Abstract
Estrone (E1) and estriol (E3) are considered “weak” estrogens, which exert suppressive effects through estrogen receptors α and β. However, recent studies have demonstrated that E1 and E3, as well as estradiol (E2), suppress gonadotropin-releasing hormone-induced luteinizing hormone secretion from bovine gonadotrophs via G-protein-coupled receptor 30, which is expressed in various reproductive organs. Currently, there is a lack of fundamental knowledge regarding E1 and E3, including their blood levels. In addition, xenoestrogens may remain in the body over long time periods because of enterohepatic circulation. Therefore, it is time to reconsider the roles of endogenous estrogens and xenoestrogens for reproduction.
Keywords: Gonadotroph, G protein-coupled estrogen receptor-1, Pituitary, Xenoestrogen, zearalenone
Estrogen receptors
Estradiol (E2), which is secreted from the ovaries, is a powerful feedback regulator, controlling the secretion of gonadotropin-releasing hormone (GnRH) from the hypothalamus and the secretion of luteinizing hormone (LH) form the anterior pituitary. Blood E2 concentrations fluctuate in heifers and cows between 0.004 nM (1 pg/ml) and 0.030 nM (8 pg/ml) during the luteal phase of the estrous cycle [1, 2]. Small concentrations exert a negative feedback effect on GnRH secretion from the hypothalamus [3,4,5], and suppress the amounts of LH mRNA in the anterior pituitary [6].
E2 binds to nuclear-localized estrogen receptors α or β (ERα or ERβ), and changes the mRNA expression of the genes that produce GnRH and LH in the hypothalamus or the pituitary, respectively [7,8,9]. However, E2 also suppresses LH production in a rapid, nongenomic manner within the pituitary via binding to the G protein-coupled receptor 30 (GPR30; or G protein-coupled estrogen receptor 1) [10,11,12,13,14]. GPR30 can bind to E2 to initiate several rapid, nongenomic signaling events in the cytoplasm [15].
Significant effects of estrone and estriol
Estrone (E1) and estriol (E3) have been explained as “weak” estrogens compared to E2, with respect to genomic actions [16]. However, according to a recent study, both E1 and E3 have been shown to be strong estrogens in the nongenomic signaling pathways, and elicit functional responses in rat lactotroph-like cells [16]. Additionally, E1 exerts a direct, nongenomic action on rat aortic metabolism [17]. However, there are no reports on the nongenomic effects of E1 and E3 on LH secretion by gonadotrophs in animals, including women and rodents.
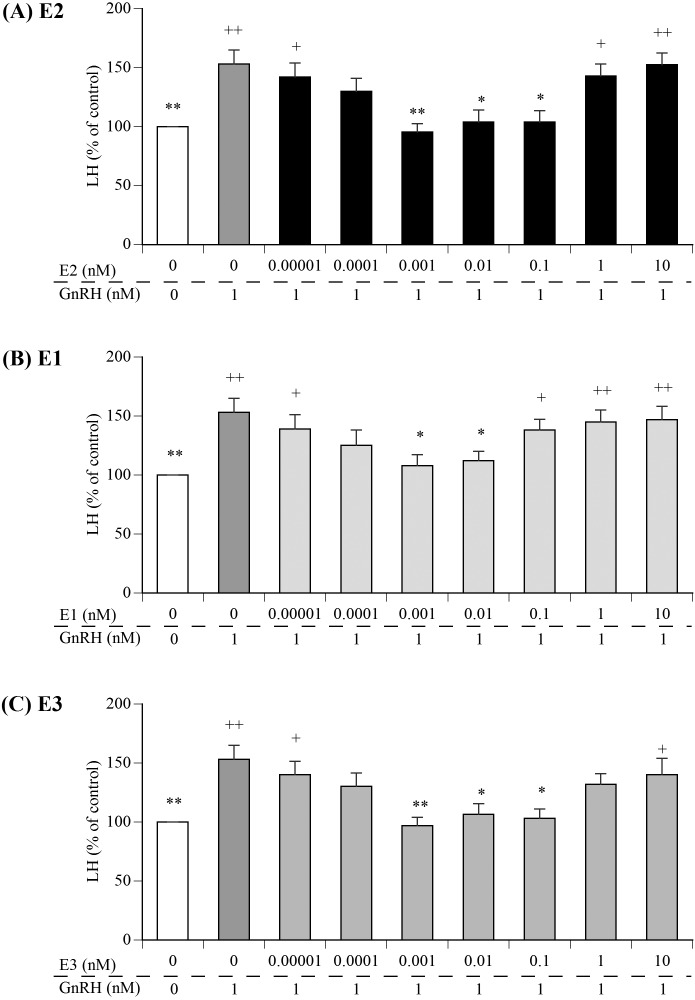
