Abstract
Background
Waldenström Macroglobulinemia (WM) is a rare subtype of indolent B-cell lymphoma, and prospective randomized studies on WM are scarce. The R-CHOP therapy [rituximab (R), cyclophosphamide, hydroxy-doxorubicin, vincristine, and prednisone] is a popular and recommended regimen for primary therapy, prescribed by several treatment guidelines for WM. However, treatment with R-CHOP is accompanied by severe myelosuppression and high rates of peripheral neuropathy. Therefore, we retrospectively evaluated the efficacy and toxicity of half-dose CHOP combined with R as a primary therapy for WM.
Methods
Patients with untreated symptomatic WM, treated at the Disaster Medical Center between April 2011 and September 2016, were retrospectively analyzed after administration of 6 cycles of half-dose R-CHOP for every 3 weeks. The response, median time to response, best response, progression-free survival, overall survival, and toxicities were evaluated.
Results
Of the 20 WM patients analyzed, 16 (80%) received half-dose R-CHOP without vincristine, and 13 (65%) responded to the treatment. With a median follow-up duration of 26.3 months, the 2-year progression-free survival and 2-year overall survival rates were 70 and 93.3%, respectively. The median time to response and best response were 6 and 9.9 weeks, respectively. Grade 3/4 leukocytopenia, neutropenia, febrile neutropenia, and Grade 1 peripheral neuropathy developed in 32, 37, 0, and 21% of patients, respectively.
Conclusion
The half-dose R-CHOP is an effective and well-tolerated primary therapy for WM. To the best of our knowledge, this is the first study reporting the use of a reduced-dose R-CHOP regimen for the primary treatment of WM.
Keywords: Waldenström Macroglobulinemia, R-CHOP, Reduced-dose, Primary therapy
INTRODUCTION
Waldenström Macroglobulinemia (WM) is one of the rare entities of low-grade B-cell lymphomas with abnormally high levels of monoclonal IgM [1,2,3,4]. Owing to its rarity, therapeutic reports from prospective randomized studies on WM are scarce [5,6,7]. Although several treatment guidelines therapeutic recommendations for WM are currently available [8,9,10], the level of evidence for each regimen is not satisfactory. Furthermore, the goal of primary therapy depends on the condition of each patient, such as the need for rapid disease control or a low tumor burden with mild symptoms [11]. The R-CHOP treatment regime, which combines CHOP [cyclophosphamide (CPA), hydroxy-doxorubicin (ADR), vincristine (VCR), and prednisone (PSL)] with the anti-CD20 antibody, rituximab (R), is widely used worldwide [5,6,12,13], and is the standard primary regimen for most subtypes of B-cell lymphomas [13]. This regimen is also one of the most recommended regimens for untreated symptomatic WM, particularly where rapid disease control is required [8,9,10]. However, severe myelosuppression and peripheral neuropathy (PN) are the main toxicities reported in WM patients treated with the standard-dose R-CHOP regimen as primary therapy [5,6,12] because the bone marrow is the main site involved in malignancy and WM patients are generally older, at least in their 60s or 70s. Furthermore, approximately 20% of patients with WM have symptomatic or occult PN [14,15]. We herein report the efficacy and toxicity of a half-dose R-CHOP regimen (half-dose R-CHOP), in which the use of VCR was omitted in 80% of the patients [half-dose R-CH(O)P]. This is the first study reporting the use of a reduced-dose R-CHOP regimen in patients with untreated symptomatic WM.
