Abstract
Background
Resource use measurement by patient recall is characterized by inconsistent methods and a lack of validation. A validated standardized resource use measure could increase data quality, improve comparability between studies, and reduce research burden.
Objectives
To identify a minimum set of core resource use items that should be included in a standardized adult instrument for UK health economic evaluation from a provider perspective.
Methods
Health economists with experience of UK-based economic evaluations were recruited to participate in an electronic Delphi survey. Respondents were asked to rate 60 resource use items (e.g., medication names) on a scale of 1 to 9 according to the importance of the item in a generic context. Items considered less important according to predefined consensus criteria were dropped and a second survey was developed. In the second round, respondents received the median score and their own score from round 1 for each item alongside summarized comments and were asked to rerate items. A final project team meeting was held to determine the recommended core set.
Results
Forty-five participants completed round 1. Twenty-six items were considered less important and were dropped, 34 items were retained for the second round, and no new items were added. Forty-two respondents (93.3%) completed round 2, and greater consensus was observed. After the final meeting, 10 core items were selected, with further items identified as suitable for “bolt-on” questionnaire modules.
Conclusions
The consensus on 10 items considered important in a generic context suggests that a standardized instrument for core resource use items is feasible.
Keywords: cost measurement, patient-reported, randomized clinical trial, resource use
Introduction
For cost-effectiveness analyses to be optimal, resource use measurement in randomized controlled trials (RCTs) must be accurate. Nevertheless, to date, considerably more research has been directed at improving outcome measurement methodologies (e.g., utilities) [1]. The methods used to measure costs are poorly reported [2], and instruments to collect data directly from patients are commonly not validated [3] (although there are studies in which the reliability/validity of self-report is considered [4]). When available, routine data sources (e.g., electronic hospital records) might reduce attrition bias, be more accurate, and minimize the burden on trial participants. Routine data may, however, not be readily available, consistent, or suitable for costing purposes [5]. Electronic systems may also be costly to access and may lack information on personal costs incurred by patients. It is therefore likely that researchers will continue to be reliant on instruments based on patient recall (e.g., diaries, logs, and questionnaires [6]) for some time, despite the fact that self-reported data on health care use are of variable accuracy [7].
A significant amount of work in recent years has focused on developing core outcome sets (COSs), which are agreed minimum sets of outcomes (often health-related) to be measured and reported in all trials for a specific condition/treatment [8]. Standardization counteracts problems with researchers selecting outcomes on the basis of their own expertise or the statistical significance of results. A standard set of outcomes also reduces heterogeneity and improves comparability across trials [9]. Although developing a core set of resource use items has much in common with COS development, there are also some important differences. A fundamental consideration of an economic analysis is the perspective, which leads to the inclusion of different types of resource use. Although COSs are specific to clinical conditions or treatments and are therefore different across trials, a core set of resource use is specific to the perspective, but could potentially be generalizable across trials. Separate measurement instruments may be required for outcomes identified in COSs (e.g., the EuroQol five-dimensional questionnaire for quality of life or the modified Health Assessment Questionnaire for patient satisfaction with activities of daily living [10]); in contrast, a core set of resource use items would generally form a single instrument.
Standardization of resource use measurement is potentially controversial among health economists. Legitimate concerns about the study perspective, nature of the intervention, and type of analysis planned may suggest that standardization is too limiting. There is a trade-off between gathering as much information as possible (with increased patient burden and possible poor response rates) and gathering less information (which may not allow an accurate analysis to be conducted). As Drummond et al. [11p253] point out, “The skill in costing is to match the level of precision (and effort) to the importance (in quantitative terms) of the cost item.” Nevertheless, standardizing outcomes using the EuroQol five-dimensional questionnaire is accepted in the United Kingdom (and indeed required by the National Institute for Health and Care Excellence [12]), despite the inevitable limitation on the flexibility of the instrument. In contrast, health economists typically generate new, or revise existing, resource use instruments for RCTs on a case-by-case basis; some standardization of cost measurement (albeit with “bolt-ons” to ensure more complete coverage of resources) would allow greater comparability between trials and would reduce the research effort required. The significant overlap between questions in instruments held in the Database of Instruments for Resource Use Measurement (www.dirum.org) [13] suggests that defining a core set may be feasible [14].
In our study (Items for a Standardised Resource-Use Measure, ISRUM), we aim to identify core items of resource use that should be included in any economic evaluation of a health care intervention conducted in the United Kingdom. We aim to identify a minimum set of items that should be measured, and not a complete set; we anticipate that health economists may measure additional items according to the particular nature of the RCT and the perspective of the analysis. We use a Delphi survey to seek consensus expert opinion.
Methods
Approval for the study was granted by the Faculty of Health Sciences Research Ethics Committee of the University of Bristol. A patient and public involvement (PPI) representative was recruited to the study team via the People in Health West of England (http://www.phwe.org.uk/) mailing list.
Phase 1: Identification of “Long List” and Development of Survey
The identification of a long list of resource use items is described in detail elsewhere [14]. In brief, a review of measurement instruments currently used in RCTs of health interventions was undertaken; individual items were extracted by two researchers and disagreements were resolved by discussion. Items were scrutinized by a single researcher and overlapping items merged. Similar types of items were combined; for example, doctor, nurse, and allied health professional were collapsed into “professional seen.” Items not relevant to a National Health Service (NHS) and personal social services (PSS) perspective (commonly taken in UK studies) were dropped. Remaining items were formulated as individual questions for a Delphi survey. The Delphi method is used increasingly for consensus in COSs [15]. It requires expert participants to provide their opinions in sequential questionnaires (rounds), with each round presenting group feedback from the previous round. Anonymity of the responses is maintained to ensure that no individual dominates the process [16]. A Web-administered “eDelphi” survey was developed using REDCap electronic data capture tools hosted at the University of Bristol [17]; items were grouped according to the location in which the care took place (e.g., hospital). The survey was piloted in the study team, and a think-aloud Web usability study (in which participants were asked to talk through their responses) was conducted with a convenience sample to ensure it was comprehensible and manageable [18].
Phase 2: Prioritization of Resource Use Items
Stakeholders
Practicing health economists with experience of RCTs in the United Kingdom were recruited to the Delphi panel. A generic email was sent to the Health Economists’ Study Group mailing list describing the preparatory work and purpose of the study and inviting participation by following a Web link. Health economists who had recently contributed to National Institute for Health Research Health Technology Assessment reports (http://www.journalslibrary.nihr.ac.uk/hta) or attended relevant workshops were approached directly. One reminder email was sent. Completion of the first questionnaire was deemed to represent informed consent to participate. Demographic details were requested in the survey including subgroups describing experience with different types of patient care (physical, mental, and public health; older adults; primary and secondary care), length of experience, and professional background.