Therefore, we evaluated whether GPR30 mediated E1 and E3 to suppress GnRH-induced LH secretion from bovine pituitary [18]. As shown in Fig. 1, pre-treatment with picomolar levels, but not femtomolar or nanomolar levels, of E2, E1, or E3 suppressed the GnRH-induced LH secretion. The GPR30 antagonist (G36), but not ERα antagonist (MPP), abrogate such suppressing effects of picomolar levels of E2, E1, and E3 on LH secretion [20]. Therefore, E1 and E3, as well as E2, suppress GnRH-induced LH secretion from anterior pituitary cells in a nongenomic manner, via GPR30 binding (Fig. 2). Previous studies reported that in ruminants, the intramuscular injection of E3 induces an LH surge earlier than an E2 injection [19, 20]. The nongenomic effect of E1 on LH secretion may have an important role in controlling the number of ovulations in heifers treated for superovulation [21]. Therefore, we need to reconsider the functions of E1 and E3, and further studies are required to evaluate the functional significance of the findings, given that E2, E1, and E3 exert similar, nongenomic suppressive effects via GPR30 binding in gonadotrophs.
Fig. 1.
The effects of pretreatment with femtomolar, picomolar, or nanomolar levels of E2, E1, or E3 on GnRH-induced LH secretion from cultured bovine anterior pituitary cells (from Otsuka and Kadokawa [18]). + indicates P < 0.05, and ++ indicates P < 0.01 compared to the control (white bar); * indicates P < 0.05, and ** indicates P < 0.01 compared to GnRH alone (dark grey bar).
Fig. 2.
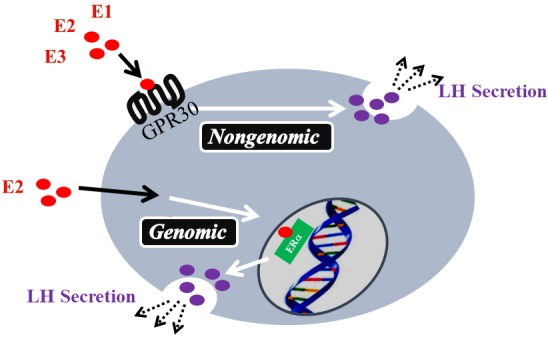
Genomic and nongenomic pathways controlling LH secretion from bovine gonadotrophs.
As shown in Fig. 1, both E1 and E3 can markedly affect the GnRH-induced LH secretion rapidly. Iqbal et al. [11] had suggested that E2 might have a biphasic effect on LH secretion by ovine gonadotropes in vivo, with a rapid suppression of LH release (negative feedback) initially, followed by a positive feedback event many hours later. Iqbal et al. [11] had also reported that E2 might activate the MAPK pathway for exerting its time-delayed positive feedback effect. Therefore, the nanomolar concentration of E1, E2, and E3 may have activated the MAPK pathway earlier than the picomolar concentrations, suggesting that both ligand dosage and duration of action are important for their suppressive effects on LH secretion by gonadotrophs.
Cytoplasmic pathway of GPR30
Unlike the cytoplasmic pathways for ERα and ERβ, which control gene expression in various cells, little is known about the cytoplasmic signaling pathway of activated GPR30. Few studies have recently revealed that protein kinase A (PKA) and phosphorylated extracellular signal-regulated kinase (pERK) are involved in the cytoplasmic signaling pathways of GPR30 in mouse trigeminal ganglia [22] and rat liver [23]. Within 15 min of E2 treatment, LH secretion from ovine AP decreases, and pERK levels increase [11, 24]. A GPR30 antagonist, G36, inhibits ERK phosphorylation by estrogen in SKBr3 cells [25]. Furthermore, we have previously reported that PKA and pERK are the intracellular mediators downstream of GPR30, which induce the nongenomic suppression of GnRH-induced LH secretion from bovine AP cells by E2 or GPR30-specific agonists [26].
