Abstract
Introduction
Treatment with selective serotonin reuptake inhibitors has been suggested to mitigate amyloid-β (Aβ) pathology in Alzheimer's disease, in addition to an antidepressant mechanism of action.
Methods
We investigated whether chronic treatment with paroxetine, a selective serotonin reuptake inhibitor, mitigates Aβ pathology in plaque-bearing double-transgenic amyloid precursor protein (APP)swe/presenilin 1 (PS1)ΔE9 mutants. In addition, we addressed whether serotonin depletion affects Aβ pathology. Treatments were assessed by measurement of serotonin transporter occupancy and high-performance liquid chromatography. The effect of paroxetine on Aβ pathology was evaluated by stereological plaque load estimation and Aβ42/Aβ40 ratio by enzyme-linked immunosorbent assay.
Results
Contrary to our hypothesis, paroxetine therapy did not mitigate Aβ pathology, and depletion of brain serotonin did not exacerbate Aβ pathology. However, chronic paroxetine therapy increased mortality in APPswe/PS1ΔE9 transgenic mice.
Discussion
Our results question the ability of selective serotonin reuptake inhibitor therapy to ameliorate established Aβ pathology. The severe adverse effect of paroxetine may discourage its use for disease-modifying purposes in Alzheimer's disease.
Keywords: Alzheimer's disease; Cerebral amyloidosis; Neocortex; Monoamine; Serotonin; Selective serotonin reuptake inhibitor; SERT occupancy; [3H]DASB; Autoradiography; Transgenic mouse model; 5,7-dihydroxytryptamine; Stereology
Highlights
-
•
Chronic paroxetine treatment does not mitigate established amyloid-β pathology in APPswe/PS1ΔE9 mice.
-
•
Chronic paroxetine treatment increases the mortality of APPswe/PS1ΔE9 but not littermate wild-type mice.
-
•
Serotonergic depletion does not influence established amyloid-β pathology in APPswe/PS1ΔE9 mice.
1. Introduction
The serotonergic system degenerates in Alzheimer's disease (AD) along with the cholinergic and noradrenergic systems [1], [2], [3]. In patients with AD, the level of serotonin (5-hydroxytryptamine [5-HT]) is significantly reduced in several cortical regions, in particular in the frontal and temporal cortex [4], [5]. This pathological change has been suggested to contribute to the depressive symptoms that frequently precede the cognitive decline in patients with AD [6], [7], [8]. It is still being disputed whether antidepressive treatment of patients with AD with selective serotonin reuptake inhibitors (SSRIs) impacts on the decline of cognition in AD [9], [10], [11], [12] or the conversion from mild cognitive impairment to AD [13]. In AD, the density of specific cortical 5-HT receptors correlates positively to amyloid β (Aβ) pathology and negatively to cognitive performance [14], with a reduced density of the 5-HT4R and 5-HT6R being observed in mild cognitive impairment [15], [16], [17]. Evidence that SSRI treatment impacts on Aβ accumulation comes from positron emission tomography studies, showing reduced uptake of Pittsburgh compound B, a proposed marker of Aβ pathology, in the brains of individuals with a history of SSRI therapy, including citalopram and fluoxetine [18]. In addition, acute, successive administration of citalopram (30 mg) to healthy individuals with 2 hours interval is reported to diminish cerebrospinal fluid levels of Aβ [19].
Interestingly, studies in transgenic mouse models of AD have suggested that SSRI treatment has major impact on Aβ pathology [18], [19], [20]. In the APPswe/PS1ΔE9 (amyloid precursor protein [APP]/presenilin 1 [PS1]) transgenic mouse, which is a well-established model of Aβ pathology [21], 4 months treatment with citalopram (8 mg/kg/day per os) from 3 months of age results in ∼50% lower Aβ plaque load in the neocortex at 7 months of age [18]. In 6-month-old APP/PS1 mice, 4 weeks of treatment with citalopram (10 mg/kg/day i.p.) impairs initial Aβ plaque formation and growth [18]. Five months treatment with paroxetine (5 mg/kg/day i.p.) from 5 months of age results in lower Aβ levels at 10 months of age in the hippocampus of 3 × Triple-Tg mice [20], a model of combined Aβ and tau pathology. In addition, 5-week treatment with fluoxetine (10 mg/kg/day i.p.) is suggested to reduce Aβ load in 18-month-old APP/PS1 mice [22], at an age when these mice show a reduction in 5-HT in the neocortex [23], [24]. Finally, infusion of 5-HT into the hippocampus of APP/PS1 mice has been reported to reduce interstitial levels of Aβ [18], by mechanisms involving stimulation of the 5-HT4R and 5-HT6R and increased processing of APP via the nonamyloidogenic route [25], [26].
Importantly, there are still no preclinical studies, where plaque-bearing APP/PS1 mice have been chronically treated with doses of SSRI, resulting in ≈80% occupancy of the serotonin transporter (SERT), which is considered therapeutic for the treatment of depression [27]. Therefore, we tested the hypothesis that chronic treatment of plaque-bearing 9-month-old APP/PS1 mice for 9 months with paroxetine (5 or 10 mg/kg/day per os) would mitigate Aβ pathology and improve behavior (locomotion, social memory). We also tested the hypothesis that a neurotoxin-induced loss of cortical 5-HT at 9 months of age would impact on Aβ pathology at 12 months. The results of this study question the proposed beneficial effect of SSRI therapy and 5-HT on Aβ pathology in mice with manifest Aβ pathology.
2. Methods
2.1. Paroxetine treatment and neurotoxin injection
Paroxetine: APPswe/PS1ΔE9 (APP/PS1) and littermate wild-type (Wt) mice [21] were bred on a B6C3 background in house or at Taconic A/S, Denmark [28]. Paroxetine (Seroxat oral solution 2 mg/mL, GSKline) was administered in the drinking water in a dose of 10 mg/kg/day or 5 mg/kg/day (Table 1) to 9-month-old male APP/PS1 and Wt mice. APP/PS1 and Wt controls received normal drinking water. Treatment efficacy was assessed by autoradiographic measurement of the SERT occupancy using [3H]3-amino-4-(2-dimethylaminomethylphenylsulfanyl)-benzonitrile, as detailed in Supplementary Methods.