MATERIALS AND METHODS
Patients
Twenty untreated symptomatic WM patients were treated at the Disaster Medical Center between April 2011 and September 2016 and retrospectively analyzed. The approval of the institutional Review Board was obtained from our hospital, and the study was performed according to the Declaration of Helsinki formulated in 1995. The diagnostic criteria of WM were selected according to the guidelines prescribed during the 2nd International Workshop on WM (IWWM) [2]. Briefly, WM was defined based on the following: 1) the detection of monoclonal IgM; 2) presence of neoplastic cells including small round cells, and lymphoplasmacytic cells positive for CD20, and some plasma cells accounting for more than 10% in the BM; 3) distinction from other IgM-secreting malignancies including indolent B-cell lymphomas and myelomas; and 4) differentiation of symptomatic WM from smoldering/asymptomatic WM and monoclonal IgM gammopathies of undetermined significance. Symptomatic WM was defined by the incidence of constitutional symptoms, cytopenia, organomegaly and/or symptoms attributed to the monoclonal IgM protein, including the hyperviscosity syndrome and cold agglutinin disease [2,8]. Clinical data were obtained from medical charts, and included the patients' age, gender, hemoglobin (Hb) levels, platelet counts, the serum levels of β2-microglobulin, IgM, and M-protein, the score obtained by the International Prognostic Scoring System for WM (IPSSWM) [16], the performance status (PS), the incidence of adenopathy, hepatomegaly, splenomegaly, cold agglutinin disease (CAD), B symptoms, and the clinical course undertaken, including primary and salvage treatments. The PS was assessed according to the scale developed by the Eastern Cooperative Oncology Group.
Assessment of responses, survival, and toxicities
Responses were defined according to the Updated Response Criteria adopted by the 6th International Workshop on WM [17]. Briefly, a complete response (CR) was defined by the resolution of all symptoms, normalization of serum IgM levels with the complete disappearance of the IgM paraprotein as detected by immunofixation, no evidence of disease in bone marrow examinations, resolution of any existing adenopathy or splenomegaly, and absence of other symptoms associated with WM. Patients who achieved a very good partial response (VGPR), partial response (PR), and minor response (MR) were defined by the reductions in serum IgM levels by >90, 50–90, and 25–50%, respectively. Patients with stable disease (SD) were defined by a <25% change in serum IgM levels in the absence of new or increasingly severe adenopathy or splenomegaly, and/or other progressive signs or symptoms of WM. Progressive disease (PD) was defined by a >25% increase in serum IgM levels from the lowest attained response value or the progression of clinically significant disease-related symptoms.
Progression-free survival (PFS) was defined as the time from the beginning of the treatment until the first recognition of disease progression, which necessitated a change in the chemotherapy regimen due to recurrence of symptoms of WM. Overall survival (OS) was measured from the beginning of the treatment until death from any cause, and surviving patients were censored on the last day of contact [18]. All survival curves were evaluated by the means, using the method of Kaplan and Meier. The log-rank test was used to evaluate the differences in PFS between the groups.
The toxicities arising following administration of the regimen were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 4.0 (CTCAE) [19].
Primary treatment
Primary treatment was administered to patients with symptomatic WM, defined by the diagnostic criteria for WM by the 2nd IWWM [2] and NCCN guidelines [8]. All WM patients who received the half-dose R-CHOP regimen as primary therapy were enrolled in the present study. Sixteen (80%) out of the 20 patients diagnosed after December 2012, received half-dose R-CH(O)P [50% dose of standard-dose CHOP (intravenous (IV) administration of CPA at a dose of 375 mg/m2 on day 1; IV administration of ADR at a dose of 25 mg/m2 on day 1; oral administration of 60 mg PSL from days 1 to 5); and IV administration of 375 mg/m2 of R (day 1 or 2)]. Four out of the 20 patients (20%) diagnosed between April 2011 and November 2012 received half-dose R-CHOP [half-dose R-CH(O)P and IV administration of VCR at a dose of 0.7 mg/m2 on day 1]. Generally, 6 cycles of R-CH(O)P or half-dose R-CHOP was administered every 3 weeks as primary therapy. The Granulocyte colony-stimulating factor was used in accordance with the guidelines of the American Society of Clinical Oncology, at the discretion of each physician.