Crosstalk between ERs and GPR30 in gonadotrophs
Whether GPR30 acts as an autonomous ER in vivo or interacts with nuclear estrogen receptor signaling pathways in response to estrogens is controversial. As reviewed by Romano and Gorelick [27], several lines of evidence support the role of GPR30 as an autonomous estrogen receptor in vivo. However, evidence supporting the interaction of GPR30 with ER signaling is also available. These reports suggest that GPER may either activate ER or form a complex with ER. Romano and Gorelick [27] concluded that the degree to which GPR30 influences nuclear estrogen receptor signaling likely depends on the cell type, developmental stage, and pathology. In our study, GPR30 antagonists, but not ERα antagonists, abrogated the suppressive effect of picomolar concentrations of E2, E1, and E3 on LH secretion from bovine gonadotrophs [18]. Our data suggested that GPR30 is an autonomous estrogen receptor that suppressed the GnRH-induced LH secretion from bovine gonadotrophs. However, any crosstalk between ER and GPR30 may have important roles in the nongenomic effects of E1, E2, and E3, which induce prolactin secretion from GH3/B6/F10 rat pituitary tumor cells [16]. Therefore, further studies are required to clarify the mechanism underlying the nongenomic effects of endogenous estrogens in ruminants and other species.
Lack of fundamental knowledge regarding E1 and E3
Little is known about blood E1 concentrations in domestic animals. The blood E1 concentration in cows is probably in the picomolar range during the estrous cycle, and increases to nanomolar levels during pregnancy [28]. However, we found no reports about blood E3 concentration in cows. In animals, E3 is synthesized from E1 and E2 [29, 30]. Thus, it is reasonable to assume that blood E3 concentrations in cows might follow the same trend as blood E1 concentrations. Although further studies are required to clarify the blood concentrations of E1 and E3 in domestic animals, the picomolar concentrations of E1 and E3, as well as E2, may exert similar negative feedback effects from the ovaries to gonadotrophs to control GnRH-induced LH suppression during the estrous cycle.
Xenoestrogens
We must be cautious that the GPR30 expressed on cells in various reproductive organs may bind xenoestrogens as well as endogenous estrogens. Xenoestrogens include plant estrogens, mycoestrogens, and environmental estrogens.
Plants have chemical mechanisms to defend against attacks by animals, including insects and vertebrate herbivores. Phytochemical options exist by which plants can modulate the fertility of herbivores [31]. Phytoestrogens are most well-known group of phytochemical mimics of vertebrate reproductive hormones [32]. However, little is known about whether phytoestrogens are GPR30 ligands. A previous study demonstrated that the phytoestrogen genistein is a GPR30 ligand in human periodontal ligament cells [33]. Although phytoestrogens have been studied in menopausal women, many of their effects remain unstudied in both human and domestic animals, as reviewed by other authors [34, 35].
α-Zearalanol (or Zeranol; α-ZAL) is a non-steroidal estrogenic compound, derived from zearalenone (ZEN), the mycoestrogen produced by Fusarium [36]. It has been established that α-ZAL implantation delays puberty, increases the incidence of non-ovulatory estrus, and retards reproductive tract development in heifers [37]. Previous studies also indicate that in ruminants, α-ZAL suppresses LH secretion in a rapid, nongenomic manner in vivo [38,39,40,41].
We previously reported that ZEN and five known ZEN metabolites rapidly suppressed GnRH-induced LH secretion, by decreasing cytoplasmic cyclic adenosine monophosphate without decreasing expression of the LHα and LHβ subunits [14, 42]. It is important to note that α-ZAL has been detected in cereal grains and animal feeds [43], cattle urine, and ruminant bodies worldwide [44].
In our study, we estimated that ZEN had the greatest nongenomic inhibiting effect on LH secretion in terms of magnitude and effective concentration range, followed by α-ZAL, zearalanone (ZAN), α-zearalenol (α-ZOL), β-zearalenol (β-ZOL), and β-zearalanol (β-ZAL) [42]. It is important to note that the relative strengths of the nongenomic inhibiting effects caused by each ZEN analog differ from the reported relative strengths of their genomic effects measured by the MCF7 human breast cell proliferation assay [45], as summarized in Fig. 3.
Fig. 3.
