Abstract
Background
The aim of this study was to evaluate the effects of darbepoetin alfa (DA) on hemoglobin (Hb) concentration and the need for transfusions in multiple myeloma (MM) patients receiving chemotherapy with novel agents.
Methods
Of 251 patients with MM who received DA therapy for at least 4 weeks, 142 who did not receive RBC transfusion during 4 weeks after DA initiation and started DA therapy at baseline Hb <10.0 g/dL were analyzed.
Results
After 4 weeks of DA therapy, 80 (60.6%) of 132 patients with evaluable data had Hb that increased ≥1.0 g/dL from baseline, while 50 (37.9%) had Hb that increased ≥2.0 g/dL from baseline. Pretreatment Hb level did not correlate with the proportion of patients with increased Hb. The median duration of DA therapy was 9.0 weeks. At the end of DA therapy, of 135 patients with evaluable data, 86 (60.6%) had Hb that increased ≥1.0 g/dL from baseline, while 67 (47.2%) had Hb that increased ≥2.0 g/dL from baseline. Stage III disease according to the International Staging System and absence of myeloma bone disease at diagnosis were independent predictors of higher Hb response during early DA therapy.
Conclusion
We demonstrated the efficacy of DA therapy in a homogeneous group of MM patients receiving chemotherapy. DA therapy significantly increased Hb concentration, regardless of baseline Hb level.
Keywords: Multiple myeloma, Anemia, Erythropoietin
INTRODUCTION
Anemia in multiple myeloma (MM) is common and results from multifactorial causes, with chronic anemia of cancer and cancer therapy being frequent causes. Kyle et al. reported hemoglobin (Hb) concentrations ≤12 g/dL in 73% of 1,027 anemic patients with newly-diagnosed MM (NDMM) [1]. The European Cancer Anaemia Survey (ECAS) of 2,316 evaluable patients (1,612 with lymphoma and 704 with MM) reported an anemia prevalence of 72.9% (MM, 85.3%; non-Hodgkin's lymphoma, 77.9%; Hodgkin's disease, 57.4%) and an incidence of 55.4% among chemotherapy patients [2].
Anemia is detrimental to physical capacity and quality of life (QoL) in cancer patients [3,4] and treatment with erythropoiesis-stimulating agents (ESA) is necessary. The ECAS report from Ludwig et al. [5] evaluated the relationship between anemia among 15,317 cancer patients and World Health Organization performance status and risk factors for its development. The report found that decreased Hb level significantly correlated with poor performance scores. The International Myeloma Working Group recommends that treatment with ESAs be considered for patients receiving chemotherapy who have Hb <10 g/dL [6]. Treatment options for anemic myeloma patients include red blood cell (RBC) transfusions and recombinant human erythropoietin (rHuEPO). RBC transfusions have an immediate effect and rapidly increase Hb. Unfortunately, the effects of RBC transfusions are transient and can be associated with risks including infections and mild to life-threatening immunologic reactions. rHuEPO is biologically equivalent to the human endogenous hormone erythropoietin (EPO). Its application increases Hb over an extended time without the risk of blood transfusions.
Since the late 1980s, rHuEPO has been critical for managing renal anemia in patients receiving maintenance dialysis, resulting in prolonged survival rates and improved QoL and decreasing the need for blood transfusions [7]. A decade later, darbepoetin alfa (DA) was developed for clinical use. The mean terminal half-life of 25.3 hours for intravenous DA is 3-fold longer than the 8.5-hour half-life of intravenous rHuEPO [8].
Results from a phase 2 study in patients with lymphoproliferative malignancies such as lymphoma or myeloma demonstrated that DA at doses of 1.0–4.5 µg/kg/week significantly increases Hb relative to placebo [9]. To further evaluate the effects of DA on Hb and RBC transfusions in patients with MM receiving novel agents, we conducted this retrospective study at a fixed dose of 120 µg/week.