Table 1.
Study design
| Experimental group | Treatment/intervention | Genotype |
Treatment period (months) | Behavioral analysis | |
|---|---|---|---|---|---|
| Wt (n) | APP/PS1 (n) | ||||
| Nine months paroxetine treatment | Vehicle | 20 | 14 | 9–18 months | Open field, elevated plus maze, social interaction, social memory∗ Y-maze† |
| Paroxetine 10 mg/kg/day | 15 | 5 | |||
| Vehicle | 15 | 7 | 9–18 months | Open field, elevated plus maze, social interaction, social memory Y-maze‡ | |
| Paroxetine 5 mg/kg/day | 11 | 7 | |||
| Three months paroxetine treatment | Vehicle | 6 | 10 | 9–12 months | Not done |
| Paroxetine 10 mg/kg/day | 6 | 7 | |||
| Three months 5,7-DHT-induced loss of 5-HT | Sham | 6 | 6 | 9–12 months | Open field, social interaction, social memory Y-maze‡ |
| 5,7-DHT | 6 | 6 | |||
Neurotoxin: To destroy 5-HT fibers and neurons, 5,7-dihydroxytryptophan (5,7-DHT) was stereotaxically injected into the lateral ventricles of 9-month-old male APP/PS1 and Wt mice (Table 1), as described elsewhere [31], but injecting a total dose of 80 μg 5,7-DHT. Mice with unsuccessful lesions, as determined by the measurement of 5-HT in neocortical tissues, were excluded from statistical analysis (Danish Veterinary & Food Administration: J.no. 2007/562-50, J.no. 2012-15-2935-00,023, J.no. 2012-DY-2934-00,008).
Tissue processing: See Supplementary Methods.
2.2. Behavior
The behavior of 18-month-old mice was assessed using the open field, the elevated plus maze, and the social interaction tests [29]. The 5,7-DHT-treated mice were assessed using the open field test [32]. All tests were performed in the light phase of 24-hr light/dark cycle. For details, see Supplementary Methods.
2.3. High-performance liquid chromatography for 5-HT
Monoamine levels were assessed in perchlorate extracts of the dissected tissues using high-performance liquid chromatography with electrochemical detection [24], [32]. The detection limit for 5-HT was 30 fmol on column. The detection level in tissues was 0.25 pmol/mg wet weight.
2.4. Immunohistochemistry for human Aβ
Cryostat and vibratome sections were demasked free floating in 70% formic acid diluted in H2O for 15 min at room temperature, followed by staining for human Aβ using biotinylated monoclonal mouse-anti-Aβ immunoglobulin G1 (6E10, Biosite), which was detected by horse radish peroxidase-Streptavidin and developed with diaminobenzidine. Sections were mounted on glass-slides, dehydrated, and coverslipped from xylene in Depex. Paraffin sections were demasked in Tris-ethylenediaminetetraacetic acid-glucose-Buffer at 480 Watts for 15 min and stained as mentioned previously. The protocol for SERT is given in Supplementary Methods.
2.5. Enzyme-linked immunosorbent assay for human Aβ
Samples were processed according to the manufacturers protocol (Invitrogen), whereafter the Aβ was measured using Invitrogen Aβ42 Human enzyme-linked immunosorbent assay Kit (KHB3441) and Aβ40 Human enzyme-linked immunosorbent assay Kit (KHB3482), as described in Supplementary Materials. Data expressed in pg/mg protein were used for calculation of the Aβ42/Aβ40 ratio.
2.6. Quantification of Aβ plaque load
The Aβ plaque load was estimated in the neocortex by the use of stereological grid counting, using the new CAST software (Visiopharm), by an observer who was blinded to the treatment of the mice. The estimation was performed in 6.2 +/− 1.5 (mean +/− standard deviation) sections per mouse in the 18-month-old APP/PS1 mice. For details, see Supplementary Methods. The percentage of neocortex covered by plaques in individual sections was calculated as: Aβ plaque load (%) = No. of Aβ+ plaques touching a cross (in a grid)/No. of crosses in neocortex × 100%. Plaque density was given by the number of plaques per area, and plaque size by the number of crosses hitting individual plaques. Data on Aβ plaque load are presented in dot diagrams, and plaque density and plaque size in bar graphs. The coefficient of error (CE) of the estimate of individual mice was used to calculate a mean CE for the group. The CE values and the coefficient of variation were calculated as done previously [33].
2.7. Statistical methods
Data sets were tested for normality of distribution. Bartlett's test was used to check the equality of variances. Binary logistic regression was used to assess the effects of genotype, age, and treatment on mouse survival. Statistically significant differences between groups were evaluated using unpaired, two-tailed Student t tests, one-way analysis of variance (ANOVA), or repeated measures two-way ANOVA, followed by least significant difference post hoc tests. Analyses were performed using GraphPad software (Prism 4.0b) for Macintosh or XLSTAT (version 2010.3.06) for Windows. In one data set, one outlier >2 SDs from the mean was excluded. Statistical difference is indicated as *P < .05, **P < .01, ***P < .001, and ****P < .0001.
3. Results
3.1. 5-HT is reduced in aging APP/PS1 mice
The 18-month-old male APP/PS1 mice showed a reduction in 5-HT levels of ≈40% in frontal cortex compared with littermate Wt mice (P < .01, Student t test) (Fig. 1). This reduction is within the range previously reported for this AD model [23] and in line with the reduction in 5-HT levels observed in frontal cortex of AD patients [5], [6].
Fig. 1.
