Abstract
Allergic rhinitis (AR) is a global health problem that causes major illnesses and disabilities worldwide. Epidemiologic studies have demonstrated that the prevalence of AR has increased progressively over the last few decades in more developed countries and currently affects up to 40% of the population worldwide. Likewise, a rising trend of AR has also been observed over the last 2–3 decades in developing countries including China, with the prevalence of AR varying widely in these countries. A survey of self-reported AR over a 6-year period in the general Chinese adult population reported that the standardized prevalence of adult AR increased from 11.1% in 2005 to 17.6% in 2011. An increasing number of original articles and imporclinical trials on the epidemiology, pathophysiologic mechanisms, diagnosis, management and comorbidities of AR in Chinese subjects have been published in international peer-reviewed journals over the past 2 decades, and substantially added to our understanding of this disease as a global problem. Although guidelines for the diagnosis and treatment of AR in Chinese subjects have also been published, they have not been translated into English and therefore not generally accessible for reference to non-Chinese speaking international medical communities. Moreover, methods for the diagnosis and treatment of AR in China have not been standardized entirely and some patients are still treated according to regional preferences. Thus, the present guidelines have been developed by the Chinese Society of Allergy to be accessible to both national and international medical communities involved in the management of AR patients. These guidelines have been prepared in line with existing international guidelines to provide evidence-based recommendations for the diagnosis and management of AR in China.
Keywords: Allergic rhinitis, China, diagnosis, treatment
1. INTRODUCTION
1.1 Chinese Guideline for allergic rhinitis (AR) workshop
To date, several international guidelines are available for the diagnosis and treatment of AR, in different parts of the world.1,2,3,4,5,6,7,8,9 Of these, 7 have been written by specialist groups from Europe, UK, USA, Canada, Japan and Australia for the management of AR patients from the respective countries, whereas 2 guidelines —Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines and the World Allergy Organization (WAO) White Book on Allergy: Update 2013 (www.worldallergy.org)—have been prepared as evidence-based documents in consultation with experts from all over the world.
Compared to the published international AR guidelines, the criteria for the diagnosis and therapeutic evaluation of AR in China were established by specialist groups and organized by the Editorial Board of the Chinese Journal of Otorhinolaryngology early in 1990. In view of the rapid growth of the prevalence and misconceptions of AR during clinical practice, these guidelines were subsequently updated by Chinese Guideline for AR workshops held in Haikou in 1997, in Lanzhou in 2004, in Wuyishan in 200910 and in Tianjin in 2015.11 Moreover, a clinical practice for children with AR was developed by a group of specialists at a workshop held in Chongqing in 2012.12 However, these Chinese guidelines for AR have been published mostly in Mandarin Chinese in the Chinese Journal of Otorhinolaryngology Head Neck Surgery. In view of the large number of original articles as well as well-controlled clinical trials of Chinese AR patients that have been published in many peer-reviewed international journals by Chinese researchers and authors over the past 2 decades, our knowledge and understanding of the epidemiology, pathophysiologic mechanisms, diagnosis, management and comorbidities of AR have been broadened substantially. Consequently, the Chinese Society of Allergy organized a workshop of the experts working in the different fields of AR management in China to develop the most updated Chinese Guidelines for the diagnosis and treatment of AR, using evidence-based models, as has been the case in the development of the ARIA international guidelines. Importantly, these guidelines have also been developed in English in order that they can be readily accessed for reference by the non-Chinese speaking international fraternity.
1.2 Traditional Chinese Medicine
AR belongs to the category of ‘Bi Qiu’ in Traditional Chinese Medicine (TCM). ‘Bi Qiu’ refers to the disease which is characterised by sudden nasal itching, sneezing, rhinorrhea and nasal blockage. Thus, AR, vasomotor rhinitis and other similar diseases are all included in the ‘Bi Qiu’ category.
In ancient Chinese literature, the word ‘Bi Qiu’ was first found in the book of ‘Huangdi Neijing’ (Western Han Dynasty, 99 B.C.–26 B.C.). ‘Bi Qiu’ had several alternative terms such as ‘Qiu,’ ‘Ti,’ ‘Qiu Bi’ and ‘Runny nose’ and the record of the disease can be dated back to the book of ‘Rites’ (Western Han Dynasty, about 100 B.C.). The article of ‘Yue Ling’ in ‘Rites’ documented that “In the last month of autumn, if the summer practices were observed, there would be great floods in the states. Then the winter stores would be affected and there would be many patients with sneezing and runny nose”; thus indicating that the ancient Chinese people recognized the existence of a close relationship between AR and natural environment/climate events. Indeed, the ancient physicians believed that the main pathophysiology of AR was the dysfunction of ‘Zang-Fu,’ including the lungs, spleen and/or kidneys, in addition to external pathogenic factors like Wind-Evil, Cold-Evil, or other unusual pathogens. Although several ancient TCM literature has discussed AR from different aspects, ‘Su Wen’ in ‘Huangdi Neijing’ suggested that “(kidney) Deficiency would result in dysfunction of 9 orifices, and deficiency in the upper part and excess in the lower part of the body would manifest as runny nose and incessant lacrimation.” ‘Tai Ping Sheng Hui Fang’ (Song Dynasty, 992 A.D.) suggested that “the lung had its specific opening in the nose. When lung Cold affected nose ascending meridian, the runny nose would happen. Thus, the cause of AR was Deficiency, Excess, Cold or Heat, involving the ‘Zang-Fu’, organs of the lungs, spleen and/or kidneys.”
While Western medicine was introduced into China about a century ago and has flourished since then, TCM has existed for almost all of China's 5,000-year history and still plays an important role in the Chinese medical system. TCM employs several treatment approaches in the management of AR including Chinese herbs taken orally or applied externally, acupuncture and the ‘Daoyin (an ancient body-mind exercise aimed at health care as well as physical and spiritual purification)’. Each treatment can be used either alone or in combination, and generally complies with the theory of ‘where there is a syndrome, there is a treatment,’ suggesting that different therapies and measures should be used according to the disease characteristics of the patient. Indeed, to date a large number of clinical studies have confirmed the effectiveness and safety of TCM in the treatment of AR.
1.3 Need for Chinese Guidelines for AR and their update
An ever increasing number of original articles and clinical trials have been published in peer-reviewed journals by Chinese researchers over the past 2 decades. This has substantially broadened our knowledge and database on the epidemiology, mechanisms, diagnoses, managements and comorbidities of AR, especially in Chinese subjects. Thus, in order to disseminate this knowledge and promote further research and clinical practice on the management of AR in China, the executive/organizing committee of the Chinese Society of Allergy decided that it was necessary to extensively review and summarize current literature, using an evidence-based model. Furthermore, it was also necessary to review the characteristics and current practice of clinical diagnosis and treatment of AR in China. Although Chinese guidelines for AR have been in existence since 1991 and subsequently updated several times, none of them have been published in English in any international peer-reviewed journal. Consensus among the Chinese professionals and practitioners indicated that the Chinese guidelines for AR had to be published in English from a Chinese viewpoint on the disease to be communicated to the international professionals and practitioners involved in the treatment of AR.
It is appreciated that since the recommendations in the Chinese guidelines were originally proposed by attendees at a workshop, these guidelines need to be validated and revised by both Chinese and international experts from all over the world. It is anticipated that the Chinese guidelines for AR published in English will serve as a reference for the treatment of AR by physicians, healthcare professionals and organizations involved in the treatment of AR in China and facilitate the development of relevant local standard of care documents for patients. Additionally, the guidelines will be updated every 2 years, adding relevant data and information from newly published papers in peer-reviewed journals and thus developing the guideline into a state-of-the-art document for specialists as well as for the general practitioner and other healthcare professionals. Consequently, it is expected that this document will encourage researchers and professionals to submit and publish their research in relevant peer-reviewed journals as well as update their knowledge of AR. Overall, the aim of this document is to provide an evidence-based document on the diagnosis and management of AR across China, using a stepwise approach in line with other AR treatment guidelines.
2. EPIDEMIOLOGY
2.1 Global prevalence
Epidemiologic studies have revealed that the prevalence of AR has increased progressively in more-developed countries and currently affects up to 40% of the population worldwide.13,14 A high prevalence of AR has also been recorded in the developed nations of the Northern Hemisphere, with 23%–30% of the population affected in Europe15,16 and 12%–30% in the US.17 The great diversity of AR prevalence is found in the non-Western populations of the Southern Hemisphere, with wide inter- and intraregional variations ranging from 2.9% to 54.1% between countries.18 The global rising trend of AR has been observed in the past few decades and the AR prevalence has varied widely particularly in developing nations.2 The increase in AR prevalence has been linked with increased urbanization and improvements in living standards, which have contributed to increased exposure to a variety of indoor and outdoor pollutants and allergens, the potentiating effects of which cannot be ignored on respiratory disorders. Although the prevalence and possible factors responsible for the etiologies of AR have been well documented in many developed countries, there is comparatively little information available for developing countries.19 Large-scale coordinated studies specifically designed to estimate the prevalence of AR in regions with different environmental factors and climates are also required.
2.2 Previous AR prevalence in China
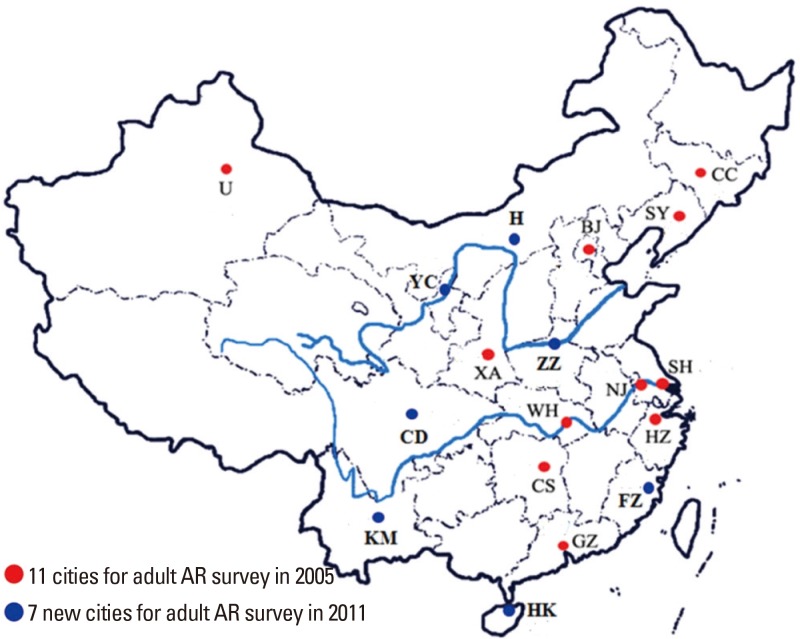
AR is one of the most common allergic disorders globally and affects 10% to 40% of the world's population.7 While the majority of epidemiologic data come from surveys of AR prevalence conducted mainly in Europe and North America, and to a lesser extent in the developed Asian countries, relatively little epidemiologic data are available on AR prevalence in especially adults in China. One nationwide population-based study has assessed self-reported AR using validated questionnaire-based telephone interviews in over 38,000 adult in 11 major cities across China.20 The interviews were conducted from September 2004 to May 2005 and the authors demonstrated that the prevalence of AR was highly variable, ranging from 8.7% in Beijing in North China to 24.1% in Urumqi in Northwest China. Compared to adults, however, more data are available from studies investigating the prevalence of AR in children in China. The majority of such studies have investigated the prevalence of AR in combination with asthma and eczema using the standardized and appropriately translated versions of the International Study of Asthma and Allergies in Childhood (ISAAC) protocols,21,22,23 with only 1 nationwide study reporting the prevalence of specifically AR in children in China.24 In this study, a total of 23,791 children aged 6–13 years in 8 metropolitan capital cities of provinces in 4 regions were surveyed between November and December of 2005, using a cluster-stratified sampling method. The study demonstrated that the mean prevalence of childhood AR was 9.8% and ranged from 3.9% in Xi'an in Central China to 16.8% in Guangzhou in South China. The published data on the prevalence of AR in children and adults in China suggest that industrialization and the gross output of industries in most of the developed cities may reflect the prevalence of AR in certain cities in China. Moreover, these studies are limited due to nonuniform standardized study methods and diagnosis systems, which lead to biased comparisons among the studies.25
2.3 Current AR prevalence and trends
Compared to availability of progressive data for AR prevalence in many countries all over the world, there are insufficient comparable epidemiologic data for AR in China. As one of the largest countries in the world with a population of around 1.3 billion citizens, China has different topographic, climatic and economic conditions, which influence the lifestyle and exposure to allergens in different regions across the country. Thus, while the epidemiologic changes in AR prevalence is not unexpected due to the topographical and climatic conditions, the transition in socioeconomic status of many regions and individuals, particularly as a consequence of rapid urbanization and changes to a Western lifestyle over the past few years, appears to have further influenced the prevalence of AR. The influence of rapid urbanization and changes to a Western lifestyle has often impacted AR prevalence adversely in China as in the developed Western countries. However, a more comprehensive study involving subjects from 18 major cities in China has recently reported that there was an overall increase in the prevalence of self-reported AR in the general Chinese adult population during a 6-year period spanning from 2005 to 2011.26 Compared to the national survey in 2005, the standardized prevalence of adult AR in the 18 major cities was 17.6% in 2011, with the highest prevalence of 23% recorded in Shanghai and the lowest prevalence of 9.8% recorded in Chengdu. These findings suggest that the prevalence of AR in China has not yet reached a plateau (Fig. 1). Other recent studies have focused on the prevalence of AR in almost 800 million people living in the rural areas of China. One study demonstrated that in North China while the prevalence of adult self-reported AR was significantly higher in the rural area than in the urban area (19.1% vs 13.5%), the prevalence of confirmable AR in these areas were 6.2% and 7.2%, respectively.27 For preschool children, the prevalence of clinical AR in Beijing was found to be 19.5% in the urban areas and 10.8% in the rural areas.28 However, these studies indicate that the limited availability of local health services and the unmet need for the diagnosis and therapy of AR in rural areas of China should be given greater consideration in the future. A multicenter investigation has evaluated the clinical features of 11,004 AR patients from 13 allergy centers in Central China.29 The study showed that 9.7% of all patients had intermittent mild AR, 3.1% persistent mild AR, 33.9% intermittent moderate-severe AR, and 53.3% persistent moderate-severe AR. Furthermore, 61.6% and 42.2% of the patients had concomitant ocular and lower respiratory symptoms, respectively.29 Collectively, these studies illustrate that AR is both a common and a growing national concern in China. They further indicate that future epidemiologic studies of AR in China performed at the national level should aim to assess the true prevalence of AR as demonstrate by a clinical diagnosis of AR confirmed by allergen-related examinations and employing standardized methodology across all centers involved in the study.
Fig. 1. Prevalence of adult AR in major cities in China in 2005 and 2011.
2.4 Comorbidities and complications
2.4.1 Bronchial asthma
AR is an independent risk factor for the onset of asthma, and 40% of AR patients have or will have asthma.30 As the upper and lower airway inflammatory responses are similar and interconnected in these individuals, this could be described as “one airway, one disease.” For AR patients, diagnosis for the coexisting asthma should be based on the patient's medical history, symptoms and lung function examination. Indeed, the 2004–2005 survey of AR patients in the 11 major cities in China showed that among all the subjects with self-reported AR, an average of 9.2% suffered from asthma31: Beijing (12.7%), Changchun (8.3%), Changsha (7.5%), Guangzhou (5.4%), Hangzhou (13.1%), Nanjing (9.2%), Shanghai (9.3%), Shenyang (8.8%), Urumqi (6.3%), Wuhan (4.3%) and Xi'an (9.6%). Similarly, in 2011, a survey consisting of total 47, 216 telephone interviews showed that the prevalence of asthma in the AR subpopulation was 28% (23).
2.4.2 Allergic conjunctivitis
Itchy/watery eyes, redness and other eye symptoms are the main symptom of AR patients with allergic conjunctivitis, especially seasonal AR patients, whose incidence could be as high as 85%.32 The AR survey during the year 2005-2011 showed that the incidence of eye symptoms in AR patients was 32%–59% based on medical history and clinical manifestation.26 It is not difficult to diagnose allergic conjunctivitis, but differential diagnosis for other common conjunctival lesions should be noticed.
2.4.3 Chronic rhinosinusitis
Allergic inflammation is a major factor related to chronic rhinosinusitis (CRS).33 The cross-sectional survey of 7 cities in China recently showed the prevalence of CRS ranging from 4.8% to 9.7%.34 Moreover, the prevalence of CRS was found to be 30% in AR patients and 23% in asthmatic patients, compared to just 6% and 7%, respectively, in subjects without AR or asthma. Similarly, larger surveys of subjects with self-reported AR from 11 and 18 major cities across China have demonstrated 13.3%31 and 10.1%, respectively, of the AR patients26 to have CRS. In another study, among all the 1,411 participants over 15 years old, 118 (8.4%) had self-reported CRS; patients with CRS had an increased prevalence of AR and chronic obstructive pulmonary disease compared to those without.35 Furthermore, the quality of life was significantly impaired in patients with CRS than in those without, with the quality of sleep being markedly impaired in CRS patients. Although this study did not assess the correlation between CRS severity and impairment of sleep, it is possible that the impairment in CRS patients with AR may indeed be correlated with the severity of CRS as shown in patients with AR.
2.4.4 Upper airway cough syndrome
AR and sinusitis are a common cause of chronic cough in children and adults.12,36 Nasal secretions reflux from the nose and the throat directly or indirectly stimulate cough. Cough resulting from chronic sinusitis may thus be the main clinical manifestation of upper airway cough syndrome (UACS). A pilot study of 393 children with cough as a chief complaint in Chengdu has recently shown that 45.8% of the children suffered from AR. Similarly, a multicenter survey investigating causes of chronic cough in China found that UACS was most frequently associated with AR (63.4%).37
2.4.5 Otitis media
Secretory otitis media (SOM) is a nonsuppurative inflammatory disease. Middle ear effusion—which includes serous fluid and pulp-like mucus—and hearing loss are the main features, and AR is regarded as one of the possible risk factors inducing SOM in children.12 Indeed, one study from the UK found that the prevalence of AR in patients with chronic or recurrent OME ranged from 24% to 89%.38 Similarly, a study from Qingdao city in China has indicated that children with SOM have increased annual frequency of AR.
3. MAJOR ALLERGENS IN CHINA
3.1 China in general
Exposure to inhalant allergens is the primary inducer of AR symptoms; with particularly the aeroallergens, which include both outdoor and indoor allergens, being the most common allergens. Outdoor allergens, which mainly include pollen and fungi, are positively associated with the development of seasonal/intermittent AR, whereas indoor allergens, which typically include mites, animal dander, cockroach and fungi, are the major cause of perennial/persistent AR. Although exposure to certain occupational allergens may also lead to AR, exposure to food allergens rarely causes isolated nasal allergy symptoms.7,11 Due to the effect of geographic, climatic and humanistic factors, the types of allergens inducing AR vary significantly among regions. Identifying major local allergens is thus the first step to AR management involving diagnosis, prevention and allergen-specific immunotherapy (AIT).
In 1964, Voorhorst39 discovered that the allergenic properties of house dust originated from the component of mites. The first mite allergen was isolated by Fain in 1966 from the genus Dermatophagoides pteronyssinus (Der p), and since then more species of mites have been discovered.40,41 In China, Chan and colleagues42 have contributed greatly to the identification of diverse Dermatophagoides farina (Der f) allergens by proteomics. The novel allergens from Der f such as Der f 25 (triosephosphate isomerase), Der f 26 (myosin alkali light chain), Der f 27 (serpin), Der f 28 (heat shock protein), Der f 29 (cyclophilin), Der f 30 (ferritin), Der f 31 (cofilin), Der f 32 (pyrophosphate) and Der f 33 (alpha-tubulin) have greatly extended the spectrum of dust mite allergens,43 and the findings from Liu and colleagues61 could be of benefit for the guidance on more effective diagnosis and AIT of HDM respiratory allergy in China.
Pollen is a common aeroallergen worldwide. Ragweed allergen was described by Carl Linnaeus in the 18th century,44 but since then more highly allergenic pollen inducing seasonal allergic symptoms in respiratory tract have been discovered all over the world including China. In the 1950s, it was first reported that the genus Artemisia was the most important source of allergenic pollen in North China.45 Many new pollen allergens have subsequently been described in China. Indeed, during the mid-1980s to early 1990s, nearly 80 provincial- and municipal-level hospitals participated in a national epidemic survey on anemophilous allergenic pollen, resulting in the publication of a book entitled “A National Survey of Airborne and Allergenic Pollen in China” in 1991. This book summarizes the geographical distribution and drift patterns of airborne allergenic pollen by regions in mainland China and is designed for reference by clinicians involved in the treatment of allergic disease. It is worth noting that increasing urbanization and alien plant invasion have led to emergence of different trends in diffusion of pollen allergen.
3.2 Current data and trends
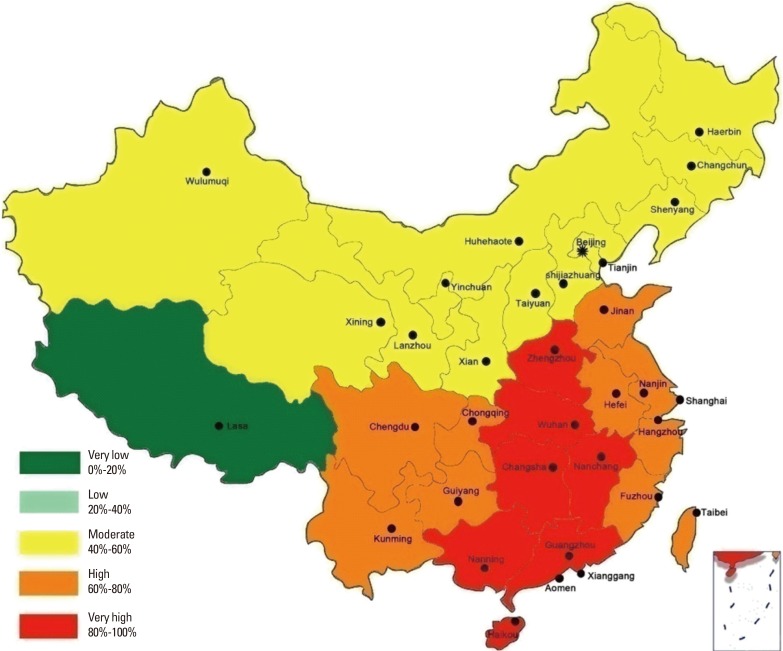
Zhang and colleagues25 reviewed the pattern of sensitization to inhalant allergens among AR patients in mainland China and found that the prevalence and type of aeroallergens were different among various cities and regions. A survey by Li and colleagues46 of 6,304 patients suffering from asthma and/or rhinitis in 17 cities from 4 regions of China showed that the overall prevalence of positive skin prick responses was highest for Der f (59.0%), Der p (57.6%), and Blomia tropicalis (40.7%), and lowest for mixed mould IV (4.4%), mixed grass pollen (3.5%), and mixed tree pollen (2.2%). The prevalence of sensitization to other allergens ranged from 16.1% for American cockroach, 14.0% for dog, 11.5% for Blatella germanica, 11.3% for Artemisia vulgaris, 10.3% for cat, 6.5% for Ambrosia artemisifolia, and 6.3% for mixed mould I.46 Moreover, this study showed that the prevalence of sensitization to allergens were different between adults and children, with Der p and Der f reported as the predominant aeroallergens in perennial/persistent AR individuals in China. The prevalence of positive skin prick test results to Der p in Qingdao, Zhengzhou, Xiamen, and Guangzhou have been reported to be 69.6% (66.4%), 86.32% (87.54%), 76.56% (77.16%), and 72.84% (76.36%), respectively.47 We still reviewed 146 published reports documenting the prevalence of sensitization to Der p and Der f among 89,779 AR patients from 7 major regions across China, and drafted a nationwide epidemiologic map to better represent the patterns of sensitization to Der p and Der f in these regions (Fig. 2). This map indicated that overall sensitization to the 2 allergens is fairly similar, although the order of regional distribution for positive sensitization rates was South> Central> East> Southwest> Northwest> Northeast> North. These data suggested an obvious geographic difference of the prevalence of sensitization to dust mites, demonstrating a trend of decrease from south and east to north and west in China. It is likely that the complicated geographic environment, climate, human activity, and air pollution contribute to these regional differences in the pattern of allergen sensitization. Nevertheless, an overall upward trend in the prevalence of sensitization to dust mites in China has been observed in recent decades, and this may be related to the rapid change towards a “Western lifestyle.”
Fig. 2. The prevalence of sensitization to dust mites in China.
Airborne pollen is the most frequent and seasonal cause of AR in the western and northern regions of China. The existence of a considerable regional difference in the distribution of pollen species and counts is due to the geographic and vegetation differences in China; thus Artemisia pollen is the most common allergenic one in the northern part of the Yangtze River (Beijing, Xinjiang, Shanxi, Shandong, Shenyang, Lanzhou, and Ningxia) in China. Tables 1, 2 show the geographic distribution of tree, grass, and atrazine pollen in different regions in China.48,49 Thus, availability of this information and establishment of national real-time monitoring of atmospheric pollen may make the prevention and treatment of AR patients with pollen allergy and seasonal migration possible.
Table 1. The main airborne tree pollens in different regions of China.
| Region | Tree pollen genus |
|---|---|
| Northeast China | Poplus, Ulmus, Pinus, Salix, Birch, Acer, Quercus |
| North China | Poplus, Platane, Pinus, Salix, Fraxinus, Birch, Ailantus |
| Northwest China | Poplus, Ulmus, Salix, Acer, Cupressaceae, Platane, Corylus, Fraxinus |
| East China | Platane, Pinus, Cupressaceae, Broussonetia, Pterocarya Kunth, Ulmus, Salix, Poplus |
| Central China | Platane, Cupressaceae, Pinus, Broussonetia, Pterocarya Kunth, Quercus, Ligustrum, Morus |
| Southwest China | Salix, Pinus, Alder, Cupressaceae, Broussonetia, Poplus, Firmiana Marsili, Cryptomeria |
| South China | Pinus, Broussonetia, Eucalyptus, Cupressaceae, Casuarina, Morus, Juglans L, Palmae |
Table 2. The main grass and atrazine pollens in different regions of China.
| Region | Grass and atrazine pollen genus |
|---|---|
| Northeast China | Artemisia Annual, Humulusl, Gramineae, Ambrosia, Chenopodiuml, Cyperaceae |
| North China | Artemisia Annual, Humulusl, Gramineae, Chenopodiuml, Amaranthaceae, Ambrosia |
| Northwest China | Artemisia Annual, Chenopodiuml, Humulusl, Gramineae, Helianthus, Amaranthaceae |
| East China | Artemisia Annual, Gramineae, Humulusl, Ambrosia, Chenopodiuml, Amaranthaceae |
| Central China | Artemisia Annual, Gramineae, Humulusl, Ambrosia, Chenopodiuml, Amaranthaceae |
| South China | Gramineae, Artemisia Annual, Chenopodiuml, Humulusl, Amaranthaceae, Ricinus |
| Southwest China | Artemisia Annual, Gramineae, Chenopodiuml, Humulusl, Helianthus, Ricinus |
With improvements in living standards, pet ownership has become more prevalent in China. The number of domestic pets in China has increased 9-fold in 2013 compared to 2003. One survey showed that the positive serum sIgE rates of cat and dog allergens in AR child patients in Shanghai were 6.9% and 28.2%, respectively.50 Wang and colleagues51 conducted a 10-year retrospective study to investigate the trends in the prevalence of sensitization to common aeroallergens among AR patients in Guangzhou, the largest city in South China. The authors showed that the prevalence of sensitization to cat hair and dog dander had increased nearly 2-fold during the past decade, suggesting the importance of controlling the pet ownership and introducing SIT for pet allergy.51
4. BURDEN OF AR IN CHINA
4.1 Health economics
The direct and indirect costs associated with the management of AR are a huge burden on the society.52 Data from the National Bureau of Statistics of China (http://www.stats.gov.cn/) indicate that there were 1.37 billion people in China at the end of 2014. A recent report showed that the standardized prevalence of self-reported AR is 17.6% in 18 major cities of China, and the prevalence of self-reported asthma 28% in the AR population.26 From these data, it is estimated that 0.24 billion people could be affected by AR and of these 67.51 million people could have AR combined with asthma (ARS).
Although there is no report of the direct cost of AR in China to date, a study by Chen and colleagues53 estimated that the direct cost of an ARS patient receiving subcutaneous immunotherapy (SCIT) or a specific medical treatment in Wuhan, China in 2013 was $982 and $259 per year, respectively. Since the income and economy of Wuhan represent the average levels of China, using the data of Chen and colleagues53 it is possible to estimate that the total societal cost in China could be $17.49 billion per year for all ARS patients if they received only medicinal therapy and no immunotherapy.
Furthermore, as the average disposable income of Wuhan was $4,451 per person in 2013 (http://www.whtj.gov.cn/), it can be estimated that as the cost of SCIT is not included in most medical insurance, the patients would have to spend 22% of their disposable income for their treatment involving SCIT.
In addition to the direct costs, there are considerable indirect costs such as decreased work productivity, workdays (adults) or school days (children) absent due to illness.54 Expenses managing the comorbidities of AR such as sinusitis, bronchitis and otitis media should also be considered “hidden” costs of AR.55
4.2 Effects of AR on life quality
AR is an important and serious public health problem not just because of its high prevalence but also because it adversely impacts patients' quality of life (QOL) with respect to work productivity, school performance, social life, and mental and psychologic states. The disease burden comes from the morbidity of nasal symptoms, numerous comorbidities, and the impairment of multiple domains of QOL. Patients report that the disorder has a marked detrimental effect on their sleep, social life, and attendance and functioning at school and work,56 and patients also experience other psychologic symptoms that include fatigue, mood changes, anxiety, and depression.57,58 Yin and colleagues59 found that symptoms of AR could cause great discomfort in the patient's daily functioning, including playing a satisfactory role in family, and professional and social life. Furthermore, nasal symptoms were significantly associated with anxiety, and emotion and behavior problems. A previous study on QOL of AR patients, using several instruments including the Medical Outcomes Study Short-Form 36-Item Health Survey (SF-36), Eysenck personality questionnaire (EPQ), self-rating anxiety scale (SAS) and self-rating depression scale (SDS), showed that the patients' health status declined in all domains, especially in general health perceptions, physical role functioning, and emotional role functioning.60 Furthermore, although the patients had no obvious differences in personality characteristics, the tendency to develop anxiety, but not depressive emotion, was observed. A study by Liu and colleagues61 using the Chinese version of SF-36 has also demonstrated pronounced decrements in general health, role-emotional, and role-physical dimensions in mild AR patients, whereas significant impairments were noted in all domains in patients with moderate to severe AR. In the latter group, the impairments were most pronounced in general health, role-emotional and social function domains. Similarly, in a more recent prospective cohort study of patients with moderate/severe AR, using visual analogue scale (VAS) and AR Control Test (ARCT) demonstrated impairments in sleep in 86.9%, work life in 84.9%, social activities in 81%, and physical activities in 90.1% of the patients.62 Indeed, nasal symptoms including stuffy/blocked nose, runny nose, sneezing and post nasal drip, as well as the consequential practical problems, including inconvenience of having to carry tissues or handkerchief, need to rub nose/eyes, and need to blow the nose repeatedly, have been shown to be the most troublesome aspects of AR.63 Li and colleagues64 employed the rhinoconjunctivitis quality of life questionnaire (RQLQ) to assess the QOL in AR patients according to the sensitization profile for relevant aeroallergens in North China and showed that this was worse in patients sensitized to tree pollens or weed pollens than in those sensitized to HDMs. Although QOL of the patients was not significantly correlated with the level of specific IgE to the causative allergen, the QOL varied with the allergen responsible for symptoms.64 A study using Symptom Checklist-90 (SCL-90) has shown the SCL-90 scores to be significantly higher for the obsessive-compulsive, hostility, somatisation, and psychoticism dimensions in AR patients than in healthy controls.65 This study further indicated that the psychologic status of AR patients worsens with comorbid asthma. However, the effect of gender is somewhat unclear. A study by Xi and colleagues65 did not demonstrated effect of gender on SCL-90 scores of AR patients, whereas a study by Lv and colleagues66 demonstrated poorer psychologic functioning in female patients with moderate-to-severe persistent AR than in nonallergic women. VAS and RQLQ have also been employed to assess symptom severity and QOL, respectively, in Chinese children with AR.67 As for the adults, nasal symptoms were the most impairing aspect of QOL, with nasal itching and sneezing the main factors affecting the quality of sleep. While the quality of sleep may affect non-hay fever symptoms and emotions, rhinorrhea appeared to be the main factor causing embarrassment to the child. VAS was significantly correlated with RQLQ, and skin prick tests (SPTs) results correlated with both VAS and RQLQ, suggesting a close relationship between the allergen level and symptom severity/QOL.67 Song and colleagues58 investigated the effect of AR in 814 middle school students aged 10 to17 years enrolled from 4 schools in Changsha city, using VAS, to assess the effect of AR on sleep, emotion, and memory of these students. The rates of students reporting a moderate-to-severe impact of AR symptoms on sleep, emotion, and memory were 47.14%, 14.29%, and 27.14%, respectively, compared to 21.96%, 6.83%, and 11.28%, respectively, for children without AR. The authors suggested that AR significantly decreased the sleep quality and memory of these students, while emotional issues were increased with the onset of AR.58 AR has also been shown to impact on the sleep and attention in children and to decrease the QOL of children.68 Similar to these findings in Chinese AR patients, the symptoms of AR have also been shown to impair the QOL of patients from diverse regions of the world by adversely impacting on sleep, daily activities, physical and mental status, and social functioning.69 Poor sleep leads to fatigue and daytime somnolence, resulting in decreased performance, productivity, and social functioning as well as increased risk of associated diseases. A study from Europe has recently suggested that the severity of AR adversely impacts on patients' QOL to a greater degree than the duration of disease.70 Effects of gender, marital status, residential area, and duration of symptoms have also been shown to significantly impact on the patient's well-being.71 QOL of children with AR has been shown to be severely compromised due to frequent night awakenings, easy fatigue, defects of language, and irritability, which all have a negative influence on learning abilities. Indeed, AR may negatively impact on the QOL of the whole family because it could interfere with social life and financial costs.72
4.3 Psychologic impact
Although AR is not life-threatening, the serious negative influence of the disease on the patient's quality of life and psychologic status has received more attention in recent years. Several studies have investigated the influence of psychosocial factors on atopic disorders and the effect of atopic disorders on mental health, demonstrating that there is a significant bidirectional relationship between psychosocial factors and future atopic disorders as well as between atopic disorders and future poor mental health.73 Cuffel and colleagues74 carried out a questionnaire survey of 85,298 people and reported that the incidences of anxiety and depression in AR patients were 1.41 and 1.7 times, respectively, that of the general population. Several studies have shown an association between the risk of suicide during the hay fever season and seasonal pollen counts.75,76 Sansone and colleagues76 reviewed the studies investigating the relationships between allergies and anxiety/mood syndrome and found that the majority of studies (9 of 11 studies on anxiety syndromes, and 10 of 12 studies on depressive syndromes) indicated associations between allergies and anxiety/mood syndromes. One population-based study has suggested that AR may even be a risk factor for suicide.77 Similarly, some studies have investigated the psychologic effects of AR in Chinese subjects. Xi and colleagues65 used the SCL-90 to study psychologic characteristics between AR patients and nonallergic individuals and demonstrated that there were significant differences between the 2 groups; with the SCL-90 scores for somatization, compulsion, interpersonal sensitivity, hostility, and psychosis being higher in the AR patients. In another study, Lv and colleagues61 assessed the psychologic aspects of Chinese female outpatients with moderate-to-severe persistent AR and nonpsychometric adult females, using the Minnesota Multiphasic Personality Inventory (MMPI). The authors concluded that women with AR have poor psychologic functioning as indicated by poorer MMPI scores for hypochondriasis, depression, hysteria, psych asthenia, schizophrenia and social introversion. Moreover, the women with AR felt depressed and unhappy, and were more likely to be pessimistic about the future. Some exhibited apprehensive behavior, and even anger and resentment because they had likely experienced many hours in hospital and felt misunderstood by physicians as well as by family members or others, and had a greater tendency to be alone. Another study by these authors has indicated that the psychologic status of seasonal AR patients was likely to be influenced markedly by the symptoms of AR, such as nasal obstruction and nasal itching.78 Collectively, these findings suggest that allergists should target both allergic diseases and subsequent psychologic disorders as a whole, rather than treat them as separate disease entities.
5. DEFINITION AND CLASSIFICATION
5.1 AR
AR is a symptomatic disorder of the nose, which is defined as an infectious inflammation associated with IgE-mediated inflammatory response to allergens. The symptoms of AR includes paroxysmal sneezing, rhinorrhea, nasal congestion and itchin.4,7,8,11,79
The classification of AR in China has been adjusted continually following a long period of research and discussion. According to the original classification in 1997, AR was divided into 2 categories: perennial (the onset of symptoms is all year round and symptoms last for at least 6 months per year) and seasonal (the onset of symptom is seasonal). In order to adapt to the situation in China, the classification was subsequently modified into 4 categories by combining the traditional classification with the classified standard recommended by ARIA in 2004: seasonal intermittent, seasonal persistent, perennial intermittent, and perennial persistent.80 Furthermore, the severity of AR was classified as mild (the symptoms are not interfering with sleep, daily activities, physical exercise, entertainment, work and study) and moderate-severe (the symptoms are disturbing and severely affected patients' life mentioned above) in accordance with the ARIA classification. Thus, the classification was modified to being intermittent (<4 days/week or <4 weeks/year) or persistent (≥4 days/week and ≥4 weeks/year) by the frequency of symptoms and to being mild (the symptoms are not interfering with quality of the patient's life) or moderate-severe (the symptoms cause severe impairments in quality of life of patients) according to the severity of symptoms.10 More recently, the classification of AR has been further modified by the addition of the type of allergen in 2016.11
Taking the Chinese patients' situation and international common classification into consideration, AR can thus be classified in 3 ways as shown in Table 3.
Table 3. Classification of AR.
| Type of allergen |
| i. Seasonal AR |
| ii. Perennial AR |
| Frequency of symptoms |
| i. Intermittent AR (<4 days/week or <4 weeks/year) |
| ii. Persistent AR (≥4 days/ week and ≥4 weeks/year) |
| Severity of symptoms |
| i. Mild AR |
| ii. Moderate-severe AR |
-
1) The type of allergen
- a) SAR: the onset of symptoms is seasonal. Aeroallergens (pollen and fungi) are the most common allergens.
- b) Perennial AR: the onset of symptoms is year-round. The allergens include dust mites, animal dander, tree pollen, etc.
-
2) The frequency of symptoms4,10,11,80
- a) Intermittent AR: <4 days/week or <4 weeks/year
- b) Persistent AR: ≥4 days/week and ≥4 weeks/year
-
3) The severity of symptoms4,10,11,80
- a) Mild AR: the symptoms are not interfering with quality of the patient's lifestyle including daily life, work, study, etc.
- b) Moderate-severe AR: the symptoms cause severe trouble in quality of the patient' life.
As the duration of aeroallergen pollen season depends on climatic conditions and geographic location, in some areas where the aeroallergen pollen season is year-round, this causes difficulty in classifying AR by the type of allergen. Thus, in such areas AR is divided into the seasonal, perennial or the mixed (perennial with seasonal exacerbations) types.79
The classification of AR by the frequency of symptoms also has some limitations.4 It is particularly difficult to sort out patients with perennial symptoms, but for less than 4 days/week (more like “persistent”), into “intermittent” AR type.
The appropriate classification of AR should thus take into consideration different geographic conditions and patients' situations, and needs to be continuously improved accordingly.
