Abstract
Vertebrate oocytes arrested at the first meiotic prophase must proceed to the second meiotic metaphase (MII) before fertilization. This meiotic process requires the precise control of protein degradation. Part of the protein degradation in oocytes is controlled by members of the ubiquitin-conjugating enzyme family, UBE2C and UBE2S, which are known to participate in mono-ubiquitination and poly-ubiquitination, respectively. Although UBE2 enzymes have been well studied in mitosis, their contribution to mammalian oocyte meiosis is relatively unknown and has been studied only in mice. Here, we investigated the contribution of UBE2C and UBE2S to porcine oocyte maturation using an RNA injection method. Overexpression of UBE2S prevented MII arrest of oocytes and led to the formation of a pronucleus (PN) at 48 h of culture. This effect was also observed for prolonged cultures of UBE2C-overexpressing oocytes, suggesting the effectiveness of poly-ubiquitination in the rapid escape from M-phase in porcine oocytes. Although the inhibition of either UBE2C or UBE2S by antisense RNA (asRNA) injection had no effect on oocyte maturation, asRNA-injected oocytes showed inhibited PN formation after parthenogenetic activation. These results indicated that ubiquitination of certain factors by UBE2S and UBE2C plays a role in the escape from MII arrest in porcine oocytes. Further investigations to identify the factors and how mono- and/or poly-ubiquitination contributes to protein degradation could provide a better understanding of UBE2 roles in oocyte maturation.
Keywords: APC/C, CCNB, E2, Oocyte maturation, Ubiquitin-proteasome pathway
In vertebrate ovarian follicles, oocytes are arrested at the first meiotic prophase and must proceed through the second meiotic metaphase (MII) for fertilization. This meiotic process called oocyte maturation is initiated by the activation of maturation/M-phase promoting factor (MPF) [1], which is stimulated by cyclin B (CCNB) accumulation [2,3,4,5] and chromosome segregation triggered by securin degradation [6]. Thus, the progression of oocyte maturation is controlled in large part by the degradation of a specific protein mediated by the ubiquitin-proteasome pathway. The ubiquitination of a target protein is mediated by the ubiquitin-activating enzyme (E1), the ubiquitin-conjugating enzyme (E2), and the ubiquitin ligase (E3). The E2 enzyme binds E1-activated ubiquitin through a thioester bond, and then, ubiquitin is transferred to E3 for binding to the target substrates [7,8,9,10]. E4 enzymes also exist to promote multi-ubiquitination [9, 11]. Presently, while only two enzymes are known as E1, approximately 40 enzymes are known as E2 and many more as E3 in humans, and each enzyme contributes to specific types of ubiquitin linkages [10, 12].
Degradation of part of the mammalian oocytes proteins is mediated by the UBE2 family members UBE2C and UBE2S, as E2 enzymes, and by anaphase-promoting-complex/cyclosome (APC/C), as the E3 enzyme [13,14,15]. Although both UBE2C and UBE2S are E2 enzymes, their roles are not interchangeable: UBE2C is known to be involved in mono-ubiquitin activation and passes the first ubiquitin to be conjugated to target proteins (mono-ubiquitination), and UBE2S is mainly involved in ubiquitin chain elongation (poly-ubiquitination) [14,15,16,17]. As an in vitro study has revealed, UBE2S alone cannot initiate ubiquitin chain formation; it only elongates the chain that is created by UBE2C [16, 18].
UBE2 enzymes have been fairly well studied in mitosis, but little is known about their contribution in meiosis, and only Xenopus and mouse oocytes have been used in studies addressing the roles of the UBE2 family in oocyte maturation. It was shown by Ben-Eliezer and co-workers that mouse UBE2C and UBE2S are necessary for completion of the first meiotic division [19]. This report further examined MII arrest and demonstrated that UBE2C- or UBE2S-overexpressing oocytes did not arrest at MII but extruded a second polar body. In Xenopus oocytes, CCNB is shown to be degraded by not only poly-ubiquitination but also by either mono-ubiquitination or multi-mono-ubiquitination [13]. This indicates that CCNB can be degraded by ubiquitination performed via only UBE2C, without UBE2S; this mechanism was not found in mouse oocytes. Since so little is known about UBE2 enzymes in oocyte maturation, and, currently, mice are the only mammalian species in which these factors have been examined, further investigations are needed to reveal the function of those factors in oocyte maturation of mammalian species other than mice. Herein, we investigated UBE2 effects in porcine oocyte maturation and CCNB accumulation by regulating UBE2C and UBE2S expression within maturing porcine oocytes.