5-HT levels (pmol/mg wet weight) are reduced in frontal cortex of old APP/PS1 mice. HPLC measurement of 5-HT in the frontal cortex of 18-month-old male APP/PS1 Tg and Wt mice. Data are expressed as mean ± SEM. Student t test; Tg, n = 6; Wt, n = 5. **P < .01. Abbreviations: 5-HT, 5-hydroxytryptamine; APP, amyloid precursor protein; HPLC, high-performance liquid chromatography; SEM, standard error of the mean; PS1, presenilin 1; ww, wet weight of dissected brain tissue.
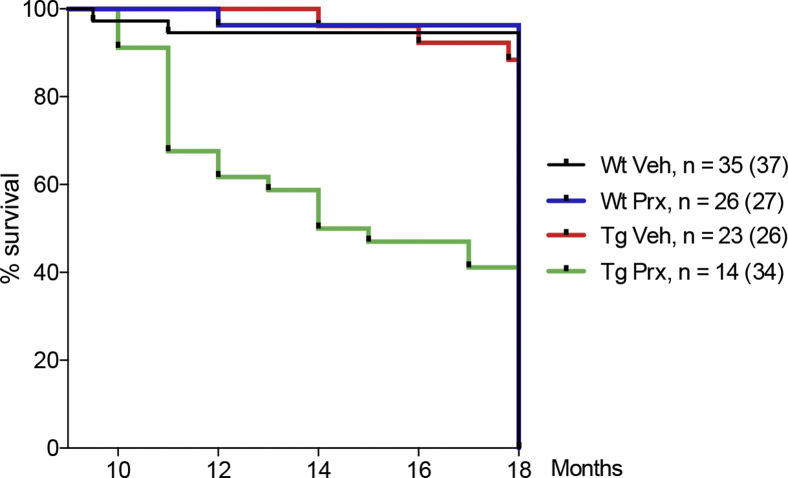
3.2. Chronic paroxetine treatment reduces the survival of APP/PS1 mice
Individually caged 9-month-old male APP/PS1 and Wt mice were treated per os with paroxetine (Seroxat oral solution 2 mg/mL, GSKline) for up to 9 months, whereas vehicle-treated mice received normal drinking water (Table 1). Paroxetine treatment at 10 mg/kg/day per os reduced the survival of APP/PS1 mice (Fig. 2). Binary logistic regression showed that the effect of treatment, genotype, and age on premature death was statistically significant (χ2 [3] = 166.2, P < .001). The model explained 27.8% of the variance in premature deaths (Nagelkerke R2), and correctly classified 88.3% of cases. The independent factors treatment (Wald score = 39.3; P < .001), genotype (Wald score = 46.6; P < .001), and age (Wald score = 41.2; P < .001) added significantly to the model and increased the likelihood of death by 4.8, 5.6, and 1.3, respectively. Because of the reduced survival rate, the dose of paroxetine was reduced to 5 mg/kg/day (Table 1), resulting in paroxetine serum levels of 40–50 nmol/L. Binary logistic regression of the effects of genotype, age, and 5 mg/kg/day paroxetine treatment was significant (χ2 [3] = 56.7, P < .001), with the independent factors age (Wald score = 41.2; P < .001) and paroxetine (Wald score = 5.3, P < .05), but not genotype (Wald score = 0.0; P > .05), adding significantly to the model. Age and paroxetine treatment at 5 mg/kg/day per os increased the likelihood of premature death by 2.0 and 0.2, respectively.
Fig. 2.
Peroral paroxetine treatment reduces the survival of aging APP/PS1 mice. The survival was recorded for mice treated with paroxetine (prx) or vehicle (veh) for 9 months and shown in a Kaplan-Meier diagram. The APP/PS1 mice treated with paroxetine had a lower survival than all other groups after 9 months of treatment. Numbers of mice before and after treatment are shown, with the number before initiation of treatment indicated in brackets. Abbreviations: APP, amyloid precursor protein; PS1, presenilin 1.
3.3. Clinically relevant occupancy is achieved by oral paroxetine treatment
To ensure that all mice, including those treated with 5 mg/kg/day per os, were treated with a clinically relevant dose of paroxetine, we assessed the SERT occupancy in 18-month-old Wt mice treated with paroxetine in a dose of 5 mg/kg/day for 3 months. Vehicle-treated mice showed a high specific [3H]3-amino-4-(2-dimethylaminomethylphenylsulfanyl)-benzonitrile binding in all brain regions analyzed, except the cerebellum (data not shown). In neocortex, the occupancy of SERT in paroxetine-treated mice was 86% (Fig. 3). These results confirmed that the APP/PS1 and Wt mice were treated with paroxetine doses considered therapeutic in humans [27].
Fig. 3.
Assessment of SERT occupancy by [3H]DASB radioligand binding. (A) Autoradiograms showing the binding of the [3H]DASB radioligand in sagittal brain sections from Wt mice administered paroxetine in the drinking water (5 mg/kg/day) or vehicle from 15 to 18 months of age. Nonspecific binding (NSB) is shown at the bottom. (B) Graphic presentation showing that the SERT occupancy (%) of the paroxetine-treated Wt mice (5 mg/kg/day) is 86% in the neocortex. Data are expressed as mean ± SEM. n = 4/group. Abbreviations: [3H]DASB, [3H]3-amino-4-(2-dimethylaminomethylphenylsulfanyl)-benzonitrile; SEM, standard error of the mean.
3.4. Long-term paroxetine treatment has no effect on established Aβ pathology
The effect of the 9-month oral SSRI treatment was examined by comparing the Aβ plaque load in 18-month-old paroxetine- and vehicle-treated APP/PS1 mice. The Aβ plaque load was estimated by the use of a stereological point counting technique. The plaque load in the neocortex showed to be comparable in paroxetine- and vehicle-treated mice (Student t test: Veh, 18.0 ± 0.7% (mean ± standard error of the mean) vs. Prx, 16.5 ± 1.2 %, P = .27) (Fig. 4A and 4B). The mean CE and coefficient of variation of the estimated plaque loads are shown in Table 2. In addition, no difference was noted in plaque density (P = .66, Student t test), and plaque size (P > .05, all comparisons, Student t test), between vehicle- and paroxetine-treated mice (Fig. 4C and 4D). The majority of the plaques (>99%) were smaller than 7.0 × 103 μm2, a few ranged from 7.0–13.9 × 103 μm2 (0.5%), and almost no plaques were larger than 14.0 × 103 μm2 (Fig. 4D).