5.2 Local AR
Local AR (LAR) is a newly described form of AR. LAR patients have typical clinical symptoms of AR, but without classic systemic atopy.81 In LAR patients, nasal symptoms, local sIgE production, and type 2 response-dominated inflammation in nasal mucosa can be induced during natural exposure to aeroallergens or by nasal provocation test (NPT).82
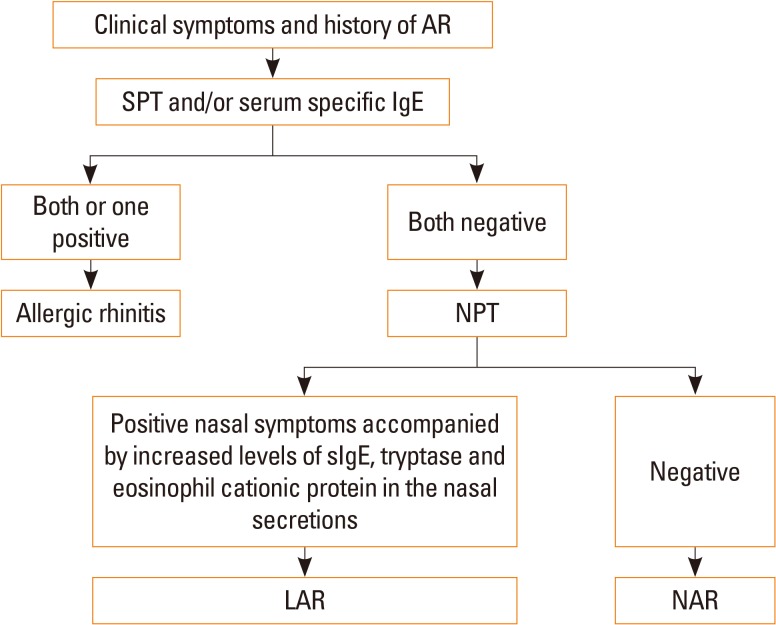
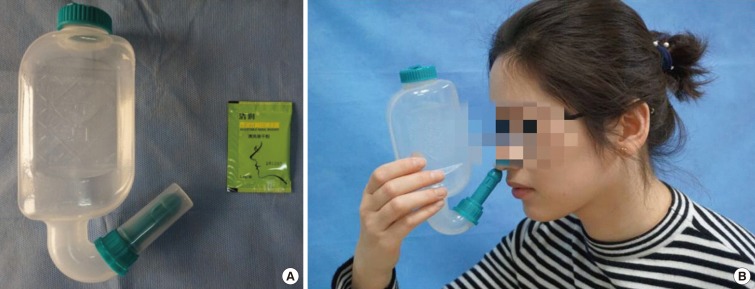
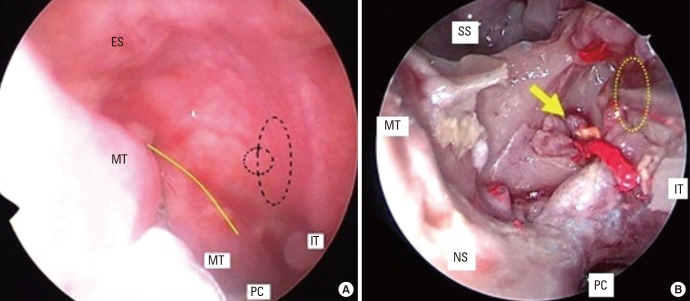
LAR affects about 47% of patients previously diagnosed as non-AR.81 Key features for the differential diagnosis of LAR and AR are shown in Table 4. LAR shares similar clinical symptoms, including rhinorrhea, nasal obstruction and itching, sneezing, and associated ocular symptoms, with AR; however, SPT and serum sIgE are negative for LAR patients. On the contrary, similar to AR patients, NPT is able to induce positive immediate, late, and dual nasal symptoms accompanied by increased levels of sIgE, tryptase, and eosinophil cationic protein (ECP) in the nasal secretions of LAR patients.83 NPT can be evaluated by assessing the change in nasal volume (NV) using acoustic rhinometry, and nasal symptoms can be score using a VAS system. A 30% increase in the total VAS plus a 30% decrease in the volume of nasal cavity from 2 to 6 cm (NV 2–6 cm) is considered a positive response.84 The release of ECP and tryptase is significantly up-regulated in the nasal secretion as early as 15 minutes after challenge and can last for 24 hours with a gradual increase. In some patients, sIgE can be detected in nasal secretion at baseline, and local sIgE levels can be further rapidly increased after NPT.85 Due to the lack of standardized provocation reagents in China and the potential side effects of NPT, NPT is only carried out in a laboratory, and seldom in the clinic. Thus, the prevalence of LAR in China is not clear and this disease entity has not been widely recognized by Chinese physicians. Patients with LAR may present persistent or intermittent symptoms, with severity classified as mild, moderate or severe, similar to AR patients.81 Indeed, a prospective follow-up study has recently shown that only a small number of individuals with LAR may evolve to typical AR with systemic atopy, suggesting that LAR is more likely a distinct entity.86
Table 4. Diagnosis of AR and LAR.
| AR | LAR | |
|---|---|---|
| Symptoms | Rhinorrhea, nasal obstruction, nasal itching, sneezing with or without ocular symptoms | Rhinorrhea, nasal obstruction, nasal itching, sneezing with or without ocular symptoms |
| Disease duration | Persistent or intermittent | Persistent or intermittent |
| Laboratory test | SPT and/or serum sIgE antibody positive | SPT and serum sIgE antibody negative |
| Aeroallergen nasal provocation test | Positive | Positive |
A diagnostic flowchart for LAR is summarized in Fig. 3.
Fig. 3. Diagnostic flowchart for LAR. AR, allergic rhinitis; SPT, skin prick test; IgE, immunoglobulin E; NPT, nasal provocation test; LAR, local allergic rhinitis; NAR, non-allergic rhinitis; sIgE, serum-specific IgE.
6. MECHANISMS
6.1 Genetic factors
6.1.1 Genetics
AR, like other allergic diseases, is an inflammatory disease with a complex genetic component in the etiology of AR. Based on the European studies of twins, it was estimated that AR exhibited a heritability ranging between 33% and 91%.87,88 In China, a genetic epidemiologic study involving 23,825 families from Jiangsu province reported that the average AR heritability of the first, the second, and third generations was 81.86%.89 Earlier studies using genome-wide linkage scans for AR in affected-sib-pair families from European and Japanese populations demonstrated that the chromosomes 1p31, 2q32, 3p24-p14, 4q32.2, and 9q22-q34 were likely to contain the candidate gene loci associated with the development of AR.90,91,92
With the rapid development of genotyping techniques, large population-based association strategies have been carried out more widely to investigate specific susceptibility genes for AR. Because of the important role in antigen presentation, the human leukocyte antigen (HLA) is known to be an important genetic susceptibility locus for a variety of allergic diseases. Moreover, several HLA alleles have been shown to be associated with AR in different ethnic groups. In an earlier study, Lin and colleagues93 first investigated the association between several HLA alleles (HLA-B27, RR of A31, A28, B12, and A33) and the genetic susceptibility of AR in a Chinese population. Subsequently, several studies have been performed in different ethnic groups from different parts of China (northeastern area,94 Beijing,95,96,97 Xinjiang98) and demonstrated a strong association between HLA class II alleles (DR and DQ) and AR. However, these studies have been limited by the complicated nature of the genotyping procedures employed and the small sample sizes (<100 AR patients) investigated. More recently, Zhao and colleagues99 have employed polymerase chain reaction sequence-based typing (PCR-SBT), a form of higher resolution HLA typing, to assess the HLA-II gene alleles associated with AR in HDM-sensitive Han Chinese subjects and control subjects. The authors reported that HLA-DQB1*06:01:01 and HLA-DRB1*08:03:02 were significantly increased in HDM-sensitive AR patients compared to healthy controls, suggesting that these alleles may confer a risk of AR in Han Chinese subjects sensitized to HDM.
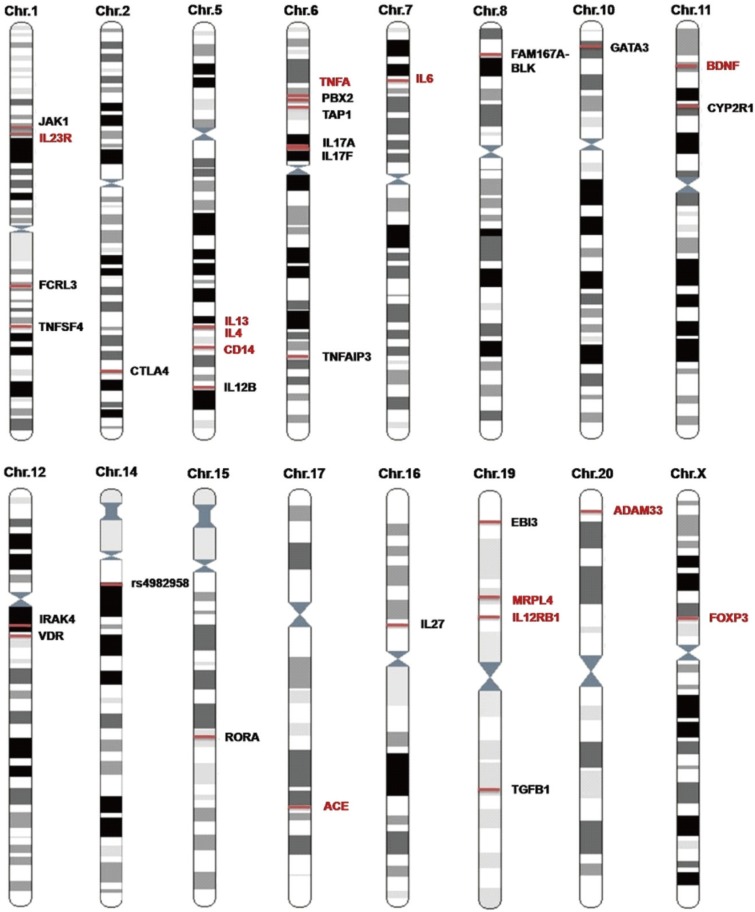
Candidate gene studies have also implicated a number of several other susceptibility genes related to AR in Chinese subpopulations (Fig. 4). These include cytokines (IL13,100,101 IL4,102 IL12B,103 IL17A,103,104 17F,102 IL6,105 IL27106) cytokine receptors (IL23R,107 IL12RB1,108 and EBI3109) immunity pathway molecules (JAK1,110 FCRL3,111 TNFSF4,112 CTLA4,113 CD14,114 TNFA,115 PBX2,116 TAP1,117 TNFAIP3,118 FAM167A-BLK,112 GATA3,119 IRAK4,120 RORA,103 TGFB1,121 and FOXP3109), allergic airway inflammation and airway remodelling molecular genes (BDNF,122 CYP2R1,123 VDR,123 ACE,124,125,126 MRPL4,115 ADAM33127,128), and others (rs4982958 at 14q11.2).129 Fig. 4 shows that Chinese subpopulations and other ethnic groups, including white European, Koreans and Japanese, share a large number of these genetic susceptibility loci.
Fig. 4. Susceptibility loci of AR in Chinese population studies. Some loci were reported only in Chinese populations (loci shown in black) or in both Chinese populations and other ethnic populations (loci shown in red).
Loci on the chromosome 5q31-33 have been shown to be hotspots for AR susceptibility. These loci contain a cluster of cytokines and immune-related genes such as IL-13, IL-4, CD14, and IL-12B. Ying and colleagues101 conducted a meta-analysis involving Asian (China, Japan, and Korea) and Caucasian (Spain Germany and UK) subjects, and found that the functional single nucleotide polymorphism (SNP) rs20541 in the IL-13 gene was significantly associated with AR, particularly in Asians. However, inconsistent data from other studies suggest that rs20541 may explain little of the heritability of AR.130 Recently, Li and colleagues131 found that the DNA hypomethylation status of specific CpG islands located ~2 kb upstream of the IL-13 gene may be an independent risk factor for HDM-sensitive AR. Likewise, IL-4, another important Th2 cytokine gene, has multiple polymorphisms, including rs2243250, which regulates IL-4 gene expression and has been reported to be associated with AR in many ethnic groups (Chinese, Caucasian, and others).130 The best gene-environment interaction presented in allergy to date should be SNPs in the CD14 gene, which plays a critical role in the innate immune response to microbial invasion. However, the association between CD14 genotypes and AR was found to be controversial in different studies in the Chinese population.114 Except for HLA loci, chromosome 6 has several susceptible loci (TNFA, PBX, TAP1, IL17A, IL17F, and TNFAIP3) for AR. The study of Zhang and colleagues115 demonstrated a genotype-dependant association pattern with regard to the SNP rs1799964 in the TNFA gene vs AR development in a Chinese population.
Some allergic airway inflammation and airway remodelling molecular genes have been shown to be as important as immune-related genes for AR susceptibility. Jin and colleagues,122 have identified an association of a common functional SNP rs6265 in the brain-derived neurotrophic factor (BDNF) gene with AR risk and disease severity in 2 independent populations of Chinese patients with moderate-to-severe AR. A series of studies have focused on the correlation of the polymorphism based on the presence (insertion/deletion) of a non-sense DNA fragment in the angiotensin-converting enzyme (ACE) gene, exerting an anti-inflammatory effect by inactivating different proinflammatory peptides with and AR risk in different populations (also including Chinese populations).124,125,126 Similarly, the association of T1, T2, V4, and Q-1 polymorphisms in Disintegrin with the metalloproteinase 33 (ADAM33) gene encoded for a protein important for airway remodelling and AR have also been investigated in some Chinese studies.127,128
To date, 3 genome-wide association studies (GWAS) have been performed specifically for the AR phenotype. Andiappan and colleagues132 first employed GWAS strategy in a cohort of 4,461 ethnic Chinese individuals in Singapore and demonstrated that SNPs in mitochondrial ribosomal protein L4 (MRPL4) and B-cell adaptor for phosphatidylinositol 3-kinase (BCAP) were suggestively associated with AR. A recent study in a Han Chinese population demonstrated that SNPs in the MRPL4 was strongly associated with the risk of AR.115 However, other association signals have not yet replicated in other populations.
Despite the overlapping genetic susceptibility to AR in the Chinese population and other ethnic populations, genetic heterogeneity also plays an important role in explaining the apparent discrepancy in the genetic studies between races.
Although remarkable progress has been made in the genetics of AR and allergy, several limitations of these studies remain to be overcome; in particular, the confounding effects of endophenotyping, sample size, unmapping variants, epigenetic effects, gene-gene/gene-environment interactions, and functional validation. Furthermore, Zhang and colleagues103 recently provided evidence that there are wide interactions among the crucial genes involved in the effector T-cell pathways and that the T helper 17 (Th17) pathway is a key player in developing susceptibility to AR. Therefore, future research should systematically integrate “overall data” from genomics, proteomics, epigenomics, and metabolomics to provide new insights into precision medical treatments for AR.
6.1.2 microRNA
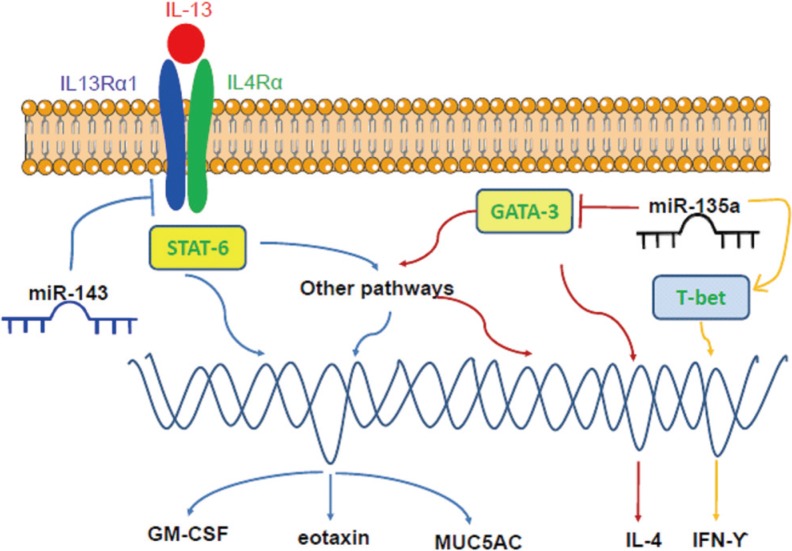
Genetic regulation plays an undoubted role in the pathogenesis of AR. Because they function as endogenous inhibitors of translational processes, microRNA (miRNA/miR) is a class of short, noncoding RNAs that have emerged as important regulators of gene expression in the immune system.133 Altered miRNA expression profiles have been identified in AR. In this regard, 7 up-regulated and 10 down-regulated miRNAs were recently identified in activated bone marrow-derived mast cells following IgE-FcεRI cross-linking with antigen, suggesting that these miRNAs may exert considerable influence on core signalling pathways and biologic behaviors.134 Among these altered miRNAs, miR-21a-3p and miR-3113-5p were the most remarkably up-regulated and down-regulated miRNAs according to the bioinformatics algorithm.134 Some miRNAs can modify the messenger RNA (mRNA) and protein expression of the chemokines and transcription factors directly. The miRNA microarray chip analysis has shown that miR-224, miR-187, and miR-143 were down-regulated in AR patients,135 among which miR-143 has been shown to decrease the mRNA and protein expression levels of granulocyte-macrophage colony-stimulating factor (GM-CSF), eotaxin, and mucin 5AC (MUC5AC) in IL-13-stimulated nasal epithelial cells through direct suppression of IL-13 receptor α1 chain (IL13Rα1).136 This is particularly relevant as IL-13 plays an important role in the pathogenesis of allergic inflammation. In addition, miR-135a can down-regulate the mRNA and protein expression levels of GATA- binding protein-3 and IL-4, and up-regulate the expression levels of T-bet and IFN-γ, thus correcting the Th1/Th2 imbalance in AR mice.137 The potential effects of miR-143 and miR-135a on signalling pathways in AR development are briefly illustrated in Fig. 5.
Fig. 5. The potential effects of miR-143 and miR-135a on signaling pathways. While miR-143 can inhibit the expression of GM-CSF, eotaxin, and MUC5AC by suppressing the IL13Rα1 signalling pathway, miR-135a can down-regulate the mRNA and protein expression levels of GATA-3 and IL-4 and up-regulate the expression levels of T-bet and IFN-γ, thereby correcting the Th1/Th2 imbalance.
Some miRNAs could predict the onset of AR. Suojalehto and colleagues138 have reported that miR-205, miR-155, and miR-498 were up-regulated in the nasal mucosa of currently symptomatic AR, whereas let-7e was down-regulated in currently nonsymptomatic AR. Notably, Chen and colleagues139 found that miRNA-21 expression levels were significantly low in mononuclear leucocytes from cord blood samples with elevated cord blood IgE (CBIgE) and in monocytes from AR children, indicating that miRNA-21 may be an early predictor of AR and a possible therapeutic target for treating AR. However, further studies are needed reveal the full impact of miRNAs in the development of AR as well as to reveal their potential as therapeutic targets and noninvasive biomarkers in AR.
6.2 Immunopathogenesis
6.2.1 General concept
The symptoms of AR are a result of inhaled allergen-induced inflammation in the nasal mucosa, which is characterized by a Th2-dominated immune response associated with increased levels of serum IgE.140,141
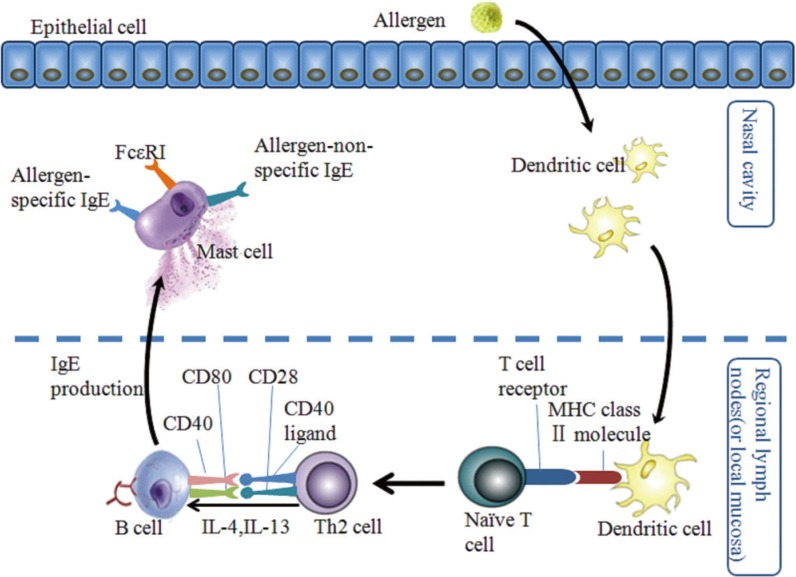
Similar to other allergic diseases, the immune response in AR begins with sensitization. When the nasal mucosa is exposed to allergens, the allergens are captured and processed by antigen-presenting cells (mainly dendritic cells) and presented to naïve T cells. Naïve T cells then differentiate into Th2 cells which produce IL-4, IL-5, and IL-13. The IL-4 and IL-13 cytokines, together with the ligation of matched co-stimulatory molecules in Th2 cells and B cells, promote B cell phenotype switching to produce allergen specific IgE. Thereafter, the allergen-specific IgE binds to its high-affinity receptors (FcεRI) on the surface of mast cells and basophils, causing sensitization of these 2 cell types (Fig. 6).142
Fig. 6. The process of allergen sensitization in AR. Following exposure of nasal mucosa to allergens, allergens are captured, taken up, and processed by dendritic cells (DCs). Subsequently, DCs are activate and, mature, and migrate to regional lymph nodes or to sites in the local mucosa, where they present allergen-derived peptides in the context of MHC class II molecules to naïve T cells. Naïve T cells then differentiate into Th2 cells, which produce IL-4 and IL-13 in the presence of early IL-4. In the presence of these Th2-derived cytokines, together with ligation of the suitable co-stimulatory molecules (CD40 ligand with CD40 and CD28 with CD80), B cells undergo immunoglobulin class-switch recombination to produce IgE antibodies. The locally and/or systemically diffused IgE binds to the high-affinity receptors (FcεRI) on mast cells and basophils (not shown), and results in sensitization of these cells (adapted from Galli and colleagues [142]).
Re-exposure of sensitized individuals to the sensitizing allergens leads to a cascade of pathologic events, and subsequently the symptoms of AR. Allergic responsiveness can generally be divided into 2 phases: the immediate or early-phase and the late-phase responses.140
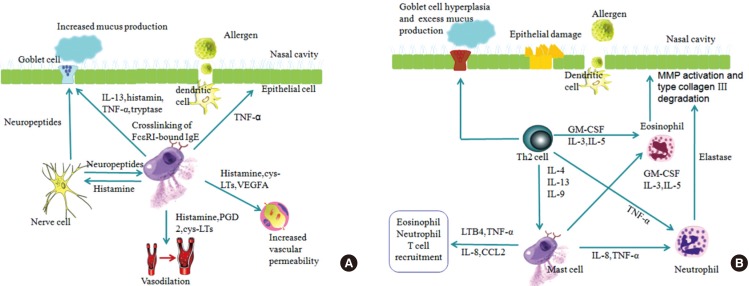
The early phase response occurs in sensitized individuals within minutes of allergen exposure, with mast cells and basophils being the best-known effector cells in this phase. Mast cells are abundant in the epithelial compartment of nasal mucosa in the sensitized individuals and can be easily activated upon re-exposure to the allergens. Cross-linking of the allergen-specific IgE-FcεRI complexes on the mast cell and basophil surfaces by specific allergen triggers secretion of 3 classes of biologic products: those stored in cytoplasmic granules, lipid-derived mediators, and newly synthesized cytokines, chemokines, and growth factors as well as other products.140 These mediators collectively result in vasodilation, increased vascular permeability, and mucus production, as well as stimulation of sensory nerves, which evoke the symptoms of nasal itching, rhinorrhea, sneezing and congestion (Fig. 7).142
Fig. 7. Early and late phase reactions in AR. (A) Early phase reaction: Ligation of allergen-specific IgE-FcεRI complexes by the corresponding allergen on mast cells activates mast cells to secrete preformed mediators (e.g. histamine and tryptase) and lipid-derived mediators (e.g. PGD2, LTB4 and PAF), which increase vascular permeability, mucus secretion, and blood vessel dilation. This results in watery rhinorrhea, mucosal edema, and nasal congestion. Stimulation of sensory nerves in the nose results in sneezing and sensations of nasal itch and congestion (adapted from Galli and colleagues [142]). (B) Late phase reaction: Ligation of IgE-FcεRI complexes by allergen on mast cells results in release of newly synthesized cytokines, chemokines and growth factors, which contribute to the late phase reaction. Mast cells promote the influx and activation of inflammatory leukocytes (such as neutrophils, eosinophils and T cells) by producing TNF-α, LTB4, IL-5, IL-8, and CCL2. T cells that recognize allergen-derived peptides also release products (e.g. IL-4, IL-13, and IL-9) and contribute to late-phase reactions. IL-4 and IL-13 released by Th2 cells can stimulate mast cells to produce more IgE and induce goblet cell hyperplasia, which results in excess mucus production. The recruited immune cells have some downstream effects. For example, elastase released by neutrophils promotes activation of matrix metalloproteinases and degradation of type III collagen. Basic proteins released by eosinophils can cause epithelial cell damage (adapted from Galli and colleagues [142]).
Late-phase reaction typically develops at 2 to 6 hours after allergen exposure and is characterized by a prolongation of sneezing, rhinorrhea and a predominantly sustained nasal congestion. A variety of mediators and cells are involved in this phase. Some mast-cell products such as TNF-α, LTB4, IL-5, and IL-8/CXCL8 have the potential to recruit and activate other immune cells including monocytes, T cells, eosinophils and basophils. The released products of mast cells (e.g. histamine, LTB4, PGD2, and TNF-α) can also modulate the activity of dendritic cells, T cells and B cells, or influence structural cells (including vascular endothelial cells, epithelial cells and nerve cells). On the other hand, some mast cell products (e.g. IL-10 and TGF-β) have anti-inflammatory or immunosuppressive functions. The recruited immune cells, however, may lead to some tissue damage and remodelling, for example, eosinophil basic protein induces epithelial cells injury, and Th2 cytokines (IL-4, IL-5, and IL-9) provoke more IgE production, goblet cell hyperplasia, and excess mucus production (Fig. 7).142
In addition to the common pathophysiologic pathways detailed above, other mechanisms are also likely to be involved in AR. Although epithelial cells are important structural cells playing major roles in providing an effective barrier to entry of foreign particles, secretion of mucus, and removal of foreign agents by virtue of possessing cilia, increasing evidence shows that epithelial cells also have potent immunomodulatory activities through synthesizing and releasing cytokines and chemokines (e.g. CCL2, CCL20, GM-CSF, IL-1β, TSLP, IL-25, and IL-33).143 The epithelial cytokines and chemokines mediate the cross-talk between epithelial cells and immune cells,144 and thus bridge the innate and adaptive immunity in nasal tissues. Nasal epithelial cells in patients with AR may also play a role in antigen presentation through enhanced expression of HLA-DR and CD86.145 It has been proposed that diesel exhaust particles disrupt tight junctions and increase the paracellular permeability in RPMI 2,650 cells (a human nasal epithelial cell line) in vitro.146
Evidence from some recent studies suggests that regulatory T cells (Treg)147 and Th17 cells,148 type 2 innate lymphoid cells,149 miRNA,137,150 follicular Th cells,151 and regulatory B cells151 may also play a role in AR. Indeed, apart from the well-documented classic immunopathologic mechanisms of AR, the following potential immunologic mechanisms may play key roles in AR.
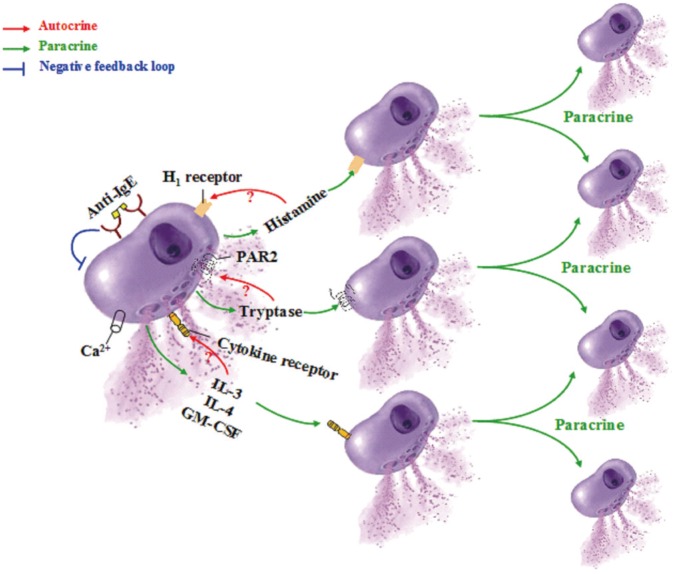
1) Self-amplification mechanisms of mast cell activation
It has previously been advocated that at least 2 pathways exist in humans for mast cells to amplify their own activation-degranulation signals in an autocrine or paracrine manner,152 which may partially explain the phenomena that when a sensitized individual contacts allergen only once, the local allergic response in the involved tissue or organ may last for days or weeks. These pathways include the tryptase-protease-activated receptor (PAR)-2 pathway and the histamine-H1 receptor pathway (Fig. 8).
Fig. 8. Self-amplification mechanisms of mast cell degranulation. IL, interleukin; GM-CSF, granulocyte/macrophage colony-stimulating factor; PAR, protease activated receptor (adapted from He and colleagues [152]).
Several self-amplification mechanisms of mast cell activation have been reported. For example, while IL-36 released from the mast cells153 can selectively induce retinaldehyde dehydrogenase-II release154 from mast cells, GM-CSF secreted from mast cells153,155 is able to induce IL-4 release from mast cells.156 IL-4 secreted from mast cells157 can in turn amplify the classic Fc epsilonRI-dependent mast cell activation and release of cysteinyl leukotrienes,158 and in synergy with stem cell factor (SCF), IL-4 strongly enhances mast cell proliferation and shifts IgE-dependent cytokine production in mature human mast cells toward an increased release of Th2 cytokines IL-5, and IL-13.159
2) Self-amplification mechanisms of mast cell accumulation
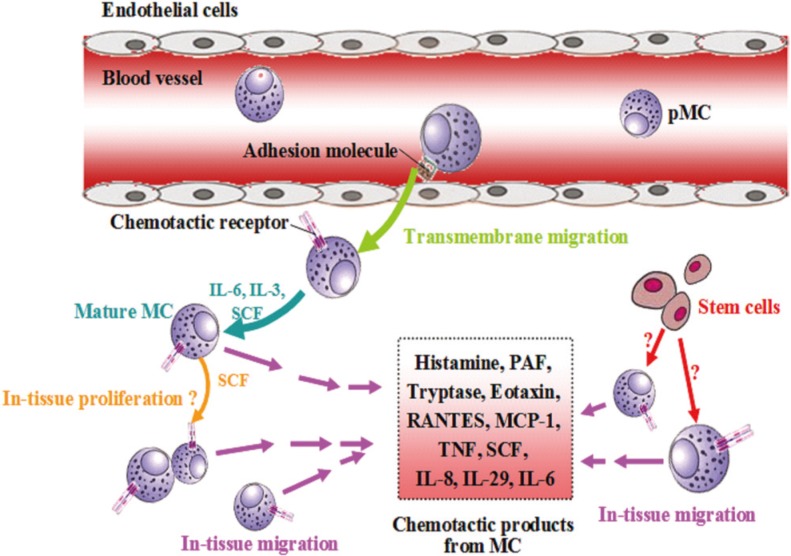
The fundamental requirement for paracrine self-amplification mechanisms of mast cell activation is the presence of a relatively high density of mast cells in the involved tissues. It has long been recognized that the numbers of mast cells in allergic tissues such as lung160 and skin161 are dramatically increased, but the mechanisms through which mast cells are accumulated remain obscure. Generally speaking, mast cells can be accumulated by 2 mechanisms: (1) migration from adjacent tissues or from blood and (2) local generation in the tissue (Fig. 9).
Fig. 9. Self-amplification mechanisms of mast cell accumulation. IL, interleukin; pMC, pregenitor mast cell; MC, mast cell; SCF, stem cell factor; TNF, tumor necrosis factor; RANTES, regulated upon activation normal T cell expressed and secreted; MCP-1, monocyte chemotactic protein-1; PAF, platelet-activating factor (adapted from He and colleagues [152]).
Numerous mast cell products have been found to be able to induce mast cell migration. Thus, while histamine has been shown to induce chemotaxis of mouse mast cells through histamine H4 receptor,162 PAF has been identified as a potent chemoattractant of both murine and human mast cells.163 Interactions of eotaxin, RANTES, and MCP-1 with CCR3 on basophils and mast cells are responsible for the recruitment of these cells.164 While IL-6165 and TNF166 stimulate migration of mast cells in the presence of laminin, IL-4 induces homotypic aggregation of human cord blood mononuclear progenitor cells (hCBMCs) in the presence of SCF and IL-6.167 SCF by itself is capable of inducing the migration of mast cells via its receptor c-Kit.168 Moreover, IL-29 has been found to be released from mast cells and is able to induce mast cell infiltration in mouse peritoneum by a CD18- and ICAM-1-dependent mechanism.169 Mast cells are found to express and release significantly higher concentration of IL-8 and expression of IL-8 receptors CXCR1 and CXCR2, through which IL-8 recruits mast cells.170
Little is known about whether mast cells can be generated in tissue, but a report that two-thirds of freshly dispersed mast cells from skin cultured with recombinant human SCF showed evidence of proliferation suggests that mast cells may have the ability to proliferate in skin tissue.171 Although there is a lack of direct evidence that mast cells can be derived from tissue stem cells, the finding that mast cells can be obtained from bone marrow and cord blood CD34172- or CD133- positive progenitor cells173 in the presence of IL-6 and SCF,174 strongly suggests that tissue stem cells could possibly be driven to differentiate into mast cells under inflammatory conditions.
3) Influence of mast cell mediators on secondary effector cells of allergy
While activation of primary effector cells, including mast cells and basophils, is a key element of allergic disease, stimulation of secondary effector cells of allergy such as eosinophils and neutrophils also plays also a crucial role in particularly the late-phase reactions. A review of studies investigating the role of human mast cell-derived cytokines has substantially described the pivotal interaction between mast cells and eosinophils in eosinophil-mediated inflammatory responses.175 In addition, TSLP, IL-25,176 and IL-31177 have recently been shown to be able to activate eosinophils and to contribute to allergic inflammation. Mast cell products, including tryptase,178 chymase,179 MMP-9,180 heparin,181 IL-8 and TNF,182 are also potent chemoattractants for neutrophils and may be responsible for the cross-talk between mast cells and neutrophils. As large numbers of eosinophils and neutrophils can reside in the involved tissue and are able to release an array of proinflammatory mediators, these cells also play an important role in the etiology of allergic disease. However, the mechanisms underlying allergens to selectively accumulate and activate eosinophils and neutrophils via mast cells remain obscure.
4) Contribution of Tregs to AR
In recent years, Tregs have emerged as key cells involved during the sensitization phase of the pathogenesis of allergy.183 It is recognized that acquired immunity is controlled by Tregs that suppress responses of effector T cells. Tregs can be classified into natural Tregs (nTreg)184 including inducible costimulator (ICOS)(+) Tregs,185 inducible/adaptive Tregs (iTreg),186 IL-10-producing type 1 Tregs (Tr1 cells),187 CD8(+) Tregs188 and IL-17-producing Tregs.189 These cells share some common features including expression of Foxp3 (except for Tr1 cells) and secretion of inhibitory cytokine IL-10 and/or TGF-β (Table 5). It is apparent that Tregs are likely contribute to allergic disorders and play a crucial role in the treatment of allergy through their actions on suppression of effector T cells and inhibition of activation of mast cells and basophils. Thus, modulation of the functions of Tregs may provide a novel strategy for preventing and treating allergic diseases.
Table 5. Characteristics of subsets of Treg cell.
| Subset | Specific markers | Secretory products | Actions | Location |
|---|---|---|---|---|
| nTreg | CD4, CD25, Foxp3 | IL-10, TGF-β | Block T cell proliferation, suppression of DCs, inhibition of effector Th1, Th2 and Th17 cells; eliminate production of allergen-specific IgE, induce IgG4 secretion; suppress mast cells, basophils and eosinophils; interact with resident tissue cells and participate tissue remodelling | Thymus [188] |
| ICOS(+) Treg | CD4, CD25, Foxp3, ICOS | IL-10, IL-17, IFN-γ | Suppress hapten-reactive CD8(+) T cells | Generated from nTregs |
| iTreg | CD4, Foxp3 | IL-10, TGF-β | Similar to nTreg | Periphery |
| Tr1 | CD4, CD25 | IL-10 | Suppress effector Th cell migration and functions [186]; suppress mast cells, basophils, and eosinophils [187] | Generated from non-Treg cell precursors and home lungs, and draining lymph nodes |
| CD8(+)Treg | CD8, Foxp3, CD25 (not for tonsil origin), CD28 | IL-10, TNF-α, IFN-γ, GB | Block activation of naïve or effector T cells; suppress IgG/IgE antibody responses [188], IL-4 expression, and the proliferation of CD4(+) T cells. | Generated from OT-1 CD8 cells [188] and tonsils |
| IL-17-producing Foxp3 (+) Treg | CD4, Foxp3, CCR6, ROR- GTF | IL-17 | Inhibit the proliferation of CD4(+) effector T cells [189]. | Differentiated from CD4(+)Foxp3(+) CCR6(−) Tregs in peripheral blood and lymphoid tissue [189] |
nTreg, natural regulatory T cell; ICOS, inducible costimulator; iTreg, inducible/adaptive regulatory T cell; Tr1 cell, IL-10-producing type 1 regulatory T cell; GB, granzyme B; RORGTF, ROR gammat transcription factor (adapted from Zhang and colleagues [183]).
