ABSTRACT
Mosquito-borne flaviviruses are a group of RNA viruses that constitute global threats for human and animal health. Replication of these pathogens is strictly dependent on cellular lipid metabolism. We have evaluated the effect of the pharmacological activation of AMP-activated protein kinase (AMPK), a master regulator of lipid metabolism, on the infection of three medically relevant flaviviruses, namely, West Nile virus (WNV), Zika virus (ZIKV), and dengue virus (DENV). WNV is responsible for recurrent outbreaks of meningitis and encephalitis, affecting humans and horses worldwide. ZIKV has caused a recent pandemic associated with birth defects (microcephaly), reproductive disorders, and severe neurological complications (Guillain-Barré syndrome). DENV is the etiological agent of the most prevalent mosquito-borne viral disease, which can induce a potentially lethal complication called severe dengue. Our results showed, for the first time, that activation of AMPK using the specific small molecule activator PF-06409577 reduced WNV, ZIKV, and DENV infection. This antiviral effect was associated with an impairment of viral replication due to the modulation of host cell lipid metabolism exerted by the compound. These results support that the pharmacological activation of AMPK, which currently constitutes an important pharmacological target for human diseases, could also provide a feasible approach for broad-spectrum host-directed antiviral discovery.
KEYWORDS: AMPK, West Nile virus, Zika virus, antiviral agents, dengue fever, flavivirus, lipid synthesis
INTRODUCTION
Climate change, increase in global travel and trade, and urbanization are facilitating the spread of pathogenic mosquito-borne flaviviruses. Flaviviruses (genus Flavivirus, family Flaviviridae) are single-stranded, positive-sense RNA viruses responsible for a variety of illnesses that range from mild flu-like symptoms to birth defects or severe neurological diseases and hemorrhagic fevers (1). Examples of flavivirus-caused diseases are yellow fever, dengue, West Nile fever, and Zika congenital syndrome. Currently there are no specific antiviral therapies licensed for any flavivirus. However, the therapeutic victory of antivirals against Hepacivirus (2), a genus within Flaviviridae closely related to flaviviruses, reinforces the future of medicinal chemistry for the development of effective antivirals against flaviviruses. Here, we have focused on three medically relevant mosquito-borne flaviviruses, namely, West Nile virus (WNV), Zika virus (ZIKV), and dengue virus (DENV). The first (WNV), is responsible for recurrent outbreaks of febrile illness, meningitis, encephalitis, and acute flaccid paralysis, affecting humans and horses worldwide (3), and it constitutes the leading cause of arboviral encephalitis in the United States (4). The second (ZIKV), has caused a recent pandemic associated with birth defects (microcephaly), testis damage, male infertility, and severe neurological diseases, such as Guillain-Barré syndrome (5). The third (DENV), is the etiological agent of the most prevalent mosquito-borne viral disease, causing about 100 million cases of disease each year (6).
An alternative approach for the development of antiviral therapies, including those to combat flaviviruses, is the search for drugs that target the host cell machinery components that are coopted by the viruses to complete their life cycle (7–9). This strategy should entail a higher genetic barrier against the development of viral resistance relative to that in the case of directly acting antivirals targeted against the virus. Because the same host factors are usually coopted by closely related viruses, this approach also could lead to the identification of potential broad-spectrum antivirals (10). The flavivirus life cycle is strictly dependent on host cell lipid metabolism. Specifically, flavivirus infection requires fatty acid and cholesterol biosynthesis for membrane rearrangements and proper development of viral replication complexes (11, 12). Moreover, sphingolipid metabolism is also important for flavivirus entry (13, 14), replication (15, 16), and particle biogenesis (13, 17). Hence, lipid-targeting antiviral strategies are currently being explored as an alternative host-directed approach to fight flaviviruses (18, 19). Accordingly, the blockage of fatty acid synthesis through inhibition of fatty acid synthase (FASN) or acetyl coenzyme A (acetyl-CoA) carboxylase (ACC), as well as the inhibition of sphingolipid metabolism or cholesterol biosynthesis, reduces flavivirus multiplication (11–13, 15–17, 20, 21). In this context, AMP-activated protein kinase (AMPK) has been recently involved in flavivirus infection through regulation of lipid metabolism (22–24). AMPK is a heterotrimeric enzyme that constitutes a master regulator of lipid metabolism (25), and its activation positively regulates fatty acid oxidation, whereas it negatively regulates lipid synthesis, becoming an attractive therapeutic target for the treatment of multiple human diseases (26, 27). For instance, there is supporting evidence that for DENV and ZIKV, the activation of this enzyme impairs viral replication (22, 23). On the contrary, another study supports that DENV infection is reduced by inhibition of this enzyme (24).
In this work, we evaluated the effect of 6-chloro-5-[4-(1-hydroxycyclobutyl)phenyl]-1H-indole-3-carboxylic acid (PF-06409577), a selective activator of AMPK (28, 29), on WNV, ZIKV, and DENV infections. Our results show an antiviral effect of this AMPK activator against these three medically relevant flaviviruses. The antiviral effect was presumably associated with the modulation of host cell lipid metabolism exerted by the compound. These results support that the activation of this protein kinase could constitute a feasible target for host-directed antiviral discovery.
RESULTS
Impact of AMPK activation by PF-06409577 on host cell lipid metabolism.