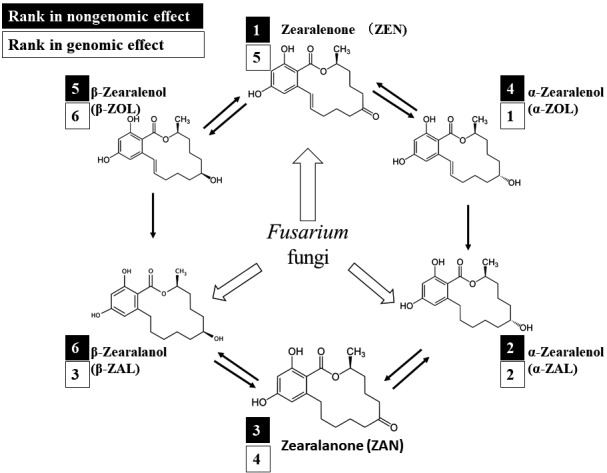
. Relative strengths of the nongenomic inhibiting effects of ZEN analogs on bovine gonadotroph LH secretion (white font in black boxes) [42] and genomic effects of the ZEN analogs measured by the MCF7 human breast cell proliferation assay (black font in white boxes) [45]. White and black arrows indicate mycoestrogens synthesized by the Fusarium fungi and their metabolic pathways according to Erasmuson et al. [44], respectively.
Biehl et al. reported that ZEN administered orally was rapidly and efficiently absorbed and metabolized by pigs, reaching maximum plasma concentrations within 2 or 3 h [46]. These authors also reported that 45% of the administered ZEN was excreted in bile, of which 85% was reabsorbed into the systemic circulation [45]. It is unknown whether ZEN analogs are subject to this type of “enterohepatic circulation” in ruminants. However, E2 undergoes enterohepatic circulation [47]; the bile of ruminants contains ZEN, α-ZOL, and β-ZOL [48]. The half-life for the elimination of ZEN from the blood of ruminants is quite long (28.6 h) [49]. Therefore, ZEN remains in ruminant bodies fed with ZEN-contaminated feed for long periods; it is possible that ZEN concentrations attain values at which LH secretion is inhibited in vivo.
Thomas and Dong [50] reported that bisphenol A (BPA) and nonylphenol, both well-known environmental estrogens and endocrine disruptors, imperfectly mimic the effects of physiologic estrogens via binding to ERα, ERβ, and GPR30. These authors also reported that the binding affinity of BPA and ZEN to GPR30 is less than 3% relative to that of E2, and that BPA shows a higher affinity to GPR30 than to ERα and ERβ. If both ligand dosage and duration of action are important for the suppressive effect on LH secretion by gonadotrophs, the low affinity of xenoestrogens to GPR30 may modulate the duration of action to allow the biphasic effect on LH secretion by gonadotrophs. Thus, there is a need to test the hypothesis that the rapid negative feedback from low-affinity xenoestrogens may appear before the effects of E2, while the positive feedback may appear after the effects of E2.
Bisphenol S (BPS) is an alternative to BPA in plastic consumer products and thermal paper. However, Viñas and Watson [51] reported that BPS, once considered a safe substitute for BPA, disrupts membrane-initiated E2-induced cell signaling, leading to altered proliferation, cell death, and prolactin release in a rat cell line. Substituting BPA with BPS in consumer products is a fast process, especially in the U.S. [52]; BPS is now ubiquitous in the environment (including water, sediment, sludge, indoor dust and air, consumer products, food, and human urine) worldwide [52]. Recent studies have demonstrated that BPS may have adverse effects on the reproductive, endocrine, and nervous systems of animals and humans, and may trigger oxidative stress [52]. Further studies are required to clarify the effects of environmental estrogens mediated by GPR30 in domestic animals.
GPR30 is expressed in various reproductive organs
Recently, GPR30 expression has been shown to be important in various reproductive functions such as testosterone production in human testes [53], lordosis behavior in rats [54], SBD-1 gene expression in ovine oviduct epithelial cells [55], mouse oocyte maturation [56], and mediation of the direct effects of E2 in immortalized GnRH neurons [57]. The GPR30 gene is expressed in the granulosa and theca cells, although its role in the ovary is not yet clear [58]. GPR30 rapidly stimulates GnRH secretion from GnRH neurons [59]. Therefore, further studies are required to evaluate the hypothesis that various endogenous estrogens and xenoestrogens are risk factors for reproductive suppression in domestic animals.