Survey round 1
In round 1 of the survey, participants were asked to rate the importance of retaining each item in the core standardized resource use set on a scale of 1 (not important) to 9 (very important). Participants were asked to think in terms of resource use relevant to an NHS and PSS perspective for adult patients of any age, living with wide-ranging physical and/or mental health conditions of variable severity (see Appendix 1 in Supplemental Materials found at doi:10.1016/j.jval.2017.06.011). They were asked to assume that there may be differences between trial arms in any item and that they have no access to any other source of resource use data (such as medical records). Participants were encouraged to comment on their ratings and suggest additional items. After completion of the questionnaire, items for which the participant had scored 7 to 9 were presented back to them, with a request to select their “top 10” items for the core set. Round 1 item scores were summarized across participants, and items to retain for round 2 were identified using prespecified criteria; items suggested by participants were added if they met prespecified criteria (see Analysis section).
Survey round 2
All participants who had completed round 1 of the survey were emailed a Web link to the round 2 questionnaire. Feedback from round 1 was presented for each round 2 item in the form of the median score along with a reminder of the individual’s own score. Comments in round 1 that were relevant to selection choice were also summarized and presented, and changes were made to the wording for a small number of items on the basis of some of the comments. Participants were asked to rerate each item (see Appendix 1 in Supplemental Materials) and were given further opportunity to comment on their choices. A reminder invitation was sent after 2 weeks, and a further reminder specifying a closing date was issued 1 week later. Shortly after the closing date, nonresponders were contacted by telephone to request reasons for noncompletion.
Analyses
Statistical analyses were carried out in Stata 14 (StataCorp LP, College Station, TX) [19] and were conducted according to a prespecified analysis plan.
Criteria for retaining items
At the end of round 1, the percentage of participants scoring 7 to 9 (high priority) and 1 to 3 (low priority) was calculated for each item, both for participants overall and for each of the “type of experience” subgroups separately. Items were retained if scored 7 to 9 by more than 50% and 1 to 3 by less than 15% by participants overall or within two or more subgroups of participants; these prespecified criteria were deliberately inclusive. Items were also retained if 15% or more of the participants prioritized the item in their top 10 list. Items not meeting any of these criteria were closely examined for overlap with retained items; if there was no overlap, the item was further considered for retention. New items were added to round 2 if suggested by more than 10% of participants.
After round 2, items were retained if scored 7 to 9 by more than 70% and 1 to 3 by less than 15% of all participants. Because further Delphi rounds were beyond the scope of this study, more stringent criteria were also set (>70% scoring an item 8 or 9 and <15% scoring 1–3) to aid discussions in a final item selection meeting so that a pragmatic core set could be identified.
Attrition
Nonresponders to round 2 were examined in terms of years of experience; mean scores were compared with those from round 2 responders.
Assessment of consensus
It is not a requirement of the Delphi process to achieve consensus for all items (e.g., when all participants agreed on the high/low priority grouping); it is, however, essential that participants agree on a reduced number of items to be most important. It is therefore informative to consider the level of agreement across participants in both rounds and the degree of stability in scores.
For each round, the percentage of participants scoring 7 to 9 and 1 to 3 was examined for evidence of bimodality (defined as >40% rating an item 7–9 and >40% rating it 1–3) for each item, because this could indicate an irreconcilable difference of opinion. The intraclass correlation coefficient (two-way random effects model) was calculated for both rounds, to give an indication of agreement within the survey [20].
For each item, the mean absolute change in score between rounds was also calculated; a large change (defined as ≥3 points) could indicate instability. The percentage of people changing their score by a small amount (1 or 2 points) and a large amount (≥3 points) was calculated for each item to give an indication of the stability of the results. Variation in changes to scores with length of experience (categorized as <5 years, 5–10 years, 10–20 years, and >20 years) was explored through linear regression. Finally, the SD of scores was calculated for each item (separately for each round) as a measure of the spread in responses across participants (and degree of agreement) and was used to calculate the change in each item’s variability between rounds [21].
Analysis of comments
Content analysis (a systematic approach to studying text that aims to categorize and quantify content) was conducted for comments by using nVivo software (QSR International Ltd. London) [22], [23]. Suggestions in round 1 for new items were extracted, and broad themes were identified for both rounds.
Phase 3: Final Item Selection Meeting
The project team met to determine the final core items to include in a standardized “short form” resource use measure. Participants who had commented extensively during the Delphi process or were associated with the Medical Research Council (MRC) Network of Hubs for Trials Methodology Research were invited to attend the meeting. Each item included in round 2 was discussed in detail. The two prespecified criteria were applied to the round 2 data to identify the items considered most crucial (more stringent criteria) and very important (less stringent criteria) for inclusion in the final core set. Items reaching the more stringent criteria were included in the final set if considered relevant, by the team, to all trials and patient populations. If relevant only to specific settings, items were included in suggested bolt-on modules. Items reaching the less stringent criteria were then discussed and merged with those already in the final set when appropriate or were considered as separate items for the core set or as items in bolt-on modules. Remaining items were examined to ensure that nothing vital was overlooked.
Results
Phase 1
Items were extracted from 59 resource use instruments. After the deduplication and merging processes, the long list contained 60 items, categorized as hospital care (n = 15), emergency care (n = 5), care at a general practitioner (GP) surgery or health clinic (n = 7), care at home (n = 7), remote access care (n = 4), other community care (n = 6), residential care (n = 10), and medication (n = 6). Usability studies with both a native and a non-native English speaker indicated that the Delphi survey was comprehensible, and completion was manageable.
