Recent emergence of antimicrobial resistance of Neisseria gonorrhoeae worldwide has resulted in limited therapeutic choices for treatment of infections caused by this organism. We performed global transcriptomic analysis of N. gonorrhoeae in subjects with gonorrhea who attended a Nanjing, China, sexually transmitted infection (STI) clinic, where antimicrobial resistance of N. gonorrhoeae is high and increasing. We found that N. gonorrhoeae transcriptional responses to infection differed in genital specimens taken from men and women, particularly antibiotic resistance gene expression, which was increased in men. These sex-specific findings may provide a new approach to guide therapeutic interventions and preventive measures that are also sex specific while providing additional insight to address antimicrobial resistance of N. gonorrhoeae.
KEYWORDS: Neisseria gonorrhoeae, RNA-seq, antibiotic resistance, human mucosal infection
ABSTRACT
Neisseria gonorrhoeae is a bacterial pathogen responsible for the sexually transmitted infection gonorrhea. Emergence of antimicrobial resistance (AMR) of N. gonorrhoeae worldwide has resulted in limited therapeutic choices for this infection. Men who seek treatment often have symptomatic urethritis; in contrast, gonococcal cervicitis in women is usually minimally symptomatic, but may progress to pelvic inflammatory disease. Previously, we reported the first analysis of gonococcal transcriptome expression determined in secretions from women with cervical infection. Here, we defined gonococcal global transcriptional responses in urethral specimens from men with symptomatic urethritis and compared these with transcriptional responses in specimens obtained from women with cervical infections and in vitro-grown N. gonorrhoeae isolates. This is the first comprehensive comparison of gonococcal gene expression in infected men and women. RNA sequencing analysis revealed that 9.4% of gonococcal genes showed increased expression exclusively in men and included genes involved in host immune cell interactions, while 4.3% showed increased expression exclusively in women and included phage-associated genes. Infected men and women displayed comparable antibiotic-resistant genotypes and in vitro phenotypes, but a 4-fold higher expression of the Mtr efflux pump-related genes was observed in men. These results suggest that expression of AMR genes is programed genotypically and also driven by sex-specific environments. Collectively, our results indicate that distinct N. gonorrhoeae gene expression signatures are detected during genital infection in men and women. We propose that therapeutic strategies could target sex-specific differences in expression of antibiotic resistance genes.
IMPORTANCE Recent emergence of antimicrobial resistance of Neisseria gonorrhoeae worldwide has resulted in limited therapeutic choices for treatment of infections caused by this organism. We performed global transcriptomic analysis of N. gonorrhoeae in subjects with gonorrhea who attended a Nanjing, China, sexually transmitted infection (STI) clinic, where antimicrobial resistance of N. gonorrhoeae is high and increasing. We found that N. gonorrhoeae transcriptional responses to infection differed in genital specimens taken from men and women, particularly antibiotic resistance gene expression, which was increased in men. These sex-specific findings may provide a new approach to guide therapeutic interventions and preventive measures that are also sex specific while providing additional insight to address antimicrobial resistance of N. gonorrhoeae.
INTRODUCTION
Neisseria gonorrhoeae is responsible for the sexually transmitted infection (STI) gonorrhea. Rates of gonococcal infection in the United States have increased 46% since 2011, and approximately 450,000 cases of gonorrhea were reported to the U.S. Centers for Disease Control and Prevention in 2015. N. gonorrhoeae is the second most common reportable bacterial STI in the United States (1). Infection with N. gonorrhoeae also contributes significantly to global STI morbidity and is responsible for 78 million cases each year (2). For the past 8 decades, gonorrhea has been treated successfully with antibiotics, despite patterns of resistance, but over the last several years, multidrug-resistant and untreatable strains have begun to emerge worldwide. Resistant strains include isolates from both Eastern and Western Europe (3, 4), Asia (5–7), and recently the United States (8–10). This rise in antibiotic-resistant strains may be linked, in part, to an increase in availability of over-the-counter antibiotics, particularly in many parts of Asia. New strategies for combating this disease are necessary, as evidenced by increased efforts to develop gonococcal vaccines (11, 12) and new drug regimens (7, 13, 14).
In humans, the only natural host for N. gonorrhoeae, symptomatic responses to infection are unique to men and women. While most infected men remain asymptomatic (15), those who develop symptoms often show robust inflammation characterized by purulent urethral discharge accompanied by large numbers of polymorphonuclear leukocytes (PMNs). In women, gonococcal infections are asymptomatic 50 to 80% of the time (16) or are accompanied by nonspecific symptoms such as vaginal discharge (17). The presence of cervical mucus or microscopic PMN counts of ≥10 seen microscopically on an oil immersion field does not correlate well with gonococcal infection in the absence of coinfecting agents (18). Manifestations of gonococcal infection may be driven by environmental factors specifically associated with the genital tracts of men and women: these may include biofilm formation (19, 20); the influence of the microbiome, particularly in women (21–25); and both direct and indirect molecular interactions between gonococci and specific host cells from men or women (26–30).
Gonococcal pathogenesis and host immune responses have been studied principally in vitro and in animal models (31), both of which may not faithfully replicate differences in infection between men and women. Historically, the only human model of gonococcal infection is based on genital experimental challenge of male volunteers, which has provided valuable information on gonococcal virulence factors, but is limited by the duration of the experimental infection and is not applicable to women (32, 33). A better understanding of gonococcal pathogenesis during natural mucosal infection, which is less well studied, is critical to implement biological strategies to treat and prevent infection. Previous work from our group using a gene-specific microarray and reverse transcription-quantitative PCR (qRT-PCR) analysis demonstrated that at least 20 gonococcal iron-regulated genes were expressed during human mucosal infection in men and women (34, 35). Recently, we also examined the complete N. gonorrhoeae transcriptome using cervicovaginal lavage specimens of infected women attending an STI clinic in China where antibiotic-resistant N. gonorrhoeae is prevalent (36, 37). We reported that a large portion of the gonococcal genome is expressed (65% of protein-coding genes) and regulated during infection (305 genes regulated compared to in vitro growth) (38). In the present study, we examined gonococcal transcriptomes from men attending the same clinic and compared bacterial gene expression and regulation in infected men with expression and regulation in infected women. The resulting data revealed sex-specific adaptation of genes involved in host-pathogen interactions and phage and antibiotic resistance.
RESULTS
Description of subjects.
The male cohort comprised six male subjects (average age, 34 years) attending the Nanjing (China) Sexually Transmitted Disease (STD) Clinic, who presented with urethral discharge and/or dysuria and were diagnosed with N. gonorrhoeae infection (Table 1). Four men were coinfected with Chlamydia trachomatis and/or Mycoplasma genitalium, three had a history of prior gonococcal infection, and three had self-administered antibiotics prior to their clinic visit (Table 1). In contrast, none of the seven enrolled women had a history of gonococcal infection or had taken antibiotics prior to their clinic visit (see Table S1 in the supplemental material). Two gonococcal strains were isolated from a man and a woman who comprised a single dyad (subjects M-4 and F-6, respectively).
TABLE 1 .
Characteristics of male subjects with gonococcal urethritis
| Subject | Age (yr) |
No. of days with symptoms |
Other STI microbe(s) |
Urethral PMN scorea |
Prior gonococcal infection |
ABX in last 30 daysb |
|---|---|---|---|---|---|---|
| M-1 | 27 | 4 | C. trachomatis | +++ | Yes | Yes |
| M-2 | 37 | 21 | None | +++ | No | No |
| M-3 | 46 | 3 | None | +++ | Yes | No |
| M-4 | 28 | 3 | M. genitalium | +++ | No | Yes |
| M-5 | 34 | 15 |
M. genitalium, C. trachomatis |
+++ | Yes | Yes |
| M-6 | 32 | 1 | C. trachomatis | ++ | No | No |
Number of PMNs per oil immersion field indicated: ++, 5 to 9; +++, ≥10.
Self-administered antibiotics (ABX) prior to diagnosis in the STD clinic.
Characteristics of female subjects. Download TABLE S1, DOCX file, 0.02 MB (16.1KB, docx) .
Copyright © 2018 Nudel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Gonococcal gene expression in the human genital tract.
RNA was extracted from male urethral or cervicovaginal lavage specimens, and individual cDNA reads were aligned to N. gonorrhoeae strain FA1090 using Rockhopper (39). Total gene expression was reported in reads per kilobase per million (RPKM). On average, 8.1% of the total RNA from male urethral specimens aligned to the N. gonorrhoeae FA1090 genome and 42.5% to the human genome; 4.3% of total RNA from cervicovaginal lavages aligned to FA1090 and 45% to the human genome.
Expression of 1,725 gonococcal genes (approximately 65% of the gonococcal genome) was detected in male urethral specimens. High expression of a core set of genes that encoded oxidative stress products (e.g., peroxiredoxin/glutaredoxin), housekeeping genes (e.g., the gene coding for elongation factor Tu), outer membrane protein genes (e.g., genes coding for Rmp [Omp3] and porin P.IB), and hypothetical protein genes were identified across male specimens (see Table S3 in Data Set S1 in the supplemental material). A more detailed analysis of the top 200 gonococcal genes (approximately 10% of total genome) that were expressed in all six male urethral secretions (in aggregate) was carried out by determining the ratio of gene enrichment and segregation into broad categories. Despite some variability in RPKM values among subjects, significantly enriched categories included genes coding for pilin and oxidative stress and regulatory proteins (Fig. 1), which reflected a common pattern of high gene expression during natural infection.
FIG 1 .
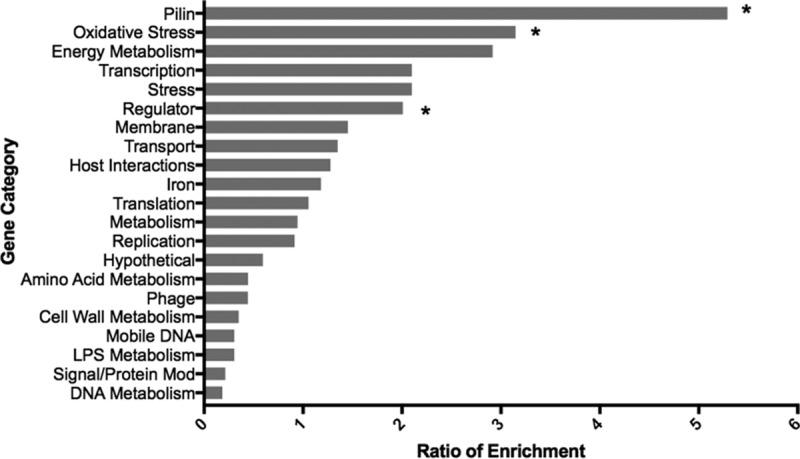
Categorization of the top 200 N. gonorrhoeae genes expressed during natural infection in men. Expression of gonococcal genes in 6 infected men was averaged, and the top 200 genes were categorized based on characteristics indicated for N. gonorrhoeae strain FA1090 available in NCBI. Categories are shown on the y axis, and the ratio of enrichment (% of genes of a given functional category in RNA-seq data set/% of genes assigned to that functional category in whole gonococcal genome) is shown on the x axis. For clarity, ribosomal protein, tRNA, and rRNA genes were removed. Asterisks indicate that enrichment was significant (P ≤ 0.05) by Fisher’s exact test.