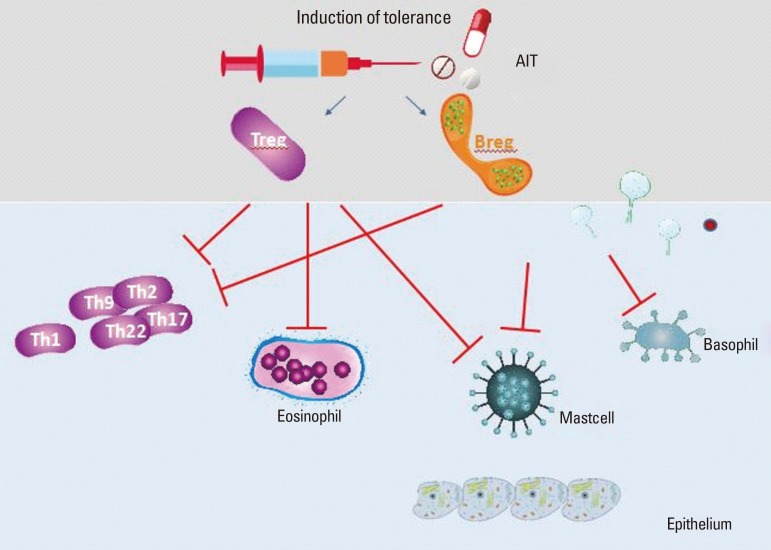
There is increasing interest in the role of both nTreg and iTreg populations in preventing hypersensitive immune responses and the underlying sensitization to allergens. It was speculated as early as 2006 that Tregs may actively prevent Th2 responses to allergens occurring in healthy nonatopic individuals and that their functions may be impaired in allergic patients.190 It has been suggested that peripheral T-cell tolerance to environmental antigens is crucial for the avoidance of allergy and that aberrant activation of Th2 cells in allergy is secondary to impaired mechanisms of peripheral T-cell tolerance normally mediated by antigen-specific T-cell anergy, Tregs and the suppressive cytokines IL-10 and TGF-β. Therefore, the most appealing therapy for allergic diseases would be allergen-specific immunotherapy191 that reduces Th2 cytokine production and promotes induction of anergy, Treg, and suppressor cytokines.192
A study which investigated allergen-induced Th2, Th1 and Treg immune responses in peripheral blood mononuclear cells (PBMC), and their association with symptom improvement in AD patients after 3 years of AIT showed that both IL-4 expression and the IL-4/IFN-gamma ratio were decreased in patients with a good therapeutic outcome after 1 year of AIT, whereas the induced Treg and Th1 responses persisted over 3 years after AIT.193
6.2.2 Innate type 2 immune response
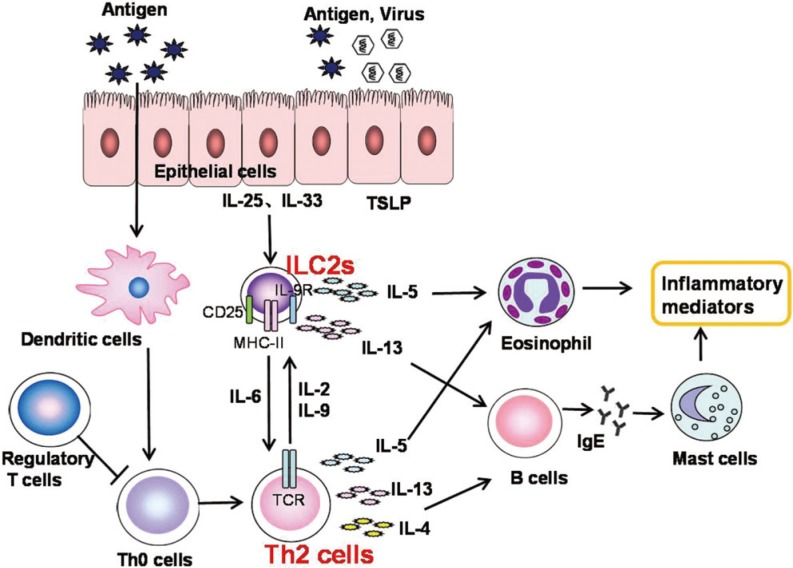
CD4+ Th2 cells play a significant role in AR. Indeed, type 2 cytokines produced by Th2 cells such as IL-4, IL-5 and IL-13 drive many features of allergic rhinitis. Group 2 innate lymphoid cells (ILC2s) are a newly recognized subset of the innate lymphoid cell family, which rapidly and dramatically produces IL-5 and IL-13 in response to IL-25 or IL-33194,195,196 and is likely to play a role in the etiology of AR (Fig. 10). ILC2s are morphologically similar to, but smaller than lymphocytes. ILC2s lack T-cell, B-cell, natural-killer cell or other cell lineage markers, but express the IL-7 receptor α-chain (CD127), c-Kit, Sca-1, etc.194,195,197,198,199 ILC2s produce dramatic amounts of IL-5 and IL-13, and some IL-4 in response to the Th2 cell-stimulating cytokines IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) produced by epithelial cells.200,201,202,203,204 The discovery of ILC2s puts forward a new challenge to the traditional opinions that T helper type 2 (Th2) cells play a dominant role in Th2-skewed allergic diseases. There may, however, be some interactions between ILC2 and Th2 cells via some cytokines as shown in Fig. 10. ILC2s may interact with T cells through both cytokine secretion and specific molecules on the cell surface. ILC2s may regulate T cell differentiation through antigen presentation via major histocompatibility complex (MHC) class II and through the production of IL-6. In turn, T cells support the maintenance and proliferation of ILCs through production of IL-2 and IL-9.205
Fig. 10. The role of ILC2 in AR. Type 2 responses are initiated by allergens that disrupt the epithelial barriers and induce secretion of IL-25, IL-33, and TSLP. IL-25 and IL-33 activate ILC2s to produce the type 2 cytokines IL-5 and IL-13. Epithelial cytokines also activate DCs to induce Th2 responses. Secretion of IL-5 by ILC2s leads to the recruitment and activation of mast cells and eosinophils. The activation of T cells further amplifies the secretion of type 2 cytokines, and the production of IL-4 and IL-13 by T cells leads to the production of IgE by B cells. Together, the responses triggered by secretion of type 2 cytokines from ILC2s and Th2 cells play an important role in inducing allergic inflammation.
ILC2s have been reported to be involved in the pathology of both asthma and AR in animals and humans. Studies of adaptive immunodeficiency mice have demonstrated that influenza-,206 protease-,207 ryegrass-208 or mite-induced209 airway hyperreactivity or asthmatic inflammation is mediated via ILC2s. In addition, ILC2s have also been found to be increased in several allergic immune diseases in humans, such as AD,210,211 active eosinophilic esophagitis212 and CRS with nasal polyps or eosinophilia.213,214 Moreover, high ILC2 levels have been found in patients with even moderate-to-severe asthma,215 persistent airway eosinophilia215 or rhinovirus-induced asthma exacerbations.216 Importantly, increased peripheral ILC2s have been reported in AR patients during the grass pollen season217 or after the challenge with cat antigen.149
More recent evidence from a study in Chinese subjects has indicated that the percentage of ILC2s was significantly elevated in HDM-sensitized AR patients, compared to mugwort-sensitized AR patients and healthy controls, with no significant difference between the latter 2 groups.218 Importantly, peripheral ILC2 levels in HDM-sensitized AR patients were strongly correlated positively with the severity of the clinical VAS score and with the plasma levels of their functional cytokine IL-13.219 Moreover, stimulation with IL-25 and IL-33 induced significantly greater production of IL-5 and IL-13 in peripheral blood mononuclear cells (PBMCs) of HDM-sensitized AR patients than in those of mugwort-sensitized AR patients or healthy controls.218 The levels of IL-5 and IL-13 were also higher following stimulation with IL-25 and IL-33 compared to stimulation with DerP1 stimulation.219 Similarly, sorted ILC2s from AR patients produced large amounts of IL-5 and IL-13 after stimulation with IL-25 and IL-33. Furthermore, a prospective study has investigated the effects of glucocorticoid treatment on the levels and function of ILC2s in patients with asthma or asthma plus AR.220 The study showed high frequency of ILC2s in human PBMCs from both groups of patients with asthma or asthma plus AR, and demonstrated that ILC2 levels significantly decreased to normal levels 3 months after glucocorticoid treatment. Collectively, these findings suggest that sensitizing allergen type may be an important factor determining the functional profile and frequency of ILC2s in AR patients and that high levels of innate type 2 immune responses in AR may provide a potential strategy for mediating the immunopathogenesis and therapy of this disease.
6.3 Inflammatory mediators
6.3.1 Chemokines and receptors
Chemokines are a group of cytokines responsible for the activation of leukocytes, such as T/B lymphocytes, monocytes, neutrophils, eosinophils and basophils, which are associated with allergic inflammation. It is now generally accepted that several chemokines and their receptors play essential roles in the pathogenesis of AR.
Eotaxin is thought to be associated with allergic inflammation as it is involved in the recruitment and activation of eosinophils by chemotaxis after antigen challenge in AR patients.221 Eotaxin exists in 3 isoforms: eotaxin-1 (CCL11), eotaxin-2 (CCL24) and eotaxin-3 (CCL26). Eotaxin can accelerate basophilic cell degranulation, accumulate eosinophils, and then further induce an allergic reaction through the IgE-mast-cell-FεRI cascade.222 Regulated upon activation normal T cell expressed and secreted factor (RANTES/CCL5) is another chemokine closely associated with allergic inflammation and is found to be highly expressed in the epithelial and endothelial cells of the lower nasal mucosa in AR.223 RANTES contributes to eosinophil-mediated inflammatory responses in the earlier period of allergic reaction.224
Cytokines and chemokines produce marked effects via the receptors of chemokines. Among them, the most important eosinophil chemokine receptor is CCR3, which ligands with eotaxin-1, eotaxin-2, eotaxin-3, and RANTES to activate eosinophils to release granules.225,226 Other receptors such as CCR4 and CCR8 expressed on eosinophils, mast cells and basophils are also associated with AR.227 Thus, this subset of chemokines and chemokine receptors are potentially important in modulating immune responses by amplifying Th2 cell responses in AR.
6.3.2 Nasal nitric oxide (NO) and gasotransmitters
The oxidant/antioxidant imbalance is also an important part of the pathogenesis of AR as allergens can stimulate the generation of reactive oxygen species, which can cause nasal mucosal epithelial damage. This induces inflammatory cells to release inflammatory mediators such as cytokines, chemokines and adhesion molecules, and lead to the development of further inflammation and damage. Additionally, some small gaseous molecules known as gasotransmitters have also been shown to play important roles in regulating the oxidation process in AR.228
The first gasotransmitter identified was nitric oxide (NO).229 An increasing body of evidence has indicated NO production to be increased in both perennial and seasonal AR,194 with many studies indicating nasally exhaled NO in humans to be generated mainly within the mucosal epithelium of the paranasal sinuses.230 Furthermore, increased local concentrations of NO in AR tend to increase Th2 cell-synthesized interleukins, including IL-4, IL-5, and IL-10, which promote the production of IgE and accumulation of eosinophils. NO also tends to increase edema and plasma exudation, and cause denudation and desquamation of the epithelial lining.231
Carbon monoxide (CO) and hydrogen sulfide (H2S) have also been identified as gasotransmitters, which participate in the resolution of allergy-induced airway inflammation.232 While exhaled CO has been implicated as a likely gasotransmitter in the development of asthma, its role in the development of AR is less clear, despite being generated in the nasal mucosa of AR.233 Similarly, the role of H2S in the pathogenesis of AR is also unclear. A previous study in humans has suggested that this gasotransmitter may have multiple functions in human nasal mucosa and contribute to the development of allergic symptoms such as rhinorrhea, sneezing and nasal stuffiness.234 However, other studies on the regulation of the endogenous H2S pathway in the nasal mucosa of an AR guinea pig model has suggested that H2S may exert anti-inflammatory as well as antioxidant effects in the nasal mucosa and therefore may serve a protective function in AR patients.235
6.3.3 Substance P
Substance P (SP) is a neuropeptide neurotransmitter that belongs to the tachykinin family of peptides, which can be released by C-nerve fibers in the nasal mucosa. It has been found to have a variety of proinflammatory and prosecretory effects in epithelial, glandular and vascular tissues in the nasal cavity and thus play an important role in neurogenic inflammation.236 The nasal mucosa is densely innervated by sensory nerve fibers that release SP, which leads to vascular permeability, plasma extravasation, glandular secretion and proinflammatory cell influx.237 Stimulation of the nasal mucosa by SP induces the release of histamine and thereby influences the physiologic and pathophysiologic nasal conditions, especially during allergic inflammatory processes. Using capsaicin as a blocking agent of SP experiments on AR animal models, Zhang and colleagues238 have shown that capsaicin could effectively deplete the concentration of SP in the nasal mucosa and thus relieve the various symptoms of AR in these animals. Intranasal administration of capsaicin in the treatment of patients with AR has also demonstrated to relieve clinical symptoms of AR and to markedly reduce the concentration of SP in the nasal secretions.239 These studies indicated that the therapeutic mechanism of capsaicin in AR was related to the blocking of axon reflex, via which stimulation of allergen on sensory nerve fibers may lead to the release of SP (Fig. 11).
Fig. 11. Stimulation of allergen on C fibers may lead to the release of substance P via axon reflex.
There is some evidence that the expression of endogenous SP mRNA and peptide is significantly increased under IgE-activated conditions and that small hairpin RNA (shRNA)- mediated knockdown of endogenous SP can reduce the ability of IgE-activated mast cells to undergo degranulation.240 This finding suggests that endogenous SP plays an important role in mast cell antigen-mediated degranulation and thus enhances the progression of allergic inflammation.240 Indeed, it is notable that SP is expressed not only by neurons, but also in immune cells such as mast cells240 which release allergic mediators such as histamine,241 thereby exacerbating allergic symptoms. It is possible that widespread expression of SP in diverse cell types may indicate a multifunctional role for SP in a wide variety of physiologic and pathophysiologic conditions by activating a multitude of signalling pathways. Moreover, SP acts via neurokinin-1 receptors (NK-1R) to stimulate the release of inflammatory mediators including histamine and chemokines, which recruit inflammatory cells. Knockdown of NK-1R expression effectively inhibits NK-1R expression, and alleviates AR-related clinical symptoms and eosinophil inflammation in the nasal mucosal tissues of rats, suggesting that NK-1R may play an important role in the development of AR.242
6.4 Environmental factors
6.4.1 Pollen
Pollens are important inhalant allergens as a cause of allergic disease. Pollen grains deposit on the nasal mucosa and release allergenic proteins to cause specific IgE and responses to pollen allergen, which are characterized by increased expression of Th2 cytokines. Allergic disease caused by pollen allergens is known as “pollinosis”. According to a study by Zhang and colleagues,20 the prevalence of pollen-related AR in China is 47.8%. The main airborne allergic pollens come from wind-pollinated plants rather than insect-pollinated plants and have their own characteristics to facilitate wind dispersion. These pollens are small (diameter 10–100 µm), light, and prolific with some conifer pollen developing air sacs to make it easy to become airborne. The allergic pollens also demonstrate seasonality and regionalism. The florescence of allergic plants is influenced by meteorological factors.
Tree and weed pollens are the main pollen allergens in China.243 Qiao and colleagues244 first collected the common airborne pollens and -plants in China and documented these as a compilation of “Airborne pollens and plants in China.” Most tree plants flower from March to May, and weed plants from July to October. The differences in the geography and climate result in the diversification of florescence in different regions of China. Artemisia, Humulus, and Amaranthaceae are the major weeds that cause late summer and fall seasonal pollinosis. The principal tree pollens in North China come from the cold-resistant trees such as Populus and Salix, whereas in South China the tree pollens are mainly from Broussonetia, Melia, and Casuarinatrees.243
A study conducted between November 2003 to October 2004 to investigate the general and seasonal distribution of airborne pollens and their relationship with pollinosis in 16 areas in 12 cities in Hubei province, Central China, identified 61 pollen genera within the 257, 520 pollen samples collected.245 The peak airborne pollen distribution occurred in 2 seasons each year, spring (March and April) and autumn (August to October), and pollinosis corresponded to the peak pollen distribution. Similarly, another study of airborne pollens performed in Beijing indicated that the summer-autumn pollen concentration peaked from August 20 to September 15, with the major pollens being Artemisia L, Chenopodium album, and Humulus scandens.246 There was a significant correlation between specific pollen concentration and the number of patients sensitized to a particular pollen as well as between pollen exposure and the onset of symptoms of AR.246
6.4.2 Traditional pollutants
In urban and suburban areas, industrial facilities and transportation-related emissions are major sources of air pollution. The main air pollutants include carbon oxides (COx), nitrogen oxides (NOx), ozone (O3), sulphur dioxide (SO2), and PM—a complex mixture of chemicals and particles of which diesel exhaust particles (DEPs) are the largest single component.247
Increasing evidence shows that air pollution is associated with respiratory diseases, particularly AR. A study assessed the potential effects of PM10, SO2, and NO2 on outpatient visits caused by AR in Beijing during the period 2009–2010 and found strong associations between the daily concentrations of the 3 air pollutants and the daily number of outpatients for AR.248 Similarly, another study of 32,143 Taiwanese school children indicated that persistent exposure to NOX, CO, and SO2 may increase the prevalence of AR.249
Several molecular mechanisms underlying the effects of air pollution on allergic respiratory disease have been explored in animal and human studies. First, air pollution may enhance IgE production by stimulating B lymphocytes. Diaz-Sanchez and colleagues250 showed that exposure to DEPs significantly increased IgE levels in human nasal fluids by greatly increasing the number of IgE-secreting cells and by altering the expression of IgE mRNA isoforms. This suggests that DEP exposure in vivo induces both a quantitative increase in IgE production and a shift in the type of IgE produced. Secondly, air pollution may increase the levels of some proinflammatory cytokines. The study of Diaz-Sanchez and colleagues250 also showed that nasal challenge in healthy humans with 0.15 mg DEPs suspended in 200 µL of saline expressed the TH2-type cytokines IL-4, IL-5, IL-6, and IL-10 in their nasal mucosal cells 18-24 hours after exposure. Indeed, studies have demonstrated that DEPs may enhance the symptoms of allergic rhinitis by synergistic action with pollen resulting in increased production and secretion of inflammatory cytokines such as IL,4, IL-5, and IL-13251 as well as enhanced local IgE production accompanied by isotype switching from IgM or IgD to IgE antibody in nasal lavage cells.252 Moreover, nasal histamine levels after challenge with dust mite allergen in dust mite-sensitive subjects were increased 3-fold when DEPs were coadministered with the allergen.253 Thirdly, air pollution may enhance reactive oxygen species (ROS) production. ROS such as superoxide, hydrogen peroxide and hydroxyl radical react with proteins, lipids, and DNA, and then lead to cellular damage. Finally, air pollution may enhance the immune response to allergens by physically binding with them. For example, one study has demonstrated that incubation of DEPs with purified natural grass pollen allergen Lol p1 for 30 minutes resulted in the binding of Lol p1 to DEPs with sufficient strength, which could not be removed by different washing methods.254 However, by employing this mechanism, DEPs may be transported with allergens, such as pollen grain fragments, into human airways, where both agents may be deposited on the mucosa at the same location and the close proximity of the DEPs and allergens would facilitate synergistic immunologic responses and respiratory symptoms.255
6.5 Nutrition and intestinal flora
In recent years, nutrition has been thought to play a role in the pathogenesis of AR,256,257,258 with several studies from China and other countries demonstrating a relationship between AR and nutrition.123,256,259,260
The mechanisms underlying the effects of nutrition in the pathogenesis of AR are poorly understood, but epigenetic modulation261 and immunobiologic processes258,262 may be involved. Epigenetic modulation (including DNA methylation, histone modifications and miRNA) refers to chemical reactions that switch parts of the genome on and off, thereby changing gene expression,261 which may influence AR-related immune responses. Evidence of nutrition-induced epigenetic changes in patients with allergy and atopy has been found for nutrients, including folic acid and fish oil, and in obesity itself.261 As for immunobiologic processes, immunoregulatory effects and airway epithelial cell responses appeared to be involved when AR patients or AR animal models were exposed to some nutrients such as ginger,258 vitamin D and vitamin E.262
On the other hand, epidemiologic studies have noted associations between deficiency in microbial exposure in early life and increasing risk of allergies in childhood. After neonates are born, microbes begin to colonize in their oral, respiratory and gut tracts. The microbial communities, in turn, are influenced by an array of environmental factors, early life local microbial exposures, delivery mode,263 diet,259 antimicrobial administration, physiologic factors, mental stress, etc, which are characterized by fluctuating microbial diversity. The neonatal gut microbiome plays an instrumental role in the development and function of childhood immune system,264 with the interaction between intestinal flora in early life and the mucosal immune system influencing the induction of immune tolerance or immune imbalance.
Microbial colonization patterns in very early life may influence immune development and function in key-time mode.265 Neonatal immune cells are different from mature cells and can learn to tolerate the symbiotic bacteria in an age-dependent manner. Once the critical ‘window of opportunity’ of immunologic development is missed, intestinal immune development cannot be fully achieved in the adult and subsequently allergic disease develops.
6.6 Pathogenesis and syndrome differentiation in TCM
6.6.1 Theories related to TCM
The origin of TCM can be traced back to remote antiquity. In its long course of development, TCM has gradually evolved into a unique and integrated system of medicine and an important part of Chinese culture. Huangdi Neijing (Huangdi's Canon of Medicine), the “Bible” for TCM published over 2000 years ago, is the earliest and greatest medical classic extant in China. The content of Huangdi Neijing covers the theories of yin-yang and Zangxiang. The theory of yin-yang permeates through all aspects of the theoretical system of TCM. Physiologically, the theory of yin-yang holds that the normal life activities of the human body result from the coordination between yin and yang in a unity of opposites, and pathologically TCM considers that the imbalance between yin and yang is one of the basic causes for the pathogenesis of disease. Based on this determination, right herbs are selected according to their yin or yang properties to rectify the imbalance of yin and yang, eventually achieving the purpose of curing the disease.
The theory of Zangxiang studies the physiologic and pathologic changes of viscera and their relationships. It plays an important role in the theoretical system of TCM and is significant in both expounding the physiology and pathology of the human body and guiding clinical practice. Viscera, which are the basis of the theory of Zangxiang, include the 5 Zang-organs: the heart, lungs, spleen, liver and kidneys. According to the theory of TCM, the lungs open into the nose. The main physiologic functions of the lung are to dominate Qi, control respiration, govern dispersing and descending activities, and regulate water passage. The fact that the nose is the pathway for respiration is why it is believed in TCM that the lung opens to the nose. The 5 Zang organs interact with each other in both the physiologic functions and the pathologic features.
Treatment based on syndrome differentiation is the basic principle guiding clinical diagnosis and treatment of disease. Syndrome is a pathologic generalization of a disease at a certain stage in the course of its development. Since syndrome includes the location, cause and nature of a disease as well as the relation between pathogenic factors and healthy Qi, it reveals the nature of disease more comprehensively and accurately. A patient's symptoms and signs collected with the 4 diagnostic methods are analyzed and generalized, and finally diagnosed with some syndrome or disease. Generally speaking, syndrome is a reflection of waning and waxing of yin and yang of Zangfu-organs or invasion of exogenous evils. Since physiologic functions of every Zang-fu organs are different, the reflections of pathology are different. The theory of physiology and pathology of Zang-fu organs is the theoretical basis of Visceral Syndrome Differentiation.
6.6.2 Syndrome differentiation
According to the theory of TCM, AR falls under the category of Biqiu. The etiology is based on the deficiency of a healthy-Qi, and external evils take advantage of a weak point. In terms of syndrome differentiation of solid and hollow viscera, AR is induced by the inconsistent function of the lungs, spleen and kidneys, especially because of the deficiency of the kidney-yang. According to the “Standards for the diagnosis and curative effect of Chinese medical symptom” issued by the State Administration of TCM in 1994266 and Chinese Otorhinolaryngology edited by Wang Shizhen,267 AR is divided into 4 types: kidney-yang deficiency, lung-Qi deficiency, spleen-Qi deficiency, and lung heat.
Kidney-yang deficiency.Kidney-yang deficiency and the lung's suffering from cold can lead to lung-Qi deficiency, which makes the body vulnerable to external evils and causes nasal itching and sneezing. The kidney fails to keep Qi and Qi descends to the top, causing frequent sneezing. Fading life gate fire and Qi transformation failure lead body fluids to ascend to the nose, which triggers constant rhinorrhea. Studies have shown that the kidney plays an important role in the regulation of cAMP/cGMP proportions.268,269 Pharmacologic studies have found that yang-warming herbal medicinal formulae are instrumental in the IgE regulation system.270 Seasonal allergic diseases can be prevented by taking the medication in advance, effectively reducing seasonal allergic incidences.
Lung-Qi deficiency.The lung-Qi governs the lung and its opening at the nose. If the lung-Qi is deficient, there is insecurity of defense Qi, the lung will suffer from wind-cold evil invasion, and the lung fails to diffuse and descend, which causes stagnation of body fluids, leading to overflow rhinorrhea and Biqiu. Patients often have shortness of breath and fatigue, aversion to wind and cold, pale and fat tongue, thin and pale tongue coating, and weakened pulse. The Wind tends to move and change rapidly by nature, so does the symptoms of AR. The occurrence of lung-Qi deficiency is closely related to AR. Zhao Jiangyun and colleagues271 have shown that lung-Qi deficiency is related to lower than normal indicators of peripheral T-lymphocyte subpopulation, indicating lower cell immune function of patients, which affects the immune regulation of T cells. It has also been demonstrated that cAMP levels in plasma272 and nasal secretions273 are lower in patients with lung-Qi deficiency than in normal subjects. With a decreased cAMP and cAMP/cGMP ratio, the release of chemical medium is strengthened and accelerated, thus affecting the response of the nasal mucosa.
Spleen-Qi Deficiency.The spleen is the source of engendering transformation. A healthy spleen is full of Qi and blood, defending the body surface against the invasion of exogenous pathologic factors. When the spleen is deficient, there is no growth of Qi or blood, and leads to lung-Qi deficiency and malnutrition to the nose. Therefore, spleen-Qi deficiency also affects water transportation and regulation, and fluid retention invades nasal orifices, causing nasal mucosal edema and constant rhinorrhea. Moreover, a study has shown that patients suffering from lung-Qi deficiency and spleen-Qi deficiency have the highest level of serum IgE and a higher proportion of eosinophils in nasal secretions,274 suggesting that AR is more easily induced by spleen deficiency.
Lung heat.Apart from the above-mentioned syndrome differentiation typing, the lung-heat pattern for AR is generally considered one of the most common types.267,275 As the lung suffers from heat evil, it fails to diffuse and descend, which causes stagnation of body fluids, leading to overflow rhinorrhea. Meanwhile, the heat invades nasal orifices, causing itching and constant sneezing.
7. DIAGNOSIS
7.1 Diagnosis criteria
7.1.1 Clinical history
Clinical history is essential for the accurate diagnosis of AR and for the assessment of its severity as well as its response to treatment. The interview begins with a thorough general medical history and should be followed up by questions more specific for allergy including environmental and occupational information. It is also common to collect information on personal and familial histories of patients with allergic disease.
The most frequent symptoms include sneezing, anterior rhinorrhea, bilateral nasal obstruction and nasal pruritus in patients with AR. In addition, most patients with pollen-induced rhinitis have eye symptoms. It is also important to distinguish between allergy and nonallergy symptoms.56 Subjective assessment of symptoms of AR is generally based on 4 nasal symptoms (sneezing, rhinorrhoea, nasal itching and nasal obstruction) and 2 ocular symptoms (ocular itching/grittiness/redness and ocular tearing).276 In China, VAS is most commonly used to quantify the above-mentioned assessments.276
7.1.2 Nasal examinations
In patients with AR, a nasal examination is necessary. Use of anterior rhinoscopy and nasal endoscopy is the widely used approaches, and nowadays the majority of hospitals in China have been equipped with an endoscopic examination system. Generally speaking, anterior rhinoscopy needs to be paired with the use of a speculum and mirror, and will provide the primary information. Nasal endoscopy is the next step, which is useful for patients with treatment failure or for excluding other conditions.
Nasal examination should describe: 1) the anatomical situation in the nose (e.g. the septum, the size of the inferior turbinate, and if possible the structures in the middle meatus); 2) the color of the mucosa; and 3) the amount and aspect of the mucus. An endoscopic image of nasal mucosa in a patient suffering from AR is exhibited in Fig. 12, demonstrating pale and edematous nasal mucosa, watery nasal discharge, and swollen inferior turbinates.
Fig. 12. A reprehensive endoscopic image of nasal mucosa suffering from AR.
7.1.3 Skin tests
Skin tests are widely used to demonstrate an IgE-mediated allergic reaction in the skin. These tests represent a major diagnostic tool in the field of allergy. As there are many complexities in their performance and interpretation, it is recommended that they be carried out by trained health professionals.277
1) Methods
a) Skin test methods
Two methods of skin testing, including intradermal skin tests and skin prick tests (SPTs), are available in China. Intradermal skin tests may be employed for allergy diagnosis in some instances (e.g. weak allergen solution). They are not widely used because they correlate less well with symptoms.278 Additionally, they may induce some false-positive reactions and systemic reactions.279,280 SPTs are recommended for the diagnosis of immediate-type allergy because there is a high degree of correlation between symptoms and provocative challenges as recommended by the Position Papers of the European Academy of Allergology and Clinical Immunology (EAACI),281 WHO,282 and the US Joint Council of Allergy Asthma and Immunology.283,284 In accordance with this recommendation, SPTs have also been widely used in China since 2000 to 2010. The inhaled antigens tested include HDMs) (Der f and Der p), seasonal grass pollens (giant ragweed, mugwort, lamb's quarters, Humulus, Chenopodium album), animal hair (dog and cat), mold (indoor and outdoor mustiness or floricultural environment) and cockroach. Due to the Food and Drug Administration policy restrictions concerning foreign SPT diagnostic reagents, few domestic SPT diagnostic reagents are currently used for allergen identification.
b) Negative and positive control solutions
Due to inter-patient variability in skin reactivity, it is necessary to include negative and positive controls in every skin test. Negative control solutions are diluents used to preserve allergen vaccines. The rare dermographic patient will produce wheal-and-erythema reactions to the negative control. Any reaction at the negative control test site will hinder the interpretation of the allergen site.283 Positive control solutions are used to detect suppression by medications or disease and to determine variations in technician performance. The usual positive control for prick-puncture testing is histamine dihydrochloride.285,286
2) Criteria of positivity
Skin tests should be read at the peak of their reaction by measuring the wheal and the flare approximately 15 minutes after performance of the tests. For prick tests, when the control site is completely negative, wheals of >3 mm represent a positive skin response.287,288
3) Factors affecting skin testing
Skin reaction is dependent on a number of variables that may alter performance of the skin tests. The quality of the allergen extract is of importance. If possible, standardized allergens should be used.281 Drugs may also affect skin tests, and it is therefore always necessary to ask the patient questions about the drugs they have taken. This is particularly the case for oral H1-antihistamines. Montelukast neither appears to reduce skin test reactivity289,290 nor needs to be discontinued before skin testing.
7.1.4 Serum specific IgE measurements
In contrast to the low predictive value of total serum IgE measurements in the diagnosis of allergic diseases, the measurement of allergen-specific IgE in serum is of importance. Furthermore, specific IgE measurements are not influenced by drugs or skin diseases.
1) Methods
The first technique ever used to accurately measure serum-specific IgE was the radioallergosorbent test (RAST).291,292 New techniques are now available using either radio-or enzyme-labelled anti-IgE.293,294 Enzyme-labelled anti-IgE measurement has been widely used in China. Results are expressed in terms of units of IgE (IU/mL, KU/L). ImmunoCAP system (Pharmacia, Uppsala, Sweden) and Euroline Allergy Diagnostics (Beijing Oumeng Biotechnology Co., Ltd., Beijing, China) are currently the most widely used manufacturers in China. The assay spectrum of Euroline comprises different profiles guided by diagnostic requirements (food, inhalation and pediatric allergy) with up to 54 parameters tested in one determination, basically meeting the needs of the inhaled allergens for AR diagnosis. In comparison, ImmunoCAP has only finished the registrations of 30 allergens, which involved total IgE, phadiatop, food, indoor inhaled allergens and a portion of outdoor pollens, thereby greatly limiting its application in China.
2) Criteria for positivity
The IgE level above 0.35 KU/L is usually testified as a positive result. However, some sensitized subjects have an IgE level below this cutoff, and the measurement of serum specific IgE is usually less sensitive than SPTs.295 Although a low specific IgE titer may not be clinically relevant, the titer of serum specific IgE is usually unrelated to the severity of symptoms.
3) Screening tests using serum specific IgE
Some methods use either a mixture of several allergens in a single assay296 or test several different allergens during a single assay.297 These tests can therefore be used as screening tests for the diagnosis of allergic diseases. Certainly, using most of these tests, the patient is defined only as allergic or nonallergic and more extensive investigations for rhinitis are needed if the test is positive.
7.1.5 Imaging
Computerized tomography (CT) is the principal radiologic investigation for most sinonasal disorders, but it is of limited use in the diagnosis of AR.298,299,300,301,302 CT scans should be carried out only in the following instances: to exclude other conditions, in patients who do not respond to treatment, and in patients with unilateral rhinitis.
7.1.6 Nasal provocation tests (NPTs)
NPTs elicit a response from the nasal mucosa by controlled and standardized exposure to allergens. Interpretation of nasal response includes symptom assessment and nasal patency evaluation. Rhinomanometry and acoustic rhinometry, 2 widely used complementary techniques, can evaluate nasal patency objectively.303 To date, NPTs are mostly used in research and seldom in clinical practice in China due to the lack of the registered drugs for NPTs. Additionally, they are important in the diagnosis of occupational AR and sometimes performed before immunotherapy for AR.304,305
7.1.7 Fractional exhaled NO
Fractional exhaled NO (FeNO) can be used as a reproducible and noninvasive biomarker for asthma and other lower respiratory diseases.306,307 The possible use of nasal NO measurements in the diagnosis and treatment of AR still needs to be further evaluated because of variable as well as contradictory findings of nasal NO concentrations in this disease.308,309,310 Likewise, nasal NO test was carried out in China in just recent years as there was not enough consistency in available data to obtain the range of normal value, which is necessary for the diagnosis of AR or other upper airway inflammatory diseases.311,312,313,314
7.1.8 Diagnosis of AR in TCM
TCM employs unique methods for the diagnosis of AR, which are completely different from those employed in Western medicine. Looking, listening, questioning and feeling the pulse are the 4 main ways of diagnosing AR, by determining 4 types of syndromes for AR as detailed above in section (6.6.2) on “Syndrome Differentiation”.
7.2 Differential diagnosis
Differential diagnosis of AR is broad and somewhat complex. Inflammation of nasal mucosa and the symptoms of sneezing, itching, rhinorrhea and nasal congestion need to be identified appropriately based on a careful history and targeted examinations.
7.2.1 Vasomotor Rhinitis
Vasomotor rhinitis, also known as nonallergic rhinitis and idiopathic rhinitis, is rhinitis with an unclear etiology, which may possibly be associated with neuroendocrine dysfunction.315,316 The symptoms of vasomotor rhinitis, particularly sneezing and watery rhinorrhea, can be induced typically by cold air, irritant odors, tobacco smoke, alcohol, sports and emotional reaction.317 There is no unique finding with tests for sensitization to allergens and eosinophil granulocyte in nasal secretions.
7.2.2 Nonallergic rhinitis with eosinophilia syndrome (NARES)
NARES is a clinical syndrome characterized by hypereosinophilia. Patients may exhibit similar symptoms to AR, but more serious and often associated with olfactory dysfunction.318 Allergen sensitization tests and nasal provocation tests are negative. Hypereosinophilia is notable both in nasal discharge and blood, with the estimated standards showing >20% more eosinophils than granular cells and monocytes in the nasal discharge and >5% eosinophils in peripheral blood.317
7.2.3 Infectious rhinitis
Infectious rhinitis is caused by a viral or bacterial infection over a short course of 7–10 days and presents with similar symptoms compared to AR initially, accompanied by fever, headache, weakness and limb pain.319 Tests for sensitization to allergens and eosinophils in nasal secretions are negative, but high lymphocyte counts are noted after acute bacterial infection.320
7.2.4 Hormonal rhinitis
Hormonal rhinitis may typically presents nasal congestion and rhinorrhea caused by abnormal levels of endocrine hormones including sex hormones, thyroxine and pituitary hormones. Tests for sensitization to allergens and eosinophils in nasal secretions are negative.
7.2.5 Medicamentous rhinitis (Rhinitis medicamentosa)
Nasal congestion caused by long-term use of a decongestant nasal spray is the marked feature of medicamentous rhintis. Tests for sensitization to allergens and eosinophils in nasal secretions are negative.321
7.2.6 Aspirin intolerance triad
Rhinitis symptoms are accompanied with asthma and nasal polyps, perhaps with urticaria and angioedema provoked by acetylsalicylic or other analgesics. Nasal polyps with high recurrence and uncontrolled asthma are the challenges. Positive aspirin provocation test, identified history, hypereosinophilia and negative allergen sensitization test are the identified features.322
7.2.7 Cerebrospinal fluid rhinorrhea
Physicians should be alert to significant trauma history, watery rhinorrhea, and absence of itching and sneezing in the diagnosis of cerebrospinal fluid leaks.323,324
Rarely, nasal symptoms similar to those of AR can also occur as a consequence of a foreign body325 or nasal tumor.326 A detailed history and examinations are the key to differential diagnosis of this condition.
7.3 QOL measurements
Rhinitis severity is based on the impact of disease on QOL. QOL measurements are the best approximation of the burden of disease on the patient with AR, particularly as a quantifiable measure of a patient's perception of the impact of his/her disease and its treatment on his/her daily life, physical/psychological/social functioning and general well-being.69,327 QOL appears to be moderately correlated with the more classic outcome variables used in clinical trials, such as daily symptom scores and nasal hyperreactivity. The effect of AR on QOL also runs parallel with the effect on conventional medical outcome measures. Generic or disease-specific questionnaires estimate QOL and are widely used both in clinical practice and in research. The choice of questionnaire should depend on the task at hand. The findings of different specific instruments of questionnaire can be combined for scheduled purpose.
7.3.1 Generic QOL questionnaire
Generic QOL questionnaires are used to measure physical, mental and psychosocial functions in all health conditions, and allow comparisons between different diseases and health populations. The most widely tested and used instrument for AR is Short-Form 36-item health survey questionnaire (SF-36), which provides summary scores in 2 domains: physical health and mental health.7 This questionnaire allows comparisons between different diseases and health populations, which makes them less suitable for measuring individual specific clinical outcomes.327 A Chinese version of SF-36 has been widely used in evaluation the QOL of adult AR and is especially suitable for comparison with other diseases. (The Chinese version of SF-36 is shown in Supporting information 1).
7.3.2 Disease-specific QOL questionnaires
Specific instruments have been designed for assessing problems patients experience from AR or conjunctivitis, and have the advantage of describing the disease-associated problems of the patients more accurately.7
1) Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ)
The RQLQ has been employed as a gold standard instrument of QOL in adult patients with both AR and rhinoconjunctivitis, and is widely used to assess the symptoms and effects of therapy.328 The RQLQ contains a list of 28 health-related items in 7 domains including sleep, non-hay fever symptoms, practical problems, nose symptoms, eye symptoms, activities and emotional function (Table 6). The RQLQ has also been adapted specifically for pediatric patients (PADQLQ)329 and adolescent patients330 according to health-related items and domains particularly relevant to these groups of patients (Table 6). The Chinese version of RQLQ is now available and can be acquired by authorization from Juniper (Shown in Supporting information 2). Currently, RQLQ is widely used in the clinic and for research purposes, especially to assess the effects of therapy including drug therapy and allergen immunotherapy.
Table 6. Different versions of RQLQ for different age groups.
| Questionnaire | Application | Number of Item | Domains |
|---|---|---|---|
| RQLQ | Adult | 28 | Sleep |
| Non-hay fever symptoms | |||
| Practical problems | |||
| Nose symptoms | |||
| Eye symptoms | |||
| Activities | |||
| Emotional function | |||
| PADQLQ [329] | Children 6 to 12 years old with SAR | 23 | Nose symptoms |
| Eye symptoms | |||
| Pratical problems | |||
| Other symptoms | |||
| Activities | |||
| RQLQ for Adolescents [330]. | 12- to 17-year-old adolescents | 25 | Sleep |
| Emotions | |||
| Nose symptoms | |||
| Eye symptoms | |||
| Other hay fever symptoms | |||
| Non-hay fever symptoms |
RQLQ, The Rhinoconjuncitivitis Quality of Life Questionnaire; PADQLQ, The Pediatric Allergic Disease Quality of Life Questionnaire.
2) Other forms of the RQLQ
Different versions of the RQLQ have been developed for special purposes. The Standardized Version of the RQLQ (RQLQ(S)) was developed by replacing the 3 “patient-specific” activity questions of RQLQ with generic activities (regular activities at home and at work, social activities and outdoor activities) to provide better evaluation for activities.331 The Mini RQLQ, which contains only 14 questions, was developed particularly for large clinical trials.332 The Nocturnal Rhinoconjunctivitis Quality of Life Questionnaire (NRQLQ) was designed to measure the functional problems that are most troublesome to patients with nocturnal AR. The NRQLQ consists of 16 items over 4 domains (sleep problems, symptoms during sleep time, symptoms on waking and practical problems).333
7.4 Psychologic status evaluation
Psychologic status is evaluated by the Self-reporting Inventory, which has been recognized as having satisfactory reliability, validity and utility. Psychologic status evaluation methods can be divided into 2 sides, which involve mental health evaluation and personality tests.
Mental health evaluation has been widely applied in the field of clinical medicine and includes the following items:
1) SCL-90 is one of the most famous psychologic measuring scales. It is sourced from the Cornell Medical Index and is widely used in psychiatric departments and psychologic counselling clinics. SCL-90 includes somatization, compulsion, interpersonal sensitivity, depression, anxiety, hostility, phobic disorder, paranoid personality and psychosis. Although Symptom Checklist-90 (SCL-90) can reflect the patient's mental health dimension relatively comprehensively, it is not a diagnostic tool, but just a type of screening checklist.
2) Self-rating Depression Scale (SDS) and Self-rating anxiety Scale (SAS) are specific screening checklists for assessing the patient's subjective feeling of depression and anxiety, respectively. In addition to early screening of patients suffering from depression and anxiety, these screens can also be used to observe changes in the degree of severity and development of the state of the illness during therapy.
3) State-Trait Anxiety Inventory (STAI) can not only directly reflect the level of the anxiety of patients, but also distinguish the state of anxiety from the consistent trait anxiety, thus offering the precise direction for further intervention and treatment.
4) Hospital Anxiety and Depression Scale (HADS) is suitable for rough screening of the tendency for depression and anxiety among general patients.