MATERIALS AND METHODS
Patient selection and treatment procedures
In our institution, DA therapy (120 µg s.c., once weekly) was considered in MM patients receiving chemotherapy who had Hb <10.0 g/dL and discontinued in case Hb levels increased beyond 12.0 g/dL. Two hundred and fifty-one MM patients received DA therapy (120 µg s.c., once weekly) for at least 4 weeks at a single institution between January 2011 and December 2015. Among these patients, 142 who did not receive RBC transfusion during 4 weeks after DA initiation and who started DA therapy at baseline Hb <10.0 g/dL were eligible for analysis. Of 82 NDMM patients, 29 (35%) were eligible for autologous stem cell transplantation (ASCT). Of these, 27 patients received novel agent-based induction chemotherapy (bortezomib+dexamethasone in 8 patients, thalidomide+dexamethasone in 18 patients, and lenalidomide+dexamethasone in 1 patient) and two received high-dose dexamethasone. Of 53 patients (65%) ineligible for ASCT who received chemotherapy alone, 30 received novel agents as frontline therapy (bortezomib+melphalan+prednisolone in 28 patients, melphalan+prednisolone+thalidomide in 1 patient, and lenalidomide+dexamethasone in 1 patient) and 23 received melphalan+prednisolone or cyclophosphamide+prednisolone. Included were 60 relapsed/refractory MM patients who received salvage chemotherapy such as bortezomib-based (N=15), thalidomide-based (N=18), lenalidomide-based (N=12), or other (N=15) therapy. The Institutional Review Board of The Catholic University of Korea approved the research protocol. This study was conducted in accordance with the tenets of the Declaration of Helsinki.
Definitions and evaluation of response
Efficacy was assessed by Hb response and incidence of RBC transfusion. To assess Hb response, the percentage of patients who, in the absence of RBC transfusion during the preceding 28 days, had increased Hb ≥1.0 and ≥2.0 g/dL from baseline was calculated after 4 weeks and the percentage with increased Hb ≥1.0 and ≥2.0 g/dL from baseline was calculated at the end of DA therapy. To evaluate the effect of DA therapy on RBC transfusion, the percentage of patients receiving RBC transfusions from week 5 to the end of DA therapy and the amount of transfused RBC were analyzed. Changes in Hb from baseline to 4 weeks and the end of DA therapy were also calculated. In addition, clinical data on adverse events such as venous thromboembolism were collected.
Statistical analysis
We analyzed data to summarize the effectiveness of DA when used according to revised prescribing information and recommendations with Hb treatment initiation threshold ≤10 g/dL for chemotherapy-induced anemia in MM [6]. The study objectives were 1) to evaluate the effect of DA on improved Hb and reduced need for transfusion during week 5 to the end of DA therapy, and 2) to identify predictive factors for increases of ≥2.0 g/dL in Hb after 4 weeks of DA therapy. For these endpoints, patients who did not complete the first 4 weeks of treatment or received RBC transfusion during the first 4 weeks were excluded from the analysis. Means (±standard error of the mean, SEM) of Hb concentration were compared via the two-tailed Student's t-test in two ways: between baseline Hb and 4 weeks of treatment, and between baseline Hb and last value during treatment, excluding Hb within 28 days after RBC transfusion. Hb levels were collected at baseline, every 4 weeks, and at the end of DA therapy, and the cumulative incidences (CI) of Hb response were plotted using the Kaplan-Meier method. Potential predictive factors for ≥2.0 g/dL increases in Hb after 4 weeks of DA therapy were assessed using logistic regression. Covariates with P-values less than 0.1 in the univariate analyses were added to the multivariate analysis model.
RESULTS
Patient characteristics
A total of 142 patients were analyzed (Table 1), including 51 men and 91 women with a median age of 65.5 years (range, 37–87). By the International Staging System (ISS), 13.4%, 29.6% and 35.9 had stage I, stage II and stage III disease, while 21.1% had unknown stages at diagnosis. The median time from diagnosis to initiation of DA therapy was 12.4 months (0–136.4). Included were 82 (58%) patients with NDMM and 60 (42%) with relapse and/or refractory multiple myeloma (RRMM). Previous exposure to more than two therapeutic lines was seen in 29% of total patients, with 39 (28%) having received prior ASCT. Hematological evaluation revealed a mean white blood cell count of 4.3×106 cells/mL (range, 0.9–16.4), Hb 8.8 g/dL (range, 6.9–9.9), and platelet count 147.5×106 cells/mL (range, 9–615).
Table 1. Patient characteristics.
a)Induction+ASCT was considered in one therapeutic line.
