ABSTRACT
Cutaneous and superficial fungal infections affecting the skin, nails, and hair of humans are caused primarily by dermatophytes of the genera Trichophyton and Epidermophyton or by yeasts of the genera Candida and Malassezia. Onychomycosis is a common fungal infection of the nail that frequently coexists with tinea pedis, the most prevalent mycotic skin infection. Efficacy rates for current topical onychomycosis therapies are hampered by low drug penetration across the nail plate, which is theoretically obviated with nitric oxide (NO)-based topical therapies. The Nitricil technology platform is comprised of polysiloxane-based macromolecules that stably release therapeutic levels of NO. In the reported studies, NVN1000, the lead candidate of the platform, was assessed for its spectrum of in vitro activity against a broad range of filamentous fungi and yeast species commonly associated with cutaneous fungal infections. Time-kill assays demonstrated that NVN1000 exhibited fungicidal activity as early as 4 h. Additionally, the penetration of several unique NVN1000 NO-releasing drug product formulations (gel, cream, and lacquer) was evaluated following a single topical application in an in vitro infected human nail assay, with all formulations showing similar inhibition of fungal growth. Repeated topical application in this model demonstrated that a lower-strength dose of NO could achieve the same efficacy as a higher-strength dose after 7 days. Together, these in vitro results demonstrate that NO-releasing treatments rapidly penetrate the nail plate and eradicate the fungal infection, representing promising novel topical therapies for the treatment of onychomycosis and other cutaneous fungal infections.
KEYWORDS: nitric oxide, Nitricil, NVN1000, onychomycosis, tinea pedis, dermatophyte, SB208
INTRODUCTION
Superficial and cutaneous fungal infections are caused by pathogenic fungi that are limited to the hair, nails, and epidermis. Most prevalent are dermatophytosis, candidiasis, and pityriasis versicolor (1). Dermatophytosis is classified by the involved body area (tinea capitis, tinea corporis, or tinea pedis). Candidiasis is caused by Candida fungi (most frequently Candida albicans), with skin infections posing a formidable threat to immunocompromised hosts (2). Pityriasis versicolor results from mycelial growth of the Malassezia fungal species, most often Malassezia furfur. These superficial infections are rarely life-threatening; however, they occur frequently and with significant morbidity (1).
Onychomycosis, a chronic superficial mycosis of the nail bed or plate, accounts for one-third of cutaneous fungal infections and is frequently associated with clinical presentations of tinea pedis (3, 4). The dermatophytes Trichophyton rubrum and Trichophyton mentagrophytes are responsible for 80 to 90% of onychomycosis cases (5). Onychomycosis relapse or reinfection occurs frequently, with reported rates as high as 57% (6). Recent studies suggest that the nail plate, interdigital space, and surrounding cutaneous tissue may serve as an overlooked reservoir of dermatophytes (7). Reinfection via this reservoir may thus require nail-directed therapy and simultaneous treatment of the entire forefoot for the effective treatment of tinea and infection of the surrounding cutaneous tissue leading to chronic onychomycosis (7).
The use of topical antifungal agents for the treatment of onychomycosis has been limited by poor nail penetration and failure to achieve therapeutic drug levels. Systemic therapies have been associated with hepatotoxic adverse events that necessitate monitoring, and a risk of drug-drug interactions, particularly with itraconazole (8). Additionally, resistance to the current armamentarium of antifungal drugs is rising (9). Recent clinical studies have demonstrated enhanced complete clearance rates of onychomycosis when tinea pedis and onychomycosis are treated concurrently with different medications (10). Currently, there is no approved single topical therapeutic agent that provides for the simultaneous treatment of the nail plate, nail bed, and surrounding cutaneous tissue.
Nitric oxide (NO) is a short-lived hydrophobic and reactive diatomic gas that is produced in the human body by a variety of cell types. It serves a multitude of functions, including vasodilation, neurotransmission, angiogenesis, and modulation of wound healing, and as a major component of innate immunity against a broad range of invading pathogens (11). NO exhibits broad spectrum reactivity with demonstrated antimicrobial efficacy against bacteria, yeast, fungi, and viruses, both in vitro and in vivo (12–18). The oxidative and nitrosative species generated upon NO exposure provide numerous pathways for microbial damage. These species interact with a variety of molecular targets, including iron-sulfur thiols, tyrosine residues, membrane lipids, and DNA bases, providing numerous fungal targets to act upon. The mechanisms through which NO kills pathogens appear to vary depending upon the organism (19, 20). Unlike the single intracellular target of traditional antibiotics, the multiple biochemical targets of NO lead to the general hypothesis that microbes are unlikely to develop resistance to NO (12, 21–23). Its development as a topical therapeutic agent, however, has been hampered by the inability to stably store and controllably release NO.
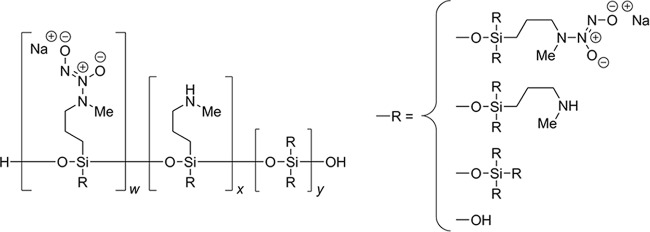
NVN1000 is a co-condensed, diazeniumdiolate-modified polysiloxane molecule, which is designed to stably store therapeutic quantities of NO via the incorporation of a N-diazeniumdiolate NO donor throughout a silica-based polymeric backbone (Fig. 1). The rate of NO release can be modulated as a function of particle size and functional composition, such that timed release of NO can be controlled. The NVN1000 drug substance is stably stored in a gel or ointment formulation, with the release of NO from the macromolecule occurring through proton-initiated hydrolysis upon admixture with a hydrogel. The combined chemical properties of the two components of the drug product admixture formulation confer additional tunability to the NO release kinetics. For the potential topical treatment of cutaneous fungal infections, the NVN1000 drug substance can be prepared in a variety of topical formulations (gels, creams, or lacquers). In this study, the broad-spectrum in vitro antifungal activity of NVN1000 was evaluated against a panel of fungal and yeast species commonly associated with superficial and cutaneous infections, utilizing growth inhibition and time-kill assays. Several NVN1000-containing topical formulations were then assessed for penetration of the nail plate and subsequent fungicidal efficacy, using the ChubTur infected human nail model, at concentrations that proved to be safe and well tolerated following 28 days of repeated dermal exposure in miniature swine.