Species difference in GPR30
As mentioned previously, further studies are required to clarify the physiological significance of E1 and E2 in other animals. Matthews et al. [60] reported that ERs from different species exhibit differential ligand preferences and relative binding affinities for estrogenic compounds due to the differences in their amino acid sequences. The GPR30 gene is conserved in Homo (H.) sapiens, Macaca (M.) mulatta, Bos (B.) taurus, Canis (C.) lupus, Mus (M.) musculus, Rattus (R.) norvegicus, Gallus (G.) gallus, Xenopus (X.) tropicalis, and Danio (D.) rerio (https://www.ncbi.nlm.nih.gov/homologene/15855). The amino acid sequence of B. taurus GPR30 (XP_002698215.1) has 7 transmembrane helices, similar to the GPR30 of other animals (determined by the SOSUI algorithm; http://harrier.nagahama-i-bio.ac.jp/sosui/). However, the pairwise alignment scores of GPR30 of H. sapiens with that of B. taurus is lower (84.6% of identity) than that with those of other mammals [M. mulatta (98.1%), C. lupus (89.7%), M. musculus (86.9%), and R. norvegicus (86.1%)]. This could be because the amino acid sequences of the extracellular N-terminus region of GPR30 of different species differ substantially from each other. Therefore, it can be assumed that GPR30 plays a wide variety of roles in different organs of carnivores, omnivores, and herbivores that consume large quantities of phytoestrogens.
Conclusion
Endogenous estrogens and xenoestrogens may play important roles in the reproduction process via binding to GPR30 in various cells; however, little is known about the mechanism by which activated GPR30 mediates these effects. Therefore, we need to reconsider the roles of endogenous estrogens and xenoestrogens in different cells in the reproduction process of various species.
Acknowledgments
We mourn the loss of Dr Faidiban O Rudolf. We are grateful to Ms Ayumi Murakami, Ms Haruna Kubo, Ms Urara Nakamura, and Ms Midori Otsuka for their assistance.
References
- 1.Endo N, Nagai K, Tanaka T, Kamomae H. Comparison between lactating and non-lactating dairy cows on follicular growth and corpus luteum development, and endocrine patterns of ovarian steroids and luteinizing hormone in the estrous cycles. Anim Reprod Sci 2012; 134: 112–118. [DOI] [PubMed] [Google Scholar]
- 2.Spicer LJ, Echternkamp SE. Ovarian follicular growth, function and turnover in cattle: a review. J Anim Sci 1986; 62: 428–451. [DOI] [PubMed] [Google Scholar]
- 3.Clarke IJ. Evidence that the switch from negative to positive feedback at the level of the pituitary gland is an important timing event for the onset of the preovulatory surge in LH in the ewe. J Endocrinol 1995; 145: 271–282. [DOI] [PubMed] [Google Scholar]
- 4.Evans NP, Dahl GE, Glover BH, Karsch FJ. Central regulation of pulsatile gonadotropin-releasing hormone (GnRH) secretion by estradiol during the period leading up to the preovulatory GnRH surge in the ewe. Endocrinology 1994; 134: 1806–1811. [DOI] [PubMed] [Google Scholar]
- 5.García-Galiano D, Pinilla L, Tena-Sempere M. Sex steroids and the control of the Kiss1 system: developmental roles and major regulatory actions. J Neuroendocrinol 2012; 24: 22–33. [DOI] [PubMed] [Google Scholar]
- 6.Mercer JE, Phillips DJ, Clarke IJ. Short-term regulation of gonadotropin subunit mRNA levels by estrogen: studies in the hypothalamo-pituitary intact and hypothalamo-pituitary disconnected ewe. J Neuroendocrinol 1993; 5: 591–596. [DOI] [PubMed] [Google Scholar]
- 7.Gieske MC, Kim HJ, Legan SJ, Koo Y, Krust A, Chambon P, Ko C. Pituitary gonadotroph estrogen receptor-alpha is necessary for fertility in females. Endocrinology 2008; 149: 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hewitt SC, Korach KS. Oestrogen receptor knockout mice: roles for oestrogen receptors alpha and beta in reproductive tissues. Reproduction 2003; 125: 143–149. [DOI] [PubMed] [Google Scholar]