Abbreviations: ANC, absolute neutrophil count; ASCT, autologous stem cell transplantation; CR, complete response; ECOG, Eastern Cooperative Oncology Group; F, female; Hb, hemoglobin; M, male; MM, multiple myeloma; NA, not available; PD, progressive disease; SD, stable disease; VGPR, very good partial response; WBC, white blood cell.
Effects on hemoglobin
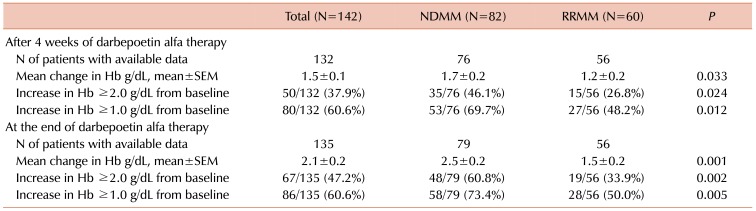
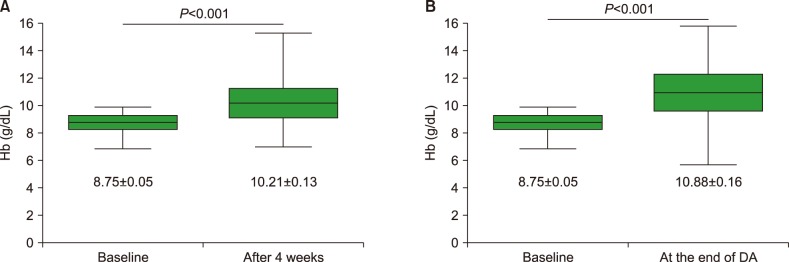
After 4 weeks of DA therapy, among 132 patients with evaluable data, 80 (60.6%) showed increases in Hb ≥1.0 g/dL from baseline, while 50 (37.9%) patients showed increases ≥2.0 g/dL (Table 2). The percentage of patients with increased Hb was similar for subgroups of patients with pretreatment Hb <8.0 g/dL, ≥8.0 to <9.0 g/dL, and ≥9.0 to <10.0 g/dL (Supplementary Table 1). The median duration of DA therapy was 9.0 weeks (range, 4–74). At the end of DA therapy, of 135 patients with evaluable data, increased Hb ≥1.0 g/dL from baseline was observed in 86 (60.6%) patients and ≥2.0 g/dL in 67 (47.2%) (Table 2), with 9.3% of additional patients showing Hb improvement ≥2.0 g/dL. The CIs of Hb response (≥2.0 g/dL) were 35.2±4.0%, 50.1±4.5%, and 57.5±4.6% at 4, 8, and 16 weeks, respectively (Fig. 1). The mean change in Hb during DA therapy is as shown in Fig. 2. DA therapy resulted in increased mean Hb from baseline 8.75 g/dL to 10.21 g/dL 4 weeks later (P<0.001) and to 10.88 g/dL at the end of DA therapy (P<0.001). The increase in Hb with DA therapy was observed in patients with NDMM, with a mean change of 1.7±0.2 g/dL 4 weeks later, and 2.5±0.2 g/dL at the end of therapy. The mean increase in Hb among patients with RRMM was 1.2±0.2 g/dL at 4 weeks and 1.5±0.2 g/dL at the end of therapy. The magnitude of the increase in Hb was larger for patients with NDMM than for those with RRMM.
Table 2. Hemoglobin endpoints.
Abbreviations: Hb, hemoglobin; NDMM, newly-diagnosed multiple myeloma; RRMM, relapsed/refractory multiple myeloma; SEM, standard error of the mean.
Fig. 1. Cumulative incidences of Hb response (≥2.0 g/dL).
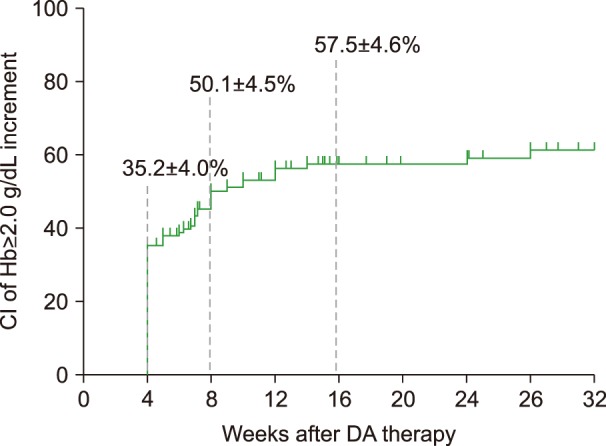
Fig. 2. Mean (SEM) change in hemoglobin during darbepoetin alfa therapy. (A) Mean (SEM) changes in hemoglobin from baseline to 4 weeks and (B) to the end of darbepoetin alfa therapy.