FIG 1.
Chemical structure of NVN1000, a polysiloxane nitric oxide-releasing macromolecule.
RESULTS
The broad-spectrum antifungal activity of NVN1000 was assessed by evaluating the MIC against 40 different fungal strains in a modified macrodilution broth test method. The MIC values for NVN1000 are summarized in Table 1 and ranged from 0.5 mg/ml to 8.0 mg/ml, equating to 75.5 to 1,208 μg NO/ml and demonstrating a 16-fold range for all fungal species tested. The ketoconazole comparator MIC values for all fungal species had a >500-fold range under the same assay conditions (<0.063 to >32.0 μg/ml). There was no correlation between the susceptibility profiles of NVN1000 and ketoconazole.
TABLE 1.
Antifungal activity of NVN1000 and ketoconazole against a panel of fungal isolates
| Fungal species and strain | NVN1000 MIC (μg NO/ml) | Ketoconazole MIC (μg/ml) |
|---|---|---|
| Trichophyton rubrum | ||
| NCPF 0113 | 75.5 | >32.0 |
| NCPF 0118 | 302 | <0.063 |
| NCPF 0295 | 604 | >32.0 |
| NCPF 5025 | 302 | >32.0 |
| ATCC MYA-4438 | 302 | >32.0 |
| ATCC 10218 | 75.5 | <0.063 |
| ATCC 22402 | 151 | <0.063 |
| ATCC 28188 | 604 | 0.125 |
| ATCC 44697 | 302 | >32.0 |
| Trichophyton mentagrophytes | ||
| NCPF 0224 | 604 | 0.25 |
| NCPF 5024 | 1,208 | 0.5 |
| ATCC 9533 | 151 | 1.0 |
| ATCC 18749 | 75.5 | 0.125 |
| ATCC 28939 | 75.5 | 1.0 |
| Epidermophyton floccosum | ||
| NCPF 5011 | 1,208 | >32.0 |
| NCPF 5012 | 302 | >32.0 |
| ATCC 38826 | 151 | 4.0 |
| ATCC 44685 | 604 | 0.125 |
| ATCC 52061 | 604 | 1.0 |
| ATCC 52063 | 302 | >32.0 |
| ATCC 52066 | 604 | >32.0 |
| Fusarium species | ||
| Fusarium keratoplasticum ATCC 36031 | 604 | >32.0 |
| Fusarium oxysporum ATCC 62705 | 302 | >32.0 |
| Fusarium solani ATCC 56480 | 604 | >32.0 |
| Malassezia furfur | ||
| NCPF 3349 | 75.5 | >32.0 |
| NCPF 8211 | 151 | 16.0 |
| ATCC 14521 | 151 | >32.0 |
| Candida albicans | ||
| ATCC 10231 | 151 | <0.063 |
| ATCC 58716 | 151 | 16.0 |
| ATCC 96901 | 151 | >32.0 |
| BLSI 112613Ca1 | 151 | >32.0 |
| BLSI 112613Ca2 | 151 | >32.0 |
| Candida species | ||
| Candida lusitaniae ATCC 200950 | 151 | <0.063 |
| Candida tropicalis NCPF 8760 | 1,208 | 32.0 |
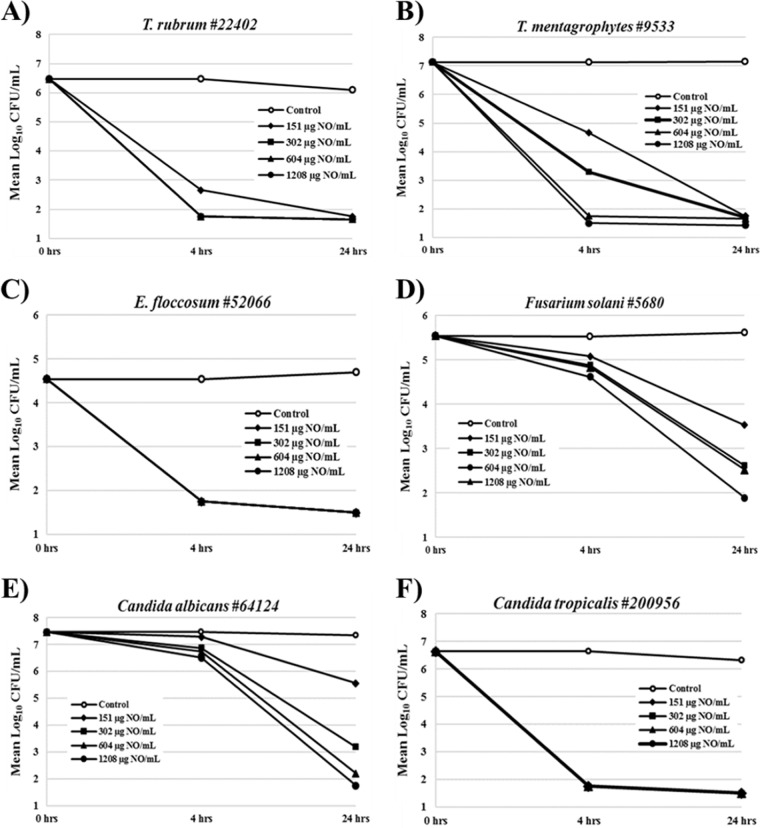
The fungicidal activity of NVN1000 against a panel of fungal species was examined, utilizing the time-kill assay to assess the rate and extent of fungal reduction following exposure to NVN1000 under static growth conditions. The time-kill curves following 24 h of exposure to NVN1000 are shown in Fig. 2. The dermatophytes T. mentagrophytes, Epidermophyton floccosum, and T. rubrum were especially susceptible to NVN1000, exhibiting a 99.9% fungal reduction following challenge with 2 mg/ml of NVN1000 (302 μg NO/ml) in as little as 4 h (Table 2). Following 24 h of exposure, fungicidal activity (99.9% fungal reduction) was observed for all tested species, apart from Fusarium solani, challenged with 302 μg NO/ml, revealing the lingering antimicrobial effect of nitrosative species despite the short half-life of NO.