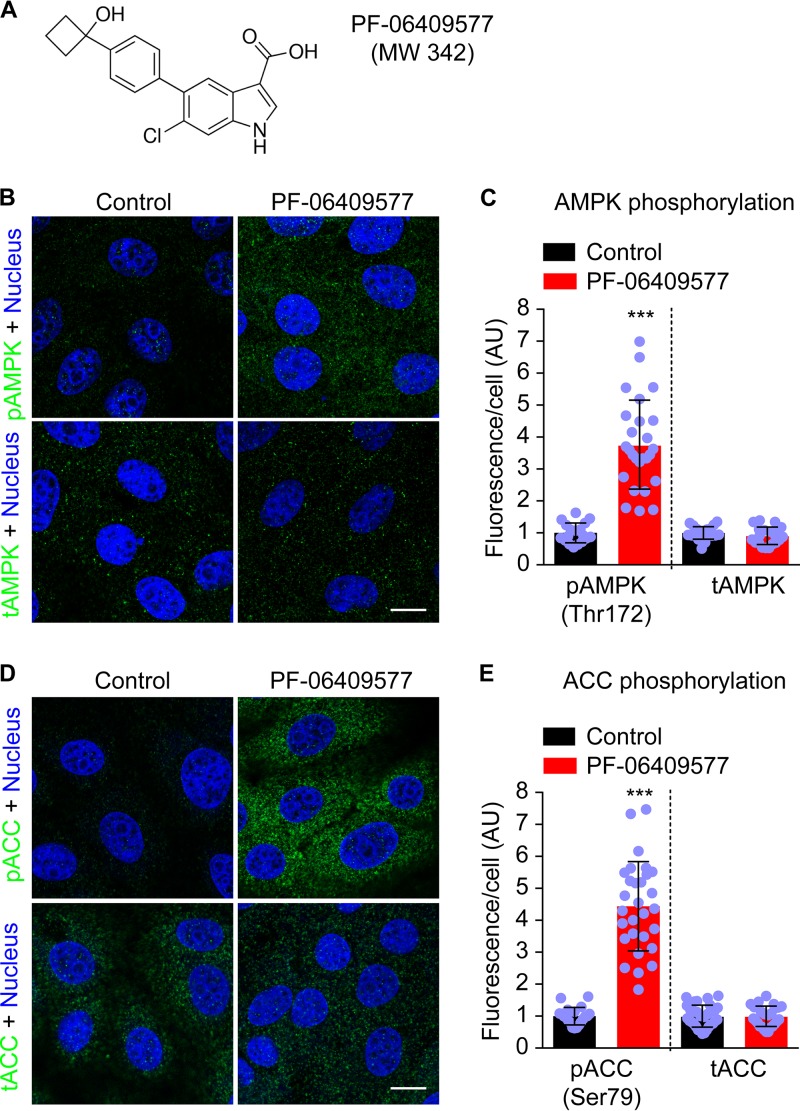
The phosphorylation of a key threonine (Thr172) of the catalytic (α) subunit of AMPK (pAMPK) has been shown to increase the kinase activity of AMPK by 500- to 1000-fold (30, 31). PF-06409577 is a recently developed direct small molecule activator of AMPK that increases AMPK activity through promoting phosphorylation of this residue (28). Therefore, the degree of phosphorylation of Thr172 of the catalytic subunit of AMPK (AMPKα) was investigated in Vero cells treated with this compound. Immunofluorescence analysis showed that, as expected, treatment with PF-06409577 (Fig. 1A) resulted in a significant increase of the cellular pAMPK (Thr172) without affecting total AMPK (tAMPK) levels (Fig. 1B and C). One of the major target proteins of AMPK is the acetyl-CoA carboxylase (ACC). Remarkably, ACC activity is necessary for flavivirus multiplication because this enzyme catalyzes the pivotal step of the fatty acid synthesis pathway and hence regulates host cell lipid metabolism (21). Considering that the phosphorylation of ACC at serine 79 (Ser79) by AMPK inhibits the enzymatic activity of ACC (32), the levels of phosphorylated (Ser79) ACC (pACC) were investigated in cells treated with PF-06409577. AMPK activation by PF-06409577 resulted in an increase in the phosphorylation of ACC without affecting the levels of total ACC (tACC), supporting an inhibition of ACC activity in PF-06409577-treated cells (Fig. 1D and E).
FIG 1.
Treatment with PF-06409577 results in the phosphorylation of AMPK and ACC. (A) Chemical structure of compound PF-06409577. MW, molecular weight. (B) Immunofluorescence detection of the phosphorylated (Thr 172) catalytic (α) subunit of AMPK (pAMPK) and total AMPK (tAMPK) in Vero cells treated or not treated (control) with PF-06409577 (50 μg/ml) for 24 h. pAMPK or tAMPK are shown in green and cell nuclei in blue. (C) Quantification of the fluorescence of pAMPK and tAMPK in cells treated as described in panel B. (D) Immunofluorescence detection of phosphorylated (Ser79) ACC (pACC) and total ACC (tACC) in cells treated or not treated as described in panel B. pACC and tACC are shown in green and cell nuclei in blue. (E) Quantification of the fluorescence of pACC and tACC in cells treated as described in panel D. (C and E) Data are presented as mean ± SD (n = 20 to 41). Points represent individual cells analyzed. AU, arbitrary units. Bar, 10 μm. In the case of different variances, two-tailed Student's t test P values between control and treated cells were calculated applying Welch's correction. ***, P < 0.005.
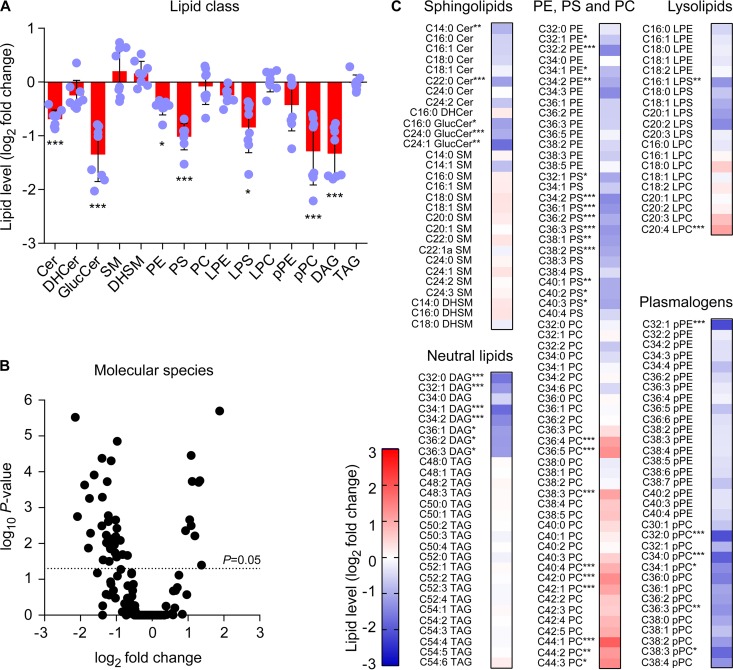
To evaluate the effect of AMPK activation by PF-06409577 on host cell lipids, Vero cells were treated with the compound, and their lipid content was analyzed by mass spectrometry and compared to that of control cells treated with the same amount of drug solvent. In these analyses, we included 15 lipid classes (5 sphingolipids, 8 glycerophospholipids, and 2 neutral lipids) (Fig. 2A). Treatment with PF-06409577 provoked a reduction in multiple lipid classes, being statistically significant for several sphingolipids (ceramide [Cer] and glucosylceramide [GlucCer]), glycerophospholipids (phosphatidylethanolamine [PE], phosphatidylserine [PS], lyso-phosphatidylserine [LPS], and plasmalogen-phosphatidylcholine [pPC]), and one neutral lipid (diacylglycerol [DAG]). From the 171 molecular species identified in the analysis, 43 (25%) were significantly altered (Fig. 2B and C). Most of them (33 molecular species), including 5 sphingolipids (Cer and GlucCer), 21 glycerophospholipids (PE, PS, LPS, plasmalogen-PE [pPE], and pPC), and 7 neutral lipids (DAG) were reduced in PF-06409577 cells. On the contrary, only 9 PC and 1 lyso-PC (LPC) were significantly elevated in cells treated with the compound. Several lipids that were significantly altered in cells treated with PF-0409577, such as Cer and glycerophospholipids, have been previously demonstatrated to be involved in different steps of the flavivirus life cycle (16, 17, 33). In this way, these results support that the modulation of host cell lipid metabolism exerted by PF-06409577 could interfere with flavivirus multiplication.