The common personality tests involve the following 2 categories:
1) Minnesota Multiphasic Personality Inventory (MMPI) is as a diagnostic tool in psychiatry for assessing personality disorder and can fully reflect the degree of subjects' physical and mental dimensions as well as clinical symptoms. MMPI covers 2 parts: 4 validity scales and 10 clinical scales. The validity scales include Question (Q), Lie scale (L), Infrequency (F) and Correction (k), while the clinical scales involve Hypochondriasis (Hs), Depression (D), Hysteria (Hy), Psychopathic deviate (Pd), Masculinity/femininity (Mf), Paranoia (Pa), Psychasthenia (Pt), Schizophrenia (Sc), Hypomania (Ma) and Social introversion (Si).
2) Eysenck Personality Questionnaire (EPQ) is a type of simplified personality inventory test that has been categorized in terms of 3 personality dimensions, which involve introversion-extroversion, psychoticism and nervousness, and exert a great effect on the outcome of the treatment for long-term chronic diseases and tumors.
All the above-mentioned mental assessment scales are in Chinese and can be found at http://www.bnufr.com/ceping/test. The physician should issue the username and password to subjects completing these assessment scales.
8. TREATMENT
8.1 Therapeutic strategy
The therapeutic principle of AR involves a comprehensive approach including environmental control, pharmacotherapy, AIT and patient education.7
These 4 key elements combine the prevention and treatment of AR. As an important part of prevention and treatment strategy, environmental control should focus on avoidance or reduction of various allergens as well as air pollutants; however, existing measures are often difficult to achieve complete control of AR. Pharmacotherapy and AIT are the main treatments for AR. Although AR is currently not completely curable, standardized comprehensive therapy can achieve optimal symptom control and significant improvement in the QOL of patients.334 Individualized approach to the education of the patient, as well as disease management and follow-up, appears to be of importance.
8.2 Allergen avoidance
Individuals exposed to high concentrations of indoor allergens (e.g. HDMs and animal dander) may benefit from multifaceted avoidance measures after environmental counseling.7,333 A Cochrane systematic review has shown that house dust mite avoidance measures can reduce allergen load and improve symptoms of perennial AR.335 However, the authors of this review concluded that due to the small sample size of clinical trials and the poor quality of the evidence, it is still difficult to provide precise recommendations. A Chinese multicenter, randomized, placebo-controlled, crossover study has demonstrated that pollen blocker cream is effective in relieving nasal symptoms and improving QOL in both adults and children with perennial AR due to HDM.336 Moreover, a systematic review and meta-analysis suggests that interventions to prevent and remediate indoor dampness and mold may reduce the risk of AR.337
During outdoor activities in season with a high load of pollens, patients sensitive to pollen should avoid the peak of allergenic pollens spread in the air to reduce AR symptoms attack.
For individuals exposed to pollens in a natural environment, we recommend some allergen-controling tools (e.g. special masks, glasses, nasal filters, pollen blocker cream, nasal cellulose powder), which can reduce nasal inhalation or conjunctival contact of the allergenic pollen and relieve nasal and ocular symptoms.7,338,339,340,341
8.3 Pharmacotherapy
8.3.1 H1-antihistamines
A large number of H1-antihistamines have been available worldwide since 1942 when the antihistamines were introduced for clinical applications for the first time. Antihistamines act as inverse agonists that combine with and stabilize the inactive conformation of the H1-receptor, shifting the equilibrium toward the inactive state. Antihistamines are functionally classified into 2 groups, first- or second-generation antihistamines, according to whether or not they enter the blood brain barrier (BBB) readily. First-generation antihistamines can cross the BBB readily and also have anticholinergic effects, which limits their use as a consequence of the side effects of sedation and mucosal dryness.342
1) Oral antihistamines
Oral antihistamines are effective against nasal symptoms, particularly rhinorrhea, sneezing and nasal itching, which are mainly mediated by histamine, but are less effective against nasal congestion.343 First-generation antihistamines often cause adverse effects such as sleepiness, impaired performance and dry mouth. In contrast, second-generation antihistamines are effective to some extent for nasal congestion aside from sneezing and watery rhinorrhea, and also cause less adverse side effects. Thus, in almost all situations, the use of oral second-generation antihistamines is proposed as first-line therapy for intermittent and persistent AR.7 Commonly used second-generation antihistamines include cetirizine, loratadine, levocetirizine and desloratadine. Some of these are marketed as OTC medications.
These agents have a rapid onset of action, occurring from 1 to 2 hours after oral administration. Most of them have duration of action of more than 24 hours, and patients can take them once daily. During regular daily dosing, little or no tolerance occurs towards their effects and they can also relieve some symptoms of eyes.344
The clinical effect for the control of nasal symptoms in trials of intermittent allergic rhinitis lasting 2 weeks and of persistent allergic rhinitis lasting 4 weeks has been extensively demonstrated; however, the sample- size has usually been small.345
The use of second-generation antihistamines prior to pollen exposure can improve nasal symptoms and activity impairment in pollinosis. Treatment is better on a regular basis than on as-needed basis for symptom relief.344
In contrast to first-generation antihistamines, second-generation antihistamines are relatively safe. The long-term safety of second-generation antihistamines, including cetirizine, levocetirizine, fexofenadine, loratadine and desloratadine, have been validated in randomized controlled trials (RCTs) lasting 6 to 18 months, in both adults and in children as young as 1 to 2 years old.342,343,346 Currently, numerous oral antihistamine products are available for use in Chinese AR patients (Table 7).
Table 7. Market share of the main oral antihistamine products used in China.
| Product | Component | Side effect | Market ratio (%) |
|---|---|---|---|
| ZYRTEC and Chinese analog | Cetirizine | Slight sedation | 8 |
| KAISITING | Ebastine | Slight sedation | 10 |
| CLARITYNE and Chinese analog | Loratadine | Slight sedation | 12 |
| ENLISI and Chinese analog | Desloratadine | Minor sedation | 12 |
| XYAL and Chinese analog | Levocetirizine | Minor sedation | 8 |
| BEIXUE | Desloratadine Citrate Disodium | Minor or no sedation | 31 |
| LUSU | Rupatadine | Minor or no sedation | 1 |
2) Intranasal antihistamines
Intranasal antihistamines are also proposed as first-line therapy. The intranasal preparations are targeted delivery that can increase dosage to nasal tissues while decreasing systemic effects.347 Intranasal antihistamines have a more rapid onset of action than oral antihistamines, ranging from 15 to 30 minutes versus 150 minutes of average onset time. Due to the rapid onset of action and targeted delivery of intranasal formulations, they are especially useful in patients with episodic nasal symptoms or as a pretreatment before inhaled allergen exposure.348 However, because of washout from the nasal mucosa, intranasal formulations require administration at 6 to 12 hours intervals.
Intranasal antihistamines also have the advantage for the relief of nasal congestion, which is much more efficacious than oral preparations. Intranasal administration shows benefit even in patients who fail oral treatment. A recent meta-analysis has shown that the efficacy of intranasal antihistamines was superior to that of oral antihistamines with respect to total nasal symptom scores (TNSS), and there was no significant difference in TNSS between patients treated intranasal antihistamine and those treated with intranasal corticosteroids.349,350,351
Currently, only 4 intranasal antihistamine products (AZEP, MIN QI, LIVOSTIN and SHUNTUOMIN) are available for the treatment of AR patients in China, of which AZEP (azelastine hydrochloride) has the largest share of the Chinese market (Table 8). In a multicenter, randomized, double-blind, parallel-group trial, Han and colleagues352 have demonstrated that azelastine and levocabastine nasal sprays, which are commonly used worldwide for the treatment of AR, are comparably safe and effective in the treatment of Chinese patients with moderate-to-severe persistent AR.
Table 8. Intranasal antihistamine products available in China.
| Product | AZEP | MIN QI | LIVOSTIN | SHUNTUOMIN |
|---|---|---|---|---|
| Component | Azelastine hydrochloride | Azelastine hydrochloride | Levocabastine Hydrochloride | Sodium Cromoglicate, Naphazoline hydrochlo- ride and Chlorphenamine Maleate |
| Age | >6 years | >12 years | Not suitable for <12 years | >7 years |
| Manufacturer | Meda, Germany | Guizhou Yunfeng, China | Janssen Pharmaceutica NV, USA | Shandong Tianshun, China |
| Side effect | Bitter | Dozy | Dozy, renal insufficiency need to follow physician's advice | Dozy |
| Market ratio | 53.65% | 27.12% | 14.23% | 5% |
The most common adverse events related to intranasal formulations use are local side effects such as epistaxis/nasal burning, poor taste, sedation, more frequent dosing and increased cost relative to oral formulations. Although the incidence of side effects is low, caution should be exercised at the initiation of intranasal antihistamines for signs of adverse events, and follow-up with a clinician is advised to assess response and side effects.
8.3.2 Leukotriene receptor antagonists
Leukotrienes are among the major mediators that are involved in the pathogenesis of AR and act by attracting eosinophils, increasing microvascular leakage and elevating mucous gland secretion.353 Leukotriene modifiers include leukotriene synthesis inhibitors and leukotriene receptor antagonist (LTRA). Singulair (Montelukast from MSD) is the patient's favourite choice of the LTRA products and occupies 11.7% of the total market in China (data taken from IMS CHPA (http://www.imshealth.com).
Montelukast works by blocking the leukotriene receptor and thus blocks the end organ response of leukotrienes. Montelukast has been shown to improve both allergen-induced nasal and ocular symptoms354 and early intervention with montelukast before pollen season significantly improves AR symptoms, compared to post-onset treatment with topical steroids only.355 Furthermore, LTRA is better suited for night-time symptoms and contributes to improvements in sleeping disorders.356
Compared to nonsedating antihistamines, LTRAs tend to provide more relief of nasal congestion. However, a meta-analysis by Xu and colleagues357 comparing the efficacy and safety of selective antihistamines and LTRAs for the treatment of seasonal AR, and a clinical trial by Liu and colleagues358 comparing the effect of treatment with montelukast or loratadine in patients with AR indicated that antihistamines and LTRAs had similar effects for seasonal AR. Indeed, a combined use of montelukast and loratadine has been suggested to provide the most effective treatment for seasonal AR and associated eye symptoms.353 Furthermore, the addition of an antihistamine to montelukast is reported to be equivalent to intranasal corticosteroids (INS).354 Montelukast in combination with intranasal steroids has also been shown to obtain faster improvement in TNSS,359 and in AR patients with steroid resistance LTRA can be used as an adjunct therapy.360
Montelukast 5 or 10 mg once daily has been reported to be a well-tolerated and safe therapeutic option for children. Alternative forms such as liquids or oral disintegrating tablets are available. Montelukast is pregnancy category B medication, allowing ease of administration to pregnant woman.
LTRAs constitute good therapeutic options in AR, with acknowledged underlying mechanisms,361 and are thus likely to provide greater benefits to AR patients in clinical practice in China.
8.3.3 Nasal corticosteroids
INS is the most efficacious anti-inflammatory medications available for the treatment of AR.362 The choice of INS in the treatment of AR is due to the attainment of high drug concentrations in the local mucosa, with a fairly small risk of systemic adverse effects.
INSs directly modulate the pathophysiology of AR, as they have been shown to significantly inhibit recruitment of basophils, eosinophils, neutrophils and mononuclear cells to nasal secretions in nasal allergen challenge models.363,364,365
One recent study has indicated that INS delivered 86% of metered dose to the nasal cavity, with approximately 60% of metered dose to the posterior nasal cavity.366 These delivery characteristics contributed to sustained nasal contact time, which improved the outcomes. For seasonal AR patients, inflammatory cells and cytokines within the nasal mucosa and secretions have been shown to be significantly reduced after use of INS.367,368 Similarly, INSs tend to decrease the SP concentration in tears of patients with seasonal AR, which is correlated with the severity of ocular and nasal symptoms.369 The efficacy of INS appears after 3–5 hours of dosing, but maximum efficacy may develop over a period of up to 2 weeks.
INS has proved to be effective in improving all symptoms of AR.370 If nasal congestion is present or symptoms are frequent, INS is the most appropriate first-line treatment as it is more effective than any other treatment.371 INS is not only effective for all nasal symptoms, but also for ocular symptoms, including itching, tearing, redness and puffiness, with no significant difference compared to intranasal antihistamine.372
High drug concentrations of INSs can be achieved at receptor sites in the nasal mucosa, with a minimal risk of systemic adverse effects, in the treatment of AR. An efficient topical delivery system also ensures fewer systemic side effects and leads to a better treatment result, which benefits the AR patient significantly. INSs are well tolerated and the adverse effects are few in number. Safety and efficacy are proved for all INSs available by multiple studies, with the side effects being mild in severity and similar in incidence as for placebo.
Currently, only 2 clinical trials investigating the effects of INS in Chinese subjects have been published in international peer-review journals. Zhang and colleagues373 assessed the effect of mometasone furoate nasal spray 200 µg once daily in a multicenter open-label study involving 500 patients with moderate-to-severe AR. The authors reported that mometasone furoate nasal spray reduced symptoms and improved quality of life of the patients. Han and colleagues374 also assessed the effect and safety of fluticasone furoate nasal spray 110 µg once daily or placebo in a multicenter, randomized, double-blind, placebo-controlled, parallel-group study involving 363 adult and adolescent subjects with intermittent or persistent AR. The authors reported that fluticasone furoate nasal spray was safe and significantly more effective than placebo in improving nasal symptoms as well as in decreasing rhinoscopy score and activities of daily living (ADL) score in patients with intermittent or persistent AR. Currently, however, fluticasone furoate nasal spray is not in general clinical use in China.
Currently, there are 4 multinational corporation and 4 domestic corporation INS products in clinical use in China, of which the multinational corporation (MNC) INS have 85% of the market share, compared to only 15% of the market share for domestic INS. Nasonex continues to be the No. 1 product among China INS market. All data are from IMS China Hospital Pharmaceutical Analysis (CHPA) (http://www.imshealth.com). Table 9 exhibits the main characteristics of the INSs available in China.
Table 9. INSs available in China.
| INS | Onset of action | Receptor affinity | Minimum age | Recommended dosage | Patients' preference | Safety |
|---|---|---|---|---|---|---|
| Mometasone furoate | In subjects with SAR, mometasonefuroate nasal spray significantly improved nasal symptomsscores compared with placebo, in as little as 7 hours after a single 200 µg dose and total symptom scores as soon as 5 hours | 2,244 | 2 years | Adults and children >12 years of age: 2 sprays (100 µg) per nostril q.d. | >Fluticasone propionate | Good |
| Children 3–11 years of age: 1 spray (50 lg) per nostril q.d. | ||||||
| Mometasone furoate (Generic) | N/A | N/A | 3 years | Adults and children >12 years of age: 2 sprays (100 µg) per nostril q.d. | N/A | Good |
| Children 3–11 years of age: 1 spray (50 lg) per nostril q.d. | ||||||
| Triamcinolone acetonide | 1–2 days | 233 | 6 years | Adults and children >12 years of age: 2 sprays (110 µg) per nostril q.i.d. | N/A | Good |
| Children 6–12 years of age: 1 spray (55 µg) per nostril or 110 µg q.i.d., up to 2 sprays (110 µg each) per nostril or 220 lg q.d. | ||||||
| Budesonide | 855 | 6 years | Adults and children >6 years of age: 1 spray (32 µg/spray) per nostril q.i.d. up to a maximum of 256 lg/day (>12 years of age) or 128 µg/day (6 to <12 years of age) | N/A | Good | |
| Fluticasone propionate | 1,775 | 4 years | Adults: 2 sprays (100 µg) per nostril q.i.d. or 1 spray (50 µg) b.i.d. | <Mometasone furoate | Good | |
| Adolescents and children > 4 years of age: 1 spray (50 µg) per nostril per day up to, but not in excess of, 2 sprays (100 µg) per nostril per day | ||||||
| Beclomethasone dipropionate | N/A | 1,345 | 6 years | Adults and children >12 years of age: 1 or 2 sprays (42–84 µg) per nostril b.i.d. (total dose 168–336 µg/day) | N/A | Good |
| Children 6–12 years: 1 spray (42 µg) per nostril b.i.d. for total of 168 lg/day up to 2 sprays per nostril b.i.d. for total of 336 µg/day |
8.3.4 Mast cell stabilizers
Mast cell stabilizers can stabilize the membrane of mast cells and basophils to prevent degranulation, thereby inhibiting the release of a variety of proinflammatory mediators, including histamine. The mast cell stabilizers are commonly used in clinical practice and include cromolyn sodium, tranilast, pemirolast potassium and so on. In China, tranilast is more widely used. These drugs partly relieve the symptoms of nasal itching, sneezing and rhinorrhea, but are not very effective in controlling nasal obstruction. In AR patients with allergic conjunctivitis, cromolyn sodium eye drops can improve ocular symptoms. Based on the mechanism of action of the mast cell stabilizers, these drugs can be used as prophylactic treatment. Indeed, patients with pollinosis (seasonal AR) are usually required to use mast cell stabilizers for about 2 weeks before the spread of pollen to reduce nose and eye symptoms.8,375
Although mast cell stabilizers have good safety and tolerance, they belong to second-line drugs376 because they have some limitations such as slow onset and short action, and also because they may not be effective in the treatment of existing symptoms.
8.3.5 Nasal decongestants
Topical decongestants can constrict blood vessels in the nasal mucosa and improve nasal patency, thus relieving the symptom of nasal obstruction in patients with AR. In clinical therapy, the most frequently used nasal decongestants are ephedrine hydrochloride, pseudoephedrine hydrochloride, oxymetazoline hydrochloride, xylometazoline hydrochloride and so on. In China, the commonly used nasal decongestant is 0.05% oxymetazoline. It has been shown that the clinical concentration of 0.05% oxymetazoline has no obvious inhibitory effects on human nasal ciliary beat frequency in vitro377; however, it should be highlighted that the nasal decongestants relieve the symptom of nasal congestion only temporarily in AR patients. When used in conjunction with INS, decongestants can benefit AR patients with stuffy noses by improving even and effective distribution of the INS.4,378
It has been pointed out that topical decongestants can cause rebound nasal congestion after continuous use for 5 days and drug-induced rhinitis after long-term use, and pose a risk to fluctuating blood pressure in elderly patients.379 As drugs belonging to a second-line therapy, nasal decongestants are generally not used continuously for more than 7 days.376 It is important to remember that a lower dose (half the concentration of the drug for adults) of nasal decongestant spray should be chosen for children.380
Oral decongestants could cause systemic side effects including hypertension, myocardial ischemia, arrhythmia and tachycardia, and are therefore not recommended for the treatment of AR patients.
8.3.6 Nasal anticholinergics
Intranasal anticholinergics can inhibit both watery secretion of nasal glands and vasodilatation of airway blood vessels. They are recommended as second-line drugs.376 Ipratropium bromide, a quaternary derivative of isopropyl noratropine, has proved to be effective in controlling watery nasal discharge of AR patients.7 Currently, however, there is no approved intranasal anticholinergic in the Chinese market.
Although systemic side effects of nasal anticholinergics are rare, care should nevertheless be exercised when these drugs are prescribed to patients suffering from prostatic hypertrophy or glaucoma.376
8.3.7 Nasal saline irrigation
Nasal saline irrigation (NSI) is a simple and inexpensive treatment for AR as well as other nasal disorders (e.g. CRS and atrophic rhinitis). Several studies have demonstrated NSI to be effective. In China, numerous types of irrigation equipment are available, and a patented product of nasal irrigation has recently been developed and extensively used by Beijing TongRen Hospital as shown in Fig. 13.
Fig. 13. Nasal irrigation equipment developed and patented by Beijing Tongren Hospital. (A) Nasal irrigator and salt package. (B) A patient performing nasal irrigation.
The exact mechanism underlying the efficacy of NSI is not well understood, but at least 3 have been proposed. First, NSI has a direct cleaning effect. As the saline passes through the nasal cavity, it can humidify and remove obstructive mucus and crusts. This can likely improve the sense of breathing immediately. Secondly, NSI can remove or reduce the inflammatory mediators and allergenic proteins such as histamine, prostaglandins, leukotrienes, eosinophil-released major basic protein and pollen. Thirdly, NSI can restore impaired nasal mucociliary function. Improved mucociliary clearance with increased ciliary beat frequency has been shown in patients receiving nasal rinsing.381,382,383,384,385
Furthermore, NSI may improve the efficacy of topical medications, such as INS and intranasal antihistamines, via clearing excessive nasal secretions and decreasing pre-existing edema. It is crucial that NSI is proposed as a good adjunctive treatment option to maintain the effectiveness of other treatment, particularly in children and pregnant women.386
8.3.8 Chinese herbal medicines
Treatment based on syndrome differentiation, an important feature of TCM, is the basic principle guiding clinical diagnosis and treatment of disease. According to the theory of Zangxiang, the treatment of AR emphasizes syndrome differentiation. TCM in the treatment of “AR” involves “Toning” the kidney, thus benefiting the lung and invigorating the spleen simultaneously. The kidney can fully play the controlling and astringing role by nourishing kidney and invigorating yang, and therefore bring sneezing to a halt. The transportation and transformation function of the spleen is strengthened; therefore, the Qi and blood are vigorous. With adequate lung-Qi and firm interstitial striae, the body surface is protected from the invasion of exogenous pathologic factors such as wind evil. Therefore, AR can be effectively prevented.
The treatment of the kidney-yang deficiency involves warming yang and invigorating the kidney; commonly used formulas are Jin Gui Shen Qi Wan and You Gui Wan. Sun and colleagues387 adopted YouGui Soup to treat AR with kidney-yang deficiency and showed that YouGui treatment had a remarkable effectiveness on symptoms, and the total effective rate was 89.4%.
The treatment of lung-Qi deficiency involves warming the lung to dissipate cold. The common prescriptions are Xiao Qing Long decoction, Guizhi decoction and Yupingfeng granule, Cangerzi granule, etc. Lin and colleagues388 used the method of invigorating Qi to consolidate the exterior to treat AR, adjusting cyclic nucleotide levels in the body, inhibiting IgE and mast cell degranulation, and restoring blood flow in nasal mucosa. This suggests invigorating Qi and benefiting the lung in the treatment of lung-Qi deficiency have great effects on AR.
Treatment of spleen-Qi deficiency is replenishing Qi to invigorate the spleen. The commonly used prescriptions are decoction of 4 noble, Bu Zhong Yi Qi decoction, Shenling Baizhu decoction, etc. Qiu and colleagues389,390 have shown that the onset of AR is closely related to spleen-Qi deficiency and that Bu Zhong Yi Qi decoction plays a role in the inhibition of cellular infiltration of acidophilic granulocytes and mastocytes in nasal mucosa in experimental spleen-Qi deficiency AR.
Treatment of lung heat is clearing lung-heat and relieving a stuffy nose. Xin Yi Qing Fei Yin is a commonly used prescription Zuwang Gan, a well-known Chinese otorhinolaryngology expert and one of the founders of modern otorhinolaryngology discipline in TCM, proposed that the treatment of lung-heat pattern AR is clearing away heat and desensitization and that Qingre Tuomin Decoction is the effective compound formulae for the pattern.391 The common Chinese herbs include: folium mori, peppermint, periostracum cicada, earthworm, beautiful sweetgum fruit, radix lithospermi, radix rubiae, Yerbadetajo Herb, etc.
Apart from the syndrome differentiation-oriented treatments for AR mentioned above, several randomized controlled clinical trials have investigated the efficacy and safety of Chinese herbal medicine (CHM) in Chinese patients with AR.392,393,394,395,396 Chan and colleagues392 reported that the Chinese herbal formulae (Cure-allergic-rhinitis Syrup [CS] and Yu-ping-feng San [YS]) were effective in reducing symptoms and enhancing the quality of life in AR patients with ‘yang- and/or Qi-deficiency’ body constitution. Similarly, Min and colleagues393 provided evidence that moxibustion in combination with a Chinese herbal preparation, comprising white mustard seed, euphorbia, corydalis tuber and ginger juice, was significantly more effective than loratadine in reducing symptoms and enhancing the quality of life in patients with AR. Another randomized controlled trial demonstrated that the Chinese herbal formula Shi-Bi-Lin was also significantly more effective than placebo in relieving symptoms of nasal blockage and improving quality of life in patients with PAR.394 Chui and colleagues395 compared the efficacy of a Chinese herbal nose drop preparation in patients with PAR and demonstrated that this preparation also significantly improved clinical symptom scores and several quality of life indices compared to placebo. A study by Hsu and colleagues396 investigated the effect of herbal point-patch treatment in AR patients and showed that acupoint herbal point-patches were an effective treatment for improvement in quality of life in AR. Besides studies in Chinese patients, some randomized trial also assessed the efficacy of CHM in Korean,397 Australian398,399,402 and German400 patients with AR. Although 2 meta-analysis of clinical trials on traditional CHM for the treatment of AR revealed that CHM interventions appeared to have beneficial effects in patients with AR,321,402 some clinical trials subsequently provided conflicting data for the potential efficacy of CHM for AR399,402 Particularly when the effect of CHM was assessed in combination with acupuncture for the treatment of SAR. While one study from Germany400 demonstrated that CHM combined with acupuncture significantly improved both symptoms and quality of life scores in SAR patients compared to acupuncture treatment alone, another study from Australia402 failed to show any significant differences in symptomatic relief or improvement in quality-of-life scores in SAR patients treated with CHM + acupuncture or acupuncture alone. While it is possible that the differences in the findings between these studies may attributed to a variety of inherent differences, the true potential of CHM as an effective therapy for AR needs to be assessed and confirmed by larger well-controlled multicenter trials in well-characterized patients treated for longer periods.
8.4 AIT
8.4.1 Mechanisms of immunotherapy
AIT represents a sole potentially curative and specific method of allergy treatment. AIT results in the restoration of immune tolerance toward the allergen of interest (Fig. 14). Numerous studies have shown that the mechanisms underlying AIT include desensitization effects (very early phase), modulation of effector cell responses and related antibody isotypes, modulation of migration of inflammatory cells (e.g. mast cells, basophils and eosinophils) and release of their mediators, and induction of Treg cells.403
Fig. 14. Allergen tolerance: changes in cells of allergic inflammation during allergen tolerance. AIT, allergen-specific immunotherapy; Breg, B regulatory cell; Treg, T regulatory cell.
The desensitization effect of AIT is similar to rapid desensitization for hypersensitivity reactions to drugs.403,404,405,406 In the very early phase, the suppression of mast cells and basophils might be affected by changes in other immune factors such as Treg cells and specific IgE levels.407 Following AIT, dendritic cells can induce T cells with a regulatory phenotype and function (Treg 1 [TR1] cells), which secrets IL-10. Such Treg cells inhibit subsequent inflammatory responses that might be important mediators of the beneficial action of AIT.408,409,410 Depletion and adoptive transfer of pulmonary plasmacytoid dendritic cells in a mouse model demonstrated that plasmacytoid dendritic cells played a central role in protection against sensitization to allergen and development of asthma.411 In addition, several clinical trials have shown that antigen presenting cells (APCs), including B cells, monocytes and macrophages, produce more IL-10 following AIT, which might lead to increased generation of IL-10-secreting TR1-like cells.412,413
In allergic diseases, the activity of both allergen-specific IL-10-secreting TR1-like cells and CD4+CD25+ Treg cells is compromised, but can be ameliorated by AIT.412,414,415,416,417 Modulation of T-cell responses to allergen following AIT occurs in several ways. These include 1) increasing the allergen-induced ratio of Th1 cytokines to Th2 cytokines,418,419 2) induction of epitope-specific T-cell anergy that can be blocked by neutralization of IL-10,422 3) generation of allergen-specific Treg cells that can suppress the responses of effector T cells following delivery of either whole allergen extracts or synthetic peptides that contain or consist of a T-cell epitope,416,417 and 4) increasing the production of cytokines with regulatory activity. Induction of mRNA that encodes IL-10 and increased production of IL-10 protein have also been reported to occur in both the blood and the tissues following AIT.404,416,417,421,422,423
When exposed to high concentrations of allergens, the levels of allergen-specific IgG4, IgG1 and IgA, but not specific IgE, are increased.416,424 Allergen-specific IgG was found to prevent immediate allergic skin inflammation following AIT. IgG isotypes could compete with IgE for the same epitopes, resulting in the binding of allergen and were therefore termed blocking antibodies.425,426,427,428,429 Studies analyzing IgG isotypes induced by AIT have shown that the concentrations of IgG1 and particularly IgG4 were increased 10- to 100-fold following AIT, influencing the blocking activity on IgE-mediated responses.430,431,432,433
8.4.2 Indications and contraindications
AIT is indicated in patients with AR/conjunctivitis and/or allergic asthma with the evidence of specific IgE antibodies to clinically relevant allergens434,435 (Table 10). SPT is the preferred method of testing for specific IgE antibodies. In vitro allergen-specific IgE testing is a reasonable alternative to SPT.
Table 10. Indications for and contraindications to AIT.
| Indications |
| Patients with AR/conjunctivitis and/or allergic asthma who have demonstrable evidence of specific IgE antibodies to clinically relevant allergens; includes patients who: |
| - Do not achieve control of symptoms with avoidance measures and pharmacotherapy |
| - Do not want ongoing or long-term pharmacotherapy |
| - Experience undesirable side effects with pharmacotherapy |
| Contraindications |
| - Patients on beta-blockers (relative contraindication with venoms) |
| - Patients with uncontrolled or severe asthma |
| - Significant co-morbid diseases, such as cardiovascular disability |
| Special considerations |
| - Children <5 years of age |
| - Pregnancy |
| - The elderly |
| - Patients with malignancy, immunodeficiency or autoimmune diseases |
1) Contraindications
AIT is contraindicated to patients with the conditions that increase their risk of dying from treatment-related systemic reactions such as severe or poorly controlled asthma, or significant cardiovascular diseases (e.g., unstable angina, recent myocardial infarction, significant arrhythmia and uncontrolled hypertension)434,435,436 (Table 10). Patients using beta-blockers are also contraindicated to AIT because these agents can amplify the severity of the reaction and make the treatment of systemic reactions more difficult.
2) Special considerations
Physicians should weigh the risks and benefits of AIT for children less than 5 years of age, since they may have difficulty in complaining about the potential side effects, especially the systemic effects. Generally, AIT is not initiated in pregnant women; however, it can be safely continued in women who have been on treatment prior to becoming pregnant. Other populations needing special consideration include the elderly who have comorbid medical conditions that may increase the risk of experiencing immunotherapy-associated adverse events, patients with autoimmune disorders, those with immunodeficiency syndromes and those with malignant disease (Table 10).434,435
8.4.3 Routes of administration
AIT carries the risk of anaphylactic reactions and should therefore only be prescribed by physicians who are trained in the treatment of allergy and the use of immunotherapy.
1) SCIT
a) Location
The injections must be given by trained personnel, in clinical settings, who are equipped to manage any possible systemic adverse reactions or anaphylaxis.434,435,436 All patients receiving AIT should generally be observed for at least 30 minutes after injection to ensure proper management of side reactions.436
b) Allergen vaccines
Allergen vaccines are commercially available for most of the commonly recognized allergens (e.g. house dust mites and grass/tree pollen). Standardized extracts should be utilized whenever possible, since the efficacy and safety of AIT depend on the quality of the allergen extracts.434,437
c) Treatment phases
Generally, AIT consists of 2 phases: a build-up phase (also known as up-dosing or induction) and a maintenance phase.434,435,436,437,438 During the build-up phase, the patient receives weekly injections, starting with a very low dose, with gradual increases in dose over the course of 4–5 months. Accelerated schedules, such as cluster/rush immunotherapy and administration of several injections at increasing doses on a single visit, may also be used. During a cluster schedule, multiple injections (usually 2–3) are administered per visit once a week, reaching maintenance in several weeks, e.g. 6 weeks. In a rush protocol, multiple injections are administered on consecutive days, generally reaching maintenance within 1 week. It has been documented that there is no increase in systemic reactions (SRs) and more rapid achievement of symptomatic improvement for the cluster schedule.437,438 A rush schedule is associated with an increase in SRs sometimes, but can also be well tolerated.435 During the maintenance phase, the patient receives injections of the maintenance dose every 6 to 8 weeks, usually for a period of 3 years. After that, many patients experience a prolonged, protective effect, and consideration can therefore be given to stopping therapy.
d) Maintenance period follow-up
Maintenance immunotherapy follow-ups include assessment of the efficacy of treatment, monitoring of adverse reactions, assessment of patient compliance, and determining whether immunotherapy can be discontinued or if dose/schedule adjustments are required when the patient is exposed to increased allergen levels or when he/she is experiencing an exacerbation of symptoms.434
e) Efficacy assessment
Symptom and medication scores are generally recommended to assess the clinical efficacy.434,435,436,437,438 At present, there are no other generally acceptable specific tests or clinical markers that can be used to assess the efficacy. Allergen specific IgG4 and IL-10-secreting type I Treg cells have been measured in some studies and found to be increased gradually.439
f) Safety
AIT is generally safe and well-tolerated when used in appropriately selected patients.437,438 However, local and systemic reactions may occur. Local reactions such as wheal, subcutaneous induration and redness/itching at the injection site can generally be managed with local treatment (e.g., cool compresses or topical corticosteroids) or oral antihistamines. Systemic reactions, generally grade 1 or 2, occur in about 1% of injections on AIT. The most severe reaction is anaphylaxis, although fatal anaphylactic reactions are rare.
2) Sublingual immunotherapy
a) Location
Although sublingual immunotherapy (SLIT) is approved for home use, guidelines indicate that the first 1 or more SLIT doses should be administered in a physician-supervised setting with a 30-minute observation period and thereafter at home.440
b) Allergen vaccines
There are 2 forms of SLIT preparations—aqueous and tablet.441 Currently, there is only 1 aqueous formulation for HDM that is used in China.376
c) Treatment phases
The HDM SLIT vaccine approved by the China Food and Drug Administration is used in the form of drops (No. 1, 1 µg/mL; No. 2, 10 µg/mL; No. 3, 100 µg/mL; No. 4, 333 µg/mL; and No. 5, 1,000 µg/mL). In the up-dosing phase of SLIT, patients are administered with increasing doses starting from No.1 to No.3 during the first 3 weeks. Children (4–14 years old) are maintained with the No.4 and adult (≥14 years old) maintained with the No.5. Patients are instructed to keep the drops under the tongue for 1–3 minutes before swallowing. The whole treatment period is 3–5 years.
d) Maintenance period follow-up
It is important and necessary to establish a normalized patient management system which includes patient records and patient follow-up information. It can assist the clinician to 1) assess the efficacy of treatment, 2) record the clinical data and scores of the patients, 3) monitor adverse reactions, 4) improve patient compliance, and 5) determine whether immunotherapy can be discontinued or dose/schedule adjustments are required when the patient is exposed to increased allergen levels or when he/she is experiencing an exacerbation of symptoms.
e) Efficacy assessment
SLIT has been established as an evidence-based effective treatment in AR. Meta-analyses confirm its efficacy in reducing both symptoms and medication scores.442,443 There are also increasing studies which show the efficacy of SLIT in Chinese AR patients.444,445,446,447 These studies have assessed various aspects of SLIT including efficacy, safety, adherence, mechanism, mono- or polysensitized patients, children or adults, and AR or asthma.
f) Safety
SLIT has a better safety profile than SCIT. The most common adverse effects are minor local reactions in the mouth and the gastrointestinal tract, with few cases of anaphylaxis, but no fatality. Adherence is more favorable for SLIT, since it is safe, non-invasive and easily taken at home, which is especially well suited for children.448,449
8.4.4 Adverse reactions and management
1) Classification
The adverse reaction of specific allergen immunotherapy is classified as local reaction (LR) or systemic reaction (SR) according to its range and severity.
SCIT-related LR is defined as swelling, erythema or pruritus around the injection site (Fig. 15), involving 26%–82% of the patients and 0.7%–16% of the shots.450,451,452,453 Chinese researchers have reported 62.9% of injections to be accompanied by LRs.454 Traditionally, SCIT-related LR can be differentiated as early local or late local reaction depending on whether the reaction happens within 30 minutes after injection.455 LRs seem to be just little bother or no bother to patients, and 96% of patients indicate that they would not discontinue the treatment because of local reactions.456
Fig. 15. SCIT-related local reaction.
SLIT-related LR is defined as oral pruritus or swelling, gastrointestinal symptoms, dizziness, etc. The WAO has classified SLIT-related LRs as mild, moderate, severe, and unknown severity, according to the severity of symptoms, if patients require symptomatic treatment and discontinuation of SLIT is required. Most of the LRs tend to disappear after the initial dose and discontinuation because the side effects are almost always less than 5%.457 According to Chinese studies, 5.1% patients reported LRs with their treatment454; however, the rate appeared to be higher (7%–9.6%) in children with combined allergic rhinitis and asthma syndrome.458
SCIT-related SRs can range in severity from mild rhinitis or urticaria symptoms to life-threatening anaphylaxis. According to the WAO SCIT SR grading system, SCIT-related SRs are divided into 5 grades459 based on the organ involved and severity of the SR. The percentage of SR in conventional schedules is approximately 0.1%–0.2% per injection,459,460 with fatal anaphylaxis reported at a rate of approximately 1 in 1–2.5 million injections.279,280,461 A recently completed multicenter study in China reported that SRs occurred in 0.47% of injections and that the occurrence of SRs was significantly higher in children than in adults.462 Risk factors for anaphylaxis include uncontrolled asthma, previous reactions to allergen immunotherapy, dosing errors, hypersensitivity, use of beta-blockers, dosing during the peak pollen season, inadequate waiting time after injections, epinephrine delayed or not given, and accelerated build-up regimens. Uncontrolled asthma is the most important risk factor therein,279,280,461,463 thus making assessment of asthma severity especially important for the prevention of anaphylaxis.
Compared to SCIT-related SRs, SLIT-related SRs are rarer and occur at a rate of around 0.056% per dose administered.464 There is still no confirmed report of SLIT-related fatality.
2) Management
Premedication could be effective in decreasing LRs during allergen immunotherapy. Oral antihistamines have been demonstrated to be effective in preventing LRs in cluster465 and rush regimens,466 whereas leukotriene antagonists have been shown to be effective in rush protocols.467 However, routine dose adjustments for LRs are not supported by the current literature.453
Most of the SCIT-related SRs appear within 30 minutes after injection, although some SRs are biphasic and could occur during 2–48 hours after the injection is administered.468 However, the second phase reaction is generally mild and additional epinephrine is unnecessary in most cases. Prompt administration of epinephrine is vital when severe SRs occur469 as a lot of anaphylaxis-related fatalities are due to the delay of epinephrine use.
The specific management of adverse events is shown below (Table 11).376,470
Table 11. The specific management of adverse events resulting from immunotherapy.
| Grade | Symptoms | Management |
|---|---|---|
| Large local reaction | Induration diameter > 4 cm (erythema, pruritus, pseudopodium) | Tourniquet usage; Adrenaline (1 mg/mL) 0.1–0.2 mL sealed injection; Steroids topically; Antihistamine orally (i.m. or i.v. when necessary) |
| Mild to moderate systemic reaction | Induration diameter > 4 cm (erythema, pruritus, pseudopodium), reaction spreading along lymphatics and blood vessels, with rhinitis, conjunctivitis, asthma or urticaria symptoms | Tourniquet usage; Adrenaline (1 mg/mL) 0.1–0.2 mL sealed injection, repeat when necessary (every 15 min); Venous channel; Steroids topically; Antihistamine i.m.; Beta-2 agonist inhalation or i.v. or s.c.; Aminophyline i.v. when necessary; Steroids i.v.; Check blood pressure and pulse rate |
| Severe systemic reaction (non life-threaten) | Pruritus of palms and soles, pruritus of scalp, skin flush, urticaria rash, dyspnea, tachypnoea, Hoarseness, abdominal pain, nausea, emesis | Adrenaline (1 mg/mL) 0.3–0.5 mg deeply i.m.; Venous channel; Steroids i.v., repeat when necessary; Antihistamine i.m.; Check blood pressure and pulse rate; Beta-2 agonist when necessary; Aminophyline i.v. when necessary |
| Oxygen; Other symptomatic treatment | ||
| Anaphylactic shock | Paleness, skin clamminess, blood pressure decreasing, mental status altering, gatism | Adrenaline (1 mg/mL) 0.5–0.8 mg deeply i.m. or (diluted 0.1 mg/mL) 0.3–0.5 mg i.v. (slowly in fractionated doses) may be repeated after 10-20 min; Place patient in supine position; Venous channel, i.vi line (saline); Vasoactive agents to maintain blood pressure; Steroids i.v. or i.v.gtt; Mechanical ventilation when necessary; Check blood pressure, pulse rate and oxygen saturation; Beta-2 agonist when necessary; Aminophyline i.v. when necessary |
For children: Adrenaline (1 mg/mL) 0.01 mg/kg (0.01 mL/kg) i.m. If needed (diluted 0.1 mg/mL) i.v. Antihistamine Clemastine (1 mg/mL) 0.0125–0.025 mg/kg i.m. Corticosteroid methylprednisolone 2 mg/kg i.v
8.5 Surgical treatment
Medical therapy is successful in a majority of patients with AR, but there are still a large number of patients who fail medical treatment. The effectiveness of surgical treatment for refractory AR has been demonstrated in several studies.471,472,473,474 There is still no gold standard surgical treatment for intractable AR, but the following rules are suggested: 1) strictly follow indications and contraindications of operations, and 2) select the proper surgical procedures and techniques based on a patient's anatomy, severity of disease and comorbidities.