- 9.Sánchez-Criado JE, Trudgen K, Millán Y, Blanco A, Monterde J, Garrido-Gracia JC, Gordon A, Aguilar R, de Las Mulas JM, Ko C. Estrogen receptor (ESR) 2 partially offsets the absence of ESR1 in gonadotropes of pituitary-specific Esr1 knockout female mice. Reproduction 2012; 143: 549–558. [DOI] [PubMed] [Google Scholar]
- 10.Arreguin-Arevalo JA, Nett TM. A nongenomic action of 17beta-estradiol as the mechanism underlying the acute suppression of secretion of luteinizing hormone. Biol Reprod 2005; 73: 115–122. [DOI] [PubMed] [Google Scholar]
- 11.Iqbal J, Latchoumanin O, Clarke IJ. Rapid in vivo effects of estradiol-17beta in ovine pituitary gonadotropes are displayed by phosphorylation of extracellularly regulated kinase, serine/threonine kinase, and 3,5-cyclic adenosine 5-monophosphate-responsive element-binding protein. Endocrinology 2007; 148: 5794–5802. [DOI] [PubMed] [Google Scholar]
- 12.Rudolf FO, Kadokawa H. Expression of estradiol receptor, GPR30, in bovine anterior pituitary and effects of GPR30 agonist on GnRH-induced LH secretion. Anim Reprod Sci 2013; 139: 9–17. [DOI] [PubMed] [Google Scholar]
- 13.Rudolf FO, Kadokawa H. Effects of STX, a novel estrogen membrane receptor agonist, on GnRH-induced luteinizing hormone secretion from cultured bovine anterior pituitary cells. J Vet Med Sci 2014; 76: 1623–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura U, Rudolf FO, Pandey K, Kadokawa H. The non-steroidal mycoestrogen zeranol suppresses luteinizing hormone secretion from the anterior pituitary of cattle via the estradiol receptor GPR30 in a rapid, non-genomic manner. Anim Reprod Sci 2015; 156: 118–127. [DOI] [PubMed] [Google Scholar]
- 15.Maggiolini M, Picard D. The unfolding stories of GPR30, a new membrane-bound estrogen receptor. J Endocrinol 2010; 204: 105–114. [DOI] [PubMed] [Google Scholar]
- 16.Watson CS, Jeng YJ, Kochukov MY. Nongenomic actions of estradiol compared with estrone and estriol in pituitary tumor cell signaling and proliferation. FASEB J 2008; 22: 3328–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selles J, Polini N, Alvarez C, Massheimer V. Novel action of estrone on vascular tissue: regulation of NOS and COX activity. Steroids 2005; 70: 251–256. [DOI] [PubMed] [Google Scholar]
- 18.Otsuka M, Kadokawa H. GPR30 mediates estrone, estriol, and estradiol to suppress gonadotropin-releasing hormone-induced luteinizing hormone secretion in the anterior pituitary of heifers. J Reprod Dev 2017; 63: 519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forrest DW, Kaltenbach CC, Dunn TG. Estriol- and estradiol-17 beta-induced luteinizing hormone release in ovariectomized cows and ewes. J Anim Sci 1981; 52: 1106–1113. [DOI] [PubMed] [Google Scholar]
- 20.Schoenemann HM, Humphrey WD, Crowder ME, Nett TM, Reeves JJ. Pituitary luteinizing hormone-releasing hormone receptors in ovariectomized cows after challenge with ovarian steroids. Biol Reprod 1985; 32: 574–583. [DOI] [PubMed] [Google Scholar]
- 21.Saumande J, Lopez-Sebastian A. Changes in the plasma concentrations of free and conjugated oestrogens in heifers after treatment to induce superovulation and the relationship with number of ovulations. J Reprod Fertil 1982; 66: 411–416. [DOI] [PubMed] [Google Scholar]
- 22.Yue J, Zhang Y, Li X, Gong S, Tao J, Jiang X. Activation of G-protein-coupled receptor 30 increases T-type calcium currents in trigeminal ganglion neurons via the cholera toxin-sensitive protein kinase A pathway. Pharmazie 2014; 69: 804–808. [PubMed] [Google Scholar]
- 23.Zucchetti AE, Barosso IR, Boaglio AC, Basiglio CL, Miszczuk G, Larocca MC, Ruiz ML, Davio CA, Roma MG, Crocenzi FA, Pozzi EJ. G-protein-coupled receptor 30/adenylyl cyclase/protein kinase A pathway is involved in estradiol 17ß-D-glucuronide-induced cholestasis. Hepatology 2014; 59: 1016–1029. [DOI] [PubMed] [Google Scholar]
- 24.Iqbal J, Latchoumanin O, Sari IP, Lang RJ, Coleman HA, Parkington HC, Clarke IJ. Estradiol-17beta inhibits gonadotropin-releasing hormone-induced Ca2+ in gonadotropes to regulate negative feedback on luteinizing hormone release. Endocrinology 2009; 150: 4213–4220. [DOI] [PubMed] [Google Scholar]