FIG 2.
Impact of PF-06409577 on cellular lipids. (A) Changes in the lipidome of Vero cells after 24 h of treatment with PF-06409577 (50 μg/ml). Fold change in lipid levels were calculated as the log2(PF-06409577/control) for each lipid class. Data are expressed as the mean ± SD (n = 8). Points represent independent replicates. (B) Volcano plot (corrected P value versus log2 fold change) for individual molecular species identified in the analysis. Dashed line indicates P = 0.05. (C) Heatmap displaying the effect of PF-06409577 at the individual lipid species analyzed. Red and blue denote an increase and a decrease in lipid species relative to control cells, respectively (n = 8). Student's t test P values were Sidak-Bonferroni corrected for multiple comparisons. *, P < 0.05; **, P < 0.01; ***, P < 0.005. For lipid abbreviations, see Materials and Methods.
AMPK activator PF-06409577 reduces flavivirus multiplication.
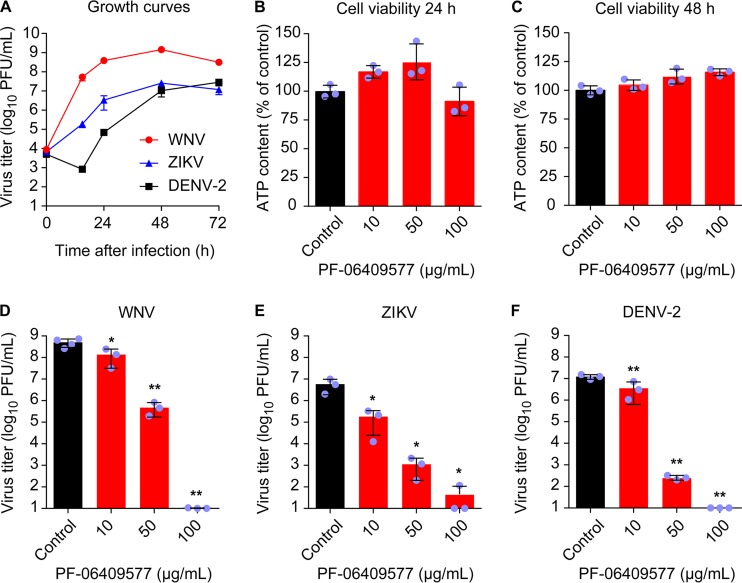
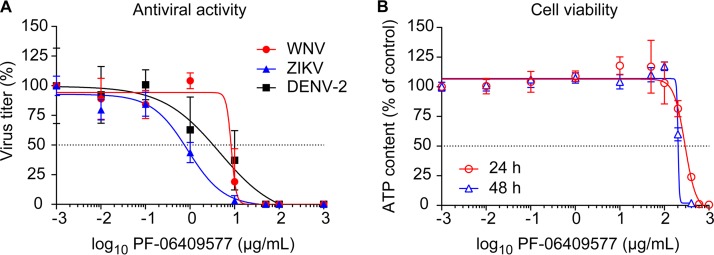
To analyze the effect of PF-06409577 on flavivirus infection, the highly neurovirulent WNV strain NY99, representative of the virus that is currently circulating in the American continent (34), ZIKV strain PA259459, isolated from a human patient in Panama in 2015 and representative of the recent ZIKV pandemic (35), and a DENV serotype 2 (DENV-2) isolate (36) were used. Based on single-step growth curves of the three viruses in Vero cells (Fig. 3A), we selected 24 h postinfection (p.i.) for further analysis of WNV and ZIKV and 48 h p.i. for analysis of DENV-2. The lack of toxicity of PF-06409577 was verified by quantification of cellular ATP content in Vero cells (Fig. 3B and C). Results showed no statistically significant reduction of the cellular ATP content at either 24 (Fig. 3B) or 48 h (Fig. 3C) posttreatment, excluding major cytotoxic effects of the drug within the interval of concentrations tested (0 to 100 μg/ml). Accordingly, cells were infected (multiplicity of infection [MOI] of 1 PFU/cell) and treated with PF-06409577 (0 to 100 μg/ml) at 1 h postinfection (h p.i.), and the virus yield in the supernatant of infected cultures was determined by plaque assay at 24 h p.i. for WNV and ZIKV and at 48 h p.i. for DENV-2. Treatment with PF-06409577 significantly reduced the virus yield in a dose-dependent manner for WNV (Fig. 3D), ZIKV (Fig. 3E), and DENV-2 (Fig. 3F). Overall, these results indicate that PF-06409577 is a potent inhibitor of flavivirus multiplication at concentrations that do not exert major cytotoxic effects.
FIG 3.
Treatment with AMPK activator PF-06409577 inhibits flavivirus infection. (A) Single-step growth curve of WNV, ZIKV, and DENV-2. Vero cells were infected with the corresponding flavivirus (MOI of 1 PFU/cell), and virus yield in supernatant was determined by plaque assay at the indicated times p.i. (B and C). Evaluation of the cytotoxicity of PF-06409577 on Vero cells by determination of ATP content at 24 h posttreatment (B) or 48 h posttreatment (C). (D, E and F) Reduction of WNV (D), ZIKV (E), and DENV-2 (F) infection in Vero cells treated with PF-06409577. Cells were infected with the corresponding flavivirus (MOI of 1 PFU/cell), and the drug was added 1 h after virus inoculation and maintained during the assay. Virus yield in supernatant was determined by plaque assay at 24 h p.i. for WNV and ZIKV or 48 h p.i. for DENV-2. Data are presented as mean ± SD (n = 3 or 4). Points represent independent biological replicates. Two-tailed Student's t test P values between control and each compound concentration were Bonferroni-corrected for multiple comparisons.*, P < 0.05; **, P < 0.01.
PF-06409577 reduces flavivirus replication.