Materials and Methods
Porcine oocyte culture
Prepubertal guilt ovaries were collected from a commercial slaughterhouse. Cumulus-oocyte complexes (COCs) were aspirated from follicles of 2–5 mm in diameter and washed in culture medium, which consisted of a modified Krebs-Ringer bicarbonate solution (TYH) containing 1.0 IU/ml eCG (Serotropin; ASKA Pharmaceutical, Tokyo, Japan), and in 20% porcine follicular fluid, prepared as previously described [20]. Approximately 25 COCs were cultured in culture medium (100 µl), at 38°C, saturated humidity, and 5% CO2. After culture, cumulus cells were removed by pipetting in saline containing 0.1% polyvinylpyrrolidone (PVP) and 0.1% hyaluronidase (Sigma-Aldrich, MO, USA). Denuded oocytes were subjected to nucleus observation and immunoblotting analysis. Nuclear states were observed by mounting denuded oocytes onto a glass slide, fixing with acetic acid-ethanol (1:3), and staining with 0.75% acetoorcein solution.
Cloning of UBE2C and UBE2S cDNAs and vector construction
Total RNA was extracted from non-cultured porcine oocytes using Trizol reagent (Invitrogen, CA, USA), and first-strand cDNA was synthesized using SuperScript III (Invitrogen), according to the manufacturer’s instruction. Porcine UBE2C and UBE2S cDNAs were amplified by PCR using the primer sets designed from NCBI Expressed Sequence Tag (EST) database and porcine database (National Center Biotechnology Information, Bethesda, MD, USA) (Table 1), respectively, and the PCR products were cloned into pGEM-T EASY vector (A1360; Promega, WI, USA). These vectors were used for the synthesis of antisense RNAs (asRNAs). For the synthesis of Flag-tagged mRNAs, forward primers were designed to omit a start codon and to add a BglII or BamHI site before the UBE2C or UBE2S sequence, respectively. The reverse primers were designed downstream of the stop codon and carried a BglII site or BamHI site for UBE2C and UBE2S mRNAs, respectively (Table 1). PCR fragments were cloned into the pGEM-T Easy vector, and then, digested with BglII or BamHI and inserted into the BamHI-digested pCMV-Tag1 vector (Agilent Technologies, CA, USA) in order to add a Flag tag to the N-terminal. These vectors were referred to as Flag-UBE2C-vector and Flag-UBE2S-vector. Cloned products were sequenced with a commercial sequencing kit (Applied Biosystems, CA, USA), using a DNA sequencer (Applied Biosystems), following the manufacturer’s instructions.
Table 1. Primers used for cloning of UBE2C and UBE2S cDNAs and preparation of Flag-tagged vectors.
| Product | Sequence | ||
|---|---|---|---|
| UBE2C cloning | Forward | 5'- | CCAGATGGCTTCCCAGAA |
| Reverse | 5'- | AACACAGGGAGAGAGCTGGA | |
| Flag-UBE2C-vector | Forward | 5'- | AGATCTCCGCTTCCCAGAACCGCGA |
| Reverse | 5'- | GGAGATCTACACAGGGAGAGAGCTG | |
| UBE2S cloning | Forward | 5'- | CCGCAGTCATGAACTCCAA |
| Reverse | 5'- | GAGGAGAGCCCGCTACAG | |
| Flag-UBE2S-vector | Forward | 5'- | GGATCCCCAACTCCAATGTGGAGAA |
| Reverse | 5'- | GGGGATCCAGGAGAGCCCGCTACAG | |
In vitro RNA synthesis
RNAs were synthesized in vitro from the above vectors linearized by restriction enzymes, using either T3- or Sp6- RNA polymerase (Promega) in the presence of m7G(5’)ppp(5’)G to synthesize capped RNA transcripts, as described previously [21]. Synthesized RNAs were precipitated with ethanol, washed, dried, and resuspended in RNase-free water (Gibco, NY, USA). Resuspended RNAs were stored at −80°C until further use. Enhanced green fluorescent protein (EGFP) mRNA was synthesized from the EGFP vector in a similar manner [21].
Microinjection
A single UBE2 RNA or a mixture of UBE2 RNAs were added to EGFP mRNA, as an indicator of microinjection and oocyte viability, and the concentration of each RNA was adjusted to 250 ng/µl. The RNA solutions were microinjected to the COCs placed in the culture medium, using microinjectors (MO-202U; Narishige, Tokyo, Japan) equipped with manipulators (GJ-8; Narishige) mounted on an inverted microscope (Diaphot 200; Nikon, Tokyo, Japan). Approximately 50 pl of RNA solution were injected into the cytoplasm of non-cultured GV oocytes by continuous pneumatic pressure. The injected oocytes were cultured as described above for up to 72 h. Oocytes showing EGFP fluorescence were selected under a fluorescent microscope and subjected to further analysis.