Phase 2
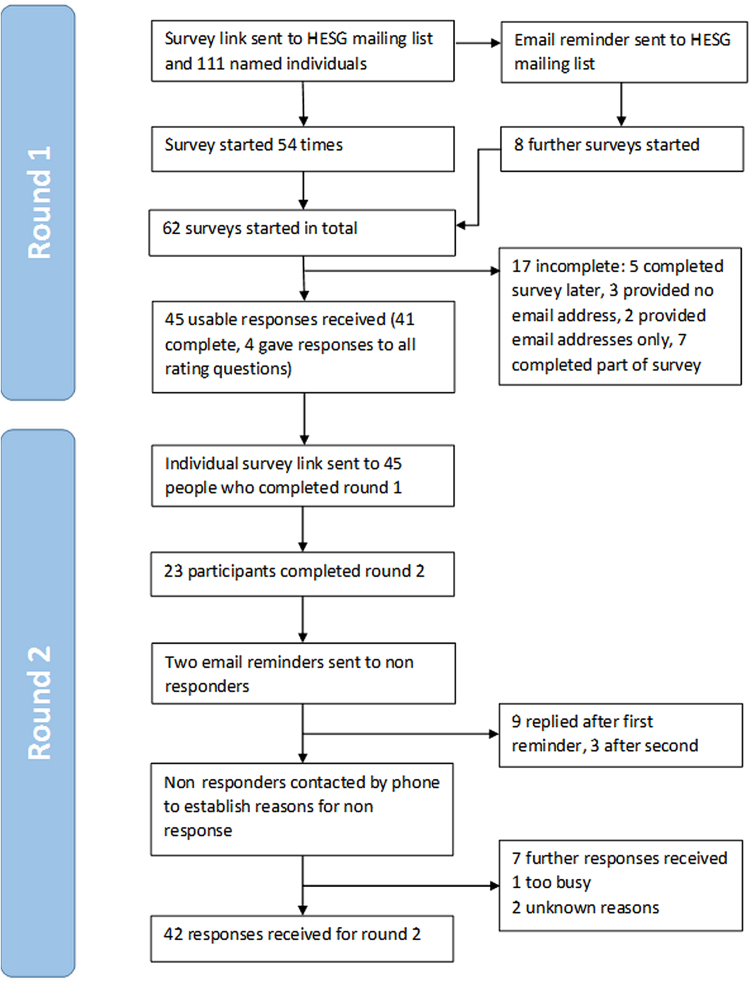
Forty-five participants provided usable responses to round 1; 41 completed the whole survey, whereas 4 supplied ratings for all items, but did not select their top 10 (Fig. 1). Participants with a range of experience were represented (Table 1), although almost all (42 of 45) were working in academia. Application of the predefined consensus criteria identified 27 items to be retained for round 2, considered to be of high priority by participants overall. Four additional items were considered important by two or more subgroups: minor surgery (important to participants with experience of primary care, physical health, public health, or older adults), living in either a residential home or a supported accommodation (rated highly by participants with experience of primary care, mental health, or older adults), and the period over which medication is taken (important to respondents with experience in primary care and public health). Type of ward and scans were added because more than 15% of respondents cited them in their top 10. Finally, equipment was identified as a suitable addition because it came close to meeting several of the aforementioned criteria and no other similar items were included. No new items met the inclusion criteria. Thirty-four items were therefore included for round 2 (Table 2) and 26 items were dropped (Table 3). Engagement with the project in round 1 was good, with broadly positive comments indicating that achieving consensus was feasible.
Fig. 1.
Flowchart of Delphi study participants through the study. HESG, Health Economists’ Study Group.
Table 1.
Characteristics of 45 Delphi participants from round 1
| Characteristic | n (%) |
|---|---|
| Length of experience | |
| <5 y | 11 (24.4) |
| 5–10 y | 12 (26.7) |
| 10–20 y | 11 (24.4) |
| >20 y | 11 (24.4) |
| Trial experience | |
| Adults | 44 (97.8) |
| Children | 21 (46.7) |
| Older adults | 26 (57.8) |
| Physical health conditions | 38 (84.4) |
| Mental health conditions | 28 (62.2) |
| Public health interventions | 20 (44.4) |
| Primary care | 33 (73.3) |
| Secondary care | 39 (86.7) |
| Background | |
| Academia | 42 (93.3) |
| Other | 3 (6.7) |
Table 2.
Items retained at the end of round 1
| Item description | % rating 7–9 | % rating 1–3 | Median (IQR) | Inclusion reason |
|---|---|---|---|---|
| Hospital care | ||||
| (1) Number of hospital admissions (inpatient stay or day case) | 95.56 | 0.00 | 9 (9–9) | Consensus |
| (2) Number of hospital outpatient appointments | 91.11 | 2.22 | 9 (8–9) | Consensus |
| (3) Length of stay (e.g., dates or number of nights) | 84.44 | 0.00 | 9 (8–9) | Consensus |
| (4) Number of operations/procedures undergone | 64.44 | 4.44 | 8 (6–9) | Consensus |
| (5) Type of operation/procedure undergone | 64.44 | 4.44 | 8 (6–9) | Consensus |
| (6) Type of professional seen (e.g., consultant/nurse) | 53.33 | 13.33 | 7 (5–8) | Consensus |
| (7) Number of imaging scans undergone (e.g., x-ray/MRI) | 42.22 | 13.33 | 6 (5–8) | Top 10* |
| (8) Type of ward stayed in | 37.78 | 11.11 | 6 (5–8) | Top 10* |
| Emergency care | ||||
| (9) Number of visits to A&E | 91.11 | 0.00 | 9 (7–9) | Consensus |
| (10) Number of admissions to hospital, after A&E | 80.00 | 2.22 | 9 (7–9) | Consensus |
| (11) Number of times paramedic care received | 53.33 | 4.44 | 7 (5–9) | Consensus |
| Care at a GP surgery or health clinic | ||||
| (12) Number of appointments at a GP surgery or health clinic | 95.56 | 2.22 | 9 (9–9) | Consensus |
| (13) Type of professional seen (e.g., GP/nurse/counselor) | 80.00 | 0.00 | 9 (7–9) | Consensus |
| (14) Number of minor surgery/procedures/treatments undergone | 46.67 | 6.67 | 6 (5–9) | Subgroup† |
| Care at home | ||||
| (15) Number of health care or social care professional visits at home (e.g., health visitor/GP) | 86.67 | 2.22 | 9 (8–9) | Consensus |
| (16) Type of professional seen at home | 80.00 | 0.00 | 9 (7–9) | Consensus |
| (17) Number of professional visits for help with daily activities (e.g., washing/dressing) | 55.56 | 6.67 | 7 (5–9) | Consensus |
| (18) Equipment (e.g., wheelchairs/portable oxygen/specialist clothing) or home adaptation (e.g., grab rails/ramp) supplied | 44.44 | 13.33 | 6 (5–8) | Close‡ |
| Remote access care | ||||
| (19) Number of real-time telephone/computer contacts with health or social care professional (e.g., with GP or telephone helpline) | 68.89 | 4.44 | 7 (6–9) | Consensus |
| (20) Type of professional contacted (e.g., doctor/nurse/social worker) | 53.33 | 6.67 | 7 (5–9) | Consensus |
| Other community care | ||||
| (21) Number of visits to health care professional in the community (e.g., dentist, pharmacist, nurse, counselor, and therapist) | 80.00 | 2.22 | 9 (7–9) | Consensus |