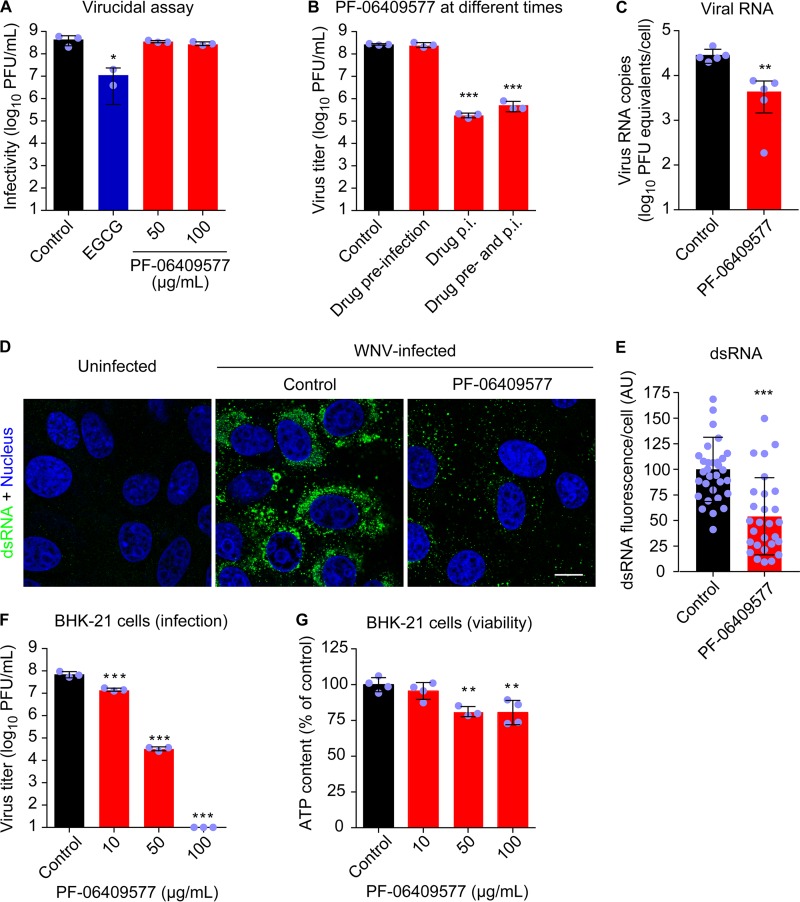
In order to identify the step of the flavivirus life cycle that was affected by AMPK activation, we selected WNV as a model. To exclude the possibility that the inhibition of flavivirus production was the result of a virucidal effect of the drug, the direct effect of the compound on the infectivity of WNV was evaluated (Fig. 4A). To this end, the virus was preincubated with the compound and then titrated to determine the remaining infectivity. (−)-Epigallocatechin gallate (EGCG), a drug with known virucidal effect against flaviviruses (36, 37), was included as a positive control for the assay. Whereas EGCG significantly reduced the infectivity of WNV, PF-06409577 did not affect the infectivity of the virus, supporting that the inhibitory effect of virus yield observed was not related to a virucidal effect of the compound (Fig. 4A). Cells were then treated with the drug at different times (1 h prior to infection, 1 h after virus inoculation, or from 1 h prior to infection and throughout the rest of the assay). Treatment of cells prior to infection did not reduce WNV multiplication, suggesting that the antiviral effect of PF-06409577 was produced at a postentry step (Fig. 4B). Thus, the effect of the compound on WNV replication was analyzed by quantitative reverse transcriptase PCR (RT-PCR) (Fig. 4C). Treatment with PF-06409577 significantly reduced the amount of cell-associated WNV RNA, supporting that the drug interfered with viral genome replication. Replication complex development and hence genome replication are linked to membrane rearrangements and lipid synthesis in flavivirus-infected cells (11, 17, 21). Accordingly, the presence of double-stranded RNA (dsRNA) intermediates, which provide well-characterized markers of flavivirus replication complexes (38), was investigated. Treatment with PF-06409577 significantly reduced the accumulation of dsRNA intermediates, further supporting impairment of flavivirus replication (Fig. 4D and E). The antiviral effect of PF-06409577 against WNV was also observed in BHK-21 cells, supporting that this compound worked as an antiviral agent in cell lines of different origin (Fig. 4F) at concentrations that did not exert major cytotoxic effects (Fig. 4G). Taken together, these results suggest that AMPK activation by PF-06409577 reduces flavivirus infection by interfering with replication complex development and genome replication.
FIG 4.
PF-06409577 inhibits replication of WNV. (A) Lack of virucidal effect of PF-06409577. WNV (∼4 × 108 PFU) was treated with EGCG (5 μg/ml), PF-06409577 (50 or 100 μg/ml), or drug vehicle (control) for 1 h at 37°C in culture medium. Then the infectivity in each sample was determined by plaque assay. EGCG was included in the experiments as a positive-control compound with virucidal activity (n = 2 or 3). (B) PF-06409577 inhibits WNV multiplication when added after virus infection. Vero cells were infected with WNV (MOI of 1 PFU/cell) and treated with PF-06409577 (50 μg/ml) only 1 h before virus inoculation (drug preinfection), 1 h after virus inoculation (drug p.i.), 1 h before virus inoculation, and during infection (drug pre- and p.i.), or left untreated (control). Virus yield in supernatant was determined at 24 h p.i. by plaque assay (n = 3 or 4). (C) Amount of cell-associated viral RNA in cell cultures infected with WNV (MOI of 1 PFU/cell) and treated with PF-06409577 (50 μg/ml) from 1 h p.i. Viral RNA was determined by quantitative RT-PCR at 24 h p.i. (n = 5). (D) Accumulation of dsRNA intermediates in WNV-infected cells. Vero cells were infected with WNV (MOI of 1 PFU/cell) and treated with PF-06409577 (50 μg/ml). Cells were fixed and processed for immunofluorescence using a monoclonal antibody to detect dsRNA at 24 h p.i. Uninfected cells are shown as a control of the specificity of the antibody against dsRNA. Nuclei are shown in blue. Bar, 10 μm. (E) Quantification of dsRNA fluorescence in WNV-infected cells treated or not (control) with PF-06409577 as described in panel D (n = 28 to 32). AU, arbitrary units. (F) Reduction of WNV infection in BHK-21 cells treated with PF-06409577. Cells were infected with WNV (MOI of 1 PFU/cell), and drug was added 1 h after virus inoculation and maintained during the assay. Virus yield in supernatant was determined by plaque assay at 24 h p.i. (n = 3). (G) Evaluation of the cytotoxicity of PF-06409577 on BHK-21 cells by determination of ATP content at 24 h posttreatment (n = 3 or 4). Data are expressed as mean ± SD. Points represent independent biological replicates or individual cells analyzed. Two-tailed Student's t test P values between each condition and control were Bonferroni-corrected in the case of multiple comparisons shown in panels A, B, F, and G. Welch's correction was applied in the case of different variances in panel C. *, P < 0.05; **, P < 0.01; and ***, P < 0.005.
Efficacy, safety, and resistance profile of PF-06409577.