FIG 2.
Time-kill curves for representative fungal isolates following exposures to NVN1000 from 4 to 24 h. Reported values are the means of two independent assays and are reported as the mean log CFU/ml at the designated time point. Open circles, untreated control; closed symbols, various concentrations of NVN1000.
TABLE 2.
Percent fungal kill achieved for representative fungal isolates following 4 h of exposure to NVN1000
| Species | NVN1000 exposure at: |
|||
|---|---|---|---|---|
| 151 μg NO/ml (%) | 302 μg NO/ml (%) | 604 μg NO/ml (%) | 1,208 μg NO/ml (%) | |
| T. rubrum | 99.97 | 99.99 | 99.99 | 99.99 |
| T. mentagrophytes | 99.63 | 99.98 | 99.999 | 99.999 |
| E. floccosum | 99.70 | 99.70 | 99.70 | 99.70 |
| Fusarium solani | 64.21 | 77.35 | 87.83 | 78.30 |
| C. albicans | 31.49 | 73.96 | 81.29 | 87.94 |
| C. tropicalis | 99.99 | 99.99 | 99.99 | 99.99 |
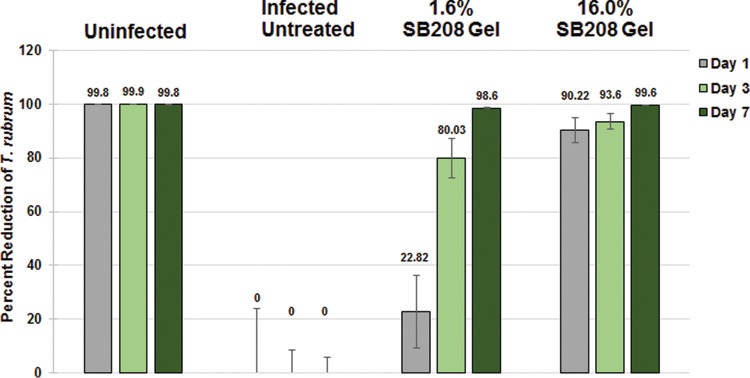
The ability of various NVN1000-containing topical formulations to effectively penetrate the nail and exhibit antifungal activity was evaluated in vitro, utilizing the infected human nail assay (ChubTur test system) developed by MedPharm Ltd., as this assay most accurately mimics the conditions of onychomycosis (24). As a surrogate for fungal viability, ATP levels were assessed following topical application of candidate test formulations, as ATP levels are inversely proportional to the percent fungal kill. In the T. rubrum-infected nail assay (ChubTur test system) the various formulations of NVN1000 (gel, cream, or lacquer) and the positive control, efinaconazole, all showed a high level of fungicidal efficacy after a single 24-h application, ranging from a mean percent fungal kill of 82% with efinaconazole to 99% with 16% NVN1000 lacquer (Table 3). The percentage of ATP recovery from each formulation was statistically reduced compared to that of the infected control (P < 0.05). However, comparison of fungicidal efficacy between active treatment groups failed to show statistical significance differences in this single-dose study, likely due to the small number of nails (n = 6) used per group. Based on the results of the in vitro infected human nail assay, the optimized SB208 gel formulation was further characterized, utilizing the ChubTur cells to determine the efficacy of low- (1.6%) and high (16%)-strength formulations against T. rubrum after repeat daily dosing for up to 7 days. Figure 3 presents the percent fungal kill observed when T. rubrum-infected nails were treated with a low (1.6% NVN1000) and high (16% NVN1000) daily dose of an optimized SB208 gel formulation for up to 7 days. The mean percentages of ATP recovered from the T. rubrum-infected nails compared to that of the infected control after a single dose and 24-h exposure were 77.15% for the low- (1.6%) and 9.78% for the high (16%)-strength SB208 gel formulation, equating to a 23% and 90% inhibition of fungal growth, respectively. There was a statistically significant (P ≤ 0.05) decrease in ATP recovery observed following a single topical application with both strengths of SB208 gel formulation compared to ATP recovery from untreated infected nails. Despite the potent antifungal activity observed with high-strength (16%) SB208 gel on day 1, quantifiable ATP recovery was still higher than that of the day 1 noninfected control, indicating that complete fungal killing had not yet been achieved. Following 3 days of daily treatment, fungal growth inhibition following topical application of low- (1.6%) and high (16%)-strength SB208 gels was 80% and 93.6%, respectively (Fig. 3). However, only the ATP recovery from the high-strength (16.0%) SB208 gel group was statistically indistinguishable (P > 0.05) from that of the day 3 noninfected control group, indicating that complete fungal killing had been achieved. After 7 days of daily dosing, both the low- (1.6%) and the high (16.0%)-strength SB208 gels achieved complete fungal killing, as determined by ATP recovery values that were equivalent to those of the day 7 noninfected controls (P > 0.05) (Fig. 2).
TABLE 3.
