SE, standard error; CI, confidence interval.
NOTE. N represents the number of participants with values for the designated test at the designated time point; all participants in the full analysis set (N = 239) were included in the analysis.
Mean (SE) for ADCS-CGIC.
Derived using a constrained longitudinal data analysis model (or a mixed-effects repeated-measures model for ADCS-CGIC) including the categorical factors of treatment, APOE4 genotype (positive, negative), AD medication stratum (donepezil, other acetylcholinesterase inhibitors), gender, the use of memantine (use, no use), the time-by-treatment interaction, and age as continuous covariate.
Fig. 2.
ADAS-Cog11 mean (SE) change from baseline scores over 24 weeks (a negative score indicates improvement, and a positive score indicates worsening relative to baseline); dashed line = MK-7622 and solid line = placebo. Abbreviations: ADAS-Cog, Alzheimer's Disease Assessment Scale–Cognitive Subscale; SE, standard error.
Fig. 3.
Estimated difference (95% CI) versus placebo in change from baseline ADAS-Cog11 score by subgroups (a negative score indicates improvement, and a positive score indicates worsening relative to baseline). Abbreviations: APOE4, apolipoprotein E ε-4; ADAS-Cog, Alzheimer's Disease Assessment Scale–Cognitive Subscale; AchEIs, acetylcholinesterase inhibitors; CI, confidence interval.
Fig. 4.
ADCS-ADL mean (SE) change from baseline scores at week 12 and 24 (a negative score indicates worsening relative to baseline); dashed line = MK-7622 and solid line = placebo. Abbreviations: ADCS-ADL, Alzheimer's Disease Cooperative Study–Activities of Daily Living Inventory; SE, standard error.
3.3. Safety
Adverse events are summarized in Table 3. There were no deaths. Although not statistically significant (i.e., the 95% confidence interval for the difference included zero), participants receiving MK-7622 reported more adverse events and serious adverse events than participants treated with placebo. Participants on MK-7622 had more discontinuations due to adverse events than participants on placebo. Serious adverse events resulted in treatment discontinuation in six participants on MK-7622 group versus none on placebo; the serious adverse events resulting in discontinuation were lung neoplasm, ischemic stroke, normal pressure hydrocephalus, fatigue/mental status change, cholangiocarcinoma, and bipolar I disorder. One serious adverse event (bipolar I disorder) was considered to be related to drug by the investigator. Participants on MK-7622 reported significantly more cholinergically related adverse events than participants on placebo (P = .006). The most common cholinergically related event, and the most common specific adverse event, was diarrhea, occurring in 15.1% of the MK-7622 45-mg group versus 5.8% in the placebo group. There were no relevant differences in vital signs, ECGs, or chemistry or hematology laboratory measures.
Table 3.
Summary of adverse events (AE) within 14 days after dose administration
| AE type | MK-7622 45 mg (N = 119) | Placebo (N = 120) | Estimated difference (95% CI)∗ |
|---|---|---|---|
| AE Summary | |||
| Any AE | 83 (69.7) | 71 (59.2) | 10.6 (−1.6 to 22.5) |
| Drug-related AE | 34 (28.6) | 24 (20.0) | 8.6 (−2.3 to 19.4) |
| Serious AE | 9 (7.6) | 4 (3.3) | 4.2 (−1.7 to 10.9) |
| Serious drug-related† AE | 1 (0.8) | 0 (0.0) | 0.8 (−2.3 to 4.6) |
| Death | 0 (0.0) | 0 (0.0) | 0.0 (−3.1 to 3.1) |
| Discontinued due to AE | 19 (16.0) | 7 (5.8) | 10.1 (2.3 to 18.5) |
| Discontinued due to drug-related† AE | 10 (8.4) | 6 (5.0) | 3.4 (−3.2 to 10.4) |
| Discontinued due to serious AE | 6 (5.0) | 0 (0.0) | 5.0 (1.8 to 10.6) |