RESULTS
Clinical characteristics
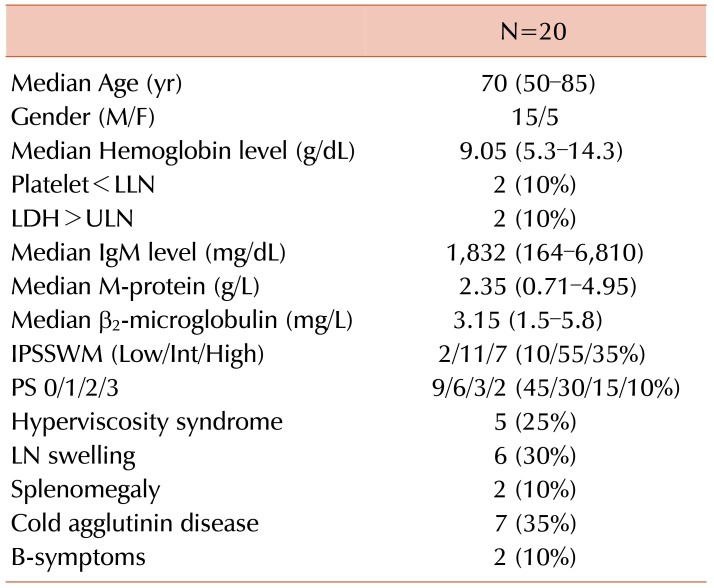
The clinical characteristics of the 20 patients during diagnosis are presented in Table 1. The median age was 70 years (range, 50–85). Fifteen of the 20 patients were males. The median Hb level was 9.05 g/dL (range, 5.3–14.3), and the median serum M-protein level was 2.35 g/L (range, 0.71–4.95). Two out of 20 patients (10%) had B-symptoms and 7 (35%) had CAD. Five patients showed the incidence of the hyperviscosity syndrome (25%), and underwent plasma exchange prior to chemo-immunotherapy. According to the IPSSWM, 2 (10%), 11 (55%), and 7 (35%) patients were classified as being at low, intermediate, and high risk, respectively.
Table 1. Patient characteristics.
Abbreviations: IPSSWM, International Prognostic Scoring System for Waldenstroöm Macroglobulinemia; LLN, lower limit of normal; PS, performance status; ULN, upper limit of normal.
Responses and survival
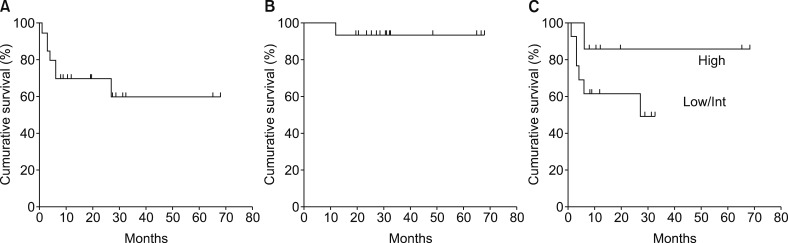
All the 20 patients were assessable for measuring responses to the primary treatment, PFS, and OS. The median follow-up for all the 20 patients was 26.3 months. Two patients (10%) achieved CR, 1 (5%) achieved VGPR, 10 (50%) were classified as PR, and 4 (20%) were MR. Thus, the overall response rate (ORR) was 65%. The remaining 3 patients (15%) showed SD. Two out of the 3 patients with SD were 79 years-old, and the primary treatment for one of the patients had to be discontinued owing to traumatic subarachnoid hemorrhage. The other patients with SD had high levels of serum IgM (5,730 mg/dL) during diagnosis of WM. The median times to a response and best response were 6 and 9.9 weeks, respectively. The patients did not reach median PFS, and the 2-year PFS and 3-year PFS rates were 70 and 60%, respectively (Fig. 1A). The median OS was not reached, and the 2-year OS rate was 93.3% (Fig. 1B). Patients aged >65 years, and with Hb <11.5 g/dL, plt <100×109/L, serum β2-microglobulin >3.0 mg/L, serum monoclonal protein concentration >7.0 g/dL, and IPSSWM score (low/int vs. high) did not affect the shorter PFS (Fig. 1C) in the present study. Thirteen patients received a maintenance dose of R after the initial therapy, while none of the patients received high-dose chemotherapy followed by autologous peripheral blood stem cell transplantation.
Fig. 1. Survival curves showing (A) progression-free survival (PFS). Median PFS was not reached, and the estimated 2-year PFS and 3-year PFS rates were 70% and 60%, respectively; (B) overall survival. The estimated 2-year OS rate was 93.3%; (C) PFS between the high-risk and low/intermediate-risk groups of IPSSWM (P=0.20).