Percent kill of T. rubrum in the ChubTur infected nail investigation following a single topical application of various NVN1000-containing topical formulations
| Test formulation | Mean percent kill of T. rubrum vs infected control ± SEMa |
|---|---|
| 16% NVN1000 gel | 88.67 ± 4.72 |
| 16% NVN1000 cream | 91.62 ± 2.09 |
| 16% NVN1000 lacquer | 99.02 ± 0.30 |
| Jublia (10% efinaconazole solution) | 82.49 ± 6.71 |
Infected control (n = 6).
FIG 3.
Mean percent fungal reduction following repeated topical application of SB208 gel in the ChubTur infected nail assay.
Results from the in vivo mammalian toxicology study demonstrated that twice-daily dermal administration of SB208 gel for 28 consecutive days was well tolerated. There were no macroscopic test article-related findings, other than a transient erythema at the dose application site, which was attributable to the vasodilation of the cutaneous microvasculature following NO exposure. There were no SB208 gel-related effects on food consumption, body weight, hematology, clinical chemistry and coagulation parameters, ophthalmologic and electrocardiographic measurements, and organ weight. Histologically, the dose site skin tissues of 8% and 16% SB208 gel twice-daily dose group animals displayed nonadverse, minimal to mild alterations, including hyper/parakeratosis, epidermal hyperplasia, epidermal crusts, dermal edema, and mononuclear cell infiltration in the dermis. Further evidence of cutaneous nitric oxide bioavailability was assessed through the quantification of neovascularization in the dermis. CD31 quantification of blood vessels from immune-stained sections showed increased dermal blood vessel counts in dose site skin sections compared to their respective control skin sections. At the end of the 28-day treatment period with 16% SB208 gel, female and male animals showed a mean of 33% and 66% increase in vessels counted per objective field compared to those of vehicle-treated control animals. Reversibility for all findings was noted in the recovery phase. Under the conditions of this study, the no observed adverse effect level (NOAEL) for SB208 gel was considered to be equal to or greater than the highest dose used, 16% SB208 gel twice daily (BID) (96 mg/kg/day NVN1000), for which the local application site NVN1000 dose was 3.4 mg/cm2/day.
Discussion/conclusion.
MIC assay results indicated broad-spectrum NVN1000 and ketoconazole antifungal activity against a wide panel of yeast and filamentous fungi. However, all NVN1000 MIC values listed in Table 1 fell within a 16-fold range, demonstrating a narrower susceptibility profile than that of ketoconazole (>500-fold) over a diverse panel of fungi that are responsible for a large percentage of cutaneous infections in humans. This narrow susceptibility range of fungal species to NVN1000 may provide a clinical advantage, particularly when associated with mixed fungal infections.
There are multiple reasons why the millimolar concentration range of nitric oxide (75.5 to 1,208 μg NO/ml) from NVN1000 needed to inhibit fungal growth was higher than the micromolar concentration range (0.06 to 32 μg/ml) of ketoconazole. First, NVN1000 is a polymeric macromolecule, with only approximately 15% of its overall weight being NO. Second, unlike ketoconazole, NO is a highly reactive gaseous species that exhibits a short half-life, measured in seconds, before it decomposes to inactive metabolites (e.g., nitrite and nitrate) in the physiological milieu, with most of the nitric oxide being released from the macromolecule in a relatively short period of time (>90% within 40 min). Without sustained pressure of the direct antifungal agent, doses of nitric oxide that do not exhibit a strong bactericidal effect cannot effectively inhibit the propagation of fungal species in optimal growth conditions.
As both tinea pedis and onychomycosis have high rates of recurrence, the fungicidal activity represented by time-kill studies of a novel potential therapy provides perhaps even more meaningful information about the potential for treatment success than the growth inhibitory concentration determined by MIC assay. Therefore, the fungicidal efficacy of NVN1000 was evaluated against six representative dermatophytes and yeasts via time-kill studies, using a similar concentration range to that employed in the MIC experiments. As shown in Fig. 2 and Table 2, despite the short half-life of NO, three of the six representative dermatophyte and yeast species were effectively killed (>4-log reduction in CFU/ml) by NVN1000 concentrations of ≤604 μg NO/ml at the earliest 4-h time point, whereas the other three species showed complete killing by 24 h of exposure at NVN1000 concentrations of ≤1,208 μg NO/ml. These results demonstrate the rapid and broad fungicidal potential of NVN1000.
Successful treatment of onychomycosis with a topical agent is fraught with difficulty due to several factors, including the location of the fungal infection within the nail bed, the unique physical and chemical properties of the nail, adequate nail penetration, and the coexistence of tinea pedis, which aids in recurrence of fungal infection (7, 25). Among these factors, difficulty in finding compounds with the pharmacologic/pharmacokinetic profile to allow for adequate nail penetration to achieve potentially therapeutic levels after topical administration is the most challenging. The newest topical antifungal treatments, efinaconazole 10% solution and tavaborole 5% solution, specifically developed for the treatment of dermatophyte onychomycosis, still only achieved complete cure rates of approximately 17% and 9%, respectively, in pivotal phase 3 clinical trials (26, 27).
The results of the MIC and time-kill studies with NVN1000 demonstrated that T. rubrum and T. mentagrophytes, the primary pathogens associated with both onychomycosis and tinea pedis, were particularly susceptible to NO exposure. The limitations of a gaseous antifungal agent in the in vitro setting become advantages when translating the findings toward clinical application for onychomycosis. Most topical antifungal drugs that successfully treat cutaneous fungal infections do not effectively penetrate the nail plate and bed, thus limiting the ability to concurrently treat tinea pedis and onychomycosis with a single topical antifungal agent (25). Given the rapid fungicidal activity of NVN1000 observed in time-kill assays against dermatophyte species, further effectiveness of rapidly penetrating nitric oxide-releasing drug product candidates in an infected nail assay (ChubTur test system) that accurately mimics the conditions of onychomycosis was explored (24). This assay has been previously utilized to compare the effectiveness of Penlac, Loceryl, and a terbinafine spray formulation (24). In this assay, fungicidal efficacy could only result from penetration of the applied topical agents through the nail to the resident fungal infection established beneath the nail.