Effects on red blood cell transfusions
The percentages of patients receiving RBC transfusions from week 5 to the end of DA therapy are shown in Table 3. During week 5 to the end of DA therapy, 25% of patients received RBC transfusions with a mean amount of 6.1±0.9 units. A higher percentage of patients with RRMM (22/60, 36.7%) required RBC transfusion from week 5 to the end of DA therapy than patients with NDMM (14/82, 17.1%) (P=0.008).
Table 3. Percentage of patients receiving red blood cell transfusions.
Abbreviations: Hb, hemoglobin; NDMM, newly-diagnosed multiple myeloma; RBC, red blood cell; RRMM, relapsed/refractory multiple myeloma; SEM, standard error of the mean.
Predictive factors affecting increases in hemoglobin concentration
The results of univariate analysis for potential factors affecting increases in Hb ≥2.0 g/dL after 4 weeks of DA therapy are shown in Table 4. In detail, Hb endpoints according to response to chemotherapy are shown in Supplementary Table 2. After adjusting for potential factors affecting increases in Hb, multivariate analyses revealed that ISS stage III disease compared to ISS stage I–II disease [relative risk (RR) of 2.73, P=0.026] and absence of myeloma bone disease at diagnosis (RR of 2.86, P=0.022) were associated with a higher Hb response ≥2.0 g/dL after 4 weeks of DA therapy (Table 4).
Table 4. Univariate and multivariate analyses of variables affecting hemoglobin responsea).
a)Factors affecting increases in Hb ≥2.0 g/dL after 4 weeks of darbepoetin alfa therapy were analyzed using logistic regression.
Abbreviations: ANC, absolute neutrophil count; ASCT, autologous stem cell transplantation; CI, confidential interval; F, female; Hb, hemoglobin; IMiDs, immunomodulatory drugs; M, male; NDMM, newly-diagnosed multiple myeloma; PR, partial response; RR, relative risk; RRMM, relapsed/refractory multiple myeloma; WBC, white blood cell.
DISCUSSION
This study demonstrated that DA at a fixed dose of 120 µg once weekly was an effective and well-tolerated treatment for chemotherapy-induced anemia in patients with MM receiving novel agents. After excluding the patients for whom DA therapy was initiated during the early period of chemotherapy (<1 mo) to distinguish the effect on MM-related anemia and chemotherapy-induced anemia, similar results were observed (Supplementary Table 3). At the end of DA therapy, among 135 evaluable patients, increases in Hb ≥1.0 from baseline were observed in 86 (60.6%) and ≥2.0 g/dL in 67 (47.2%). Patients with ISS stage III disease and without bone lesion seemed to have higher Hb responses to DA treatment. The mean change in Hb observed from baseline to 4 weeks later was 1.46 g/dL (P<0.001) and 2.13 g/dL at the end of DA therapy (P<0.001). These endpoints are commonly used as measurements of efficacy in studies of ESA [10].
Currently, using the single weekly schedule, DA is administered at 2.25 µg/kg (150 mg fixed dose), and increased to 4.50 µg/kg (300 µg fixed dose) if response is not observed at week 4 [11]. In two large studies of MM, patients received DA at 2.25 µg/kg/week (approximately 150 µg/kg/wk) [9,12]. DA at 150 µg, increasing to 300 µg if no response is observed, results in a substantial response in patients with low-risk myelodysplastic syndrome [13,14]. Our study showed improvements in anemia with a relatively low dose of DA for MM patients; this may offer the advantage of less frequent administration.