| Discontinued due to serious drug-related† AE | 1 (0.8) | 0 (0.0) | 0.8 (−2.3 to 4.6) |
| Cholinergically related AEs | |||
| Any cholinergically related AE‡ | 25 (21.0) | 10 (8.3) | 12.7 (3.8 to 21.9)‡ |
| Diarrhea | 18 (15.1) | 7 (5.8) | 9.3 (1.6 to 17.6) |
| Hyperhidrosis | 5 (4.2) | 0 (0.0) | 4.2 (1.0 to 9.5) |
| Vomiting | 3 (2.5) | 1 (0.8) | 1.7 (−2.3 to 6.4) |
| Nausea | 2 (1.7) | 2 (1.7) | 0.0 (−4.4 to 4.5) |
| Salivary hypersecretion | 1 (0.8) | 0 (0.0) | 0.8 (−2.3 to 4.6) |
| Bradycardia | 1 (0.8) | 0 (0.0) | 0.8 (−2.3 to 4.6) |
| Abdominal pain | 1 (0.8) | 2 (1.7) | −0.8 (−5.1 to 3.1) |
| Salivary gland pain | 0 (0.0) | 0 (0.0) | 0.0 (−3.1 to 3.1) |
| Atrioventricular block | 0 (0.0) | 0 (0.0) | 0.0 (−3.1 to 3.1) |
| Common AEs ≥5% in either group | |||
| Diarrhea | 18 (15.1) | 7 (5.8) | 9.3 (1.6 to 17.6) |
| Headache | 11 (9.2) | 6 (5.0) | 4.2 (−2.5 to 11.5) |
| Rhinorrhea | 7 (5.9) | 1 (0.8) | 5.0 (0.6 to 10.9) |
| Urinary incontinence | 6 (5.0) | 0 (0.0) | 5.0 (1.8 to 10.6) |
| Weight decreased | 6 (5.0) | 2 (1.7) | 3.4 (−1.5 to 9.1) |
| Urinary tract infection | 6 (5.0) | 7 (5.8) | −0.8 (−7.2 to 5.5) |
| Fall | 2 (1.7) | 6 (5.0) | −3.3 (−9.0 to 1.6) |
Abbreviation: CI, confidence interval.
NOTE. Data are represented as number (%) of participants.
Difference versus placebo based on the Miettinen and Nurminen method.
Determination made by investigator while blinded.
P value = .006 versus placebo based on the Miettinen and Nurminen method; statistical testing (P value) was only prespecified for the “any cholinergically related AE” category.
3.4. Pharmacokinetics
Generally, exposures were within the expected confidence intervals for MK-7622 as predicted from internal dose-exposure modeling. No individuals were below the level of quantification, suggesting no serious adherence concerns.
4. Discussion
This phase-2 proof-of-concept study was terminated based on results from a futility analysis which indicated that the selective M1 positive allosteric modulator MK-7622, when given as adjunctive therapy to acetylcholinesterase inhibitors, was not effective in improving cognition at 12 weeks in individuals with mild-to-moderate AD. It is important to consider whether the results indicate that the drug mechanism is ineffective (a negative study) or instead were noninformative because of study design/implementation issues (a failed study). Placebo responses occur in AD cognition studies, and consequently, studies need to be sufficiently long—at least 12 weeks in early phase studies—to be sure that the placebo response is exhausted [21]. Fig. 2 shows that at week 4, there was a 1- to 1.5-point placebo benefit on the ADAS-Cog11, but by week 8, the placebo response was no longer evident. This response trajectory in the placebo group argues against a failed study.
Another issue to consider is whether the participants recruited did indeed have AD, given that there was no biomarker confirmation of the clinical diagnosis of AD (i.e., no cerebrospinal fluid or amyloid imaging). The inclusion of non-AD participants is less likely in studies such as this that focus on those with mild-to-moderate severity dementia compared with studies that focus on milder participants. Although some non-AD participants may have been included, the distribution of APOE4 (54%–61% ApoE4 positive), as well as other baseline characteristics, suggests that the population was similar to that of studies of participants with confirmed AD diagnoses [22] and similar to the populations enrolled in other recent AD clinical trials (e.g., study by Doody et al. [23]). Furthermore, the effect of MK-7622 on APOE4 carriers or memantine, where the diagnosis is more likely to be AD, was not different from that of placebo.