The results of the preliminary in vitro study assessing penetration of various NVN1000-containing topical formulations indicated that all of the tested topical NO-releasing formulations were equally effective at reducing T. rubrum viability after a single application, with no statistically significant differences observed in fungicidal efficacy between the gel, cream, or lacquer topical formulations. The encouraging results from preliminary experiments with different NVN1000 topical formulations prompted additional investigation of dose dependency in achieving fungicidal efficacy. SB208 gel was selected for further characterization, based on its physiochemical properties, which allowed for concurrent application to cutaneous soft tissues, as well as to the nail plate, and the ability of this formulation to be used for the potential treatment of large surface areas. SB208 gel was developed using findings from the preliminary infected nail assay to maximize NO release, improve cosmetic elegance, and reduce potential cutaneous intolerability. In the dose-response study using the ChubTur model, both the high- (16%) and low (1.6%)-strength SB208 gel formulations exhibited fungicidal activity as early as 24 h following the first topical application, with complete eradication of the T. rubrum infection by 3 and 7 days, respectively. These findings indicate that repeated topical applications, as would be expected to occur clinically, of lower-strength doses may achieve comparable antifungal efficacy to higher doses following multiple applications of SB208 gel.
As to the safety of SB208 gel, dermal application of supratherapeutic doses of SB208 gel were evaluated in a repeat-dose, dermal good laboratory practices (GLP) toxicology study in miniature swine. The miniature swine model has been well accepted for cutaneous absorption and toxicity studies, due to swine integument being morphologically and functionally similar to human skin (28). Due to these similarities, this model has become the animal model of choice for drug development programs aiming to assess dermal absorption, local tolerance, and systemic toxicity following dermal exposures. Following macroscopic and microscopic evaluation of the skin and systemic organs, SB208 was found to be nonadverse with the no observed adverse effect level (NOAEL) being the highest dose used (96 mg/kg/day NVN1000). The dermal exposure of NVN1000 at the local application site was 3.4 mg/cm2/day, which was approximately 7-fold higher than the highest well tolerated clinical dose of SB208 gel (0.49 mg/cm2/day; up to 2.7 mg/kg/day NVN1000) used in a phase 2a study in subjects with tinea pedis (ClinicalTrials.gov registration no. NCT02860052). Collectively, these results suggest that the unique mechanism of action and molecular characteristics of a topical NO-releasing gel, coupled with a good tolerability profile, may produce high rates of fungicidal efficacy against a wide range of fungal species. Furthermore, the ability of NO and its associated gaseous species to rapidly migrate through the nail plate and through microbial lipid membranes supports the conclusion that SB208 gel may potentially be an effective topical treatment for cutaneous fungal infections, such as onychomycosis. The potential to simultaneously treat both the nail plate and the surrounding cutaneous tissues with a single topical formulation could improve the clinical outcome compared to the standard of care for both tinea pedis and onychomycosis, which is particularly important, as the prevalence of onychomycosis is increasing in elderly and diabetic populations where more advanced disease occurs, with associated morbidities such as wounds, cellulitis, and compromised ambulation (29).
MATERIALS AND METHODS
Synthesis of NVN1000 nitric oxide-releasing macromolecules.
NVN1000 is a copolymer derived from aminoalkoxysilanes and alkylalkoxysilanes terminated with silanonals. NVN1000 is formed from the cocondensation of N-methylaminopropyltrimethoxysilane (MAP3), tetraethoxysilane (TEOS), and N,N-methylaminopropyldiazeniumdiolate-trimethoxysilane sodium salt (MAP3-NONOate) via a sol-gel process, as described previously (30), to create a network of siloxane bonds with organic functionality throughout (Fig. 1). This process yields 5 μmol NO/mg of NVN1000 drug substance, as determined via assessment of NO content under acidic conditions (pH 3) with a Sievers nitric oxide analyzer (Boulder, CO). Consistent with previously reported release rates for MAP3/TEOS NO-releasing macromolecules, more than 90% of the nitric oxide is released after 40 min (31). Following jet milling, the median particle size distribution (D50) value was 5.04 μm, as determined by a Mastersizer 2000 instrument (Malvern).
NVN1000 drug product formulations.
NVN1000 can be formulated for topical delivery in a variety of drug product compositions. As the release of nitric oxide from the silica-based backbone is proton initiated, exposure to moisture directly affects drug stability. To ensure stability, NVN1000 is formulated in anhydrous compositions. To determine the most efficacious topical formulation for the delivery of nitric oxide to the nail bed and cutaneous tissues, NVN1000 was formulated into a topical gel, ointment, or lacquer. Prior to topical application, the stable, active phase was mixed in a 1:1 ratio with a hydrogel phase providing the proton source necessary for initiation of nitric oxide release from the drug product. The NVN1000 gel is an alcohol-based gel consisting of anhydrous ethanol, hexylene glycol, cyclomethicone, hydroxypropyl cellulose, Transcutol, Elastomer 10, and titanium dioxide. The NVN1000 cream results from the admixture of an NVN1000 petrolatum-based ointment with a benzoic acid-preserved potassium phosphate-based aqueous hydrogel phase. The NVN1000 lacquer formulation results from the admixture of the ethylcellulose polymer-based NVN1000 lacquer and a benzoic acid-preserved potassium phosphate-based aqueous hydrogel phase. SB208 gel is the drug product admixture formulation that results from the 1:1 mixing of the NVN1000 in a silicon-based gel with a carbomer homopolymer hydrogel (pH 4).