Parthenogenetic activation
After 48 h of maturation, COCs were denuded and placed in a mannitol solution (0.3 M Mannitol, 0.1 mM MgSO4, 0.05 mM CaCl2, 0.01% PVA). Activation was performed with a single DC pulse (150 V/mm for 99 μsec), and the oocytes were cultured for an additional 24-h period in the culture medium.
Immunoblotting analysis
Micro-Western blot was performed as described in a previous report [22]. The oocyte numbers used in each experiment are indicated in the figure legends. The antibodies used were: anti-CCNB1 (ADI-KAM-CC195-E, mouse IgG monoclonal antibody; Enzo Life Sciences, NY, USA), anti-Cdc2 (sc-54, mouse IgG monoclonal antibody; Santa Cruz, TX, USA), and anti-Flag (F1804, mouse IgG monoclonal antibody; Sigma-Aldrich). Horseradish peroxidase (HRP)-conjugated anti-mouse IgG (Jackson ImmunoResearch Laboratories, PA, USA) was used as secondary antibody. Signals were detected using ImmunoStar LD (Wako, Tokyo, Japan) and C-Digit (Li-Cor; Lincoln, NE, USA), as instructed by manufacturers. The relative intensity of the endogenous CCNB1 was quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data were analyzed with the Chi-square test, and a probability of P < 0.05 was considered as statistically significant.
Results
Effects of UBE2 asRNA injection on oocyte maturation
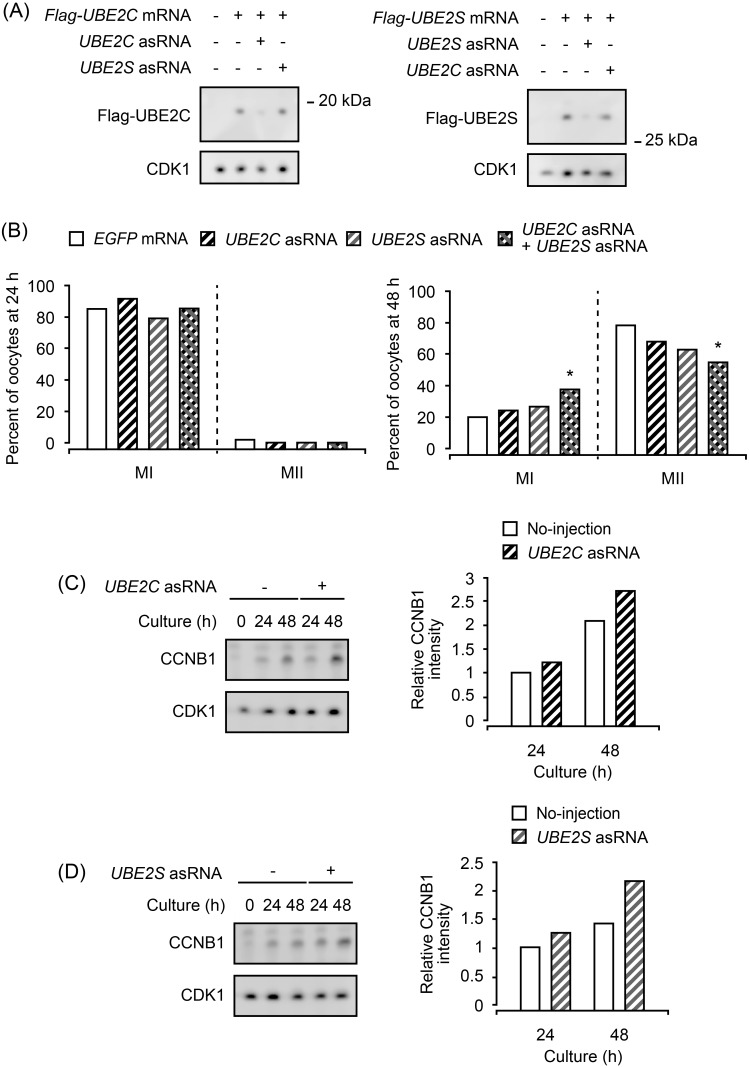
First, we confirmed the specificity of UBE2 asRNAs for the inhibition of UBE2 expression by co-injecting each asRNA with Flag-UBE2 mRNA. The protein expression of injected mRNAs was detected by an anti-Flag antibody. Six hours after the injection, each asRNA had decreased the signal intensity of its target UBE2, solely, demonstrating the effectiveness and specificity of asRNA in the inhibition of UBE2 expression (Fig. 1A).