Thinking of the potential for clinical application of AMPK activators like PF-06409577 as antiviral agents, the balance between efficacy and safety of the drug was evaluated. The antiviral potency against WNV, ZIKV, and DENV-2 was estimated by calculating the half-maximal effective concentration (EC50) (Fig. 5A and Table 1). EC50s were within the μM range for all the flaviviruses assayed, being this value the lowest for ZIKV. The cytotoxicity was explored by calculating the half-maximal cytotoxic concentration (CC50) (Fig. 5B and Table 1). Using these values, the selectivity index (SI) was calculated for WNV, ZIKV, and DENV-2 (Table 1). This index determines the relative effectiveness of the drug in inhibiting viral replication compared to inducing cytotoxicity (39). SIs of PF-06409577 evidenced a good relationship between efficacy and safety of the drug, especially for ZIKV. The selection of resistant mutants is one of the most important causes for the failure of antiviral therapies. To evaluate this possibility, WNV and ZIKV were subjected to 10 serial passages in the presence of the compound and their sensitivity to the drug was analyzed. No increase in the resistance to the treatment was observed in viral populations passaged in the presence of the drug (data not shown). Overall, these results support a good balance between antiviral activity and safety that, together with the lack of selection of resistant variants, make PF-06409577 an interesting lead compound for the development of host-directed antivirals against flaviviruses.
FIG 5.
Antiviral activity of PF-06409577 against WNV, ZIKV, and DENV-2. (A) Dose-response curves for Vero cells infected with WNV, ZIKV, and DENV-2 (MOI of 1 PFU/cell) and treated with increasing amounts of PF-06409577. Virus yield in supernatant was determined at 24 h p.i. by plaque assay (n = 4). (B) Vero cells were treated with increasing amounts of PF-06409577, and the cellular ATP content was determined as an estimation of cell viability at 24 or 48 h posttreatment (n = 4). Dashed lines denote a 50% reduction. Data are expressed as mean ± SD.
TABLE 1.
Selectivity indexes of PF-06409577 against WNV, ZIKV, and DENV-2
| Variable | Virus | PF-06409577 values after treatment for: |
|
|---|---|---|---|
| 24 h | 48 h | ||
| CC50a (μg/ml [μM]) | 315.7 (923.7) | 201.4 (586.4) | |
| EC50b (μg/ml [μM]) | WNV | 8.2 (24.1) | |
| ZIKV | 0.9 (2.6) | ||
| DENV-2 | 4.6 (13.4) | ||
| SI (CC50/EC50) | WNV | 38.5 | |
| ZIKV | 350.8 | ||
| DENV-2 | 43.4 | ||
The half-maximal cytotoxic concentration (CC50) is the concentration that results in the reduction of 50% of the amount of cellular ATP after 24 or 48 h of treatment of uninfected cells.
The half-maximal effective concentration (EC50) is the concentration of drug at which virus yield (MOI of 1 PFU/cell) is inhibited by 50%. Virus yield was determined by plaque assay 24 h p.i. for WNV and ZIKV and 48 h p.i. for DENV-2.
DISCUSSION
The current therapeutic results obtained with a variety of antivirals justifies the search for novel compounds to treat viral threats for which there are still no licensed therapies (40). One of the approaches that is a growing force for antimicrobial discovery is the identification of host-directed antivirals (10). The advantages of this approach include the potential identification of broad-spectrum compounds and that these therapies are less prone to therapy resistance than antivirals that directly target pathogens. These advantages make this kind of approach very suitable for discovery of antivirals against flaviviruses. In fact, the genus Flavivirus encompasses a high number of closely related viral species (1). However, most of these are still neglected pathogens, which has reduced the efforts to develop specialized therapies and makes desirable the identification of broad-spectrum compounds (8). Following this line of thought, in a search for potential host-directed antivirals, the effect of direct activation of AMPK by PF-06409577 was explored in this study. The crystal structure of PF-06409577 bound to AMPK, revealing that activation of AMPK by this compound is mediated by its binding at the interface of α and β subunits in a pocket termed the allosteric drug and metabolite site (28).
Treatment with PF-06409577 induced AMPK activation and resulted in inhibitory phosphorylation of the ACC, one of the main regulators of lipid synthesis involved in flavivirus replication (21). Moreover, lipidomics analysis evidenced that AMPK activation altered host cell lipid metabolism, and specifically reduced the amounts of several sphingolipids and glycerophospholipids important for flavivirus multiplication (13, 16, 17, 33). Remarkably, PF-06409577 reduced WNV, ZIKV, and DENV-2 multiplication at noncytotoxic concentrations, supporting the importance of AMPK regulation in flavivirus-infected cells. This reduction in virus infection, analyzed using WNV as a model, was not related to virucidal effect of the compound and was most probably associated with an impairment of proper replication complex development due to an imbalance of specific cellular lipids required for this purpose. This mechanism of action has been also proposed for other inhibitors of flavivirus multiplication that target lipid metabolism (11, 21, 41, 42).
As commented in the introduction, in the case of the flaviviruses DENV and ZIKV, the involvement of AMPK during infection has recently been analyzed by other authors (22–24). Using other AMPK activators that are different from PF-06409577, two previous studies independently found that activation of AMPK decreased ZIKV and DENV infection (22, 23). This is consistent with our results, described here, using the direct AMPK activator PF-06409577. In addition to these studies, the inhibition of AMPK activation using compound C has been also reported to reduce DENV infection (24). These results may suggest that flavivirus replication depends on a finely tuned regulation of AMPK function, and that either sustained activation or inhibition of this enzyme provokes a dysregulation in lipid metabolism that impairs proper flavivirus replication.
The antiviral potential of pharmacological activation of AMPK against DENV and ZIKV was previously explored using indirect activators of AMPK, such as metformin and 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR or acadesine) (22, 23). Metformin indirectly activates AMPK by increasing the levels of intracellular AMP (43), and AICAR is intracellularly converted to an AMP mimetic (44). However, both drugs also exert AMPK-independent effects, which complicate the interpretation of these results (44, 45). In this work, we have evaluated for the first time, the antiviral potential of a selective AMPK activator (PF-06409577) against flavivirus infection. This drug exhibits minimal off-target pharmacology (29) and has been even advanced to first-in-human trials for the treatment of diabetic nephropathy (28). Thus, the preclinical characteristics of PF-06409577 make this drug and related compounds good candidates for repurposing AMPK activators as host-targeting antivirals against flaviviruses. In this context, the antiviral activity of PF-06409577 was observed within the low μM range, and the low cytotoxicity and SI values fell inside commonly accepted medicinal chemistry criteria for antiviral design. Specifically, the EC50s for ZIKV were close to drug levels measured in the plasma of animals treated with an oral dose of the compound (28). In addition, the lack of resistance observed upon serial passage of WNV and ZIKV in the presence of the drug also sustains the repositioning of this compound for antiviral therapies. Regarding the safety concerns that should arise upon in vivo administration of AMPK activators for the treatment of flaviviral infections, the clinical success of other AMPK activators (i.e., metformin and aspirin) for human pathologies supports the feasible utilization in vivo of this class of compounds (46). Our results suggest that selective activators of AMPK, like PF-06409577, may exhibit better characteristics for potential antiviral therapies than do indirect activators (metformin and AICAR) because PF-06409577 exhibits antiviral activity within the μM range, whereas metformin and AICAR only reduce flavivirus multiplication in the mM range (22, 23).