| (22) Number of visits to social care professional in the community (e.g., social worker/housing worker/drug and alcohol worker) | 75.56 | 2.22 | 9 (7–9) | Consensus |
| (23) Type of health care professional seen | 71.11 | 4.44 | 8 (5–9) | Consensus |
| (24) Type of social care professional seen in the community | 62.22 | 4.44 | 8 (5–9) | Consensus |
| Residential care | ||||
| (25) Stay in hospice | 77.78 | 6.67 | 9 (7–9) | Consensus |
| (26) Length of time spent in the hospice | 75.56 | 8.89 | 8 (7–9) | Consensus |
| (27) Use of short-term respite or rehabilitation care | 66.67 | 6.67 | 7 (5–9) | Consensus |
| (28) Length of stay in short-term respite or rehabilitation care | 62.22 | 8.89 | 7 (5–9) | Consensus |
| (29) Living in a nursing home | 53.33 | 11.11 | 7 (5–9) | Consensus |
| (30) Living in a residential home | 48.89 | 11.11 | 6 (5–9) | Subgroup† |
| (31) Living in supported accommodation/sheltered housing | 46.67 | 11.11 | 6 (5–9) | Subgroup† |
| Medication | ||||
| (32) Number of prescribed medications | 68.89 | 6.67 | 8 (5–9) | Consensus |
| (33) Name of medication | 64.44 | 4.44 | 8 (5–9) | Consensus |
| (34) Period taken for (e.g., dates or number of days) | 46.67 | 11.11 | 6 (5–9) | Subgroup† |
A&E, accident and emergency; GP, general practitioner; IQR, interquartile range; MRI, magnetic resonance imaging.
Item included because >15% of participants listed it in their “top 10” choices.
Item included because >1 subgroup rated it highly.
Item included because it came close to meeting several criteria.
Table 3.
Items dropped at the end of round 1
| Item description | % rating 7–9 | % rating 1–3 | Median (IQR) |
|---|---|---|---|
| Hospital care | |||
| Number of other procedures undergone | 35.56 | 20.00 | 6 (4–7) |
| Number of laboratory tests undergone | 35.56 | 24.44 | 5 (4–7) |
| Type of imaging scans undergone | 31.11 | 15.56 | 6 (5–8) |
| Type of other procedures undergone | 26.67 | 17.78 | 5 (4–7) |
| Type of laboratory tests undergone | 22.22 | 26.67 | 5 (3–6) |
| Length of outpatient appointment | 15.56 | 55.56 | 3 (2–5) |
| Number of hospital transport journeys (nonemergency) | 13.33 | 42.22 | 5 (2–5) |
| Emergency care | |||
| Number of ambulance journeys | 28.89 | 17.78 | 5 (4–7) |
| Time spent in A&E | 15.56 | 46.67 | 4 (3–5) |
| Care at a GP surgery or health clinic | |||
| Number of laboratory tests undergone | 40.00 | 22.22 | 5 (4–7) |
| Type of minor surgery/procedures/treatments undergone | 33.33 | 11.11 | 5 (5–7) |
| Timing of appointments (office hours or out of hours) | 33.33 | 31.11 | 5 (3–9) |
| Type of laboratory tests undergone | 26.67 | 28.89 | 5 (3–7) |
| Care at home | |||
| Type of equipment or adaptation supplied | 35.56 | 22.22 | 5 (4–7) |
| Time spent with professional at home | 33.33 | 22.22 | 5 (4–7) |
| Time spent by professional for help with daily activities | 31.11 | 15.56 | 5 (5–7) |
| Remote access care | |||
| Duration of contact with professional | 24.44 | 33.33 | 5 (3–6) |
| Number of email or SMS (text) communications with health care professional | 13.33 | 42.22 | 4 (3–5) |
| Other community care | |||
| Use of patient support services in the community (e.g., self-help groups/lunch clubs/day center) | 28.89 | 15.56 | 5 (4–7) |
| Type of support service used | 24.44 | 22.22 | 5 (4–6) |
| Residential care | |||
| Date moved to nursing home | 46.67 | 13.33 | 6 (5–9) |
| Date moved to residential home | 42.22 | 13.33 | 6 (5–9) |
| Date moved to supported accommodation/sheltered housing | 40.00 | 13.33 | 5 (5–9) |
| Medication | |||
| Frequency taken | 42.22 | 15.56 | 6 (5–9) |
| Dose taken | 42.22 | 15.56 | 5 (5–9) |
| Route taken (e.g., oral/suppository/intravenous) | 24.44 | 35.56 | 5 (3–6) |
A&E, accident and emergency; GP, general practitioner; IQR, interquartile range.
Out of 45 participants, 42 (93.3%) responded to round 2 (Fig. 1). The three nonresponders each came from a different level of experience. Nonresponders had a mean score of 8.53 ± 0.33 in round 1 compared with 7.13 ± 1.09 for responders (P = 0.03). There was no evidence of bimodality for any item in either round. All responding participants changed at least one rating between rounds, and all items were changed by at least one participant. Participants changed their scores by a mean of 0.70 ± 0.36 points between rounds.
The intraclass correlation coefficient (95% confidence interval) increased from 0.85 (0.77–0.91) in round 1 to 0.93 (0.89–0.96) in round 2, suggesting increased consensus in round 2. Between rounds, SDs reduced for all individual items except for hospital admission items and prescribed medication (Table 4), again suggesting movement toward increased consensus in round 2. As anticipated, 100% concordance on the priority group (high/low) was not achieved for any item in either round. No relationship was observed between changes to mean scores and length of experience.
Table 4.