Supplemental Tables S3 to S7. Table S3 shows the top 10 most highly expressed genes in each male subject. Table S4 contains the composite RPKM values for each gene in men in vivo versus in vitro. Table S5 contains the composite RPKM values for significantly different noncoding RNA genes in men in vivo versus in vitro. Table S6 contains the composite RPKM values for significantly different predictive sRNAs in men in vivo versus in vitro and men in vivo versus women in vivo. Table S7 contains the composite RPKM values for each gene in men versus women. Download DATA SET S1, XLSX file, 0.2 MB (224.5KB, xlsx) .
Copyright © 2018 Nudel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Gonococcal gene regulation in the male genital tract.
Our earlier studies had demonstrated that approximately 17% of gonococcal genes were regulated in vivo during cervical infection in women (38). Gonococcal gene expression in the male genital tract was compared to expression of matched infecting strains grown in vitro by analysis of transcriptome sequencing (RNA-seq) data sets for individual strains and for the composite data sets. Genes that displayed a significant difference in expression under in vivo versus in vitro conditions were selected for further analysis based on the following criteria: a ≥2-fold difference in RPKM value, a q (false-discovery rate) value of ≤0.05, and expression greater than 10 RPKM under at least one condition (see Table S4 in Data Set S1). The majority of genes were expressed similarly in vivo and in vitro, but approximately 4.2% of the total number of genes detected (79 genes) revealed increased expression in vivo compared to that observed in vitro; expression of 5.2% (99 genes) was decreased in vivo compared to that observed in vitro (Fig. 2A).
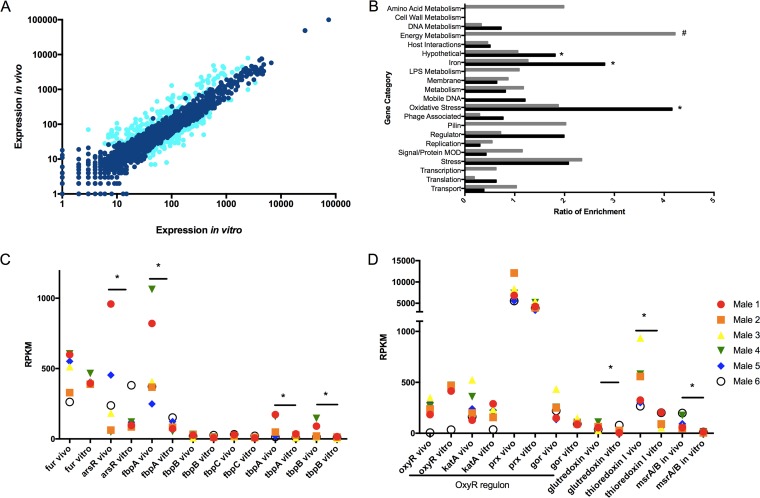
FIG 2 .
Comparison of N. gonorrhoeae gene expression in infected men in vivo and isolates grown in vitro. (A) Composite gene expression levels in urethral specimens (n = 6) on the y axis plotted against composite expression of corresponding gonococcal isolates (n = 6) grown in vitro (x axis). Data are shown as log10 expression (RPKM) levels. The genes indicated in light blue had q values of ≤0.05 and ≥2-fold changes in vivo (versus in vitro). (B) Functional enrichment of genes with statistically significant changes in expression under in vivo and in vitro conditions. Genes were differentially expressed if the q value was ≤0.05 and the fold change was ≥2. Black bars represent functional enrichment of genes with increased expression in vivo. Gray bars represent functional enrichment of genes with decreased expression in vivo. Categories are shown on the y axis, and the ratio of enrichment (% of genes of a given functional category in RNA-seq data set/% of genes assigned to that functional category in whole gonococcal genome) is shown on the x axis. Ribosomal protein, tRNA, and rRNA genes were removed for clarity. *, significant enrichment among genes increased in vivo (versus in vitro), and #, significant enrichment among genes decreased in vivo, with P ≤ 0.05, by Fisher’s exact test. (C and D) Expression levels of iron genes (C) and oxidative stress genes (D) under in vivo and in vitro conditions. (Six male specimens [in vivo] and the corresponding 6 isolates [in vitro] are shown in the same color in adjacent columns.) *, q ≤ 0.05.
Regulation of gonococcal gene expression was then examined by functional enrichment analysis (Fig. 2B). Genes involved in iron and oxidative stress pathways and genes encoding hypothetical proteins were among those significantly enriched and were more highly expressed in vivo than in vitro. For example, expression of certain iron-scavenging genes (e.g., fbpA, tbpA, and tbpB) and of the fur-controlled regulator arsR was significantly higher in vivo than in vitro, although variance in arsR in vivo expression was large (Fig. 2C). This observation suggests that the male genital tract is iron depleted (similar to the female genital tract [38]). Genes involved in energy metabolism were also significantly enriched but had decreased expression in vivo (Fig. 2B). Among the downregulated energy metabolism genes was the nuoA–N operon (NGO1737–1751) (40), involved in aerobic respiration; its overall decreased expression in vivo may suggest a mechanism for bacterial survival in an anaerobic environment (41).
Among the enriched oxidative stress genes increased in vivo, we observed a significant increase in a glutaredoxin family protein gene (NGO0031), the thioredoxin I (trx1) gene (NGO0652), and msrAB (NGO2059) (Fig. 2D). The family of glutaredoxin proteins function as glutathione-dependent reductases (42); msrAB plays a role in reducing oxidized methionines to reactive peptides (43) and trx1 has been shown to respond to oxidation and is under control of the Fur regulon (44, 45). While expression of the well-studied OxyR regulon (oxyR, prx, kat, and gor) was not significantly different in in vivo samples compared to cultures grown in vitro, we observed decreased expression of the OxyR repressor in vivo, with increased expression of genes within the OxyR regulon (prx, kat, and gor). This notable trend further suggests that the gonococcus is exposed to reactive oxygen species during mucosal infection in men (Fig. 2D) (46, 47).
Several gonococcal noncoding RNAs were also regulated during infection in men. These included 4 rRNAs and 29 tRNAs, which were expressed at higher levels during infection compared to growth in vitro, except for NGO t31, an arginine tRNA that was expressed more highly during in vitro growth (see Table S5 in Data Set S1). Putative small RNAs (sRNAs: defined as expression representing an intergenic region of the complementary strand of a known open reading frame [ORF] and 30 to 250 nucleotides in length) were also regulated during infection; 29 sRNAs were increased in vivo compared to in vitro, and 21 sRNAs were decreased in vivo (see Table S6 in Data Set S1). Among these sRNAs, several have been described previously (48, 49).
Comparison of gonococcal gene signatures in the male and female genital tracts.
Building on gonococcal transcriptome data in infected men and women, we examined whether site-specific, environmental conditions influenced expression of gonococcal genes during infection. A comparative transcriptome analysis was carried out using the 6 urethral and 7 cervicovaginal specimens. We performed principal-component analysis (PCA) to assess whether global in vivo gene expression revealed transcriptional signatures specific for infections in men or women (Fig. 3A). PCA showed a clear distinction between gonococcal genes expressed in specimens from men and women, with high variance among the 7 specimens from women compared to low variance in men, as evidenced by tight clustering when the first two principal components were plotted against each other (Fig. 3A). Gene expression analysis of the composite data sets was also performed; parameters set for defining differential gene regulation were a ≥2-fold change in RPKM, a q value of ≤0.05, and expression greater than 10 RPKM under at least one condition (see Table S5 in Data Set S1). Of 1,879 genes expressed, 176 (9.4%) were increased in men compared to women and 80 (4.3%) were decreased in men, indicating that nearly 14% of gonococcal genes were differentially regulated during gonococcal infection in the male and female genital tracts (see Table S7 in Data Set S1).
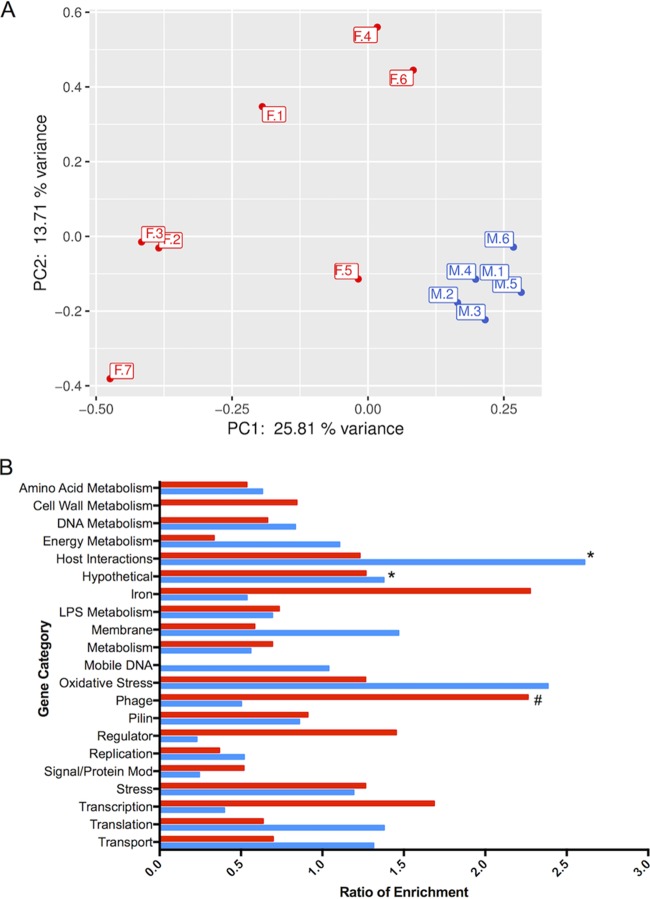
FIG 3 .