- 25.Dennis MK, Field AS, Burai R, Ramesh C, Petrie WK, Bologa CG, Oprea TI, Yamaguchi Y, Hayashi S, Sklar LA, Hathaway HJ, Arterburn JB, Prossnitz ER. Identification of a GPER/GPR30 antagonist with improved estrogen receptor counterselectivity. J Steroid Biochem Mol Biol 2011; 127: 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudolf FO, Kadokawa H. Cytoplasmic kinases downstream of GPR30 suppress gonadotropin-releasing hormone (GnRH)-induced luteinizing hormone secretion from bovine anterior pituitary cells. J Reprod Dev 2016; 62: 65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romano SN, Gorelick DA. Crosstalk between nuclear and G protein-coupled estrogen receptors. Gen Comp Endocrinol 2018; 261: 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel OV, Takenouchi N, Takahashi T, Hirako M, Sasaki N, Domeki I. Plasma oestrone and oestradiol concentrations throughout gestation in cattle: relationship to stage of gestation and fetal number. Res Vet Sci 1999; 66: 129–133. [DOI] [PubMed] [Google Scholar]
- 29.Thomas MP, Potter BV. The structural biology of oestrogen metabolism. J Steroid Biochem Mol Biol 2013; 137: 27–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright JV, Schliesman B, Robinson L. Comparative measurements of serum estriol, estradiol, and estrone in non-pregnant, premenopausal women; a preliminary investigation. Altern Med Rev 1999; 4: 266–270. [PubMed] [Google Scholar]
- 31.Hughes CL., Jr. Phytochemical mimicry of reproductive hormones and modulation of herbivore fertility by phytoestrogens. Environ Health Perspect 1988; 78: 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams NR. Detection of the effects of phytoestrogens on sheep and cattle. J Anim Sci 1995; 73: 1509–1515. [DOI] [PubMed] [Google Scholar]
- 33.Luo LJ, Liu F, Lin ZK, Xie YF, Xu JL, Tong QC, Shu R. Genistein regulates the IL-1 beta induced activation of MAPKs in human periodontal ligament cells through G protein-coupled receptor 30. Arch Biochem Biophys 2012; 522: 9–16. [DOI] [PubMed] [Google Scholar]
- 34.Abdi F, Alimoradi Z, Haqi P, Mahdizad F. Effects of phytoestrogens on bone mineral density during the menopause transition: a systematic review of randomized, controlled trials. Climacteric 2016; 19: 535–545. [DOI] [PubMed] [Google Scholar]
- 35.Wocławek-Potocka I, Mannelli C, Boruszewska D, Kowalczyk-Zieba I, Waśniewski T, Skarżyński DJ. Diverse effects of phytoestrogens on the reproductive performance: cow as a model. Int J Endocrinol 2013; 2013: 650984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minervini F, DellAquila ME. Zearalenone and reproductive function in farm animals. Int J Mol Sci 2008; 9: 2570–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moran C, Prendiville DJ, Quirke JF, Roche JF. Effects of oestradiol, zeranol or trenbolone acetate implants on puberty, reproduction and fertility in heifers. J Reprod Fertil 1990; 89: 527–536. [DOI] [PubMed] [Google Scholar]
- 38.Elsasser TH, Bolt DJ, Bradley BD, Roper M. Acute and chronic changes in adenohypophyseal hormone secretion in sheep during zeranol administration. Am J Vet Res 1983; 44: 1068–1071. [PubMed] [Google Scholar]
- 39.Elsasser TH, Bolt DJ, Bradley BD, Roper M. Luteinizing hormone, follicle stimulating hormone and prolactin secretion in ewes and wethers after zeranol or estradiol injection. J Anim Sci 1983; 57: 443–448. [DOI] [PubMed] [Google Scholar]
- 40.Cooper RA. Some aspects of the use of the growth promoter zeranol in ewe lambs retained for breeding. III. Effect on plasma LH levels. Br Vet J 1985; 141: 424–426. [DOI] [PubMed] [Google Scholar]
- 41.Fabry J, Renaville R, Halleux V, Burny A. Plasma testosterone and LH responses to LHRH in double-muscled bulls treated with trenbolone acetate and zeranol. J Anim Sci 1983; 57: 1138–1145. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura U, Kadokawa H. The nonsteroidal mycoestrogen zearalenone and its five metabolites suppress LH secretion from the bovine anterior pituitary cells via the estradiol receptor GPR30 in vitro. Theriogenology 2015; 84: 1342–1349. [DOI] [PubMed] [Google Scholar]