Indicators of response to feedback from round 1
| Item description |
Mean ± SD |
Round 2 − Round 1 |
% rating an item 7–9 |
% changing score by |
||||
|---|---|---|---|---|---|---|---|---|
| Round 1 | Round 2 | Change in mean | Change in SD | Round 1 | Round 2 | 1 or 2 | ≥3 | |
| GP appointments | 8.5 ± 1.1 | 8.7 ± 0.9 | 0.13 | −0.23 | 95.56 | 97.62 | 21.43 | 0.00 |
| GP surgery/procedure | 6.3 ± 2.1 | 5.8 ± 1.9 | −0.52 | −0.25 | 46.67 | 35.71 | 52.38 | 2.38 |
| GP professional seen | 7.9 ± 1.5 | 8.2 ± 1.2 | 0.30 | −0.35 | 80.00 | 92.86 | 23.81 | 2.38 |
| Equipment | 6.1 ± 2.2 | 6.0 ± 1.7 | −0.13 | −0.48 | 44.44 | 33.33 | 45.24 | 4.76 |
| Home visits | 8.3 ± 1.3 | 8.5 ± 0.8 | 0.19 | −0.49 | 86.67 | 97.62 | 11.90 | 2.38 |
| Help with activities | 6.9 ± 2.1 | 6.8 ± 1.5 | −0.13 | −0.58 | 55.56 | 59.52 | 59.52 | 4.76 |
| Professional seen at home | 7.8 ± 1.6 | 8.0 ± 1.4 | 0.20 | −0.23 | 80.00 | 88.10 | 21.43 | 7.14 |
| Admissions after A&E | 7.8 ± 1.7 | 7.9 ± 1.8 | 0.06 | 0.06 | 80.00 | 88.10 | 21.43 | 16.67 |
| Paramedic care | 6.8 ± 2.0 | 6.7 ± 1.3 | −0.07 | −0.68 | 53.33 | 50.00 | 52.38 | 7.14 |
| A&E | 8.2 ± 1.2 | 8.5 ± 0.8 | 0.25 | −0.40 | 91.11 | 95.24 | 26.19 | 2.38 |
| Length of stay | 8.2 ± 1.5 | 8.4 ± 1.4 | 0.20 | −0.02 | 84.44 | 92.86 | 23.81 | 2.38 |
| Hospital admissions | 8.7 ± 0.8 | 8.6 ± 1.2 | −0.14 | 0.38 | 95.56 | 97.62 | 9.52 | 2.38 |
| Hospital outpatients | 8.2 ± 1.5 | 8.5 ± 0.9 | 0.21 | −0.52 | 91.11 | 97.62 | 26.19 | 2.38 |
| Imaging scans | 6.2 ± 2.3 | 5.6 ± 1.8 | −0.58 | −0.51 | 42.22 | 23.81 | 33.33 | 9.52 |
| Operation/procedure | 7.2 ± 2.1 | 7.5 ± 1.4 | 0.28 | −0.75 | 64.44 | 78.57 | 45.24 | 7.14 |
| Specialty/ward | 6.2 ± 2.0 | 6.1 ± 1.7 | −0.08 | −0.26 | 37.78 | 38.10 | 45.24 | 7.14 |
| Operation type | 7.1 ± 2.0 | 7.3 ± 1.4 | 0.17 | −0.59 | 64.44 | 71.43 | 35.71 | 7.14 |
| Professional seen outpatient | 6.2 ± 2.2 | 6.4 ± 1.7 | 0.16 | −0.49 | 53.33 | 57.14 | 47.62 | 7.14 |
| Name of medication | 7.1 ± 2.1 | 7.3 ± 1.9 | 0.17 | −0.20 | 64.44 | 73.81 | 42.86 | 14.29 |
| Prescribed medication | 7.2 ± 2.3 | 6.7 ± 2.3 | −0.51 | 0.03 | 68.89 | 64.29 | 35.71 | 9.52 |
| Period taken for | 6.4 ± 2.3 | 6.3 ± 1.8 | −0.09 | −0.41 | 46.67 | 40.48 | 42.86 | 9.52 |
| Community health care | 7.8 ± 1.7 | 8.1 ± 1.3 | 0.29 | −0.41 | 80.00 | 88.10 | 33.33 | 4.76 |
| Community social care | 7.7 ± 1.8 | 8.0 ± 1.2 | 0.22 | −0.55 | 75.56 | 85.71 | 35.71 | 4.76 |
| Health professional seen | 7.2 ± 2.0 | 7.5 ± 1.5 | 0.30 | −0.56 | 71.11 | 78.57 | 33.33 | 7.14 |
| Social care professional seen | 7.0 ± 2.2 | 7.4 ± 1.6 | 0.38 | −0.56 | 62.22 | 73.81 | 35.71 | 9.52 |
| Telephone/computer contacts | 7.2 ± 1.9 | 7.0 ± 1.5 | −0.27 | −0.43 | 68.89 | 64.29 | 50.00 | 2.38 |
| Professional contacted | 6.6 ± 2.0 | 6.8 ± 1.5 | 0.16 | −0.51 | 53.33 | 54.76 | 52.38 | 2.38 |
| Respite length of stay | 6.9 ± 2.3 | 6.7 ± 1.9 | −0.24 | −0.36 | 62.22 | 64.29 | 57.14 | 2.38 |
| Hospice length of stay | 7.4 ± 2.1 | 7.4 ± 2.1 | −0.02 | −0.02 | 75.56 | 78.57 | 33.33 | 7.14 |
| Nursing home | 6.8 ± 2.4 | 6.6 ± 2.0 | −0.14 | −0.42 | 53.33 | 57.14 | 57.14 | 4.76 |
| Residential home | 6.7 ± 2.4 | 6.2 ± 2.1 | −0.50 | −0.28 | 48.89 | 40.48 | 42.86 | 11.90 |
| Supported accommodation | 6.4 ± 2.5 | 5.9 ± 2.0 | −0.52 | −0.52 | 46.67 | 30.95 | 40.48 | 9.52 |
| Stay in hospice | 7.5 ± 2.1 | 7.6 ± 2.1 | 0.13 | −0.05 | 77.78 | 80.95 | 19.05 | 11.90 |
| Respite care | 7.0 ± 2.2 | 6.9 ± 1.9 | −0.16 | −0.32 | 66.67 | 73.81 | 47.62 | 7.14 |
A&E, accident and emergency; GP, general practitioner.
Twenty-eight respondents commented in round 1, with two not completing the survey. The content analysis showed that the hospital and home care categories attracted the highest number of comments (15 and 11, respectively). Some comments indicated that the task was cognitively challenging. The most common theme was that the inclusion of a particular item depended on another factor including perspective, intervention, setting, condition, patient group, level of detail, recall period, time horizon, and comparator. Potential issues with patient recall and practical aspects of administering a resource use questionnaire were also raised. Seventeen respondents commented in round 2; comments largely focused on useful suggestions for developing an instrument, with seven individuals suggesting a modular approach.