Fig. 1.
Effects of UBE2 asRNA injection on meiotic maturation of oocytes. Non-cultured oocytes were injected with UBE2 asRNA and cultured for the indicated periods. (A) The specificity of asRNA was examined by injecting Flag-UBE2 mRNA with each asRNA. UBE2 was detected by an anti-Flag antibody, 6 h after injection. The experiments were repeated twice, and representative results are shown. Thirty (left panel) and 15 oocytes (right panel) were used in each lane. (B) asRNA-injected oocytes were cultured for 24 or 48 h, and their nuclear states were observed. MI, first meiotic metaphase; MII, second meiotic metaphase. At least two independent experiments were conducted, and more than 40 oocytes were observed for each condition. * P < 0.05. (C, D) Accumulation of endogenous CCNB1 and CDK1 was examined for UBE2C asRNA-injected oocytes (C) and UBE2S asRNA-injected oocytes (D) by immunoblotting. Twenty oocytes were used in each lane. The experiments were repeated twice, and representative results are shown in the left panel. The levels of CCNB1 in asRNA-injected oocytes relative to that in non-injected oocytes are quantified from two independent immunoblotting analyses, and the averages are shown in the right panels.
Next, the effects of UBE2 asRNAs on meiotic maturation of oocytes were evaluated (Fig. 1B). After 24 h of culture, neither UBE2C nor UBE2S asRNA affected the progression of oocyte maturation, and the percentages of oocytes at first meiotic metaphase (MI) (92.5% and 78.7%, respectively) were not significantly different from those of EGFP mRNA-injected oocytes (85.9%). In addition, the co-injection of both asRNAs also had no effect on the progression of oocyte maturation (85.7% of oocytes were at MI). After 48 h of culture, however, co-injection of both asRNAs resulted in a significant increase of MI-arrested oocytes and a decrease of oocytes reaching MII (72.2% compared with 85.9% in EGFP mRNA-injected oocytes), while the injection of each asRNA alone had no effect on oocyte maturation. The accumulation of CCNB and the states of CDK1 were examined by immunoblotting analysis (Fig. 1C, D). CCNB1 was not detected in non-cultured oocytes, and its protein level was gradually increased in accordance with the culture period, as reported previously [23]. In agreement with the nuclear state observation, accumulation of CCNB was not affected at 24 h but was slightly higher at 48 h of culture by either UBE2C or UBE2S asRNA injection. CDK1 level remained unchanged during maturation and was unaffected by UBE2 asRNA injection.
Effects of Flag-UBE2 mRNA injection on oocyte maturation
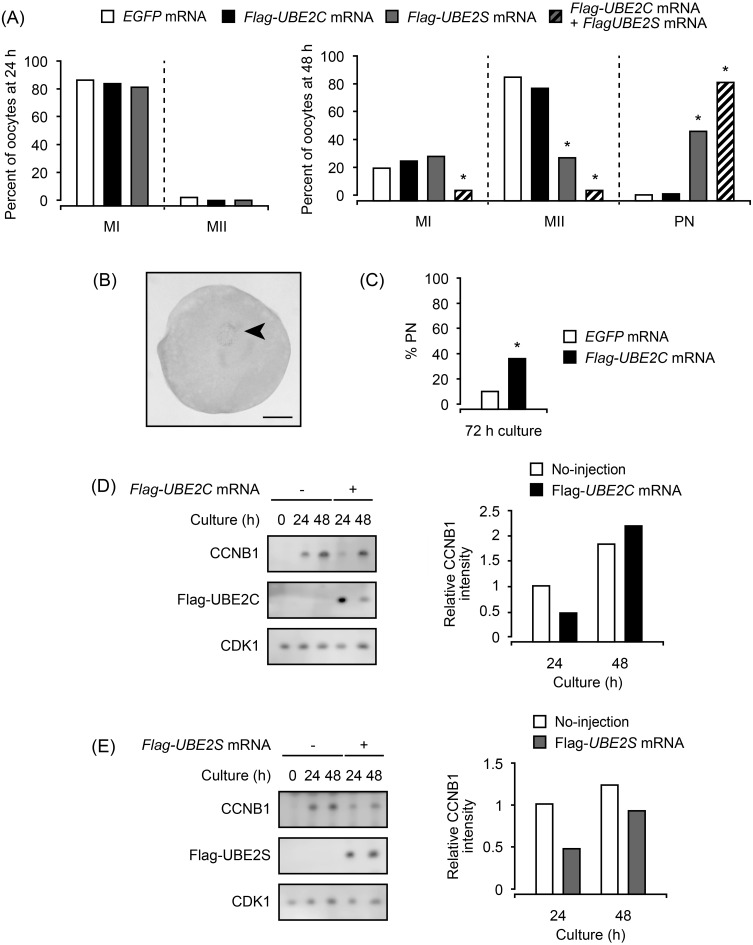