- 43.Cortinovis C, Pizzo F, Spicer LJ, Caloni F. Fusarium mycotoxins: effects on reproductive function in domestic animalsa review. Theriogenology 2013; 80: 557–564. [DOI] [PubMed] [Google Scholar]
- 44.Erasmuson AF, Scahill BG, West DM. Natural zeranol (alpha-zearalanol) in the urine of pasture-fed animals. J Agric Food Chem 1994; 42: 2721–2725. [Google Scholar]
- 45.Shier WT, Shier AC, Xie W, Mirocha CJ. Structure-activity relationships for human estrogenic activity in zearalenone mycotoxins. Toxicon 2001; 39: 1435–1438. [DOI] [PubMed] [Google Scholar]
- 46.Biehl ML, Prelusky DB, Koritz GD, Hartin KE, Buck WB, Trenholm HL. Biliary excretion and enterohepatic cycling of zearalenone in immature pigs. Toxicol Appl Pharmacol 1993; 121: 152–159. [DOI] [PubMed] [Google Scholar]
- 47.Hoffmann B, Goes de Pinho T, Schuler G. Determination of free and conjugated oestrogens in peripheral blood plasma, feces and urine of cattle throughout pregnancy. Exp Clin Endocrinol Diabetes 1997; 105: 296–303. [DOI] [PubMed] [Google Scholar]
- 48.Dänicke S, Keese C, Meyer U, Starke A, Kinoshita A, Rehage J. Zearalenone (ZEN) metabolism and residue concentrations in physiological specimens of dairy cows exposed long-term to ZEN-contaminated diets differing in concentrate feed proportions. Arch Anim Nutr 2014; 68: 492–506. [DOI] [PubMed] [Google Scholar]
- 49.Dong M, He XJ, Tulayakul P, Li JY, Dong KS, Manabe N, Nakayama H, Kumagai S. The toxic effects and fate of intravenously administered zearalenone in goats. Toxicon 2010; 55: 523–530. [DOI] [PubMed] [Google Scholar]
- 50.Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol 2006; 102: 175–179. [DOI] [PubMed] [Google Scholar]
- 51.Viñas R, Watson CS. Bisphenol S disrupts estradiol-induced nongenomic signaling in a rat pituitary cell line: effects on cell functions. Environ Health Perspect 2013; 121: 352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu LH, Zhang XM, Wang F, Gao CJ, Chen D, Palumbo JR, Guo Y, Zeng EY. Occurrence of bisphenol S in the environment and implications for human exposure: A short review. Sci Total Environ 2018; 615: 87–98. [DOI] [PubMed] [Google Scholar]
- 53.Vaucher L, Funaro MG, Mehta A, Mielnik A, Bolyakov A, Prossnitz ER, Schlegel PN, Paduch DA. Activation of GPER-1 estradiol receptor downregulates production of testosterone in isolated rat Leydig cells and adult human testis. PLoS ONE 2014; 9: e92425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anchan D, Gafur A, Sano K, Ogawa S, Vasudevan N. Activation of the GPR30 receptor promotes lordosis in female mice. Neuroendocrinology 2014; 100: 71–80. [DOI] [PubMed] [Google Scholar]
- 55.Wen S, Cao G, Bao T, Cheng L, Li H, Du C, Tu Y, Li Q, Jian R, Zhao P, Wuriliga Modulation of ovine SBD-1 expression by 17beta-estradiol in ovine oviduct epithelial cells. BMC Vet Res 2012; 8: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li YR, Ren CE, Zhang Q, Li JC, Chian RC. Expression of G protein estrogen receptor (GPER) on membrane of mouse oocytes during maturation. J Assist Reprod Genet 2013; 30: 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacobi JS, Martin C, Nava G, Jeziorski MC, Clapp C, Martínez de la Escalera G. 17-Beta-estradiol directly regulates the expression of adrenergic receptors and kisspeptin/GPR54 system in GT17 GnRH neurons. Neuroendocrinology 2007; 86: 260–269. [DOI] [PubMed] [Google Scholar]
- 58.Wang C, Prossnitz ER, Roy SK. Expression of G protein-coupled receptor 30 in the hamster ovary: differential regulation by gonadotropins and steroid hormones. Endocrinology 2007; 148: 4853–4864. [DOI] [PubMed] [Google Scholar]
- 59.Terasawa E, Noel SD, Keen KL. Rapid action of oestrogen in luteinising hormone-releasing hormone neurones: the role of GPR30. J Neuroendocrinol 2009; 21: 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matthews J, Celius T, Halgren R, Zacharewski T. Differential estrogen receptor binding of estrogenic substances: a species comparison. J Steroid Biochem Mol Biol 2000; 74: 223–234. [DOI] [PubMed] [Google Scholar]