Comparison of N. gonorrhoeae gene expression during infection in men and women in vivo. (A) Variance in global expression in each specimen from men and women. The first principal component (PC1 [x axis]) had a variance of ~26%, and the second principal component (PC2 [y axis]) had a variance of ~14%. (B) Functional enrichment of genes with significant changes in expression levels in men and women. Genes were differentially expressed if the q value was ≤0.05 and the fold change was ≥2. Blue bars represent genes with increased expression in vivo in men compared to women. Red bars represent genes with increased expression in vivo in women compared to men. *, significant enrichment among genes increased in vivo in men; #, significant enrichment among genes increased in vivo in women.
A functional enrichment analysis of gene categories performed on male and female specimens revealed that phage-associated genes were significantly enriched in female specimens (Fig. 3B). While female specimens had higher expression of a broad range of phage types (e.g., double-stranded and filamentous single-stranded phages), male specimens had higher expression in the subset of double-stranded DNA prophages (see Fig. S1A in the supplemental material) (50). Other highly expressed categories enriched in male specimens included genes that encoded hypothetical proteins and proteins involved in host interactions (Fig. 3B). Iron and oxidative stress genes that were differentially expressed in male and female specimens included the ferredoxin gene (NGO1859), a TonB receptor protein gene (NGO1205), and fetA (NGO2093). (fetA is regulated by MpeR and induced under low-iron conditions [51].) These genes all manifested increased expression in women (Fig. S1B). In contrast, expression of the oxidative stress gene bfrB was increased in male specimens; other oxidative stress genes were similarly expressed in both men and women (Fig. S1C), suggesting that exposure to oxidative stress occurs in both the male and female genital tracts.
Phage expression, iron regulation, and oxidative response genes in the male and female genital tracts. (A) Expression levels of phage genes are shown on the x axis and represent male urethral samples (blue) and female cervicovaginal lavage samples (red). (B and C) Expression levels of significantly differentially expressed iron genes (B) and oxidative stress genes (C). *, gene more highly expressed in the male genital tract; #, gene more highly expressed in the female genital tract (q value of <0.05). Red and blue X’s indicate the male and female counterparts of the dyad. Download FIG S1, TIF file, 0.8 MB (845KB, tif) .
Copyright © 2018 Nudel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Regulation of several gonococcal sRNAs was also observed. A significant change in expression of 119 sRNAs was observed in specimens obtained from men and women: 28 were expressed at higher levels in male specimens and 91 in female specimens. Previously identified sRNAs showing regulation based on sex included smRNA4, smRNA5, and smRNA8 (49), fnrS (a small RNA induced under anaerobic conditions [44]), and the iron-repressed sRNA encoding NrrF (52) (see Table S6 in Data Set S1). In addition, 45 tRNAs showed differential regulation, with all being more highly expressed in female patients than in male patients (see Table S5 in Data Set S1).
Expression of antimicrobial resistance genes.
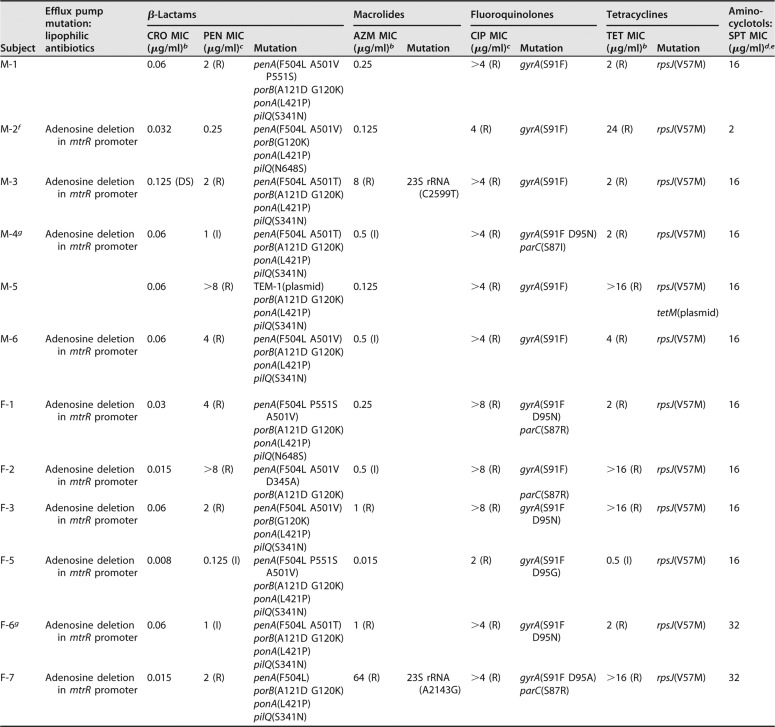
Gonococcal antimicrobial resistance (AMR) is a growing concern and is common in strains from the eastern regions of the world, including China. We examined phenotypic and genotypic AMR patterns from our cohort and found that all the isolates exhibited resistance to penicillin, tetracycline, and ciprofloxacin, with one isolate showing resistance to azithromycin (Table 2). One isolate also showed reduced susceptibility to ceftriaxone. Strains isolated from women exhibited similar antimicrobial susceptibility patterns (Table 2). These results were similar to AMR patterns of male urethritis strains reported earlier (2011 to 2012) from the Nanjing STD Clinic (53).
TABLE 2 .
Phenotypic resistance and AMR genes identified in male and female N. gonorrhoeae isolates (n = 13) by WGSa
| Subject | Efflux pump mutation: lipophilic antibiotics |
β-Lactams |
Macrolides |
Fluoroquinolones |
Tetracyclines |
Amino- cyclotols: SPT MIC (μg/ml)d,e |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CRO MIC (μg/ml)b |
PEN MIC (μg/ml)c |
Mutation | AZM MIC (μg/ml)b |
Mutation | CIP MIC (μg/ml)c |
Mutation | TET MIC (μg/ml)b |
Mutation | |||
| M-1 | 0.06 | 2 (R) |
penA(F504L A501V P551S) |
0.25 | >4 (R) | gyrA(S91F) | 2 (R) | rpsJ(V57M) | 16 | ||
| porB(A121D G120K) | |||||||||||
| ponA(L421P) | |||||||||||
| pilQ(S341N) | |||||||||||
| M-2f | Adenosine deletion in mtrR promoter |
0.032 | 0.25 | penA(F504L A501V) | 0.125 | 4 (R) | gyrA(S91F) | 24 (R) | rpsJ(V57M) | 2 | |
| porB(G120K) | |||||||||||
| ponA(L421P) | |||||||||||
| pilQ(N648S) | |||||||||||
| M-3 | Adenosine deletion in mtrR promoter |
0.125 (DS) | 2 (R) | penA(F504L A501T) | 8 (R) | 23S rRNA (C2599T) |
>4 (R) | gyrA(S91F) | 2 (R) | rpsJ(V57M) | 16 |
| porB(A121D G120K) | |||||||||||
| ponA(L421P) | |||||||||||
| pilQ(S341N) | |||||||||||
| M-4g | Adenosine deletion in mtrR promoter |
0.06 | 1 (I) | penA(F504L A501T) | 0.5 (I) | >4 (R) | gyrA(S91F D95N) | 2 (R) | rpsJ(V57M) | 16 | |
| porB(A121D G120K) | parC(S87I) | ||||||||||
| ponA(L421P) | |||||||||||
| pilQ(S341N) | |||||||||||
| M-5 | 0.06 | >8 (R) | TEM-1(plasmid) | 0.125 | >4 (R) | gyrA(S91F) | >16 (R) | rpsJ(V57M) | 16 | ||
| porB(A121D G120K) | |||||||||||
| ponA(L421P) | tetM(plasmid) | ||||||||||
| pilQ(S341N) | |||||||||||
| M-6 | Adenosine deletion in mtrR promoter |
0.06 | 4 (R) | penA(F504L A501V) | 0.5 (I) | >4 (R) | gyrA(S91F) | 4 (R) | rpsJ(V57M) | 16 | |
| porB(A121D G120K) | |||||||||||
| ponA(L421P) | |||||||||||
| pilQ(S341N) | |||||||||||
| F-1 | Adenosine deletion in mtrR promoter |
0.03 | 4 (R) |
penA(F504L P551S A501V) |
0.25 | >8 (R) |
gyrA(S91F D95N) |
2 (R) | rpsJ(V57M) | 16 | |
| porB(A121D G120K) | parC(S87R) | ||||||||||
| ponA(L421P) | |||||||||||
| pilQ(N648S) | |||||||||||
| F-2 | Adenosine deletion in mtrR promoter |
0.015 | >8 (R) |
penA(F504L A501V D345A) |
0.5 (I) | >8 (R) | gyrA(S91F) | >16 (R) | rpsJ(V57M) | 16 | |
| porB(A121D G120K) | parC(S87R) | ||||||||||
| F-3 | Adenosine deletion in mtrR promoter |
0.06 | 2 (R) | penA(F504L A501V) | 1 (R) | >8 (R) |
gyrA(S91F D95N) |
>16 (R) | rpsJ(V57M) | 16 | |
| porB(G120K) | |||||||||||
| ponA(L421P) | |||||||||||
| pilQ(S341N) | |||||||||||
| F-5 | Adenosine deletion in mtrR promoter |
0.008 | 0.125 (I) |
penA(F504L P551S A501V) |
0.015 | 2 (R) |
gyrA(S91F D95G) |
0.5 (I) | rpsJ(V57M) | 16 | |
| porB(A121D G120K) | |||||||||||
| ponA(L421P) | |||||||||||
| pilQ(S341N) | |||||||||||
| F-6g | Adenosine deletion in mtrR promoter |
0.06 | 1 (I) | penA(F504L A501T) | 1 (R) | >4 (R) |
gyrA(S91F D95N) |
2 (R) | rpsJ(V57M) | 32 | |
| porB(A121D G120K) | |||||||||||
| ponA(L421P) | |||||||||||
| pilQ(S341N) | |||||||||||
| F-7 | Adenosine deletion in mtrR promoter |
0.015 | 2 (R) | penA(F504L) | 64 (R) | 23S rRNA (A2143G) |
>4 (R) | gyrA(S91F D95A) | >16 (R) | rpsJ(V57M) | 32 |
| porB(A121D G120K) | parC(S87R) | ||||||||||
| ponA(L421P) | |||||||||||
| pilQ(S341N) | |||||||||||
CRO, ceftriaxone; PEN, penicillin; AZM, azithromycin; CIP, ciprofloxacin; TET, tetracycline; SPT, spectinomycin; S, susceptible; DS, decreased susceptibility; I, intermediate resistance; R, resistant.