Fig. 4.
No effect on Aβ pathology in 18-month-old APP/PS1 mice treated with paroxetine for 9 months. (A) Immunohistochemistry of coronal section for the human Aβ using the 6E10 antibody suggesting that the distribution and density of plaques is comparable in the neocortex of 18-month-old APP/PS1 mice treated with vehicle or paroxetine for 9 months. Bar: 200 μm. (B) Dot-diagram showing the % Aβ plaque load in APP/PS1 mice treated with vehicle or paroxetine for 9 months. The horizontal line indicates the mean and the error bars, the SEM. Statistical comparison shows no difference in % Aβ plaque load between groups (Student t test, P = .27). (C) Bar diagram showing the density of plaques in APP/PS1 treated with paroxetine (n = 12) or vehicle (n = 18). Student t test; P = .66. (D) Bar diagram showing the size distribution in % of plaques counted in APP/PS1 mice treated with paroxetine (n = 12) or vehicle (n = 18). Student t test; P < .05, all comparisons. Abbreviations: Aβ, amyloid-β; APP, amyloid precursor protein; NCx, neocortex; SEM, standard error of the mean; Str, Striatum; Veh, vehicle; Prx, paroxetine; PS1, presenilin 1.
Table 2.
Precision of stereological estimates of the % Aβ plaque load in paroxetine- and vehicle-treated 18-month-old APP/PS1 transgenics
| Treatment | n | % Aβ plaque load Mean ± SEM |
CE | CV |
|---|---|---|---|---|
| Vehicle | 18 | 18.0 ± 0.7 | 0.07 | 0.17 |
| Paroxetine | 12 | 16.5 ± 1.2 | 0.06 | 0.24 |
Abbreviations: SEM, standard error of the mean; Aβ, amyloid-β; CE, coefficient of error; CV, coefficient of variation; APP, amyloid precursor protein; PS1, presenilin 1.
NOTE. The CE and CV are calculated as in West et al. [29].
To evaluate if paroxetine treatment might reduce plaque load compared with baseline at the time when the paroxetine treatment was initiated, groups of 3- and 9-month-old male APP/PS1 mice were processed in parallel with the 18-month-old APP/PS1 and Wt mice. As previously shown for female APP/PS1 mice [28], the plaque load in the male APP/PS1 mice increased steadily from 3 months (0.04 ± 0.04%) through 9 months (8.1 ± 0.7%), to 18 months of age (19.8 ± 0.9%) [One-way ANOVA: F(2,28) = 110.1, P < .0001]. The plaque load in the paroxetine-treated 18-month-old APP/PS1 mice was thereby statistically higher than that in 9-month-old APP/PS1 mice (Student t test 16.5 ± 1.2% vs. 8.1 ± 0.7%, P < .0001).
In conclusion, 9-month oral paroxetine treatment (10 and 5 mg/kg/day) does not mitigate Aβ pathology in the neocortex of 18-month-old male APP/PS1 mice.
3.5. Paroxetine treatment impacts mouse behavior in both APP/PS1 and Wt mice
Effects of paroxetine treatment (5 mg/kg/day) were evaluated using the open field test, the elevated plus maze test, and the social interaction test. Repeated measures two-way ANOVA showed no significant effect of genotype in the open field (F[1,33] = 0.76, P > .05), no genotype by treatment interaction (F[1,33] = 0.18, P > .05), and only a tendency toward an effect of the paroxetine treatment (F[1,33] = 3.22, P = .08). Still, there was a significant effect of the paroxetine treatment that reduced locomotion given by the number of horizontal entries in both Wt and APP/PS1 mice (data not shown) (F[5,165] = 2.86, P = .02). No significant differences were observed in any parameters in the elevated plus maze. There was an effect of treatment on social interaction (two-way ANOVA: F[1,31] = 4.70, P = .04). However, comparison with vehicle- and paroxetine-treated mice showed no effect of treatment on individual behaviors, such as total contact time, and latency. There was no effect of genotype on any parameters, and no significant effect was observed in the social memory test (data not shown).
3.6. Short-term paroxetine treatment has no effect on Aβ pathology
In contrast to the studies by Cirrito et al. [18] and Nelson et al. [20] in which mice were treated with SSRI for 4 to 5 months and brains were processed for Aβ pathology at a younger age, we treated mice with paroxetine (10 mg/kg/day per os) or vehicle for 3 months and analyzed Aβ pathology in 12-month-old male APP/PS1 mice (Table 1) by quantifying plaque load in the right neocortex, and by enzyme-linked immunosorbent assay for Aβ42 and Aβ40 in the left neocortex (Fig. 5A and 5B). There was no difference in plaque load between the paroxetine- and vehicle-treated APP/PS1 mice (P > .05, Student t test) (Fig. 5A). In addition, levels of Aβ42 and Aβ40 were unaffected by the treatment (P > .05, Student t test) (data not shown) as was the ratio of Aβ42/Aβ40 (P > .05, Student t test) (Fig. 5B), a risk parameter for AD [34].
Fig. 5.