Given that these observations do not suggest a failed study or an inappropriate target population, it is important to consider whether the dose of MK-7622 (45 mg) was adequate to test the target mechanism. Dose selection was based on phase-1 data that showed tolerability up to 80 mg in short dosing regimens; with accumulation on daily dosing, 45 mg exhibited similar plasma exposure as 80- to 90-mg doses in healthy-volunteer studies (young and elderly). Phase-1 pharmacokinetic studies of drug levels in the cerebrospinal fluid also suggested that the drug was entering the central nervous system in adequate amounts [9]. Indirect evidence of target engagement at the 45-mg dose was obtained in qEEG and scopolamine models in phase-1 studies in healthy volunteers [9]. Furthermore, the adverse event profile of the 45-mg dose in the present study suggested that it was having pharmacological activity and precludes the evaluation of meaningfully higher doses in any future studies.
A potential reason for the observed lack of efficacy was that MK-7622 was studied on the background of concomitant treatment with acetylcholinesterase inhibitors and memantine in some participants. We hypothesized that acetylcholinesterase inhibitors and MK-7622 may have synergistic effects. However, it is also possible that there may be a ceiling effect for the cholinergic mechanism or that insufficient acetylcholine remains at the synapses in mild-to-moderate AD patients to allow a modulator the opportunity to achieve its effect, in which case different results could theoretically be observed in patients at an earlier stage of the disease. Another limitation of the add-on design is that the amount of incremental efficacy required to observe clinical benefit over current standard of care remains undefined in the literature. Thus, the assumptions around expected effect size may be less robust than those in a monotherapy trial.
The question of whether MK-7622 might be effective as monotherapy remains unanswered. However, monotherapy trials are increasingly impractical to execute, and to add meaningful value compounds should be able to demonstrate clear benefit over the standard of care. The apparent failure of the selective M1 allosteric modulator mechanism does not necessarily mean that M1 selective receptor agonists would be ineffective; as noted previously, a modulator may require sufficient acetylcholine at the synapses in AD patients to achieve its effect, whereas this may not be the case for an agonist.
We hypothesized that M1 modulation would be free of cholinergic side effects, but this did not appear to be the case. That is, we saw an increase in what appeared to be cholinergically mediated side effects, particularly those of a gastrointestinal nature (e.g., diarrhea). Our findings suggest that activation of M1 receptors alone is sufficient to produce unwanted cholinergic side effects. This supports recent preclinical observations [24] and calls into question part of the rationale for developing treatments that selectively target the M1 receptor; that is, this mechanism would have an improved tolerability profile over nonselective cholinergic treatments. However, it may be premature to conclude that our tolerability findings with an allosteric modulator extrapolate to all M1 receptor–targeted therapeutics.
Finally, we note that the futility approach adopted for this study, while not new (e.g., as seen in the study of Galasko et al. and Haig et al. [25], [26]), is an important learning experience for the field. The design allowed the study to be stopped when there was little chance of demonstrating efficacy, and as a result, it saved participants from needless exposure, which was particularly important, given the drug's side effects profile. Researchers should consider incorporating a futility approach for future proof-of-concept studies of novel-mechanism agents.
Research in Context.
-
1.
Systematic review: The M1 muscarinic receptor is thought to mediate the procognitive effects of cholinergic agents, whereas other acetylcholine receptors, particularly M2 and M3, may account for their side effects. Multiple muscarinic agonists have been developed, several have produced cognitive or behavioral benefits, but these compounds were not selective for M1 and produced intolerable peripheral cholinergic side effects. We evaluated MK-7622, a novel selective M1-positive allosteric modulator, in participants with Alzheimer's disease.
-
2.
Interpretation: MK-7622 did not demonstrate an improvement versus placebo in cognition at 12 weeks or function at 24 weeks. Participants taking MK-7622 were more likely to experience cholinergically related adverse events, particularly diarrhea, than those taking placebo.
-
3.