S = ≤0.25 µg/ml.
S = ≤0.06 µg/ml.
S = ≤32 µg/ml.
No mutation was found to confer resistance to aminocyclotols.
Isolate did not grow in agar dilution media, and MICs were determined by Etest on chocolate agar.
Male and female partners in the dyad (M-4 and F-6).
Whole-genome DNA sequencing (WGS) was performed on all available strains (6 male and 6 female) to define strain relatedness and genotypic determinants of antibiotic resistance. A single-nucleotide polymorphism (SNP) distance tree was constructed that used SNP distances (differences) that ranged between 81 and 3,913 separating the genomes (see Fig. S2 in the supplemental material). The male and female dyad (M-4 and F-6) yielded genomic sequences that were separated by only 81 SNPs. Strain NCCP11945 isolated from a subject with gonococcal disease in South Korea (average SNP distance of 2,828 SNPs compared to strain FA1090) shared considerable homology with the isolates obtained from our cohort compared to the commonly used laboratory strain, FA1090 (average distance of 4,212 compared to FA1090).
Hamming distance tree and SNP distance matrix of N. gonorrhoeae isolates. Tree visualizing Hamming distances of total pairwise SNPs identified across strains. The scale bar shows a distance of 300 SNPs. Download FIG S2, TIF file, 0.9 MB (898.4KB, tif) .
Copyright © 2018 Nudel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Genomic analyses based on publicly available, curated, antibiotic resistance databases (CARD and GC-MLST) identified multiple AMR genes (Table 2) and SNP-level variants that likely contributed to phenotypic resistance. All gonococcal strains exhibited SNP mutations in gyrA and/or parC (which confer resistance to fluoroquinolones), SNP mutations in penA, ponA, porB, and pilQ (β-lactam resistance), and SNP mutations in rpsJ (tetracycline resistance) (54). Only one isolate (M-5) carried plasmid-based resistance genes (tem-1 and tetM). Interestingly, among the male subjects, M-3 was the only isolate to exhibit azithromycin resistance and a corresponding mutation in the 23S rRNA gene (55). Three isolates had an adenosine deletion in the promoter region of mtrR, the repressor of the mtrCDE efflux pump, a system that exports bactericidal host-derived compounds and hydrophobic antibiotics (β-lactams and macrolides) from the bacterial cell (56–58). Isolates from women exhibited similar genomic patterns of resistance: by and large, the same mutations identified in the male isolates were also present in the female isolates (Table 2). Among 6 SNPs associated with β-lactam resistance in the dyad isolates (subjects M-4 and F-6, who were infected by the same strain), 5 SNPs were shared in the dyad isolates. Two SNPs in gyrA were shared (S91F and D95N), and a Ser SNP in parC [parC(S87I)] was detected only in the male isolate. Finally, while several SNP mutations coincided with phenotypic resistance to azithromycin, ciprofloxacin, and penicillin (Table 2), the single isolate with decreased sensitivity to ceftriaxone (M-3) did not possess the mosaic penA XXXIV allele (59) or any additional curated mutations that distinguished it from the phenotypically sensitive strains (Table 2) (59, 60).
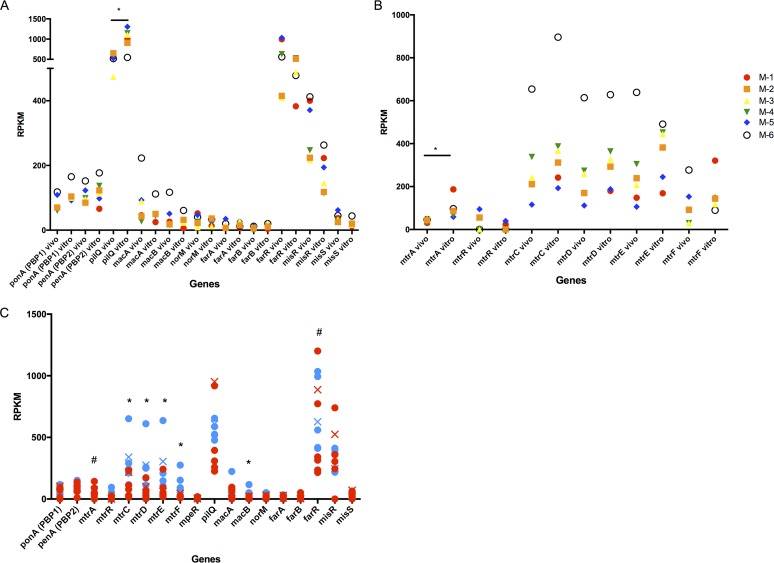
Expression levels of genes associated with antibiotic resistance in men were also compared to expression of corresponding genes in vitro (Fig. 4A and B). Expression of the pilQ gene, associated with antibiotic permeability (14), was significantly decreased by 1.78-fold in vivo compared to in vitro (Fig. 4A). The MisRS two-component regulatory system is important for resistance to host-derived antimicrobial peptides and antibiotics (61), and though not statistically significant, misR had 2.08-fold higher expression in vivo compared to in vitro, supporting its important role in vivo. The expression of the Mtr efflux pump was also closely examined; expression of mtrA, which can influence expression of mtrCDE (62), was significantly decreased by 2.75-fold in vivo (Fig. 4B). Gonococcal strains from male urethral specimens (M-2, M-3, M-4, and M-6) contained an adenosine deletion in the mtrR promoter that was accompanied by the lowest expression of mtrR and highest expression of mtrCDE and mtrF, confirming that the adenosine deletion directly affects expression of the Mtr locus (63) in vivo. Expression of mpeR was also investigated, as it has been shown to regulate the Mtr locus; however, expression was below 10 RPKM in all in vitro and in vivo samples.
FIG 4 .
Expression of gonococcal antibiotic resistance genes. Expression levels of antibiotic resistance genes (A) and the Mtr efflux pump (B) in the male genital tract (in vivo) and the corresponding strain in CDM (in vitro); genes are identified on the x axis. (Six male specimens [in vivo] and the corresponding 6 isolates [in vitro] are shown in the same color.) *, q ≤ 0.05. (C) Expression levels of antibiotic resistance genes from the male genital tract (blue) or the female genital tract (red). *, higher gene expression in men; #, higher gene expression in women (q ≤ 0.05). Red and blue X’s indicate the male (M-4) and female (F-6) counterparts of the dyad.
Transcriptomes from male urethral specimens were also compared with female cervicovaginal lavage specimens to determine if expression of AMR genes was influenced by sex (Fig. 4C). Because of the adenosine deletion in the mtrR promoter in 4/6 males (described above) and all female isolates (Table 2), expression of mtrR was low in both groups and resulted in significantly higher expression of mtrCDE and mtrF in men, as predicted (63), but not in women. Even within the dyad, where both M-4 and F-6 contained the adenosine deletion in the mtrR promoter, M-4 had approximately 2× higher expression levels of mtrCDE than F-6. Interestingly, the expression of mtrA, an activator of mtrCDE (62), was significantly higher during infection in women compared to men, despite lower expression of mtrCDE in women (Fig. 4C). The farR gene, which negatively controls expression of the farAB operon by directly binding to the farAB promoter (64) to prevent excess expression of gonococcal efflux pumps, also showed significantly higher expression in cervicovaginal lavages. Collectively these results suggest that, although strains isolated from infected men and women exhibit comparable AMR genotypes and in vitro phenotypes, expression of AMR genes is different during infection in the male and female genital tracts.
DISCUSSION
N. gonorrhoeae infects the male and female genital tracts, two very distinct environments in humans. As there is little doubt about the intrinsic tissue, cellular, and molecular differences that define these host environments, it is reasonable to assume that the gonococcus will adapt to these environmental differences during infection (65). Building on our previous results on the gonococcal transcriptome profile during infection in women (37), we extended our analysis to specimens from infected men. In the present study, we not only report a distinct gonococcal transcriptome profile in vivo compared to in vitro in infected men, but also, for the first time, describe a comparison of N. gonorrhoeae gene expression during infection in the male and female genital tracts. Our results also demonstrate distinct gonococcal gene expression signatures in men and women, consistent with the intrinsically different makeups and natures of the two sites of infection. Furthermore, our approach based on both whole-genome and RNA sequencing enabled us to evaluate expression of antibiotic resistance determinants during infection in both the male and female genital tracts.
Currently, the only human model of gonococcal infection is experimental urethral challenge of male volunteers. Those studies have examined gonococcal virulence factors and have provided valuable information on opacity proteins and genes involved in iron acquisition, structural modification of lipooligosaccharides, and a variety of other established virulence factors (32, 66, 67) but are limited by the short duration of experimental infection. The small number of infecting strains used experimentally in men (mostly laboratory-adapted strains FA1090 and MS11mkC [32]) have precluded an in-depth understanding of the range of adaptions employed during gonococcal infections generally. Using a panel of N. gonorrhoeae strains isolated from infected subjects, we compared the gonococcal in vivo transcriptome expressed during infection of the male genital tract to that of the corresponding infecting strains grown in vitro. Our results revealed increased expression of genes involved in oxidative stress and iron scavenging in vivo, accompanied by decreased expression of genes involved in metabolism-associated categories (i.e., energy and amino acid metabolism). In particular, we observed that gonococci expressed high levels of trx1, msrAB, and glutaredoxin genes during infection in male subjects, in agreement with the large PMN influx that occurs during infection in symptomatic men. Our results also demonstrated increased expression of iron-regulated genes (tbpAB [66]), indicating that the male genital tract is an iron-depleted environment. We observed lower expression of genes involved in gonococcal aerobic energy metabolism processes and higher expression of oxidative stress response genes in urethral specimens compared to that of the corresponding infecting strains grown in vitro. It is established that the genital tract is anaerobic or microaerobic (68, 69). Thus, we would expect that a comparison of RNA-seq data sets from urethral and cervicovaginal lavage specimens to RNA-seq data sets from in vitro growth conditions would identify differential regulation of gonococcal genes utilized for aerobic energy production. However, the presence of neutrophils, especially in male subjects during infection, likely requires a corresponding N. gonorrhoeae oxidative defense response. Such immune cells are obviously absent under our in vitro conditions, and thus these oxidative stress pathways are expressed at lower levels. While we recognize that in vitro culture conditions differ compared to the conditions in the human genital tract beyond metabolite content, this study provides a gene-specific view of the response of N. gonorrheoae to infection and leads to a number of hypotheses regarding infection mechanisms that can be explored in more detail in future studies.