The effects of UBE2 on oocyte maturation were examined by UBE2 overexpression. Injection of Flag-UBE2C or Flag-UBE2S mRNA did not affect the maturation progression after 24 h of culture, and the percentages of MI oocytes (83.8% and 81.3%, respectively) were not different from that of control EGFP mRNA-injected oocytes (85.9%; Fig. 2A). On the other hand, at 48 h of culture, 42.9% of Flag-UBE2S mRNA-injected oocytes formed a pronucleus (PN) and only 23.9% remained at MII, while the majority of Flag-UBE2C mRNA-injected oocytes were at MII (71.0%), as well as EGFP mRNA-injected oocytes (78.4%) (Fig. 2A). A representative image of a PN is shown in Fig. 2B. Co-injection of Flag-UBE2C mRNA with Flag-UBE2S mRNA increased the percentage of PN-forming oocytes to 76.4%. Then, we checked the ability of UBE2C to induce PN formation by culturing Flag-UBE2C mRNA-injected oocytes for 72 h. The result showed that 36.2% of the oocytes formed PNs (Fig. 2C), indicating the significant PN-forming ability of UBE2C. Accumulation of CCNB1 was low after 24 h of culture in both UBE2-overexpressing oocytes, despite the unaffected nuclear states. In contrast, CCNB1 accumulation level at 48 h of culture was low in UBE2S-overexpressing oocytes, while it was comparable to the control in UBE2C-overexpressing oocytes (Fig. 2D, E).
Fig. 2.
Effects of Flag-UBE2 mRNA injection on meiotic maturation of oocytes. Non-cultured oocytes were injected with Flag-UBE2 mRNA and cultured for the indicated periods. (A) The injected oocytes were cultured for 24 or 48 h, and their nuclear states were observed. MI, first meiotic metaphase; MII, second meiotic metaphase; PN, pronucleus-forming oocytes. At least two independent experiments were conducted, and more than 45 oocytes were observed for each condition. * P < 0.05. (B) Representative image of PN observed in Flag-UBE2S mRNA-injected oocytes cultured for 48 h. Arrow: PN. Scale bar: 50 μm. (C) The percentages of PN-forming oocytes injected with Flag-UBE2C mRNA were examined after 72 h of culture. At least two independent experiments were conducted, and more than 45 oocytes were observed. * P < 0.05. (D, E) Accumulation of endogenous CCNB1 and CDK1 was examined for Flag-UBE2C mRNA-injected oocytes (D) and Flag-UBE2S mRNA-injected oocytes (E) by immunoblotting. Twenty oocytes were used in each lane. The experiments were repeated twice, and representative results are shown in the left panels. The levels of CCNB1 in mRNA-injected oocytes relative to those in non-injected oocytes are quantified from two independent immunoblotting analyses, and the averages are shown in the right panels.
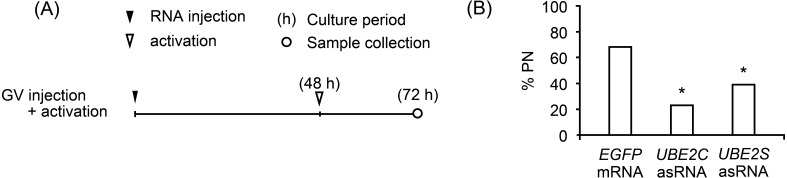
Parthenogenetic activation of UBE2 asRNA-injected oocytes
Since overexpression of UBE2 stimulated PN formation in MII oocytes, the effects of UBE2 on the escape from meiosis were examined by UBE2 asRNA injection and parthenogenetic activation (Fig. 3A). The percentage of PN formation in either UBE2C asRNA- or UBE2S asRNA-injected oocytes was significantly lower than that of EGFP mRNA-injected oocytes (22.9%, 39.0%, and 68.3%, respectively) (Fig. 3B), suggesting the requirement of UBE2 for the escape from MII arrest.
Fig. 3.
Effects of UBE2 asRNA injection on PN formation after parthenogenetic activation. (A) Schematic drawing of the experimental time course. (B) Percentage of oocytes that formed pronucleus (PN). At least two independent experiments were conducted, and more than 50 oocytes were examined for each condition. * P < 0.05.