During the follow-up, 7 patients developed refractory disease or progression after the initial treatment, 4 patients required a change of the chemotherapy regime due to constitutional symptoms during initial chemotherapy, and 3 patients showed recognizable symptomatic elevation of IgM after completion of initial chemotherapy. All the patients received a regimen containing Bendamustine (Benda). All the 7 patients responded well to this treatment, with 2 achieving VGPR and 5 achieving PR. During the median follow-up of 26.0 months, none of the patients showed mortality due to disease progression.
Toxicities
The toxicities that developed following primary therapy in 19 patients are represented in Table 2. Grade 3/4 leukocytopenia was detected in 6 patients (32%) and neutropenia in 7 (37%). Febrile neutropenia did not occur in any of the patients. Grade 1 PN was observed in 4 patients (21%), who subsequently received half-dose R-CHOP therapy. No IgM flares were observed in any of the patients [20]. Thus, none of the patients required further dose reduction or omission of the half-dose R-CHOP therapy.
Table 2. Toxicities (N=19).
Each number indicates numbers of cases who suffering from each toxicity.
DISCUSSION
WM is one of the rare disease subtypes of indolent B-cell lymphoma [1,2]. In the United States (US) the overall annual incidence of WM is 2 to 3 per one million individuals [3,4]. Due to the rarity of its occurrence, only a few prospective randomized studies have been completed to date. Therefore, the level of evidence of the recommended regimens for each guideline is still low. The primary regimens recommended by the National Comprehensive Cancer Network [8], European Society for Medical Oncology [9], and 7th IWWM [10] are similar: R monotherapy, R±Benda, R±a purine analog, R±a proteasome inhibitor, and R±an alkylating agent-containing regimen including R-CHOP therapy. The ORR of R alone was previously reported to be approximately 44 to 55%, with the median PFS estimated to be 16 to 30 months [20,21]. On the other hand, chemo-immunotherapy is generally accepted to achieve higher ORRs and longer PFS than those by R alone, based on the findings of several randomized studies on other subtypes of indolent B-cell lymphoma [22,23].
Although the efficacy of each regimen has mainly been reported in phase II trials and a few randomized trials [5,6,7], reliable data on epidemiological treatment patterns and efficacy outcomes in the population are limited. Buske and coworkers analyzed the treatment and outcome patterns of 454 patients with symptomatic WM, diagnosed between 2,000 and 2014 in 10 European countries [24]. The median age was 65 years and median PFS of frontline therapy was 29 months. They also reported that the most popular treatment regime is an initial combination therapy with R (40%), followed by R monotherapy (31%). The agents most commonly combined for therapy with R were CPA (43%) and VCR (24%). Chlorambucil was mainly used as a monotherapy (12%). Fludarabine (Flu) and Benda are also popular regimens for the treatment of WM; however, Flu is used in the 2nd line (total: 24%, monotherapy: 6%, combination: 18%), while Benda is used in the 3rd line (total: 20%, monotherapy: 1%, combination: 19%). Olszewski and coworkers reported trends in the treatment patterns and outcomes of 2,666 patients with WM, aged ≥65 years, between 1994 and 2011, using the results of the linked data of the Medical and Surveillance, Epidemiology and End program in the US [25,26]. They reported that the median age was 78 years and median OS was 4.6 years from diagnosis. A focus on treatment regimens between 2009 and 2011 revealed that 56% patients received R alone (more frequently in patients >80 years old), 18% received an alkylating agent with or without R, and 10% received Bor with or without R. These findings indicated that a combined regimen of R with an alkylating agent remains the most popular treatment regimen.