While we identified both expected and novel differences in gonococcal gene expression during human infection compared to in vitro growth, the most revealing aspect of our study was the differential gonococcal expression profiles observed in men and women. Our results showed that nearly 14% of gonococcal genes that were expressed in human infection were differentially expressed in men and women. Principal-component analysis demonstrated that gonococcal expression in men and women separated into distinct gene expression signatures, with high variance among the 7 female specimens and low variance among the 6 male specimens. We acknowledge that high variance could be due to the small sample size, but it may also be due to other female-specific environmental factors that could alter gonococcal gene expression, such as menstrual cycle or the microbiome, which exhibits diversity among individuals (70). We also observed differences in the presence and type of coinfecting organisms in infected men and women. Several male subjects had a coinfection of Chlamydia trachomatis or Mycobacterium genitalium, and while some female subjects were also infected with these pathogens, several females additionally carried Ureaplasma urealyticum. In addition, 3/7 of the female subjects had bacterial vaginosis (BV), suggesting differences in their natural microflora in combination with these coinfections. We hypothesize that changes in the microflora and the presence of coinfecting organisms have a direct effect on the transcriptomic response of N. gonorrhoeae, as does the variable host response during coinfection compared to infection with N. gonorrhoeae alone. Both of these factors most likely impact the overall differences observed between samples of the same gender as well as differences observed in the gonococcal transcriptomic response during infection in men and women. When genomic DNA sequence analysis was also considered, there was no clustering among strains derived from men or women (except for strains in the single dyad), which strongly suggests that the observed differences in transcriptomic signatures were driven by exposure of gonococci, generally, to the male or the female genital tract and not by strain relatedness. Furthermore, this is also supported in the single dyad by differences in gene expression manifested by the same strain in each partner, which reflected changes in gene expression that were seen overall in the other men and women. In addition to the small sample size, it is important to also consider other limitations of the study when interpreting sex-specific and growth-specific aspects of N. gonorrhoeae infection. Due to the design of the study, men are recruited because they enter the clinic with a symptomatic response: thus, men may have a similar stage of infection and gonococcal growth stage. This is in contrast to the matched females, who are partners of the recruited men and who were at various stages of infection and therefore gonococcal growth.
Among the most relevant gonococcal transcriptome differences that emerged in male and female specimens were those in genes encoding antimicrobial resistance determinants. The rise of antibiotic resistance in N. gonorrhoeae is now a global concern (71). Patterns of resistance vary among countries because of population mobility and geographically localized increases in antimicrobial resistance—such as those observed in China (72); these patterns are predictive of future resistance patterns in countries that currently have lower levels of resistance, such as the United States (73, 74). Whole-genome DNA sequencing (WGS) of the gonococcal strains isolated from our male and female cohort revealed similarity of genetic determinants of resistance to strains isolated in the United States (59). In all strains, regardless of the origin of the specimens (men or women), the presence of an adenosine deletion in the mtrR promoter (also detected by WGS) correlated with increased expression of the Mtr regulon. Nevertheless, strains isolated from men showed a significantly higher expression of mtrCDE and mtrF than those from women. While a direct explanation for these results remains speculative, it is possible that the higher expression of mtrCDE observed in men may be due to the readily accessible antibiotics in China; approximately half of the male subjects (but none of the female subjects) in this study had self-administered antibiotics prior to seeking care at the STD clinic. Future studies including a larger cohort may help resolve these differences. An additional explanation for gender-specific differences is that biofilm formation in the female genital tract (and apparent lack of it in the male genital tract) is a contributing factor (19, 20). Biofilms can provide protection against harsh environments, and their absence in the male genital tract may lead to increased expression of antibiotic-resistant efflux pumps as a defense mechanism (75, 76).
N. gonorrhoeae contains phage DNA inserted in the genome: five regions comprising double-stranded DNA (dsDNA) lysogenic phage genomes (50) and four additional regions comprising filamentous phages (77). The role of phage genes in N. gonorrhoeae infection has not been studied extensively. Previous work from our group has identified a phage gene, npr, involved in invasion of female epithelial cells in vitro, which correlates with disease progression in a female mouse model of gonococcal infection (78). Our finding that expression of phage genes was increased in female subjects may also relate to the formation of biofilms in the female genital tract (20). We propose that biofilm formation may facilitate DNA exchange via phages among bacteria, thus contributing to differences in infections of men and women. Additional transcriptional patterns that differentiated organisms obtained during infection in men and women included genes encoding tRNAs with higher expression observed in women compared to men. However, apparent expression of tRNAs may be partially affected by reads aligning to additional microbes present in the genital tract: tRNA genes are highly conserved across species, and the women in the study may have a larger population of microbial organisms. Six of seven gonococcus-infected women reported in this study had BV and/or other bacterial genital infections. Some studies have suggested a role for tRNAs in bacterial infection (79, 80), and it is possible that differences observed in men and women also correlate with the enhanced microbial environment in women. Small RNA expression in vivo was also different; NrrF and FnrS (an sRNA induced under anaerobic conditions) were expressed at higher levels in women than in men.
Collectively, our results provide the first global view of gonococcal gene expression during infection in humans and define gene signatures specific to infections in men and women. We report important differences related to antibiotic resistance and gonococcal pathogenesis that can be extrapolated to improve understanding of gonococcal disease outcomes and potential treatments. Our analysis also highlights shortfalls of studying bacterial infections using in vitro models and systems. It is critical that studies designed to identify targeted therapies for gonococcal infections consider sex-specific differences in gene expression profiles that may impact treatment outcomes. Furthermore, addressing how expression of antimicrobial resistance genes are driven by environmental cues in the male and female genital tracts has important implications for the use of targeted antibiotics.
MATERIALS AND METHODS
Ethics statement.
All subjects provided written informed consent in accordance with requirements by Institutional Review Boards from Tufts University, Boston, MA (protocol no. 11710), the University of Massachusetts Medical School, Worcester, MA, and Boston University School of Medicine, Boston, MA, and the Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, Nanjing, China.
Identification of subjects and characterization of urethral infections.
Urethral swab specimens were collected from six men attending the Nanjing Sexually Transmitted Disease (STD) Clinic, chosen at random, who were participants in a study of gonococcal transmission (81). Male subjects presented with symptoms and signs of urethritis (dysuria and/or urethral discharge). Gram stains of urethral exudates showed PMNs and Gram-negative intracellular diplococci, criteria that are highly specific (99%) for gonococcal infection (82). Urethral swab specimens from men with urethritis were also inoculated onto Thayer-Martin medium (DL Biotech, China) and cultured in candle jars at 36°C. Gonococcal isolates were identified by colonial morphology, Gram stain, and oxidase testing. Urethral swab specimens were also tested for Chlamydia trachomatis and Mycoplasma genitalium by PCR (83, 84) and Ureaplasma urealyticum and Mycoplasma hominis by culture using the Mycoplasma IST2 test (BioMérieux, France).
Collection of cervicovaginal lavage specimens (n = 4) was described previously (38), and three additional specimens were obtained for the present study (total n = 7). PCR testing using primers directed against the gonococcal porA pseudogene (85) had previously identified N. gonorrhoeae infection in one woman (F-4) whose cervical culture was negative. Women reported that they were monogamous (3/7 were married), having been the sole sex partner of a man with gonococcal urethritis. One woman (F-6) and one man (M-4) were members of a single dyad.
Antimicrobial testing of N. gonorrhoeae.
N. gonorrhoeae strains from urethral swab and cervicovaginal lavage specimens were identified and processed as previously described (38). Antimicrobial sensitivity testing of N. gonorrhoeae isolates was determined for penicillin, tetracycline, spectinomycin, azithromycin, ceftriaxone, cefixime, and ciprofloxacin at the Nanjing STD Clinic. Mean inhibitory concentrations (MICs) were determined by the agar dilution method, and current Clinical and Laboratory Standards Institute cutoffs were used, except for azithromycin, in which we followed the European Committee on Antimicrobial Susceptibility Testing (http://www.eucast.org) (86). A single isolate (from M-2) that did not grow in agar dilution media or GC agar had MICs determined on chocolate agar by Etest: thus, MIC values may be one dilution lower due to enriched agar (87).
RNA isolation.
Total RNA was isolated from urethral swab and cervicovaginal lavage specimens using TRIzol (Invitrogen) as described previously (38). Briefly, specimens were washed twice with 70% ethanol and treated with Turbo DNase (Ambion), the RiboMinus kit (Invitrogen) (to deplete eukaryotic rRNA), and the Microbe Express kit (Ambion) (to deplete bacterial rRNA) using diethylpyrocarbonate (DEPC)-treated EDTA-free water. Gonococcal isolates that corresponded to each specimen were grown overnight on chocolate agar plates at 37°C in 5% CO2 prior to inoculation in chemically defined medium (CDM) containing ferric nitrate (100 µM). Liquid cultures were inoculated at an optical density at 600 nm (OD600) of 0.1, harvested after 3 h, and pelleted, and RNA was extracted as described above.
RNA sequencing and analysis.
RNA sequencing was performed as previously described (38). cDNA libraries were prepared with TruSeq RNA preparation kit and sequenced on an Illumina HiSeq 2500 instrument using high-output V3 chemistry in single-read 100 formats. Analysis of RNA-seq data was performed using the Rockhopper program, aligning the reads to the NCBI genome sequence of N. gonorrhoeae strain FA1090 and then reporting the RNA-seq data in reads per kilobase per million (RPKM) (37). The cutoff for gene expression was set at an RPKM value of at least 10. A composite set of RPKM values was derived from RNA-seq data from the six male urethral samples and another from the three newly collected cervicovaginal samples added to the four reported earlier (in the Gene Expression Omnibus database under GenBank accession no. GSE71151) (38). These are referred to as “in vivo” RNA-seq data. Data from gonococcal strains isolated from each subject and cultured in vitro were also obtained (referred to as “in vitro” data).