Discussion
In the present study, we investigated the involvement of the ubiquitin-conjugating enzymes UBE2C and UBE2S in porcine oocyte maturation by injecting their mRNA or asRNA into immature porcine oocytes. Although the percentages of MI oocytes after 24 h of culture were not affected by the injection of RNAs, the results revealed that the PN formation rate in UBE2-overexpressing oocytes cultured for 48 h or more was significantly increased. Similar results have been reported in mouse oocytes, which extruded a second polar body after UBE2C or UBE2S overexpression [16]. UBE2C and UBE2S have been known to work with ubiquitin ligase APC/C to ubiquitinate target substrates for degradation by proteasomes [24]. Thus, the results from both the present and the mouse study suggest that the factors required for the maintenance of MII arrest are degraded through a UBE2-mediated mechanism. This, in turn, can be supported by the result that the injection of UBE2C or UBE2S asRNA alone significantly inhibited the PN formation of porcine oocytes after parthenogenetic activation. These results provide, for the first time, experimental evidence for the UBE2-mediated protein degradation playing a role in the release of porcine oocytes from MII arrest.
It is known that UBE2C contributes mainly to mono-ubiquitination, or the initial ubiquitination of substrates, while UBE2S contributes to the subsequent poly-ubiquitination, or ubiquitin chain formation [11, 13,14,15]. In the case of Xenopus, depending on the protein, mono-ubiquitination or multiple mono-ubiquitination can trigger protein degradation [10]. In the present study, PN formation at 48 h of culture was observed in UBE2S- but not in UBE2C-overexpressing oocytes, and PN formation in UBE2C-overexpressing oocytes was observed only after 72 h of culture. This indicates that mono-ubiquitination by UBE2C alone is not enough for the immediate degradation of the factors functioning in MII arrest in porcine oocytes and that poly-ubiquitination by UBE2S is more effective in the degradation of these factors, even though UBE2C and UBE2S have almost the same effects on mouse oocytes [16]. Therefore, unlike in mouse oocytes, the immediate degradation of these factors in porcine oocytes might depend on poly-ubiquitination more than on mono-ubiquitination. As for the asRNA injection experiment, it is plausible that UBE2C asRNA showed stronger inhibition of M-phase escape than UBE2S asRNA as the inhibition of mono-ubiquitination would automatically inhibit poly-ubiquitination. However, the remaining UBE2 levels after asRNA injection should be analyzed.
From the present study, the identity of the protein(s) that is ubiquitinated via UBE2C and UBE2S in porcine oocytes remains elusive. Several proteins are required for MII maintenance in mature oocytes and need to be degraded when oocytes are activated, including the MPF regulatory subunit CCNB, the separase inhibitor securin, and the cytostatic factors MOS and EMI2 [25,26,27,28]. The ubiquitination required for EMI2 degradation is performed not by APC/C but by SCFβTrCP, and, thus, EMI2 could not be the target substrate of UBE2C and UBE2S [29]. As for MOS, ubiquitination of Lysine 34 is known to be a prerequisite for degradation; however, the exact factor involved in this ubiquitination is unknown [30, 31]. In contrast to these factors, the requirement of UBE2C and UBE2S for the degradation of CCNB and securin has been reported [10, 11, 16]. Since the present experiments showed a decrease of CCNB accumulation in UBE2C- or UBE2S-overexpressing oocytes cultured for 24 h, it is undoubtable that CCNB is one of the substrates of UBE2C and UBE2S. As the present study was unable to detect the ubiquitination state of CCNB, further investigation is required to identify the protein(s) that is/are ubiquitinated by UBE2 and to discover the ubiquitination state required for degradation.
It was unexpected that the percentages of oocytes injected with asRNA or mRNA of either UBE2C or UBE2S in MI did not differ from that of the control at 24 h of culture and thereafter. It might be possible that UBE2s are very stable proteins and that asRNA, which inhibits the translation of new UBE2s, cannot decrease the already existing UBE2 at MI induction and MI escape, or that germinal vesicle (GV) stage oocytes contain excessive amounts of UBE2 enzymes, even after degradation of some UBE2. The immunoblotting result showing the unaffected CCNB level in UBE2 asRNA-injected oocytes suggests that asRNA injection could not sufficiently inhibit the function of UBE2 after 24 h of culture. On the other hand, although UBE2 overexpression showed a decrease in the CCNB level, the oocytes were able to reach MI, similar to the controls. In the initiation of porcine oocyte maturation, the activation of MPF is attributed to the dephosphorylation of pre-MPF, and the initiation of germinal vesicle breakdown (GVBD) can take place even with low MPF activity [23, 32]. It was also reported in mice that inhibition or overexpression of UBE2 did not have an impact on the meiotic resumption of oocytes [19]. In mouse oocytes, extrusion of the first polar body was enhanced by UBE2 overexpression and spindle disturbance induced by UBE2 inhibition [19]. Throughout the present experiments, a slight inhibition of the first polar body extrusion was observed only in both UBE2C and UBE2S asRNA-injected oocytes, and no abnormalities were detected in the single asRNA-injected oocytes. This result may indicate some differences among species regarding UBE2 dependence during oocyte maturation.