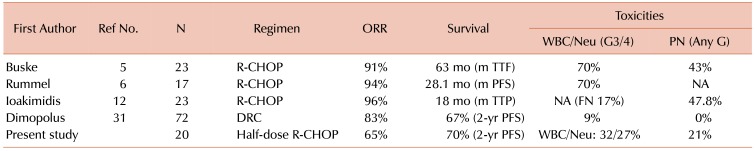
It is generally accepted that a regimen combining R with an alkylating agent is one of the most appropriate primary therapies for WM patients requiring urgent treatment [11]. R monotherapy is preferred for patients who do not require immediate disease control or those aged ≥80 years [25]. A literature review on the combination regimens of R with alkylating agents is presented in Table 3. The R-CHOP regimen is the most commonly used and highly effective primary therapy for patients with WM [5,6,12]. Buske and coworkers reported a longer time to treatment failure in the R-CHOP arm than in the CHOP arm, in a small-sized prospective randomized study [5]. Ioakimidis and coworkers performed a retrospective analysis on R-CHOP, R-CVP (R, CPA, VCR, and PSL), and R-CP (R, CPA, and PSL) in untreated and previously treated patients with WM [12]. The ORRs among the groups were similar, being approximately 80 to 90%, whereas the median reduction rate in serum IgM levels was superior in the R-CHOP group. Dimopolus and coworkers performed a single-arm prospective study using a DRC regimen [Dexamethasone (Dex), R, and CPA], and demonstrated its high efficacy and low toxicity [27]. In that study, the ORR was 83% and the 2-year PFS rate was 67%. This regimen did not contain anthracycline agents including ADR, and the median time to a response was relatively slow, being 4.1 months. In the present study, the median age was 70 years, and 90% of patients were grouped into the intermediate risk and high-risk categories, according to the scores by the IPSSWM. The efficacy of the half-dose R-CHOP regimen was favorable compared to that of the DRC therapy; the ORR was 65%, the 2-year PFS rate was 70%, and the 2-year OS rate was 93.3%. Furthermore, the median times to a response and the best response were 6.0 and 9.9 weeks, respectively, which were earlier than those with the DRC regimen. Thus, the half-dose R-CHOP therapy is highly effective and achieves quick responses.
Table 3. Literature review on rituximab plus alkylating regimens.
Abbreviations: DRC, dexamethasone, rituximab, and cyclophosphamide; FN, febrile neutropenia; G, grade; M, median; NA, not applicable; ORR, overall response rate; PFS, progression-free survival; PN, peripheral neuropathy; R-CHOP, rituximab, cyclophosphamide, hydroxy-doxorubicin, vincristine, and prednisone; Ref No., reference number; TTF, time to treatment failure; TTP, time to progression.
One of the major toxicities of a combination regimen of R with an alkylating agent is severe myelosuppression because the bone marrow is the chief site involved in malignancy and patients with WM are generally elderly. Rummel and coworkers reported in a prospective study of R-Benda versus R-CHOP as a first-line treatment for indolent B-cell lymphomas including WM, that if grade 4 leukocytopenia or thrombocytopenia was recognized, the dose of CPA and ADR needs to be reduced by 80% [6]. There are few studies on the reduced-dose R-CHOP therapy for the treatment of B-cell lymphomas, except WM [28,29]. Kikuchi and coworkers evaluated the efficacy and toxicity of a two-thirds dose of R-CHOP chemotherapy in B-cell lymphomas, including primarily the diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma in patients aged 70 years or older, and they concluded that the two-thirds dose of R-CHOP has high efficacy and tolerable toxicity [28]. Peyrade and coworkers reported the high efficacy and safety of a dose of CHOP that was close to the half-dose CHOP, in combination with 375 mg/m2 of R, in a prospective phase II study in patients with DLBCL, who were older than 80 years [29]. Considering these reports, doses between 50% to two-thirds of R-CHOP chemotherapy are suitable for patients with lymphomas without bone marrow invasion, who are older than 70 or 80 years. Focusing on WM, more than 50% of patients develop severe leukocytopenia and neutropenia when treated with standard-dose R-CHOP therapy [5,6,12], because the main site of neoplastic growth is the bone marrow, and the malignancy occurs in patients in their 70s to 80s. Therefore, although further dose-reductions of R-CHOP therapy in WM compared to lymphomas without bone marrow invasion might be reasonable, no useful data pertaining to this have been available so far. On the other hand, myelosuppression is mild in patients treated with the DRC regimen with only 9% of patients showing grade 3/4 neutropenia in a previous study [27].
Another specific toxicity is PN, with approximately 20% of WM patients demonstrating symptomatic or occult IgM paraproteinemic neuropathy including the anti-myelin-associated glycoprotein neuropathy [5,6,12]. Dimopolus and coworkers identified PN in 43–48% of patients treated with the standard-dose R-CHOP regimen [5,12]. PN developed in 46% of patients treated using a combined regimen of Bortezomib, R, and Dex [30]. Therefore, agents that may affect neuropathy need to be avoided. In the present study, 37% of patients had grade 3/4 neutropenia, whereas none had febrile neutropenia. Grade 1 PN was detected in 21% of the patients, and they were all treated with half-dose R-CHOP therapy. Therefore, the half-dose R-CH(O)P therapy may offer a better strategy for avoiding PN.