No effect on Aβ pathology in neocortex of 12-month-old APP/PS1 mice treated with paroxetine or subjected to a 5,7-DHT-induced depletion of 5-HT. (A, B) Dot diagram showing the % Aβ plaque load in the neocortex of 6E10-stained sections of the left hemisphere (A), and bar diagram showing the Aβ42/Aβ40 ratio in the right neocortex (B) of the same vehicle- and paroxetine-treated, 12-month-old APP/PS1 mice (Veh: n = 9 (one outlier > 2 standard deviations from the mean was excluded), Prx: n = 6 (one sample not included in the ELISA for Aβ40), Student t test, P = .95). (C, D) Dot diagram showing the % Aβ plaque load in 6E10-stained sections of the left hemisphere (C), and bar diagram showing the Aβ42/Aβ40 ratio in the right neocortex (D) of the same 5,7-DHT- and sham-injected 12-month-old APP/PS1 mice. (Student t test, n = 6/group, P = .67). Horizontal lines and bar height indicate the means and the error bars, the SEM. Abbreviations: Aβ, amyloid-β; 5,7-DHT, 5,7-dihydroxytryptophan; APP, amyloid precursor protein; ELISA, enzyme-linked immunosorbent assay; Veh, vehicle; Prx, paroxetine; PS1, presenilin 1; 5-HT, 5-hydroxytryptamine; SEM, standard error of the mean.
3.7. Chronic loss of 5-HT has no effect on Aβ pathology or mouse behavior
We finally examined whether 5,7-DHT-induced serotonergic deafferentation of the forebrain of 9-month-old male APP/PS1 mice might impact Aβ pathology at 12 months of age (Table 1). The serotonergic deafferentation was validated by demonstration of undetectable levels of 5-HT and loss of SERT-immunopositive fibers in the neocortex (data not shown). The plaque load was comparable in sham-injected and deafferented APP/PS1 mice (2.4 ± 0.9% vs. 2.7 ± 0.6%, P = .29, Student t test) (Fig. 5C). Similarly, the levels of soluble Aβ42 and Aβ40 in the neocortex from the same mice were unaffected by the treatment (data not shown, P > .05, Student t test), as was the ratio of Aβ42/Aβ40 (P > .05, Student t test) (Fig. 5D). The behavioral testing at baseline in the open field test showed higher locomotor activity of APP/PS1 compared with Wt mice (P < .01, Student t test. APP/PS1: n = 22; Wt: n = 16). However, no significant differences were observed in the open field and social interaction tests between sham-injected and deafferented APP/PS1 mice at 12 months (data not shown, Sham: n = 6; 5,7-DHT: n = 6). These results suggest that 3-month impairment of 5-HT signaling has no effect on neither Aβ pathology nor behavior of 12-month-old APP/PS1 mice.
4. Discussion
4.1. Main findings
The main results of this study showed that chronic per os treatment with paroxetine of plaque-bearing 9-month-old APP/PS1 mice for up till 9 months was unable to mitigate Aβ pathology. However, treatment with paroxetine increased mortality for transgenic mice exclusively and reduced locomotion in both transgenic and littermate control mice. In addition, neurotoxin-induced serotonergic deafferentation of 9-month-old APP/PS1 mice had no impact on Aβ pathology in 12-month-old male APP/PS1 mice. These results were unexpected as previous studies have suggested that SSRIs mitigate Aβ pathology [18], [19], [20], [22]. Considering the therapeutic implications of the potential option to impact directly on Aβ pathology in individuals developing AD, it is important to understand the background for these apparently contradictory results.
4.2. What is already known
Previous studies have reported reduced Aβ pathology after shorter periods of SSRI treatment in younger APP/PS1 and 3 × TgAD mice [18], [19], [20]. In Cirrito et al. [18], citalopram treatment of APP/PS1 mice from 3 to 7 months of age, resulted in a lower plaque load in neocortex of APP/PS1 mice. In the study by Sheline et al. [19], 4 weeks of citalopram treatment of 6-month-old APP/PS1 mice impaired the formation and growth of cortical plaques at 7 months [18]. Another study using 17-month-old APP/PS1 mice suggested that 5-week treatment with fluoxetine (10 mg/kg/day i.p.) was sufficient to mitigate plaque load; however, as acknowledged by the authors, the plaque load was not quantified [22]. In contrast, a study from our own group showed no or minor effect on the Aβ levels in neocortex and hippocampus in APP/PS1 mice treated from 3 to 9 months of age with escitalopram in a dose of 5 mg/kg/day per os, leading to ≈80% occupancy of SERT [31]. This suggests that the differences in results cannot simply be explained by differences in the time of treatment start, but that other factors, such as sex, genetic background, and type of SSRI should be taken into consideration. Studies suggest that the R-enantiomer present in citalopram but not in escitalopram may reduce levels of Aβ by SERT-independent mechanisms, possibly by increasing the γ-secretase activity, increasing the nonamyloidogenic processing of APP, and reducing the production of Aβ [35], [36].
4.3. Strengths and limitations
The fact that the APP/PS1 and Wt mice were all littermates, of the same sex, and housed identically during their entire life span affords credibility to our findings of (1) a lack of effect of chronic paroxetine treatment on established Aβ pathology and (2) premature death by paroxetine treatment in APP/PS1 mice. APP/PS1 mice are known to exhibit seizure activity [37], [38], which also occurs frequently in patients with AD [39]. Recently, fluoxetine administered in a dose of 15 mg/kg/day i.p. for 55 days to 19-week-old male APP/PS1 mice was found to increase seizure activity and mortality [40]. Although, the lowest paroxetine dose used in our study was 5 mg/kg/day, which is higher than the 0.3 mg/kg/day considered therapeutic in humans [27], it would be important to know if paroxetine in a dose of 0.3 mg/kg/day and therapeutic concentrations of e.g. citalopram increase seizure activity and mortality of AD transgenic mice. Importantly, we did not observe any mortality in the APP/PS1 mice treated with escitalopram (5 mg/kg/day per os) from 3 to 9 months of age [31]. It is also possible that chronic SSRI treatment, especially when administered i.p., may enhance stress and reduce survival in APP/PS1 mice. Under stressful conditions, SSRI treatment exaggerates, rather than ameliorates depressive-like symptoms in C57BL/6 mice [41]. In the present study, APP/PS1 and littermate Wt mice were treated with a clinically relevant paroxetine dose starting at an age when Aβ pathology is well established, but still increasing [28], similar to the progression of Aβ pathology in patients with AD [42]. Despite these similarities, the present results have been obtained in a mouse model and cannot be directly extrapolated to AD, which is a complex, polygenic neurodegenerative disease. Finally, it is increasingly recognized that Aβ pathology and associated molecular pathways represent only one out of several pathogenic mechanisms in AD, including tau pathology [43], [44], brain inflammation [45], and cholinergic deficits [46], that can be modulated by serotonergic signaling in AD [47], [48].