Phase 3
In addition to the project team, three Delphi participants were invited to attend the final item selection meeting; because of other commitments, only one was available. The selection group identified community health care questions that could be combined with GP questions for consistency. Items asking about details of hospital operations or procedures were considered less important by the more stringent set of consensus rules and were rejected for the core set of items for the short form (Table 5). These items could be included in an extended hospital care module for trials in which admissions (or re-admissions) for procedures are prevalent. Similarly, most residential care items (with the exception of hospice stays) did not meet the stringent consensus rules. Although residential care was thought to be extremely important in some trials, it was judged by the selection meeting group to be not relevant in most trials and was therefore identified as a suitable candidate for a bolt-on module. Items on social care did not meet the more stringent consensus rules, potentially because they were considered to be more relevant to particular groups, such as older adults; these items could therefore be included in a bolt-on social care module. Perhaps surprisingly, items on medication use were not identified as important by the more stringent criterion rules. The selection committee group felt that medication use was relevant to participants in most of the trials and should therefore remain on the included list; nevertheless, future work will look at the practical aspects of collecting medication data, and medication may form a separate module in the future.
Table 5.
Final outcomes for items after round 2
| Item description | % rating 7–9 | % rating 8 or 9 | % rating 1–3 | Outcome: pre-agreed rules | Outcome: more stringent rules | Final outcome after item selection meeting |
|---|---|---|---|---|---|---|
| Hospital outpatients | 97.62 | 80.95 | 0.00 | Include | Include | Short form |
| Hospital admissions | 97.62 | 90.48 | 2.38 | Include | Include | Short form |
| Length of stay | 92.86 | 85.71 | 2.38 | Include | Include | Short form |
| Operation/procedure | 78.57 | 61.90 | 0.00 | Include | Exclude | Extended hospital care module |
| Operation type | 71.43 | 54.76 | 0.00 | Include | Exclude | Extended hospital care module |
| A&E | 95.24 | 88.10 | 0.00 | Include | Include | Short form |
| Admissions after A&E | 88.10 | 76.19 | 7.14 | Include | Include | Short form |
| GP appointments | 97.62 | 95.24 | 0.00 | Include | Include | Short form |
| GP professional seen | 92.86 | 78.57 | 0.00 | Include | Include | Short form |
| Home visits | 97.62 | 85.71 | 0.00 | Include | Include | Short form |
| Professional seen at home | 88.10 | 69.05 | 2.38 | Include | Exclude | Short form |
| Community health care | 88.10 | 78.57 | 2.38 | Include | Include | Combined with GP appointments in short form |
| Community social care | 85.71 | 69.05 | 0.00 | Include | Exclude | Social care module |
| Health professional seen | 78.57 | 57.14 | 2.38 | Include | Exclude | Combined with GP appointments in short form |
| Social care professional seen | 73.81 | 52.38 | 2.38 | Include | Exclude | Social care module |
| Stay in hospice | 80.95 | 73.81 | 9.52 | Include | Include | Residential care module |
| Hospice length of stay | 78.57 | 66.67 | 11.90 | Include | Exclude | Residential care module |
| Respite care | 73.81 | 40.48 | 7.14 | Include | Exclude | Residential care module |
| Name of medication | 73.81 | 59.52 | 4.76 | Include | Exclude | Short form |
| Professional seen outpatient | 57.14 | 26.19 | 4.76 | Exclude | Exclude | |
| Specialty/ward | 38.10 | 16.67 | 9.52 | Exclude | Exclude | |
| Imaging scans | 23.81 | 11.90 | 9.52 | Exclude | Exclude | |
| Paramedic care | 50.00 | 23.81 | 0.00 | Exclude | Exclude | |
| GP surgery/procedure | 35.71 | 16.67 | 9.52 | Exclude | Exclude | |
| Help with activities | 59.52 | 30.95 | 2.38 | Exclude | Exclude | |
| Equipment | 33.33 | 21.43 | 2.38 | Exclude | Exclude | |
| Telephone/computer contacts | 64.29 | 38.10 | 2.38 | Exclude | Exclude | |
| Professional contacted | 54.76 | 35.71 | 2.38 | Exclude | Exclude | |
| Respite length of stay | 64.29 | 40.48 | 7.14 | Exclude | Exclude | |
| Nursing home | 57.14 | 35.71 | 9.52 | Exclude | Exclude | |
| Residential home | 40.48 | 28.57 | 11.90 | Exclude | Exclude | |
| Supported accommodation | 30.95 | 19.05 | 11.90 | Exclude | Exclude | |
| Prescribed medication | 64.29 | 47.62 | 14.29 | Exclude | Exclude | |
| Period taken for | 40.48 | 28.57 | 4.76 | Exclude | Exclude |
A&E, accident and emergency; GP, general practitioner.
Discussion
On the basis of consensus among health economists, we have identified a minimum core set of 10 resource use items that should be considered for inclusion in a standardized questionnaire for patients (Table 6). We have identified additional items that are suitable for inclusion as bolt-on or extended modules covering further details about hospital procedures, residential care, and social care. Agreement among participants was excellent [24] and moved toward consensus in the second round. Results were reasonably stable, suggesting that a third round would not have significantly altered the outcome. Although the survey was conducted from the viewpoint of the NHS and PSS, the key inclusions are all items commonly provided by the NHS. Social services care could therefore form a separate bolt-on module for trial populations in which it is thought to be prevalent.
Table 6.
Items included in the final core set
| Type of care | Item |
|---|---|
| 1. Hospital care | Number of hospital admissions (inpatient stay or day case) |
| 2. Hospital care | Length of stay (e.g., dates or number of nights) |
| 3. Hospital care | Number of hospital outpatient appointments |
| 4. Emergency care | Number of visits to A&E |
| 5. Emergency care | Number of admissions to hospital, after A&E |
| 6. Care at a GP surgery or health clinic or other community setting | Number of appointments |
| 7. Care at a GP surgery or health clinic or other community setting | Type of professional seen |
| 8. Health care at home | Number of health care professional visits at home |
| 9. Health care at home | Type of health care professional seen at home |
| 10. Medication | Name/class of medication |
A&E, accident and emergency; GP, general practitioner.