In summary, our results support the feasibility of repurposing the selective activator of AMPK PF-06409577 and structurally related compounds as starting points for the development of feasible host-directed anti-flaviviral therapeutics.
MATERIALS AND METHODS
Cells, viruses, infections, and virus titrations.
Infectious virus manipulations were performed in biosafety level 3 (BSL-3) facilities. Vero cells (catalog no. CCL-81; ATTC, Manassas, VA) and BHK-21 cells (catalog no. CCL-10; ATCC) were cultured as described previously (42, 47). WNV strain NY99 (34, 48), ZIKV strain PA259459 (49), and DENV-2 (36) were used. Unless otherwise specified, for infections in liquid medium, the viruses were incubated with cell monolayers for 1 h at 37°C and the inoculum was removed and replaced by fresh medium containing the drugs or by drug vehicle, supplemented with 1% fetal bovine serum. Virus titration was performed by standard plaque assay in semisolid agarose medium (50). The multiplicity of infection (MOI) was expressed as PFU/cell and is indicated in the figure legends.
Drug treatments.
PF-06409577 (catalog no. PZ0319) was from Sigma (St. Louis, MO). EGCG (catalog no. 0981 S) was from Extrasynthese (Genay, France). Drugs were dissolved in dimethyl sulfoxide (DMSO), and control cells were treated in parallel with the same amount of drug vehicle. As mentioned above and unless otherwise specified, drugs were added to infected monolayers 1 h after virus inoculation. Cell viability was estimated in uninfected cells by ATP measurement using the Cell Titer Glo luminescent cell viability assay (catalog no. G7579) from Promega (Madison, WI).
Immunofluorescence and confocal microscopy.
Mouse monoclonal anti-dsRNA (J2; catalog no. 10010200) was from English and Scientific Consulting (Szirák, Hungary). Rabbit monoclonal antibodies against phospho-AMPKα (Thr172) (40H9; catalog no. 2535), total AMPKα (D5A2; catalog no. 5831), phospho-ACC (Ser79) (D7D11; catalog no. 11818) or total ACC (C83B10; catalog no. 3676) were form Cell Signaling Technology (Danvers, MA). Alexa Fluor 488 goat anti-mouse IgG (catalog no. A11001), Alexa Fluor 488 goat anti-rabbit IgG (catalog no. A11008), and TO-PRO-3 (catalog no. T3605) were from Invitrogen Molecular Probes (Eugene, OR). Immunofluorescence and confocal microscopy were performed as described previously (20). Quantification of fluorescence was performed using MBF ImageJ (51).
Lipidomics.
Vero cells treated with PF-06409577 (50 μg/ml), or drug vehicle (DMSO), were harvested after 24 h of treatment and subjected to lipid extractions (106 cells/determination). Lipid extractions, identification, and quantification by mass spectrometry were performed as described previously (17, 52, 53). Fold change in lipid levels between control and treated cells was calculated as log2(PF-06409577/control). Sphingolipids (Cer, ceramide; SM, sphingomyelin), glycerophospholipids (PC, phosphatidylcholine; PE, phosphatidylethanolamine; and PS, phosphatidylserine), diacylglycerol (DAG), and triacylglycerol (TAG) were annotated using “C followed by the total fatty acyl chain length:total number of unsaturated bonds” and the “lipid subclass” (e.g., C32:2 PC). If the sphingoid base residue was dihydrosphingosine, the name contained a “DH” prefix. For monohexosylceramides (glucosylceramides) a “Gluc” prefix was added to indicate the presence of the sugar moiety. Plasmalogens and lysophospholipids were annotated as described above, except that a “p” or “L” prefix was included, respectively.
Quantitative PCR.
RNA was extracted from infected cell monolayers using TRIzol reagent (Life Technologies, Carlsbad, CA). The amount of viral RNA, calculated as genomic equivalents to PFU by comparison with previously titrated samples, was determined by real-time fluorogenic reverse transcriptase PCR (RT-PCR) (21).
Data analysis.
Statistical analyses were performed using GraphPad Prism 7 for Windows (GraphPad Software Inc., San Diego, CA). For multiple comparisons, analysis of variance (ANOVA) and a t test applying Bonferroni's correction was used. For single comparisons, an unpaired t test was used, and Welch's correction was applied in the case of differences in the variance between groups. Lipidomic data were analyzed using multiple t tests and Sidak-Bonferroni correction for multiple comparisons. The number of biological replicates or individual cells analyzed in each experiment is denoted by n in the figure legends. Unless otherwise specified, data are presented as the mean ± standard deviation (SD). Statistically significant differences are indicated in the figures as follows: *, P < 0.05; **, P < 0.01, ***, P < 0.001.
ACKNOWLEDGMENTS
We thank M. Calvo and A. García for technical assistance.