4.4. What does this study add and how to move forward?
In contrast to previous short-term studies using the same mouse model, this extended study demonstrated that chronic, long-term treatment with paroxetine did not ameliorate already established Aβ pathology in old APP/PS1 transgenic mice and selectively increased mortality in the APP/PS1 transgenic mice, but not in controls. Future studies, examining the effect of chronic treatment with newer generation SSRIs in APP/PS1 and tau transgenic mice, should be performed to determine whether the observed lack of beneficial effects and the serious adverse effect is specific for paroxetine. Knowing the answer to these questions is important for adjusting or reconsidering the wide-spread use of SSRIs in individuals with mild cognitive impairment and AD.
Research in Context.
-
1.
Systematic review: Chronic treatment with selective serotonin reuptake inhibitors (SSRIs) has been suggested to mitigate amyloid-β (Aβ) pathology in mouse models of Alzheimer's disease and in patients with Alzheimer's disease, in addition to having an antidepressant mechanism of action.
-
2.
Interpretation: We tested the hypothesis that chronic treatment with paroxetine, a selective serotonin reuptake inhibitor, would mitigate Aβ pathology in plaque-bearing double-transgenic APPswe/PS1ΔE9 mutants. In addition, we addressed whether serotonin depletion influences Aβ pathology. Contrary to our hypothesis, chronic, long-term therapy with paroxetine did not mitigate Aβ pathology. However, the therapy increased mortality for transgenic mice exclusively. Depletion of serotonin did not exacerbate Aβ pathology.
-
3.
Future directions: Future studies, examining the effect of chronic treatment with newer generation selective serotonin reuptake inhibitors in APP/PS1 and tau transgenics, should be performed to determine whether the observed lack of beneficial effects and the serious adverse effect are specific for paroxetine.
Acknowledgments
The authors thank Sussanne Petersen, Lene Jørgensen, and Inge Nielsen for their excellent technical assistance. The project was funded by the Lundbeck Foundation (A.A.B., B.F., and O.W.); the Danish Alzheimer's Society (B.F. and O.W.); the Foundation Fonden til Lægevidenskabens Fremme (M.Se.), the Velux Foundation (B.F.), the University of Southern Denmark (SDU2020 B.F.), and the Danish Medical Research Council (A.A.B., B.F., O.W.).
Authors' contribution: M.Se., M.Si., L.Ø.O., A.M., J.B.G., O.W., and B.F. designed the experiments, were responsible for carrying out the experiments, data analysis, and wrote the manuscript. For the behavioral experiments E.B. and K.L.L. helped with design and data analysis. A.M.K. assisted with surgery, and C.U.L. with histology in the same experiment. A.A.B. contributed to carrying out the experiments, and A.M. performed the SERT occupancy studies and assisted with statistical analysis. All authors approved the manuscript.
Footnotes
Conflicts of interests: No conflicts of interest for any of the authors.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2018.04.005.
Supplementary data
References
- 1.Hendricksen M., Thomas A.J., Ferrier I.N., Ince P., O'Brien J.T. Neuropathological study of the dorsal raphe nuclei in late-life depression and Alzheimer's disease with and without depression. Am J Psych. 2004;161:1096–1102. doi: 10.1176/appi.ajp.161.6.1096. [DOI] [PubMed] [Google Scholar]
- 2.Sierksma A.S., van den Hove D.L., Steinbusch H.W., Prickaerts J. Major depression, cognitive dysfunction and Alzheimer's disease: Is there a link? Eur J Pharmacol. 2010;626:72–82. doi: 10.1016/j.ejphar.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Ramirez M.J., Lai M.K., Tordera R.M., Francis P.T., Bowen D.M. Serotonergic therapies for cognitive symptoms in Alzheimer's disease: Rationale and current status. Drugs. 2014;74:729–736. doi: 10.1007/s40265-014-0217-5. [DOI] [PubMed] [Google Scholar]
- 4.Arai H., Kosaka K., Iizuka R. Changes of biogenic amines and their metabolites in postmortem brains from patients with Alzheimer-type dementia. J Neurochem. 1984;43:388–393. doi: 10.1111/j.1471-4159.1984.tb00913.x. [DOI] [PubMed] [Google Scholar]
- 5.Palmer A.M., Wilcock G.K., Esiri M.M., Francis P.T., Bowen D.M. Monoaminergic innervation of the frontal and temporal lobes in Alzheimer's disease. Brain Res. 1987;401:231–238. doi: 10.1016/0006-8993(87)91408-9. [DOI] [PubMed] [Google Scholar]
- 6.Geerlings M.I., den Heijer T., Koudstaal P.J., Hofman A. History of depression, depressive symptoms, and medial temporal lobe atrophy and the risk of Alzheimer disease. Neurol. 2008;70:1258–1264. doi: 10.1212/01.wnl.0000308937.30473.d1. [DOI] [PubMed] [Google Scholar]
- 7.Harrington K.D., Gould E., Lim Y.Y., Ames D., Pietrzak R.H., Rembach A., AIBL Research Group Amyloid burden and incident depressive symptoms in cognitively normal older adults. Int J Geriatr Psych. 2017;32:455–463. doi: 10.1002/gps.4489. [DOI] [PubMed] [Google Scholar]
- 8.Ye Q., Bai F., Zhang Z. Shared genetic risk factors for late-life depression and Alzheimer's disease. J Alz Dis. 2016;52:1–15. doi: 10.3233/JAD-151129. [DOI] [PubMed] [Google Scholar]