Knapp and Beecham [25] identified “reduced lists” of key services that could be measured to capture over 90% of the total costs of health and social care in patient groups with mental health conditions. The study indicated that, in principle, capturing a fairly small number of key items of resource use can lead to adequate cost information, with diminishing returns gained by further data collection. Nevertheless, although there was some overlap with the items we identified in this study (hospital inpatient and outpatient, residential care, and GP care), the nature of the patient group meant that social services played a considerably more prominent role.
Generic resource use measures developed to date include the Annotated Patient Cost Questionnaire [26] and the Client Service Receipt Inventory [27]. The former was designed as a generic patient-reported instrument. Although empirical evidence suggests that the questionnaire performs well [28], it has not been widely adopted (possibly because of the length of the questionnaire necessitating substantial work to generate an instrument for a trial). The latter has been tested extensively, demonstrating good consistency, reliability, and validity [29], [30], [31], [32] and is well used. Nevertheless, it was developed in the context of psychiatric care, was designed for interview administration rather than patient self-completion, and has been subject to uncontrolled modification over the years. Standardization of data collection has also been attempted in the context of cancer care [33], and a generic Dutch language instrument has been developed [34]. Nevertheless, neither implementation combines full standardization across all disease areas with a concise instrument and neither attempts to determine relevant content through a documented consensus process involving health economists.
Strengths of the study include the recruitment of the panel of expert participants, who were representative of a wide range of experience and had extensive NHS research experience. The stability of the panel was good with less than 10% attrition, and the study benefited from patient involvement in the study team. Established methods for conducting Delphi surveys were followed, with consensus criteria defined in advance of conducting each round. There was clear consensus for items ultimately included in the core set. Nevertheless, there may also be some limitations. Almost all the respondents came from an academic background; wider participation from industry representatives may have been beneficial in terms of generalizability, although their experience of NHS research would have been more limited. A larger sample participating in the Delphi survey would have been preferable; there is, however, no statistical basis on which to determine necessary sample size for a Delphi survey, and previous studies including fewer participants have been shown to produce reliable results [35]. Respondents were asked to rate the type of resource use (e.g., hospital or GP care) as well as the measurement information (such as the number of nights or appointments) simultaneously. The task was therefore cognitively challenging, with a large set of factors to bear in mind while responding; it is possible that participants may not have taken everything relevant into account.
The items identified are those considered most important by professional health economists for inclusion in a core set of resource use items. Work is now needed to identify the most appropriate way to measure these items to ensure patient acceptability and comprehensibility. There was evidence from the comments that some participants were considering patient ability to respond to questions. For example, one respondent commented that “… many patient groups are very confused about which services and professionals have visited them at home.” This requires further investigation with patient groups. Patients were not recruited to the Delphi panel, because the task was not meaningful in the context of the UK health care system in which patients do not pay for services at the point of use. The patient perspective was, however, represented during the study by the PPI member of the project team. Translation of the questionnaire to other languages (and other health care systems) also requires further investigation; given the common nature of the items included, it is possible that it will extend readily to other health care systems.
In this project, we have focused on an NHS and PSS perspective. There will commonly be requirements for additional data to be collected; any future instrument should take this into account through modularization, allowing modifications in a controlled fashion only, with alterations recorded. It is also likely that the resource use associated with the intervention itself will need to be collected separately. The developed instrument should be reviewed regularly to ensure that it remains current; for example, remote access care does not feature in our short form, but may become more pertinent in future if online consultations become common. We plan to develop a core module based on the 10 items identified in this study, working with PPI representatives to convert the items into questions that are meaningful and straightforward to answer.
Conclusions
The consensus on which items are important to health economists working on clinical trials in a generic context suggests that a standardized instrument for core items is feasible. The list of items identified forms a coherent set that is potentially relevant to most trials, conditions, and patient groups; it is therefore suitable for further development into a flexible instrument with additional extended and bolt-on modules. Collecting cost data in a manner that is simultaneously concise, understandable for patients, valid, precise, consistent between trials, and generalizable is challenging. We have provided much needed evidence that it may be possible to develop a standardized instrument that goes some way to meeting those challenges, on the basis of the most important cost items.
Acknowledgments
We thank the health economists who responded to the Delphi survey, whose expert participation enabled us to conduct this study, and Ed Wilson in particular. We also thank Mai Baquedano for help with setting up the REDCap survey software and Leila Rooshenas for guidance with the qualitative content analysis. An earlier version of the article was discussed at the Health Economists’ Study Group meeting in Gran Canaria in June 2016. We thank Rachael Hunter and other attendees for useful comments.
Source of financial support: This work was undertaken with the support of the MRC Collaboration and innovation for Difficult and Complex randomized controlled Trials In Invasive procedures (grant no. MR/K025643/1), the MRC North West Hub for Trials Methodology Research (grant no. MR/K025635/1), and the MRC Network of Hubs for Trials Methodology Research (grant no. MR/L004933/1-N57).
Footnotes
Gail Thornton, Patient and Public Involvement Representative.
Supplemental data associated with this article can be found in the online version at doi:10.1016/j.jval.2017.06.011.