8.5.1 Indications
1) Patients with persistent AR are dissatisfied with medical therapy for at least 2 years which consists of antihistamines, corticosteroid, leukotriene inhibitors and immunotherapy, and their symptoms significantly impact their quality of life. 2) The physician feels that surgical therapy may significantly improve symptoms and quality of life beyond what medical management has been able to accomplish.475
8.5.2 Contraindications
1) Patients have not ever been implemented medical therapy or immunotherapy. 2) Symptoms of concurrent asthma are not controlled. 3) Coagulation dysfunction of patients appears to be a tendency for bleeding. 4) Patients with poor health conditions hardly tolerate surgical procedures. 5) Patients with mental illness may be contraindicated to surgical treatment.376
8.5.3 The main procedures
1) Inferior turbinate reduction surgery
Severe nasal obstruction is a common symptom of AR that is resistance to medical therapy, and it is the most amenable to surgical intervention. Enlargement of the inferior turbinate is the main cause of nasal obstruction in intractable AR. Numerous techniques and procedures have been reported for reducing the volume of the inferior turbinate including submucosal resection, partial turbinectomy, radiofrequency ablation/coblation, microdebrider and laser turbinoplasty. Septalplasty may be used for patients with severe nasal septum deviation. The improvement rate of nasal obstruction has reported to be 80%–98% 3–12 months after treatment,476,477,478 and approximately 50% 3 years after treatment.479,480,481,482
2) Endoscopic vidian neurectomy
The 4 main symptoms that distress persistent AR patients are associated with dysfunction of the autonomic and sensory nerve imbalance in the nasal mucosa. The mechanism underlying neuronal-immune modulation is related to the symptoms of AR.483 The inhibition of parasympathetic nerve significantly relieves the symptoms of AR.484 Golding-Wood485 first developed transantral vidian neurectomy for treating vasomotor rhinitis and AR, with the transseptal and transpalatal approaches being reported a decade following Golding-Wood's report.475 However, none of these approaches offer entirely satisfactory outcomes, likely due to difficulties in nerve identification without magnification, risk of significant complications, including the palate fistula and damage to vision, and troublesome bleeding within a small surgical field.475 Recent advancements in endoscopic techniques of vidian neurectomy have made surgery safer and more effective (Fig. 16). Clinical studies reported that over 90% of patients with refractory AR were satisfied with surgical results during the 12 months follow-up period after endoscopic vidian neurectomy,486,487 and approximately 65% of patients undergoing bilateral endoscopic vidian neurectomy categorized their symptoms as much improved at the end of the 3 to 6 years follow-up period.471,482 Temporary dry eyes were reported in 23% to 72.9% of patients, and palatal numbness in 3% to 9%, which were resolved without any special treatment.471,486,487 No severe complications were noted in the studies.
Fig. 16. Vidian neurectomy diagram. (A) The area of incision and anatomical surroundings of interest in endoscopic vidian neurectomy. Yellow curved line indicates an incision; the oval, the location of sphenopalatine foramen; and the circle, the location of the pterygoid canal. (B) The yellow arrow indicates the horn-shaped pterygoid canal and nerve. E, indicates ethmoid sinus; IT, inferior turbinate; MT, middle turbinate; SS, sphenoid sinus; NS, nasal septum; PC, posterior choana.
3) Endoscopic posterior nasal neurectomy
The posterior nasal nerve consists of postganglionic fibers of pterygoid nerve and maxillary nerve sensory fibers, and it passes through the sphenopalatine foramen into the nasal cavity. Resection of the posterior nasal nerve lowers the hypersensitivity of nasal mucosa and reduces secretion, and alleviates inflammatory reaction.488 As a modified technique of vidian neurectomy, it has been demonstrated to have satisfactory short-term efficacy in severe allergic rhinitis, without the complication of dry eyes.473,474,489 However, the long-term efficacy of this procedure has not been noted in the published reports.
8.6 Acupuncture
Acupuncture is a traditional form of Chinese medicine, which can be traced back to 2,500 years ago, and more recently it has been widely used as a therapeutic modality for various otolaryngologic disorders.490,491 The latest American Clinical Practice Guideline for AR published in 2015, recommends that acupuncture be offered as an option for patients with interest in nonpharmacologic approaches to management of AR.492,493 Several recent international randomized controlled trials have also confirmed the efficacy of acupuncture in the treatment on AR.492,493
In conventional acupuncture for nasal inflammatory disease, needles are punctured at specific acupoints (termed Xuewei in Chinese) in the body and gently manipulated until the patient feels “de-qi sensation” (i.e. subjective feeling for patients are soreness, numbness heavy or distension, and objective feeling for acupuncturist is heavy and tight feeling under the fingers).
8.6.1 Acupuncture therapies on general acupoints to treat AR
Some Chinese acupuncturists have demonstrated that acupuncture works in the treatment of AR and achieves similar efficacy in moderate and severe AR as Western medicine. Acupuncture therapy is the safe and has no apparent adverse reactions.494 Indeed, a randomized controlled trial comparing the clinical efficacy and safety of acupuncture treatment with Western medicine in patients with moderate and severe persistent AR has recently concluded that acupuncture is not only a safe and effective intervention in these patients, but also the efficacy presents much more advantageous at its durability in the acupuncture group compared to the Western medicine group.495 An earlier study has also shown that penetration needling at various points has an obvious therapeutic effect on AR, which is even better than oral administration of Biyankang, a patented Chinese herbal medicine formulated for rhinitis.496 The basic acupoints for AR patients are Fengchi (GB 20), Yingxiang (LI 20), Feishu (BL 13) and Taiyuan (LU 9) for different causative factors.497 Acupuncture sometimes has a definite therapeutic effect on AR, especially with the anterior and posterior acupoint association method.498 The possible mechanisms underlying the efficacy of acupuncture treatment in AR are as follows: acupuncture may help improve the blood indices with an increased volume of blood flow and regulate the immunologic function of the human body which bring out therapeutic effects for AR.497 There is some evidence that acupuncture treatment can also reduce plasmatic level of IL-10 in chronic AR patients.499
8.6.2 Acupuncture therapies on special acupoints to treat AR
Acupuncture at 3 nasal points and the acupoints selected by syndrome differentiation have been shown to achieve a similar short-term efficacy on perennial AR compared to oral administration of loratadine.500 However, acupuncture therapy appears to be more advantageous for long-term efficacy compared to loratadine. A systematic review has recently indicated acupoint application for AR as follows: acupoints in lung and bladder meridians are mainly selected to assist exterior and resist the pathogenic Qi, and points in spleen and kidney meridians can treat AR fundamentally by joint use.501 Warm acupuncture in both summer and winter has been shown to improve the QOL of AR patients to a significantly greater extent than cetirizine, and this effect was also accompanied by a significantly greater decrease in serum IgE levels in acupuncture-treated patients, compared to cetirizine-treated patients.502 Jin's 3-needle therapy achieves superior efficacy on AR of lung-Qi deficiency and cold syndrome than administration of desloratadine oral suspension.503 The therapy of penetrating needling at head acupoints is safe for patients with PAR, and its effects can be found both in the short and long term.504 Compared to Western medication, the better efficacy of acupuncture and auricular pressing therapy in improvement of symptoms and signs of AR appear to be achieved by inhibition of the differentiation from Th cells to Th2 cells, adjustment of Th1/Th2 cells imbalance and reduction of IgE synthesis.505
In recent years, some acupuncturists have penetrated the needle at one special acupoint to reach the sphenopalatine ganglion (SPG) and get a definite effect (Fig. 17).506 It was concluded that SPG acupuncture could help improve nasal ventilation by increasing sympathetic nerve excitability in healthy volunteers. Two recent studies have also indicated that stimulation of the SPG by acupuncture can improve nasal symptoms and quality of life in nasal inflammatory diseases.507,508 Thus, it is possible that acupuncture-stimulated neural regulation may offer an alternative form of therapy for the management of nasal inflammatory disease in the future.
Fig. 17. Acupuncture site. (A) Common acupuncture sites. (B) Site of sphenopalatine ganglion acupuncture for AR. (C) High- resolution CT scan 3-dimensional reconstruction of the sphenopalatine ganglion acupuncture site.
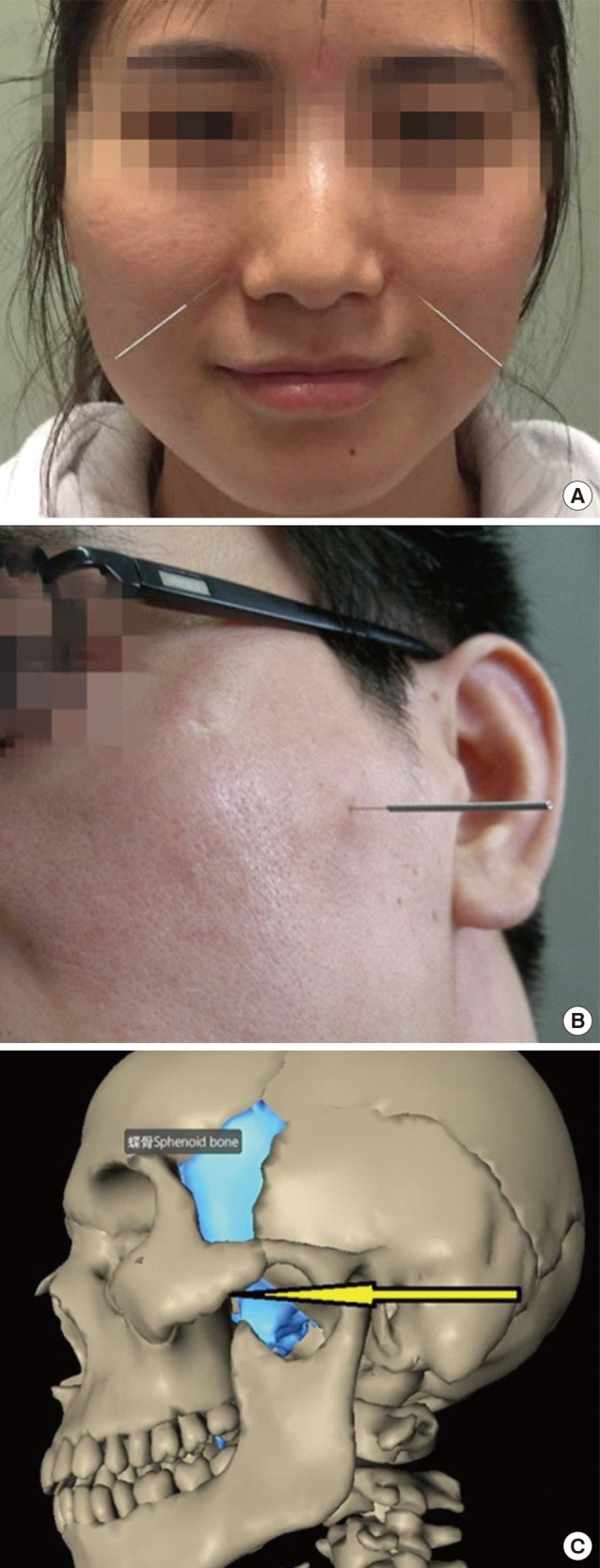
8.6.3 Connected therapies on acupoints to treat AR
Application of the dog days plaster at Dazhui (GV 14), Feishu (BL 13), Pishu (BL 20) and Shenshu (BL 23) has been shown to achieve obvious effects with good safety for AR.509 In the same season, both acupoint sticking therapy in dog days and in non-dog days can improve the symptoms of AR, but the former is better than the latter.510 Sometimes, dog-days moxibustion could be considered an enhanced method for the prevention and treatment of PAR.511 A test involving plastered and blistered application of 10% Cantharides extracton Dazhui, Neiguan point has shown that its effectiveness rate was 88%.512 In particular, nasal allergen provocation test-induced-symptoms were significantly alleviated by the therapy, and there was a significant decrease in both the numbers of eosinophils and basophils in nasal secretion and serum total IgE levels. However, compared to acupoint plaster therapy, triple-strong stimulation therapy at Dazhui (GV 14) has been shown to achieve a superior effect on the prevention and treatment of AR in children, and has a good long-term effect in preventing recurrence.513 The medicinal vesiculation combined with quick cupping at Shenque (CV 8) has a better effect for AR with syndrome of yang deficiency than oral administration of loratadine and nasal spray of budesonide.514 Acupoint catgut embedding therapy combined with acupuncture-moxibustion therapy is also safe and effective in the treatment of AR and displays more advantages for the long-term efficacy.515 However, the effect of catgut implantation needs to be substantiated in high-quality studies and in larger sample sizes in the future.516 Acupoint autohemotherapy has also been shown to significantly relieve clinical symptoms of AR, and this effect is probably associated with an increase in serum IL-12 content and the promotion of IFN-γ synthesis.517 Point-injection plus TDP radiation is an ideal therapy with a short therapeutic course, with no adverse effect and reliable therapeutic effect for AR.518
To sum up, an increasing body of evidence indicates that acupuncture is a safe treatment option, and most of the acupuncture methods employed can improve AR symptoms of nasal itching, sneezing, rhinorrhea, and especially nasal stuffiness. Acupuncture at either general or special acupoints needs to be continued over several weeks to observe significant beneficial and stable effects on symptom improvement. Dog days plaster and acupoint catgut embedding therapy can provide long-term regulation for AR patients. SPG puncturing method is a new and unusual technique, with great potential for development.
8.7 Probiotics
The interest in probiotic and its potential benefits in the prevention and treatment of diseases, including allergic diseases, are significantly increasing worldwide. Regrettably, apart from data from a few animal experiments519,520 and investigations of some other diseases,521,522 there is relatively little information on the use of probiotics in the treatment of allergic disease in China. Moreover, the available evidence is not strong enough to verify a preventive and therapeutic role of probiotics in allergy,523 and studies from other parts of the world have provided widely different data,524 probably due to differences in many aspects of study design, for example, the use of different probiotic strains/combinations and different dosage/timing as well as different demographic and genetic backgrounds. A recent meta-analysis has shown that the use of multistrain probiotics appeared to be most effective for eczema prevention, although no cogent evidence of its preventive effect has been shown for other allergic manifestations.525 To date, experts have not reached a consensus regarding the recommendation of probiotics for allergy prevention and treatment. There are still needs strong evidence based on adequately powered, well-designed, randomized, controlled trials and a more standardized approaches to support their use before final clinical recommendations on specific strains, dosage and timing can be given.
9. CLINICAL OUTCOME ASSESSMENT
Assessments of clinical outcomes of AR should be made both in the short (recent assessment) and long term. The assessments are mainly composed of subjective assessments,11 which include symptom scores, medication scores and QOL questionnaire of the patients.
9.1 Symptom scores
Subjective assessment of symptoms of AR is generally based on scores for 4 nasal symptoms (sneezing, rhinorrhea, nasal itching and nasal obstruction) and 2 ocular symptoms (ocular itching/grittiness/redness and ocular tearing).276 For patients with concomitant asthma, symptom scores for wheezing, cough, shortness of breath and chest distress are also evaluated.
Four-point scale or VAS is used to quantify the above assessments.276
9.1.1 Four-point scale:
0: No symptoms
1: Mild symptoms (symptoms present but not troublesome)
2: Moderate symptoms (troublesome symptoms but tolerable)
3: Severe symptoms (intolerable symptoms with impairment of daily activities and/or sleep)
9.1.2 VAS:
Patients grade their symptoms by putting a vertical line on a 0- to 10-cm line representing severity score from 0, ‘no symptoms’ to 10, ‘highest level of symptoms.’
9.2 Medication scores
Medication scores are usually used in AR patients that are undergoing AIT or surgical treatment. The assessment should also be reported on a daily basis and scored per day276 as follows:
1: Oral and/or topical H1-antihistamines (intranasal or intraocular)
2: INS
3: Oral corticosteroids
When the AR patient also suffers from asthma, the score should also be calculated per day as follows:
1: β2-agonist
2: Inhaled corticosteroids
9.3 QOL
The authorized Chinese version of RQLQ327 is suggested for use in the subjective assessment of the QOL in AR patients. Different versions of the RQLQ are available for use according to different ages as follows: (1) adults ≥18 years: Standard RQLQ328,331; (2) 13–17 years: Adolescent RQLQ329; and (3) 6–12 years: Pediatric RQLQ.330
10. PATIENT EDUCATION
Patient education regarding environmental control, pharmacotherapy, immunotherapy and surgical treatment is essential for the management of AR. The education must be carried out based on good communication between physicians and patients. Patients need to know not only what to do, but also why and how to do towards the disease at the outset.
Generally, a stepwise education would help patients realize the characterization of AR and its detrimental effects on QOL, understand the related treatment strategy, and complete physical and emotional preparation for accepting a long-lasting treatment. In this sense, active participation of patients can be helpful in reducing occurrence of adverse reactions and concomitance, save financial cost, and improve QOL.526
The implementation of individualized education is as important as personalized treatment. Except for distinct symptoms, results of the examinations, outcomes of the treatment, economic status and life circumstances of each patient still need to be taken into account.11 Physicians should educate patients in accordance with their own situation, involving the following items: allergen avoidance, medication use, immunotherapy choice and possible treatment outcomes.
More patience and attention need to be offered to patients who cannot understand the therapeutic strategy or those who find it hard to fulfil self-management. Although these patients might have poor educational attainment, heavy economic burden or other reasons, their desirability of relieving symptoms is very strong. Therefore, relevant dissemination requires covering patients' family members in order to get sufficient support and cooperation.
Moreover, it is notable that children with AR are likely to present symptoms with the involvement of the lungs, throat and ears. Meanwhile, QOL impairment often leads to poor- quality sleep and consequent fatigue. More seriously, poor concentration and school performance in children should be given extra concern.527 Thus, the children and their guardians' education in regard to early diagnosis and careful treatment are crucial to AR control.
On the other hand, popular science knowledge of AR could be disseminated by using traditional and novel media in an easy-to-understand fashion. However, regional disparity caused by the economic development and/or educational level should not be neglected during the implementation process. AR education might be easily acceptable to patients who live in relatively developed cities. Relatively powerful education for both patients and physicians are extremely urgent in rural and poor regions. Practicable and available methods should be applied according to local conditions such as network education as well as public assistance and government support. Moreover, better communication and close follow-up are important for patients' confidence building and will consequently improve the compliance and outcomes of the therapy.528
11. PROSPECT
In conclusion, today much research has been done aiming at the epidemiology of AR, the characteristics of genetic inheritance and the mechanism of AR. The diagnosis and treatment system suitable for the Chinese national condition have been established. However, more work is needed in the future.
In the aspect of epidemiology of AR in China, the data we have now obtained are mainly from the big cities. The extensive epidemiologic characteristic of AR for the whole population in China is unknown. With the rapid development of our economy, not only the lifestyle of the Chinese people, but also the degree of industrialization has seen dramatic changes; the distribution of allergens and the prevalence of AR are also in flux. Thus, we need more longitudinal studies about the regional prevalence of AR. On the other hand, studies about regional distribution of allergens should be continued to be performed, with the aim of determining specific allergens in certain regions.
We have made remarkable progress in the process of uncovering the mechanisms of AR and allergy, but there are still many challenging fields, which deserve further studies.
TCM, including herbal TCM and acupuncture, is a precious wealth passed down by ancient physicians and has shown to have a significant clinical effect in the treatment of AR. In spite of this, the Western therapy system occupies a primary position in the treatment of AR in China. Currently, antihistamines and INS are used as the cornerstone of AR therapy. New drugs such as specific agonists, antagonists and biologics represent a new field in AR therapy. However, basic and clinical research about biologic agents is relatively backward in our country.
For AIT, only the standardized dust mite allergen is currently used in China. Production and application of other allergen vaccines are hysteretic for the complicated procedure of approval and registration, and some allergen vaccines for immunotherapy, such as pollen, can only be used in a city like Beijing. Thus, the application and registration of new standardized allergen vaccines for the diagnosis and treatment of AR need to be promoted as soon as possible. Genetically engineered vaccines, which are expected to improve the efficacy of immunotherapy and shorten the course of treatment, have recently been investigated by researchers and some of these are at the clinical trials stage. Li and colleagues529 constructed a recombinant vaccine containing T-cell epitopes derived from Der p1 and Der p2 and showed that it effectively alleviated the allergic inflammation of the airways and lungs in experimental mice. The efficacy of some DNA vaccines was also validated in AR animal models.530
The traditional Chinese therapy should be encouraged for the treatment of AR, but faces challenges. Natural herbal medicines have complicated chemical compositions, and the effective constituents are difficult to separate accurately and standardize quantitatively. Further studies are needed to elaborate the effective components of herbal medicine and the mechanism underlying the drug's benefit. Acupuncture also suffers severely from the absence of high-quality clinical research. Integration of traditional Chinese and Western medicine is also an issue that requires much work in the future.
Footnotes
This work was supported by grants from National Key R & D Program of China (2016YFC20160905200), national natural science foundation of China (81630023, 81100704, 81441029, 81441031 and 8157089481630023, 81100704, 81441029, 81441031 and 8157089481630023, 81100704, 81441029, 81441031 and 81570894 and 81420108009), the program for Changjiang scholars and innovative research team (IRT13082), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201310), Beijing health bureau program for high level talents (2014-3-017) and Beijing Municipal Administration of Hospitals' Mission Plan (SML20150203).
References
- 1.Bousquet J, Van Cauwenberge P, Khaltaev N Aria Workshop Group; World Health Organization. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108:S147–S334. doi: 10.1067/mai.2001.118891. [DOI] [PubMed] [Google Scholar]
- 2.Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466–476. doi: 10.1016/j.jaci.2010.06.047. [DOI] [PubMed] [Google Scholar]
- 3.Roberts G, Xatzipsalti M, Borrego LM, Custovic A, Halken S, Hellings PW, et al. Paediatric rhinitis: position paper of the European Academy of Allergy and Clinical Immunology. Allergy. 2013;68:1102–1116. doi: 10.1111/all.12235. [DOI] [PubMed] [Google Scholar]
- 4.Seidman MD, Gurgel RK, Lin SY, Schwartz SR, Baroody FM, Bonner JR, et al. Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg. 2015;152:S1–S43. doi: 10.1177/0194599814561600. [DOI] [PubMed] [Google Scholar]
- 5.Ellis AK, Soliman M, Steacy L, Boulay ME, Boulet LP, Keith PK, et al. The Allergic Rhinitis - Clinical Investigator Collaborative (AR-CIC): nasal allergen challenge protocol optimization for studying AR pathophysiology and evaluating novel therapies. Allergy Asthma Clin Immunol. 2015;11:16. doi: 10.1186/s13223-015-0082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallace DV, Dykewicz MS, Bernstein DI, Blessing-Moore J, Cox L, Khan DA, et al. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122:S1–S84. doi: 10.1016/j.jaci.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 8.Okubo K, Kurono Y, Fujieda S, Ogino S, Uchio E, Odajima H, et al. Japanese guideline for allergic rhinitis 2014. Allergol Int. 2014;63:357–375. doi: 10.2332/allergolint.14-RAI-0768. [DOI] [PubMed] [Google Scholar]
- 9.Walls RS, Heddle RJ, Tang ML, Basger BJ, Solley GO, Yeo GT. Optimising the management of allergic rhinitis: an Australian perspective. Med J Aust. 2005;182:28–33. doi: 10.5694/j.1326-5377.2005.tb06551.x. [DOI] [PubMed] [Google Scholar]
- 10.Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery; Subspecialty Group of Rhinology, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association. Guidelines for diagnosis and treatment of allergic rhinitis (2009, Wuyishan) Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2009;44:977–978. [PubMed] [Google Scholar]
- 11.Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery; Subspecialty Group of Rhinology, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association. Chinese guidelines for diagnosis and treatment of allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016;51:6–24. doi: 10.3760/cma.j.issn.1673-0860.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery; Subspecialty Group of Rhinology and Pediatrics, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association; Editorial Board of Chinese Journal of Pediatrics. Guidelines for diagnosis and treatment of pediatric allergic rhinitis (2010, Chongqing) Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011;46:7–8. [PubMed] [Google Scholar]
- 13.Long A, McFadden C, DeVine D, Chew P, Kupelnick B, Lau J. Management of allergic and nonallergic rhinitis. Evid Rep Technol Assess (Summ) 2002:1–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Yorgancioğlu A, Kalayci O, Kalyoncu AF, Khaltaev N, Bousquet J. Allergic rhinitis and its impact on asthma update (ARIA 2008). The Turkish perspective. Tuberk Toraks. 2008;56:224–231. [PubMed] [Google Scholar]
- 15.Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J. 2004;24:758–764. doi: 10.1183/09031936.04.00013904. [DOI] [PubMed] [Google Scholar]
- 16.Bachert C, van Cauwenberge P, Olbrecht J, van Schoor J. Prevalence, classification and perception of allergic and nonallergic rhinitis in Belgium. Allergy. 2006;61:693–698. doi: 10.1111/j.1398-9995.2006.01054.x. [DOI] [PubMed] [Google Scholar]
- 17.Nathan RA, Meltzer EO, Derebery J, Campbell UB, Stang PE, Corrao MA, et al. The prevalence of nasal symptoms attributed to allergies in the United States: findings from the burden of rhinitis in an America survey. Allergy Asthma Proc. 2008;29:600–608. doi: 10.2500/aap.2008.29.3179. [DOI] [PubMed] [Google Scholar]
- 18.Katelaris CH, Lee BW, Potter PC, Maspero JF, Cingi C, Lopatin A, et al. Prevalence and diversity of allergic rhinitis in regions of the world beyond Europe and North America. Clin Exp Allergy. 2012;42:186–207. doi: 10.1111/j.1365-2222.2011.03891.x. [DOI] [PubMed] [Google Scholar]
- 19.Choi BC, McQueen DV, Puska P, Douglas KA, Ackland M, Campostrini S, et al. Enhancing global capacity in the surveillance, prevention, and control of chronic diseases: seven themes to consider and build upon. J Epidemiol Community Health. 2008;62:391–397. doi: 10.1136/jech.2007.060368. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Han D, Huang D, Wu Y, Dong Z, Xu G, et al. Prevalence of self-reported allergic rhinitis in eleven major cities in china. Int Arch Allergy Immunol. 2009;149:47–57. doi: 10.1159/000176306. [DOI] [PubMed] [Google Scholar]
- 21.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–491. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 22.Weiland SK, Björkstén B, Brunekreef B, Cookson WO, von Mutius E, Strachan DP, et al. Phase II of the International Study of Asthma and Allergies in Childhood (ISAAC II): rationale and methods. Eur Respir J. 2004;24:406–412. doi: 10.1183/09031936.04.00090303. [DOI] [PubMed] [Google Scholar]
- 23.Ellwood P, Asher MI, Beasley R, Clayton TO, Stewart AW ISAAC Steering Committee. The international study of asthma and allergies in childhood (ISAAC): phase three rationale and methods. Int J Tuberc Lung Dis. 2005;9:10–16. [PubMed] [Google Scholar]
- 24.Li F, Zhou Y, Li S, Jiang F, Jin X, Yan C, et al. Prevalence and risk factors of childhood allergic diseases in eight metropolitan cities in China: a multicenter study. BMC Public Health. 2011;11:437. doi: 10.1186/1471-2458-11-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Zhang L. Prevalence of allergic rhinitis in china. Allergy Asthma Immunol Res. 2014;6:105–113. doi: 10.4168/aair.2014.6.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang XD, Zheng M, Lou HF, Wang CS, Zhang Y, Bo MY, et al. An increased prevalence of self-reported allergic rhinitis in major Chinese cities from 2005 to 2011. Allergy. 2016;71:1170–1180. doi: 10.1111/all.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng M, Wang X, Bo M, Wang K, Zhao Y, He F, et al. Prevalence of allergic rhinitis among adults in urban and rural areas of china: a population-based cross-sectional survey. Allergy Asthma Immunol Res. 2015;7:148–157. doi: 10.4168/aair.2015.7.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang YM, Zhang J, Liu SL, Zhang X, Yang SN, Gao J, et al. Prevalence and associated risk factors of allergic rhinitis in preschool children in Beijing. Laryngoscope. 2013;123:28–35. doi: 10.1002/lary.23573. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Zhao Y, Li B, Zhang Q, Wan L, Liu J, et al. A multicenter study of the clinical features of allergic rhinitis in central China. Am J Rhinol Allergy. 2014;28:392–396. doi: 10.2500/ajra.2014.28.4075. [DOI] [PubMed] [Google Scholar]
- 30.Wheatley LM, Togias A. Clinical practice. Allergic rhinitis. N Engl J Med. 2015;372:456–463. doi: 10.1056/NEJMcp1412282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han DM, Zhang L, Huang D, Wu YF, Dong Z, Xu G, et al. Self-reported prevalence of allergic rhinitis in eleven cities in China. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007;42:378–384. [PubMed] [Google Scholar]
- 32.Zhang L, Jin T, Han DM. Allergic rhinoconjunctivitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012;47:173–176. [PubMed] [Google Scholar]
- 33.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012:1–298. 3 p preceding table of contents. [PubMed] [Google Scholar]
- 34.Shi JB, Fu QL, Zhang H, Cheng L, Wang YJ, Zhu DD, et al. Epidemiology of chronic rhinosinusitis: results from a cross-sectional survey in seven Chinese cities. Allergy. 2015;70:533–539. doi: 10.1111/all.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu QL, Ma JX, Ou CQ, Guo C, Shen SQ, Xu G, et al. Influence of self-reported chronic rhinosinusitis on health-related quality of life: a population-based survey. PLoS One. 2015;10:e0126881. doi: 10.1371/journal.pone.0126881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bousquet J, Schunemann HJ, Fonseca J, Samolinski B, Bachert C, Canonica GW, et al. MACVIA-ARIA Sentinel NetworK for allergic rhinitis (MASK-rhinitis): the new generation guideline implementation. Allergy. 2015;70:1372–1392. doi: 10.1111/all.12686. [DOI] [PubMed] [Google Scholar]
- 37.Lai K, Chen R, Lin J, Huang K, Shen H, Kong L, et al. A prospective, multicenter survey on causes of chronic cough in China. Chest. 2013;143:613–620. doi: 10.1378/chest.12-0441. [DOI] [PubMed] [Google Scholar]
- 38.Lack G, Caulfield H, Penagos M. The link between otitis media with effusion and allergy: a potential role for intranasal corticosteroids. Pediatr Allergy Immunol. 2011;22:258–266. doi: 10.1111/j.1399-3038.2010.01130.x. [DOI] [PubMed] [Google Scholar]
- 39.Spieksma FT, Dieges PH. The history of the finding of the house dust mite. J Allergy Clin Immunol. 2004;113:573–576. doi: 10.1016/j.jaci.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 40.Stewart GA. Dust mite allergens. Clin Rev Allergy Immunol. 1995;13:135–150. doi: 10.1007/BF02758098. [DOI] [PubMed] [Google Scholar]
- 41.Fernández-Caldas E, Puerta L, Caraballo L, Lockey RF. Mite allergens. Clin Allergy Immunol. 2008;21:161–182. [PubMed] [Google Scholar]
- 42.Chan TF, Ji KM, Yim AK, Liu XY, Zhou JW, Li RQ, et al. The draft genome, transcriptome, and microbiome of Dermatophagoides farinae reveal a broad spectrum of dust mite allergens. J Allergy Clin Immunol. 2015;135:539–548. doi: 10.1016/j.jaci.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 43.Thomas WR. Hierarchy and molecular properties of house dust mite allergens. Allergol Int. 2015;64:304–311. doi: 10.1016/j.alit.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Smith M, Cecchi L, Skjøth CA, Karrer G, Šikoparija B. Common ragweed: a threat to environmental health in Europe. Environ Int. 2013;61:115–126. doi: 10.1016/j.envint.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Tang R, Sun JL, Yin J, Li Z. Artemisia allergy research in China. Biomed Res Int. 2015;2015:179426. doi: 10.1155/2015/179426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Sun B, Huang Y, Lin X, Zhao D, Tan G, et al. A multicentre study assessing the prevalence of sensitizations in patients with asthma and/or rhinitis in China. Allergy. 2009;64:1083–1092. doi: 10.1111/j.1398-9995.2009.01967.x. [DOI] [PubMed] [Google Scholar]
- 47.Lin H, Lin R, Li N. Sensitization rates for various allergens in children with allergic rhinitis in Qingdao, China. Int J Environ Res Public Health. 2015;12:10984–10994. doi: 10.3390/ijerph120910984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gu ZY, Li Y, Zhao CQ. Allergology ear nose throat head and neck disease. 1st ed. Beijing: People's Medical Publishing House; 2012. [Google Scholar]
- 49.Han DM, Zhang L, Bachert C, Dong Z, Lin XP. Allergic rhinitis. 2nd ed. Beijing: People's Medical Publishing House; 2014. [Google Scholar]
- 50.He S, Li YJ, Chen J. Clinical features of allergic rhinitis in children of Shanghai, China. Genet Mol Res. 2016;15:1–13. doi: 10.4238/gmr.15028118. [DOI] [PubMed] [Google Scholar]
- 51.Wang W, Huang X, Chen Z, Zheng R, Chen Y, Zhang G, et al. Prevalence and trends of sensitisation to aeroallergens in patients with allergic rhinitis in Guangzhou, China: a 10-year retrospective study. BMJ Open. 2016;6:e011085. doi: 10.1136/bmjopen-2016-011085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simoens S, Laekeman G. Pharmacotherapy of allergic rhinitis: a pharmaco-economic approach. Allergy. 2009;64:85–95. doi: 10.1111/j.1398-9995.2008.01909.x. [DOI] [PubMed] [Google Scholar]
- 53.Chen J, Xiang J, Wang Y, Shi Q, Tan H, Kong W. Health economics analysis of specific immunotherapy in allergic rhinitis accompanied with asthma. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013;27:925–928. [PubMed] [Google Scholar]
- 54.Nathan RA. The burden of allergic rhinitis. Allergy Asthma Proc. 2007;28:3–9. doi: 10.2500/aap.2007.28.2934. [DOI] [PubMed] [Google Scholar]
- 55.Blaiss MS. Allergic rhinitis: direct and indirect costs. Allergy Asthma Proc. 2010;31:375–380. doi: 10.2500/aap.2010.31.3329. [DOI] [PubMed] [Google Scholar]
- 56.Bachert C, van Cauwenberge P, Khaltaev N World Health Organization. Allergic rhinitis and its impact on asthma. In collaboration with the World Health Organization. Executive summary of the workshop report. 7–10 December 1999, Geneva, Switzerland. Allergy. 2002;57:841–855. doi: 10.1034/j.1398-9995.2002.23625.x. [DOI] [PubMed] [Google Scholar]
- 57.Ozdoganoglu T, Songu M, Inancli HM. Quality of life in allergic rhinitis. Ther Adv Respir Dis. 2012;6:25–39. doi: 10.1177/1753465811424425. [DOI] [PubMed] [Google Scholar]
- 58.Song Y, Wang M, Xie J, Li W, Zhang X, Wang T, et al. Prevalence of allergic rhinitis among elementary and middle school students in Changsha city and its impact on quality of life. J Laryngol Otol. 2015;129:1108–1114. doi: 10.1017/S0022215115002492. [DOI] [PubMed] [Google Scholar]
- 59.Yin Y, Lu QY. Analysis of quality of life and emotion symptoms in adolescents with allergic rhinitis in middle area of Jiangsu province. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016;51:86–89. doi: 10.3760/cma.j.issn.1673-0860.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 60.Ke X, Qian D, Zhu L, Hong S. Analysis on quality of life and personality characteristics of allergic rhinitis. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010;24:200–202. [PubMed] [Google Scholar]
- 61.Liu G, Zhu R, Wang Z, Huang A, Li W, Zhang W, et al. Assessment of quality of life in allergic rhinitis patients with Chinese version of SF-36. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2005;19:541–542. [PubMed] [Google Scholar]
- 62.Wang Y, Zhu R, Liu G, Li W, Chen H, Daurès JP, et al. Prevalence of uncontrolled allergic rhinitis in Wuhan, China: a prospective cohort study. Am J Rhinol Allergy. 2014;28:397–403. doi: 10.2500/ajra.2014.28.4079. [DOI] [PubMed] [Google Scholar]
- 63.Huang ZZ, Zhang GH, Zhao G, Ye J, Liu X, Chen YL, et al. Clinical research on the quality of life in patients with allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010;45:450–454. [PubMed] [Google Scholar]
- 64.Li L, Guan K. Quality of life in 164 allergic rhinitis patients caused by different aeroallergens. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015;29:226–229. [PubMed] [Google Scholar]