Statistical analysis was performed on pooled data from each category of specimens: in vivo urethral, in vivo cervicovaginal lavage, and in vitro. Genes with ≥2-fold change in expression and a q value of ≤0.05 in each category were deemed as regulated. Gene assignments and categorization were performed using the KEGG and NCBI RefSeq public databases. Functional enrichment ratios were determined by dividing the percentage of genes in a functional category for a particular set of genes (e.g., highly expressed genes or differentially regulated genes) by the percentage of genes in the same category present in the gonococcal genome as a whole. This ratio was used to interpret enrichment of a particular set of genes that possessed a specific function. Fisher’s exact test was used to assign a P value to the enrichment. Principal-component analysis (PCA) (88) was performed in R using singular value decomposition on the scaled and centered data set of RPKM values for all genes in each subject, and the resulting data were visualized by sex on a colored plot.
Whole-genome sequencing and analysis.
N. gonorrhoeae isolates were grown on GC agar plates supplemented with 1% IsoVitaleX and 0.6% fetal bovine serum at 37°C and 5% CO2 for 16 to 18 h. Genomic DNA was extracted from a 10-µl inoculum of each bacterial culture using a 5Prime DNA extraction kit (5Prime, San Francisco, CA), following the manufacturer’s recommendations with slight modifications. Whole-genome sequencing (WGS) was conducted using a PacBio RSII platform (Pacific Biosciences, Menlo Park, CA) with P5-C3 chemistry. De novo genome assembly was conducted using the hierarchical genome assembly process workflow (HGAP3; SMRTAnalysis 2.3.0), which included consensus polishing using Quiver (89). Single-nucleotide polymorphisms (SNPs) across sequenced isolates were called de novo-assembled contigs using kSNP3 without a reference strain (90). Concatenated SNPs were used to construct phylogenetic trees using the approximate-maximum-likelihood-based approach; trees were visualized in FigTree (http://tree.bio.ed.ac.uk/software/figtree/). Antibiotic resistance elements were identified using the Comprehensive Antibiotic Resistance Database (91) and the PubMLST Neisseria database (https://pubmlst.org/neisseria) (92).
Data availability.
New RNA-seq data from this study have been deposited in the Gene Expression Omnibus (GSE113290). Genomic data from isolates have been deposited into NCBI under project no. PRJNA329501 (see Table S2 in the supplemental material).
Deposited genomic data. Download TABLE S2, DOCX file, 0.01 MB (14.4KB, docx) .
Copyright © 2018 Nudel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ACKNOWLEDGMENTS
This work was supported by NIH/NIAID grants U19AI084048 (P. A. Rice and C. A. Genco) and R01AI116969 (C. A. Genco). PNNL is operated for the DOE by Battelle Memorial Institute under contract DE-AC05-76RLO 1830.
The conclusions, findings, and opinions expressed by the authors do not necessarily reflect the official position of the U.S. Centers for Disease Control and Prevention (CDC).
Footnotes
This paper was submitted via the mSphereDirect™ pathway.
Contributor Information
Sarah E. F. D'Orazio, University of Kentucky.
Alison Criss, University of Virginia.
Joseph Dillard, University of Wisconsin—Madison.
REFERENCES
- 1.Centers for Disease Control and Prevention 2016. 2015 sexually transmitted disease surveillance. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 2.Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, Stevens G, Gottlieb S, Kiarie J, Temmerman M. 2015. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 10:e0143304. doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunner A, Nemes-Nikodem E, Jeney C, Szabo D, Marschalko M, Karpati S, Ostorhazi E. 2016. Emerging azithromycin-resistance among the Neisseria gonorrhoeae strains isolated in Hungary. Ann Clin Microbiol Antimicrob 15:53. doi: 10.1186/s12941-016-0166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. 2012. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother 56:1273–1280. doi: 10.1128/AAC.05760-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng CW, Li LH, Su CY, Li SY, Yen MY. 2016. Changes in the six most common sequence types of Neisseria gonorrhoeae, including ST4378, identified by surveillance of antimicrobial resistance in northern Taiwan from 2006 to 2013. J Microbiol Immunol Infect 49:708–716. doi: 10.1016/j.jmii.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Shimuta K, Unemo M, Nakayama S, Morita-Ishihara T, Dorin M, Kawahata T, Ohnishi M, Antibiotic-Resistant Gonorrhea Study Group . 2013. Antimicrobial resistance and molecular typing of Neisseria gonorrhoeae isolates in Kyoto and Osaka, Japan, 2010 to 2012: intensified surveillance after identification of the first strain (H041) with high-level ceftriaxone resistance. Antimicrob Agents Chemother 57:5225–5232. doi: 10.1128/AAC.01295-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su XH, Wang BX, Le WJ, Liu YR, Wan C, Li S, Alm RA, Mueller JP, Rice PA. 2016. Multidrug-resistant Neisseria gonorrhoeae isolates from Nanjing, China, are sensitive to killing by a novel DNA gyrase inhibitor, ETX0914 (AZD0914). Antimicrob Agents Chemother 60:621–623. doi: 10.1128/AAC.01211-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gose S, Nguyen D, Lowenberg D, Samuel M, Bauer H, Pandori M. 2013. Neisseria gonorrhoeae and extended-spectrum cephalosporins in California: surveillance and molecular detection of mosaic penA. BMC Infect Dis 13:570. doi: 10.1186/1471-2334-13-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen D, Gose S, Castro L, Chung K, Bernstein K, Samuel M, Bauer H, Pandori M. 2014. Neisseria gonorrhoeae strain with reduced susceptibilities to extended-spectrum cephalosporins. Emerg Infect Dis 20:1211–1213. doi: 10.3201/eid2007.131396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu A, Buono S, Katz KA, Pandori MW. 2011. Clinical Neisseria gonorrhoeae isolates in the United States with resistance to azithromycin possess mutations in all 23S rRNA alleles and the mtrR coding region. Microb Drug Resist 17:425–427. doi: 10.1089/mdr.2010.0199. [DOI] [PubMed] [Google Scholar]
- 11.Cash DR, Noinaj N, Buchanan SK, Cornelissen CN. 2015. Beyond the crystal structure: insight into the function and vaccine potential of TbpA expressed by Neisseria gonorrhoeae. Infect Immun 83:4438–4449. doi: 10.1128/IAI.00762-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piekarowicz A, Kłyż A, Majchrzak M, Stein DC. 2016. Oral immunization of rabbits with S. enterica typhimurium expressing Neisseria gonorrhoeae filamentous phage Phi6 induces bactericidal antibodies against N. gonorrhoeae. Sci Rep 6:22549. doi: 10.1038/srep22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cybulska P, Thakur SD, Foster BC, Scott IM, Leduc RI, Arnason JT, Dillon JA. 2011. Extracts of Canadian First Nations medicinal plants, used as natural products, inhibit Neisseria gonorrhoeae isolates with different antibiotic resistance profiles. Sex Transm Dis 38:667–671. doi: 10.1097/OLQ.0b013e31820cb166. [DOI] [PubMed] [Google Scholar]
- 14.Nandi S, Swanson S, Tomberg J, Nicholas RA. 2015. Diffusion of antibiotics through the PilQ secretin in Neisseria gonorrhoeae occurs through the immature, sodium dodecyl sulfate-labile form. J Bacteriol 197:1308–1321. doi: 10.1128/JB.02628-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Detels R, Green AM, Klausner JD, Katzenstein D, Gaydos C, Handsfield H, Pequegnat W, Mayer K, Hartwell TD, Quinn TC. 2011. The incidence and correlates of symptomatic and asymptomatic Chlamydia trachomatis and Neisseria gonorrhoeae infections in selected populations in five countries. Sex Transm Dis 38:503–509. [PMC free article] [PubMed] [Google Scholar]
- 16.Hook EW, Holmes KK. 1985. Gonococcal infections. Ann Intern Med 102:229–243. doi: 10.7326/0003-4819-102-2-229. [DOI] [PubMed] [Google Scholar]
- 17.McCormack WM, Stumacher RJ, Johnson K, Donner A. 1977. Clinical spectrum of gonococcal infection in women. Lancet i:1182–1185. doi: 10.1016/S0140-6736(77)92720-9. [DOI] [PubMed] [Google Scholar]
- 18.Brunham RC, Paavonen J, Stevens CE, Kiviat N, Kuo CC, Critchlow CW, Holmes KK. 1984. Mucopurulent cervicitis—the ignored counterpart in women of urethritis in men. N Engl J Med 311:1–6. doi: 10.1056/NEJM198407053110101. [DOI] [PubMed] [Google Scholar]
- 19.Greiner LL, Edwards JL, Shao J, Rabinak C, Entz D, Apicella MA. 2005. Biofilm formation by Neisseria gonorrhoeae. Infect Immun 73:1964–1970. doi: 10.1128/IAI.73.4.1964-1970.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steichen CT, Shao JQ, Ketterer MR, Apicella MA. 2008. Gonococcal cervicitis: a role for biofilm in pathogenesis. J Infect Dis 198:1856–1861. doi: 10.1086/593336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikolaitchouk N, Andersch B, Falsen E, Strömbeck L, Mattsby-Baltzer I. 2008. The lower genital tract microbiota in relation to cytokine-, SLPI- and endotoxin levels: application of checkerboard DNA-DNA hybridization (CDH). APMIS 116:263–277. doi: 10.1111/j.1600-0463.2008.00808.x. [DOI] [PubMed] [Google Scholar]
- 22.Graver MA, Wade JJ. 2011. The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Ann Clin Microbiol Antimicrob 10:8. doi: 10.1186/1476-0711-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng HY, Alcorn TM, Cohen MS. 1994. Effects of H2O2-producing lactobacilli on Neisseria gonorrhoeae growth and catalase activity. J Infect Dis 170:1209–1215. doi: 10.1093/infdis/170.5.1209. [DOI] [PubMed] [Google Scholar]
- 24.Montagnini Spaine D, Mamizuka EM, Pereira Cedenho A, Srougi M. 2000. Microbiologic aerobic studies on normal male urethra. Urology 56:207–210. doi: 10.1016/S0090-4295(00)00615-4. [DOI] [PubMed] [Google Scholar]