MIC determinations.
The MIC of NVN1000 was determined in vitro for 40 strains of fungi to determine the spectrum of antifungal activity. Challenge fungal species included both clinical isolates, as well as strains obtained from the American Type Culture Collection and National Collection of Pathogenic Fungi, representing T. rubrum, T. mentagrophytes, and Epidermophyton floccosum, as well as Fusarium, Candida, and Malassezia species.
Except for the Malassezia species, which used modified Dixon's agar (MDA), all strains were grown on Emmon's Sabouraud agar (ESDA), malt extract agar (MEA), or potato dextrose agar (PDA). All MIC testing was performed using modifications of the broth macrodilution method outlined in the Clinical and Laboratory Standards Institute (CLSI) guidelines Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts (M27-A2) and Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi (M38-A2) (32, 33). Challenge suspensions of each species were created to have a final population of approximately 103. The challenge strains were exposed to a series of doubling dilutions of NVN1000 ranging from 16 mg/ml to 0.032 mg/ml, prepared in RPMI 1640 medium in triplicate. Malassezia species were prepared in modified RPMI (dehydrated RPMI with extra dextrose, ox bile, polysorbate 60, glycerin, and glyceryl monooleate) for susceptibility testing. Fungal species were also challenged with ketoconazole, prepared in dimethyl sulfoxide (DMSO), as a comparator. In the United States, ketoconazole is one of the most widely used antifungal agents for cutaneous fungal infections, with approximately 7.4 million prescriptions written in 2016 (34). Tubes were inoculated with fungal suspensions to a final concentration of 0.5 to 2.5 × 103 cells/ml for yeasts and 0.4 to 5.0 × 104 CFU/ml for filamentous fungi. The MIC was determined following a 1- to 4-day incubation period. The MIC was determined visually on the basis of turbidity and recorded as the lowest concentration (highest dilution) that completely inhibited growth of the organism, as detected by the unaided eye. Additionally, when an MIC could not be visually determined, a minimum fungicidal concentration (MFC) test was performed for 14 of the 40 strains by transferring a 0.1-ml aliquot from each of the three likely MIC endpoints into a neutralization solution.
Time-kill evaluations.
Time-kill evaluations were determined following ASTM method E2783-11, Standard Test Method for Assessment of Antimicrobial Activity for Water Miscible Compounds Using a Time-Kill Procedure (35). Challenge fungi and yeast species included strains obtained from the American Type Culture Collection, representing, with strain and corresponding growth media indicated in parentheses, T. rubrum (ATCC 22402, MEA/MEA+), T. mentagrophytes (ATCC 9533, ESDA/ESDA+), and Epidermophyton floccosum (ATCC 52066, ESDA/ESDA+), as well as Fusarium solani (ATCC 56480, PDA/PDA+), Candida albicans (ATCC 64124, ESDA/ESDA+), and Candida tropicalis (ATCC 200956, ESDA/ESDA+). Prior to initiation of testing, inoculum from a lyophilized vial of each microorganism was grown on the appropriate agar and incubated at the appropriate temperature until sufficient growth was observed. For the fungi, conidia were used to prepare challenge suspensions. Briefly, a 0.1 ml aliquot of the fungal challenge was prepared in 0.9% sodium chloride irrigation solution with 0.05% Triton X-100 by suspending conidia from the solid media to achieve a concentration of 1 × 109 CFU/ml and was added at the time of testing to test tubes containing the appropriate concentration of NVN1000 test article dissolved in 10 ml of Tris (pH 7.5 to 7. 7) and vortexed. On the day of testing, a suspension of each yeast was prepared from solid media in 0.9% sodium chloride irrigation solution to a final concentration of 1 × 109 CFU/ml and added to the NVN1000 test article suspensions. The challenge suspensions of fungi and yeast were exposed to each test article concentration for 4 h ± 5 min and 24 h ± 5 min at 25°C ± 2°C. After exposure, a 0.1-ml aliquot of the challenge suspension/test article was transferred to a new test tube containing 9.9 ml of neutralizing agent, Butterfield's phosphate buffer solution with product neutralizers (BBP++) and mixed thoroughly via vortex. Additionally, 10-fold dilutions were prepared in BBP++ as described. Duplicate aliquots (10−1 to 10−5) were plated on the appropriate agar, and plates were incubated at 25°C ± 2°C until sufficient growth was observed, at which time viable counts were determined manually using a hand-tally counter. Counts in the range of 20 to 200 CFU/ml for the fungi or 30 to 300 CFU/ml for yeast were used in the data calculations. The fungicidal effects (≥3-log reduction in the original inoculum) were determined from the control population (CFU/ml) and the postexposure population (CFU/ml) at each timed exposure.
In vitro antifungal activity under the human nail plate.