MM patients, particularly those with chemotherapy-refractory or chemotherapy-resistant disease, often require correction of disease-related or chemotherapy-related anemia. A prospective study conducted among patients with lymphoproliferative malignancies demonstrated a significant reduction in the percentage of patients requiring RBC transfusion from week 5 to the end of the treatment phase, among patients receiving DA (31%) compared with those receiving placebo (48%) (P<0.001) [12]. Consistent with these results, 25% of patients in our study received RBC transfusion during week 5 to the end of DA therapy. DA treatment could reduce the side effects of blood transfusion by significantly reducing RBC transfusions. A significantly higher percentage of patients with RRMM required RBC transfusion than patients with NDMM. This result suggested compromised bone marrow reserves because of extensive prior cytotoxic chemotherapy. Previous studies of ESA using rHuEPO (three times weekly) suggest that Hb response rates may differ between patients with MM and those with other types of lymphoproliferative malignancies [15]. However, a large, randomized, placebo-controlled study conducted among patients with MM did not report higher response rates than expected for other lymphoproliferative malignancies [16]. This overall effect on RBC transfusions was also observed in our study of DA in patients with MM receiving novel agents.
Anemia is a common complication for patients with MM, occurring in more than two-thirds of all patients. In the ECAS study, a significant correlation was found between WHO performance score and mean Hb at enrollment [5]. The MM population is generally old and comorbidity is common, compromising the functional capacity and mental reserves of patients. Whether increased Hb is a goal in itself for these patients remains controversial. Anemia affects virtually all organ systems in the body [3], and it stands to reason that cancer patients would benefit from correcting anemia. QoL has been studied in a large number of studies on ESA treatment for cancer and the results are consistently positive. Improvement in QoL is strongly correlated to increased Hb, a correlation that strongly supports the hypothesis that anemia is an important factor in symptom development and the well-being of cancer patients. We did not investigate objective measures of effects on physical performance, cognitive function, or cardiovascular effects in this study.
Whether measurement of endogenous EPO levels is a useful tool for predicting response in patients with solid cancers or lymphoproliferative disorders is questionable [17,5]. EPO levels are inappropriately low in approximately 25% of all patients with MM, and increases to 50% in patients with stage III disease and 60% in those with renal impairment [18]. For individual patients, a cutoff identified as an optimal group predictor has little value. Our study did not examine baseline serum EPO levels, which have been identified as predictors of increased Hb after DA treatment. Instead, advanced ISS stage and absence of bone lesion may predict Hb response to DA treatment.
Unexplained disease regression in patients with MM and anti- myeloma effects in murine systems have been reported [19]. However, in spite of these reports, meta-analyses failed to show improved survival or tumor control in ESA-treated patients in randomized studies [20], and no single large study had shown a significant survival advantage. No indications of negative effects of ESA have been reported for patients with MM, unlike patients with solid tumors. In this study, it was not clear if DA treatment had anti-myeloma effects. In summary, our results further support other clinical studies that documented the efficacy of DA for increasing Hb and reducing transfusion requirements in anemic patients with MM receiving novel agents.
Footnotes
This study was supported by Kyowa Hakko Kirin Korea Co., Ltd. and the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Korea (A120175).
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
SUPPLEMENTARY MATERIALS
Hemoglobin endpoints according to baseline hemoglobin levels.
Hemoglobin endpoints according to responsea) to chemotherapy.
Hemoglobin endpoints according to duration from diagnosis to initiation of DA treatment.