- 25.Ketterer MR, Rice PA, Gulati S, Kiel S, Byerly L, Fortenberry JD, Soper DE, Apicella MA. 2016. Desialylation of Neisseria gonorrhoeae lipooligosaccharide by cervicovaginal microbiome sialidases: the potential for enhancing infectivity in men. J Infect Dis 214:1621–1628. doi: 10.1093/infdis/jiw329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards JL, Brown EJ, Ault KA, Apicella MA. 2001. The role of complement receptor 3 (CR3) in Neisseria gonorrhoeae infection of human cervical epithelia. Cell Microbiol 3:611–622. doi: 10.1046/j.1462-5822.2001.00140.x. [DOI] [PubMed] [Google Scholar]
- 27.Edwards JL, Brown EJ, Uk-Nham S, Cannon JG, Blake MS, Apicella MA. 2002. A co-operative interaction between Neisseria gonorrhoeae and complement receptor 3 mediates infection of primary cervical epithelial cells. Cell Microbiol 4:571–584. doi: 10.1046/j.1462-5822.2002.t01-1-00215.x. [DOI] [PubMed] [Google Scholar]
- 28.Edwards JL, Butler EK. 2011. The pathobiology of Neisseria gonorrhoeae lower female genital tract infection. Front Microbiol 2:102. doi: 10.3389/fmicb.2011.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caron E, Hall A. 1998. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- 30.Quillin SJ, Seifert HS. 2018. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat Rev Microbiol 16:226–240. doi: 10.1038/nrmicro.2017.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jerse AE, Deal CD. 2013. Vaccine research for gonococcal infections: where are we? Sex Transm Infect 89(Suppl 4):iv63–iv68. doi: 10.1136/sextrans-2013-051225. [DOI] [PubMed] [Google Scholar]
- 32.Hobbs MM, Sparling PF, Cohen MS, Shafer WM, Deal CD, Jerse AE. 2011. Experimental gonococcal infection in male volunteers: cumulative experience with Neisseria gonorrhoeae strains FA1090 and MS11mkC. Front Microbiol 2:123. doi: 10.3389/fmicb.2011.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Handsfield HH, Lipman TO, Harnisch JP, Tronca E, Holmes KK. 1974. Asymptomatic gonorrhea in men. Diagnosis, natural course, prevalence and significance. N Engl J Med 290:117–123. doi: 10.1056/NEJM197401172900301. [DOI] [PubMed] [Google Scholar]
- 34.Agarwal S, King CA, Klein EK, Soper DE, Rice PA, Wetzler LM, Genco CA. 2005. The gonococcal Fur-regulated tbpA and tbpB genes are expressed during natural mucosal gonococcal infection. Infect Immun 73:4281–4287. doi: 10.1128/IAI.73.7.4281-4287.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agarwal S, Sebastian S, Szmigielski B, Rice PA, Genco CA. 2008. Expression of the gonococcal global regulatory protein Fur and genes encompassing the Fur and iron regulon during in vitro and in vivo infection in women. J Bacteriol 190:3129–3139. doi: 10.1128/JB.01830-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su X, Jiang F, Ge Q, Dai X, Sun H, Ye S. 2007. Surveillance of antimicrobial susceptibilitites in Neisseria gonorrhoeae in Nanjing, China, 1999–2006. Sex Transm Dis 34:995–999. [DOI] [PubMed] [Google Scholar]
- 37.Wan C, Li Y, Le W, Liu Y, Li S, Wang BX, Rice PA, Su X-H. 2018. Increasing resistance to azithromycin of Neisseria gonorrhoeae in eastern Chinese cities: mechanisms and genetic diversity of resistant Nanjing isolates. Antimicrob Agents Chemother 62:e02499-17. doi: 10.1128/AAC.02499-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McClure R, Nudel K, Massari P, Tjaden B, Su X, Rice PA, Genco CA. 2015. The gonococcal transcriptome during infection of the lower genital tract in women. PLoS One 10:e0133982. doi: 10.1371/journal.pone.0133982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McClure R, Balasubramanian D, Sun Y, Bobrovskyy M, Sumby P, Genco CA, Vanderpool CK, Tjaden B. 2013. Computational analysis of bacterial RNA-Seq data. Nucleic Acids Res 41:e140. doi: 10.1093/nar/gkt444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wackwitz B, Bongaerts J, Goodman SD, Unden G. 1999. Growth phase-dependent regulation of nuoA-N expression in Escherichia coli K-12 by the Fis protein: upstream binding sites and bioenergetic significance. Mol Gen Genet 262:876–883. doi: 10.1007/s004380051153. [DOI] [PubMed] [Google Scholar]
- 41.Seib KL, Wu HJ, Kidd SP, Apicella MA, Jennings MP, McEwan AG. 2006. Defenses against oxidative stress in Neisseria gonorrhoeae: a system tailored for a challenging environment. Microbiol Mol Biol Rev 70:344–361. doi: 10.1128/MMBR.00044-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rouhier N, Jacquot JP. 2003. Molecular and catalytic properties of a peroxiredoxin-glutaredoxin hybrid from Neisseria meningitidis. FEBS Lett 554:149–153. doi: 10.1016/S0014-5793(03)01156-6. [DOI] [PubMed] [Google Scholar]
- 43.Antoine M, Gand A, Boschi-Muller S, Branlant G. 2006. Characterization of the amino acids from Neisseria meningitidis MsrA involved in the chemical catalysis of the methionine sulfoxide reduction step. J Biol Chem 281:39062–39070. doi: 10.1074/jbc.M608844200. [DOI] [PubMed] [Google Scholar]
- 44.Isabella VM, Clark VL. 2011. Deep sequencing-based analysis of the anaerobic stimulon in Neisseria gonorrhoeae. BMC Genomics 12:51. doi: 10.1186/1471-2164-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu C, Genco CA. 2012. Fur-mediated activation of gene transcription in the human pathogen Neisseria gonorrhoeae. J Bacteriol 194:1730–1742. doi: 10.1128/JB.06176-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stohl EA, Criss AK, Seifert HS. 2005. The transcriptome response of Neisseria gonorrhoeae to hydrogen peroxide reveals genes with previously uncharacterized roles in oxidative damage protection. Mol Microbiol 58:520–532. doi: 10.1111/j.1365-2958.2005.04839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seib KL, Wu HJ, Srikhanta YN, Edwards JL, Falsetta ML, Hamilton AJ, Maguire TL, Grimmond SM, Apicella MA, McEwan AG, Jennings MP. 2007. Characterization of the OxyR regulon of Neisseria gonorrhoeae. Mol Microbiol 63:54–68. doi: 10.1111/j.1365-2958.2006.05478.x. [DOI] [PubMed] [Google Scholar]
- 48.Jackson LA, Day M, Allen J, Scott E II, Dyer DW. 2017. Iron-regulated small RNA expression as Neisseria gonorrhoeae FA 1090 transitions into stationary phase growth. BMC Genomics 18:317. doi: 10.1186/s12864-017-3684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McClure R, Tjaden B, Genco C. 2014. Identification of sRNAs expressed by the human pathogen Neisseria gonorrhoeae under disparate growth conditions. Front Microbiol 5:456. doi: 10.3389/fmicb.2014.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piekarowicz A, Kłyz A, Majchrzak M, Adamczyk-Popławska M, Maugel TK, Stein DC. 2007. Characterization of the dsDNA prophage sequences in the genome of Neisseria gonorrhoeae and visualization of productive bacteriophage. BMC Microbiol 7:66. doi: 10.1186/1471-2180-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hollander A, Mercante AD, Shafer WM, Cornelissen CN. 2011. The iron-repressed, AraC-like regulator MpeR activates expression of fetA in Neisseria gonorrhoeae. Infect Immun 79:4764–4776. doi: 10.1128/IAI.05806-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ducey TF, Jackson L, Orvis J, Dyer DW. 2009. Transcript analysis of nrrF, a Fur repressed sRNA of Neisseria gonorrhoeae. Microb Pathog 46:166–170. doi: 10.1016/j.micpath.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li S, Su XH, Le WJ, Jiang FX, Wang BX, Rice PA. 2014. Antimicrobial susceptibility of Neisseria gonorrhoeae isolates from symptomatic men attending the Nanjing sexually transmitted diseases clinic (2011–2012): genetic characteristics of isolates with reduced sensitivity to ceftriaxone. BMC Infect Dis 14:622. doi: 10.1186/s12879-014-0622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ilina EN, Vereshchagin VA, Borovskaya AD, Malakhova MV, Sidorenko SV, Al-Khafaji NC, Kubanova AA, Govorun VM. 2008. Relation between genetic markers of drug resistance and susceptibility profile of clinical Neisseria gonorrhoeae strains. Antimicrob Agents Chemother 52:2175–2182. doi: 10.1128/AAC.01420-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matejková P, Flasarová M, Zákoucká H, Borek M, Kremenová S, Arenberger P, Woznicová V, Weinstock GM, Smajs D. 2009. Macrolide treatment failure in a case of secondary syphilis: a novel A2059G mutation in the 23S rRNA gene of Treponema pallidum subsp. pallidum. J Med Microbiol 58:832–836. doi: 10.1099/jmm.0.007542-0. [DOI] [PubMed] [Google Scholar]
- 56.Hagman KE, Shafer WM. 1995. Transcriptional control of the mtr efflux system of Neisseria gonorrhoeae. J Bacteriol 177:4162–4165. doi: 10.1128/jb.177.14.4162-4165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jerse AE, Sharma ND, Simms AN, Crow ET, Snyder LA, Shafer WM. 2003. A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect Immun 71:5576–5582. doi: 10.1128/IAI.71.10.5576-5582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shafer WM, Qu X, Waring AJ, Lehrer RI. 1998. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc Natl Acad Sci U S A 95:1829–1833. doi: 10.1073/pnas.95.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grad YH, Harris SR, Kirkcaldy RD, Green AG, Marks DS, Bentley SD, Trees D, Lipsitch M. 2016. Genomic epidemiology of gonococcal resistance to extended-spectrum cephalosporins, macrolides, and fluoroquinolones in the United States, 2000–2013. J Infect Dis 214:1579–1587. doi: 10.1093/infdis/jiw420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seike K, Yasuda M, Hatazaki K, Mizutani K, Yuhara K, Ito Y, Fujimoto Y, Ito S, Tsuchiya T, Yokoi S, Nakano M, Deguchi T. 2016. Novel penA mutations identified in Neisseria gonorrhoeae with decreased susceptibility to ceftriaxone isolated between 2000 and 2014 in Japan. J Antimicrob Chemother 71:2466–2470. doi: 10.1093/jac/dkw161. [DOI] [PubMed] [Google Scholar]
- 61.Kandler JL, Holley CL, Reimche JL, Dhulipala V, Balthazar JT, Muszyński A, Carlson RW, Shafer WM. 2016. The MisR response regulator is necessary for intrinsic cationic antimicrobial peptide and aminoglycoside resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother 60:4690–4700. doi: 10.1128/AAC.00823-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rouquette C, Harmon JB, Shafer WM. 1999. Induction of the mtrCDE-encoded efflux pump system of Neisseria gonorrhoeae requires MtrA, an AraC-like protein. Mol Microbiol 33:651–658. doi: 10.1046/j.1365-2958.1999.01517.x. [DOI] [PubMed] [Google Scholar]