The growth inhibition of T. rubrum under the human nail plate was determined as previously described (24). Distal nail clippings obtained from healthy human volunteers were individually infected on the underside of the nail with 5 μl of the T. rubrum suspension. The suspension was allowed to dry on the underside of the nail for 30 min in a laminar flow cabinet and subsequently was mounted onto the nail gasket within the ChubTur cell. The receiver chamber was then filled with an inert humidity control fluid (sterile Ringer's solution) and incubated at 25°C for 14 days to establish the fungal nail infection. Following fungal incubation, the cells were dosed with 50 μl of the test article admixture formulation, which was applied to the surface of the nail opposite to the T. rubrum inoculation. In repeat dose experiments, after 24 h of topical treatment, the nails were cleaned of any residual formulation with a dry cotton swab and gently rinsed with deionized water. The nails were air-dried for 30 min to remove any moisture from the nail prior to the application of the next topical dose. After the completion of dosing, the ChubTur cells were removed from incubation and dismantled. The nails were completely cleaned of all residual test article, as described above. Once clean, the nails were subsequently placed in individual wells of a 96-well plate containing the ATP standard (55.0 ng/ml) and analyzed for the presence of ATP from the viable fungi via an ATP luminescence assay (BacTiter-Glo), as previously described (24). ATP calibration standards of known concentrations were prepared by sequential dilution of a stock ATP standard (1 mg/ml) in Ringer's solution. The wells of the 96-well plate were analyzed for the presence of ATP and its recovery was compared to those of ATP standards and untreated infected positive controls. The mean percent ATP recovery from each test article formulation was compared to that of the infected control, and statistical analyses were performed using a one-way analysis of variance (ANOVA) with a post hoc Tukey's test, using a 95% confidence interval. This model was utilized in a preliminary experiment to assess the nail penetration and subsequent fungicidal activity of NVN1000 when formulated into a variety of topical drug products formulations (gel, cream, or lacquer). In the preliminary experiment, the fungicidal efficacy of each NVN1000-containing drug product formulation was assessed following a single topical application, as this was hypothesized to allow the greatest differentiation among the given drug product formulations. A 10% efinaconazole solution was included as the positive control in this single-application experiment. A second experiment was conducted with the infected nail model to assess the time to complete fungal eradication following repeat dosing with various strengths of SB208 gel. In this experiment, 1.6% and 16.0% SB208 gels were applied topically to infected nail mounted in ChubTur cells, and fungal viability was assessed via ATP luminescence as described, following 1, 3, or 7 days of daily topical application and compared to the ATP levels of noninfected and infected control nails incubated for the same durations.
In vivo mammalian toxicology.
A GLP repeat-dose dermal toxicology study was conducted to evaluate the dermal toxicity of SB208 gel, following 28 consecutive days of twice-daily (BID) topical administration to the backs of Hanford miniature swine, followed by a 14-day treatment-free observation period. The study was conducted by Sinclair Research Center, LLC (Auxvasse, MO) in compliance with U.S. Food and Drug Administration Good Laboratory Practice for Nonclinical Laboratory Studies, 21 CFR, Part 58. A total of 36 animals (18 males and 18 females) was divided into three treatment groups, including a vehicle gel control and two different dose levels of SB208 gel, 8% or 16% (24 or 48 mg/kg NVN1000) as a BID dose regimen (48 or 96 mg/kg/day NVN1000), with 6 animals/sex assigned to each group (4 main animals plus an additional 2 animals per sex as recovery animals). A constant dose amount of 0.3 g/kg/dose was employed for all groups to cover approximately 10% of the total body surface area (BSA). Measurements of toxicological effect included clinical observations, physical examination findings, food consumption, weekly body weight or weekly body weight change, and clinical pathology parameters, including hematology, clinical chemistry and coagulation parameters, ophthalmologic and electrocardiographic examinations, and organ weight.
ACKNOWLEDGMENTS
N.S. is an employee and stockholder of Novan, Inc.; S.J.H., K.M., M.M., and R.D. are former employees of Novan, Inc.
REFERENCES
- 1.Kelly BP. 2012. Superficial fungal infections. Pediatr Rev 33(4):e22–e37. doi: 10.1542/pir.33-4-e22. [DOI] [PubMed] [Google Scholar]
- 2.Netea MG, Brown GD, Kullberg BJ, Gow NA. 2008. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol 6:67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- 3.Scher RK. 1999. Onychomycosis: therapeutic update. J Am Acad Dermatol. 40(6 Pt 2):S21–S26. doi: 10.1016/S0190-9622(99)70397-X. [DOI] [PubMed] [Google Scholar]
- 4.Gupta AK, Scher RK. 1998. Oral antifungal agents of onychomycosis. Lancet 351:541–542. doi: 10.1016/S0140-6736(05)78552-4. [DOI] [PubMed] [Google Scholar]
- 5.Foster KW, Ghannoum MA, Elewski BE. 2004. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J Am Acad Dermatol 50:748–752. doi: 10.1016/S0190-9622(03)02117-0. [DOI] [PubMed] [Google Scholar]
- 6.Gupta AK, Cooper EA, Pacquet M. 2013. Recurrences of dermatophyte toenail onychomycosis during long-term follow-up after successful treatments with mono- and combined therapy of terbinafine and itraconazole. J Cutan Med Surg 17:201–206. doi: 10.2310/7750.2013.12088. [DOI] [PubMed] [Google Scholar]
- 7.Lipner SR, Scher RK. 2015. Management of onychomycosis and co-existing tinea pedis. J Drugs Dermatol 14:492–494. [PubMed] [Google Scholar]
- 8.Del Rosso JQ. 2014. The role of topical antifungal therapy for onychomycosis and the emergence of newer agents. J Clin Aesthet Dermatol 7(7):10–18. [PMC free article] [PubMed] [Google Scholar]