In conclusion, the present results indicate that protein degradation via UBE2 plays a role in the escape from MII arrest and that poly-ubiquitination by UBE2S might be effective for immediate degradation in porcine oocytes. Detailed observations of first polar body extrusion and the morphology of the meiotic spindle in porcine oocytes should provide further information about the contribution of UBE2 to mammalian oocyte maturation. In addition, as the study of the contribution of UBE2 to oocyte maturation proceeds, the interaction and involvement of UBE2 function in E1 ubiquitin-activating enzymes, deubiquitinases, and proteasomes would be the next points of interest for future analyses.
Acknowledgments
This work was supported by a Grant-in-Aid for Exploratory Research from Japan Society for the Promotion of Science (No. 25252056 to KN; No. 16K15052 and 17H03900 to KS).
References
- 1.Masui Y, Markert CL. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool 1971; 177: 129–145. [DOI] [PubMed] [Google Scholar]
- 2.Draetta G, Luca F, Westendorf J, Brizuela L, Ruderman J, Beach D. Cdc2 protein kinase is complexed with both cyclin A and B: evidence for proteolytic inactivation of MPF. Cell 1989; 56: 829–838. [DOI] [PubMed] [Google Scholar]
- 3.Dunphy WG, Brizuela L, Beach D, Newport J. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell 1988; 54: 423–431. [DOI] [PubMed] [Google Scholar]
- 4.Gautier J, Minshull J, Lohka M, Glotzer M, Hunt T, Maller JL. Cyclin is a component of maturation-promoting factor from Xenopus. Cell 1990; 60: 487–494. [DOI] [PubMed] [Google Scholar]
- 5.Lévesque JT, Sirard MA. Resumption of meiosis is initiated by the accumulation of cyclin B in bovine oocytes. Biol Reprod 1996; 55: 1427–1436. [DOI] [PubMed] [Google Scholar]
- 6.Salah SM, Nasmyth K. Destruction of the securin Pds1p occurs at the onset of anaphase during both meiotic divisions in yeast. Chromosoma 2000; 109: 27–34. [DOI] [PubMed] [Google Scholar]
- 7.Ciechanover A, Elias S, Heller H, Hershko A. Covalent affinity purification of ubiquitin-activating enzyme. J Biol Chem 1982; 257: 2537–2542. [PubMed] [Google Scholar]
- 8.Hershko A, Heller H, Elias S, Ciechanover A. Components of ubiquitin-protein ligase system. Resolution, affinity purification, and role in protein breakdown. J Biol Chem 1983; 258: 8206–8214. [PubMed] [Google Scholar]
- 9.Hoppe T. Multiubiquitylation by E4 enzymes: one size doesnt fit all. Trends Biochem Sci 2005; 30: 183–187. [DOI] [PubMed] [Google Scholar]
- 10.Kleiger G, Mayor T. Perilous journey: a tour of the ubiquitin-proteasome system. Trends Cell Biol 2014; 24: 352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koegl M, Hoppe T, Schlenker S, Ulrich HD, Mayer TU, Jentsch S. A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly. Cell 1999; 96: 635–644. [DOI] [PubMed] [Google Scholar]
- 12.Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem 2009; 78: 399–434. [DOI] [PubMed] [Google Scholar]
- 13.Dimova NV, Hathaway NA, Lee BH, Kirkpatrick DS, Berkowitz ML, Gygi SP, Finley D, King RW. APC/C-mediated multiple monoubiquitylation provides an alternative degradation signal for cyclin B1. Nat Cell Biol 2012; 14: 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkpatrick DS, Hathaway NA, Hanna J, Elsasser S, Rush J, Finley D, King RW, Gygi SP. Quantitative analysis of in vitro ubiquitinated cyclin B1 reveals complex chain topology. Nat Cell Biol 2006; 8: 700–710. [DOI] [PubMed] [Google Scholar]
- 15.Peters JM. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol Cell 2002; 9: 931–943. [DOI] [PubMed] [Google Scholar]