Future directions: The apparent failure of the selective M1-positive allosteric modulator mechanism does not necessarily mean that M1 selective receptor agonists would be ineffective. A modulator may require sufficient acetylcholine at the synapses in Alzheimer's disease patients to achieve its effect, whereas this may not be the case for an agonist. However, our findings suggest that activation of M1 receptors alone is sufficient to produce unwanted cholinergic side effects.
Acknowledgments
J.C. is supported by Keep Memory Alive and an NIGMS COBRE award (P20GM109025). Christopher Lines, PhD from Merck & Co, Inc, assisted with drafting the manuscript. Sheila Erespe from Merck & Co, Inc, assisted with submission of this manuscript.
Funding: The study was funded by Merck & Co, Inc, Kenilworth, NJ, USA. The study sponsor was involved in the study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Footnotes
Conflict of interest: T.V., J.L., C.A., S.F., H.L., L.S.-A., K.B.M., M.E., and D.M. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co, Inc, Kenilworth, NJ, USA, and own stock/stock options in the company.
J.C. has received research support from Avid Radiopharmaceuticals and Teva Pharmaceuticals. He has provided consultation services to AbbVie, Acadia, ADAMAS, Alzheon, Anavex, AstraZeneca, Avanir, Biogen-Idec, Biotie, Boehringer-Ingelheim, Chase, Eisai, FORUM, Genentech, Intra-cellular Therapies, Eli Lilly, Lundbeck, Merck, Neurotrope, Novartis, Nutricia, Otsuka, Pfizer, Prana, QR Pharma, Resverlogix, Roche, Suven, Takeda, and Toyoma companies. He has provided consultation to GE Healthcare and MedAvante and owns stock in ADAMAS, Prana, Sonexa, MedAvante, Neurotrax, and Neurokos. J.C. owns the copyright of the Neuropsychiatric Inventory.
M.F. has received grant research support from Accera, Biogen, Eisai, Eli Lilly, Genentech, Roche, Lundbeck, Chase Pharmaceuticals, and Boehringer-Ingelheim and has been a consultant for or on the advisory or data and safety monitoring boards of Accera, Alltech, Avanir, Biogen, Eisai Medical Research Inc, FORUM Pharmaceuticals, Genentech Inc, Grifols, Helicon Inc Research, Lundbeck, MedAvante, Medivation Inc, Merck & Co, Inc, Medtronic, Neurotrope Biosciences, Novartis, Pfizer, QR Pharma, Axovant Sciences Inc, Roche, Sanofi-Aventis, Schering-Plough, Takeda, Toyama Pharmaceutical, Pharm, Eli Lilly and Company, and UCB Pharma.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2018.03.004.
Supplementary data
References
- 1.Bubser M., Byun N., Wood M.R., Jones C.K. Muscarinic receptor pharmacology and circuitry for the modulation of cognition. Handb Exp Pharmacol. 2012;208:121–166. doi: 10.1007/978-3-642-23274-9_7. [DOI] [PubMed] [Google Scholar]
- 2.Lombardo S., Maskos U. Role of the nicotinic acetylcholine receptor in Alzheimer's disease pathology and treatment. Neuropharmacology. 2015;96:255–262. doi: 10.1016/j.neuropharm.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Fisher A. M1 muscarinic agonists target major hallmarks of Alzheimer's disease–an update. Curr Alzheimer Res. 2007;4:577–580. doi: 10.2174/156720507783018163. [DOI] [PubMed] [Google Scholar]
- 4.Bodick N.C., Offen W.W., Levey A.I., Cutler N.R., Gauthier S.G., Satlin A. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol. 1997;54:465–473. doi: 10.1001/archneur.1997.00550160091022. [DOI] [PubMed] [Google Scholar]
- 5.Fisher A., Pittel Z., Haring R., Bar-Ner N., Kliger-Spatz M., Natan N. M1 muscarinic agonists can modulate some of the hallmarks in Alzheimer's disease: implications in future therapy. J Mol Neurosci. 2003;20:349–356. doi: 10.1385/JMN:20:3:349. [DOI] [PubMed] [Google Scholar]
- 6.McArthur R.A., Gray J., Schreiber R. Cognitive effects of muscarinic M1 functional agonists in non-human primates and clinical trials. Curr Opin Investig Drugs. 2010;11:740–760. [PubMed] [Google Scholar]