- 9.Ghannoum MA, Rice LB. 1999. Antifungal agents: modes of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev 12:501–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markinson B, Caldwell B. 2015. Efinaconazole topical solution, 10% efficacy in patients with onychomycosis and coexisting tinea pedis. J Am Podiatr Med Assoc 105:407–411. doi: 10.7547/14-088. [DOI] [PubMed] [Google Scholar]
- 11.Pacher P, Beckman JS, Liaudet L. 2007. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87:315–324. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller C, McMullin B, Ghaffari A, Stenzler A, Pick N, Roscoe D, Ghahary A, Road J, Av-Gay Y. 2009. Gaseous nitric oxide bactericidal activity retained during intermittent high-dose short duration exposure. Nitric Oxide 20:16–23. doi: 10.1016/j.niox.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Ahmadi MS, Lee HH, Sanchez DA, Friedman AJ, Tar MT, Davies KP, Nosanchuk JD, Martinez LR. 2016. Sustained nitric oxide-releasing nanoparticles induce cell death in Candida albicans yeast and hyphal cells, preventing biofilm formation in vitro and in a rodent central venous catheter model. Antimicrob Agents Chemother 60:2185–2194. doi: 10.1128/AAC.02659-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weller R, Price RJ, Ormerod AD, Benjamin N, Leifert C. 2001. Antimicrobial effect of acidified nitrite on dermatophyte fungi, Candida, and bacterial skin pathogens. J Appl Microbiol 90:648–652. doi: 10.1046/j.1365-2672.2001.01291.x. [DOI] [PubMed] [Google Scholar]
- 15.Regev-Shoshani G, Crowe A, Miller CC. 2013. A nitric oxide-releasing solution as a potential treatment for fungi associated with tinea pedis. J Appl Microbiol 114:536–544. doi: 10.1111/jam.12047. [DOI] [PubMed] [Google Scholar]
- 16.Ormerod AD, Shah AJ, Li H, Benjamin NB, Ferguson GP, Leifert C. 2011. An observational prospective study of topical acidified nitrite for killing methicillin-resistant Staphylococcus aureus (MRSA) in contaminated wounds. BMC Research Notes 4:458–464. doi: 10.1186/1756-0500-4-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finnen MJ, Hennessy A, McLean S, Bisset Y, Michell R, Megson IL, Weller R. 2007. Application of acidified nitrite to the nail renders it antifungal and causes nitrosation of cysteine groups in the nail plate. Br J Dermatol 157:494–500. doi: 10.1111/j.1365-2133.2007.08063.x. [DOI] [PubMed] [Google Scholar]
- 18.Deppisch C, Herrmann G, Graepler-Mainka U, Wirtz H, Heyder S, Engel C, Marschal M, Miller CC, Riethmuller J. 2016. Gaseous nitric oxide to treat antibiotic resistant bacterial and fungal lung infections in patients with cystic fibrosis: a phase 1 clinical study. Infection 44:513–520. doi: 10.1007/s15010-016-0879-x. [DOI] [PubMed] [Google Scholar]
- 19.Fang FC. 1997. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest 99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang FC. 2004. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol 2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 21.Ghaffari A, Miller CC, McMullin B, Ghahary A. 2006. Potential application of gaseous nitric oxide as a topical antimicrobial agent. Nitric Oxide 14:21–29. doi: 10.1016/j.niox.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Nathan C, Shiloh MU. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci U S A 97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Privett BJ, Broadnax AD, Bauman SJ, Riccio DA, Schoenfisch MH. 2012. Examination of bacterial resistance to exogenous nitric oxide. Nitric Oxide 26:169–173. doi: 10.1016/j.niox.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traynor MJ, Turner RB, Evans CRG, Khengar RH, Jones SA, Brown MB. 2010. Effect of a novel penetration enhancer on the ungula permeation of two antifungal agents. J Pharm Pharmacol 62:730–737. doi: 10.1211/jpp.62.06.0009. [DOI] [PubMed] [Google Scholar]
- 25.Hui X, Baker SJ, Wester RC, Barbadillo S, Cashmore AK, Sanders V, Hold KM, Akama T, Zhang YK, Plattner JJ, Maibach HI. 2007. In vitro penetration of a novel oxaborole antifungal (AN2690) into the human nail plate. J Pharm Sci 96:2622–2631. doi: 10.1002/jps.20901. [DOI] [PubMed] [Google Scholar]
- 26.Gupta AK, Elewski BE, Sugarman IL, Leda C, Kawabata H, Kang R, Pillai R, Olin JT, Watanabe S. 2014. The efficacy and safety of efinaconazole 10% solution for treatment of mild to moderate onychomycosis: a pooled analysis of two phase 3 randomized trials. J Drugs Dermatol 13:815–820. [PubMed] [Google Scholar]
- 27.Elewski BE, Aly R, Baldwin SL, Gonzalez Soto RF, Rich P, Weisfeld M, Wiltz H, Zane LT, Pollack R. 2015. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies. J Am Acad Dermatol 73:62–69. doi: 10.1016/j.jaad.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Krongrad A, Shoemake C, Liu J, Brocksmith D, Bouchard G. 2016. The importance of minipigs in dermal safety assessment: an overview. Cutan Ocul Toxicol 36:105–113. doi: 10.1080/15569527.2016.1178277. [DOI] [PubMed] [Google Scholar]
- 29.Scher RK, Baran R. 2003. Onychomycosis in clinical practice: factors contributing to recurrence. Br J Dermatol 149(Suppl 65):S5–S9. doi: 10.1046/j.1365-2133.149.s65.5.x. [DOI] [PubMed] [Google Scholar]
- 30.Shin JH, Metzger SK, Schoenfisch MH. 2007. Synthesis of nitric oxide-releasing silica nanoparticles. J Am Chem Soc 129:4612–4619. doi: 10.1021/ja0674338. [DOI] [PubMed] [Google Scholar]
- 31.Stevens EV, Wells A, Shin JH, Liu J, Der CJ, Schoenfisch MH. 2010. Nitric oxide-releasing silica nanoparticle inhibition of ovarian cancer cell growth. Molecular pharmaceutics. Molecular Pharmaceuticals 7:775–785. doi: 10.1021/mp9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CLSI. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 2nd edition. CLSI document M27-A2. CLSI, Wayne, PA. [Google Scholar]
- 33.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd edition. CLSI document M38-A2. CLSI, Wayne, PA. [Google Scholar]
- 34.QuintilesIMS. 2017. QuintilesIMS national prescription audit (Jan 2016–Jan 2017). QuintilesIMS, Parsippany, NJ. [Google Scholar]
- 35.ASTM International. 2016. Standard test method for assessment of antimicrobial activity for water miscible compounds using a time-kill procedure. ASTM International document E2783-11. ASTM International, West Conshohocken, PA. [Google Scholar]