- 65.Xi L, Zhang Y, Han D, Zhang L. Effect of asthma, aeroallergen category, and gender on the psychological status of patients with allergic rhinitis. J Investig Allergol Clin Immunol. 2012;22:264–269. [PubMed] [Google Scholar]
- 66.Lv X, Han D, Xi L, Zhang L. Psychological aspects of female patients with moderate-to-severe persistent allergic rhinitis. ORL J Otorhinolaryngol Relat Spec. 2010;72:235–241. doi: 10.1159/000314884. [DOI] [PubMed] [Google Scholar]
- 67.Cao R, Xu Y, Tao Z, Zhang Y, Chen W, Deng A. Analysis of symptoms and quality of life in children with allergic rhinitis. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010;24:1071–1073. 1076. [PubMed] [Google Scholar]
- 68.Sha JC, Zhu DD, Dong Z, Jiang XD, Li L, Zhu XW, et al. Survey on clinical characteristics of pediatric allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011;46:26–30. [PubMed] [Google Scholar]
- 69.Maspero J, Lee BW, Katelaris CH, Potter PC, Cingi C, Lopatin A, et al. Quality of life and control of allergic rhinitis in patients from regions beyond western Europe and the United States. Clin Exp Allergy. 2012;42:1684–1696. doi: 10.1111/j.1365-2222.2012.04025.x. [DOI] [PubMed] [Google Scholar]
- 70.Small M, Piercy J, Demoly P, Marsden H. Burden of illness and quality of life in patients being treated for seasonal allergic rhinitis: a cohort survey. Clin Transl Allergy. 2013;3:33. doi: 10.1186/2045-7022-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adebola SO, Abidoye B, Ologe FE, Adebola OE, Oyejola BA. Health-related quality of life and its contributory factors in allergic rhinitis patients in Nigeria. Auris Nasus Larynx. 2016;43:171–175. doi: 10.1016/j.anl.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 72.Miraglia Del Giudice M, Marseglia A, Leonardi S, La Rosa M, Salpietro C, Brunese FP, et al. Allergic rhinitis and quality of life in children. Int J Immunopathol Pharmacol. 2011;24:25–28. doi: 10.1177/03946320110240s406. [DOI] [PubMed] [Google Scholar]
- 73.Chida Y, Hamer M, Steptoe A. A bidirectional relationship between psychosocial factors and atopic disorders: a systematic review and meta-analysis. Psychosom Med. 2008;70:102–116. doi: 10.1097/PSY.0b013e31815c1b71. [DOI] [PubMed] [Google Scholar]
- 74.Cuffel B, Wamboldt M, Borish L, Kennedy S, Crystal-Peters J. Economic consequences of comorbid depression, anxiety, and allergic rhinitis. Psychosomatics. 1999;40:491–496. doi: 10.1016/S0033-3182(99)71187-4. [DOI] [PubMed] [Google Scholar]
- 75.Postolache TT, Stiller JW, Herrell R, Goldstein MA, Shreeram SS, Zebrak R, et al. Tree pollen peaks are associated with increased nonviolent suicide in women. Mol Psychiatry. 2005;10:232–235. doi: 10.1038/sj.mp.4001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sansone RA, Sansone LA. Allergic rhinitis: relationships with anxiety and mood syndromes. Innov Clin Neurosci. 2011;8:12–17. [PMC free article] [PubMed] [Google Scholar]
- 77.Qin P, Mortensen PB, Waltoft BL, Postolache TT. Allergy is associated with suicide completion with a possible mediating role of mood disorder - a population-based study. Allergy. 2011;66:658–664. doi: 10.1111/j.1398-9995.2010.02523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lv X, Xi L, Han D, Zhang L. Evaluation of the psychological status in seasonal allergic rhinitis patients. ORL J Otorhinolaryngol Relat Spec. 2010;72:84–90. doi: 10.1159/000297576. [DOI] [PubMed] [Google Scholar]
- 79.Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108:S2–S8. doi: 10.1067/mai.2001.115569. [DOI] [PubMed] [Google Scholar]
- 80.Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery; Chinese Otorhinolaryngology Society of Chinese Medical Association. Diagnostic and treatment principle for allergic rhinitis and a recommended scheme. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2005;40:166–167. [PubMed] [Google Scholar]
- 81.Rondon C, Fernandez J, Canto G, Blanca M. Local allergic rhinitis: concept, clinical manifestations, and diagnostic approach. J Investig Allergol Clin Immunol. 2010;20:364–371. [PubMed] [Google Scholar]
- 82.Gómez F, Rondón C, Salas M, Campo P. Local allergic rhinitis: mechanisms, diagnosis and relevance for occupational rhinitis. Curr Opin Allergy Clin Immunol. 2015;15:111–116. doi: 10.1097/ACI.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 83.Dordal MT, Lluch-Bernal M, Sánchez MC, Rondón C, Navarro A, Montoro J, et al. Allergen-specific nasal provocation testing: review by the rhinoconjunctivitis committee of the Spanish Society of Allergy and Clinical Immunology. J Investig Allergol Clin Immunol. 2011;21:1–12. [PubMed] [Google Scholar]
- 84.Rondón C, Campo P, Herrera R, Blanca-Lopez N, Melendez L, Canto G, et al. Nasal allergen provocation test with multiple aeroallergens detects polysensitization in local allergic rhinitis. J Allergy Clin Immunol. 2011;128:1192–1197. doi: 10.1016/j.jaci.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 85.Rondón C, Campo P, Togias A, Fokkens WJ, Durham SR, Powe DG, et al. Local allergic rhinitis: concept, pathophysiology, and management. J Allergy Clin Immunol. 2012;129:1460–1467. doi: 10.1016/j.jaci.2012.02.032. [DOI] [PubMed] [Google Scholar]
- 86.Rondón C, Campo P, Zambonino MA, Blanca-Lopez N, Torres MJ, Melendez L, et al. Follow-up study in local allergic rhinitis shows a consistent entity not evolving to systemic allergic rhinitis. J Allergy Clin Immunol. 2014;133:1026–1031. doi: 10.1016/j.jaci.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 87.van Beijsterveldt CE, Boomsma DI. Genetics of parentally reported asthma, eczema and rhinitis in 5-yr-old twins. Eur Respir J. 2007;29:516–521. doi: 10.1183/09031936.00065706. [DOI] [PubMed] [Google Scholar]
- 88.Feijen M, Gerritsen J, Postma DS. Genetics of allergic disease. Br Med Bull. 2000;56:894–907. doi: 10.1258/0007142001903580. [DOI] [PubMed] [Google Scholar]
- 89.Ma L, Chen DL, Zhang RX, Wang XL, Shi YJ, Ji C, et al. Genetic epidemiological study on allergic rhinitis in Nantong region of Jiangsu province. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007;42:643–646. [PubMed] [Google Scholar]
- 90.Haagerup A, Bjerke T, Schøitz PO, Binderup HG, Dahl R, Kruse TA. Allergic rhinitis--a total genome-scan for susceptibility genes suggests a locus on chromosome 4q24–q27. Eur J Hum Genet. 2001;9:945–952. doi: 10.1038/sj.ejhg.5200753. [DOI] [PubMed] [Google Scholar]
- 91.Haagerup A, Børglum AD, Binderup HG, Kruse TA. Fine-scale mapping of type I allergy candidate loci suggests central susceptibility genes on chromosomes 3q, 4q and Xp. Allergy. 2004;59:88–94. doi: 10.1111/j.1398-9995.2004.00294.x. [DOI] [PubMed] [Google Scholar]
- 92.Yokouchi Y, Shibasaki M, Noguchi E, Nakayama J, Ohtsuki T, Kamioka M, et al. A genome-wide linkage analysis of orchard grass-sensitive childhood seasonal allergic rhinitis in Japanese families. Genes Immun. 2002;3:9–13. doi: 10.1038/sj.gene.6363815. [DOI] [PubMed] [Google Scholar]
- 93.Lin S, Liu R, Guo H. Detection of antigen specificities of HLA-A, B loci in perennial allergic rhinitis. Zhonghua Er Bi Yan Hou Ke Za Zhi. 1997;32:18–20. [PubMed] [Google Scholar]
- 94.Yang L, Zhang Q, Zhang P. Analysis of HLA-DRB1 allele polymorphism for patients with allergic rhinitis. Zhonghua Er Bi Yan Hou Ke Za Zhi. 1999;34:147–149. [PubMed] [Google Scholar]
- 95.Xing Z, Yu D. Linkage of allergic rhinitis with HLA-DRB alleles polymorphism. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2001;15:199–201. [PubMed] [Google Scholar]
- 96.Xing Z, Yu D, An S. Association of hypersensitivity to wormwood pollen in patients with allergic rhinitis with HLA alleles polymorphism. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2002;16:678–680. [PubMed] [Google Scholar]
- 97.Wang M, Xing ZM, Yu DL, Yan Z, Yu LS. Association between HLA class II locus and the susceptibility to Artemisia pollen-induced allergic rhinitis in Chinese population. Otolaryngol Head Neck Surg. 2004;130:192–196. doi: 10.1016/j.otohns.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 98.Cui Z, Zhang H, Liu Y, Yang Y, Xiang Y. Analysis of HLA-DQB1 polymorphism for patients with allergic rhinitis of Uygur and Han people in Xinjiang. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011;25:645–648. [PubMed] [Google Scholar]
- 99.Zhao Y, Zhao Y, Li J, Zhang Y, Zhang L. HLA-DRB1*08:03:02 and HLA-DQB1*06:01:01 are associated with house dust mite-sensitive allergic rhinitis in Chinese subjects. Int Forum Allergy Rhinol. 2016;6:854–861. doi: 10.1002/alr.21747. [DOI] [PubMed] [Google Scholar]
- 100.Andiappan AK, Rotzschke O, Wang Y, Chew FT. Association of interleukin-13 SNP rs20541 (Arg>Gln) to allergic rhinitis in an Asian population of ethnic Chinese in Singapore. Gene. 2013;529:357–358. doi: 10.1016/j.gene.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 101.Ying XJ, Zhao SW, Wang GL, Xie J, Xu HM, Dong P. Association of interleukin-13 SNP rs20541 with allergic rhinitis risk: a meta-analysis. Gene. 2013;521:222–226. doi: 10.1016/j.gene.2013.03.088. [DOI] [PubMed] [Google Scholar]
- 102.Robertson JI. Antihypertensive drug therapy: achievements, failures and prospects. Neth J Med. 1990;37:89–93. [PubMed] [Google Scholar]
- 103.Zhang Y, Li J, Wang C, Zhang L. Association between the interaction of key genes involved in effector T-cell pathways and susceptibility to develop allergic rhinitis: a population-based case-control association study. PLoS One. 2015;10:e0131248. doi: 10.1371/journal.pone.0131248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang M, Zhang Y, Han D, Zhang L. Association between polymorphisms in cytokine genes IL-17A and IL-17F and development of allergic rhinitis and comorbid asthma in Chinese subjects. Hum Immunol. 2012;73:647–653. doi: 10.1016/j.humimm.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 105.Zhao N, Liu HJ, Sun YY, Li YZ. Role of interleukin-6 polymorphisms in the development of allergic rhinitis. Genet Mol Res. 2016;15:1–6. doi: 10.4238/gmr.15016987. [DOI] [PubMed] [Google Scholar]
- 106.Shen Y, Yuan XD, Hu D, Ke X, Wang XQ, Hu GH, et al. Association between interleukin-27 gene polymorphisms and susceptibility to allergic rhinitis. Hum Immunol. 2014;75:991–995. doi: 10.1016/j.humimm.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 107.Hu D, Hu G, Zhu J, Shen Y, Kang H, Hong S. Association between polymorphisms of the IL-23R gene and allergic rhinitis in a Chinese Han population. PLoS One. 2013;8:e63858. doi: 10.1371/journal.pone.0063858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wei P, Kou W, Sun R, Hu GH, Hu D, Feng J, et al. Erratum to: association study between interleukin-12 receptor β1/β2 genes and allergic rhinitis in the Chinese Han population. Eur Arch Otorhinolaryngol. 2015;272:895–896. doi: 10.1007/s00405-014-3204-2. [DOI] [PubMed] [Google Scholar]
- 109.Zhang Y, Duan S, Wei X, Zhao Y, Zhao L, Zhang L. Association between polymorphisms in FOXP3 and EBI3 genes and the risk for development of allergic rhinitis in Chinese subjects. Hum Immunol. 2012;73:939–945. doi: 10.1016/j.humimm.2012.07.319. [DOI] [PubMed] [Google Scholar]
- 110.Shen Y, Liu Y, Ke X, Kang HY, Hu GH, Hong SL. Association between JAK1 gene polymorphisms and susceptibility to allergic rhinitis. Asian Pac J Allergy Immunol. 2016;34:124–129. doi: 10.12932/AP0630.34.2.2016. [DOI] [PubMed] [Google Scholar]
- 111.Gu Z, Hong SL, Ke X, Shen Y, Wang XQ, Hu D, et al. FCRL3 gene polymorphisms confer autoimmunity risk for allergic rhinitis in a Chinese Han population. PLoS One. 2015;10:e0116419. doi: 10.1371/journal.pone.0116419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu Y, Ke X, Kang HY, Wang XQ, Shen Y, Hong SL. Genetic risk of TNFSF4 and FAM167A-BLK polymorphisms in children with asthma and allergic rhinitis in a Han Chinese population. J Asthma. 2016;53:567–575. doi: 10.3109/02770903.2015.1108437. [DOI] [PubMed] [Google Scholar]
- 113.Yang KD, Liu CA, Chang JC, Chuang H, Ou CY, Hsu TY, et al. Polymorphism of the immune-braking gene CTLA-4 (+49) involved in gender discrepancy of serum total IgE levels and allergic diseases. Clin Exp Allergy. 2004;34:32–37. doi: 10.1111/j.1365-2222.2004.01776.x. [DOI] [PubMed] [Google Scholar]
- 114.Han D, She W, Zhang L. Association of the CD14 gene polymorphism C-159T with allergic rhinitis. Am J Rhinol Allergy. 2010;24:e1–e3. doi: 10.2500/ajra.2010.24.3411. [DOI] [PubMed] [Google Scholar]
- 115.Wei X, Zhang Y, Fu Z, Zhang L. The association between polymorphisms in the MRPL4 and TNF-α genes and susceptibility to allergic rhinitis. PLoS One. 2013;8:e57981. doi: 10.1371/journal.pone.0057981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao Y, Zhang Y, Zhang L. Variant of PBX2 gene in the 6p21.3 asthma susceptibility locus is associated with allergic rhinitis in Chinese subjects. Int Forum Allergy Rhinol. 2016;6:537–543. doi: 10.1002/alr.21725. [DOI] [PubMed] [Google Scholar]
- 117.Chen Q, Liu Z, Zhang H. Relationship between rs1057141 and rs1135216 polymorphisms of TAP1 gene and allergic rhinitis in Xinjiang Han people. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012;26:917–920. 925. [PubMed] [Google Scholar]
- 118.Ke X, Yang Y, Shen Y, Wang X, Hong S. Association between TNFAIP3 gene polymorphisms and risk of allergic rhinitis in a Chinese Han population. Iran J Allergy Asthma Immunol. 2016;15:46–52. [PubMed] [Google Scholar]
- 119.Wang XD, Zhang L, Duan H, She WY, Zhao Y, Liu S, et al. Association of single nucleotide polymorphisms of GATA3 with allergic rhinitis phenotypes in Chinese. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008;43:494–498. [PubMed] [Google Scholar]
- 120.Zhang Y, Lin X, Desrosiers M, Zhang W, Meng N, Zhao L, et al. Association pattern of interleukin-1 receptor-associated kinase-4 gene polymorphisms with allergic rhinitis in a Han Chinese population. PLoS One. 2011;6:e21769. doi: 10.1371/journal.pone.0021769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhu XJ, Zhu LP, Lu MP, Wang ML, Qi QH, Yin M, et al. Association of TGFB1 gene polymorphism -509C/T with disease severity in childhood allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010;45:459–464. [PubMed] [Google Scholar]
- 122.Jin P, Andiappan AK, Quek JM, Lee B, Au B, Sio YY, et al. A functional brain-derived neurotrophic factor (BDNF) gene variant increases the risk of moderate-to-severe allergic rhinitis. J Allergy Clin Immunol. 2015;135:1486–1493.e8. doi: 10.1016/j.jaci.2014.12.1870. [DOI] [PubMed] [Google Scholar]
- 123.Tian HQ, Chen XY, Lu Y, Lu WM, Wang ML, Zhao HL, et al. Association of VDR and CYP2R1 polymorphisms with mite-sensitized persistent allergic rhinitis in a Chinese population. PLoS One. 2015;10:e0133162. doi: 10.1371/journal.pone.0133162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huang RF, Dong P, Zhang TZ, Ying XJ, Hu H. Angiotensin-converting enzyme insertion/deletion polymorphism and susceptibility to allergic rhinitis in Chinese populations: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2016;273:277–283. doi: 10.1007/s00405-014-3350-6. [DOI] [PubMed] [Google Scholar]
- 125.Guo M, Ma J, Han Y, Lu L. Angiotensin-converting enzyme gene insertion/deletion polymorphisms and the susceptibility to allergic rhinitis. Allergol Immunopathol (Madr) 2014;42:568–572. doi: 10.1016/j.aller.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 126.Lin H, Lin D, Zheng CQ. Angiotensin-converting enzyme insertion/deletion polymorphism associated with allergic rhinitis susceptibility: evidence from 1,410 subjects. J Renin Angiotensin Aldosterone Syst. 2014;15:593–600. doi: 10.1177/1470320313502107. [DOI] [PubMed] [Google Scholar]
- 127.Li Z, Yan F, Yang Z, Zhou J, Chen Y, Ding Z. Association between ADAM33 S2 and V4 polymorphisms and susceptibility to allergic rhinitis: a meta-analysis. Allergol Immunopathol (Madr) 2016;44:170–176. doi: 10.1016/j.aller.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 128.Chen RX, Lu WM, Zhu LP, Lu MP, Wang ML, Wang YL, et al. Association study on ADAM33 polymorphisms in mite-sensitized persistent allergic rhinitis in a Chinese population. PLoS One. 2014;9:e95033. doi: 10.1371/journal.pone.0095033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tang XF, Tang HY, Sun LD, Xiao FL, Zhang Z, Li Y, et al. Genetic variant rs4982958 at 14q11.2 is associated with allergic rhinitis in a Chinese Han population running title: 14q11.2 is a susceptibility locus for allergic rhinitis. J Investig Allergol Clin Immunol. 2012;22:55–62. [PubMed] [Google Scholar]
- 130.Lu MP, Chen RX, Wang ML, Zhu XJ, Zhu LP, Yin M, et al. Association study on IL4, IL13 and IL4RA polymorphisms in mite-sensitized persistent allergic rhinitis in a Chinese population. PLoS One. 2011;6:e27363. doi: 10.1371/journal.pone.0027363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li JY, Zhang Y, Lin XP, Ruan Y, Wang Y, Wang CS, et al. Association between DNA hypomethylation at IL13 gene and allergic rhinitis in house dust mite-sensitized subjects. Clin Exp Allergy. 2016;46:298–307. doi: 10.1111/cea.12647. [DOI] [PubMed] [Google Scholar]
- 132.Andiappan AK, Wang DY, Anantharaman R, Parate PN, Suri BK, Low HQ, et al. Genome-wide association study for atopy and allergic rhinitis in a Singapore Chinese population. PLoS One. 2011;6:e19719. doi: 10.1371/journal.pone.0019719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Baltimore D, Boldin MP, O'Connell RM, Rao DS, Taganov KD. MicroRNAs: new regulators of immune cell development and function. Nat Immunol. 2008;9:839–845. doi: 10.1038/ni.f.209. [DOI] [PubMed] [Google Scholar]
- 134.Teng Y, Zhang R, Yu H, Wang H, Hong Z, Zhuang W, et al. Altered MicroRNA expression profiles in activated mast cells following IgE-FcεRI cross-linking with antigen. Cell Physiol Biochem. 2015;35:2098–2110. doi: 10.1159/000374016. [DOI] [PubMed] [Google Scholar]
- 135.Shaoqing Y, Ruxin Z, Guojun L, Zhiqiang Y, Hua H, Shudong Y, et al. Microarray analysis of differentially expressed microRNAs in allergic rhinitis. Am J Rhinol Allergy. 2011;25:e242–e246. doi: 10.2500/ajra.2011.25.3682. [DOI] [PubMed] [Google Scholar]
- 136.Teng Y, Zhang R, Liu C, Zhou L, Wang H, Zhuang W, et al. miR-143 inhibits interleukin-13-induced inflammatory cytokine and mucus production in nasal epithelial cells from allergic rhinitis patients by targeting IL13Rα1. Biochem Biophys Res Commun. 2015;457:58–64. doi: 10.1016/j.bbrc.2014.12.058. [DOI] [PubMed] [Google Scholar]
- 137.Luo Y, Deng Y, Tao Z, Chen S, Xiao B, Ren J, et al. Regulatory effect of microRNA-135a on the Th1/Th2 imbalance in a murine model of allergic rhinitis. Exp Ther Med. 2014;8:1105–1110. doi: 10.3892/etm.2014.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Suojalehto H, Toskala E, Kilpeläinen M, Majuri ML, Mitts C, Lindström I, et al. MicroRNA profiles in nasal mucosa of patients with allergic and nonallergic rhinitis and asthma. Int Forum Allergy Rhinol. 2013;3:612–620. doi: 10.1002/alr.21179. [DOI] [PubMed] [Google Scholar]
- 139.Chen RF, Huang HC, Ou CY, Hsu TY, Chuang H, Chang JC, et al. MicroRNA-21 expression in neonatal blood associated with antenatal immunoglobulin E production and development of allergic rhinitis. Clin Exp Allergy. 2010;40:1482–1490. doi: 10.1111/j.1365-2222.2010.03592.x. [DOI] [PubMed] [Google Scholar]
- 140.Hansen I, Klimek L, Mösges R, Hörmann K. Mediators of inflammation in the early and the late phase of allergic rhinitis. Curr Opin Allergy Clin Immunol. 2004;4:159–163. doi: 10.1097/00130832-200406000-00004. [DOI] [PubMed] [Google Scholar]
- 141.Dullaers M, De Bruyne R, Ramadani F, Gould HJ, Gevaert P, Lambrecht BN. The who, where, and when of IgE in allergic airway disease. J Allergy Clin Immunol. 2012;129:635–645. doi: 10.1016/j.jaci.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 142.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012;18:684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 144.Kamekura R, Yamashita K, Jitsukawa S, Nagaya T, Ito F, Ichimiya S, et al. Role of crosstalk between epithelial and immune cells, the epimmunome, in allergic rhinitis pathogenesis. Adv Otorhinolaryngol. 2016;77:75–82. doi: 10.1159/000441878. [DOI] [PubMed] [Google Scholar]
- 145.Takizawa R, Pawankar R, Yamagishi S, Takenaka H, Yagi T. Increased expression of HLA-DR and CD86 in nasal epithelial cells in allergic rhinitics: antigen presentation to T cells and up-regulation by diesel exhaust particles. Clin Exp Allergy. 2007;37:420–433. doi: 10.1111/j.1365-2222.2007.02672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fukuoka A, Matsushita K, Morikawa T, Takano H, Yoshimoto T. Diesel exhaust particles exacerbate allergic rhinitis in mice by disrupting the nasal epithelial barrier. Clin Exp Allergy. 2016;46:142–152. doi: 10.1111/cea.12597. [DOI] [PubMed] [Google Scholar]
- 147.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–822. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 148.Louten J, Boniface K, de Waal Malefyt R. Development and function of TH17 cells in health and disease. J Allergy Clin Immunol. 2009;123:1004–1011. doi: 10.1016/j.jaci.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 149.Doherty TA, Scott D, Walford HH, Khorram N, Lund S, Baum R, et al. Allergen challenge in allergic rhinitis rapidly induces increased peripheral blood type 2 innate lymphoid cells that express CD84. J Allergy Clin Immunol. 2014;133:1203–1205.e7. doi: 10.1016/j.jaci.2013.12.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Panganiban RP, Wang Y, Howrylak J, Chinchilli VM, Craig TJ, August A, et al. Circulating microRNAs as biomarkers in patients with allergic rhinitis and asthma. J Allergy Clin Immunol. 2016;137:1423–1432. doi: 10.1016/j.jaci.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 151.Kim AS, Doherty TA, Karta MR, Das S, Baum R, Rosenthal P, et al. Regulatory B cells and T follicular helper cells are reduced in allergic rhinitis. J Allergy Clin Immunol. 2016;138:1192–1195.e5. doi: 10.1016/j.jaci.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.He S, Zhang H, Zeng X, Yang P. Self-amplification mechanisms of mast cell activation: a new look in allergy. Curr Mol Med. 2012;12:1329–1339. doi: 10.2174/156652412803833544. [DOI] [PubMed] [Google Scholar]
- 153.Law M, Morales JL, Mottram LF, Iyer A, Peterson BR, August A. Structural requirements for the inhibition of calcium mobilization and mast cell activation by the pyrazole derivative BTP2. Int J Biochem Cell Biol. 2011;43:1228–1239. doi: 10.1016/j.biocel.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Spiegl N, Didichenko S, McCaffery P, Langen H, Dahinden CA. Human basophils activated by mast cell-derived IL-3 express retinaldehyde dehydrogenase-II and produce the immunoregulatory mediator retinoic acid. Blood. 2008;112:3762–3771. doi: 10.1182/blood-2008-01-135251. [DOI] [PubMed] [Google Scholar]
- 155.Wodnar-Filipowicz A, Heusser CH, Moroni C. Production of the haemopoietic growth factors GM-CSF and interleukin-3 by mast cells in response to IgE receptor-mediated activation. Nature. 1989;339:150–152. doi: 10.1038/339150a0. [DOI] [PubMed] [Google Scholar]
- 156.Zhang H, Yang H, Zhang L, Yang X, Zhang Z, Lin Q, et al. Induction of IL-4 release and upregulated expression of protease activated receptors by GM-CSF in P815 cells. Cytokine. 2009;48:196–202. doi: 10.1016/j.cyto.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 157.Baraniuk JN. Pathogenesis of allergic rhinitis. J Allergy Clin Immunol. 1997;99:S763–S772. doi: 10.1016/s0091-6749(97)70125-8. [DOI] [PubMed] [Google Scholar]
- 158.Boyce JA. Mast cells: beyond IgE. J Allergy Clin Immunol. 2003;111:24–32. doi: 10.1067/mai.2003.60. [DOI] [PubMed] [Google Scholar]
- 159.Lorentz A, Wilke M, Sellge G, Worthmann H, Klempnauer J, Manns MP, et al. IL-4-induced priming of human intestinal mast cells for enhanced survival and Th2 cytokine generation is reversible and associated with increased activity of ERK1/2 and c-Fos. J Immunol. 2005;174:6751–6756. doi: 10.4049/jimmunol.174.11.6751. [DOI] [PubMed] [Google Scholar]
- 160.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 161.Järvikallio A, Naukkarinen A, Harvima IT, Aalto ML, Horsmanheimo M. Quantitative analysis of tryptase- and chymase-containing mast cells in atopic dermatitis and nummular eczema. Br J Dermatol. 1997;136:871–877. [PubMed] [Google Scholar]
- 162.Hofstra CL, Desai PJ, Thurmond RL, Fung-Leung WP. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J Pharmacol Exp Ther. 2003;305:1212–1221. doi: 10.1124/jpet.102.046581. [DOI] [PubMed] [Google Scholar]
- 163.Nilsson G, Metcalfe DD, Taub DD. Demonstration that platelet-activating factor is capable of activating mast cells and inducing a chemotactic response. Immunology. 2000;99:314–319. doi: 10.1046/j.1365-2567.2000.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hart PH. Regulation of the inflammatory response in asthma by mast cell products. Immunol Cell Biol. 2001;79:149–153. doi: 10.1046/j.1440-1711.2001.00983.x. [DOI] [PubMed] [Google Scholar]
- 165.Misiak-Tłoczek A, Brzezińska-Błaszczyk E. IL-6, but not IL-4, stimulates chemokinesis and TNF stimulates chemotaxis of tissue mast cells: involvement of both mitogen-activated protein kinases and phosphatidylinositol 3-kinase signalling pathways. APMIS. 2009;117:558–567. doi: 10.1111/j.1600-0463.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 166.Brzezińska-Błaszczyk E, Pietrzak A, Misiak-Tłoczek AH. Tumor necrosis factor (TNF) is a potent rat mast cell chemoattractant. J Interferon Cytokine Res. 2007;27:911–919. doi: 10.1089/jir.2006.0158. [DOI] [PubMed] [Google Scholar]
- 167.Toru H, Kinashi T, Ra C, Nonoyama S, Yata J, Nakahata T. Interleukin-4 induces homotypic aggregation of human mast cells by promoting LFA-1/ICAM-1 adhesion molecules. Blood. 1997;89:3296–3302. [PubMed] [Google Scholar]
- 168.Huang B, Lei Z, Zhang GM, Li D, Song C, Li B, et al. SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood. 2008;112:1269–1279. doi: 10.1182/blood-2008-03-147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.He S, Zhang H, Chen H, Yang H, Huang T, Chen Y, et al. Expression and release of IL-29 by mast cells and modulation of mast cell behavior by IL-29. Allergy. 2010;65:1234–1241. doi: 10.1111/j.1398-9995.2010.02349.x. [DOI] [PubMed] [Google Scholar]
- 170.Olsson N, Taub DD, Nilsson G. Regulation of mast cell migration by T and T cytokines: identification of tumour necrosis factor-alpha and interleukin-4 as mast cell chemotaxins. Scand J Immunol. 2004;59:267–272. doi: 10.1111/j.0300-9475.2004.01397.x. [DOI] [PubMed] [Google Scholar]
- 171.Kambe N, Kambe M, Kochan JP, Schwartz LB. Human skin-derived mast cells can proliferate while retaining their characteristic functional and protease phenotypes. Blood. 2001;97:2045–2052. doi: 10.1182/blood.v97.7.2045. [DOI] [PubMed] [Google Scholar]
- 172.Kempuraj D, Saito H, Kaneko A, Fukagawa K, Nakayama M, Toru H, et al. Characterization of mast cell-committed progenitors present in human umbilical cord blood. Blood. 1999;93:3338–3346. [PubMed] [Google Scholar]
- 173.Andersen HB, Holm M, Hetland TE, Dahl C, Junker S, Schiøtz PO, et al. Comparison of short term in vitro cultured human mast cells from different progenitors - Peripheral blood-derived progenitors generate highly mature and functional mast cells. J Immunol Methods. 2008;336:166–174. doi: 10.1016/j.jim.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 174.Yamaguchi M, Azuma H, Fujihara M, Hamada H, Ikeda H. Generation of a considerable number of functional mast cells with a high basal level of FcepsilonRI expression from cord blood CD34+ cells by co-culturing them with bone marrow stromal cell line under serum-free conditions. Scand J Immunol. 2007;65:581–588. doi: 10.1111/j.1365-3083.2007.01937.x. [DOI] [PubMed] [Google Scholar]
- 175.Shakoory B, Fitzgerald SM, Lee SA, Chi DS, Krishnaswamy G. The role of human mast cell-derived cytokines in eosinophil biology. J Interferon Cytokine Res. 2004;24:271–281. doi: 10.1089/107999004323065057. [DOI] [PubMed] [Google Scholar]
- 176.Wisniewski JA, Borish L. Novel cytokines and cytokine-producing T cells in allergic disorders. Allergy Asthma Proc. 2011;32:83–94. doi: 10.2500/aap.2011.32.3428. [DOI] [PubMed] [Google Scholar]
- 177.Niyonsaba F, Ushio H, Hara M, Yokoi H, Tominaga M, Takamori K, et al. Antimicrobial peptides human beta-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. J Immunol. 2010;184:3526–3534. doi: 10.4049/jimmunol.0900712. [DOI] [PubMed] [Google Scholar]
- 178.He S, Peng Q, Walls AF. Potent induction of a neutrophil and eosinophil-rich infiltrate in vivo by human mast cell tryptase: selective enhancement of eosinophil recruitment by histamine. J Immunol. 1997;159:6216–6225. [PubMed] [Google Scholar]
- 179.He S, Walls AF. Human mast cell chymase induces the accumulation of neutrophils, eosinophils and other inflammatory cells in vivo. Br J Pharmacol. 1998;125:1491–1500. doi: 10.1038/sj.bjp.0702223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Okada S, Kita H, George TJ, Gleich GJ, Leiferman KM. Migration of eosinophils through basement membrane components in vitro: role of matrix metalloproteinase-9. Am J Respir Cell Mol Biol. 1997;17:519–528. doi: 10.1165/ajrcmb.17.4.2877. [DOI] [PubMed] [Google Scholar]
- 181.Dias-Baruffi M, Pereira-da-Silva G, Jamur MC, Roque-Barreira MC. Heparin potentiates in vivo neutrophil migration induced by IL-8. Glycoconj J. 1998;15:523–526. doi: 10.1023/a:1006995222189. [DOI] [PubMed] [Google Scholar]
- 182.Thomas PS. Tumour necrosis factor-alpha: the role of this multifunctional cytokine in asthma. Immunol Cell Biol. 2001;79:132–140. doi: 10.1046/j.1440-1711.2001.00980.x. [DOI] [PubMed] [Google Scholar]
- 183.Zhang H, Kong H, Zeng X, Guo L, Sun X, He S. Subsets of regulatory T cells and their roles in allergy. J Transl Med. 2014;12:125. doi: 10.1186/1479-5876-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jutel M, Akdis M, Blaser K, Akdis CA. Are regulatory T cells the target of venom immunotherapy? Curr Opin Allergy Clin Immunol. 2005;5:365–369. doi: 10.1097/01.all.0000173784.81024.7a. [DOI] [PubMed] [Google Scholar]
- 185.Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L, et al. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28:870–880. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ray A, Khare A, Krishnamoorthy N, Qi Z, Ray P. Regulatory T cells in many flavors control asthma. Mucosal Immunol. 2010;3:216–229. doi: 10.1038/mi.2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wu K, Bi Y, Sun K, Wang C. IL-10-producing type 1 regulatory T cells and allergy. Cell Mol Immunol. 2007;4:269–275. [PubMed] [Google Scholar]
- 188.Noble A, Giorgini A, Leggat JA. Cytokine-induced IL-10-secreting CD8 T cells represent a phenotypically distinct suppressor T-cell lineage. Blood. 2006;107:4475–4483. doi: 10.1182/blood-2005-10-3994. [DOI] [PubMed] [Google Scholar]
- 189.Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci USA. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xystrakis E, Boswell SE, Hawrylowicz CM. T regulatory cells and the control of allergic disease. Expert Opin Biol Ther. 2006;6:121–133. doi: 10.1517/14712598.6.2.121. [DOI] [PubMed] [Google Scholar]
- 191.Ziora D, Sitek P, Machura E, Ziora K. Bronchial asthma in obesity--a distinct phenotype of asthma? Pneumonol Alergol Pol. 2012;80:454–462. [PubMed] [Google Scholar]
- 192.Li L, Boussiotis VA. Control and regulation of peripheral tolerance in allergic inflammatory disease: therapeutic consequences. Chem Immunol Allergy. 2008;94:178–188. doi: 10.1159/000155086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nieminen K, Laaksonen K, Savolainen J. Three-year follow-up study of allergen-induced in vitro cytokine and signalling lymphocytic activation molecule mRNA responses in peripheral blood mononuclear cells of allergic rhinitis patients undergoing specific immunotherapy. Int Arch Allergy Immunol. 2009;150:370–376. doi: 10.1159/000226238. [DOI] [PubMed] [Google Scholar]
- 194.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 196.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Brickshawana A, Shapiro VS, Kita H, Pease LR. Lineage(−)Sca1+c-Kit(−)CD25+ cells are IL-33-responsive type 2 innate cells in the mouse bone marrow. J Immunol. 2011;187:5795–5804. doi: 10.4049/jimmunol.1102242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol. 2013;132:933–941. doi: 10.1016/j.jaci.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 201.Salmond RJ, Mirchandani AS, Besnard AG, Bain CC, Thomson NC, Liew FY. IL-33 induces innate lymphoid cell-mediated airway inflammation by activating mammalian target of rapamycin. J Allergy Clin Immunol. 2012;130:1159–1166.e6. doi: 10.1016/j.jaci.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hung LY, Lewkowich IP, Dawson LA, Downey J, Yang Y, Smith DE, et al. IL-33 drives biphasic IL-13 production for noncanonical Type 2 immunity against hookworms. Proc Natl Acad Sci USA. 2013;110:282–287. doi: 10.1073/pnas.1206587110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yasuda K, Muto T, Kawagoe T, Matsumoto M, Sasaki Y, Matsushita K, et al. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proc Natl Acad Sci USA. 2012;109:3451–3456. doi: 10.1073/pnas.1201042109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lefrançais E, Duval A, Mirey E, Roga S, Espinosa E, Cayrol C, et al. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci USA. 2014;111:15502–15507. doi: 10.1073/pnas.1410700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Licona-Limón P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14:536–542. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 206.Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 208.Kim HK, Lund S, Baum R, Rosenthal P, Khorram N, Doherty TA. Innate type 2 response to Alternaria extract enhances ryegrass-induced lung inflammation. Int Arch Allergy Immunol. 2014;163:92–105. doi: 10.1159/000356341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Klein Wolterink RG, Kleinjan A, van Nimwegen M, Bergen I, de Bruijn M, Levani Y, et al. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol. 2012;42:1106–1116. doi: 10.1002/eji.201142018. [DOI] [PubMed] [Google Scholar]
- 210.Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Doherty TA, Baum R, Newbury RO, Yang T, Dohil R, Aquino M, et al. Group 2 innate lymphocytes (ILC2) are enriched in active eosinophilic esophagitis. J Allergy Clin Immunol. 2015;136:792–794.e3. doi: 10.1016/j.jaci.2015.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Miljkovic D, Bassiouni A, Cooksley C, Ou J, Hauben E, Wormald PJ, et al. Association between group 2 innate lymphoid cells enrichment, nasal polyps and allergy in chronic rhinosinusitis. Allergy. 2014;69:1154–1161. doi: 10.1111/all.12440. [DOI] [PubMed] [Google Scholar]
- 214.Ho J, Bailey M, Zaunders J, Mrad N, Sacks R, Sewell W, et al. Group 2 innate lymphoid cells (ILC2s) are increased in chronic rhinosinusitis with nasal polyps or eosinophilia. Clin Exp Allergy. 2015;45:394–403. doi: 10.1111/cea.12462. [DOI] [PubMed] [Google Scholar]
- 215.Christianson CA, Goplen NP, Zafar I, Irvin C, Good JT, Jr, Rollins DR, et al. Persistence of asthma requires multiple feedback circuits involving type 2 innate lymphoid cells and IL-33. J Allergy Clin Immunol. 2015;136:59–68.e14. doi: 10.1016/j.jaci.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Jackson DJ, Makrinioti H, Rana BM, Shamji BW, Trujillo-Torralbo MB, Footitt J, et al. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med. 2014;190:1373–1382. doi: 10.1164/rccm.201406-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lao-Araya M, Steveling E, Scadding GW, Durham SR, Shamji MH. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol. 2014;134:1193–1195.e4. doi: 10.1016/j.jaci.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 218.Fan D, Wang X, Wang M, Wang Y, Zhang L, Li Y, et al. Allergen-dependent differences in ILC2s frequencies in patients with allergic rhinitis. Allergy Asthma Immunol Res. 2016;8:216–222. doi: 10.4168/aair.2016.8.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhong H, Fan XL, Yu QN, Qin ZL, Chen D, Xu R, et al. Increased innate type 2 immune response in house dust mite-allergic patients with allergic rhinitis. Clin Immunol. 2017;183:293–299. doi: 10.1016/j.clim.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 220.Yu QN, Guo YB, Li X, Li CL, Tan WP, Fan XL, et al. ILC2 frequency and activity are inhibited by glucocorticoid treatment via STAT pathway in patients with asthma. Allergy. 2018 Mar 15; doi: 10.1111/all.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Salib RJ, Lau LC, Howarth PH. Nasal lavage fluid concentrations of eotaxin-1 (CCL11) in naturally occurring allergic rhinitis: relationship to disease activity, nasal luminal eosinophil influx, and plasma protein exudation. Clin Exp Allergy. 2005;35:995–1002. doi: 10.1111/j.1365-2222.2005.02236.x. [DOI] [PubMed] [Google Scholar]
- 222.Yan Z, Zhang R, Yu S, Wu G. Study on the expression of Eotaxin and the role of histamine in allergic rhinitis. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2009;23:1086–1088. [PubMed] [Google Scholar]