This work was supported by grant AGL2014-56518-JIN from MINECO to M.A.M.-A and grants RTA2015-00009 from INIA and S2013/ABI-2906 (PLATESA) from the Comunidad Autónoma de Madrid to J.-C.S. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Simmonds P, Becher P, Bukh J, Gould EA, Meyers G, Monath T, Muerhoff S, Pletnev A, Rico-Hesse R, Smith DB, Stapleton JT, Ictv Report C . 2017. ICTV virus taxonomy profile: Flaviviridae. J Gen Virol 98:2–3. doi: 10.1099/jgv.0.000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Webster DP, Klenerman P, Dusheiko GM. 2015. Hepatitis C. Lancet 385:1124–1135. doi: 10.1016/S0140-6736(14)62401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin-Acebes MA, Saiz JC. 2012. West Nile virus: a re-emerging pathogen revisited. World J Virol 1:51–70. doi: 10.5501/wjv.v1.i2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burakoff A, Lehman J, Fischer M, Staples JE, Lindsey NP. 2018. West Nile virus and other nationally notifiable arboviral diseases—United States, 2016. MMWR Morb Mortal Wkly Rep 67:13–17. doi: 10.15585/mmwr.mm6701a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saiz JC, Martin-Acebes MA, Bueno-Mari R, Salomon OD, Villamil-Jimenez LC, Heukelbach J, Alencar CH, Armstrong PK, Ortiga-Carvalho TM, Mendez-Otero R, Rosado-de-Castro PH, Pimentel-Coelho PM. 2017. Zika virus: what have we learnt since the start of the recent epidemic? Front Microbiol 8:1554. doi: 10.3389/fmicb.2017.01554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guzman MG, Harris E. 2015. Dengue. Lancet 385:453–465. doi: 10.1016/S0140-6736(14)60572-9. [DOI] [PubMed] [Google Scholar]
- 7.Krishnan MN, Garcia-Blanco MA. 2014. Targeting host factors to treat West Nile and dengue viral infections. Viruses 6:683–708. doi: 10.3390/v6020683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boldescu V, Behnam MAM, Vasilakis N, Klein CD. 2017. Broad-spectrum agents for flaviviral infections: dengue, Zika and beyond. Nat Rev Drug Discov 16:565–586. doi: 10.1038/nrd.2017.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saiz JC, Martin-Acebes MA. 2017. The race to find antivirals for Zika virus. Antimicrob Agents Chemother 61:e00411-17. doi: 10.1128/AAC.00411-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufmann SHE, Dorhoi A, Hotchkiss RS, Bartenschlager R. 2018. Host-directed therapies for bacterial and viral infections. Nat Rev Drug Discov 17:35–56. doi: 10.1038/nrd.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heaton NS, Perera R, Berger KL, Khadka S, Lacount DJ, Kuhn RJ, Randall G. 2010. Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis. Proc Natl Acad Sci U S A 107:17345–17350. doi: 10.1073/pnas.1010811107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackenzie JM, Khromykh AA, Parton RG. 2007. Cholesterol manipulation by West Nile virus perturbs the cellular immune response. Cell Host Microbe 2:229–239. doi: 10.1016/j.chom.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Tani H, Shiokawa M, Kaname Y, Kambara H, Mori Y, Abe T, Moriishi K, Matsuura Y. 2010. Involvement of ceramide in the propagation of Japanese encephalitis virus. J Virol 84:2798–2807. doi: 10.1128/JVI.02499-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taniguchi M, Tasaki T, Ninomiya H, Ueda Y, Kuremoto KI, Mitsutake S, Igarashi Y, Okazaki T, Takegami T. 2016. Sphingomyelin generated by sphingomyelin synthase 1 is involved in attachment and infection with Japanese encephalitis virus. Sci Rep 6:37829. doi: 10.1038/srep37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin-Acebes MA, Gabande-Rodriguez E, Garcia-Cabrero AM, Sanchez MP, Ledesma MD, Sobrino F, Saiz JC. 2016. Host sphingomyelin increases West Nile virus infection in vivo. J Lipid Res 57:422–432. doi: 10.1194/jlr.M064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aktepe TE, Pham H, Mackenzie JM. 2015. Differential utilisation of ceramide during replication of the flaviviruses West Nile and dengue virus. Virology 484:241–250. doi: 10.1016/j.virol.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Martin-Acebes MA, Merino-Ramos T, Blazquez AB, Casas J, Escribano-Romero E, Sobrino F, Saiz JC. 2014. The composition of West Nile virus lipid envelope unveils a role of sphingolipid metabolism in flavivirus biogenesis. J Virol 88:12041–12054. doi: 10.1128/JVI.02061-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin-Acebes MA, Vazquez-Calvo A, Saiz JC. 2016. Lipids and flaviviruses, present and future perspectives for the control of dengue, Zika, and West Nile viruses. Prog Lipid Res 64:123–137. doi: 10.1016/j.plipres.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Villareal VA, Rodgers MA, Costello DA, Yang PL. 2015. Targeting host lipid synthesis and metabolism to inhibit dengue and hepatitis C viruses. Antiviral Res 124:110–121. doi: 10.1016/j.antiviral.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin-Acebes MA, Blazquez AB, Jimenez de Oya N, Escribano-Romero E, Saiz JC. 2011. West Nile virus replication requires fatty acid synthesis but is independent on phosphatidylinositol-4-phosphate lipids. PLoS One 6:e24970. doi: 10.1371/journal.pone.0024970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merino-Ramos T, Vazquez-Calvo A, Casas J, Sobrino F, Saiz JC, Martin-Acebes MA. 2015. Modification of the host cell lipid metabolism induced by hypolipidemic drugs targeting the acetyl coenzyme A carboxylase impairs West Nile virus replication. Antimicrob Agents Chemother 60:307–315. doi: 10.1128/AAC.01578-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng F, Ramos da Silva S, Huang IC, Jung JU, Gao SJ. 2017. Suppression of Zika virus infection and replication in endothelial cells and astrocytes by PKA inhibitor PKI 14-22. J Virol 92:e02019-. doi: 10.1128/JVI.02019-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soto-Acosta R, Bautista-Carbajal P, Cervantes-Salazar M, Angel-Ambrocio AH, Del Angel RM. 2017. DENV up-regulates the HMG-CoA reductase activity through the impairment of AMPK phosphorylation: a potential antiviral target. PLoS Pathog 13:e1006257. doi: 10.1371/journal.ppat.1006257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jordan TX, Randall G. 2017. Dengue virus activates the AMP kinase-mTOR axis to stimulate a proviral lipophagy. J Virol 91:e02020-. doi: 10.1128/JVI.02020-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herzig S, Shaw RJ. 2018. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol 19:121–135. doi: 10.1038/nrn.2018.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinberg GR, Kemp BE. 2009. AMPK in health and disease. Physiol Rev 89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 27.Day EA, Ford RJ, Steinberg GR. 2018. AMPK as a therapeutic target for treating metabolic diseases. Trends Endocrinol Metab 28:545–560. doi: 10.1016/j.tem.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Cameron KO, Kung DW, Kalgutkar AS, Kurumbail RG, Miller R, Salatto CT, Ward J, Withka JM, Bhattacharya SK, Boehm M, Borzilleri KA, Brown JA, Calabrese M, Caspers NL, Cokorinos E, Conn EL, Dowling MS, Edmonds DJ, Eng H, Fernando DP, Frisbie R, Hepworth D, Landro J, Mao Y, Rajamohan F, Reyes AR, Rose CR, Ryder T, Shavnya A, Smith AC, Tu M, Wolford AC, Xiao J. 2016. Discovery and preclinical characterization of 6-chloro-5-[4-(1-hydroxycyclobutyl)phenyl]-1H-indole-3-carboxylic acid (PF-06409577), a direct activator of adenosine monophosphate-activated protein kinase (AMPK), for the potential treatment of diabetic nephropathy. J Med Chem 59:8068–8081. doi: 10.1021/acs.jmedchem.6b00866. [DOI] [PubMed] [Google Scholar]