- 9.Kessing L.V., Forman J.L., Andersen P.K. Do continued antidepressants protect against dementia in patients with severe depressive disorder? Int Clin Psychopharmacol. 2011;26:316–322. doi: 10.1097/YIC.0b013e32834ace0f. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg P.B., Mielke M.M., Han D., Leoutsakos J.S., Lyketsos C.G., Rabins P.V. The association of psychotropic medication use with the cognitive, functional, and neuropsychiatric trajectory of Alzheimer's disease. Int J Geriatr Psych. 2012;27:1248–1257. doi: 10.1002/gps.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones H.E., Joshi A., Shenkin S., Mead G.E. The effect of treatment with selective serotonin reuptake inhibitors in comparison to placebo in the progression of dementia: a systematic review and meta-analysis. Age Ageing. 2016;45:448–456. doi: 10.1093/ageing/afw053. [DOI] [PubMed] [Google Scholar]
- 12.Moranos J., Nwankwo C., Patten S.B., Mousseau D.D. The association of antidepressant drug usage with cognitive impairment of dementia, including Alzheimer disease: a systematic review and meta-analysis. Depress Anxiety. 2017;34:217–226. doi: 10.1002/da.22584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartels C., Wagner M., Wolfsgruber S., Ehrenreich H., Scheider A., for the Alzheimer's Disease Neuroimaging Initiative Impact of SSRI therapy on risk of conversion from mild cognitive impairment to Alzheimer's dementia in individual with previous depression. Am. J. Psychiatry. 2018;175:232–241. doi: 10.1176/appi.ajp.2017.17040404. [DOI] [PubMed] [Google Scholar]
- 14.Madsen K., Neumann W.J., Holst K., Marner L., Haahr M.T., Lehel S. Cerebral serotonin 4 receptors and amyloid-β in early Alzheimer's disease. J Alzheimers Dis. 2011;26:457–466. doi: 10.3233/JAD-2011-110056. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Alloza M., Hirst W.D., Chen C.P., Lasheras B., Francis P.T., Ramírez M.J. Differential involvement of 5-HT IB/ID and 5-HT6 receptors in cognitive and non-cognitive symptoms in Alzheimer's disease. Neuropsychopharmacol. 2004;29:410–416. doi: 10.1038/sj.npp.1300330. [DOI] [PubMed] [Google Scholar]
- 16.Hasselbalch S.G., Madsen K., Svarer C., Pinborg L.H., Holm S., Paulson O.B. Reduced 5-HT2A receptor binding in patients with mild cognitive impairment. Neurobiol Aging. 2008;29:1830–1838. doi: 10.1016/j.neurobiolaging.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Guiard B.P., Di Giovanni G. Central serotonin-2A (5-HT2A) receptor dysfunction in depression and epilepsy: the missing link? Front Pharmacol. 2015;6:46. doi: 10.3389/fphar.2015.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cirrito J.R., Disabato B.M., Restivo J.L., Verges D.K., Goebel W.D., Sathyan A. Serotonin signaling is associated with lower amyloid-β levels and plaques in transgenic mice and humans. Proc Natl Acad Sci U.S.A. 2011;108:14968–14973. doi: 10.1073/pnas.1107411108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheline Y.I., West T., Yarasheski K., Swarm R., Jasielec M.S., Fisher J.R. An antidepressant decreases CSF Aβ production in healthy individuals and in transgenic AD mice. Sci Transl Med. 2014;6:236re4. doi: 10.1126/scitranslmed.3008169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson R.L., Guo Z., Halagappa V.M., Pearson M., Gray A.J., Matsuoka Y. Prophylactic treatment with paroxetine ameliorates behavioral deficits and retards the development of amyloid and tau pathologies in 3xTgAD mice. Exp Neurol. 2007;205:166–176. doi: 10.1016/j.expneurol.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jankowsky J.L., Slunt H.H., Ratovitski T., Jenkins N.A., Copeland N.G., Borchelt D.R. Co-expression of multiple transgenes in mouse CNS: A comparison of strategies. Biomol Eng. 2001;17:157–165. doi: 10.1016/s1389-0344(01)00067-3. [DOI] [PubMed] [Google Scholar]
- 22.Ma J., Gao Y., Jiang L., Chao F.L., Huang W., Zhou C.N. Fluoxetine attenuates the impairment of spatial learning ability and prevents neuron loss in middle-aged APPswe/PSEN1dE9 double transgenic Alzheimer's disease mice. Oncotarget. 2017;8:27676–27692. doi: 10.18632/oncotarget.15398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y., Yoo M.J., Savonenko A., Stirling W., Price D.L., Borchelt D.R. Amyloid pathology is associated with progressive monoaminergic neurodegeneration in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2008;28:13805–13814. doi: 10.1523/JNEUROSCI.4218-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Von Linstow C.U., Severino M., Metaxas A., Waider J., Babcock A.A., Lesch K.P. Effect of aging and Alzheimer's disease-like pathology on brain monoamines in mice. Neurochem Int. 2017;108:238–245. doi: 10.1016/j.neuint.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Tesseur I., Pimenova A.A., Lo A.C., Ciesielska M., Lichtenthaler S.F., De Maeyer J.H. Chronic 5-HT4 receptor activation decreases Aβ production and deposition in hAPP/PS1 mice. Neurobiol Aging. 2013;34:1779–17789. doi: 10.1016/j.neurobiolaging.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 26.Fisher J.R., Wallace C.E., Tripoli D.L., Sheline Y.I. Cirrito JR Redundant G(s)-coupled serotonin receptors regulate amyloid-beta metabolism in vivo. Mol Neurodeg. 2016;11:45. doi: 10.1186/s13024-016-0112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez C., Reines E.H., Montgomery S.A. A comparative review of escitalopram, paroxetine, and sertraline: Are they all alike? Int Clin Psychopharmacol. 