- 7.Ma L., Seager M.A., Wittmann M., Jacobson M., Bickel D., Burno M. Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc Natl Acad Sci U S A. 2009;106:15950–15955. doi: 10.1073/pnas.0900903106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nickols H.H., Conn P.J. Development of allosteric modulators of GPCRs for treatment of CNS disorders. Neurobiol Dis. 2014;61:55–71. doi: 10.1016/j.nbd.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uslaner J.M., Kuduk S.D., Wittmann M., Lange H.S., Fox S.V., Min C. Preclinical to human translational pharmacology of the novel M1 positive allosteric modulator MK-7622. J Pharmacol Exp Ther. 2018;365:556–566. doi: 10.1124/jpet.117.245894. [DOI] [PubMed] [Google Scholar]
- 10.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 11.4th ed. American Psychiatric Association; Arlington, VA: 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 12.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Hachinski V.C., Iliff L.D., Zilhka E., Du Boulay G.H., McAllister V.L., Marshall J. Cerebral blood flow in dementia. Arch Neurol. 1975;32:632–637. doi: 10.1001/archneur.1975.00490510088009. [DOI] [PubMed] [Google Scholar]
- 14.Rosen W.G., Mohs R.C., Davis K.L. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 15.Mohs R., Knopman D., Petersen R., Ferris S., Ernesto C., Grundman M. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer's disease Assessment Scale that broadens its scope. Alzheimer Dis Assoc Disord. 1997;11 S13–21. [PubMed] [Google Scholar]
- 16.Galasko D., Bennett D., Sano M., Ernesto C., Thomas R., Grundman M. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S33–S39. [PubMed] [Google Scholar]
- 17.Schneider L.S., Olin J.T., Doody R.S., Clark C.M., Morris J.C., Reisberg B. Validity and reliability of the Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S22–S32. doi: 10.1097/00002093-199700112-00004. [DOI] [PubMed] [Google Scholar]
- 18.Cummings J.L., Mega M., Gray K., Rosenberg-Thompson S., Carusi D.A., Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 19.Liang K., Zeger S. Longitudinal data analysis of continuous and discrete responses for pre-post designs. Sankhyā: Indian J Stat. 2000;62:134–148. [Google Scholar]
- 20.Miettinen O., Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–226. doi: 10.1002/sim.4780040211. [DOI] [PubMed] [Google Scholar]
- 21.Ito K., Corrigan B., Romero K., Anziano R., Neville J., Stephenson D. Understanding placebo responses in Alzheimer's disease clinical trials from the literature meta-data and CAMD database. J Alzheimers Dis. 2013;37:173–183. doi: 10.3233/JAD-130575. [DOI] [PubMed] [Google Scholar]
- 22.Saunders A.M. Apolipoprotein E and Alzheimer disease: an update on genetic and functional analyses. J Neuropathol Exp Neurol. 2000;59:751–758. doi: 10.1093/jnen/59.9.751. [DOI] [PubMed] [Google Scholar]
- 23.Doody R.S., Thomas R.G., Farlow M., Iwatsubo T., Vellas B., Joffe S. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 24.Alt A., Pendri A., Bertekap R.L., Jr., Li G., Benitex Y., Nophsker M. Evidence for classical cholinergic toxicity associated with selective activation of M1 muscarinic receptors. J Pharmacol Exp Ther. 2016;356:293–304. doi: 10.1124/jpet.115.226910. [DOI] [PubMed] [Google Scholar]
- 25.Galasko D., Bell J., Mancuso J.Y., Kupiec J.W., Sabbagh M.N., van Dyck C. Clinical trial of an inhibitor of RAGE-Abeta interactions in Alzheimer disease. Neurology. 2014;82:1536–1542. doi: 10.1212/WNL.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haig G.M., Pritchett Y., Meier A., Othman A.A., Hall C., Gault L.M. A randomized study of H3 antagonist ABT-288 in mild-to-moderate Alzheimer's dementia. J Alzheimers Dis. 2014;42:959–971. doi: 10.3233/JAD-140291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.