- 16.Garnett MJ, Mansfeld J, Godwin C, Matsusaka T, Wu J, Russell P, Pines J, Venkitaraman AR. UBE2S elongates ubiquitin chains on APC/C substrates to promote mitotic exit. Nat Cell Biol 2009; 11: 1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williamson A, Wickliffe KE, Mellone BG, Song L, Karpen GH, Rape M. Identification of a physiological E2 module for the human anaphase-promoting complex. Proc Natl Acad Sci USA 2009; 106: 18213–18218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu T, Merbl Y, Huo Y, Gallop JL, Tzur A, Kirschner MW. UBE2S drives elongation of K11-linked ubiquitin chains by the anaphase-promoting complex. Proc Natl Acad Sci USA 2010; 107: 1355–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben-Eliezer I, Pomerantz Y, Galiani D, Nevo N, Dekel N. Appropriate expression of Ube2C and Ube2S controls the progression of the first meiotic division. FASEB J 2015; 29: 4670–4681. [DOI] [PubMed] [Google Scholar]
- 20.Naito K, Fukuda Y, Toyoda Y. Effects of porcine follicular fluid on male pronucleus formation in porcine oocytes matured in vitro. Gamete Res 1988; 21: 289–295. [DOI] [PubMed] [Google Scholar]
- 21.Ohashi S, Naito K, Liu J, Sheng Y, Yamanouchi K, Tojo H. Expression of exogenous proteins in porcine maturing oocytes after mRNA injection: Kinetic analysis and oocyte selection using EGFP mRNA. J Reprod Dev 2001; 47: 351–357. [Google Scholar]
- 22.Naito K, Kagii H, Iwamori N, Sugiura K, Yamanouchi K, Tojo H. Establishment of a small-scale Western blotting system named as ‘‘micro-Western blotting’’ for mammalian ova analysis. J Mamm Ova Res 1999; 16: 154–157. [Google Scholar]
- 23.Kuroda T, Naito K, Sugiura K, Yamashita M, Takakura I, Tojo H. Analysis of the roles of cyclin B1 and cyclin B2 in porcine oocyte maturation by inhibiting synthesis with antisense RNA injection. Biol Reprod 2004; 70: 154–159. [DOI] [PubMed] [Google Scholar]
- 24.Pines J. Cubism and the cell cycle: the many faces of the APC/C. Nat Rev Mol Cell Biol 2011; 12: 427–438. [DOI] [PubMed] [Google Scholar]
- 25.Fujioka YA, Onuma A, Fujii W, Sugiura K, Naito K. Analyses of EMI functions on meiotic maturation of porcine oocytes. Mol Reprod Dev 2016; 83: 983–992. [DOI] [PubMed] [Google Scholar]
- 26.Ohsumi K, Koyanagi A, Yamamoto TM, Gotoh T, Kishimoto T. Emi1-mediated M-phase arrest in Xenopus eggs is distinct from cytostatic factor arrest. Proc Natl Acad Sci USA 2004; 101: 12531–12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sagata N, Watanabe N, Vande Woude GF, Ikawa Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature 1989; 342: 512–518. [DOI] [PubMed] [Google Scholar]
- 28.Tung JJ, Jackson PK. Emi1 class of proteins regulate entry into meiosis and the meiosis I to meiosis II transition in Xenopus oocytes. Cell Cycle 2005b; 4: 478–482. [DOI] [PubMed] [Google Scholar]
- 29.Tung JJ, Hansen DV, Ban KH, Loktev AV, Summers MK, Adler JR, 3rd., Jackson PK. A role for the anaphase-promoting complex inhibitor Emi2/XErp1, a homolog of early mitotic inhibitor 1, in cytostatic factor arrest of Xenopus eggs. Proc Natl Acad Sci USA 2005a; 102: 4318–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishizawa M, Okazaki K, Furuno N, Watanabe N, Sagata N. The second-codon rule and autophosphorylation govern the stability and activity of Mos during the meiotic cell cycle in Xenopus oocytes. EMBO J 1992; 11: 2433–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishizawa M, Furuno N, Okazaki K, Tanaka H, Ogawa Y, Sagata N. Degradation of Mos by the N-terminal proline (Pro2)-dependent ubiquitin pathway on fertilization of Xenopus eggs: possible significance of natural selection for Pro2 in Mos. EMBO J 1993; 12: 4021–4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimaoka T, Nishimura T, Kano K, Naito K. Analyses of the regulatory mechanism of porcine WEE1B: the phosphorylation sites of porcine WEE1B and mouse WEE1B are different. J Reprod Dev 2011; 57: 223–228. [DOI] [PubMed] [Google Scholar]