2014;29:185–196. doi: 10.1097/YIC.0000000000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babcock A.A., Ilkjær L., Clausen B.H., Villadsen B., Dissing-Olesen L., Bendixen A.T. Cytokine-producing microglia have an altered beta-amyloid load in aged APP/PS1 Tg mice. Brain Behav Immun. 2015;48:86–101. doi: 10.1016/j.bbi.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Olesen L.Ø., Bouzinova E.V., Severino M., Sivasaravanaparan M., Hasselstrøm J.B., Finsen B. Behavioural phenotyping of APPswePS1dE9 mice: age-related changes and effect of long-term paroxetine treatment. PLosOne. 2016;11:e0165144. doi: 10.1371/journal.pone.0165144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olesen L.Ø., Sivasaravanaparan M., Severino M., Babcock A.A., Bouzinova E.V., West M.J. Neuron number and neurogenesis in the dentate gyrus of aged APPswe/PS1dE9 transgenic mice: effect of long-term treatment with paroxetine. Neurobiol Dis. 2017;104:50–60. doi: 10.1016/j.nbd.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 31.von Linstow C.U., Waider J., Grebing M., Metaxas A., Lesch K.P., Finsen B. Serotonin augmentation therapy by escitalopram has minimal effects on amyloid-β levels in early-stage Alzheimer's-like disease in mice. Alz Res Ther. 2017;9:74. doi: 10.1186/s13195-017-0298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambertsen K.L., Gramsbergen J.B., Sivasaravanaparan M., Ditzel N., Sevelsted-Møller L.M., Oliván-Viguera A. Genetic KCa3.1-Deficiency produces locomotor hyperactivity and alterations in cerebral monoamine levels. PLoS One. 2012;7:e47744. doi: 10.1371/journal.pone.0047744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.West M.J., Østergaard K., Andreassen O., Finsen B. Counting in situ hybridized neurons with modern unbiased stereological methods. J Comp Neurol. 1996;370:11–22. doi: 10.1002/(SICI)1096-9861(19960617)370:1<11::AID-CNE2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 34.Hampel H., Shen Y., Walsh D.M., Aisen P., Shaw L.M., Zetterberg H. Biological markers of amyloid β-related mechanisms in Alzheimer's disease. Exp Neurol. 2010;223:334–346. doi: 10.1016/j.expneurol.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Payton S., Cahill C.M., Randall J.D., Gullans S.R., Rogers J.T. Drug discovery targeted to the Alzheimer's APP mRNA 5'-untranslated region: the action of paroxetine and dimercaptopropanol. J Mol Neurosci. 2003;20:267–275. doi: 10.1385/JMN:20:3:267. [DOI] [PubMed] [Google Scholar]
- 36.Morse L.J., Payton S.M., Cuny G.D., Rogers J.T. FDA-preapproved drugs targeted to the translational regulation and processing of the amyloid precursor protein. J Mol Neurosci. 2004;24:129–136. doi: 10.1385/JMN:24:1:129. [DOI] [PubMed] [Google Scholar]
- 37.Minkeviciene R., Rheims S., Dobszay M.B., Zilberter M., Hartikainen J., Fülöp L. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci. 2009;29:3453–3462. doi: 10.1523/JNEUROSCI.5215-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ziyatdinova S., Gurevicius K., Kutchiashvili N., Bolkvadze T., Nissinen J., Tanila H. Spontaneous epileptiform discharges in a mouse model of Alzheimer's disease are suppressed by antiepileptic drugs that block sodium channels. Epilepsy Res. 2011;94:75–85. doi: 10.1016/j.eplepsyres.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Born H.A. Seizures in Alzheimer's disease. Neurosci. 2015;286:251–263. doi: 10.1016/j.neuroscience.2014.11.051. [DOI] [PubMed] [Google Scholar]
- 40.Sierksma A.S.R., de Nijs L., Hoogland G., Vanmierlo T., van Leeuwen F.W., Rutten B.P. Fluoxetine treatment induces seizure behavior and premature death in APPswe/PS1dE9 mice. J Alz Dis. 2016;51:677–682. doi: 10.3233/JAD-151066. [DOI] [PubMed] [Google Scholar]
- 41.Alboni S., van Dijk M., Poggini S., Milior G., Perrotta M., Drenth T. Fluoxetine effects on molecular, cellular and behavioral endophenotypes of depression are driven by the living environment. Mol Psych. 2017;22:552–561. doi: 10.1038/mp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jack C.R., Wiste H.J., Lesnick G.T., Weigand S.D., Knopman D.S., Vemuri P. Brain β-amyloid load approaches a plateau. Neurol. 2013;80:890–896. doi: 10.1212/WNL.0b013e3182840bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li C., Götz J. Tau-based therapies in neurodegeneration: Opportunities and challenges. Nat Rev Drug Disc. 2017;16:863–883. doi: 10.1038/nrd.2017.155. [DOI] [PubMed] [Google Scholar]
- 45.Heneka M.T., Carson M.J., Khoury J.E., Landreth G.E., Brosseron F., Feinstein D.L. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14:389–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramos-Rodriguez J.J., Pacheco-Herrero M., Thyssen D., Murillo-Carretero M.I., Berrocoso E., Spires-Jones T.L. Rapid β-amyloid deposition and cognitive impairment after cholinergic denervation in APP/PS1 mice. J Neuropathol Exp Neurol. 2013;72:272–285. doi: 10.1097/NEN.0b013e318288a8dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith G.S., Kramer E., Ma Y., Hermann C.R., Dhawan V., Chaly T. Cholinergic modulation of the cerebral metabolic response to citalopram in Alzheimer's disease. Brain. 2009;132:392–401. doi: 10.1093/brain/awn326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frozza R.L., Lourance M.V., De Felice F.G. Challenges for Alzheimer's disease therapy: Insights from novel mechanisms beyond memory defects. Front Neurosci. 2018;12:37. doi: 10.3389/fnins.2018.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.