- 223.Canonica GW, Compalati E. Minimal persistent inflammation in allergic rhinitis: implications for current treatment strategies. Clin Exp Immunol. 2009;158:260–271. doi: 10.1111/j.1365-2249.2009.04017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.KleinJan A, Dijkstra MD, Boks SS, Severijnen LA, Mulder PG, Fokkens WJ. Increase in IL-8, IL-10, IL-13, and RANTES mRNA levels (in situ hybridization) in the nasal mucosa after nasal allergen provocation. J Allergy Clin Immunol. 1999;103:441–450. doi: 10.1016/s0091-6749(99)70469-0. [DOI] [PubMed] [Google Scholar]
- 225.Kaplan AP. Chemokines, chemokine receptors and allergy. Int Arch Allergy Immunol. 2001;124:423–431. doi: 10.1159/000053777. [DOI] [PubMed] [Google Scholar]
- 226.Bochner BS, Bickel CA, Taylor ML, MacGlashan DW, Jr, Gray PW, Raport CJ, et al. Macrophage-derived chemokine induces human eosinophil chemotaxis in a CC chemokine receptor 3- and CC chemokine receptor 4-independent manner. J Allergy Clin Immunol. 1999;103:527–532. doi: 10.1016/s0091-6749(99)70481-1. [DOI] [PubMed] [Google Scholar]
- 227.Zhang RX, Yu SQ, Jiang JZ, Liu GJ. Complementary DNA microarray analysis of chemokines and their receptors in allergic rhinitis. J Investig Allergol Clin Immunol. 2007;17:329–336. [PubMed] [Google Scholar]
- 228.Zhuo M, Small SA, Kandel ER, Hawkins RD. Nitric oxide and carbon monoxide produce activity-dependent long-term synaptic enhancement in hippocampus. Science. 1993;260:1946–1950. doi: 10.1126/science.8100368. [DOI] [PubMed] [Google Scholar]
- 229.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 230.Lundberg JO, Rinder J, Weitzberg E, Lundberg JM, Alving K. Nasally exhaled nitric oxide in humans originates mainly in the paranasal sinuses. Acta Physiol Scand. 1994;152:431–432. doi: 10.1111/j.1748-1716.1994.tb09826.x. [DOI] [PubMed] [Google Scholar]
- 231.Hanazawa T, Antuni JD, Kharitonov SA, Barnes PJ. Intranasal administration of eotaxin increases nasal eosinophils and nitric oxide in patients with allergic rhinitis. J Allergy Clin Immunol. 2000;105:58–64. doi: 10.1016/s0091-6749(00)90178-7. [DOI] [PubMed] [Google Scholar]
- 232.Yu S, Yan Z, Che N, Zhang X, Ge R. Impact of carbon monoxide/heme oxygenase on hydrogen sulfide/cystathionine-γ-lyase pathway in the pathogenesis of allergic rhinitis in guinea pigs. Otolaryngol Head Neck Surg. 2015;152:470–476. doi: 10.1177/0194599814567112. [DOI] [PubMed] [Google Scholar]
- 233.Shaoqing Y, Ruxin Z, Yingjian C, Jianqiu C, Yanshen W, Genhong L. A meta-analysis of the association of exhaled carbon monoxide on asthma and allergic rhinitis. Clin Rev Allergy Immunol. 2011;41:67–75. doi: 10.1007/s12016-009-8195-1. [DOI] [PubMed] [Google Scholar]
- 234.Park SJ, Kim TH, Lee SH, Ryu HY, Hong KH, Jung JY, et al. Expression levels of endogenous hydrogen sulfide are altered in patients with allergic rhinitis. Laryngoscope. 2013;123:557–563. doi: 10.1002/lary.23466. [DOI] [PubMed] [Google Scholar]
- 235.Shaoqing Y, Ruxin Z, Yinjian C, Jianqiu C, Zhiqiang Y, Genhong L. Down-regulation of endogenous hydrogen sulphide pathway in nasal mucosa of allergic rhinitis in guinea pigs. Allergol Immunopathol (Madr) 2009;37:180–187. doi: 10.1016/j.aller.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 236.Lundberg JM, Brodin E, Hua X, Saria A. Vascular permeability changes and smooth muscle contraction in relation to capsaicin-sensitive substance P afferents in the guinea-pig. Acta Physiol Scand. 1984;120:217–227. doi: 10.1111/j.1748-1716.1984.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 237.Baraniuk JN, Lundgren JD, Okayama M, Goff J, Mullol J, Merida M, et al. Substance P and neurokinin A in human nasal mucosa. Am J Respir Cell Mol Biol. 1991;4:228–236. doi: 10.1165/ajrcmb/4.3.228. [DOI] [PubMed] [Google Scholar]
- 238.Zhang R, Jiang D, Li Z. Experimental study on blocking agent of substance P nerves in the treatment of allergic rhinitis. Zhonghua Er Bi Yan Hou Ke Za Zhi. 1994;29:282–285. [PubMed] [Google Scholar]
- 239.Zhang R, Jiang D, Li Z. Clinical observation and therapeutic mechanism of blocking agent of substance P nerves in the treatment of perennial allergic rhinitis. Zhonghua Er Bi Yan Hou Ke Za Zhi. 1995;30:163–165. [PubMed] [Google Scholar]
- 240.Hu H, Zhang R, Fang X, Yu M, Yu S, Zhang J, et al. Effects of endogenous substance P expression on degranulation in RBL-2H3 cells. Inflamm Res. 2011;60:541–546. doi: 10.1007/s00011-010-0301-6. [DOI] [PubMed] [Google Scholar]
- 241.Hanf G, Schierhorn K, Brunnée T, Noga O, Verges D, Kunkel G. Substance P induced histamine release from nasal mucosa of subjects with and without allergic rhinitis. Inflamm Res. 2000;49:520–523. doi: 10.1007/s000110050625. [DOI] [PubMed] [Google Scholar]
- 242.Wang H, Zhang R, Wu J, Hu H. Knockdown of neurokinin-1 receptor expression by small interfering RNA prevents the development of allergic rhinitis in rats. Inflamm Res. 2013;62:903–910. doi: 10.1007/s00011-013-0649-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Liu ZG, Song JJ, Kong XL. A study on pollen allergens in China. Biomed Environ Sci. 2010;23:319–322. doi: 10.1016/S0895-3988(10)60070-0. [DOI] [PubMed] [Google Scholar]
- 244.Qiao BS. Airborne pollens and plants in China. Beijing: Chinese Peking Union Medical Publishing House; 2005. [Google Scholar]
- 245.Liu GH, Zhu RF, Zhang W, Li WJ, Wang ZX, Chen H. Survey of airborne pollen in Hubei province of China. Chin Med Sci J. 2008;23:212–217. doi: 10.1016/s1001-9294(09)60041-9. [DOI] [PubMed] [Google Scholar]
- 246.Ouyang YH, Zhang DS, Fan EZ, Li Y, Zhang L. Correlation between symptoms of pollen allergic rhinitis and pollen grain spreading in summer and autumn. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012;47:623–627. [PubMed] [Google Scholar]
- 247.Trasande L, Thurston GD. The role of air pollution in asthma and other pediatric morbidities. J Allergy Clin Immunol. 2005;115:689–699. doi: 10.1016/j.jaci.2005.01.056. [DOI] [PubMed] [Google Scholar]
- 248.Zhang F, Wang W, Lv J, Krafft T, Xu J. Time-series studies on air pollution and daily outpatient visits for allergic rhinitis in Beijing, China. Sci Total Environ. 2011;409:2486–2492. doi: 10.1016/j.scitotenv.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 249.Hwang BF, Jaakkola JJ, Lee YL, Lin YC, Guo YL. Relation between air pollution and allergic rhinitis in Taiwanese schoolchildren. Respir Res. 2006;7:23. doi: 10.1186/1465-9921-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Diaz-Sanchez D, Tsien A, Casillas A, Dotson AR, Saxon A. Enhanced nasal cytokine production in human beings after in vivo challenge with diesel exhaust particles. J Allergy Clin Immunol. 1996;98:114–123. doi: 10.1016/s0091-6749(96)70233-6. [DOI] [PubMed] [Google Scholar]
- 251.Diaz-Sanchez D, Tsien A, Fleming J, Saxon A. Combined diesel exhaust particulate and ragweed allergen challenge markedly enhances human in vivo nasal ragweed-specific IgE and skews cytokine production to a T helper cell 2-type pattern. J Immunol. 1997;158:2406–2413. [PubMed] [Google Scholar]
- 252.Fujieda S, Diaz-Sanchez D, Saxon A. Combined nasal challenge with diesel exhaust particles and allergen induces in vivo IgE isotype switching. Am J Respir Cell Mol Biol. 1998;19:507–512. doi: 10.1165/ajrcmb.19.3.3143. [DOI] [PubMed] [Google Scholar]
- 253.Diaz-Sanchez D, Penichet-Garcia M, Saxon A. Diesel exhaust particles directly induce activated mast cells to degranulate and increase histamine levels and symptom severity. J Allergy Clin Immunol. 2000;106:1140–1146. doi: 10.1067/mai.2000.111144. [DOI] [PubMed] [Google Scholar]
- 254.Knox RB, Suphioglu C, Taylor P, Desai R, Watson HC, Peng JL, et al. Major grass pollen allergen Lol p 1 binds to diesel exhaust particles: implications for asthma and air pollution. Clin Exp Allergy. 1997;27:246–251. [PubMed] [Google Scholar]
- 255.Pandya RJ, Solomon G, Kinner A, Balmes JR. Diesel exhaust and asthma: hypotheses and molecular mechanisms of action. Environ Health Perspect. 2002;110(Suppl 1):103–112. doi: 10.1289/ehp.02110s1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Han YY, Forno E, Gogna M, Celedón JC. Obesity and rhinitis in a nationwide study of children and adults in the United States. J Allergy Clin Immunol. 2016;137:1460–1465. doi: 10.1016/j.jaci.2015.12.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Bunyavanich S, Rifas-Shiman SL, Platts-Mills TA, Workman L, Sordillo JE, Camargo CA, Jr, et al. Prenatal, perinatal, and childhood vitamin D exposure and their association with childhood allergic rhinitis and allergic sensitization. J Allergy Clin Immunol. 2016;137:1063–1070.e2. doi: 10.1016/j.jaci.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kawamoto Y, Ueno Y, Nakahashi E, Obayashi M, Sugihara K, Qiao S, et al. Prevention of allergic rhinitis by ginger and the molecular basis of immunosuppression by 6-gingerol through T cell inactivation. J Nutr Biochem. 2016;27:112–122. doi: 10.1016/j.jnutbio.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 259.Nwaru BI, Takkinen HM, Kaila M, Erkkola M, Ahonen S, Pekkanen J, et al. Food diversity in infancy and the risk of childhood asthma and allergies. J Allergy Clin Immunol. 2014;133:1084–1091. doi: 10.1016/j.jaci.2013.12.1069. [DOI] [PubMed] [Google Scholar]
- 260.Gong W, Feng Y, Yan P, Li S, Yu C, Zhou X, et al. Effect of nasal instillation of vitamin D3 on patient with allergic rhinitis symptoms. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014;28:1031–1033. [PubMed] [Google Scholar]
- 261.Sabounchi S, Bollyky J, Nadeau K. Review of environmental impact on the epigenetic regulation of atopic diseases. Curr Allergy Asthma Rep. 2015;15:33. doi: 10.1007/s11882-015-0533-1. [DOI] [PubMed] [Google Scholar]
- 262.Miller DR, Turner SW, Spiteri-Cornish D, Scaife AR, Danielian PJ, Devereux GS, et al. Maternal vitamin D and E intakes during early pregnancy are associated with airway epithelial cell responses in neonates. Clin Exp Allergy. 2015;45:920–927. doi: 10.1111/cea.12490. [DOI] [PubMed] [Google Scholar]
- 263.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Fujimura KE, Lynch SV. Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe. 2015;17:592–602. doi: 10.1016/j.chom.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352:539–544. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.The State Administration of Traditional Chinese medicine. Standards for diagnosis and curative effect of Chinese medical symptom. Nanjing University Press; 2001. [Google Scholar]
- 267.Shizhen W. Chinese otorhinolaryngology. Beijing: China Traditional Chinese Medicine Publishing House; 2003. [Google Scholar]
- 268.Guruijin Several important issues of ear nasal allergy and immunity. Chinese J Otorhinolaryngol. 1992;27:3011. [Google Scholar]
- 269.Wen Z, Tao ZD, Cao GZ. Cyclic nucleotide. Ion and the autonomic nervous system functions of patients with perennial allergic rhinitis. J Hunan Med Univ. 1990;17:551. [Google Scholar]
- 270.Shen ZY. Research on warming yang herbs to prevent seasonal asthmatic attack and its principles. J Integr Tradit West Med. 1986;6:L1. [Google Scholar]
- 271.Zhao JY, Liu ZB, Wu HQ. Observation of T-lymphocyte subsets in patients with lung qi deficiency and lung-yin deficiency. J Anhui Coll Tradit Chinese Med. 1993;12:49. [Google Scholar]
- 272.The Wenzhou Medical Sciences. Preliminary report on the relationship between chronic bronchitis of plasma cyclic nucleotide levels and TCM differentiation of Zangfu. Zhejiang J Tradit Chinese Med. 1981;16:2. [Google Scholar]
- 273.Lin WS, Xiong ZM, Zhang ZK. Relationship between nasal secretion of cyclic nucleotide levels and syndrome differentiation of chronic bronchitis. Zhejiang J Tradit Chinese Med. 1982;17:5221. [Google Scholar]
- 274.Lu DW, Wang MH. Correlation research on allergic rhinitis based on syndrome differentiation of traditional Chinese and allergy index. J Integr Chinese West Med Ear Nose Throat. 1996;4:1121. [Google Scholar]
- 275.Liao YH, Li YY, Chen H. Proven case of Zuwang Gan by using metal-clearing method for allergic rhinitis. J Guangzhou Univ Tradit Chinese Med. 2004;21:154–156. [Google Scholar]
- 276.Pfaar O, Demoly P, Gerth van Wijk R, Bonini S, Bousquet J, Canonica GW, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI Position Paper. Allergy. 2014;69:854–867. doi: 10.1111/all.12383. [DOI] [PubMed] [Google Scholar]
- 277.Demoly P, Michel F, Bousquet J. In vivo methods for study of allergy: skin tests, techniques and interpretation. In: Middleton E Jr, Reed CE, Ellis EF, Adkinson NF Jr, Yunginger JW, Busse WW, editors. Allergy: principles and practice. 5th ed. St Louis (MO): Mosby Co; 1998. [Google Scholar]
- 278.Sub-Committee on Skin Tests of the European Academy of Allergology and Clinical Immunology. Skin tests used in type I allergy testing Position paper. Allergy. 1989;44(Suppl 10):1–59. [PubMed] [Google Scholar]
- 279.Lockey RF, Benedict LM, Turkeltaub PC, Bukantz SC. Fatalities from immunotherapy (IT) and skin testing (ST) J Allergy Clin Immunol. 1987;79:660–677. doi: 10.1016/s0091-6749(87)80164-1. [DOI] [PubMed] [Google Scholar]
- 280.Reid MJ, Lockey RF, Turkeltaub PC, Platts-Mills TA. Survey of fatalities from skin testing and immunotherapy 1985–1989. J Allergy Clin Immunol. 1993;92:6–15. doi: 10.1016/0091-6749(93)90030-j. [DOI] [PubMed] [Google Scholar]
- 281.The European Academy of Allergology and Clinical Immunology. Position paper: allergen standardization and skin tests. Allergy. 1993;48(Suppl):48–82. [PubMed] [Google Scholar]
- 282.Allergen immunotherapy: therapeutic vaccines for allergic diseases. Geneva: January 27–29 1997. Allergy. 1998;53(44 Suppl):1–42. doi: 10.1111/j.1398-9995.1998.tb04930.x. [DOI] [PubMed] [Google Scholar]
- 283.Huggins KG, Brostoff J. Local production of specific IgE antibodies in allergic-rhinitis patients with negative skin tests. Lancet. 1975;2:148–150. doi: 10.1016/s0140-6736(75)90056-2. [DOI] [PubMed] [Google Scholar]
- 284.Board of Directors. American Academy of Allergy and Immunology. Allergen skin testing. J Allergy Clin Immunol. 1993;92:636–637. [PubMed] [Google Scholar]
- 285.Miadonna A, Leggieri E, Tedeschi A, Zanussi C. Clinical significance of specific IgE determination on nasal secretion. Clin Allergy. 1983;13:155–164. doi: 10.1111/j.1365-2222.1983.tb02583.x. [DOI] [PubMed] [Google Scholar]
- 286.Deuschl H, Johansson SG. Specific IgE antibodies in nasal secretion from patients with allergic rhinitis and with negative or weakly positive RAST on the serum. Clin Allergy. 1977;7:195–202. doi: 10.1111/j.1365-2222.1977.tb01442.x. [DOI] [PubMed] [Google Scholar]
- 287.Osterballe O, Weeke B. A new lancet for skin prick testing. Allergy. 1979;34:209–212. doi: 10.1111/j.1398-9995.1979.tb01700.x. [DOI] [PubMed] [Google Scholar]
- 288.Adinoff AD, Rosloniec DM, McCall LL, Nelson HS. Immediate skin test reactivity to Food and Drug Administration-approved standardized extracts. J Allergy Clin Immunol. 1990;86:766–774. doi: 10.1016/s0091-6749(05)80181-2. [DOI] [PubMed] [Google Scholar]
- 289.Simons FE, Johnston L, Gu X, Simons KJ. Suppression of the early and late cutaneous allergic responses using fexofenadine and montelukast. Ann Allergy Asthma Immunol. 2001;86:44–50. doi: 10.1016/S1081-1206(10)62354-X. [DOI] [PubMed] [Google Scholar]
- 290.Hill SL, 3rd, Krouse JH. The effects of montelukast on intradermal wheal and flare. Otolaryngol Head Neck Surg. 2003;129:199–203. doi: 10.1016/S0194-5998(03)00607-7. [DOI] [PubMed] [Google Scholar]
- 291.Wide L, Bennich H, Johansson SG. Diagnosis of allergy by an in-vitro test for allergen antibodies. Lancet. 1967;2:1105–1107. doi: 10.1016/s0140-6736(67)90615-0. [DOI] [PubMed] [Google Scholar]
- 292.Johansson SG, Bennich H, Foucard T. Quantitation of IgE antibodies and allergens by the radioallergosorbent test, RAST. Int Arch Allergy Appl Immunol. 1973;45:55–56. doi: 10.1159/000231001. [DOI] [PubMed] [Google Scholar]
- 293.Bousquet J, Chanez P, Chanal I, Michel FB. Comparison between RAST and Pharmacia CAP system: a new automated specific IgE assay. J Allergy Clin Immunol. 1990;85:1039–1043. doi: 10.1016/0091-6749(90)90048-9. [DOI] [PubMed] [Google Scholar]
- 294.Pastorello EA, Incorvaia C, Pravettoni V, Marelli A, Farioli L, Ghezzi M. Clinical evaluation of CAP System and RAST in the measurement of specific IgE. Allergy. 1992;47:463–466. doi: 10.1111/j.1398-9995.1992.tb00665.x. [DOI] [PubMed] [Google Scholar]
- 295.Chinoy B, Yee E, Bahna SL. Skin testing versus radioallergosorbent testing for indoor allergens. Clin Mol Allergy. 2005;3:4. doi: 10.1186/1476-7961-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Eriksson NE. Allergy screening with Phadiatop and CAP Phadiatop in combination with a questionnaire in adults with asthma and rhinitis. Allergy. 1990;45:285–292. doi: 10.1111/j.1398-9995.1990.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 297.Kam KL, Hsieh KH. Comparison of three in vitro assays for serum IgE with skin testing in asthmatic children. Ann Allergy. 1994;73:329–336. [PubMed] [Google Scholar]
- 298.Lloyd GA, Lund VJ, Scadding GK. CT of the paranasal sinuses and functional endoscopic surgery: a critical analysis of 100 symptomatic patients. J Laryngol Otol. 1991;105:181–185. doi: 10.1017/s0022215100115300. [DOI] [PubMed] [Google Scholar]
- 299.Mafee MF, Chow JM, Meyers R. Functional endoscopic sinus surgery: anatomy, CT screening, indications, and complications. AJR Am J Roentgenol. 1993;160:735–744. doi: 10.2214/ajr.160.4.8456654. [DOI] [PubMed] [Google Scholar]
- 300.Leipzig JR, Martin DS, Eisenbeis JF, Slavin RG. Computed tomographic study of the paranasal sinuses in allergic rhinitis. J Allergy Clin Immunol. 1996;98:1130–1131. doi: 10.1016/s0091-6749(96)80206-5. [DOI] [PubMed] [Google Scholar]
- 301.Bhattacharyya N, Fried MP. The accuracy of computed tomography in the diagnosis of chronic rhinosinusitis. Laryngoscope. 2003;113:125–129. doi: 10.1097/00005537-200301000-00023. [DOI] [PubMed] [Google Scholar]
- 302.Mafee MF, Tran BH, Chapa AR. Imaging of rhinosinusitis and its complications: plain film, CT, and MRI. Clin Rev Allergy Immunol. 2006;30:165–186. doi: 10.1385/CRIAI:30:3:165. [DOI] [PubMed] [Google Scholar]
- 303.Galassi C, De Sario M, Biggeri A, Bisanti L, Chellini E, Ciccone G, et al. Changes in prevalence of asthma and allergies among children and adolescents in Italy: 1994–2002. Pediatrics. 2006;117:34–42. doi: 10.1542/peds.2004-2709. [DOI] [PubMed] [Google Scholar]
- 304.Hytönen M, Sala E. Nasal provocation test in the diagnostics of occupational allergic rhinitis. Rhinology. 1996;34:86–90. [PubMed] [Google Scholar]
- 305.Eggleston PA, Ansari AA, Adkinson NF, Jr, Wood RA. Environmental challenge studies in laboratory animal allergy. Effect of different airborne allergen concentrations. Am J Respir Crit Care Med. 1995;151:640–646. doi: 10.1164/ajrccm.151.3.7881650. [DOI] [PubMed] [Google Scholar]
- 306.American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 307.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Struben VM, Wieringa MH, Feenstra L, de Jongste JC. Nasal nitric oxide and nasal allergy. Allergy. 2006;61:665–670. doi: 10.1111/j.1398-9995.2006.01096.x. [DOI] [PubMed] [Google Scholar]
- 309.Chen W, Purohit A, Barnig C, Casset A, de Blay F. Niox and Niox Mino: comparison of exhaled NO in grass pollen allergic adult volunteers. Allergy. 2007;62:571–572. doi: 10.1111/j.1398-9995.2007.01334.x. [DOI] [PubMed] [Google Scholar]
- 310.Olin AC, Alving K, Torén K. Exhaled nitric oxide: relation to sensitization and respiratory symptoms. Clin Exp Allergy. 2004;34:221–226. doi: 10.1111/j.1365-2222.2004.01888.x. [DOI] [PubMed] [Google Scholar]
- 311.Zhang L, Luo XR, Liu CY, Zhao Y, Han DM. Measurement of exhaled nitric oxide in healthy Chinese. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2009;44:302–306. [PubMed] [Google Scholar]
- 312.Leng G, Li Z, Wang Q. Detection of exhaled nitric oxide of healthy in Nanjing. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012;26:769–771. [PubMed] [Google Scholar]
- 313.Liu D, Huang Z, Huang Y, Yi X, Chen X. Measurement of nasal and fractional exhaled nitric oxide in children with upper airway inflammatory disease: preliminary results. Int J Pediatr Otorhinolaryngol. 2015;79:2308–2311. doi: 10.1016/j.ijporl.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 314.You S, Zhang J, Ji L, Bai Y, Wang H. Noninvasive measurement of nasal NO and fractional exhaled NO in healthy people and patients with allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014;49:323–325. [PubMed] [Google Scholar]
- 315.Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery; Subspecialty Group of Rhinology, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association. Suggestion on the diagnosis and treatment of vasomotor rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013;48:884–885. [PubMed] [Google Scholar]
- 316.Zhang L, Han DM. A brief introduction to non-allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010;45:976–981. [PubMed] [Google Scholar]
- 317.Wang H, Zhang J, You S, Ao Y, Bai Y, Shi H, et al. Diagnosis and clinical characteristics of patients with non-allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014;49:501–505. [PubMed] [Google Scholar]
- 318.Meng CD, Li L, Jiang XD, Dong Z, Zhu DD. Clinical characteristics in patients with non-allergic rhinitis and allergic rhinitis: preliminary analysis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010;45:999–1002. [PubMed] [Google Scholar]
- 319.Zhou XL, Long Y, Lin CZ, Jiang YS, Lu XD, Yang C, et al. Microbic distribution of acute rhinitis patient's nasal cavity and dependability research of respiratory infection. Chin J Prim Med Pharm. 2009;16:437–438. [Google Scholar]
- 320.Zhang CD. Comparison of eosiniphis and total IgE in nasal secretion of allergic rhinitis and acute rhinitis patients. Med Lab Sci Clin. 2009;20:63–64. [Google Scholar]
- 321.Wang S, Tang Q, Qian W, Fan Y. Meta-analysis of clinical trials on traditional Chinese herbal medicine for treatment of persistent allergic rhinitis. Allergy. 2012;67:583–592. doi: 10.1111/j.1398-9995.2012.02806.x. [DOI] [PubMed] [Google Scholar]
- 322.Aspirin ZY. Intolerance-rhinitis, sinusitis, nasal polyps and asthma. Clin Otorhinolaryngol (China) 2000;14:381–383. [Google Scholar]
- 323.Lu M, Liu HB, Zhu WH, Chen BH, Liu Y, Zhao R, et al. Spontaneous cerebrospinal fluid rhinorrhea: case report. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2001;36:69. [Google Scholar]
- 324.Huang DQ, Li WR, Ou XY. One case of posttraumatic cerebrospinal fluid rhinorrhea. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2006;41:549. [PubMed] [Google Scholar]
- 325.Ma F. 32 cases of nasal foreign body misdiagnosed as rhinitis. Clin Misdiagnosis Mistherapy. 2010;23:94. [Google Scholar]
- 326.Jiang XD, Dong Z, Li GY, Gao G, Zhu DD. Endoscopic surgery for 89 cases of nasal inverted papilloma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010;45:186–189. [PubMed] [Google Scholar]
- 327.Dietz de Loos DA, Segboer CL, Gevorgyan A, Fokkens WJ. Disease-specific quality-of-life questionnaires in rhinitis and rhinosinusitis: review and evaluation. Curr Allergy Asthma Rep. 2013;13:162–170. doi: 10.1007/s11882-012-0334-8. [DOI] [PubMed] [Google Scholar]
- 328.Juniper EF, Guyatt GH. Development and testing of a new measure of health status for clinical trials in rhinoconjunctivitis. Clin Exp Allergy. 1991;21:77–83. doi: 10.1111/j.1365-2222.1991.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 329.Juniper EF, Howland WC, Roberts NB, Thompson AK, King DR. Measuring quality of life in children with rhinoconjunctivitis. J Allergy Clin Immunol. 1998;101:163–170. doi: 10.1016/s0091-6749(98)70380-x. [DOI] [PubMed] [Google Scholar]
- 330.Juniper EF, Guyatt GH, Dolovich J. Assessment of quality of life in adolescents with allergic rhinoconjunctivitis: development and testing of a questionnaire for clinical trials. J Allergy Clin Immunol. 1994;93:413–423. doi: 10.1016/0091-6749(94)90349-2. [DOI] [PubMed] [Google Scholar]
- 331.Juniper EF, Thompson AK, Ferrie PJ, Roberts JN. Validation of the standardized version of the Rhinoconjunctivitis Quality of Life Questionnaire. J Allergy Clin Immunol. 1999;104:364–369. doi: 10.1016/s0091-6749(99)70380-5. [DOI] [PubMed] [Google Scholar]
- 332.Juniper EF, Thompson AK, Ferrie PJ, Roberts JN. Development and validation of the mini Rhinoconjunctivitis Quality of Life Questionnaire. Clin Exp Allergy. 2000;30:132–140. doi: 10.1046/j.1365-2222.2000.00668.x. [DOI] [PubMed] [Google Scholar]
- 333.Juniper EF, Rohrbaugh T, Meltzer EO. A questionnaire to measure quality of life in adults with nocturnal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2003;111:484–490. doi: 10.1067/mai.2003.137. [DOI] [PubMed] [Google Scholar]
- 334.Hellings PW, Fokkens WJ, Akdis C, Bachert C, Cingi C, Dietz de, et al. Uncontrolled allergic rhinitis and chronic rhinosinusitis: where do we stand today? Allergy. 2013;68:1–7. doi: 10.1111/all.12040. [DOI] [PubMed] [Google Scholar]
- 335.Nurmatov U, van Schayck CP, Hurwitz B, Sheikh A. House dust mite avoidance measures for perennial allergic rhinitis: an updated Cochrane systematic review. Allergy. 2012;67:158–165. doi: 10.1111/j.1398-9995.2011.02752.x. [DOI] [PubMed] [Google Scholar]
- 336.Li Y, Cheng L, Chen X, Yang B, Wang D. Efficacy evaluation of a pollen blocker cream against dust-mite allergy: a multicenter, randomized, double-blind, placebo-controlled crossover trial. Am J Rhinol Allergy. 2015;29:e129–e133. doi: 10.2500/ajra.2015.29.4218. [DOI] [PubMed] [Google Scholar]
- 337.Jaakkola MS, Quansah R, Hugg TT, Heikkinen SA, Jaakkola JJ. Association of indoor dampness and molds with rhinitis risk: a systematic review and meta-analysis. J Allergy Clin Immunol. 2013;132:1099–1110.e18. doi: 10.1016/j.jaci.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 338.Kenney P, Hilberg O, Laursen AC, Peel RG, Sigsgaard T. Preventive effect of nasal filters on allergic rhinitis: a randomized, double-blind, placebo-controlled crossover park study. J Allergy Clin Immunol. 2015;136:1566–1572.e5. doi: 10.1016/j.jaci.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 339.Schwetz S, Olze H, Melchisedech S, Grigorov A, Latza R. Efficacy of pollen blocker cream in the treatment of allergic rhinitis. Arch Otolaryngol Head Neck Surg. 2004;130:979–984. doi: 10.1001/archotol.130.8.979. [DOI] [PubMed] [Google Scholar]
- 340.Åberg N, Dahl Å, Benson M. A nasally applied cellulose powder in seasonal allergic rhinitis (SAR) in children and adolescents; reduction of symptoms and relation to pollen load. Pediatr Allergy Immunol. 2011;22:594–599. doi: 10.1111/j.1399-3038.2011.01182.x. [DOI] [PubMed] [Google Scholar]
- 341.Åberg N, Ospanova ST, Nikitin NP, Emberlin J, Dahl Å. A nasally applied cellulose powder in seasonal allergic rhinitis in adults with grass pollen allergy: a double-blind, randomized, placebo-controlled, parallel-group study. Int Arch Allergy Immunol. 2014;163:313–318. doi: 10.1159/000360734. [DOI] [PubMed] [Google Scholar]
- 342.Simons FE, Simons KJ. Histamine and H1-antihistamines: celebrating a century of progress. J Allergy Clin Immunol. 2011;128:1139–1150.e4. doi: 10.1016/j.jaci.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 343.Simons FE. Advances in H1-antihistamines. N Engl J Med. 2004;351:2203–2217. doi: 10.1056/NEJMra033121. [DOI] [PubMed] [Google Scholar]
- 344.Simons FE, Simons KJ. H1 antihistamines: current status and future directions. World Allergy Organ J. 2008;1:145–155. doi: 10.1186/1939-4551-1-9-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Zhang L, Cheng L, Hong J. The clinical use of cetirizine in the treatment of allergic rhinitis. Pharmacology. 2013;92:14–25. doi: 10.1159/000351843. [DOI] [PubMed] [Google Scholar]
- 346.Simons FE Early Prevention of Asthma in Atopic Children (EPAAC) Study Group. Safety of levocetirizine treatment in young atopic children: an 18-month study. Pediatr Allergy Immunol. 2007;18:535–542. doi: 10.1111/j.1399-3038.2007.00558.x. [DOI] [PubMed] [Google Scholar]
- 347.Nickels AS, Dimov V, Wolf R. Pharmacokinetic evaluation of olopatadine for the treatment of allergic rhinitis and conjunctivitis. Expert Opin Drug Metab Toxicol. 2011;7:1593–1599. doi: 10.1517/17425255.2011.630389. [DOI] [PubMed] [Google Scholar]
- 348.Horak F, Zieglmayer UP, Zieglmayer R, Kavina A, Marschall K, Munzel U, et al. Azelastine nasal spray and desloratadine tablets in pollen-induced seasonal allergic rhinitis: a pharmacodynamic study of onset of action and efficacy. Curr Med Res Opin. 2006;22:151–157. doi: 10.1185/030079906X80305. [DOI] [PubMed] [Google Scholar]
- 349.Ratner PH, Findlay SR, Hampel F, Jr, van Bavel J, Widlitz MD, Freitag JJ. A double-blind, controlled trial to assess the safety and efficacy of azelastine nasal spray in seasonal allergic rhinitis. J Allergy Clin Immunol. 1994;94:818–825. doi: 10.1016/0091-6749(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 350.Kaliner MA, Berger WE, Ratner PH, Siegel CJ. The efficacy of intranasal antihistamines in the treatment of allergic rhinitis. Ann Allergy Asthma Immunol. 2011;106(Suppl):S6–S11. doi: 10.1016/j.anai.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 351.Feng S, Deng C, Li L, Liao W, Fan Y, Xu G, et al. Efficacy of intranasal antihistamine in the treatment of allergic rhinitis: a meta-analysis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014;49:832–838. [PubMed] [Google Scholar]
- 352.Han D, Chen L, Cheng L, Liu S, Fu Z, Zhang W, et al. A multicenter randomized double-blind 2-week comparison study of azelastine nasal spray 0.1% versus levocabastine nasal spray 0.05% in patients with moderate-to-severe allergic rhinitis. ORL J Otorhinolaryngol Relat Spec. 2011;73:260–265. doi: 10.1159/000330269. [DOI] [PubMed] [Google Scholar]
- 353.Cobanoğlu B, Toskala E, Ural A, Cingi C. Role of leukotriene antagonists and antihistamines in the treatment of allergic rhinitis. Curr Allergy Asthma Rep. 2013;13:203–208. doi: 10.1007/s11882-013-0341-4. [DOI] [PubMed] [Google Scholar]
- 354.Kushnir NM. The role of decongestants, cromolyn, guafenesin, saline washes, capsaicin, leukotriene antagonists, and other treatments on rhinitis. Immunol Allergy Clin North Am. 2011;31:601–617. doi: 10.1016/j.iac.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 355.Ouyang Y, Fan E, Li Y, Zhang L. Onset feature and efficacy of early interventional treatment of Artemisia pollinosis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014;49:272–276. [PubMed] [Google Scholar]
- 356.Hara H, Sugahara K, Hashimoto M, Mikuriya T, Tahara S, Yamashita H. Effectiveness of the leukotriene receptor antagonist pranlukast hydrate for the treatment of sleep disorder in patients with perennial allergic rhinitis. Acta Otolaryngol. 2014;134:307–313. doi: 10.3109/00016489.2013.861926. [DOI] [PubMed] [Google Scholar]
- 357.Xu Y, Zhang J, Wang J. The efficacy and safety of selective H1-antihistamine versus leukotriene receptor antagonist for seasonal allergic rhinitis: a meta-analysis. PLoS One. 2014;9:e112815. doi: 10.1371/journal.pone.0112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Liu X, Xing Z, Gao Z. The effect of antianaphylaxis drugs on specific IgE and eosinophil in serum of patients with allergic rhinitis. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2005;19:337–339. [PubMed] [Google Scholar]
- 359.Pinar E, Eryigit O, Oncel S, Calli C, Yilmaz O, Yuksel H. Efficacy of nasal corticosteroids alone or combined with antihistamines or montelukast in treatment of allergic rhinitis. Auris Nasus Larynx. 2008;35:61–66. doi: 10.1016/j.anl.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 360.Qu S, Li T, Chen Y, Lin Z, Ou Z. The role of leukotriene D4 antagonist in allergic rhinitis with steroid resistance. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2005;19:557–559. [PubMed] [Google Scholar]
- 361.Liang M, Xu R, Xu G. Recent advances in allergic rhinitis. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015;29:202–206. [PubMed] [Google Scholar]
- 362.Panwankar R, Canonica GW, Holgate ST. WHO white book on allergy: update 2013. Milwaukee, WI: World Allergy Organization; 2013. [Google Scholar]
- 363.Bascom R, Wachs M, Naclerio RM, Pipkorn U, Galli SJ, Lichtenstein LM. Basophil influx occurs after nasal antigen challenge: effects of topical corticosteroid pretreatment. J Allergy Clin Immunol. 1988;81:580–589. [PubMed] [Google Scholar]
- 364.Pipkorn U, Proud D, Lichtenstein LM, Kagey-Sobotka A, Norman PS, Naclerio RM. Inhibition of mediator release in allergic rhinitis by pretreatment with topical glucocorticosteroids. N Engl J Med. 1987;316:1506–1510. doi: 10.1056/NEJM198706113162403. [DOI] [PubMed] [Google Scholar]
- 365.Erin EM, Leaker BR, Zacharasiewicz AS, Higgins LA, Williams TJ, Boyce MJ, et al. Single dose topical corticosteroid inhibits IL-5 and IL-13 in nasal lavage following grass pollen challenge. Allergy. 2005;60:1524–1529. doi: 10.1111/j.1398-9995.2005.00928.x. [DOI] [PubMed] [Google Scholar]
- 366.Shah SA, Berger RL, McDermott J, Gupta P, Monteith D, Connor A, et al. Regional deposition of mometasone furoate nasal spray suspension in humans. Allergy Asthma Proc. 2015;36:48–57. doi: 10.2500/aap.2015.36.3817. [DOI] [PubMed] [Google Scholar]
- 367.Christodoulopoulos P, Cameron L, Durham S, Hamid Q. Molecular pathology of allergic disease. II: upper airway disease. J Allergy Clin Immunol. 2000;105:211–223. doi: 10.1016/s0091-6749(00)90068-x. [DOI] [PubMed] [Google Scholar]
- 368.Meltzer EO, Jalowayski AA, Orgel HA, Harris AG. Subjective and objective assessments in patients with seasonal allergic rhinitis: effects of therapy with mometasone furoate nasal spray. J Allergy Clin Immunol. 1998;102:39–49. doi: 10.1016/s0091-6749(98)70053-3. [DOI] [PubMed] [Google Scholar]
- 369.Igarashi T, Nakazato Y, Kunishige T, Fujita M, Yamada Y, Fujimoto C, et al. Mometasone furoate nasal spray relieves the ocular symptoms of seasonal allergic rhinoconjunctivitis. J Nippon Med Sch. 2012;79:182–189. doi: 10.1272/jnms.79.182. [DOI] [PubMed] [Google Scholar]
- 370.Bernstein DI, Levy AL, Hampel FC, Baidoo CA, Cook CK, Philpot EE, et al. Treatment with intranasal fluticasone propionate significantly improves ocular symptoms in patients with seasonal allergic rhinitis. Clin Exp Allergy. 2004;34:952–957. doi: 10.1111/j.1365-2222.2004.01952.x. [DOI] [PubMed] [Google Scholar]
- 371.Bhatia S, Baroody FM, deTineo M, Naclerio RM. Increased nasal airflow with budesonide compared with desloratadine during the allergy season. Arch Otolaryngol Head Neck Surg. 2005;131:223–228. doi: 10.1001/archotol.131.3.223. [DOI] [PubMed] [Google Scholar]
- 372.Bielory L, Chun Y, Bielory BP, Canonica GW. Impact of mometasone furoate nasal spray on individual ocular symptoms of allergic rhinitis: a meta-analysis. Allergy. 2011;66:686–693. doi: 10.1111/j.1398-9995.2010.02543.x. [DOI] [PubMed] [Google Scholar]
- 373.Zhang L, Xu G, Wang X, Liu S, Li Y, Wang S, et al. Mometasone furoate nasal spray reduces symptoms and improves quality of life in Chinese patients with moderate to severe allergic rhinitis: a multicenter open-label study. Acta Otolaryngol. 2009;129:1463–1468. doi: 10.3109/00016480902856570. [DOI] [PubMed] [Google Scholar]
- 374.Han D, Liu S, Zhang Y, Wang J, Wang D, Kong W, et al. Efficacy and safety of fluticasone furoate nasal spray in Chinese adult and adolescent subjects with intermittent or persistent allergic rhinitis. Allergy Asthma Proc. 2011;32:472–481. doi: 10.2500/aap.2011.32.3474. [DOI] [PubMed] [Google Scholar]
- 375.Cheng L. Prophylactic treatment for seasonal allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013;48:532–534. [PubMed] [Google Scholar]
- 376.Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery; Subspecialty Group of Rhinology, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association. Chinese guidelines for diagnosis and treatment of allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016;51:6–24. doi: 10.3760/cma.j.issn.1673-0860.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 377.Song XH, Zhang L, Han DM, Wang KJ, Wang H, Zhang W. Effects of oxymetazoline hydrochloride on ex vivo human nasal cilia movement measured with high-speed digital microscopy. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008;43:268–271. [PubMed] [Google Scholar]
- 378.Meltzer EO, Bernstein DI, Prenner BM, Berger WE, Shekar T, Teper AA. Mometasone furoate nasal spray plus oxymetazoline nasal spray: short-term efficacy and safety in seasonal allergic rhinitis. Am J Rhinol Allergy. 2013;27:102–108. doi: 10.2500/ajra.2013.27.3864. [DOI] [PubMed] [Google Scholar]
- 379.Slavin RG. Special considerations in treatment of allergic rhinitis in the elderly: role of intranasal corticosteroids. Allergy Asthma Proc. 2010;31:179–184. doi: 10.2500/aap.2010.31.3342. [DOI] [PubMed] [Google Scholar]