The present study revealed that the half-dose R-CHOP therapy, or more specifically the half-dose R-CH(O)P regimen was highly effective, showing fast responses and was well-tolerated, although the study was a small-sized retrospective analysis. Therefore, this regimen is regarded as one of the reasonable options for primary therapy for patients with untreated symptomatic WM. To the best of our knowledge, this is the first study to report the use of a reduced-dose CHOP regimen combined with R for the primary treatment of WM. Further large-scale prospective studies are warranted.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Swerdlow SH, Berger F, Pikeru SA. Lymphoplasmacytic lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumour of hematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC Press; 2007. pp. 194–195. [Google Scholar]
- 2.Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol. 2003;30:110–115. doi: 10.1053/sonc.2003.50082. [DOI] [PubMed] [Google Scholar]
- 3.Issa GC, Leblebjian H, Roccaro AM, Ghobrial IM. New insights into the pathogenesis and treatment of Waldenstrom macroglobulinemia. Curr Opin Hematol. 2011;18:260–265. doi: 10.1097/MOH.0b013e3283474e5b. [DOI] [PubMed] [Google Scholar]
- 4.Vijay A, Gertz MA. Waldenström macroglobulinemia. Blood. 2007;109:5096–5103. doi: 10.1182/blood-2006-11-055012. [DOI] [PubMed] [Google Scholar]
- 5.Buske C, Hoster E, Dreyling M, et al. The addition of rituximab to front-line therapy with CHOP (R-CHOP) results in a higher response rate and longer time to treatment failure in patients with lymphoplasmacytic lymphoma: results of a randomized trial of the German Low-Grade Lymphoma Study Group (GLSG) Leukemia. 2009;23:153–161. doi: 10.1038/leu.2008.261. [DOI] [PubMed] [Google Scholar]
- 6.Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–1210. doi: 10.1016/S0140-6736(12)61763-2. [DOI] [PubMed] [Google Scholar]
- 7.Leblond V, Johnson S, Chevret S, et al. Results of a randomized trial of chlorambucil versus fludarabine for patients with untreated Waldenström macroglobulinemia, marginal zone lymphoma, or lymphoplasmacytic lymphoma. J Clin Oncol. 2013;31:301–307. doi: 10.1200/JCO.2012.44.7920. [DOI] [PubMed] [Google Scholar]
- 8.National Cancer Institute. NCCN Clinical Practice Guidelines in Oncology. Waldenstrom macroglobulinemia/lymphoplasmacytic lymphoma. Bethesda, MD: National Cancer Institute; 2017. [Accessed August 3, 2017]. at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [Google Scholar]
- 9.Buske C, Leblond V, Dimopoulos M, et al. Waldenstrom's macroglobulinaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:vi155–vi159. doi: 10.1093/annonc/mdt298. [DOI] [PubMed] [Google Scholar]
- 10.Dimopoulos MA, Kastritis E, Owen RG, et al. Treatment recommendations for patients with Waldenström macroglobulinemia (WM) and related disorders: IWWM-7 consensus. Blood. 2014;124:1404–1411. doi: 10.1182/blood-2014-03-565135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Treon SP. How I treat Waldenström macroglobulinemia. Blood. 2015;126:721–732. doi: 10.1182/blood-2015-01-553974. [DOI] [PubMed] [Google Scholar]
- 12.Ioakimidis L, Patterson CJ, Hunter ZR, et al. Comparative outcomes following CP-R, CVP-R, and CHOP-R in Waldenström's macroglobulinemia. Clin Lymphoma Myeloma. 2009;9:62–66. doi: 10.3816/CLM.2009.n.016. [DOI] [PubMed] [Google Scholar]
- 13.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 14.Nobile-Orazio E, Marmiroli P, Baldini L, et al. Peripheral neuropathy in macroglobulinemia: incidence and antigen-specificity of M proteins. Neurology. 1987;37:1506–1514. doi: 10.1212/wnl.37.9.1506. [DOI] [PubMed] [Google Scholar]