- 63.Veal WL, Nicholas RA, Shafer WM. 2002. Overexpression of the MtrC-MtrD-MtrE efflux pump due to an mtrR mutation is required for chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. J Bacteriol 184:5619–5624. doi: 10.1128/JB.184.20.5619-5624.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee EH, Rouquette-Loughlin C, Folster JP, Shafer WM. 2003. FarR regulates the farAB-encoded efflux pump of Neisseria gonorrhoeae via an MtrR regulatory mechanism. J Bacteriol 185:7145–7152. doi: 10.1128/JB.185.24.7145-7152.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Edwards JL, Apicella MA. 2004. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin Microbiol Rev 17:965–981. doi: 10.1128/CMR.17.4.965-981.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cornelissen CN, Kelley M, Hobbs MM, Anderson JE, Cannon JG, Cohen MS, Sparling PF. 1998. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol Microbiol 27:611–616. doi: 10.1046/j.1365-2958.1998.00710.x. [DOI] [PubMed] [Google Scholar]
- 67.Hobbs MM, Anderson JE, Balthazar JT, Kandler JL, Carlson RW, Ganguly J, Begum AA, Duncan JA, Lin JT, Sparling PF, Jerse AE, Shafer WM. 2013. Lipid A’s structure mediates Neisseria gonorrhoeae fitness during experimental infection of mice and men. mBio 4:e00892-13. doi: 10.1128/mBio.00892-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Larsen B, Monif GR. 2001. Understanding the bacterial flora of the female genital tract. Clin Infect Dis 32:e69–e77. doi: 10.1086/318710. [DOI] [PubMed] [Google Scholar]
- 69.Ma B, Forney LJ, Ravel J. 2012. Vaginal microbiome: rethinking health and disease. Annu Rev Microbiol 66:371–389. doi: 10.1146/annurev-micro-092611-150157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. 2011. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 108:4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Unemo M, del Rio C, Shafer WM 10 June 2016. Antimicrobial resistance expressed by Neisseria gonorrhoeae: a major global public health problem in the 21st century. Microbiol Spectr doi: 10.1128/microbiolspec.EI10-0009-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gu WM, Chen Y, Yang Y, Wu L, Hu WZ, Jin YL. 2014. Twenty-five-year changing pattern of gonococcal antimicrobial susceptibility in Shanghai: surveillance and its impact on treatment guidelines. BMC Infect Dis 14:731. doi: 10.1186/s12879-014-0731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wi T, Lahra MM, Ndowa F, Bala M, Dillon J-AR, Ramon-Pardo P, Eremin SR, Bolan G, Unemo M. 2017. Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Med 14:e1002344. doi: 10.1371/journal.pmed.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.MacPherson DW, Gushulak BD, Baine WB, Bala S, Gubbins PO, Holtom P, Segarra-Newnham M. 2009. Population mobility, globalization, and antimicrobial drug resistance. Emerg Infect Dis 15:1727–1732. doi: 10.3201/eid1511.090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Falsetta ML, Steichen CT, McEwan AG, Cho C, Ketterer M, Shao J, Hunt J, Jennings MP, Apicella MA. 2011. The composition and metabolic phenotype of Neisseria gonorrhoeae biofilms. Front Microbiol 2:75. doi: 10.3389/fmicb.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goytia M, Dhulipala VL, Shafer WM. 2013. Spermine impairs biofilm formation by Neisseria gonorrhoeae. FEMS Microbiol Lett 343:64–69. doi: 10.1111/1574-6968.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Piekarowicz A, Majchrzak M, Kłyz A, Adamczyk-Popławska M. 2006. Analysis of the filamentous bacteriophage genomes integrated into Neisseria gonorrhoeae FA1090 chromosome. Pol J Microbiol 55:251–260. [PubMed] [Google Scholar]
- 78.Daou N, Yu C, McClure R, Gudino C, Reed GW, Genco CA. 2013. Neisseria prophage repressor implicated in gonococcal pathogenesis. Infect Immun 81:3652–3661. doi: 10.1128/IAI.00298-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klein S, Lorenzo C, Hoffmann S, Walther JM, Storbeck S, Piekarski T, Tindall BJ, Wray V, Nimtz M, Moser J. 2009. Adaptation of Pseudomonas aeruginosa to various conditions includes tRNA-dependent formation of alanyl-phosphatidylglycerol. Mol Microbiol 71:551–565. doi: 10.1111/j.1365-2958.2008.06562.x. [DOI] [PubMed] [Google Scholar]
- 80.Shepherd J, Ibba M. 2013. Direction of aminoacylated transfer RNAs into antibiotic synthesis and peptidoglycan-mediated antibiotic resistance. FEBS Lett 587:2895–2904. doi: 10.1016/j.febslet.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin JS, Donegan SP, Heeren TC, Greenberg M, Flaherty EE, Haivanis R, Su XH, Dean D, Newhall WJ, Knapp JS, Sarafian SK, Rice RJ, Morse SA, Rice PA. 1998. Transmission of Chlamydia trachomatis and Neisseria gonorrhoeae among men with urethritis and their female sex partners. J Infect Dis 178:1707–1712. doi: 10.1086/314485. [DOI] [PubMed] [Google Scholar]
- 82.Sherrard J, Barlow D. 1996. Gonorrhoea in men: clinical and diagnostic aspects. Genitourin Med 72:422–426. doi: 10.1136/sti.72.6.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jensen JS, Uldum SA, Søndergård-Andersen J, Vuust J, Lind K. 1991. Polymerase chain reaction for detection of Mycoplasma genitalium in clinical samples. J Clin Microbiol 29:46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheng G, He Y, Zhou X, Li H. 2002. Development of Chlamydia trachomatis fluorescence PCR diagnostic kit and its clinical trial. Acad J Sun Yat-Sen 23:397–400. [Google Scholar]
- 85.Feavers IM, Maiden MC. 1998. A gonococcal porA pseudogene: implications for understanding the evolution and pathogenicity of Neisseria gonorrhoeae. Mol Microbiol 30:647–656. doi: 10.1046/j.1365-2958.1998.01101.x. [DOI] [PubMed] [Google Scholar]
- 86.CLSI 2017. Performance standards for antimicrobial susceptibility testing, 27th ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 87.Liao CH, Lai CC, Hsu MS, Chu FY, Wu MY, Huang YT, Hsueh PR. 2010. Antimicrobial susceptibility of Neisseria gonorrhoeae isolates determined by the agar dilution, disk diffusion and Etest methods: comparison of results using GC agar and chocolate agar. Int J Antimicrob Agents 35:457–460. doi: 10.1016/j.ijantimicag.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 88.Bibby J, Kent J, Mardia K. 1979. Multivariate analysis. Academic Press, London, United Kingdom. [Google Scholar]
- 89.Chin CS, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, Clum A, Copeland A, Huddleston J, Eichler EE, Turner SW, Korlach J. 2013. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods 10:563–569. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- 90.Gardner SN, Slezak T, Hall BG. 2015. kSNP3.0: SNP detection and phylogenetic analysis of genomes without genome alignment or reference genome. Bioinformatics 31:2877–2878. doi: 10.1093/bioinformatics/btv271. [DOI] [PubMed] [Google Scholar]
- 91.Jia B, Raphenya AR, Alcock B, Waglechner N, Guo P, Tsang KK, Lago BA, Dave BM, Pereira S, Sharma AN, Doshi S, Courtot M, Lo R, Williams LE, Frye JG, Elsayegh T, Sardar D, Westman EL, Pawlowski AC, Johnson TA, Brinkman FS, Wright GD, McArthur AG. 2017. CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res 45:D566–D573. doi: 10.1093/nar/gkw1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Harrison OB, Clemence M, Dillard JP, Tang CM, Trees D, Grad YH, Maiden MC. 2016. Genomic analyses of Neisseria gonorrhoeae reveal an association of the gonococcal genetic island with antimicrobial resistance. J Infect 73:578–587. doi: 10.1016/j.jinf.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of female subjects. Download TABLE S1, DOCX file, 0.02 MB (16.1KB, docx) .
Copyright © 2018 Nudel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Supplemental Tables S3 to S7. Table S3 shows the top 10 most highly expressed genes in each male subject. Table S4 contains the composite RPKM values for each gene in men in vivo versus in vitro. Table S5 contains the composite RPKM values for significantly different noncoding RNA genes in men in vivo versus in vitro. Table S6 contains the composite RPKM values for significantly different predictive sRNAs in men in vivo versus in vitro and men in vivo versus women in vivo. Table S7 contains the composite RPKM values for each gene in men versus women. Download DATA SET S1, XLSX file, 0.2 MB (224.5KB, xlsx) .
Copyright © 2018 Nudel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Phage expression, iron regulation, and oxidative response genes in the male and female genital tracts. (A) Expression levels of phage genes are shown on the x axis and represent male urethral samples (blue) and female cervicovaginal lavage samples (red). (B and C) Expression levels of significantly differentially expressed iron genes (B) and oxidative stress genes (C). *, gene more highly expressed in the male genital tract; #, gene more highly expressed in the female genital tract (q value of <0.05). Red and blue X’s indicate the male and female counterparts of the dyad. Download FIG S1, TIF file, 0.8 MB (845KB, tif) .
Copyright © 2018 Nudel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Hamming distance tree and SNP distance matrix of N. gonorrhoeae isolates. Tree visualizing Hamming distances of total pairwise SNPs identified across strains. The scale bar shows a distance of 300 SNPs. Download FIG S2, TIF file, 0.9 MB (898.4KB, tif) .
Copyright © 2018 Nudel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Deposited genomic data. Download TABLE S2, DOCX file, 0.01 MB (14.4KB, docx) .
Copyright © 2018 Nudel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
New RNA-seq data from this study have been deposited in the Gene Expression Omnibus (GSE113290). Genomic data from isolates have been deposited into NCBI under project no. PRJNA329501 (see Table S2 in the supplemental material).
Deposited genomic data. Download TABLE S2, DOCX file, 0.01 MB (14.4KB, docx) .
Copyright © 2018 Nudel et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.