- 380.Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery; Subspecialty Group of Rhinology and Pediatrics, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association; Editorial Board of Chinese Journal of Pediatrics. Guidelines for diagnosis and treatment of pediatric allergic rhinitis (2010, Chongqing) Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011;46:7–8. [PubMed] [Google Scholar]
- 381.Garavello W, Somigliana E, Acaia B, Gaini L, Pignataro L, Gaini RM. Nasal lavage in pregnant women with seasonal allergic rhinitis: a randomized study. Int Arch Allergy Immunol. 2010;151:137–141. doi: 10.1159/000236003. [DOI] [PubMed] [Google Scholar]
- 382.Georgitis JW. Nasal hyperthermia and simple irrigation for perennial rhinitis. Changes in inflammatory mediators. Chest. 1994;106:1487–1492. doi: 10.1378/chest.106.5.1487. [DOI] [PubMed] [Google Scholar]
- 383.Ponikau JU, Sherris DA, Kephart GM, Kern EB, Congdon DJ, Adolphson CR, et al. Striking deposition of toxic eosinophil major basic protein in mucus: implications for chronic rhinosinusitis. J Allergy Clin Immunol. 2005;116:362–369. doi: 10.1016/j.jaci.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 384.Talbot AR, Herr TM, Parsons DS. Mucociliary clearance and buffered hypertonic saline solution. Laryngoscope. 1997;107:500–503. doi: 10.1097/00005537-199704000-00013. [DOI] [PubMed] [Google Scholar]
- 385.Boek WM, Graamans K, Natzijl H, van Rijk PP, Huizing EH. Nasal mucociliary transport: new evidence for a key role of ciliary beat frequency. Laryngoscope. 2002;112:570–573. doi: 10.1097/00005537-200203000-00029. [DOI] [PubMed] [Google Scholar]
- 386.Li H, Sha Q, Zuo K, Jiang H, Cheng L, Shi J, et al. Nasal saline irrigation facilitates control of allergic rhinitis by topical steroid in children. ORL J Otorhinolaryngol Relat Spec. 2009;71:50–55. doi: 10.1159/000178165. [DOI] [PubMed] [Google Scholar]
- 387.Sun YM. 47 cases of clinical observation on kidney yang-deficiency type of allergic rhinitis with Yougui soup treatment. Tradit Chin Med Rev. 2004;10:40–41. [Google Scholar]
- 388.Lin HW, Shi ZX, Ma SM. Clinical study on the treatment of allergic rhinitis by replenishing qi and consolidating the exterior. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1989;9:2631. [Google Scholar]
- 389.Qiu Baoshan LP, Ma L. Experimental study of the correlation between allergic rhinitis and spleen deficiency. J Tradit Chin Med. 2003;21:10402–10411. [Google Scholar]
- 390.Qiu Baoshan LP, Huang K. The effect of benefiting qi decoction on spleen deficiency type of allergic rhinitis. Tradit Chin Med Clin Pharmacol. 2003;14:1472–1491. [Google Scholar]
- 391.Yuehong Liao AO, Xiang J. Clinical observation on the therapeutic effect of Metal-clearing methodon allergic rhinitis and a study on the semeiological basis for this kind of therapy. Chin Otorhinolaryngol J Integr Med. 2007;15:427–429. [Google Scholar]
- 392.Chan RY, Chien WT. The effects of two Chinese herbal medicinal formulae vs. placebo controls for treatment of allergic rhinitis: a randomised controlled trial. Trials. 2014;15:261. doi: 10.1186/1745-6215-15-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Min C, Peng C, Wei G, Huang X, Fu T, Du Y, et al. Moxibustion with Chinese herbal has good effect on allergic rhinitis. Int J Clin Exp Med. 2015;8:16480–16487. [PMC free article] [PubMed] [Google Scholar]
- 394.Zhao Y, Woo KS, Ma KH, van Hansselt CA, Wong KC, Cheng KF, et al. Treatment of perennial allergic rhinitis using Shi-Bi-Lin, a Chinese herbal formula. J Ethnopharmacol. 2009;122:100–105. doi: 10.1016/j.jep.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 395.Chui SH, Shek SL, Fong MY, Szeto YT, Chan K. A panel study to evaluate quality of life assessments in patients suffering from allergic rhinitis after treatment with a Chinese herbal nasal drop. Phytother Res. 2010;24:609–613. doi: 10.1002/ptr.3058. [DOI] [PubMed] [Google Scholar]
- 396.Hsu WH, Ho TJ, Huang CY, Ho HC, Liu YL, Liu HJ, et al. Chinese medicine acupoint herbal patching for allergic rhinitis: a randomized controlled clinical trial. Am J Chin Med. 2010;38:661–673. doi: 10.1142/S0192415X10008135. [DOI] [PubMed] [Google Scholar]
- 397.Jung JW, Kang HR, Ji GE, Park MS, Song WJ, Kim MH, et al. Therapeutic effects of fermented red ginseng in allergic rhinitis: a randomized, double-blind, placebo-controlled study. Allergy Asthma Immunol Res. 2011;3:103–110. doi: 10.4168/aair.2011.3.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Hu G, Walls RS, Bass D, Ramon B, Grayson D, Jones M, et al. The Chinese herbal formulation biminne in management of perennial allergic rhinitis: a randomized, double-blind, placebo-controlled, 12-week clinical trial. Ann Allergy Asthma Immunol. 2002;88:478–487. doi: 10.1016/s1081-1206(10)62386-1. [DOI] [PubMed] [Google Scholar]
- 399.Lenon GB, Li CG, Da Costa C, Thien FC, Shen Y, Xue CC. Lack of efficacy of a herbal preparation (RCM-102) for seasonal allergic rhinitis: a double blind, randomised, placebo-controlled trial. Asia Pac Allergy. 2012;2:187–194. doi: 10.5415/apallergy.2012.2.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Brinkhaus B, Hummelsberger J, Kohnen R, Seufert J, Hempen CH, Leonhardy H, et al. Acupuncture and Chinese herbal medicine in the treatment of patients with seasonal allergic rhinitis: a randomized-controlled clinical trial. Allergy. 2004;59:953–960. doi: 10.1111/j.1398-9995.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 401.Zhang X, Lan F, Zhang Y, Zhang L. Chinese herbal medicine to treat allergic rhinitis: evidence from a meta-analysis. Allergy Asthma Immunol Res. 2018;10:34–42. doi: 10.4168/aair.2018.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Xue CC, Thien FC, Zhang JJ, Yang W, Da Costa C, Li CG. Effect of adding a Chinese herbal preparation to acupuncture for seasonal allergic rhinitis: randomised double-blind controlled trial. Hong Kong Med J. 2003;9:427–434. [PubMed] [Google Scholar]
- 403.Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2011;127:18–27. doi: 10.1016/j.jaci.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 404.Tarzi M, Klunker S, Texier C, Verhoef A, Stapel SO, Akdis CA, et al. Induction of interleukin-10 and suppressor of cytokine signalling-3 gene expression following peptide immunotherapy. Clin Exp Allergy. 2006;36:465–474. doi: 10.1111/j.1365-2222.2006.02469.x. [DOI] [PubMed] [Google Scholar]
- 405.Alexander C, Ying S, B Kay A, Larché M. Fel d 1-derived T cell peptide therapy induces recruitment of CD4+ CD25+; CD4+ interferon-gamma+ T helper type 1 cells to sites of allergen-induced late-phase skin reactions in cat-allergic subjects. Clin Exp Allergy. 2005;35:52–58. doi: 10.1111/j.1365-2222.2005.02143.x. [DOI] [PubMed] [Google Scholar]
- 406.Woo HY, Kim YS, Kang NI, Chung WC, Song CH, Choi IW, et al. Mechanism for acute oral desensitization to antibiotics. Allergy. 2006;61:954–958. doi: 10.1111/j.1398-9995.2006.01147.x. [DOI] [PubMed] [Google Scholar]
- 407.Bussmann C, Xia J, Allam JP, Maintz L, Bieber T, Novak N. Early markers for protective mechanisms during rush venom immunotherapy. Allergy. 2010;65:1558–1565. doi: 10.1111/j.1398-9995.2010.02430.x. [DOI] [PubMed] [Google Scholar]
- 408.Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 409.Lambrecht BN, Pauwels RA, Fazekas De St Groth B. Induction of rapid T cell activation, division, and recirculation by intratracheal injection of dendritic cells in a TCR transgenic model. J Immunol. 2000;164:2937–2946. doi: 10.4049/jimmunol.164.6.2937. [DOI] [PubMed] [Google Scholar]
- 410.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.de Heer HJ, Hammad H, Soullié T, Hijdra D, Vos N, Willart MA, et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Akdis CA, Blesken T, Akdis M, Wüthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Nouri-Aria KT, Wachholz PA, Francis JN, Jacobson MR, Walker SM, Wilcock LK, et al. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J Immunol. 2004;172:3252–3259. doi: 10.4049/jimmunol.172.5.3252. [DOI] [PubMed] [Google Scholar]
- 414.Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199:1567–1575. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, et al. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–615. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 416.Jutel M, Akdis M, Budak F, Aebischer-Casaulta C, Wrzyszcz M, Blaser K, et al. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003;33:1205–1214. doi: 10.1002/eji.200322919. [DOI] [PubMed] [Google Scholar]
- 417.Verhoef A, Alexander C, Kay AB, Larché M. T cell epitope immunotherapy induces a CD4+ T cell population with regulatory activity. PLoS Med. 2005;2:e78. doi: 10.1371/journal.pmed.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Varney VA, Hamid QA, Gaga M, Ying S, Jacobson M, Frew AJ, et al. Influence of grass pollen immunotherapy on cellular infiltration and cytokine mRNA expression during allergen-induced late-phase cutaneous responses. J Clin Invest. 1993;92:644–651. doi: 10.1172/JCI116633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Ebner C, Siemann U, Bohle B, Willheim M, Wiedermann U, Schenk S, et al. Immunological changes during specific immunotherapy of grass pollen allergy: reduced lymphoproliferative responses to allergen and shift from TH2 to TH1 in T-cell clones specific for Phl p 1, a major grass pollen allergen. Clin Exp Allergy. 1997;27:1007–1015. doi: 10.1111/j.1365-2222.1997.tb01252.x. [DOI] [PubMed] [Google Scholar]
- 420.Akdis CA, Akdis M, Blesken T, Wymann D, Alkan SS, Müller U, et al. Epitope-specific T cell tolerance to phospholipase A2 in bee venom immunotherapy and recovery by IL-2 and IL-15 in vitro. J Clin Invest. 1996;98:1676–1683. doi: 10.1172/JCI118963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Francis JN, Till SJ, Durham SR. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy. J Allergy Clin Immunol. 2003;111:1255–1261. doi: 10.1067/mai.2003.1570. [DOI] [PubMed] [Google Scholar]
- 422.Akdis CA, Blaser K. IL-10-induced anergy in peripheral T cell and reactivation by microenvironmental cytokines: two key steps in specific immunotherapy. FASEB J. 1999;13:603–609. doi: 10.1096/fasebj.13.6.603. [DOI] [PubMed] [Google Scholar]
- 423.Bellinghausen I, Metz G, Enk AH, Christmann S, Knop J, Saloga J. Insect venom immunotherapy induces interleukin-10 production and a Th2-to-Th1 shift, and changes surface marker expression in venom-allergic subjects. Eur J Immunol. 1997;27:1131–1139. doi: 10.1002/eji.1830270513. [DOI] [PubMed] [Google Scholar]
- 424.Pereira EA, Silva DA, Cunha-Júnior JP, Almeida KC, Alves R, Sung SJ, et al. IgE, IgG1, and IgG4 antibody responses to Blomia tropicalis in atopic patients. Allergy. 2005;60:401–406. doi: 10.1111/j.1398-9995.2005.00738.x. [DOI] [PubMed] [Google Scholar]
- 425.Cooke RA, Barnard JH, Hebald S, Stull A. Serological evidence of immunity with coexisting sensitization in a type of human allergy (hay fever) J Exp Med. 1935;62:733–750. doi: 10.1084/jem.62.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Lichtenstein LM, Holtzman NA, Burnett LS. A quantitative in vitro study of the chromatographic distribution and immunoglobulin characteristics of human blocking antibody. J Immunol. 1968;101:317–324. [PubMed] [Google Scholar]
- 427.Golden DB, Meyers DA, Kagey-Sobotka A, Valentine MD, Lichtenstein LM. Clinical relevance of the venom-specific immunoglobulin G antibody level during immunotherapy. J Allergy Clin Immunol. 1982;69:489–493. doi: 10.1016/0091-6749(82)90172-5. [DOI] [PubMed] [Google Scholar]
- 428.Müller U, Helbling A, Bischof M. Predictive value of venom-specific IgE, IgG and IgG subclass antibodies in patients on immunotherapy with honey bee venom. Allergy. 1989;44:412–418. doi: 10.1111/j.1398-9995.1989.tb04172.x. [DOI] [PubMed] [Google Scholar]
- 429.Djurup R, Malling HJ. High IgG4 antibody level is associated with failure of immunotherapy with inhalant allergens. Clin Allergy. 1987;17:459–468. doi: 10.1111/j.1365-2222.1987.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 430.King TP, Jim SY, Monsalve RI, Kagey-Sobotka A, Lichtenstein LM, Spangfort MD. Recombinant allergens with reduced allergenicity but retaining immunogenicity of the natural allergens: hybrids of yellow jacket and paper wasp venom allergen antigen 5s. J Immunol. 2001;166:6057–6065. doi: 10.4049/jimmunol.166.10.6057. [DOI] [PubMed] [Google Scholar]
- 431.Linhart B, Hartl A, Jahn-Schmid B, Verdino P, Keller W, Krauth MT, et al. A hybrid molecule resembling the epitope spectrum of grass pollen for allergy vaccination. J Allergy Clin Immunol. 2005;115:1010–1016. doi: 10.1016/j.jaci.2004.12.1142. [DOI] [PubMed] [Google Scholar]
- 432.Hirahara K, Tatsuta T, Takatori T, Ohtsuki M, Kirinaka H, Kawaguchi J, et al. Preclinical evaluation of an immunotherapeutic peptide comprising 7 T-cell determinants of Cry j 1 and Cry j 2, the major Japanese cedar pollen allergens. J Allergy Clin Immunol. 2001;108:94–100. doi: 10.1067/mai.2001.115481. [DOI] [PubMed] [Google Scholar]
- 433.Soyer OU, Akdis M, Akdis CA. Mechanisms of subcutaneous allergen immunotherapy. Immunol Allergy Clin North Am. 2011;31:175–190. doi: 10.1016/j.iac.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 434.Moote W, Kim H. Allergen-specific immunotherapy. Allergy Asthma Clin Immunol. 2011;7(Suppl 1):S5. doi: 10.1186/1710-1492-7-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(Suppl):S1–S55. doi: 10.1016/j.jaci.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 436.Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556–568. doi: 10.1016/j.jaci.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 437.Zhang L, Wang C, Han D, Wang X, Zhao Y, Liu J. Comparative study of cluster and conventional immunotherapy schedules with dermatophagoides pteronyssinus in the treatment of persistent allergic rhinitis. Int Arch Allergy Immunol. 2009;148:161–169. doi: 10.1159/000155747. [DOI] [PubMed] [Google Scholar]
- 438.Tabar AI, Echechipía S, García BE, Olaguibel JM, Lizaso MT, Gómez B, et al. Double-blind comparative study of cluster and conventional immunotherapy schedules with Dermatophagoides pteronyssinus. J Allergy Clin Immunol. 2005;116:109–118. doi: 10.1016/j.jaci.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 439.Lou W, Wang C, Wang Y, Han D, Zhang L. Enhancement of the frequency and function of IL-10-secreting type I T regulatory cells after 1 year of cluster allergen-specific immunotherapy. Int Arch Allergy Immunol. 2012;159:391–398. doi: 10.1159/000338995. [DOI] [PubMed] [Google Scholar]
- 440.Canonica GW, Cox L, Pawankar R, Baena-Cagnani CE, Blaiss M, Bonini S, et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J. 2014;7:6. doi: 10.1186/1939-4551-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Sikora JM, Tankersley MS ACAAI Immunotherapy and Diagnostics Committee. Perception and practice of sublingual immunotherapy among practicing allergists in the United States: a follow-up survey. Ann Allergy Asthma Immunol. 2013;110:194–197.e4. doi: 10.1016/j.anai.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 442.Lin SY, Erekosima N, Kim JM, Ramanathan M, Suarez-Cuervo C, Chelladurai Y, et al. Sublingual immunotherapy for the treatment of allergic rhinoconjunctivitis and asthma: a systematic review. JAMA. 2013;309:1278–1288. doi: 10.1001/jama.2013.2049. [DOI] [PubMed] [Google Scholar]
- 443.Radulovic S, Wilson D, Calderon M, Durham S. Systematic reviews of sublingual immunotherapy (SLIT) Allergy. 2011;66:740–752. doi: 10.1111/j.1398-9995.2011.02583.x. [DOI] [PubMed] [Google Scholar]
- 444.Meng Q, Liu X, Li P, He L, Xie J, Gao X, et al. The influence of house dust mite sublingual immunotherapy on the TSLP-OX40L signaling pathway in patients with allergic rhinitis. Int Forum Allergy Rhinol. 2016;6:862–870. doi: 10.1002/alr.21743. [DOI] [PubMed] [Google Scholar]
- 445.Luo X, Hong H, Tang J, Wu X, Lin Z, Ma R, et al. Increased expression of miR-146a in children with allergic rhinitis after allergen-specific immunotherapy. Allergy Asthma Immunol Res. 2016;8:132–140. doi: 10.4168/aair.2016.8.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Lin Z, Liu Q, Li T, Chen D, Chen D, Xu R. The effects of house dust mite sublingual immunotherapy in patients with allergic rhinitis according to duration. Int Forum Allergy Rhinol. 2016;6:82–87. doi: 10.1002/alr.21657. [DOI] [PubMed] [Google Scholar]
- 447.Xu CX, Zhang ML, Li BZ, He Y, Zou ZH, Wu QR, et al. Efficacy of sublingual immunotherapy with dermatophagoides farinae extract in monosensitized and polysensitized patients with allergic rhinitis: clinical observation and analysis. Biomed Res Int. 2015;2015:187620. doi: 10.1155/2015/187620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Aboshady OA, Elghanam KM. Sublingual immunotherapy in allergic rhinitis: efficacy, safety, adherence and guidelines. Clin Exp Otorhinolaryngol. 2014;7:241–249. doi: 10.3342/ceo.2014.7.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Wang C, Zhang L. Specific immunotherapy for allergic rhinitis in children. Curr Opin Otolaryngol Head Neck Surg. 2014;22:487–494. doi: 10.1097/MOO.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 450.Prigal SJ. A ten-year study of repository injections of allergens: local reactions and their management. Ann Allergy. 1972;30:529–535. [PubMed] [Google Scholar]
- 451.Nelson BL, Dupont LA, Reid MJ. Prospective survey of local and systemic reactions to immunotherapy with pollen extracts. Ann Allergy. 1986;56:331–334. [PubMed] [Google Scholar]
- 452.Tankersley MS, Butler KK, Butler WK, Goetz DW. Local reactions during allergen immunotherapy do not require dose adjustment. J Allergy Clin Immunol. 2000;106:840–843. doi: 10.1067/mai.2000.110468. [DOI] [PubMed] [Google Scholar]
- 453.Kelso JM. The rate of systemic reactions to immunotherapy injections is the same whether or not the dose is reduced after a local reaction. Ann Allergy Asthma Immunol. 2004;92:225–227. doi: 10.1016/S1081-1206(10)61551-7. [DOI] [PubMed] [Google Scholar]
- 454.Zhu L, Lu JH, Xie Q, Wu YL, Zhu LP, Cheng L. Compliance and safety evaluation of subcutaneous versus sublingual immunotherapy in mite-sensitized patients with allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010;45:444–449. [PubMed] [Google Scholar]
- 455.Calabria CW, Stolfi A, Tankersley MS. The REPEAT study: recognizing and evaluating periodic local reactions in allergen immunotherapy and associated systemic reactions. Ann Allergy Asthma Immunol. 2011;106:49–53. doi: 10.1016/j.anai.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 456.Coop CA, Tankersley MS. Patient perceptions regarding local reactions from allergen immunotherapy injections. Ann Allergy Asthma Immunol. 2008;101:96–100. doi: 10.1016/S1081-1206(10)60841-1. [DOI] [PubMed] [Google Scholar]
- 457.Passalacqua G, Baena-Cagnani CE, Bousquet J, Canonica GW, Casale TB, Cox L, et al. Grading local side effects of sublingual immunotherapy for respiratory allergy: speaking the same language. J Allergy Clin Immunol. 2013;132:93–98. doi: 10.1016/j.jaci.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 458.Wen CJ, Zhu MF, Ren WM, Liu XY, Qian H. Clinical efficacy and safety of sublingual immunotherapy using standardized Dermatophagoides farinae extract for children with combined allergic rhinitis and asthma syndrome. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011;46:393–396. [PubMed] [Google Scholar]
- 459.Cox L, Larenas-Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: the world allergy organization subcutaneous immunotherapy systemic reaction grading system. J Allergy Clin Immunol. 2010;125:569–574. 574.e1–574.e7. doi: 10.1016/j.jaci.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 460.Epstein TG, Liss GM, Murphy-Berendts K, Bernstein DI. AAAAI/ACAAI surveillance study of subcutaneous immunotherapy, years 2008–2012: an update on fatal and nonfatal systemic allergic reactions. J Allergy Clin Immunol Pract. 2014;2:161–167. doi: 10.1016/j.jaip.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 461.Bernstein DI, Wanner M, Borish L, Liss GM Immunotherapy Committee, American Academy of Allergy, Asthma and Immunology. Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990–2001. J Allergy Clin Immunol. 2004;113:1129–1136. doi: 10.1016/j.jaci.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 462.Chen J, Li B, Zhao Y, Zhang Q, Wan L, Liu J, et al. A prospective multicenter study of systemic reactions in standardized specific immunotherapy for allergic rhinitis in China. Am J Rhinol Allergy. 2014;28:e40–e44. doi: 10.2500/ajra.2014.28.4005. [DOI] [PubMed] [Google Scholar]
- 463.Amin HS, Liss GM, Bernstein DI. Evaluation of near-fatal reactions to allergen immunotherapy injections. J Allergy Clin Immunol. 2006;117:169–175. doi: 10.1016/j.jaci.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 464.Cox LS, Larenas Linnemann D, Nolte H, Weldon D, Finegold I, Nelson HS. Sublingual immunotherapy: a comprehensive review. J Allergy Clin Immunol. 2006;117:1021–1035. doi: 10.1016/j.jaci.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 465.Berchtold E, Maibach R, Müller U. Reduction of side effects from rush-immunotherapy with honey bee venom by pretreatment with terfenadine. Clin Exp Allergy. 1992;22:59–65. doi: 10.1111/j.1365-2222.1992.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 466.Nielsen L, Johnsen CR, Mosbech H, Poulsen LK, Malling HJ. Antihistamine premedication in specific cluster immunotherapy: a double-blind, placebo-controlled study. J Allergy Clin Immunol. 1996;97:1207–1213. doi: 10.1016/s0091-6749(96)70186-0. [DOI] [PubMed] [Google Scholar]
- 467.Wöhrl S, Gamper S, Hemmer W, Heinze G, Stingl G, Kinaciyan T. Premedication with montelukast reduces local reactions of allergen immunotherapy. Int Arch Allergy Immunol. 2007;144:137–142. doi: 10.1159/000103225. [DOI] [PubMed] [Google Scholar]
- 468.Scranton SE, Gonzalez EG, Waibel KH. Incidence and characteristics of biphasic reactions after allergen immunotherapy. J Allergy Clin Immunol. 2009;123:493–498. doi: 10.1016/j.jaci.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 469.Kemp SF, Lockey RF, Simons FE World Allergy Organization ad hoc Committee on Epinephrine in Anaphylaxis. Epinephrine: the drug of choice for anaphylaxis. A statement of the World Allergy Organization. Allergy. 2008;63:1061–1070. doi: 10.1111/j.1398-9995.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- 470.Alvarez-Cuesta E, Bousquet J, Canonica GW, Durham SR, Malling HJ, Valovirta E. Standards for practical allergen-specific immunotherapy. Allergy. 2006;61(Suppl 82):1–20. doi: 10.1111/j.1398-9995.2006.01219_1.x. [DOI] [PubMed] [Google Scholar]
- 471.Tan G, Ma Y, Li H, Li W, Wang J. Long-term results of bilateral endoscopic vidian neurectomy in the management of moderate to severe persistent allergic rhinitis. Arch Otolaryngol Head Neck Surg. 2012;138:492–497. doi: 10.1001/archoto.2012.284. [DOI] [PubMed] [Google Scholar]
- 472.Jang TY, Kim YH, Shin SH. Long-term effectiveness and safety of endoscopic vidian neurectomy for the treatment of intractable rhinitis. Clin Exp Otorhinolaryngol. 2010;3:212–216. doi: 10.3342/ceo.2010.3.4.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Kobayashi T, Hyodo M, Nakamura K, Komobuchi H, Honda N. Resection of peripheral branches of the posterior nasal nerve compared to conventional posterior neurectomy in severe allergic rhinitis. Auris Nasus Larynx. 2012;39:593–596. doi: 10.1016/j.anl.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 474.Ogawa T, Takeno S, Ishino T, Hirakawa K. Submucous turbinectomy combined with posterior nasal neurectomy in the management of severe allergic rhinitis: clinical outcomes and local cytokine changes. Auris Nasus Larynx. 2007;34:319–326. doi: 10.1016/j.anl.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 475.Wu AW, Ting JY. Indications for surgery in refractory rhinitis. Curr Allergy Asthma Rep. 2014;14:414. doi: 10.1007/s11882-013-0414-4. [DOI] [PubMed] [Google Scholar]
- 476.Li PZ, Gu DS, Lu MP, Li YJ, Shen Y, Cheng L. Nasal coblation plasma surgery for the treatment of persistent allergic rhinitis: an evaluation of short-term outcomes. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013;48:891–894. [PubMed] [Google Scholar]
- 477.Cassano M, Granieri C, Del Giudice AM, Mora F, Fiocca-Matthews E, Cassano P. Restoration of nasal cytology after endoscopic turbinoplasty versus laser-assisted turbinoplasty. Am J Rhinol Allergy. 2010;24:310–314. doi: 10.2500/ajra.2010.24.3474. [DOI] [PubMed] [Google Scholar]
- 478.Caffier PP, Scherer H, Neumann K, Lück S, Enzmann H, Haisch A. Diode laser treatment in therapy-resistant allergic rhinitis: impact on nasal obstruction and associated symptoms. Lasers Med Sci. 2011;26:57–67. doi: 10.1007/s10103-010-0813-x. [DOI] [PubMed] [Google Scholar]
- 479.Chen YL, Tan CT, Huang HM. Long-term efficacy of microdebrider-assisted inferior turbinoplasty with lateralization for hypertrophic inferior turbinates in patients with perennial allergic rhinitis. Laryngoscope. 2008;118:1270–1274. doi: 10.1097/MLG.0b013e31816d728e. [DOI] [PubMed] [Google Scholar]
- 480.Lin HC, Lin PW, Friedman M, Chang HW, Su YY, Chen YJ, et al. Long-term results of radiofrequency turbinoplasty for allergic rhinitis refractory to medical therapy. Arch Otolaryngol Head Neck Surg. 2010;136:892–895. doi: 10.1001/archoto.2010.135. [DOI] [PubMed] [Google Scholar]
- 481.Lee JY, Lee JD. Comparative study on the long-term effectiveness between coblation- and microdebrider-assisted partial turbinoplasty. Laryngoscope. 2006;116:729–734. doi: 10.1097/01.mlg.0000205140.44181.45. [DOI] [PubMed] [Google Scholar]
- 482.Tan GL, Ma YH, Liu GS, Wang JJ, Li W. Therapeutic effect of endoscopic vidian neurectomy on moderate-severe persistent allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011;46:449–454. [PubMed] [Google Scholar]
- 483.Undem BJ, Taylor-Clark T. Mechanisms underlying the neuronal-based symptoms of allergy. J Allergy Clin Immunol. 2014;133:1521–1534. doi: 10.1016/j.jaci.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Daoud A, Xie Z, Ma Y, Wang T, Tan G. Changes of T-helper type 1/2 cell balance by anticholinergic treatment in allergic mice. Ann Allergy Asthma Immunol. 2014;112:249–255. doi: 10.1016/j.anai.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 485.Golding-Wood PH. Observations on petrosal and vidian neurectomy in chronic vasomotor rhinitis. J Laryngol Otol. 1961;75:232–247. doi: 10.1017/s0022215100057716. [DOI] [PubMed] [Google Scholar]
- 486.Lee JC, Kao CH, Hsu CH, Lin YS. Endoscopic transsphenoidal vidian neurectomy. Eur Arch Otorhinolaryngol. 2011;268:851–856. doi: 10.1007/s00405-010-1482-x. [DOI] [PubMed] [Google Scholar]
- 487.Su WF, Liu SC, Chiu FS, Lee CH. Antegrade transsphenoidal vidian neurectomy: short-term surgical outcome analysis. Am J Rhinol Allergy. 2011;25:e217–e220. doi: 10.2500/ajra.2011.25.3704. [DOI] [PubMed] [Google Scholar]
- 488.Ikeda K, Yokoi H, Saito T, Kawano K, Yao T, Furukawa M. Effect of resection of the posterior nasal nerve on functional and morphological changes in the inferior turbinate mucosa. Acta Otolaryngol. 2008;128:1337–1341. doi: 10.1080/00016480801935525. [DOI] [PubMed] [Google Scholar]
- 489.Liu HT, Ma RX, Yan XH, Feng NY, Zhou Y, Shen Y, et al. Clinical study on resection of the posterior nasal nerve for hyperreactive rhinopathy. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013;48:1032–1034. [PubMed] [Google Scholar]
- 490.Shakeel M, Trinidade A, Ah-See KW. Complementary and alternative medicine use by otolaryngology patients: a paradigm for practitioners in all surgical specialties. Eur Arch Otorhinolaryngol. 2010;267:961–971. doi: 10.1007/s00405-009-1098-1. [DOI] [PubMed] [Google Scholar]
- 491.Shakeel M, Trinidade A, Jehan S, Ah-See KW. The use of complementary and alternative medicine by patients attending a general otolaryngology clinic: can we afford to ignore it? Am J Otolaryngol. 2010;31:252–260. doi: 10.1016/j.amjoto.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 492.Choi SM, Park JE, Li SS, Jung H, Zi M, Kim TH, et al. A multicenter, randomized, controlled trial testing the effects of acupuncture on allergic rhinitis. Allergy. 2013;68:365–374. doi: 10.1111/all.12053. [DOI] [PubMed] [Google Scholar]
- 493.Brinkhaus B, Witt CM, Jena S, Liecker B, Wegscheider K, Willich SN. Acupuncture in patients with allergic rhinitis: a pragmatic randomized trial. Ann Allergy Asthma Immunol. 2008;101:535–543. doi: 10.1016/S1081-1206(10)60294-3. [DOI] [PubMed] [Google Scholar]
- 494.Chen Y, Jin X, Yu M, Qiu H, Fang Y, Zhang S, et al. Efficacy of acupuncture on moderate and severe allergic rhinitis. Zhongguo Zhen Jiu. 2015;35:339–343. [PubMed] [Google Scholar]
- 495.Chen S, Wang J, Bai P, Zhao Q, Tan C, Wang B, et al. Moderate and severe persistent allergic rhinitis treated with acupuncture: a randomized controlled trial. Zhongguo Zhen Jiu. 2015;35:1209–1213. [PubMed] [Google Scholar]
- 496.He TY, Li HQ, Zhao YD, Gao HY. Treatment of 60 cases of allergic rhinitis mainly with point-through-point method. Zhongguo Zhen Jiu. 2006;26:110–112. [PubMed] [Google Scholar]
- 497.Zhang YQ. Clinical experience in acupuncture treatment of allergic rhinitis. J Tradit Chin Med. 2009;29:186–189. doi: 10.1016/s0254-6272(09)60062-5. [DOI] [PubMed] [Google Scholar]
- 498.Chen ZX. Clinical observation on acupuncture for treatment of allergic rhinitis. Zhongguo Zhenjiu. 2007;27:578–580. [PubMed] [Google Scholar]
- 499.Petti FB, Liguori A, Ippoliti F. Study on cytokines IL-2, IL-6, IL-10 in patients of chronic allergic rhinitis treated with acupuncture. J Tradit Chin Med. 2002;22:104–111. [PubMed] [Google Scholar]
- 500.Liu TS, Qiu R, Lai XS. Efficacy on perennial allergic rhinitis treated with acupuncture at three nasal poinits and the acupoints selected by syndrome differentiation. Zhongguo Zhen Jiu. 2014;34:1083–1086. [PubMed] [Google Scholar]
- 501.Han D, Liu C, Qie L, Wang F, Wang Z. Acupoint selection and medication rules analysis for allergic rhinitis treated with acupoint application-based on data mining technology. Zhongguo Zhen Jiu. 2015;35:1177–1180. [PubMed] [Google Scholar]
- 502.Xie Y, Wan W, Zhao Y, Ye Z, Chen H, Hong X, et al. Impacts on the life quality of the patients with allergic rhinitis treated with warming acupuncture in winter and summer. Zhongguo Zhen Jiu. 2015;35:1215–1220. [PubMed] [Google Scholar]
- 503.Ou WX, Luo QY, Lin QM, Lin XH, Cao YM, Ma XW, et al. Efficacy observation on Jin's three-needle therapy for allergic rhinitis of lung qi deficiency and cold syndrome. Zhongguo Zhen Jiu. 2014;34:445–448. [PubMed] [Google Scholar]
- 504.Wang H, Li W, Ju XF, Yu XG. Effect of penetrating needling at head acupoints on perennial allergic rhinitis. Zhongguo Zhen Jiu. 2013;33:789–792. [PubMed] [Google Scholar]
- 505.Rao YQ, Han NY. Therapeutic effect of acupuncture on allergic rhinitis and its effects on immunologic function. Zhongguo Zhen Jiu. 2006;26:557–560. [PubMed] [Google Scholar]
- 506.Wang K, Chen L, Wang Y, Wang C, Zhang L. Sphenopalatine ganglion acupuncture improves nasal ventilation and modulates autonomic nervous activity in healthy volunteers: a randomized controlled study. Sci Rep. 2016;6:29947. doi: 10.1038/srep29947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Zhang L, Yang W, Wang KJ. Acupuncture at ganglion pterygoplatinum for 71 cases of chronic simple rhinitis. Zhongguo Zhen Jiu. 2013;33:495–496. [PubMed] [Google Scholar]
- 508.Xinwu L. The mechanism analysis of treating nasal disease by sphenopalatine ganglion (acupoint “ZhiBi 3”) stimulation with acupuncture needle and an introduction to the relevant needling method. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011;21:193–196. [Google Scholar]
- 509.Zhang H, Zhao M, Fu L. Short effect and adverse reaction of dog days plaster for allergic rhinitis. Zhongguo Zhen Jiu. 2016;36:33–36. [PubMed] [Google Scholar]
- 510.Chen J, Deng GZ, Chen F, Zhang SJ, Guo YF, Chen JQ, et al. Clinical comparative study on the influence of acupoint sticking therapy in dog days and in non-dog days to the quality of life of allergic rhinitis patients. Zhongguo Zhen Jiu. 2012;32:31–34. [PubMed] [Google Scholar]
- 511.Fang MS, Dou YC, Yao SM. Clinical research of medicinal vesiculation for perennial allergic rhinitis. Zhongguo Zhen Jiu. 2014;34:857–860. [PubMed] [Google Scholar]
- 512.Tang ZM, Chen JX, Tan JS. Therapy of cantharides extract for perennial allergic rhinitis and its effect on total IgE in serum. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1995;15:334–336. [PubMed] [Google Scholar]
- 513.Cao W, Qiao P, Pang W, Liu M, Li A. Triple-strong stimulation therapy at Dazhui (GV 14) in prevention and treatment of children allergic rhinitis: a randomized controlled trial. Zhongguo Zhen Jiu. 2015;35:38–42. [PubMed] [Google Scholar]
- 514.Ke ZH, Long SH. Medicinal vesiculation combined with quick cupping at Shenque (CV 8) for allergic rhinitis with syndrome of yang deficiency: a randomized controlled trial. Zhongguo Zhen Jiu. 2014;34:853–856. [PubMed] [Google Scholar]
- 515.Chen C, Li YC, Qiu BS, Huang XP, Zhuang LX. Observation of long-term efficacy and life quality in allergic rhinitis treated with acupoint catgut embedding therapy combined with acupuncture-moxibustion therapy. Zhongguo Zhen Jiu. 2014;34:439–443. [PubMed] [Google Scholar]
- 516.Li XR, Zhang QX, Liu M, Chen Q, Liu Y, Zhang FB, et al. Catgut implantation at acupoints for allergic rhinitis: a systematic review. Chin J Integr Med. 2014;20:235–240. doi: 10.1007/s11655-014-1748-z. [DOI] [PubMed] [Google Scholar]
- 517.Liang C, Jiang T. Acupoint autohemotherapy for allergic rhinitis and its effect on serum IL-12 and IFN-gamma. Zhongguo Zhen Jiu. 2012;32:1077–1080. [PubMed] [Google Scholar]
- 518.Li F, Liu KJ. Point-injection combined with TDP radiation for treatment of 30 cases of allergic rhinitis. Zhongguo Zhen Jiu. 2006;26:25–26. [PubMed] [Google Scholar]
- 519.Zhu LP, Zhang QZ, Shimada T, Enomoto T, Cheng L. Anti-allergic effects of the probiotic preparations of enterococcus on experimental allergic rhinitis in mice. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013;48:555–562. [PubMed] [Google Scholar]
- 520.Fan J, Hou Y, Zhou S, Cai X. Effect of Bifidobacterium on the immunity in BALB/c mice. Wei Sheng Wu Xue Bao. 2015;55:484–491. [PubMed] [Google Scholar]
- 521.Li SR, Wang HH, Wu ZY, Liu RH, Tong MH, Wang CH, et al. Efficacies of lactulose plus live combined Bacillus subtilis and Enterococcus faecium capsules in the treatment of functional constipation: a multicenter, randomized, double blind, controlled trial. Zhonghua Yi Xue Za Zhi. 2012;92:2955–2960. doi: 10.3760/cma.j.issn.0376-2491.2012.42.002. [DOI] [PubMed] [Google Scholar]
- 522.Investigating Group for Prevention of AAD in Children with Pneumonia by Clostridium Butyricum and Bifidobacterium. Multicenter, randomized, controlled clinical trial on preventing antibiotic-associated diarrhea in children with pneumonia using the live Clostridium butyricum and Bifidobacterium combined Powder. Zhonghua Er Ke Za Zhi. 2012;50:732–736. [PubMed] [Google Scholar]
- 523.Soh SE, Aw M, Gerez I, Chong YS, Rauff M, Ng YP, et al. Probiotic supplementation in the first 6 months of life in at risk Asian infants--effects on eczema and atopic sensitization at the age of 1 year. Clin Exp Allergy. 2009;39:571–578. doi: 10.1111/j.1365-2222.2008.03133.x. [DOI] [PubMed] [Google Scholar]
- 524.West CE. Probiotics for allergy prevention. Benef Microbes. 2016;7:171–179. doi: 10.3920/BM2015.0073. [DOI] [PubMed] [Google Scholar]
- 525.Rautava S, Kalliomäki M, Isolauri E. Probiotics during pregnancy and breast-feeding might confer immunomodulatory protection against atopic disease in the infant. J Allergy Clin Immunol. 2002;109:119–121. doi: 10.1067/mai.2002.120273. [DOI] [PubMed] [Google Scholar]
- 526.Chu Z, Zhang X, Meng B. Value of patient education in the treatment of allergic rhinitis. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015;29:396–399. [PubMed] [Google Scholar]
- 527.Scadding GK. Optimal management of allergic rhinitis. Arch Dis Child. 2015;100:576–582. doi: 10.1136/archdischild-2014-306300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Wang K, Wang C, Xi L, Zhang Y, Ouyang Y, Lou H, et al. A randomized controlled trial to assess adherence to allergic rhinitis treatment following a daily short message service (SMS) via the mobile phone. Int Arch Allergy Immunol. 2014;163:51–58. doi: 10.1159/000356317. [DOI] [PubMed] [Google Scholar]
- 529.Li C, Xu P, Xu H, Zhu H. Evaluation on the immunotherapy efficacies of synthetic peptide vaccines in asthmatic mice with group I and II allergens from Dermatophagoides pteronyssinus. Int J Clin Exp Med. 2015;8:20402–20412. [PMC free article] [PubMed] [Google Scholar]
- 530.Ou J, Shi W, Xu Y, Tao Z. Intranasal immunization with DNA vaccine coexpressing Der p 1 and ubiquitin in an allergic rhinitis mouse model. Ann Allergy Asthma Immunol. 2014;113:658–665.e1. doi: 10.1016/j.anai.2014.08.015. [DOI] [PubMed] [Google Scholar]