- 15.Nemni R, Gerosa E, Piccolo G, Merlini G. Neuropathies associated with monoclonal gammapathies. Haematologica. 1994;79:557–566. [PubMed] [Google Scholar]
- 16.Morel P, Duhamel A, Gobbi P, et al. International prognostic scoring system for Waldenstrom macroglobulinemia. Blood. 2009;113:4163–4170. doi: 10.1182/blood-2008-08-174961. [DOI] [PubMed] [Google Scholar]
- 17.Treon SP, Merlini G, Morra E, Patterson CJ, Stone MJ. Report from the Sixth International Workshop on Waldenström's Macroglobulinemia. Clin Lymphoma Myeloma Leuk. 2011;11:69–73. doi: 10.3816/CLML.2011.n.010. [DOI] [PubMed] [Google Scholar]
- 18.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cancer Therapy Evaluation Program (CTEP) Common Terminology Criteria for Adverse Events ver4.0. Bethesda, MD: National Cancer Institute; 2010. [Accessed August 3, 2017]. at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40. [Google Scholar]
- 20.Dimopoulos MA, Zervas C, Zomas A, et al. Treatment of Waldenström's macroglobulinemia with rituximab. J Clin Oncol. 2002;20:2327–2333. doi: 10.1200/JCO.2002.09.039. [DOI] [PubMed] [Google Scholar]
- 21.Gertz MA, Abonour R, Heffner LT, Greipp PR, Uno H, Rajkumar SV. Clinical value of minor responses after 4 doses of rituximab in Waldenström macroglobulinaemia: a follow-up of the Eastern Cooperative Oncology Group E3A98 trial. Br J Haematol. 2009;147:677–680. doi: 10.1111/j.1365-2141.2009.07892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcus R, Imrie K, Solal-Celigny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26:4579–4586. doi: 10.1200/JCO.2007.13.5376. [DOI] [PubMed] [Google Scholar]
- 23.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 24.Buske C, Sadullah S, Kastritis E, et al. Generation of a large observational pan-European data platform for treatment and outcome patterns in patients with Waldenstrom's macroglobulinemia. Blood (ASH Annual Meeting Abstracts) 2015;126(Suppl):abst 2096. [Google Scholar]
- 25.Olszewski AJ, Fallah J, Eaton CB, Treon SP, Castillo JJ. The evolution of management and survival outcomes of Waldenström macroglobulinemia (WM) in the United States (US) Blood (ASH Annual Meeting Abstracts) 2015;126(Suppl):abst 882. [Google Scholar]
- 26.Olszewski AJ, Treon SP, Castillo JJ. Evolution of management and outcomes in Waldenström macroglobulinemia: a population-based analysis. Oncologist. 2016;21:1377–1386. doi: 10.1634/theoncologist.2016-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dimopoulos MA, Anagnostopoulos A, Kyrtsonis MC, et al. Primary treatment of Waldenström macroglobulinemia with dexamethasone, rituximab, and cyclophosphamide. J Clin Oncol. 2007;25:3344–3349. doi: 10.1200/JCO.2007.10.9926. [DOI] [PubMed] [Google Scholar]
- 28.Kikuchi M, Nakasone H, Akahoshi Y, et al. Reduced-dose (two-thirds) R-CHOP chemotherapy for elderly patients with non-Hodgkin lymphoma. J Chemother. 2015;27:99–105. doi: 10.1179/1973947814Y.0000000219. [DOI] [PubMed] [Google Scholar]
- 29.Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2011;12:460–468. doi: 10.1016/S1470-2045(11)70069-9. [DOI] [PubMed] [Google Scholar]
- 30.Dimopoulos MA, García-Sanz R, Gavriatopoulou M, et al. Primary therapy of Waldenstrom macroglobulinemia (WM) with weekly bortezomib, low-dose dexamethasone, and rituximab (BDR): long-term results of a phase 2 study of the European Myeloma Network (EMN) Blood. 2013;122:3276–3282. doi: 10.1182/blood-2013-05-503862. [DOI] [PubMed] [Google Scholar]