References
- 1.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 2.Birgegård G, Gascón P, Ludwig H. Evaluation of anaemia in patients with multiple myeloma and lymphoma: findings of the European Cancer Anaemia Survey. Eur J Haematol. 2006;77:378–386. doi: 10.1111/j.1600-0609.2006.00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ludwig H, Strasser K. Symptomatology of anemia. Semin Oncol. 2001;28(2 Suppl 8):7–14. doi: 10.1016/s0093-7754(01)90206-4. [DOI] [PubMed] [Google Scholar]
- 4.Cella D. Factors influencing quality of life in cancer patients: anemia and fatigue. Semin Oncol. 1998;25(3 Suppl 7):43–46. [PubMed] [Google Scholar]
- 5.Ludwig H, Van Belle S, Barrett-Lee P, et al. The European Cancer Anaemia Survey (ECAS): A large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer. 2004;40:2293–2306. doi: 10.1016/j.ejca.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Ludwig H, Miguel JS, Dimopoulos MA, et al. International Myeloma Working Group recommendations for global myeloma care. Leukemia. 2014;28:981–992. doi: 10.1038/leu.2013.293. [DOI] [PubMed] [Google Scholar]
- 7.Lundin AP, Delano BG, Quinn-Cefaro R. Perspectives on the improvement of quality of life with epoetin alfa therapy. Pharmacotherapy. 1990;10:22S–26S. [PubMed] [Google Scholar]
- 8.Scott SD. Dose conversion from recombinant human erythropoietin to darbepoetin alfa: recommendations from clinical studies. Pharmacotherapy. 2002;22:160S–165S. doi: 10.1592/phco.22.14.160s.33398. [DOI] [PubMed] [Google Scholar]
- 9.Hedenus M, Hansen S, Taylor K, et al. Randomized, dose-finding study of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies. Br J Haematol. 2002;119:79–86. doi: 10.1046/j.1365-2141.2002.03774.x. [DOI] [PubMed] [Google Scholar]
- 10.Pirker R, Hedenus M, Vansteenkiste J, Hernandez E, Belton L, Terwey JH. Effectiveness of darbepoetin alfa for chemotherapy-induced anemia when initiated at hemoglobin ≤10 g/dL. Clin Ther. 2016;38:122–135.e6. doi: 10.1016/j.clinthera.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Grossi A, Balestri F, Santini S. Darbepoetin alpha in the treatment of cancer chemotherapy-induced anemia. Ther Clin Risk Manag. 2007;3:269–275. doi: 10.2147/tcrm.2007.3.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedenus M, Adriansson M, San Miguel J, et al. Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: a randomized, double-blind, placebo-controlled study. Br J Haematol. 2003;122:394–403. doi: 10.1046/j.1365-2141.2003.04448.x. [DOI] [PubMed] [Google Scholar]
- 13.Musto P, Lanza F, Balleari E, et al. Darbepoetin alpha for the treatment of anaemia in low-intermediate risk myelodysplastic syndromes. Br J Haematol. 2005;128:204–209. doi: 10.1111/j.1365-2141.2004.05288.x. [DOI] [PubMed] [Google Scholar]
- 14.Patton JF, Sullivan T, Mun Y, Reeves T, Rossi G, Wallace JF. A retrospective cohort study to assess the impact of therapeutic substitution of darbepoetin alfa for epoetin alfa in anemic patients with myelodysplastic syndrome. J Support Oncol. 2005;3:419–426. [PubMed] [Google Scholar]
- 15.Osterborg A, Boogaerts MA, Cimino R, et al. Recombinant human erythropoietin in transfusion-dependent anemic patients with multiple myeloma and non-Hodgkin's lymphoma--a randomized multicenter study. The European Study Group of Erythropoietin (Epoetin Beta) Treatment in Multiple Myeloma and Non-Hodgkin's Lymphoma. Blood. 1996;87:2675–2682. [PubMed] [Google Scholar]
- 16.Dammacco F, Castoldi G, Rödjer S. Efficacy of epoetin alfa in the treatment of anaemia of multiple myeloma. Br J Haematol. 2001;113:172–179. doi: 10.1046/j.1365-2141.2001.02715.x. [DOI] [PubMed] [Google Scholar]
- 17.Littlewood TJ, Zagari M, Pallister C, Perkins A. Baseline and early treatment factors are not clinically useful for predicting individual response to erythropoietin in anemic cancer patients. Oncologist. 2003;8:99–107. doi: 10.1634/theoncologist.8-1-99. [DOI] [PubMed] [Google Scholar]
- 18.Beguin Y, Yerna M, Loo M, Weber M, Fillet G. Erythropoiesis in multiple myeloma: defective red cell production due to inappropriate erythropoietin production. Br J Haematol. 1992;82:648–653. doi: 10.1111/j.1365-2141.1992.tb06939.x. [DOI] [PubMed] [Google Scholar]
- 19.Mittelman M, Neumann D, Peled A, Kanter P, Haran-Ghera N. Erythropoietin induces tumor regression and antitumor immune responses in murine myeloma models. Proc Natl Acad Sci U S A. 2001;98:5181–5186. doi: 10.1073/pnas.081275298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bohlius J, Wilson J, Seidenfeld J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst. 2006;98:708–714. doi: 10.1093/jnci/djj189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hemoglobin endpoints according to baseline hemoglobin levels.
Hemoglobin endpoints according to responsea) to chemotherapy.
Hemoglobin endpoints according to duration from diagnosis to initiation of DA treatment.