- 29.Salatto CT, Miller RA, Cameron KO, Cokorinos E, Reyes A, Ward J, Calabrese MF, Kurumbail RG, Rajamohan F, Kalgutkar AS, Tess DA, Shavnya A, Genung NE, Edmonds DJ, Jatkar A, Maciejewski BS, Amaro M, Gandhok H, Monetti M, Cialdea K, Bollinger E, Kreeger JM, Coskran TM, Opsahl AC, Boucher GG, Birnbaum MJ, DaSilva-Jardine P, Rolph T. 2018. Selective activation of AMPK β1-containing isoforms improves kidney function in a rat model of diabetic nephropathy. J Pharmacol Exp Ther 361:303–311. doi: 10.1124/jpet.116.237925. [DOI] [PubMed] [Google Scholar]
- 30.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. 1996. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem 271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 31.Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D. 2006. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem 281:32207–32216. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- 32.Ha J, Daniel S, Broyles SS, Kim KH. 1994. Critical phosphorylation sites for acetyl-CoA carboxylase activity. J Biol Chem 269:22162–22168. [PubMed] [Google Scholar]
- 33.Perera R, Riley C, Isaac G, Hopf-Jannasch AS, Moore RJ, Weitz KW, Pasa-Tolic L, Metz TO, Adamec J, Kuhn RJ. 2012. Dengue virus infection perturbs lipid homeostasis in infected mosquito cells. PLoS Pathog 8:e1002584. doi: 10.1371/journal.ppat.1002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe KE, Crabtree MB, Scherret JH, Hall RA, MacKenzie JS, Cropp CB, Panigrahy B, Ostlund E, Schmitt B, Malkinson M, Banet C, Weissman J, Komar N, Savage HM, Stone W, McNamara T, Gubler DJ. 1999. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 35.Gatherer D, Kohl A. 2016. Zika virus: a previously slow pandemic spreads rapidly through the Americas. J Gen Virol 97:269–273. doi: 10.1099/jgv.0.000381. [DOI] [PubMed] [Google Scholar]
- 36.Vazquez-Calvo A, Jimenez de Oya N, Martin-Acebes MA, Garcia-Moruno E, Saiz JC. 2017. Antiviral properties of the natural polyphenols delphinidin and epigallocatechin gallate against the flaviviruses West Nile virus, Zika virus, and dengue virus. Front Microbiol 8:1314. doi: 10.3389/fmicb.2017.01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carneiro BM, Batista MN, Braga ACS, Nogueira ML, Rahal P. 2016. The green tea molecule EGCG inhibits Zika virus entry. Virology 496:215–218. doi: 10.1016/j.virol.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Welsch S, Miller S, Romero-Brey I, Merz A, Bleck CK, Walther P, Fuller SD, Antony C, Krijnse-Locker J, Bartenschlager R. 2009. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe 5:365–375. doi: 10.1016/j.chom.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller PY, Milton MN. 2012. The determination and interpretation of the therapeutic index in drug development. Nat Rev Drug Discov 11:751–761. doi: 10.1038/nrd3801. [DOI] [PubMed] [Google Scholar]
- 40.De Clercq E, Li G. 2016. Approved antiviral drugs over the past 50 years. Clin Microbiol Rev 29:695–747. doi: 10.1128/CMR.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koutsoudakis G, Romero-Brey I, Berger C, Perez-Vilaro G, Perin PM, Vondran FW, Kalesse M, Harmrolfs K, Muller R, Martinez JP, Pietschmann T, Bartenschlager R, Bronstrup M, Meyerhans A, Diez J. 2015. Soraphen A: a broad-spectrum antiviral natural product with potent anti-hepatitis C virus activity. J Hepatol 63:813–821. doi: 10.1016/j.jhep.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Merino-Ramos T, Jimenez de Oya N, Saiz JC, Martin-Acebes MA. 2017. Antiviral activity of nordihydroguaiaretic acid and its derivative tetra-O-methyl nordihydroguaiaretic acid against West Nile virus and Zika virus. Antimicrob Agents Chemother 61:e00376-. doi: 10.1128/AAC.00376-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, Towler MC, Brown LJ, Ogunbayo OA, Evans AM, Hardie DG. 2010. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab 11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guigas B, Sakamoto K, Taleux N, Reyna SM, Musi N, Viollet B, Hue L. 2009. Beyond AICA riboside: in search of new specific AMP-activated protein kinase activators. IUBMB Life 61:18–26. doi: 10.1002/iub.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rena G, Hardie DG, Pearson ER. 2017. The mechanisms of action of metformin. Diabetologia 60:1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hardie DG. 2018. Keeping the home fires burning: AMP-activated protein kinase. J R Soc Interface 15:20170774. doi: 10.1098/rsif.2017.0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.González-Magaldi M, Martin-Acebes MA, Kremer L, Sobrino F. 2014. Membrane topology and cellular dynamics of foot-and-mouth disease virus 3A protein. PLoS One 9:e106685. doi: 10.1371/journal.pone.0106685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merino-Ramos T, Blazquez AB, Escribano-Romero E, Canas-Arranz R, Sobrino F, Saiz JC, Martin-Acebes MA. 2014. Protection of a single dose West Nile virus recombinant subviral particle vaccine against lineage 1 or 2 strains and analysis of the cross-reactivity with Usutu virus. PLoS One 9:e108056. doi: 10.1371/journal.pone.0108056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vazquez-Calvo A, Blazquez AB, Escribano-Romero E, Merino-Ramos T, Saiz JC, Martin-Acebes MA, Jimenez de Oya N. 2017. Zika virus infection confers protection against West Nile virus challenge in mice. Emerg Microbes Infect 6:e81 https://www.nature.com/articles/emi201768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin-Acebes MA, Saiz JC. 2011. A West Nile virus mutant with increased resistance to acid-induced inactivation. J Gen Virol 92:831–840. doi: 10.1099/vir.0.027185-0. [DOI] [PubMed] [Google Scholar]
- 51.Collins TJ. 2007. ImageJ for microscopy. Biotechniques 43(Suppl 1):25–30. [DOI] [PubMed] [Google Scholar]
- 52.Garanto A, Mandal NA, Egido-Gabas M, Marfany G, Fabrias G, Anderson RE, Casas J, Gonzalez-Duarte R. 2013. Specific sphingolipid content decrease in Cerkl knockdown mouse retinas. Exp Eye Res 110:96–106. doi: 10.1016/j.exer.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gorrochategui E, Casas J, Perez-Albaladejo E, Jauregui O, Porte C, Lacorte S. 2014. Characterization of complex lipid mixtures in contaminant exposed JEG-3 cells using liquid chromatography and high-resolution mass spectrometry. Environ Sci Pollut Res Int 21:11907–11916. doi: 10.1007/s11356-014-3172-5. [DOI] [PubMed] [Google Scholar]