Supplementary material
Supplementary material
References
- 1.Thorn J.C., Coast J., Cohen D. Resource-use measurement based on patient recall: issues and challenges for economic evaluation. Appl Health Econ Health Policy. 2013;11:155–161. doi: 10.1007/s40258-013-0022-4. [DOI] [PubMed] [Google Scholar]
- 2.Ridyard C.H., Hughes D. Review of resource-use measures in UK economic evaluations. In: Curtis L., Burns A., editors. Unit Costs of Health and Social Care. Personal Social Services Research Unit, University of Kent; Canterbury, UK: 2015. pp. 22–31. [Google Scholar]
- 3.Ridyard C.H., Hughes D.A. Methods for the collection of resource use data within clinical trials: a systematic review of studies funded by the UK Health Technology Assessment program. Value Health. 2010;13:867–872. doi: 10.1111/j.1524-4733.2010.00788.x. [DOI] [PubMed] [Google Scholar]
- 4.Noben C.Y., de Rijk A., Nijhuis F. The exchangeability of self-reports and administrative health care resource use measurements: assessement of the methodological reporting quality. J Clin Epidemiol. 2016;74:93–106. doi: 10.1016/j.jclinepi.2015.09.019. e102. [DOI] [PubMed] [Google Scholar]
- 5.NHS Improvement. Patient-level costing: case for change. 2016 Available from: https://improvement.nhs.uk/uploads/documents/CTP_PLICS_case_for_change.pdf. [Accessed January 24, 2017] [Google Scholar]
- 6.Ridyard C.H., Hughes D.A. Taxonomy for methods of resource use measurement. Health Econ. 2015;24:372–378. doi: 10.1002/hec.3029. [DOI] [PubMed] [Google Scholar]
- 7.Bhandari A., Wagner T. Self-reported utilization of health care services: improving measurement and accuracy. Med Care Res Rev. 2006;63:217–235. doi: 10.1177/1077558705285298. [DOI] [PubMed] [Google Scholar]
- 8.Clarke M. Standardising outcomes for clinical trials and systematic reviews. Trials. 2007;8:39. doi: 10.1186/1745-6215-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinha I.P., Smyth R.L., Williamson P.R. Using the Delphi technique to determine which outcomes to measure in clinical trials: recommendations for the future based on a systematic review of existing studies. PLoS Med. 2011;8:e1000393. doi: 10.1371/journal.pmed.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pincus T., Summey J.A., Soraci S.A. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheumatol. 1983;26:1346–1353. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 11.Drummond M.F., Sculpher M.J., Claxton K. Oxford University Press; Oxford, UK: 2015. Methods for the Economic Evaluation of Health Care Programmes. [Google Scholar]
- 12.National Institute for Health and Care Excellence Guide to the methods of technology appraisal. 2013 Available from: https://www.nice.org.uk/article/pmg9/resources/non-guidance-guide-to-the-methods-of-technology-appraisal-2013-pdf. [Accessed September 17, 2014] [PubMed] [Google Scholar]
- 13.Ridyard C.H., Hughes D.A., DIRUM team Development of a database of instruments for resource-use measurement: purpose, feasibility, and design. Value Health. 2012;15:650–655. doi: 10.1016/j.jval.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Thorn J.C., Ridyard C., Riley R. Identification of items for a standardised resource-use measure: review of current instruments. Trials. 2015;16(Suppl. 2):o26. [Google Scholar]
- 15.Gorst S.L., Gargon E., Clarke M. Choosing important health outcomes for comparative effectiveness research: an updated review and user survey. PLoS One. 2016;11:e0146444. doi: 10.1371/journal.pone.0146444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landeta J. Current validity of the Delphi method in social sciences. Technol Forecast Soc Change. 2006;73:467–482. [Google Scholar]
- 17.Harris P.A., Taylor R., Thielke R. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen J., Loranger H. New Riders; Berkeley, CA: 2006. Prioritizing Web Usability. [Google Scholar]
- 19.StataCorp L.P. StataCorp LP; College Station, TX: 2015. Stata Statistical Software: Release 14 [computer program] [Google Scholar]
- 20.Loeffen E., Mulder R., Kremer L. Development of clinical practice guidelines for supportive care in childhood cancer—prioritization of topics using a Delphi approach. Support Care Cancer. 2015;23:1987–1995. doi: 10.1007/s00520-014-2559-7. [DOI] [PubMed] [Google Scholar]
- 21.Brookes S.T., Macefield R.C., Williamson P.R. Three nested randomized controlled trials of peer-only or multiple stakeholder group feedback within Delphi surveys during core outcome and information set development. Trials. 2016;17:409. doi: 10.1186/s13063-016-1479-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.QSR International Ltd. London; 2012. QSR International Pty Ltd. NVivo Qualitative Data Analysis Software, Version 10 [computer program] [Google Scholar]
- 23.Bryman A. Social Research Methods. 2nd ed. Oxford University Press; Oxford, UK: 2004. [Google Scholar]
- 24.Cicchetti D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess. 1994;6:284. [Google Scholar]
- 25.Knapp M., Beecham J. Reduced list costings: examination of an informed short cut in mental health research. Health Econ. 1993;2:313–322. doi: 10.1002/hec.4730020404. [DOI] [PubMed] [Google Scholar]
- 26.Thompson S., Wordsworth S. HERU Discussion Paper 03/01, University of Aberdeen Health Economics Research Unit; Aberdeen, UK: 2001. An annotated cost questionnaire for completion by patients. [Google Scholar]
- 27.Beecham J., Knapp M. Costing psychiatric interventions. In: Thornicroft G., Brewin C., Wing J., editors. Measuring Mental Health Needs. Gaskell; London: 1992. pp. 179–190. [Google Scholar]
- 28.Wordsworth S. PhD Thesis, University of Aberdeen; Aberdeen, UK: 2004. Improving the transferability of costing results in economic evaluation: an application to dialysis therapy for end-stage renal disease. [Google Scholar]
- 29.Mirandola M., Bisoffi G., Bonizzato P., Amaddeo F. Collecting psychiatric resources utilisation data to calculate costs of care: a comparison between a service receipt interview and a case register. Soc Psychiatry Psychiatr Epidemiol. 1999;34:541–547. doi: 10.1007/s001270050173. [DOI] [PubMed] [Google Scholar]
- 30.Simpson S., Corney R., Fitzgerald P., Beecham J. A randomised controlled trial to evaluate the effectiveness and cost-effectiveness of counselling patients with chronic depression. Health Technol Assess. 2000;4:1–83. [PubMed] [Google Scholar]
- 31.Byford S., Leese M., Knapp M. Comparison of alternative methods of collection of service use data for the economic evaluation of health care interventions. Health Econ. 2007;16:531–536. doi: 10.1002/hec.1175. [DOI] [PubMed] [Google Scholar]
- 32.Patel A., Rendu A., Moran P. A comparison of two methods of collecting economic data in primary care. Fam Pract. 2005;22:323. doi: 10.1093/fampra/cmi027. [DOI] [PubMed] [Google Scholar]
- 33.Marti J., Hall P.S., Hamilton P. The economic burden of cancer in the UK: a study of survivors treated with curative intent. Psychooncology. 2016;25:77–83. doi: 10.1002/pon.3877. [DOI] [PubMed] [Google Scholar]
- 34.Bouwmans C., Roijen L.H.-v, Koopmanschap M. iMTA, Erasmus University Rotterdam; Rotterdam, The Netherlands: 2013. Handleiding iMTA Medical Cost Questionnaire (iMCQ) [Google Scholar]
- 35.Akins R.B., Tolson H., Cole B.R. Stability of response characteristics of a Delphi panel: application of bootstrap data expansion. BMC Med Res Methodol. 2005;5:1. doi: 10.1186/1471-2288-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material